Abstract
Six hundred sixty-seven first, second, and third orthotopic liver allografts in 520 patients were reviewed to determine the effect of recipient panel-reactive antibody (PRA) and donor-recipient antibody crossmatch on 2-year patient and liver allograft survival rates. Neither a high panel-reactive antibody nor a positive crossmatch for donor-specific preformed antibody was associated with decreased patient or liver allograft survival for primary grafts or retransplants. Two patients have been given kidney transplants immediately after a liver allograft from a donor with whom each patient had an initial strongly positive donor-specific antibody crossmatch. The liver apparently removed or neutralized circulating anti-donor antibody, since the renal allografts functioned promptly and did not experience hyperacute rejection.
Transplantation of renal allografts in the presence of preformed antibody to human leukocyte (HLA)-antigens on donor lymphocytes is associated with a high incidence of hyperacute allograft rejection.1–4 Furthermore, transplantation of a renal allograft from a crossmatch-negative donor into a recipient with a high percentage of panel-reactive antibody (PRA) at the time of transplantation is associated with decreased graft survival.5
We have previously reported that transplantation of the liver in the presence of preformed antidonor antibody is associated with neither hyperacute rejection of the liver nor with decreased graft survival.6,7 Our last report was based on an analysis of 1-year graft survival rates for 134 recipients of 174 liver transplants. We now have accumulated experience with 520 recipients of 667 grafts. In this article, the relationship between antibody crossmatch and recipient PRA at the time of transplantation and 2-year graft survival rates are examined once more.
MATERIAL AND METHODS
Case material
Six hundred sixty-seven liver grafts in 520 patients performed between March 1, 1980 and Dec. 31, 1985 with cyclosporine-prednisone are included in this study. Three primary grafts done before March 1, 1980 with azathioprine and steroids are not included. One surviving patient has received a fourth transplant that is also not included. Thus this review is based on 517 primary grafts, 123 second grafts, and 27 third grafts.
All patients have been followed through Jan. 31, 1986. Actuarial patient and graft survival were calculated by the life table method.8,9 The age range of the patients was 4 months to 67 years (mean 25.3 ± 18.1, SD years) including 310 adults given 385 grafts and 210 children given 282 grafts.
All patients were treated with cyclosporine-prednisone.10 Since December 1984, OKT3 monoclonal antibody (Ortho Pharmaceuticals, Raritan, N.J.) has been given for brief periods (10 to 21 days) to about 75 patients for treatment of acute cellular rejection or during periods of reduced cyclosporine coverage.11
The most common primary indications for liver replacement are cirrhosis (25.6%) biliary atresia (20.6%), primary biliary cirrhosis (17.2%), inborn errors of metabolism (13.0%), sclerosing cholangitis (8.1%), and primary liver tumors (3.9%).
Donor-recipient matching
Recipients were selected on the basis of medical need, estimated liver size and body weight, and ABO blood group. HLA typing and lymphocytotoxic crossmatching were done retrospectively and played no role in recipient selection. The lymphocytotoxic antibody crossmatch was done by the trypan blue dye exlusion method with recipient serum and unfractionated donor lymphocytes at 37° C. Antibodies in the recipient serum were detected with the same techniques, using a panel of lymphocytes obtained from 60 normal volunteers. PRA was derived from the results. If antibodies were present against 30 of the 60 donors, the PRA was 50%; if the reactivity was against 15 of the 60, the PRA was 25%, etc.
RESULTS
Patient and graft survival
The actuarial survival rate is 63.8% for the 520 patients at 2 years and 48.2% for all 667 grafts at 2 years (Fig. 1, A). The actuarial survival rate of primary grafts versus retransplants is shown in Fig. 1, B. Two hundred ninety-eight (57.6%) primary grafts and 53 (35.3%) retransplants are functioning. The 2-year actuarial survival rate for primary grafts is 52.3% and 34.4% for retransplants. Primary graft survival is significantly better than survival of retransplants (p < 0.001).
Fig. 1.
