Abstract
Acute or chronic mercury exposure can cause adverse effects during any period of development. Mercury is a highly toxic element; there is no known safe level of exposure. Ideally, neither children nor adults should have any mercury in their bodies because it provides no physiological benefit. Prenatal and postnatal mercury exposures occur frequently in many different ways. Pediatricians, nurses, and other health care providers should understand the scope of mercury exposures and health problems among children and be prepared to handle mercury exposures in medical practice. Prevention is the key to reducing mercury poisoning. Mercury exists in different chemical forms: elemental (or metallic), inorganic, and organic (methylmercury and ethyl mercury). Mercury exposure can cause acute and chronic intoxication at low levels of exposure. Mercury is neuro-, nephro-, and immunotoxic. The development of the child in utero and early in life is at particular risk. Mercury is ubiquitous and persistent. Mercury is a global pollutant, bio-accumulating, mainly through the aquatic food chain, resulting in a serious health hazard for children. This article provides an extensive review of mercury exposure and children’s health.
Introduction
Mercury is a silvery-white shiny heavy metal with unique chemical and physical properties. It has been used worldwide for many centuries for commercial and medicinal purposes.1,2 Mercury is a persistent and globally cycling element. Mercury occurs not only anthropogenically but also naturally.3,4 It has toxic properties and severely affects the environment and humans, especially developing fetuses and infants.3
Forms of Mercury and Chemical Behavior
There are 3 main forms of mercury that differ with respect to their toxicokinetics regarding absorption, distribution, and accumulation in the human body; related health outcomes; and the extent of cycling in the environment. Elemental mercury is liquid at room temperature, and in this form, is less toxic than inorganic or organic bound mercury. It has a high vapor pressure. If heated, mercury evaporates and becomes highly toxic. Metallic mercury is lipophilic and is stored in fatty tissues.4 Inorganic ions of mercury vary in water solubility. In general, divalent mercuric salts are soluble in water. The high toxicity of mercuric ions can be explained by the high affinity to sulfhydryl groups of amino acids, which are building blocks for enzymes. In organic mercury compounds, mercury is covalently bound to carbon. Organic mercury is the most dangerous form of mercury to human health. Methylmercury, the most predominant form of organic mercury, is the form that poses a risk through fish consumption. Methylmercury is better absorbed and shows a higher mobility in the human body than inorganic mercury. Another example of an organic mercury compound is ethyl mercury or thiomersal (referred to as thimerosal in the USA), which is used as a preservative in some vaccines.
Mercury as a Global Pollutant
Mercury is of global concern. The United Nations Environment Programme (UNEP) assessed the global mercury burden.5 Mercury is now a priority matter in the European Union.6,7 Progress has been made toward an anthropogenic mercury-free environment but it still remains a significant threat in developing countries.5 In 2006, the International Conference on Chemicals Management adopted the “Dubai Declaration on International Chemicals Management,” the “Overarching Policy Strategy,” and endorsed the “Global Plan of Action,” in which priority attention is given to mercury.8,9 These 3 documents constitute the Strategic Approach to International Chemicals Management. The intergovernmental forum on chemical safety expressed concern about mercury and other toxic metals in “The Budapest Statement on Mercury, Lead, and Cadmium.”10 The scientific community expressed their concern about mercury and other heavy metals in “The Declaration of Brescia on Prevention of the Neurotoxicity of Metals.”11 UNEP has a special ad-hoc open-ended work group on mercury (http://www.chem.unep.ch/mercury/OEWG2/Meeting.htm).
Mercury in the Environment
Mercury pollution of the environment has natural, anthropogenic, and historic sources.1,5 The proportion of anthropogenic mercury nearly doubled within the last 100 years and with about 70% distinctly out-weighed naturally released mercury.12 The mercury problem is mainly a man-made problem and therefore can be minimized by implementing efficient measures. Mercury is not only anthropogenic, it also occurs naturally. Natural mercury releases can be caused by volcanic activity, weathering of rocks, forest fires, and water movement. In all geologic media, mercury can be detected in variable concentrations.5 Anthropogenic mercury is released from numerous sources. UNEP classified anthropogenic sources into the 3 following categories: (1) mobilization of mercury impurities from, for example, coal-fired power plants, fossil burning, or cement production; (2) releases of mercury from intentional activities, such as mercury mining, artisanal gold and silver mining, chlor-alkali production in which mercury is used as a catalyst, manufacturing of mercury-containing medicinal products (thermometers, sphygmomanometers, and other measuring instruments) and other products (batteries, switches) and the use of fluorescent lamps, measuring instruments, and amalgam fillings; (3) combinations of intentional releases and mobilization of mercury impurities from, for example, waste incineration, landfills from mining tailings or waste incineration tailings, vaporizing of amalgam fillings in crematoria, or remobilization of historic sources of mercury in soil.5
Hot Spots of Mercury Pollution
Artisanal gold mining is a global activity, mainly in developing countries. Up to 15 million miners are working with mercury, and 80-100 million people depend on gold mining as the main source of family income.13 With favorable international prices, gold mining has gained increasing importance. Concerns over the impact of artisanal small-scale mining practices on the environment, occupational health of the miners, health of the local communities, and social dimensions have been investigated.14,15
Mercury-cell chlor-alkali plants have been identified as the main sources of mercury releases to the environment.16 The site in Vlora (Albania) is defined as a “hot spot of pollution.” The plant covers about 50,000 square meters and is located near the Adriatic Sea. At this site, the Vlora former chemical complex produced chlorine alkali until 1992.17 Mercury contamination due to mercury seawater electrolyzers and problems with children with low intelligence levels were noticed in South India.18 Zheng et al. found that the average and peak mercury daily intake of mercury for children resulting from the consumption of vegetables was 0.02 and 0.07 μg/kg/d, respectively, near the Huludao zinc plant in Liaoning province, Northeast China, an area with very high contamination levels in soil, water, and the atmosphere. Weekly intakes of total mercury for children were 2.8% and 9.7%, respectively, of the provisional tolerable weekly intake.19
