Abstract
Phosphatidylcholine and phosphatidylethanolamine are the most abundant phospholipids in eukaryotic cells and thus have major roles in the formation and maintenance of vesicular membranes. In yeast, diacylglycerol accepts a phosphocholine moiety through a CPT1-derived cholinephosphotransferase activity to directly synthesize phosphatidylcholine. EPT1-derived activity can transfer either phosphocholine or phosphoethanolamine to diacylglcyerol in vitro, but is currently believed to primarily synthesize phosphatidylethanolamine in vivo. In this study we report that CPT1- and EPT1-derived cholinephosphotransferase activities can significantly overlap in vivo such that EPT1 can contribute to 60% of net phosphatidylcholine synthesis via the Kennedy pathway. Alterations in the level of diacylglycerol consumption through alterations in phosphatidylcholine synthesis directly correlated with the level of SEC14-dependent invertase secretion and affected cell viability. Administration of synthetic di8:0 diacylglycerol resulted in a partial rescue of cells from SEC14-mediated cell death. The addition of di8:0 diacylglycerol increased di8:0 diacylglycerol levels 20–40-fold over endogenous long-chain diacylglycerol levels. Di8:0 diacylglcyerol did not alter endogenous phospholipid metabolic pathways, nor was it converted to di8:0 phosphatidic acid.
INTRODUCTION
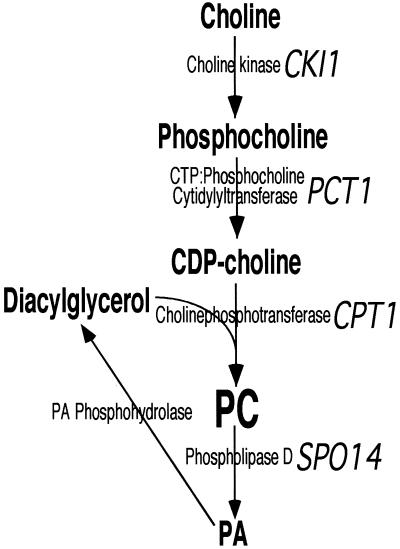
Phosphatidylethanolamine (PE) and phosphatidylcholine (PC) are the most abundant phospholipids present in eukaryotic cells, comprising ∼25 and 50% of cell phospholipid mass, respectively (White, 1973; Paltauf et al., 1992). As major membrane components PE and PC play important roles in the formation and maintenance of cellular, organellar, and vesicular membranes. PE and PC can each be synthesized through two pathways. PE can be synthesized via the decarboxylation of phosphatidylserine, or from ethanolamine to PE by Kennedy pathway enzymes (Kodaki and Yamashita, 1987; Vance, 1991; Clancey et al., 1993; Cui et al., 1993; Trotter et al., 1993; Achleitner et al., 1995; Trotter and Voelker, 1995). Hepatocytes and yeast cells have the capacity to methylate PE to PC, but all other eukaryotic cells described to date synthesize PC almost exclusively through the metabolism of choline by Kennedy pathway enzymes (Weiss et al., 1958; Vance, 1996). The Kennedy pathways for the synthesis of PE and PC phosphorylate either ethanolamine or choline to produce the phosphobase to which a CMP moiety is donated from CTP by a CTP:phosphobase cytidylyltransferase to synthesize the CDP-base. In the final reaction of the pathway ethanolaminephosphotransferase transfers a phosphoethanolamine group from CDP-ethanolamine to diacylglycerol (DAG) to form PE, whereas cholinephosphotransferase catalyzes a similar reaction using CDP-choline as the phosphobase donor for the synthesis of PC (Figure 1) (Hjelmstad and Bell, 1990, 1991a,b; Hjelmstad et al., 1994; McMaster and Bell, 1997a,b).
Figure 1.
Synthesis and turnover of phosphatidylcholine. The Kennedy pathway for the synthesis of phosphatidylcholine and its turnover by phospholipiase D are illustrated. Known yeast genes are in indicated in italics.
In the yeast Saccharomyces cerevisiae, CPT1 and EPT1 code for this organism's complement of cholinephosphotransferase and ethanolaminephosphotransferase activities. In vitro, the CPT1 gene product acted almost exclusively as a cholinephosphotransferase, whereas the EPT1 gene product catalyzed both cholinephosphotransferase and ethanolaminephosphotransferase reactions with similar efficiencies (McGee et al., 1994; McMaster and Bell, 1994; Williams and McMaster, 1998). In vivo metabolic analysis of yeast disrupted for either the CPT1 or EPT1 genes revealed that the CPT1 gene product synthesized 90–95% of Kennedy pathway-derived PC, with the EPT1 product synthesizing the remaining 5–10% of PC and all of the Kennedy pathway-derived PE (McGee et al., 1994; McMaster and Bell, 1994). Recent cloning of the human complement of cholinephosphotransferase and ethanolaminephosphotransferase enzymes revealed similar activity profiles. The human CPT1 product could synthesize only PC in vitro, and in metabolic labeling experiments in yeast devoid of their endogenous activities due to genomic inactivation of the CPT1 and EPT1 loci, expression of human CPT1 reconstituted PC synthesis, but not PE synthesis (Henneberry et al., 2000). The human CEPT1-encoded activity synthesized PC and PE in vitro, and in vivo CEPT1 reconstituted the synthesis of both PC and PE in yeast devoid of their endogenous cholinephosphotransferase and ethanolaminephosphotransferase activities (Henneberry and McMaster, 1999).
SEC14 is an essential gene that codes for the major PC/phosphatidylinositol (PI) transfer protein in yeast (Aitken et al., 1990; Bankaitis et al., 1990; Cleves et al., 1991). Ablation of SEC14 function prevented Golgi-mediated protein transport and resulted in cell death (Cleves et al., 1991; Kearns et al., 1998; Phillips et al., 1999). Utilization of a temperature-sensitive allele of SEC14 (sec14ts) allowed for a search for mutations in other genes that would allow for survival in the face of a nonfunctional SEC14 gene product. This screen resulted in the isolation of several sec14ts bypass suppressor genes (Cleves et al., 1991). Three of the inactivated genes were found to code for each of the Kennedy pathway enzymes: choline kinase (CKI1), CTP:phosphocholine cytidylyltransferase (PCT1), and cholinephosphotransferase (CPT1). Interestingly, none of the enzymes of the Kennedy pathway enzymes for the synthesis of PE were isolated during the screen, and intentional disruption of the yeast EKI1 (ethanolamine kinase) or EPT1 gene products did not rescue cells from the requirement for a functional SEC14 (Cleves et al., 1991; Kim et al., 1999). Hence, the cellular requirement for SEC14 could only be bypassed by inactivating genes in the Kennedy pathway for the synthesis of PC, but not by inactivating genes specific for the synthesis of PE.
The ability of the SEC14 bypass mutants to allow for cell survival in the absence of a functional SEC14 gene product is currently postulated to be due to alterations in Golgi DAG pool sizes, with increased DAG allowing for growth in the absence of SEC14. However, both the CPT1- and EPT1-derived gene products directly consume DAG, and yet inactivation of CPT1 bypassed the cellular requirement for SEC14, whereas inactivation of EPT1 did not. This observation appears to be paradoxical with the DAG pool size hypothesis. The current study explores the metabolic partitioning between the Kennedy pathways for the synthesis of PC and PE in yeast, and the impact of endogenous DAG consumption and exogenous DAG administration on lipid metabolism and subsequent SEC14-mediated vesicle trafficking events.