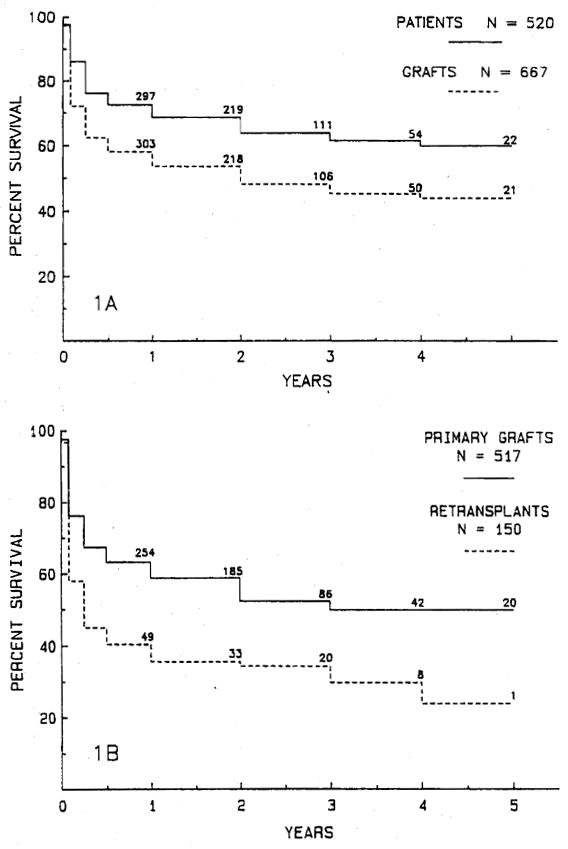
A, The actuarial survival rate for 520 patients and 667 first, second, and third liver allografts. B, Actuarial graft survival rate for 517 primary grafts and 150 retransplants. The primary graft survival rate is significantly better than the survival rate for retransplanted grafts (p < 0.001).
Graft survival and PRA
The actuarial survival rates for 505 grafts (75.7%) for which PRA analysis is available are shown Fig. 2, A and are the same as survival rate for the entire series of 667 transplants. Sixty-seven patients had a PRA of 30% or more at transplantation including 39 patients with a PRA greater than 60%. The 2-year graft survival rate in patients with a PRA under 30% is 48.9%, with a PRA of more than 30% is 51.4%, and with a PRA greater than 60% is 61.5% (Fig. 2, B). These survival rates are not significantly different.
Fig. 2.
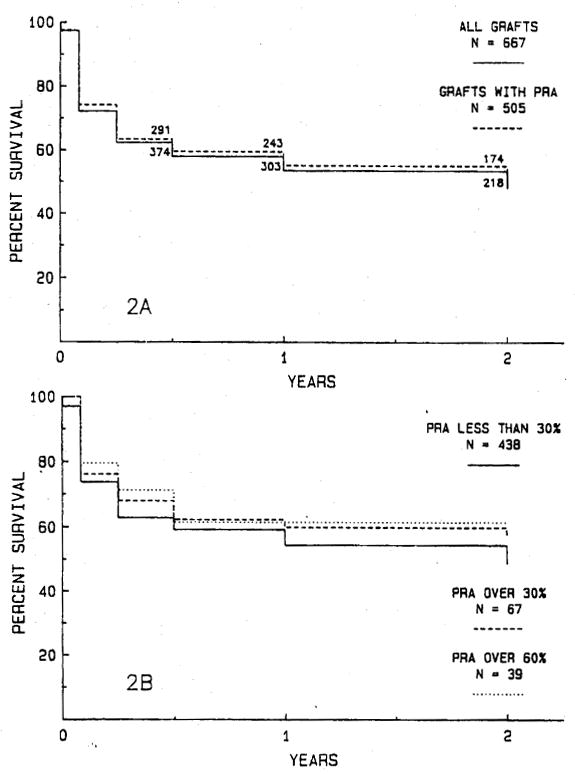
A, The actuarial survival rate for 505 grafts for which PRA data are available is compared with the survival rate for the entire series of 667 transplants. There is no significant difference. B, The actuarial survival rate for 67 grafts in patients with a PRA greater than 30%, including 39 grafts in patients with a PRA greater than 60%, compared with 438 grafts in patients with a PRA less than 30%. There are no significant differences in graft survival rates.