The former 13 large-scale mercury mines located at Wanshan, Guizhou Province, China are the largest mercury deposits, accounting for 60% of the mercury in total in China. Twenty thousand tons of were produced in Wanshan between the 1950s and the 1990s. It is classified among the top 10 of the World’s Worst Polluted Places.20 The surface water systems, air, and soil in Wanshan are highly contaminated.21-23 Mercury has contaminated rice in this region.23 The long-term dietary consumption of mercury-contaminated rice induces the aggravation of free radicals and exerts oxidative stress for humans, based on findings of the oxidative stress damage induced by consumption of Wanshan mercury-contaminated rice in rats.24
Another hot spot is at Huancavelica (Peru) where the largest mercury processing district has been present since the Spanish colonial period. This former mine provided the mercury used to extract silver from ore. The residents have been living with mercury for nearly 400 years and the effects of mercury exposure are now present.20,25 These “hot spots of pollution” pose a threat to the environment and to the health of children living near the former industrial sites. Environmental and human exposure assessments are needed in these regions.26
Environmental Sources of Exposure
Mercury Sources
There are numerous environmental sources of mercury that contribute to global mercury pollution. Some of these industries include the following: (1) the health care sector, in which mercury is used in measuring instruments or as a disinfectant and in dentistry; (2) the mining industry; power plants, crematoria; (3) and the charcoal industry. A matter of serious concern is mercury exposure via environmentally contaminated food, mainly seafood, where mercury bio-accumulates in the food chain. Efforts have been made to mitigate the global mercury burden. In some sectors mercury has been successfully phased out. For example, in the health care sector, mercury-free measuring products and disinfectants have been adopted in the last few years.27 Another achievement is the stepwise conversion and implementation of new technologies in the chlorine alkali industry.5 The last 2 European mercury mines in Almaden/Spain and Idrija/Slovenia were recently closed, with the goal of reducing the amount of mercury on the international market.28-30 Mercury is still mined in Kyrgyzstan and China. Particularly effective methods have been implemented in developed countries to reduce mercury burden. In many developing countries mercury is still a big problem and action is urgently needed. The main focus should be on removal of anthropogenic sources of mercury and prevention of exposure.31
Children are exposed to mercury through primary and secondary pollution. Children are exposed through air, water, food, and soil (Fig 1). The following sections discuss the various exposure routes. Mercury circulates in the environment such that exposure is a global problem rather than a local issue; in addition, it is able to circulate through the atmosphere, as well as through the aquatic environment. Most of the emitted mercury is in the form of gaseous elemental mercury and can be transported over thousands of kilometers.32,33 Mercury exposure can occur in saltwater or freshwater environments. Exposure can be through direct discharges from industry and households, indirect releases via waste water treatment systems, deposition of mercury from air, surface runoff of soil with mercury depositions, and leakage of water from soil and landfill contaminated with mercury.5
FIG 1.
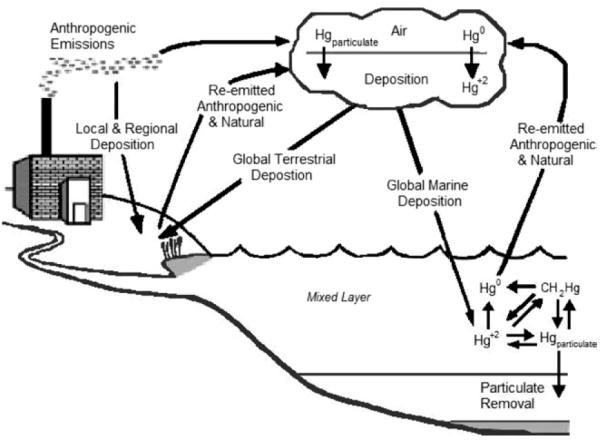
The global cycle of mercury (from US-EPA, 2004,257 adapted from Mason RP, et al. The biogeochemical cycling of elemental mercury: Anthropogenic influences. Geochim Comochim Acta 1994;58:3191-98).
Oceans, rivers, and other water bodies are dynamic sinks of mercury and therefore the aquatic environment has a crucial role the global cycle of mercury. Certainly, mercury in water can be a source of human exposure but of main concern is the biotransformation of mercury in the aquatic environment. In this process, mercury in an aquatic environment can be converted into the organic bound form methylmercury by certain bacteria and abiotic chemical processes. This process, called biomethylation, is influenced by ambient factors, including the temperature, the pH of the surrounding water, the redox potential, and complexing substances.34
Methylmercury accumulates in fish, shellfish, and sea mammals and biomagnifies in the aquatic food chain. The concentration of methylmercury is greater in the predator than in its prey, and the mercury accumulation increases up the food chain.4
Food
For nonoccupationally exposed individuals, the main source of methylmercury exposure is through consumption of contaminated fish and shellfish.35 Mercury cannot be eliminated by cooking. Inorganic mercury is also accumulated along with methylmercury in food. In 1990, the World Health Organization (WHO) estimated a human daily intake of inorganic mercury of about 4 μg in the European and North American general population. In total, 6.6 μg total mercury is taken up per day. From this, 0.6 μg is from methylmercury in fish.36 In mammals, methylmercury from fish products is in part converted into inorganic mercury and therefore might be partially relevant for the consumption of meat and poultry products.5
Tables 1 and 2 summarize types of fish with the highest levels of mercury and seafood with expected low levels of mercury. Data on mercury levels in other types of fish and seafood are available on the US Food and Drug Administration web site, which was last updated in 2006 (http://www.cfsan.fda.gov/~frf/seamehg.html). There are several sites and articles that give expanded mercury values of fish in their local regions. It may be necessary to consult local advisories for specific fish that are only located in 1 locality. Additional mercury concentration data on specific types of locally consumed fish and seafood are necessary in all countries to describe the mercury levels in commercial and noncommercially available fish so that people can make informed choices.
TABLE 1.
Fish with the highest observed mercury concentrations (source of data: FDA 2000248)
| Species | Mercury concentration (p.p.m.) |
Number of samples | Source of data | ||||
|---|---|---|---|---|---|---|---|
| Mean | Median | STDEV | Min | Max | |||
| Mackerel king | 0.730 | N/A | N/A | 0.230 | 1.670 | 213 | Gulf of Mexico report 2000 |
| Shark | 0.988 | 0.830 | 0.631 | ND | 4.540 | 351 | FDA 1990-02 |
| Swordfish | 0.976 | 0.860 | 0.510 | ND | 3.220 | 618 | FDA 1990-04 |
| Tilefish (Gulf of Mexico) | 1.450 | N/A | N/A | 0.650 | 3.730 | 60 | NMFS report 1978 |
TABLE 2.
Fish/seafood with low observed mercury concentrations (source of data: FDA 2000248)
| Mercury concentration (p.p.m.) |
Number of samples | Source of data | |||||
|---|---|---|---|---|---|---|---|
| Mean | Median | STDEV | Min | Max | |||
| Tuna (canned, light) species | 0.118 | 0.075 | 0.119 | ND | 0.852 | 347 | FDA 2002-04 |
| Shrimp* | ND | ND | ND | ND | 0.050 | 24 | FDA 1990-02 |
| Salmon (fresh/frozen)* | 0.014 | ND | 0.041 | ND | 0.190 | 34 | FDA 1990-02 |
| Salmon (canned)* | ND | ND | ND | ND | ND | 23 | FDA 1990-02 |
| Haddock (Atlantic) | 0.031 | 0.041 | 0.021 | ND | 0.041 | 4 | FDA 1990-02 |
| Scallop | 0.050 | N/A | N/A | ND | 0.220 | 66 | NMFS report 1978 |
Standard deviation data generated for new data 2004 or later only.