MATERIALS AND METHODS
Materials
[methyl-14C]Choline chloride (52 mCi/mmol) and phosphorus 32 were purchased from DuPont/NEN (Boston, MA). [methyl-14C]CDP-choline was a product of American Radiolabeled Chemicals (St. Louis, MO). Lipids were products of Avanti Polar Lipids (Alabaster, AL) except for di8:0 phosphatidic acid, which was purchased from Sigma Chemical Co. (St. Louis, MO) Reagents for invertase assays, and glass beads for yeast disruption, were also obtained from Sigma Chemical Co. Thin-layer chromatography (TLC) plates were products of Whatman (Fisher, Nepean, Ont, Canada). All other reagents were of the highest quality commercially available.
Yeast Strains and Media
Yeast strains used were CTY182 (a ura3-52 his3-200 lys2-801 CPT1 EPT1) (Cleves et al., 1991), CTY1-1A (a ura3-52 his3-200 lys2-801 sec14-1ts) (23), CTY160 (a ura3-52 his3-200 lys2-801 sec14-1ts cki1−) (23), CTY468 (a ura3-52 his3-200 lys2-801 sec14-1ts pct1::URA3) (this study), CTY434 (a ura3-52 ade2-101 leu2-3112 his4-519 sec14-1ts cpt1::LEU2) (this study) (the kind gifts of Dr. Vytas Bankaitis, University of Alabama at Birmingham, Birmingham, Al), YPP649.7 (a ura3 sec7ts), YPP649.13 (a ura3 sec13ts), and YPP649.15 (a ura3 sec15ts) (the kind gifts of Dr. Gerald Johnston, Dalhousie University, Halifax, NS, Canada). Plasmids YEp352 (vector), pRH150 (CPT1), and pRH507 (EPT1) (Hjelmstad and Bell, 1990; Hjelmstad and Bell, 1991b) were propagated in DH5α Escherichia coli and transformed into S. cerevisiae using lithium acetate with transformants selected on synthetic dextrose plates containing the appropriate nutritional supplements (Kaiser et al., 1994). YPD medium was made as described (Kaiser et al., 1994).
Enzyme Assays
CTY434 (sec14-1ts cpt1::LEU2 EPT1) cells transformed with plasmids were grown to mid-log phase at 25°C in synthetic dextrose media containing the appropriate nutrients, and microsomal membranes prepared as described (McMaster and Bell, 1994). CPT1- and EPT1-derived cholinephosphotransferase activities were measured using the Triton X-100 mixed micelle protocol (Hjelmstad and Bell, 1991a).
Metabolic Labeling
All metabolic labeling experiments were performed using yeast minimal media plus appropriate nutritional supplements. This media is choline and ethanolamine free unless supplemented exogenously as required for radiolabeling studies. Cells were radiolabeled with [14C]choline at 25°C as previously described (McMaster and Bell, 1994). Inorganic phosphorus 32 (2.5 mCi) was added to 20 ml of early log phase CTY434 cells grown at 25°C, and at 4, 12, and 24 h a 5-ml aliquot was removed and centrifuged at 3000 × g for 5 min to pellet cells. Cells were washed twice with 5 ml of ice-cold water, resuspended in 1 ml of CHCl3/CH3OH (1/1, vol/vol), and transferred to 2-ml screw cap tubes containing 0.5 g of 0.5-mm glass beads. For the efficient extraction of di8:0 phosphatidic acid, perchloric acid was added to the extraction mixture at 0.7% (vol/vol). Cells were disrupted using a BioSpec bead beater for 1 min at 4°C. The beads were washed with 1.5 ml of CHCl3/CH3OH (2:1, vol/vol) and 0.5 ml of CHCl3 and 1.5 ml of H2O were added to the combined homogenate. Tubes were vortexed and centrifuged at 2000 × g for 10 min to facilitate phase separation. The organic phase was washed once with an equal volume of 40% CH3OH (vol/vol) and an aliquot of the organic phase was dried under nitrogen gas in the presence of phospholipid standards. Phospholipids were separated by two-dimensional TLC with CHCl3/CH3COCH3/CH3OH/CH3COOH/H20 (60:24:12:12:6, vol/vol) in the first dimension and CHCl3/CH3OH/CH3COOH/H20 (50:37.5:3.5:2, vol/vol) in the second dimension. Plates were exposed to x-ray film for 24–48 h and subsequently stained with iodine vapor. Iodine stained spots corresponding to the mobility of known standards were scraped into scintillation vials and counted.
Diacylglycerol Pool Size Determination
CTY434 cells ± plasmids were grown overnight at 25°C in synthetic dextrose media containing the appropriate nutrients. Optical densities were measured at 600 nm and each strain was diluted to A600 of 0.150. Cells were grown for 1 h at 25°C and the media was subsequently supplemented with di8:0 DAG to a final concentration of 200 μM. Five-milliliter aliquots were removed at the indicated time points and lipids were extracted as described above. DAG levels were measured using the DAG kinase assay of Preiss et al. (1986), and lipids separated on by TLC using the solvent system CHCl3/CH3OH/CH3OOH (65:15:5, vol/vol). This solvent system efficiently separated short-chain and long-chain phosphorylated DAG products.
Suppression of cpt1−-mediated Bypass of sec14ts
CTY434 cells were grown overnight at 25°C in synthetic dextrose media containing the appropriate nutrients for plasmid maintenance. Optical densities were measured at 600 nm and each strain was diluted to the identical cell number. Serial dilutions of 1:10, 1:100, and 1:1000 were made in water and 1 μl of each was spotted onto a synthetic dextrose plate containing the appropriate nutrients for plasmid maintenance ± 200 μM di8:0 DAG (diacylglycerol stock was dried under nitrogen gas to evaporate the CHCl3, resuspended in sterile water by probe sonication, and added to plates after allowing autoclaved media to cool to 55°C). Plates were incubated at 25°C or 37°C for 4–7 d. Invertase secretion was measured as described (Goldstein and Lampen, 1975; Bankaitis et al. 1989). The invertase secretion index of each sample was determined by dividing external invertase by total invertase.
Routine Procedures
Lipid phosphorus was determined using the method of Ames and Dubin (1960). Protein was quantitated by the method of Lowry et al. (1951)
RESULTS
Rationale
One of the current hypotheses for the ability of certain gene inactivations to allow for survival in the absence of the normally essential SEC14 gene is that these mutations increase the Golgi DAG pool size (Kearns et al., 1997). Part of the reasoning behind this hypothesis was the observation that inactivation of the yeast CPT1 gene allowed for cell survival in the absence of the normally essential SEC14 gene product (Cleves et al., 1991). However, inconsistent with the DAG pool size hypothesis was the observation that inactivation of the yeast EPT1 gene did not allow for bypass of SEC14 function, even though both the CPT1 and EPT1 gene products directly consume DAG for the synthesis of PC, and PC/PE, respectively (Cleves et al., 1991). To further explore the relationship between the two Kennedy pathways for the synthesis of PC and PE, and their interaction with SEC14-mediated vesicle trafficking events, we instituted an in-depth metabolic, enzymatic, and vesicle transport analysis of each of these pathways.