PRA data are available for 417 (80.7%) of the 517 Primary grafts and 88 (58.7%) of the 150 retransplants. Fig. 3, A and C shows no difference in graft survival rates for the primary grafts or retransplants for which PRA data are available and the entire series of 517 primary grafts and 150 retransplants. PRA was greater than 30% for transplantation of 48 primary grafts, including 31 patients with a PRA of more than 60%. PRA was greater than 30% for 19 patients with retransplants, including eight patients with retransplants with PRA greater than 60%. There are no significant differences in graft survival rates for primary grafts (Fig. 3, B) or retransplants (Fig. 3, D) with high or low PRA.
Fig. 3.
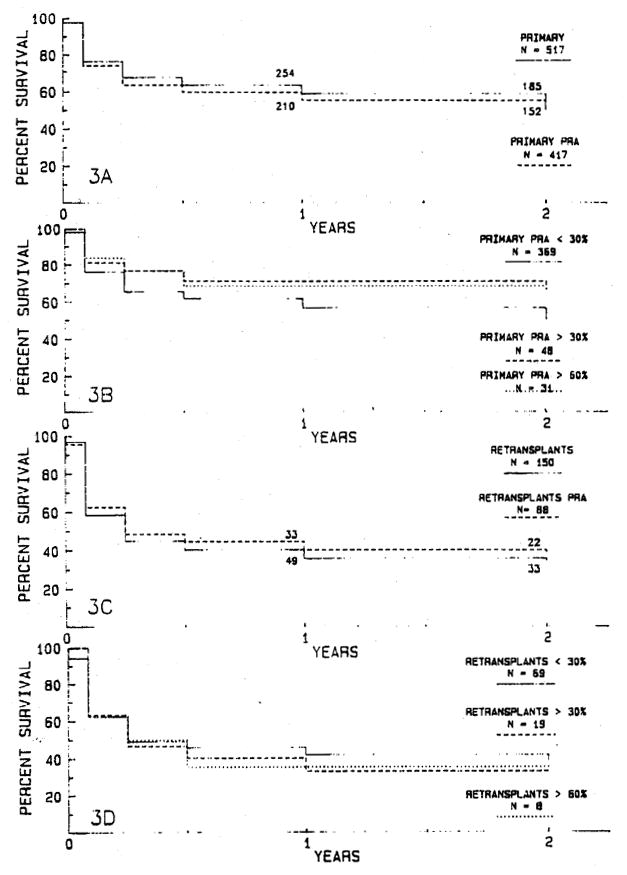
There are no significant differences in actuarial survival rates for 417 primary grafts (A) and 88 retransplants (C) for which PRA data are available compared with all 517 primary grafts (A) and all 150 retransplants (C). There are no significant differences in graft survival rates for 48 primary grafts in patients with a PRA greater than 30% (B), including 31 grafts in patients with a PRA greater than 60%, compared with 369 primary grafts in patients with a PRA under 30% (B) or for survival of 51 retransplants with a PRA greater than 30% (D), including eight retransplants in patients with a PRA greater than 60%, compared with 69 retransplants in patients with a PRA less than 30% (D).
Graft survival and antibody crossmatch
Antibody crossmatch data are available for 433 (64.9%) grafts that show no difference in survival from the complete series of 667 grafts (Fig. 4, A). The 2-year survival rate for 62 grafts transplanted with a positive crossmatch is 55.8% and 49.8% for 371 grafts with a negative crossmatch (Fig. 4, B). There is no statistical difference in graft survival rates for patients with positive and negative crossmatches at transplantation.
Fig. 4.