Mercury was measured as total mercury except for species (*), in which only methylmercury was analyzed.
ND, mercury concentration below detection level (level of detection (LOD) = 0.01 ppm) data not available.
Products from mercury cell chlor-alkali industry are widely used. Some of these products are used in the food industry as food ingredients, eg, citric acid, sodium benzoate, and high fructose corn syrup. Mercury was found as a contaminant in high fructose corn syrup, which may be part of children’s diets.37
While methylmercury-containing fungicides are no longer in use, mercury may still be present in rice. A study conducted in Saudi Arabia found that while the concentration in rice was below the 43 μg/d intake of mercury set by the Food and Agriculture Organization/WHO provisional tolerable weekly intake values, these values are for the contribution of rice only. Taking into consideration other dietary sources of mercury exposure, rice may contribute to an elevated dietary exposure.38 In addition to previous fungicide use, mining activities introduce another route of mercury exposure into the food chain through rice consumption in some regions of the world. A study conducted in the Wanshan mercury mining area in the Guizhou province of China demonstrated that rice from that region contained elevated levels of total mercury and methylated mercury22 and was a staple food in the population’s diet.21,23
Soil—Terrestrial Environment
Sources of mercury depositions in soil and soil surfaces can be the deposition of mercury from air, diffuse releases from waste products, such as batteries, switches, and medicinal waste, intended or unintended local releases from industry, spreading of sewage sludge containing contaminants on areas under cultivation, disposal on landfills, use of solid products from waste incineration, and coal combustion as construction material or decomposition of bodies with amalgam fillings.5
Fluorescent Light Bulbs
The use of compact fluorescent light bulbs has dramatically increased over the past few years. The appeal of compact fluorescent light bulbs is due to their significant increased energy efficiency (75%) compared with incandescent light bulbs and their greater lifespan of use. A compact fluorescent light bulb reportedly has 10 times the lifespan of use compared with an incandescent light bulb.39 During the hour immediately following the break of a compact fluorescent light bulb, mercury gas concentrations near the bulb shards are between 200 and 800 μg/m3. The average 8-hour occupational exposure limit allowed by the US Occupational Safety and Health Administration is 100 μg/m3. Within 4 days, a new 13-watt compact fluorescent light bulb releases about 30% of its mercury with the remaining mercury staying in the bulb debris. Cleaning up the glass shards after breakage reduced mercury release by approximately two thirds. Used bulbs followed similar patterns as brand-new bulbs but with lower rates.40 The risk can be put into perspective somewhat by considering that a power plant produces 10 mg of mercury to produce the electricity needed to light an incandescent bulb, while a compact fluorescent bulb contains 2.4 mg of mercury. In essence, the switch to compact fluorescent light bulbs over incandescent bulbs is a lower net effect of overall mercury in the environment.41,42 There is no dispute over the life cycle analysis in terms of a net reduction of environmental impact; however, there is the public health issue of preventing direct exposure to children in a home if a bulb breaks in the household.43
Health Care
There are 3 main sources of mercury in health care. The first source is dental amalgam, which contains up to 50% elemental mercury. Studies have not associated the exposure from amalgams with health outcomes among children; however, it contributes to the contamination of air when the bodies are cremated.1 In some countries, amalgam is being replaced due to the precautionary principle by mercury-free filling materials. In other countries dental amalgam is still in use, mainly due to financial aspects.35 The second source of mercury in health care is multidose activated vaccines containing ethyl mercury as a preservative. The third source of mercury in health care is the ongoing use of mercury-containing measuring devices, such as thermometers and other devices. Mercury-containing thermometers, sphygmomanometers, some barometers, manometers, switches and gauges used in medical instruments, thermostats, and some medical tubes are a concern in hospital environments because they can release elemental mercury vapor when broken. The production of mercury thermometers is decreasing,27 but they are still in demand. Mercury-free thermometers are now widely accepted.
Traditional Practices
Some traditional practices use mercury, but the extent of use is unknown.1,44 Elemental and inorganic mercury are used in some traditional therapies and religious practices, for example, Santeria or Espritismo or Ayurvedic medicine. For ritual reasons, mercury might be burned in a candle, spread in the room, carried as a talisman, or used in another manner.45,46 There are numerous reports of heavy metal poisoning with mercury from Ayurvedic medicine, which is used for children and adults.45 The use of mercury containing skin lightening creams and soaps, hair treatment, and other cosmetic products is an important source in some cultures, although the extent of exposure is difficult to estimate.47-49
Children’s Exposure
In this section, the specific exposure of children will be described (Table 3).
TABLE 3.
Overview of mercury exposure sources
| Mercury | Sources | Routes of exposure | Elimination | Toxicity |
|---|---|---|---|---|
| Elemental (metallic) | Artisanal gold mining | Inhalation | Urine and feces | CNS |
| Dental amalgams | Kidney | |||
| Crematoria | Lungs | |||
| Thermometers and other measuring devices | Skin (acrodynia in children) | |||
| Folk remedies | ||||
| Volcanoes | ||||
| Combustion | ||||
| Waste incineration | ||||
| Housing on former tailings | ||||
| Inorganic (mercuric chloride) |
Food grown in contaminated sites | Ingestion | Urine | CNS |
| Thiomersal | Dermal | Kidney | ||
| Cosmetics | Gastrointestinal tract | |||
| Folk medicine | Skin (acrodynia in children) | |||
| Lamps | ||||
| Photography | ||||
| Disinfectants | ||||
| Organic (methyl; ethyl) |
Fish | Ingestion | Feces | CNS |
| Preservatives | Parenteral | Cardiovascular | ||
| Fungicides | Transplacental |
Vulnerability of Children
Children are considered especially vulnerable to environmental threats. There are specific periods in their development when the exposure to a chemical, physical, or biological agent may result in adverse health outcomes.50-52 In addition to being especially susceptible due to their growth and development, exposures are often higher due to body weight and certain childhood behaviors make them more vulnerable to exposures (playing outside in the sand or soil, putting their hands in their mouths, etc).