In Vivo and In Vitro Cholinephosphotransferase Activities
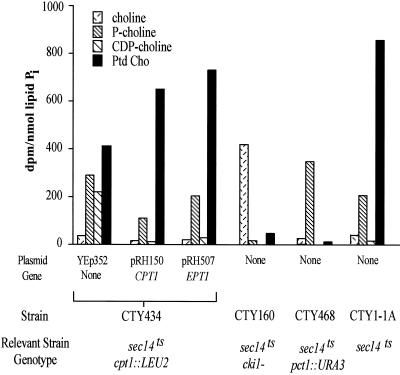
We first pulse-labeled yeast containing an inactivated CPT1 gene (cpt1::LEU2), but with an intact EPT1 gene, with radiolabeled choline. This yeast strain synthesized PC at levels 60% of those expressing an intact CPT1 gene (Figure 2), and overexpression of EPT1 reconstituted PC biosynthetic levels to 100% those provided by CPT1. Disruption of the other two genes coding for enzymes within the Kennedy pathway for PC synthesis, CKI1 and PCT1, reduced PC biosynthesis levels to 5 and 1% of wild-type levels, respectively. Analysis of the metabolites within the Kennedy pathway for PC synthesis were consistent with complete blocks at the choline kinase step for yeast containing an inactivated choline kinase gene (cki1−), an inactivated CTP:phosphocholine cytidylyltransferase gene (pct1::URA3), and a partial block in yeast containing an inactivated cholinephosphotransferase gene (cpt1::LEU2). These results demonstrated that the partitioning of PC synthesis through the Kennedy pathway enzymes was not mutually exclusive. The Kennedy pathway enzymes could significantly overlap in substrate usage in vivo at the ultimate CPT1- and EPT1-encoded phosphotransferase step.
Figure 2.
Phosphatidylcholine synthesis via the CDP-choline pathway. The CTY434 cells (sec14ts cpt1::LEU2 EPT1) were transformed with high copy plasmids containing CPT1, EPT1, or the YEp352 vector. CTY160, CTY468, and CTY1-1A yeast contained only the YEp352 vector. [14C]Choline (10,000 dpm/nmol, 10 μM) was added to 5 ml of mid-log phase yeast cells. Cells were grown at 25°C in yeast minimal media plus appropriate nutritional supplements. At 1 h cells were pelleted by centrifugation at 3000 × g for 5 min and washed with 5 ml of ice-cold water and lipids extracted as described in “Experimental Procedures.” Phospholipids and CDP-choline pathway metabolites were separated by TLC. Results are the mean of n = 6 experiments. SEs were <15% for each mean.
To ensure the high copy plasmid-borne EPT1 and CPT1 vectors were indeed altering cholinephosphotransferase activity levels, yeast cells were analyzed using an in vitro mixed micelle assay reconstitution system (Table 1) (Hjelmstad and Bell, 1991b; McMaster and Bell, 1994; Williams and McMaster, 1998). Because the yeast cells used in these studies contain an inactivated CPT1 gene, the endogenous measurable cholinephosphotransferase activity was due solely to the remaining EPT1-encoded enzyme. Endogenous EPT1-encoded cholinephosphotransferase activity was determined to be 0.92 nmol min−1 mg−1 when di18:1 DAG was used as the phosphocholine acceptor substrate, 0.28 nmol min−1 mg−1 for di16:1 DAG, and no activity was detectable when the short-chain di8:0 DAG was provided as substrate. Overexpression of EPT1 increased these activities 10–40-fold to 17.42, 9.79, and 0.17 nmol min−1 mg−1 for di18:1, di16:1, and di8:0 DAGs, respectively. As expected, overexpression of CPT1 also increased cholinephosphotransferase activity to 13.01 nmol min−1 mg−1 for di16:1 DAG, versus 4.11 nmol min−1 mg−1 for di18:1 DAG and 0.20 nmol min−1 mg−1 for di8:0 DAG.
Table 1.
Cholinephosphotransferase activity
| Diacylglycerol substrate | Plasmid/gene | Cholinephosphotransferase activity (nmol min−1 mg−1) |
|---|---|---|
| di16:1 | YEp352/none | 0.28 ± 0.02 |
| pRH150/CPT1 | 13.01 ± 0.51 | |
| pRH507/EPT1 | 9.79 ± 0.45 | |
| di18:1 | YEp352/none | 0.92 ± 0.16 |
| pRH150/CPT1 | 4.11 ± 0.22 | |
| pRH507/EPT1 | 17.42 ± 0.74 | |
| di8:0 | YEp352/none | undetectable |
| pRH150/CPT1 | 0.20 ± 0.06 | |
| pRH507/EPT1 | 0.17 ± 0.01 |
S. cerevisiae strain CTY434 (sec14ts cpt1∷LEU2 EPT1) was grown to log phase at 25°C in synthetic dextrose media containing appropriate nutritional supplements required for the maintenance of the high copy plasmid YEp352, YEp352 containing CPT1 (pRH150), of YEp352 containing EPT1 (pRH507). Cells were disrupted and microsomal membranes were isolated. Cholinephosphotransferase activities were determined using the mixed micelle assay described in “Experimental Procedures.” Results are mean ± SE (n = 4).
Phosphatidylcholine Synthesis and Vesicle Trafficking
The study of the essential SEC14-encoded PC/PI transfer protein has been facilitated by the isolation of a conditional temperature-sensitive SEC14 allele, sec14ts (Cleves et al., 1991). At the permissive temperature of 25°C cells containing the sec14ts allele possess normal SEC14-mediated PC/PI transfer activity and thus can transport vesicles from the Golgi and grow normally. Upon raising cell culture conditions to 37°C, a nonpermissive temperature for the sec14ts allele, cells can no longer catalyze the PC/PI transfer in vitro and in vivo cells cease Golgi-mediated vesicle transport and eventually die (Bankaitis et al., 1990; Cleves et al., 1991). It has been previously observed that mutations within the cpt1 gene bypassed the requirement for a functional SEC14 gene product and allowed sec14ts cells to grow at 37°C, but mutations in the ept1 gene did not restore secretory competence or cell viability (Cleves et al., 1991).
Previous in vivo metabolic analysis of the partitioning of the Kennedy pathways revealed that CPT1-encoded activity synthesized the majority of PC and barely detectable levels of PE, whereas EPT1-encoded activity synthesized a small amount of PC and the bulk of Kennedy pathway-derived PE (McMaster and Bell, 1994). In addition, increased expression of EPT1 did not affect its contribution toward the synthesis of PC (McMaster and Bell, 1994). In the current study, we were surprised by our observation that in the yeast strain used here, which contained an inactivated CPT1 gene but intact EPT1 gene, the level of PC synthesis was 60% that of wild-type, and overexpression of EPT1 restored PC synthesis to 100% wild-type levels. These data imply that there are as yet uncharacterized cellular events that can alter the ability of EPT1 to significantly contribute to the synthesis of PC. However, these same observations now provided an experimental system to examine how alterations in consumption of endogenous DAG by yeast enzymes affected SEC14-mediated vesicle transport processes and cell viability.