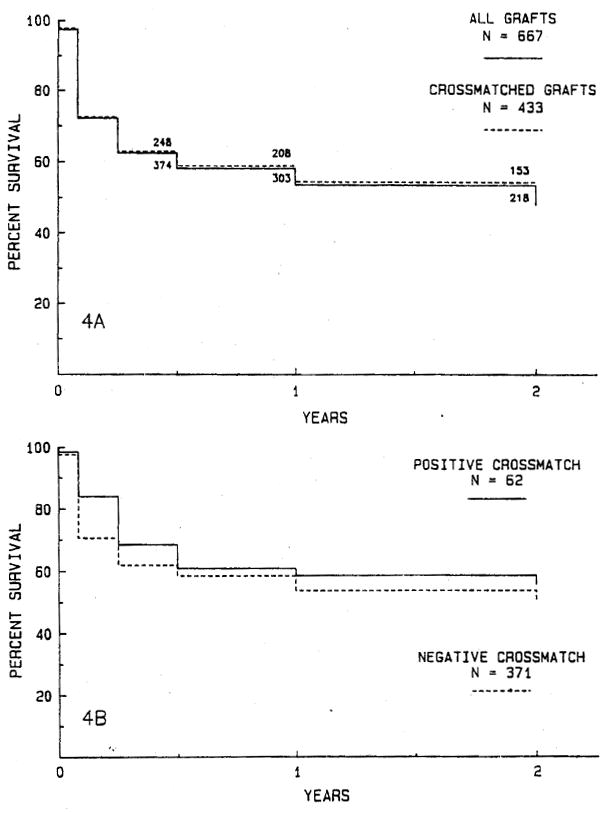
A, The actuarial survival rate for 433 grafts for which antibody crossmatch data are available compared with the survival rate for the entire series of 667 transplants. There is no significant difference. B, Actuarial survival for 62 grafts in patients with a positive crossmatch compared with 371 grafts in patients with a negative crossmatch. There are no significant differences in graft survival rates.
There are no differences in graft survival rates for the 337 (65.2%) primary grafts (Fig. 5, A) or 96 (64.0%) retransplants (Fig. 5, C) for which crossmatches are available and the entire series of 517 primary grafts and 150 retransplants. Antibody crossmatch was positive for 38 (11.3%) of the 337 crossmatched primary grafts and for 24 (25.0%) of the 96 crossmatched retransplants. There is no significant difference in graft survival between positive and negative crossmatched primary grafts (Fig. 5, B) or between positive and negative crossmatched retransplants (Fig. 5, D).
Fig. 5.
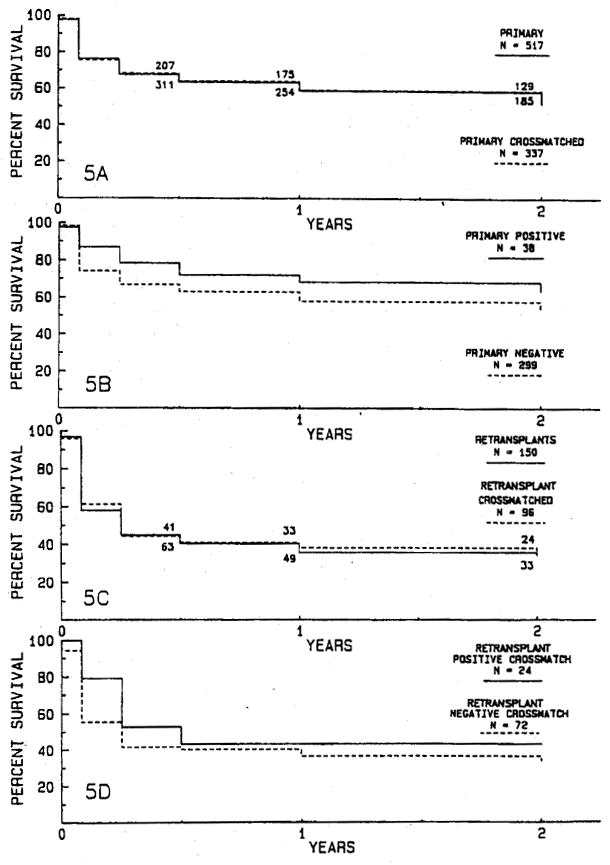
There are no significant differences in actuarial survival rates for 337 primary grafts (A) and 96 retransplants (C) for which crossmatch data are available compared with all 517 primary grafts (A) and all 150 retransplants (C). There are no significant differences in graft survival rates for 38 primary grafts in patients with a positive crossmatch (B) compared with 299 primary grafts in patients with a negative crossmatch (B) or for survival of 24 retransplants with a positive crossmatch (D) compared with 72 retransplants in patients with a negative crossmatch (D).