Physiological differences between children and adults are not only manifest in immature metabolic pathways. Because important systems are still differentiating and growing, children have unique susceptibilities not seen in adults—and critical time windows for those susceptibilities.53,54 The critical times are preconception, gestation, and postnatal. More than 1 system can be susceptible and different pathology may occur depending on the dose and timing of exposure. The fetus and infant are especially vulnerable to mercury exposures. Of special interest is the development of the central nervous system. With the formation of neuronal cells and the subsequent stages of development, the central nervous system is created.55 Damage of the nervous system caused by mercury is likely to be permanent.56,57 Neurotoxic effects can result from prenatal or early postnatal exposure.58
Sources of Children’s Exposure
Sources of Children’s Exposure to Mercury Vapor and Metallic Mercury
Children’s 3 main pathways of exposure to mercury vapor are exposure from dental amalgam, take-home exposure from occupationally exposed adults, and accidental exposure. Elemental mercury is widely used in industrial production processes (for example, in chlor-alkali production, in the fabrication of measuring instruments, such as thermometers and manometers, and in batteries and fluorescent light bulbs) with resulting pollution of the working environment of adults. Another occupational source of mercury exposure is mercury mining and smelting and artisanal gold mining. This is no longer a big issue in Europe and the USA but is an issue in areas of the world in which children may be involved in the gold extraction process.5,59
The International Labor Organization has expressed concern about child labor in gold mining.60 Up to 1 million children are involved worldwide in any kind of mining (http://www.ilo.org/ipec/areas/Miningandquarrying/lang--en/index.htm). Many of these children have direct occupational contact with mercury.60 A study to assess the health of children in artisanal gold mining areas documented that children working with mercury had high levels of mercury and symptoms of mercury intoxication.61
Although gold mining is extremely dangerous work for children, tens of thousands of children can be found in the small-scale gold mines of Africa, Asia, and South America. Children work both above and under ground. Mercury is mixed with the crushed ore or sediments to separate out the gold. Mercury is very often mishandled by small-scale miners. It can be absorbed through the skin or through inhalation of mercury vapor. Seeping into the soil or water supply, it can contaminate food and drinking water. Informal gold miners often do not wear protective clothing and most do not know about the proper handling of mercury. In some countries mercury amalgamation is done at home by women, which exposes other family members, including very young children, to mercury (http://www.ilo.org/ipec/areas/Miningandquarrying/lang--en/index.htm).
Another pathway of exposure is the use of mercury in ethnic and religious practices and also in folk remedies.45
Of concern is the accidental exposure from broken thermometers,62 and other medicinal measuring devices.63,64 Children have been exposed to mercury vapor after the application of interior latex paints.65,66
The principal form of children’s exposure to mercury in school is elemental mercury (Hg). The numerous sources of elemental mercury include thermometers, old barometers and electrical switches, and the liquid metal used in school laboratories.67,68 Moreover, children are often attracted to elemental mercury because of its unique physical properties, including silver appearance, density, and tendency to form beads.69
Today, in most developed countries children’s exposure to elemental mercury commonly occurs by accident. In the USA, elemental mercury was found to be 1 of the 10 most frequently released hazardous substances; numerous spills occurred in schools during the period 1993-1998.70,71 In the same period, the US Agency for Toxic Substances and Disease Registry analyzed mercury releases that occurred in 15 states and found that, among the 405 events in which mercury was the only substance released, schools and universities were the most frequent locations involved in fixed-facility events (n = 79, 20.3%). Five victims of these events were students visiting elementary or secondary school (36%, the same percentage of occupational lethal cases).71
Sources of Children’s Exposure to Inorganic Mercury
Inorganic mercury compounds show antiseptic, laxative, and diuretic properties. The medicinal use of mercury salts has nearly disappeared since a ban was placed on distributing consumer products containing mercury salts. Thiomersal, with ethyl mercury as a decomposition product, was formerly used as a topical antiseptic and is still found in some multidose inactivated vaccines.
Skin-lightening creams and soaps are still widely applied in developing countries.47,72 There is at least 1 case report that children in a refugee camp were exposed through use of cosmetics containing mercury salts within the families.64 The use of mercury in folk medicine, for example, in Ayurvedic medicine, is not uncommon, especially when the Ayurvedic formulation is produced in developing countries with lower requirements for quality and safety.73,74
Sources of Children’s Exposure to Organic Mercury
The main source of children’s exposure to organic mercury is the consumption of methylmercury-contaminated seafood. Methylmercury is formed by bacteria out of elemental or inorganic mercury industrial discharges into the environment or natural releases.3 Methylmercury accumulates in the aquatic food chain. In general, the bigger the carnivore fish, the higher the methylmercury content.
Methylmercury was also used as a fungicide for the treatment of seed grain. This led to a mass intoxication among people in Iraq in the 1970s.75-80
Routes of Children’s Exposure
The pathways of mercury entering the body are described. The absorption routes for mercury are ingestion, inhalation, transdermal absorption, and transplacental absorption (Fig 2).
FIG 2.
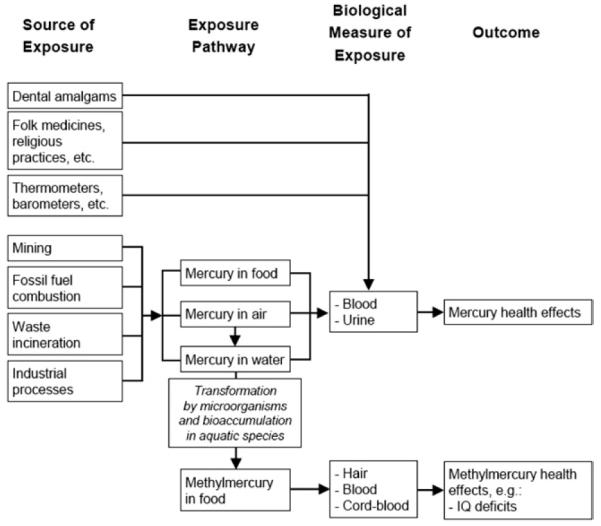
Framework of mercury exposure.175
Ingestion
Ingestion is the main route of exposure for methylmercury. This organic-bound mercury from food, especially fish, is very well absorbed from the gastrointestinal tract.81,82 Also inorganic mercury can be absorbed from the gastrointestinal tract after ingestion. However, the extent varies by solubility of the inorganic mercury compound. In general, the extent of absorption is higher with increasing solubility. Inorganic mercury salts can be found in some Ayurvedic remedies or traditional medicine. Liquid mercury is not well absorbed from the gastrointestinal tract. The reason for the very low absorption rate is that mercury first must be vaporized. The absorption of this vaporized mercury is also limited because mercury vapor is quickly bound to sulfhydryl groups in the gastrointestinal tract. Therefore, ingestion of liquid mercury has a lower impact on human health than the ingestion of organic and inorganic mercury, respectively. The predominant route of exposure to methylmercury for children in most countries in the European Union, North America, and Japan is via fish consumption. Epidemiologic studies in many countries consistently report that fish intake is the single most influential predictor of blood or hair mercury levels. Two scenarios of concern involve persons with high or particular consumption patterns of fish, and anglers and others who consume wild catch. High-level fish consumers are of particular concern, those who select fish from the higher trophic levels of food webs, such as tuna, bass, mackerel, or swordfish, as these are known to carry elevated levels of methylmercury in edible tissues. A case study of such a scenario was published by Hightower and Moore. There were 7 children in the study whose parents reported frequent consumption of tuna in sushi and sashimi. One of these children, a 7-year-old boy (who also consumed mackerel), had a hair mercury level of 15 μg/g. After 32 weeks without fish in his diet, his hair mercury level was below 1 μg/g.83 Fish is a good dietary source of lean protein and omega-3 fatty acids and fish should be part of a healthy diet. These fish ingredients are important for a child’s proper development. These beneficial effects may obscure adverse effects of prenatal methylmercury exposure.