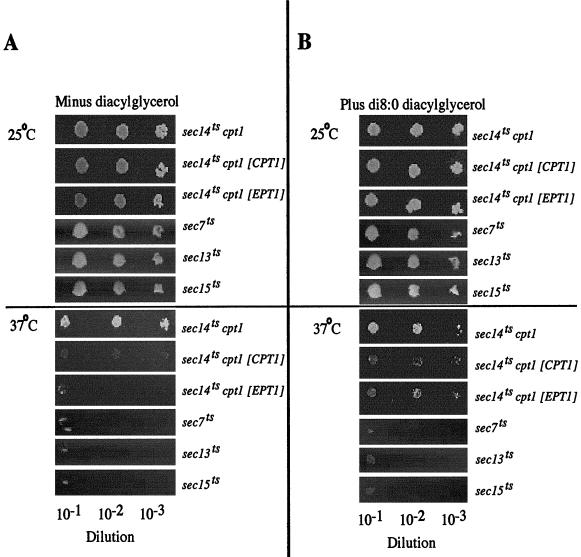
The yeast strain that contained the active EPT1 gene and inactivated CPT1 gene used in the metabolic labeling experiments described above also contained a conditional temperature-sensitive sec14ts allele. Normally, an inactivated CPT1 gene (cpt1::LEU2) would be predicted to allow for a bypass of the essential requirement for SEC14 and allow the yeast to survive and successfully transport vesicles from the Golgi apparatus at both the sec14ts-permissive temperature of 25°C and the sec14ts-nonpermissive temperature of 37°C (Cleves et al., 1991). We tested whether disruption of the CPT1 gene in the current strain, which resulted in the surprisingly high level of PC synthesis (to 60% of wild-type levels) due to the remaining EPT1 gene, was still capable of bypassing the cellular requirement for a functional SEC14 gene product (Figure 3A). The 40% decrease in Kennedy pathway-derived PC synthesis in the EPT1 cpt1::LEU2 yeast strain was sufficient to allow for survival in the face of a nonfunctioning SEC14 gene product. Upon overexpression of EPT1 or CPT1, PC synthesis was restored to 100% wild-type levels, and these yeast cells could no longer survive at 37°C, indicating the reimposition of the requirement for a functional SEC14 gene product.
Figure 3.
EPT1 and di8:0 diacylglycerol affect SEC14-mediated cell growth. (A) CTY434 yeast (sec14ts cpt1::LEU2 EPT1) transformed with CPT1 or EPT1 in the high copy number YEp352 vector, or YPP649.7 (sec7ts), YPP649.13 (sec13ts), and YPP649.15 (sec15ts) transformed with YEp352 were grown overnight at 25°C in appropriate selective medium to ensure plasmid maintenance and diluted to identical cell number as determined spectrophotometrically by culture absorbance at 600 nm. Serial dilutions (1:10) were performed, and 1 μl of each dilution was plated onto SC minus uracil plates. (B) The identical experiment except 1 μl of each dilution was plated onto SC minus uracil plates containing 200 μM di8:0 diacylglycerol. All plates were incubated for 4 d at 25°C or 7 d at 37°C. [CPT1] and [EPT1] indicate the presence of YEp352 plasmid-borne genes.
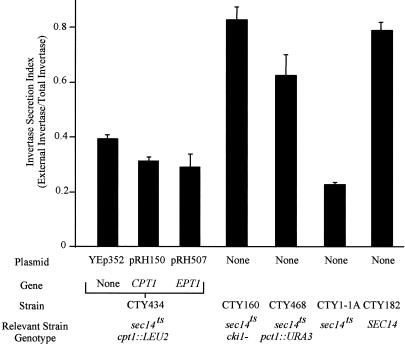
To correlate the observed affects on cell growth with alterations in vesicle transport, the ability of each of the cells to secrete invertase was measured after cells were shifted to the sec14ts-nonpermissive temperature of 37°C. Cells containing the sec14ts mutation alone displayed a significant decrease in their ability to secrete invertase compared with wild-type yeast. In cells containing the sec14ts allele, inactivation of the first two enzymes of the Kennedy pathway for the synthesis of PC, choline kinase (CKI1) and CTP:phosphocholine cytidylyltransferase (PCT1), resulted in an increase in invertase secretion to levels at or near those of wild-type cells (Figure 4). Interestingly, the ability to secrete invertase was only moderately increased in sec14ts cells containing an inactivated cholinephosphotransferase gene (CPT1). Overexpression of EPT1 or CPT1 in the sec14ts cpt1::LEU2 cells decreased invertase secretion to near those observed for cells containing the sec14ts allele alone. Total invertase enzyme activities were not affected by ablation of Sec14p function compared with wild-type yeast, and the restoration of secretion competency through inactivation of the genes of the CDP-choline pathway did not significantly alter total invertase enzyme activity. Compared with wild-type yeast (100%), the sec14ts strain possessed total invertase activity of 96% wild-type, whereas the bypass suppressors, containing both the sec14ts mutation and disruptions of each of the genes of the CDP-choline pathway, had invertase activities ranging from 65 to 103% those of wild-type yeast.
Figure 4.
Invertase secretion indices. Yeast strains CTY182 (wild-type, SEC14 CPT1 EPT1), CTY1-1A (sec14ts CPT1 EPT1), CTY160 (sec14-1ts cki1−), CTY468 (sec14-1ts pct1::URA3), or CTY434 (sec14ts cpt1::LEU2 EPT1) transformed with the vector control YEp352, or CPT1 or EPT1 in the high copy number YEp352 vector, were grown to mid-log phase in YPD (2% glucose) medium at 25°C. Cells were pelleted (2000 × g for 1 min), washed twice with water, and resuspended in 5 ml of YPD containing 0.1% glucose. Cultures were subsequently grown at 37°C to impose the temperature-sensitive phenotype and invertase activities were determined as described in “Experimental Procedures. ” Results are expressed as the mean ± SE (n = 6), except for CTY160 and CTY468 (n = 3).
To sum, complete abolition of PC synthesis in cells grown at the nonpermissive temperature for the sec14ts allele resulted in invertase secretion at wild-type levels (3–4-fold above those observed in cells containing the sec14ts allele alone), whereas PC synthesis at 60% wild-type levels increased invertase secretion to 1.5–2-fold (compared with the sec14ts allele alone) but was sufficient to allow for life, whereas restoration of PC synthesis to 100% wild-type levels reimposed the requirement for functional SEC14 on the cells. Hence, the rate of invertase secretion correlated directly with the rate to which PC synthesis was decreased. These results are consistent with the notion that the rate of endogenous DAG consumption was directly affecting the requirement for SEC14; however, lipid pathways downstream of DAG consumption or PC synthesis may also be impacting on SEC14-mediated events.
Effect of Di8:0 Diacylglycerol on Cell Growth and Lipid Metabolism
Di8:0 DAG is a synthetic lipid not produced in eukaryotic cells, but its increased solubility compared with long-chain DAGs has been exploited to allow for its use as a pharmacological tool to demonstrate DAG-specific regulatory and signaling events (Davis et al., 1985; Kearns et al., 1997). Di8:0 DAG was added to the medium of sec14ts cpt1::LEU2 cells, which contained plasmid-borne EPT1, CPT1, or a vector control. The addition of di8:0 DAG rescued cells via a reproducible partial suppression of the sec14ts growth phenotype at 37°C in sec14ts cpt1::LEU2 cells and this was independent of whether cells were overexpressing CPT1 or EPT1 (Figure 3B). The addition of di8:0 DAG did not rescue growth at the nonpermissive temperature for sec7ts and sec13ts yeast, which are defective in endoplasmic reticulum to Golgi transport, and sec15ts-containing cells, which are defective in secretion at the Golgi-to-plasma membrane step, implying the rescue of cell growth by di8:0 DAG was specific to Sec14p-mediated events. In addition, the lack of correlation between CPT1 and EPT1 expression and the ability of di8:0 DAG to alter SEC14-mediated events is consistent with our in vitro observation that di8:0 DAG was a poor substrate for these enzymes. The above-mentioned data indicate di8:0 DAG is likely not metabolized by either CPT1- or EPT1-derived activities, but if we are to effectively examine the role of DAG on SEC14-mediated vesicle transport events the effect of di8:0 DAG on DAG pool sizes and lipid metabolism parameters need to be measured.