Among the 667 grafts were 382 (57.3%) grafts for which both antibody crossmatch and PRA data are available. Fig. 6, A shows no significant difference in graft survival for these 382 grafts compared with the entire series of 667 transplants. Twenty-eight transplants were done in recipients with a positive donor antibody crossmatch and a current PRA of more than 30%, including 20 patients with a PRA greater than 60%. There is no difference in graft survival rates for these high antibody, positive crossmatch transplants and 305 grafts done with a negative crossmatch and PRA less than 30% (Fig. 6, B).
Fig. 6.
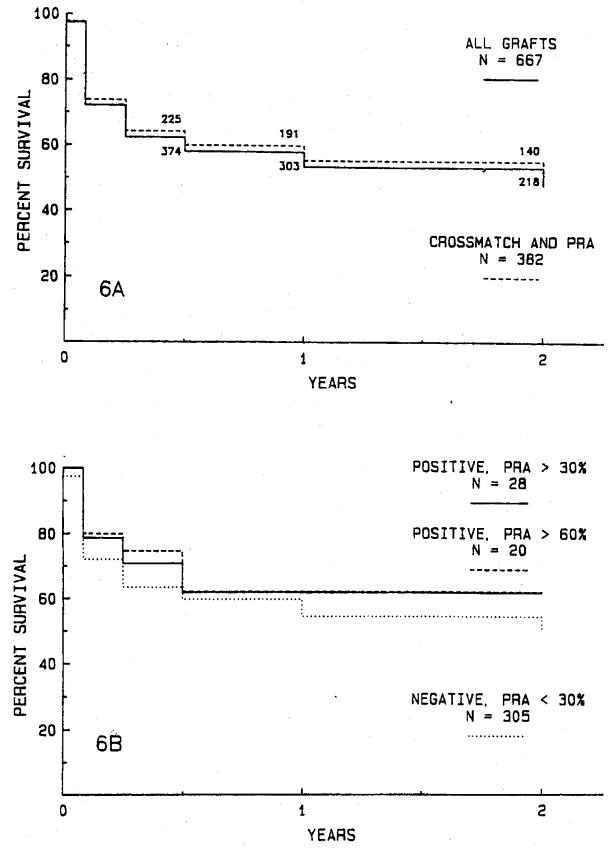
A, The actuarial survival rate for 382 grafts for which both antibody crossmatch and PRA data are available is compared with survival rate for the entire series of 667 transplants. There is no significant difference. B, The actuarial survival rate for 282 grafts in patients with a positive crossmatch and a PRA greater than 30% (including 20 patients with a PRA more than 60%) is compared with survival rate for 305 grafts in patients with a negative crossmatch and PRA less than 30%. There are no significant differences in graft survival rates.
Incidence of rejection and retransplantation
Antibody crossmatches were done in 53 of 78 patients whom a graft was lost and retransplantation was done because of allograft rejection. The incidence of retransplantation for rejection of a graft with a positive crossmatch was seven of 62 grafts (11.3%) and with a negative crossmatch 46 (12.4%) of 371 grafts. There is no significant difference between these rates of retransplantation for rejection of crossmatch-positive and crossmatch-negative grafts.