Women who may become pregnant, pregnant women, nursing mothers, and young children should avoid some types of fish and eat fish and shellfish that are lower in mercury. These susceptible subgroups should not consume shark, swordfish, king mackerel, or tilefish because they contain high levels of mercury. Women of childbearing years and children are urged to eat local panfish and gamefish sparingly, and to avoid all consumption of muskellunge, a top predator species.84,85 They are advised to consume up to 12 oz. (2 average meals) a week of fish/shellfish that are known to have lower mercury concentrations. People often consume noncommercially purchased fish (including fish caught locally by family and friends). In these cases they are advised to check local advisories about the safety of fish caught in local lakes, rivers, and coastal areas. If no advice is available, they can consume up to 6 oz. (1 average meal) per week of fish caught from local waters, but not any other fish during that week.86
Methylmercury is excreted into breast milk.87 Less is known about the excretion of inorganic mercury but animal studies have demonstrated that mercury from mercury vapor exposure is excreted into milk. Organ distribution of sucklings suggested that they were exposed to inorganic mercury via milk.88,89 Nevertheless, the advantages of breastfeeding outweigh the possible risks. Consequently, mothers should still be encouraged to breastfeed.90
Inhalation
The respiratory tract is the main absorption route of mercury vapor. Human studies indicate that about 70%-85% of inhaled mercury vapor is absorbed by the lungs into the bloodstream.91 Furthermore, the migration of mercury vapor from the pharynx to the brain via olfactory neurons has been demonstrated.92 Inhalation of mercury vapor occurs in children with amalgam fillings.
Also methylmercury vapor is absorbed by the lungs after inhalation. Data on animal studies have shown that methylmercury vapor is rapidly and almost completely absorbed into the bloodstream.93
Transplacental
Elemental as well as organic mercury can easily pass the placenta and can accumulate in the fetus because the fetus is not able to excrete mercury. Methylmercury can be detected in umbilical cord blood.94 The transplacental route of methylmercury exposure to the fetus via maternal fish consumption was first observed in Minamata Bay in Japan.94,95
Transdermal
Cosmetic preparations containing inorganic mercury compounds, such as mercuric chloride, have been used for their skin-lightening effect.47,49,96 Phenyl mercury absorbed through the skin from contaminated diapers affected urinary excretion in infants in Buenos Aires.97
Mercury-containing preparations are used in many areas of the world, including China, Central and South America, Africa, and the Middle East. The mercury in these preparations is absorbed through the skin to cause systemic mercury toxicity and there are reports of nephrotoxicity (including nephritic syndrome), dermal toxicity, and neurological toxicity associated with their use.
Toxic Effects
Mercury Toxicity
Historically, high exposures, such as those that occurred near Minamata Bay, Japan and Basra, Iraq have contributed to our understanding of the toxicity of mercury. Studies have since focused on assessing the impact of methylmercury on children’s health. Three large-scale, prospective epidemiologic studies assessed the effects of low-dose in utero exposure to methylmercury. These studies were conducted in New Zealand, the Faroe Islands, and the Seychelles. In the New Zealand study98,99 and the Faroe Islands study100-102 associations between prenatal mercury exposure and the neurological development of the children were demonstrated. Outcomes associated with prenatal mercury exposure included the loss of IQ points, and decreased performance of tests, including memory, attention, language, and spatial cognition. Prenatal mercury exposure was measured as mercury concentration in maternal hair, cord blood, or children’s hair. In the Seychelles study adverse effects on neuropsychological development and IQ were not observed.103-109 The mercury exposure levels observed in children in the Seychelles study were similar to the levels among children in the Faroe Islands study.
The use of mercury goes back to ancient times. It was used for medicinal purposes, including for the treatment of skin diseases and syphilis. Serious side effects were common, including death. The medicinal use was widespread until the 20th century when more became known about the harmful effects of mercury exposure.
Concerns were raised in 1999 about the cumulative amount of mercury in infant immunization schedules. Beginning in 1930, thiomersal, which contains 49.6% ethyl mercury, was added in some multidose vaccines for preservation. Ethyl mercury can also be a contaminant of pretreatment procedures. Unlike methylmercury, ethyl mercury does not accumulate in the fatty tissues of the body and is actively excreted via the gut. In 2006, the WHO Global Advisory Committee on Vaccine Safety concluded that there were no reasons to change current immunization practices.1,110-112 The use of mercury in vaccines is, however, still very controversial.113-121 WHO continues to review the evidence for preterm and malnourished infants.1
The use of mercury amalgam is still an established dental practice in many countries,35 although questions have been raised about children’s exposure to mercury from amalgam fillings. Mercury forms an amalgam when combined with other metals, such as gold, silver, and copper. There is an association between the number of dental amalgam fillings and mercury concentrations in urine and blood.122,123 Recent longitudinal studies on the use of amalgam fillings in children did not observe any negative effects on neuropsychological function within a 5-year follow-up period.124,125 The use of amalgam fillings for children has been discontinued in several countries due to the precautionary principle. For example, since 1997 the use of amalgam fillings for children is no longer permitted in Germany.126
In addition to its medicinal use, liquid mercury has been used for centuries in the recovery of gold and silver from ore. To date, this simple method is still applied in artisanal gold mining, a poverty driven and predominantly illegal activity in developing countries. A particular concern is that child labor is not uncommon in artisanal gold mining. Not only is this work physically demanding, but these children are also highly exposed to mercury.61
Many international studies have been conducted to investigate the impact of various sources of mercury exposure on children’s health. However, in contrast with lead, studies examining the cost of mercury exposure are rarely found.127,128 A study in the USA assessed the impact of industrial mercury emissions on children’s health and found that an estimated 300,000-600,000 American children could have reductions in IQ related to mercury.128 Estimates are that the loss of productivity due to loss of intelligence caused by methylmercury are an average 8.7 billion USD (US Dollars) annually, with emissions from American power plants accounting for 1.3 billion USD.127 Another study assessed globally the societal damages caused by ingestion of methylmercury for the year 2020. The estimate is that the annual cost will be approximately 3.7 billion USD due a loss of IQ. The corresponding cost of damages due to inhalation of methylmercury is estimated with 2.9 million USD.129