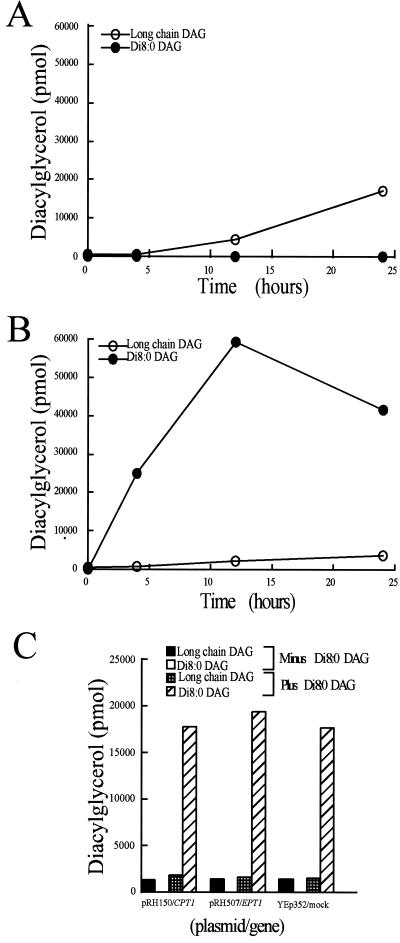
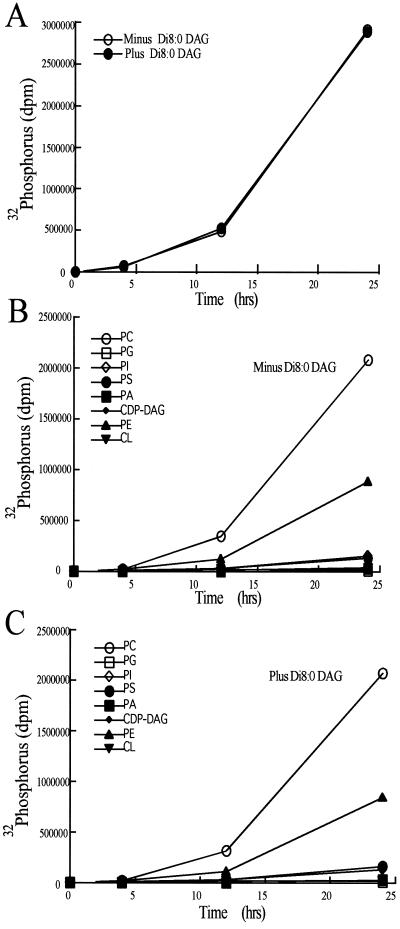
The addition of 200 μM di8:0 DAG to early log phase yeast resulted in an accumulation of di8:0 DAG to 20–40 times those of endogenous long-chain DAG levels (Figure 5). Overexpression of CPT1 or EPT1 did not affect the levels of di8:0 DAG, indicating these enzymes were not effectively consuming di8:0 DAG, consistent with the in vitro substrate specificity data and the di8:0 DAG cell growth assays (Table 1 and Figure 3). Although the combined results indicate di8:0 DAG was not effectively metabolized by yeast, and almost certainly not via CPT1- or EPT1-derived activities, the vast increase in intracellular di8:0 DAG levels prompted us to examine whether di8:0 DAG administration affected gross lipid metabolism. The incorporation of phosphorus 32 into yeast in the presence and absence of 200 μM di8:0 DAG was determined. Total net lipid synthesis was not affected by the addition of di8:0 DAG (Figure 6). Phospholipids within the lipid fraction were separated by two-dimensional TLC and the plates were exposed to x-ray film. The addition of di8:0 DAG did not affect the incorporation of phosphorus 32 into PC, PE, PI, phosphatidylserine, phosphatidic acid, CDP-diacylglycerol, phosphatidylglycerol, or cardiolipin. Importantly, we also noted that there was no conversion of the di8:0 DAG to di8:0 phosphatidic acid because there was no radiolabel associated with the mobility of this lipid in the two-dimensional TLC system used. We demonstrated that the lipid extraction protocol used in this study efficiently extracted the majority of di8:0 phosphatidic acid (our unpublished results). In addition, a visual analysis of the x-ray film did not reveal any uniquely labeled regions upon the addition of di8:0 DAG. Hence, the data are consistent with di8:0 DAG entering cells and dramatically increasing cellular DAG levels; however, di8:0 DAG did not appear to alter phospholipid metabolic pathways, or to be converted to di8:0 phosphatidic acid.
Figure 5.
Effect of exogenous di8:0 diacylglycerol administration on diacylglycerol pool sizes. CTY434 cells ± plasmids were grown overnight at 25°C in synthetic dextrose media containing the appropriate nutrients. Optical densities were measured at 600 nm and each strain was diluted to A600 of 0.150. Cells were grown for 1 h at 25°C and the medium was subsequently supplemented with di8:0 DAG to a final concentration of 200 μM. Five-milliliter aliquots were removed at the indicated time points and diacylglycerols were extracted and quantified as described in “Experimental Procedures.” (A) CTY434 cells grown without di8:0 DAG supplementation. (B) CTY434 cells grown with di8:0 DAG supplemented to 200 μM di8:0 DAG. (C) CTY434 cells containing high copy plasmids carrying the CPT1 or EPT1 genes supplemented to 200 μM di8:0 DAG for 4 h. Results are the mean of n = 4 experiments. SEs were <15% for each mean.
Figure 6.
Effect of exogenous di8:0 diacylglycerol administration on phospholipid synthesis. Inorganic 32 phosphorus (2.5 mCi) was added to 20 ml of mid-log phase CTY434 cells grown at 25°C in yeast minimal media plus appropriate nutritional supplements. At 4, 12, and 24 h a 5-ml aliquot was removed and centrifuged at 3000 × g for 5 min to pellet cells. Cells were washed twice with 5 ml of ice-cold water and lipids extracted as described in “Experimental Procedures.” Phospholipids were separated by two-dimensional TLC with CHCl3/CH3COCH3/CH3OH/CH3COOH/H20 (60:24:12:12:6, vol/vol) in the first dimension and CHCl3/CH3OH/CH3COOH/H20 (50:37.5:3.5:2, vol/vol) in the second dimension. (A) Incorporation of 32 phosphorus into total phospholipid. (B) Incorporation of 32 phosphorus into individual lipid classes. (C) Incorporation of 32 phosphorus into individual lipid classes in the presence of 200 μM di8:0 DAG. Results are the mean of n = 4 experiments. SEs were <15% for each mean.