Renal transplantation after liver transplantation with a positive crossmatch
Five patients with chronic liver and kidney failure have been given a renal allograft immediately after a liver transplant from the same donor. In two cases, the antidonor lymphocyte crossmatch was strongly positive immediately before liver transplantation. However, within a few hours after implantation of the liver, repeat crossmatches could no longer detect significant levels of circulating preformed antidonor antibody. Renal allografts then implanted in these two patients functioned promptly and did not undergo hyperacute rejection. The postoperative course of one of these patients is shown in Fig. 7. All five patients continue to survive with functioning liver and renal allografts 2 weeks to 18 months after transplantation.
Fig. 7.
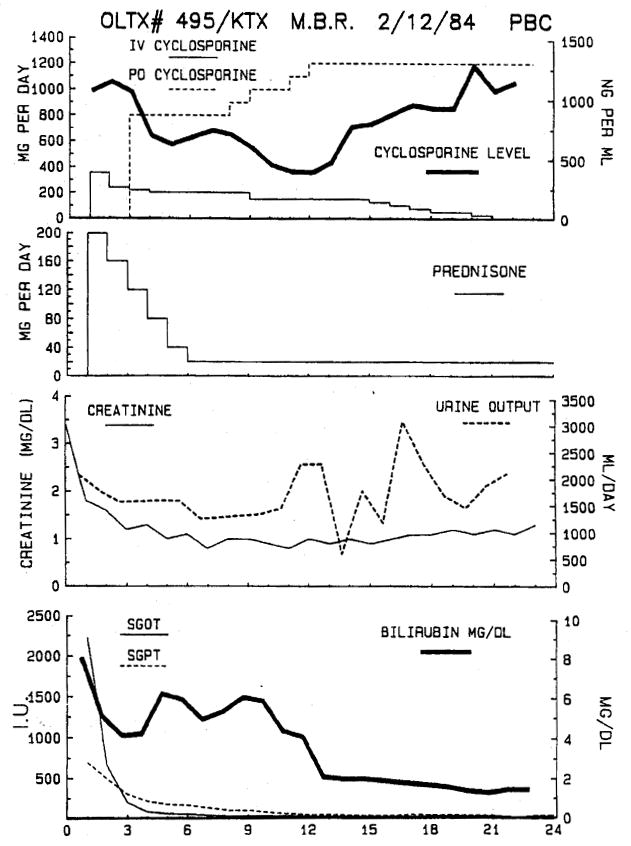
A 43-year-old woman with chronic liver and renal failure from end-stage primary biliary cirrhosis received a liver transplant from a cadaver donor with whom she had a positive antibody crossmatch. A kidney was implanted from the same donor immediately after completion of the liver transplant. The postoperative course, including immunosuppression with cyclosporine and prednisone, renal function, and liver function, is shown. Hyperacute rejection of either graft did not occur and both grafts continue to function well 18 months after transplantation.
DISCUSSION
In a previous report on the results of liver transplantation in 36 patients with a positive cytotoxic crossmatch,7 we used current recipient sera with fractionated donor T-lymphocytes assayed at 37° C. The results in the current series are based on crossmatches done with sera collected just before transplantation and unfractionated donor lymphocytes at 37° C by means of the trypan blue dye exclusion technique. In our laboratory’s experience the results for this method correlate strongly with the assay using fractionated T-lymphocytes at 37° C if kill of cells is complete. Therefore it is reasonable to believe that the positive crossmatches reported here represent mostly preformed antibody to HLA antigens expressed on donor T-lymphocytes.
This study again confirms our previous observation that the liver allograft is not subject to hyperacute rejection in the presence of preformed antidonor antibody and also shows that liver allograft survival as long as 2 years is not adversely affected by a positive antibody crossmatch for primary grafts or retransplants. In further contrast to events in renal transplantation, a high panel–reactive antibody at the time of liver transplantation is also not associated with decreased graft survival.