Neurodevelopmental Toxicity
Neurodevelopmental effects in the fetus are associated with maternal exposure. Mercury can also cause neurocognitive deficits and neuromotor disabilities. As mentioned earlier, 3 extensive epidemiologic studies among fish-eating populations have assessed mother– child pairs for prenatal methylmercury exposure and the resulting impact on child development. The Seychelles child development study examined 779 mother– child pairs with a permanent low-dose prenatal exposure to methylmercury.103,108,130,131 The exposure was due to continuous seafood consumption. The exposure was monitored by mercury levels in maternal hair. At the age of 9 neuropsychological tests were performed. Developmental milestones and neurodevelopmental outcomes using standardized testing batteries were investigated across 5 stages of age of the children. However, no convincing evidence was found to support the study thesis of adverse effects on children due to consumption of fish contaminated with methylmercury. A detailed summary of the studies has been published.132
The New Zealand study investigated 38 children of mothers who showed a mercury level higher than 6 p.p.m. (6 μg/g hair) during pregnancy and matched them with children from mothers with lower mercury levels in hair.98,99 A total of 237 children were assessed at an age of 6 years with a method similar to the Seychelles study.99 Correlations between dose and neuropsychological endpoints could be detected. A similar result was obtained from the study in the Faroe Islands in which dose-related effects were found.133
The Faroe Islands cohort included mother– child pairs but in contrast to the other 2 studies they were reported to eat whale meat episodically.100 Mercury exposure was determined by cord blood and maternal hair. At 1 year of age, children were tested for milestones101 and at 7 years of age the children were comprehensively neuropsychological assessed. A cohort of 1022 children born 1986-1987 was exposed to methylmercury. The mothers episodically ate pilot whale meat, which is potentially high in methylmercury, and continuously ate fish with a comparably lower methylmercury concentration. At age of 7 and 14, neuropsychological tests were performed, showing neuropsychological dysfunctions mainly for language, attention, and memory, and less for visuospatial and motor functions. Neurophysiologic tests showed delayed brainstem auditory-evoked potentials,57 decreased autonomic heart rate variability, both attributed to prenatal exposure. The association remained after adjusting for confounding variables and excluding children from mothers with increased hair mercury concentrations (>10 μg/g), indicating that negative effects can be found at levels previously considered safe.134
Some have hypothesized that the risk of neurological damage might be higher in the case of infrequent meals high in mercury content than in the case of continuous low-dosed meals.132 A study by Lederman et al. confirmed the association between low-dose mercury exposure and negative neuro-development.135 Reports from the Amazonian area confirm the negative effects of methylmercury exposure on the neurodevelopment, eg, visuospatial capacities.136 Breastfeeding seems to have a neurodevelopmentally protective effect even in these highly exposed areas.137 Freire et al. examined preschool children regarding methylmercury contaminated nutrition and cognitive development in Spain.138 A positive association between mercury exposition due to ingestion and delay of cognitive development was identified.94 Effects on behavioral functions, like attention, activity, and emotional outcomes were not associated with prenatal and postnatal mercury exposure in Canadian 5-year old Inuit children.139
The Minamata outbreak, in which the population was heavily burdened with methylmercury by seafood consumption, showed that besides neurodevelopmental and neurocognitive impairment, other symptoms, such as vision impairment, paresthesias, neuralgias, dermographism and impairments of taste, smell, and hearing, as well as seizures and in some cases coma and death can occur during fetal exposure to a high dose of methylmercury. Intrauterine and early neonatal death have been observed.94 Similar symptoms in adult patients were observed after the outbreak of mercury poisoning in Iraq caused by contaminated seed grains.79
Nephrotoxicity
Inorganic mercury compounds are nephrotoxic and can cause kidney damage in children. The main target in the kidneys is the proximal tubules. To some extent, the tubular cells are able to regenerate. However, in severe cases of inorganic mercury intoxication, the function of the kidneys can be limited and death might occur due to acute kidney failure.132 Phenyl mercury skin absorption via contaminated diapers showed an effect on the urinary excretion for Argentinian infants.97 A study among 403 children in China revealed no nephrotoxic effects for mercury exposure from dental amalgam fillings.140 A study among 534 children in the US showed an increase of microalbumin among the amalgam-exposed group. Microalbuminuria excretion is an indicator of adverse kidney effects. However the other biomarkers did not show an effect (alpha-1-microglobulin, gamma-glutamyl transpeptidase, and N-acetyl-beta-d-glucosaminidase).124,141
A study with adults and children in gold mining areas showed a correlation between mercury exposure and proteinuria.142
Teratogenicity
In toxicologic studies using high doses of inorganic mercury compounds or methylmercury, teratogenicity seems possible. However at regularly occurring exposure these effects have not been found.70,143
Cardiovascular Toxicity
Heart function alteration has been described in children associated with methylmercury exposure from seafood.134 The association of methylmercury exposure and cardiac effects was observed with decreased sympathetic and parasympathetic modulation of the heart rate variability. This might be due to methylmercury neurotoxicity to brainstem nuclei. A study among 274 Korean children revealed an association between urinary mercury concentration and an increase of cholesterol as a risk factor for myocardial infarction and coronary or cardiovascular disease.144 Another study from Korea indicates that the cardiac autonomic activity through parasympathetic dysfunction might be influenced by mercury even at low exposure levels in the first and second decade of life.145 Data from the Seychelles study indicate that prenatal methylmercury exposure might predict elevated blood pressure levels for teenage boys.146 A 4-year-old boy developed acrodynia, including tachycardia and hypertension due to exposure from mercury-containing interior latex paint in the US.147 Among adults methylmercury exposure is associated with increased blood pressure.148
Carcinogenity
High exposure to methylmercury is associated with leukemia among adults.149 The International Agency for Research on Cancer evaluated the strength of evidence for carcinogenity of mercury in a standardized manner using data from animal and human studies. Methylmercury compounds are classified as possible carcinogens to humans (group 2B). Metallic mercury and inorganic mercury compounds were not classifiable with regard to their carcinogenicity in humans (group 3).150 No specific data on the cancer risk for children are available.