DISCUSSION
The metabolic partitioning of the Kennedy pathways for the synthesis of PC and PE has been controversial. In vitro analysis of purified enzymes and cloned gene products has demonstrated that several enzymes of the Kennedy pathway possess the capacity to use both ethanolamine and choline pathway components. Thus, it has been hypothesized that the two metabolic pathways may contain the same enzyme components, or that the dual substrate specificity of some of the enzymes may at a minimum result in overlap between enzymes that possess substrate specificity and those that are promiscuous in their use of substrates. Most notable among these in vitro results are the ability of some mammalian kinases to phosphorylate both choline and ethanolamine (Porter and Kent, 1990; Kent, 1995), and the ability of some phosphotransferases to use CDP-choline and CDP-ethanolamine for the synthesis of PC and PE (Hjelmstad et al., 1994; Henneberry and McMaster, 1999; Henneberry et al., 2000). Recent in vivo observations have resulted in the conclusion that the ability of the CDP-ethanolamine pathway enzymes to use CDP-choline is restricted to the in vitro situation, because radiolabeling experiments in yeast carrying inactivated genes for various enzymes within the Kennedy pathways revealed that there was strict metabolic pathway partitioning (McGee et al., 1994; McMaster and Bell, 1994; Kim et al., 1999). However, this observation was recently challenged by the cloning of the human complement of cholinephosphotransferase and ethanolaminephosphotransferase enzymes. The human CPT1 product specifically used CDP-choline as its phosphobase donor in vitro and metabolic experiments demonstrated that hCPT1 reconstituted only PC synthesis in vivo in yeast devoid of their endogenous cholinephosphotransferase and ethanolaminephosphotransferase activities (Henneberry et al., 2000). However, the human CEPT1 product could use both CDP-choline and CDP-ethanolamine in vitro and was capable of reconstituting the synthesis of both PC and PE in yeast in vivo (Henneberry and McMaster, 1999). Our current study supports and extends this observation. We have found a yeast strain whereby endogenous yeast EPT1-derived cholinephosphotransferase activity can contribute to 60% of net Kennedy pathway-derived PC synthesis and overexpression of EPT1 reconstituted PC synthesis to wild-type levels. The precise mechanisms that allow for the overlap in substrate specificity by EPT1 remain to be identified, but it is clear that the Kennedy pathways are not strictly partitioned and can significantly overlap at the ultimate step in the synthesis of PC and PE.
SEC14 codes for an essential yeast PC/PI transfer protein. Previous experimentation had demonstrated that inactivation of the CPT1 gene, and the other enzymes within the Kennedy pathway for PC synthesis, allowed cells to survive in the absence of a functional SEC14 gene product, but inactivation of the EKI1 and EPT1 genes for the synthesis of PE did not bypass the cellular requirement for SEC14 (Cleves et al., 1991; Kim et al., 1999). The current paradigm whereby SEC14 serves to mediate Golgi DAG levels to maintain secretory competence was consistent with the inactivation of CPT1, but not EPT1, to bypass the essentiality of SEC14, because both CPT1 and EPT1 gene products directly consume DAG. However, the present study demonstrated that the rate of endogenous PC synthesis, and hence DAG consumption, directly correlated with the level of SEC14-dependent invertase secretion. Thus, these data predict that the rate of endogenous DAG consumption by EPT1 is normally sufficiently low so as not contribute significantly to DAG metabolism. Our recent cloning of the human CPT1 and CEPT1 gene products allowed us to test the role of each of these gene products in their ability to interact with the SEC14 secretory apparatus. We observed that expression of human CEPT1, but not CPT1, was able to mimic endogenous yeast CPT1 and prevent cell growth in the absence of a functional SEC14 gene product in the same sec14ts cpt1− yeast used in the current study (Henneberry et al., 2000). We also noted that expression of human CPT1 restored PC synthesis to levels 70% that provided by expression of human CEPT1. Thus, a positive correlation exists between endogenous DAG consumption and restoration of SEC14-dependent vesicle trafficking to levels required for cell viability.
Consistent with the variations in endogenous DAG consumption affecting SEC14-mediated cell growth and secretory capacity was our demonstration of the ability of exogenous di8:0 DAG to rescue sec14ts-mediated cell death. This observation was complemented by the first assessment of the effects of di8:0 DAG on DAG pool sizes and lipid metabolism in yeast. Di8:0 DAG entered yeast cells and accumulated to levels 20–40-fold higher than endogenous long-chain DAG. In addition, di8:0 DAG did not alter the rate of synthesis of PC, PE, PI, phosphatidylserine, phosphatidic acid (PA), CDP-DAG, phosphatidylglycerol, or cardiolipin (Patton-Vogt et al., 1997; Siddhanta and Shields, 1998). However, it should be noted that the shift in temperature from 25°C to 37°C did not affect the cellular long-chain DAG pool size. How this observation fits with the DAG pool size paradigm has yet to be effectively reconciled, except by proposing a Golgi-specific DAG pool that does not significantly contribute to total cellular DAG levels.
The hypothesis that SEC14 impacts on the regulation of Golgi DAG pool sizes is consistent with the observation that PC-bound SEC14 protein inhibits PC synthesis by inhibition of CTP:phosphocholine cytidylyltransferase (PCT1), the rate-limiting step in PC synthesis (Skinner et al., 1995). Inhibition of Pct1p activity decreases the availability of CDP-choline for use by Cpt1p and Ept1p for PC synthesis and DAG consumption. Inactivation of the yeast phospholipase D gene (SPO14/PLD1) (Waksman et al., 1996; Rudge and Engebrecht, 1999; Li et al., 2000), in each of the sec14− bypass mutants reimposed the requirement for a functional SEC14 gene product (Sreenivas et al., 1998; Xie et al., 1998). If the DAG consumption hypothesis is correct, then the PA generated by SPO14-derived activity would be predicted to be metabolized to DAG, however, the PA phosphatase genes required to directly test this hypothesis have yet to be identified in yeast.
An interesting observation during the SPO14 inactivation analysis revolved around the SAC1 gene. The SAC1 gene codes for a PI-4-P phosphatase (Guo et al., 1999; Hughes et al., 2000) and was one of the original sec14− bypass suppressors. Inactivation of SAC1 resulted in a fivefold increase in PI-4-P levels (Hama et al., 1999; Rivas et al., 1999; Stock et al., 1999) and hence it was proposed that PI-4-P levels directly correlated with the ability of SEC14 to function during vesicle trafficking (Hama et al., 1999; Stock et al., 1999). However, the inactivation of SPO14 in the sec14ts sac1 yeast reversed the bypass effect of sac1 and resulted in decreased secretion and eventual cell death, however PI-4-P levels remained high (Rivas et al., 1999). Thus, the role of PI-4-P in mediating SEC14 function is still unclear. In one of these studies it was noted that sac1 mutants displayed a dramatic increase in flux through the CDP-choline pathway for PC synthesis (Rivas et al., 1999). Inactivation of SPO14 in sac1− yeast restored PC synthesis to normal rates, indicating that SPO14-generated products directly affected PC synthesis. The most likely explanation for these results are that the PA generated by phospholipase D hydrolysis is normally converted to DAG for consumption by CPT1- and EPT1-derived phosphotransferase activities.
The challenge in analyzing alterations in lipid metabolism in the face of SEC14 disfunction, and the accompanying bypass suppressors, is addressing which lipids play a direct role in modulating protein secretion and cell growth. The data presented in this study include correlations of endogenous DAG consumption with invertase secretion indices and cell viability, and exogenous DAG administration affecting SEC14-mediated cell death. In addition, exogenous DAG did not affect the metabolism of any phospholipid tested, nor was it converted to di8:0 phosphatidic acid, implying DAG itself was likely the mediator allowing for SEC14-dependent alterations in protein secretion and cell growth. The most obvious steps in the elucidation of the precise mechanisms of SEC14-mediated protein secretion will be an assessment of whether the lipids themselves promote vesicle fusion (Ruiz-Arguello et al., 1996), or whether there are protein targets for lipid activation or inhibition, and an assessment of how these impact on SEC14-mediated vesicle trafficking.