The hyperacute rejection of renal allografts in patients who receive kidneys in the presence of preformed antidonor antibodies has been well described.1–4 Such grafts do not sustain an effective renal blood flow and angiography shows that the small vessels of the excised kidneys can be closed. Histopathologically, the arterioles and capillaries are plugged with formed blood elements, particularly erythrocytes and platelets. Our experience with combined liver-kidney transplantation in two patients with a positive antibody crossmatch suggests that a liver allograft is able to clear or neutralize quickly sufficient circulating preformed antidonor antibody to permit transplantation of a renal allograft from the same donor without hyperacute rejection. Elucidation of the mechanism by which the liver allograft is able to inactivate or eliminate preformed antibody may be important in developing clinical methods for the prevention or abrogation of hyperacute rejection in renal transplantation.
The clinical circumstances of liver allograft loss are often complicated and the causes of graft loss are often multifactorial. Therefore it is difficult in the large and complex series of patients reported here to assess the relationship between antibody crossmatch and the incidence of graft loss from rejection. The best data are available for patients having retransplantation for rejection, since thorough examination of the removed graft is possible. We found that the incidence of retransplantation for rejection of a prior transplant with a negative or a positive crossmatch was not significantly different.
In nearly every group of patients analyzed, survival was slightly better for grafts in patients with a positive antibody crossmatch or a high PRA at transplantation. However, this advantage in graft survival was not statistically significant in any case. Nevertheless, even in this large series of cases, less than 10% of the grafts were performed with a positive antibody crossmatch, and just 10% of the grafts involved recipients with a current PRA greater than 30%. It will require an even larger experience to determine if preformed antibody has a significant protective or tolerogenic effect in liver transplantation.
Acknowledgments
Supported by National Institutes of Health research project grant No. AM-29961.
Footnotes
Presented at the Forty-third Annual Meeting of the Central Surgical Association, Chicago, Ill., March 6–8, 1986.
References
- 1.Terasaki PI, Marchioro TL, Starzl TE. Serotyping of human lymphocyte antigens: Preliminary trials on long term kidney homograft survivors. In: Russell PS, Winn HJ, Amos DB, editors. Histocompatibility testing. Washington D.C: National Academy of Science—National Research Council; 1965. pp. 83–96. [Google Scholar]
- 2.Kissmeyer-Nielsen F, Olsen S, Peterson VP, Fjeldborg O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966;2:662–5. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 3.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. New Engl J Med. 1969;14:735–9. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 4.Ting A. The lymphocytotoxic crossmatch test of clinical renal transplantation. Transplantation. 1983;5:403–7. doi: 10.1097/00007890-198305000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Opelz G. Effect of HLA matching, blood tranfusions, and presensitization in cyclosporine-treated kidney transplant recipients. Transplant Proc. 1985;6:2179–83. [Google Scholar]
- 6.Starzl TE, Putnam CW, Ishikawa M, Porter KA, Piache R, Husberg BS, Halgrimson CG, Schroter G. Progress in and deterrents to orthotopic liver transplantation; with special reference to survival, resistance to hyperacute rejection, and biliary duct reconstruction. Transplant Proc. 1974;6:129–39. [PMC free article] [PubMed] [Google Scholar]
- 7.Iwatsuki S, Rabin BS, Shaw BW, Jr, Starzl T. Liver transplantation against T-cell positive warm crossmatches. Transplant Proc. 1984;16:1427–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Peto R, Pike MC, Armitage P. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colton T. Statistics in medicine. Boston: Little Brown & Co; 1974. pp. 237–50. [Google Scholar]
- 10.Starzl TE, Iwatsuki S, Shaw BW, Jr, Gordon RD. Orthotopic liver transplantation in 1984. Transplant Proc. 1985;17:250–8. [Google Scholar]
- 11.Fung JJ, Demetris AJ, Porter KA, Iwatsuki S, Gordon RD, Esquivel C, Jaffe R, Shaw BW, Jr, Starzl TE. Use of OKT3 with cyclosporine and steroids for reversal of acute kidney and liver allograft rejection. Nephron. doi: 10.1159/000184431. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]