Genotoxicity, Mutagenesis
Mercury seems to have a weak mutagenic potential.3,143 Thimerosal induces significantly sister chromatid exchanges, indicating a genotoxic and cytotoxic effect of thimerosal in cultured human peripheral blood lymphocytes.151
Reproductive Toxicity
One retrospective study examined the effect of methylmercury contamination on the sex ratio of offspring at birth and of fetuses at stillbirth. Due to the severe methylmercury pollution in Minamata, lower numbers of male offspring at birth were found. An increase in the quantity of male stillborn fetuses in Minamata was described. This observation indicates that male fetuses could be more susceptible.152 The Iraqi outbreak of organic mercury poisoning was associated with an abnormally low number of pregnancies.79 Exposure of dental assistants to mercury vapor was associated with spontaneous abortions, stillbirths, and congenital malformations.153
Immunotoxicity
Mercury is likely to be immunotoxic, as shown in animal models.3,154 Studies of mercury exposure in the Amazonian region due to gold mining activities showed a positive association between mercury and malaria.155 The New England children’s Amalgam trial showed a nonsignificant negative immunotoxic effect in the form of a decline in responsiveness of T cells and monocytes at 5-7 days after treatment.156
Clinical Presentation of Children With Mercury Exposure
Prenatal Chronic Methylmercury Exposure
Prenatal chronic methylmercury intoxication can occur when the mother is exposed to high levels of methylmercury. The placenta is not an effective barrier against mercury. Mercury can have a negative effect on the fetus even if the mother does not show symptoms.94 The central nervous system of the fetus is especially vulnerable during periods of rapid maturation.55
Low-dose in utero exposure to methylmercury has been assessed through prospective epidemiologic studies. The New Zealand study and the Faroe Islands study showed correlations between prenatal mercury exposure and the neurological development of children.98-102 The main observation was loss of IQ points, decreased performance on tests, including memory, attention, language, and spatial cognition. In contrast, the Seychelles study did not show adverse effects on neuropsychological development and IQ.103
Knowledge about the extreme vulnerability of the fetus to methylmercury began with the Minamata Bay, Japan experience. High exposure to methylmercury occurred in Minamata. A chemical company released mercury into Minamata Bay and polluted the bay heavily for decades. Mercury accumulated in the aquatic food chain. The released mercury was methylated in the aquatic food chain leading to high levels of mercury in fish. The local fish was very high in methylmercury, and the local population consumed high amounts of the fish. Eating the fish, pregnant mothers did not only burden themselves, but methylmercury was transferred in utero to the fetus. This caused severe neurological complex symptoms and severe birth defects. While the mothers were usually without symptoms of mercury poisoning, their babies were born severely damaged with microcephaly, cerebral palsy, severe mental retardation, seizure disorders, blindness, deafness, and other malformations.94,95
Depending on the dose and timing of exposure during gestation, the effects may be severe and immediately obvious, or subtle and delayed, as shown in Figure 3. Neurological symptoms include mental retardation, ataxia and cerebral palsy, seizures, vision and hearing loss, delayed developmental milestones, language disorders, and problems with motor function, visual spatial abilities, and memory. The newest findings from long-term cohort studies suggest that the cardiovascular system is also at risk—with increased incidence of high blood pressure and decreased heart rate variability as methylmercury exposure increases.134,146 The full expression of these health effects of methylmercury can be delayed and deficits are often irreversible.
FIG 3.
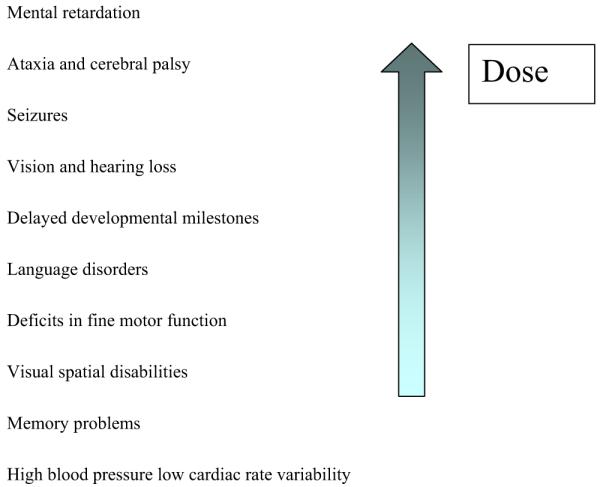
Effects of prenatal exposure. (Color version of figure is available online.)
Once the exposure has occurred in these severe cases, no effective treatment is possible. In other cases the children may be treated with early stimulation and other psychological treatment. Prevention is essential to avoid exposure.
Chronic Mercury Exposure and Skin Reactions
Mercury compounds, including inorganic and organic forms, can induce dermatotoxic reactions ranging from a chronic dermatitis to acrodynia. Acrodynia, Pink’s disease, and Morbus Feer are synonyms used for a specific clinical picture of mercury intoxication. Acrodynia is a toxic reaction to elemental or inorganic mercury exposure that occurs mainly in young children, rarely in adults.157-160 A special susceptibility may be present, because the symptoms can occur at low levels of mercury exposure. Among 32 published cases the urinary mercury concentrations were below 50 μg/L and in 4 children even below 10 μg/L.157 It is characterized by pinkish discoloration and desquamation (Figs 4-6) [desquamation of hands and feet, morbiliform, rubeoliform or scarlatiniform exanthum, erthyema, symmetrical, mainly hands, feet, and nose, predominantly distal, volar, and plantar specially in cold surroundings (Pink’s disease), bluish, cold, wet extremities], itchiness, pain in the extremities, loss of hair, loose teeth, loss of teeth, hypertension, sweating, insomnia, irritability, and apathy.
FIG 4.
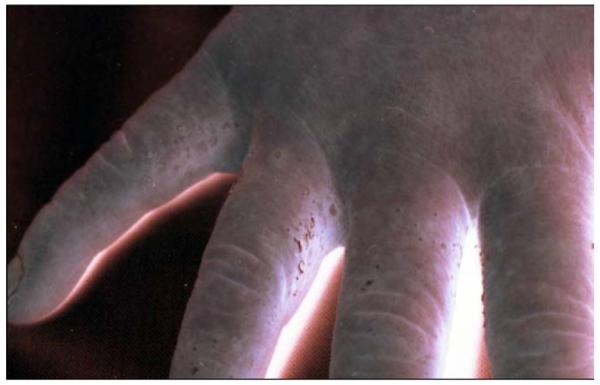
Acrodynia, scaling of the skin between the fingers.158 (Color version of figure is available online.)
FIG 6.
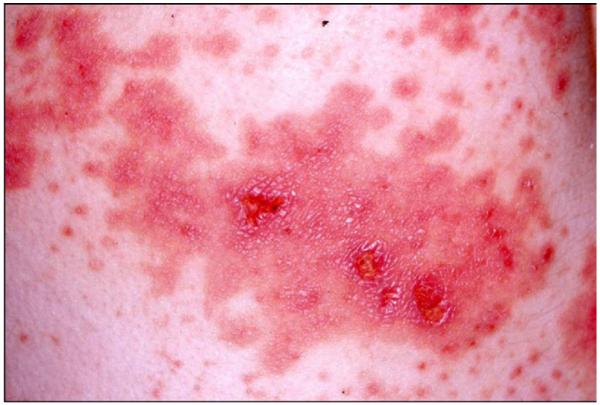
Acrodynia: Exanthema due to mercury intoxication from a mercury thermometer broken in the children’s room 4 months previously. Photo taken 3 weeks after the first pictures.158 (Color version of figure is available online.)
Tremor Mercurialis
Mercury exposure can cause tremor, the so-called “tremor Mercurialis.” Tremor is a very typical symptom of acute and chronic mercury intoxication.