ACKNOWLEDGMENTS
We thank Harold Cook and David Byers (Dalhousie University, K. Halifax, NS, Canada) for helpful comments during the course of these studies. This work was supported by a Canadian Institutes of Health Research Doctoral Award (A.L.H.), CIHR Scholarship and Operating Awards (C.R.M.), CIHR Scientist and Operating Awards (N.D.R.), and a CIHR Group Grant (N.D.R. and C.R.M.).
Abbreviations used:
- CDP-DAG
CDP-diacylglycerol
- DAG
diacylglycerol
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PI
phosphatidylinositol
REFERENCES
- Achleitner G, Zweytick D, Trotter PJ, Voelker DR, Daum G. Synthesis and intracellular transport of aminoglycerophospholipids in permeabilized cells of the yeast, Saccharomyces cerevisiae. J Biol Chem. 1995;270:29836–29842. doi: 10.1074/jbc.270.50.29836. [DOI] [PubMed] [Google Scholar]
- Aitken JF, van Heusden GPH, Temkin M, Dowhan W. The gene encoding the phosphatidylinositol transfer protein is essential for cell growth. J Biol Chem. 1990;265:4711–4717. [PubMed] [Google Scholar]
- Ames BN, Dubin DT. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960;235:769–775. [PubMed] [Google Scholar]
- Bankaitis VA, Aitken JF, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Bankaitis VA, Malehorn DE, Emr SD, Greene R. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J Cell Biol. 1989;108:1271–1281. doi: 10.1083/jcb.108.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancey CJ, Chang S-C, Dowhan W. Cloning of a gene (PSD1) encoding phosphatidylserine decarboxylase from Saccharomyces cerevisiae by complementation of an Escherichia coli mutant. J Biol Chem. 1993;268:24580–24590. [PubMed] [Google Scholar]
- Cleves AE, McGee TP, Whitters EA, Champion KM, Aitken JR, Dowhan W, Goebl M, Bankaitis VA. Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell. 1991;64:789–800. doi: 10.1016/0092-8674(91)90508-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Vance JE, Chen MH, Voelker DE, Vance DE. Cloning and expression of a novel phosphatidylethanolamine N-methyltransferase. A specific biochemical and cytological marker for a unique membrane fraction in rat liver. J Biol Chem. 1993;268:16655–16663. [PubMed] [Google Scholar]
- Davis RJ, Ganong BR, Bell RM, Czech MP. sn-1,2-Dioctanoylglycerol. A cell-permeable diacylglycerol that mimics phorbol diester action on the epidermal growth factor receptor and mitogenesis. J Biol Chem. 1985;260:1562–1566. [PubMed] [Google Scholar]
- Goldstein A, Lampen JL. β-D-Fructofuranoside fructohydrolase from yeast. Methods Enzymol. 1975;42:504–511. doi: 10.1016/0076-6879(75)42159-0. [DOI] [PubMed] [Google Scholar]
- Guo S, Stolz LE, Lemrow SM, York JD. SAC1-like domains of yeast SAC1, INP52, and INP53 and of human synaptojanin encode polyphosphoinositide phosphatases. J Biol Chem. 1999;274:12990–12995. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- Hama H, Schnieder EA, Thorner J, Takemoto JY, DeWald DB. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:34294–34300. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- Henneberry AL, McMaster CR. Cloning and expression of a human choline/ethanolaminephosphotransferase: synthesis of phosphatidylcholine and phosphatidylethanolamine. Biochem J. 1999;339:291–298. [PMC free article] [PubMed] [Google Scholar]
- Henneberry AL, Wistowe G, McMaster CR. Cloning, genomic organization, and biochemical characterization of a human cholinephosphotransferase. J Biol Chem. 2000;275:29808–29815. doi: 10.1074/jbc.M005786200. [DOI] [PubMed] [Google Scholar]
- Hjelmstad RH, Bell RM. The sn-1,2-diacylglycerol cholinephosphotransferase of Saccharomyces cerevisiae. Nucleotide sequence, transcriptional mapping, and gene product analysis of the CPT1 gene. J Biol Chem. 1990;265:1755–1764. [PubMed] [Google Scholar]
- Hjelmstad RH, Bell RM. sn-1,2-Diacylglycerol choline- and ethanolaminephosphotransferases in Saccharomyces cerevisiae. Mixed micellar analysis of the CPT1 and EPT1 gene products. J Biol Chem. 1991a;266:4357–4365. [PubMed] [Google Scholar]
- Hjelmstad RH, Bell RM. sn-1,2-Diacylglycerol choline- and ethanolaminephosphotransferases in Saccharomyces cerevisiae. Nucleotide sequence of the EPT1 gene and comparison of the CPT1 and EPT1 gene product. J Biol Chem. 1991b;266:5094–5103. [PubMed] [Google Scholar]
- Hjelmstad RH, Morash SA, McMaster CR, Bell RM. Chimeric enzymes. structure-function analysis of segments of sn-1,2- diacylglycerol choline- and ethanolaminephosphotransferases. J Biol Chem. 1994;269:20995–21002. [PubMed] [Google Scholar]
- Hughes WE, Woscholski R, Cooke FT, Patrick RS, Dove SK, McDonald NQ, Parker PJ. SAC1 encodes a regulated lipid phosphoinositide phosphatase, defects in which can be suppressed by the homologous Inp52p and Inp53p phosphatases. J Biol Chem. 2000;275:801–808. doi: 10.1074/jbc.275.2.801. [DOI] [PubMed] [Google Scholar]
- Kaiser C, Michaelis S, Mitchell A. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Kearns BG, Alb JG, Bankaitis VA. Phosphatidylinositol transfer proteins: the long and winding road to physiological function. Trends Cell Biol. 1998;8:276–282. doi: 10.1016/s0962-8924(98)01281-1. [DOI] [PubMed] [Google Scholar]
- Kearns BG, McGee TP, Mayinger P, Gedvilaite A, Phillips SE, Kagiwada S, Bankaitis VA. Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature. 1997;387:101–105. doi: 10.1038/387101a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent C. Eukaryotic phospholipid biosynthesis. Annu Rev Biochem. 1995;64:315–343. doi: 10.1146/annurev.bi.64.070195.001531. [DOI] [PubMed] [Google Scholar]
- Kim K, Kim K-H, Storey MK, Voelker DR, Carman GM. Isolation and characterization of the Saccharomyces cerevisiae EKI1 gene encoding ethanolamine kinase. J Biol Chem. 1999;274:14857–14866. doi: 10.1074/jbc.274.21.14857. [DOI] [PubMed] [Google Scholar]
- Kodaki T, Yamashita S. Yeast phosphatidylethanolamine methylation pathway. Cloning and characterization of two distinct methyltransferase genes. J Biol Chem. 1987;262:15428–15435. [PubMed] [Google Scholar]
- Li X, Routt SM, Xie Z, Cui X, Fang M, Kearns MA, Bard M, Kirsch DR, Bankaitis VA. Identification of a novel family of nonclassic yeast phosphatidylinositol transfer proteins whose function modulates phospholipase D activity and Sec14p-dependent cell growth. Mol Biol Cell. 2000;11:1989–2005. doi: 10.1091/mbc.11.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- McGee TP, Skinner HB, Bankaitis VA. Functional redundancy of the CDP-ethanolamine and CDP-choline pathway enzymes in phospholipid biosynthesis: ethanolamine-dependent effects on steady-state membrane phospholipid composition in Saccharomyces cerevisiae. J Bacteriol. 1994;176:6861–6868. doi: 10.1128/jb.176.22.6861-6868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster CR, Bell RM. Phosphatidylcholine biosynthesis in Saccharomyces cerevisiae. Regulatory insights from studies employing null and chimeric sn-1,2-diacylglycerol choline- and ethanolaminephosphotransferases. J Biol Chem. 1994;269:28010–28016. [PubMed] [Google Scholar]
- McMaster CR, Bell RM. CDP-choline: 1,2-diacylglycerol cholinephosphotransferase. Biochim Biophys Acta. 1997a;1348:100–110. doi: 10.1016/s0005-2760(97)00097-0. [DOI] [PubMed] [Google Scholar]
- McMaster CR, Bell RM. CDP-ethanolamine: 1,2-diacylglycerol ethanolaminephosphotransferase. Biochim Biophys Acta. 1997b;1348:117–123. doi: 10.1016/s0005-2760(97)00098-2. [DOI] [PubMed] [Google Scholar]
- Paltauf F, Kohlwein SD, Henry SA. In: The Molecular and Cellular Biology of the Yeast Saccharomyces. Jones EW, Pringle JR, Broach JR, editors. Plainview, NY: Cold Spring Harbor Laboratory Press; 1992. pp. 415–500. [Google Scholar]
- Patton-Vogt JL, Griac P, Sreevinas A, Bruno V, Dowd S, Swede MJ, Henry SA. Role of the yeast phosphatidylinositol/phosphatidylcholine transfer protein (Sec14p) in phosphatidylcholine turnover and INO1 regulation. J Biol Chem. 1997;272:20873–20883. doi: 10.1074/jbc.272.33.20873. [DOI] [PubMed] [Google Scholar]
- Phillips SE, et al. Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Mol Cell. 1999;4:187–197. doi: 10.1016/s1097-2765(00)80366-4. [DOI] [PubMed] [Google Scholar]
- Porter TJ, Kent C. Purification and characterization of choline/ethanolamine kinase from rat liver. J Biol Chem. 1990;265:414–422. [PubMed] [Google Scholar]
- Preiss J, Loomis CR, Bishop WR, Stein R, Niedel JE, Bell RM. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986;261:8597–8600. [PubMed] [Google Scholar]
- Rivas MP, Kearns BG, Guo S, Xie Z, Sekar C, Hosaka K, Kagiwada S, York JD, Bankaitis VA. Pleiotropic alterations in lipid metabolism in yeast sac1 mutants: relationship to “bypass Sec14p” and inositol auxotrophy. Mol Biol Cell. 1999;10:2235–2250. doi: 10.1091/mbc.10.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge SA, Engebrecht J. Regulation and function of PLDs in yeast. Biochim Biophys Acta. 1999;1439:167–174. doi: 10.1016/s1388-1981(99)00092-x. [DOI] [PubMed] [Google Scholar]
- Ruiz-Arguello MB, Basanez G, Goni FM, Alonso A. Different effects of enzyme-generated ceramides and diacylglycerols in phospholipid membrane fusion and leakage. J Biol Chem. 1996;271:26616–26621. doi: 10.1074/jbc.271.43.26616. [DOI] [PubMed] [Google Scholar]
- Siddhanta A, Shields D. Secretory vesicle budding from the trans-Golgi network is mediated by phosphatidic acid levels. J Biol Chem. 1998;273:17995–17998. doi: 10.1074/jbc.273.29.17995. [DOI] [PubMed] [Google Scholar]
- Skinner HB, McGee TP, McMaster CR, Fry MR, Bell RM, Bankaitis VA. The Saccharomyces cerevisiae phosphatidylinositol-transfer protein effects a ligand-dependent inhibition of choline-phosphate cytidylyltransferase activity. Proc Natl Acad Sci USA. 1995;92:112–116. doi: 10.1073/pnas.92.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivas A, Patton-Vogt JL, Bruno V, Griac P, Henry SA. A role for phospholipase D (Pld1p) in growth, secretion, and regulation of membrane lipid synthesis in yeast. J Biol Chem. 1998;273:16635–16638. doi: 10.1074/jbc.273.27.16635. [DOI] [PubMed] [Google Scholar]
- Stock SD, Hama H, DeWald DB, Takemoto JY. SEC14-dependent secretion in Saccharomyces cerevisiae. Nondependence on sphingolipid synthesis-coupled diacylglycerol production. J Biol Chem. 1999;274:12979–12983. doi: 10.1074/jbc.274.19.12979. [DOI] [PubMed] [Google Scholar]
- Trotter PJ, Pedretti J, Voelker DR. Phosphatidylserine decarboxylase from Saccharomyces cerevisiae. Isolation of mutants, cloning of the gene, and creation of a null allele. J Biol Chem. 1993;268:21416–21424. [PubMed] [Google Scholar]
- Trotter PJ, Voelker DR. Identification of a non-mitochondrial phosphatidylserine decarboxylase activity (PSD2) in the yeast Saccharomyces cerevisiae. J Biol Chem. 1995;270:6062–6070. doi: 10.1074/jbc.270.11.6062. [DOI] [PubMed] [Google Scholar]
- Vance JE. Newly made phosphatidylserine and phosphatidylethanolamine are preferentially translocated between rat liver mitochondria and endoplasmic reticulum. J Biol Chem. 1991;266:89–97. [PubMed] [Google Scholar]
- Vance DE. In: Biochemistry of Lipids, Lipoproteins and Membranes. Vance DE, Vance JE, editors. Amsterdam: Elsevier Scientific; 1996. pp. 153–182. [Google Scholar]
- Waksman M, Eli Y, Liscovitch M, Gerst JE. Identification and characterization of a gene encoding phospholipase D activity in yeast. J Biol Chem. 1996;271:2361–2364. doi: 10.1074/jbc.271.5.2361. [DOI] [PubMed] [Google Scholar]
- Weiss SB, Smith SW, Kennedy EP. The enzymatic formation of lecithin from cytidine diphosphate choline and D-1,2-diglyceride. J Biol Chem. 1958;233:53–64. [PubMed] [Google Scholar]
- White DA. In: Form and Function of Phospholipids. Ansell GB, Hawthorne JN, Dawson RMC, editors. Amsterdam: Elsevier Scientific; 1973. pp. 441–482. [Google Scholar]
- Williams JG, McMaster CR. Scanning alanine mutagenesis of the CDP-alcohol phosphotransferase motif of Saccharomyces cerevisiae cholinephosphotransferase. J Biol Chem. 1998;273:13482–13487. doi: 10.1074/jbc.273.22.13482. [DOI] [PubMed] [Google Scholar]
- Xie Z, Fang M, Rivas MP, Faulkner AJ, Sternweis PC, Engebrecht J, Bankaitis VA. Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc Natl Acad Sci USA. 1998;95:12346–12351. doi: 10.1073/pnas.95.21.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]