After an accidental intake over months of inorganic mercury-containing seed preservatives, a 9-year-old girl developed severe neurological symptoms. The symptoms increased over time, leading to tremor, dysdiadochokinesia, ataxic movements, ptosis, hypersalivation, aphasia, stupor, kachexia, and incontinence. The development of the tremor was seen in her handwriting (Fig 7). The mercury levels were 9.6 μg/L in blood and 18.5 μg/L in urine. The specimens were taken approximately 3 months after the onset of the symptoms and several weeks after the end of the exposure. An antidote therapy with chelating agents (2,3-dimercapto-1-propanesulfonic acid [DMPS]) was successful. Mercury levels decreased to background levels and symptoms faded until full recovery after 2 years.161
FIG 7.
Handwriting example of a 9-year-old girl in monthly intervals after an accidental intake of mercury, showing the increasing tremor in her handwriting (© Stephan Boese-O’Reilly).161
Mercury Vapor Inhalation
Inhalation of elemental mercury vapor can cause acute and chronic intoxication. Depending on the dose and time, several symptoms can be observed. The diagnosis of mercury intoxication is based on the prevalence of typical symptoms and an elevated mercury level.142 The management is to reduce or eliminate the exposure; a medical treatment with antidotes should be considered. Typical symptoms of mercury vapor intoxication include airway symptoms, such as cough, dyspnea; fever, ill-being, headaches; central nervous system problems (tremor, ataxia, coordination disturbances, dysdiadochokinesia); peripheral nervous system problems (polyneuropathy with sensation difficulties, abnormal reflexes); gingivitis, stomatitis; mercurial erethism (excitability, loss of memory, insomnia, extreme shyness); neurocognitive disorders; kidney problems (proteinuria); and skin symptoms (acrodynia with painful, swelling of extremities, pinkish discoloration, pealing, erythema). There may be a lack of correlation between the symptoms and the level of exposure.2,33,61
In many cases, the correlation between the typical severe symptoms and the measured levels of mercury in urine, blood, or hair are poor.157,160-162 Studies in gold mining areas with high exposure scenarios showed a good correlation between symptoms and scenario, but not with the mercury levels.61 One possible reason is the individual susceptibility to mercury.77-79 There are genetic regulatory mechanisms for the toxicity of mercury.151,163-166 Specimens, such as urine, blood, or hair, do not necessarily reflect the concentration of mercury at the main target organs, such as brain or kidney.167 Mercury exposure can show delayed effects, months and years after the exposure, or get more severe, meaning that the time of exposure and the time of onset of effects can differ.132 Mercury is excreted with a half-time of about 3 months. Effects can be persistent. For example, an 8-year-old boy was hospitalized with a 1-month history of bilateral lower extremity pain resulting in abnormal gait, burning sensation and pain in both hands and feet, headache, dizziness, nausea, constipation, decrease in appetite, and mood lability. He was tachycardic and hypertensive at admission. Slightly increased mercury levels were found in the 24-hour urine (12 μg/L) and the mercury/creatinine ratio was 42.9 μg/g. The source of exposure was presumably a “silvery liquid” observed on the kitchen counter 4 months prior. The source of this liquid remains unknown. The boy recovered completely after treatment with dimercaptosuccinic acid (DMSA). The severity of symptoms did not correlate with the urinary levels of mercury.160
Mercury Spills
From 1999 until the end of 2005, the state of Kentucky experienced 15 mercury spills, 10 of which were associated with schools. In November 2004, a 15-year-old student brought a vial of liquid mercury onto a school bus and into a high school in Kentucky. Mercury had been in the student’s possession for more than 1 year and large amounts had been spilled in multiple places, including the mobile home in which he lived with his family. Blood concentrations, obtained from this student and 7 family members, ranged from 32 to 72 μg/L and the 24-hour urine levels from 28 to 496 μg/L. Among the members of the examined family, the student had the highest mercury levels in both blood and urine. Urine mercury concentrations were directly associated with the amount of time spent in the mobile home.168
In the same year, an elemental mercury release occurred in a middle school in Nevada, where a student took a vial of elemental mercury (about 60 mL) from a storage shed and played with the mercury at home, in the school bus, and in the classroom. The mercury exposure was minimized due to the rapid identification of the problem and decontamination procedures applied. Only the student who brought the mercury had an elevated urine mercury concentration (11.4 μg/L).169
In October 2003 in Washington, DC students stole a container with 250 mL of liquid mercury from a science laboratory and spread it around the school and grounds. The school was shut down and decontaminated. More than 100 homes were found to be contaminated; city buses had to be cleaned because of the mercury contamination, and 1300 students were evacuated in temporary classrooms. Due to the rapid intervention, only 5 people showed symptoms of mercury exposure, but the cleanup and investigation costs were in the millions of dollars.170 Mercury intoxication in 3 Turkish adolescent students with a history of exposure to elemental mercury from broken barometers taken from school laboratories 2-4 months earlier was reported. One of the students died; the others recovered over a period of 1-4 months.171 The lack of data from other areas in the world could testify to the lack of awareness of the symptoms of acute mercury toxicity in children.
Environmental History
To identify exposure with mercury, it is necessary to take an environmental history. It is important to be aware of the sources. The health care provider taking the environmental history should be aware of the typical exposure situations for mercury.
A careful environmental history should be recorded in the patient record.172,173 The American Academy of Pediatrics book Pediatric Environmental Health describes how to take an environmental history.174 Specific questions should be asked, including the following: (1) use of herbal medicines, (2) use of interior latex paint, (3) playing with mercury brought home from school, and (4) occupational exposure of parents or adolescents.
Burden of Disease
The environmental burden of disease from certain mercury exposure settings has been estimated and175 is available at the following link: http://whqlibdoc.who.int/publications/2008/9789241596572_eng.pdf. To achieve these estimates, the methylmercury level in the hair of pregnant women or women at child-bearing age in exposed areas was used to assess exposure. The measured outcome of mild mental retardation of the exposed infants was used as a marker for neurodevelopmental toxicity. Cognitive development has been shown to be negatively influenced by prenatal methylmercury exposure. The most markedly affected group is children with IQ scores just above 69 points. If they “lose” IQ points due to exposure to methylmercury, the development of these infants can be affected and they are classified as having mild mental retardation (IQ between 50 and 69 points). The number of disability-adjusted life years (DALYs) depends on the rate of mild mental retardation caused by methylmercury exposure calculated from the exposure distribution. DALYs are a way to measure population-wise the health impact according to the number of healthy years of life lost caused by the severity and duration of the disease. The calculation was based on the approximation for the outcome (loss of IQ points) by Axelrad (Table 4).
TABLE 4.
Health effects of prenatal exposure to methylmercury175
| Outcome | Group | Biomarker | Threshold | Relationship |
|---|---|---|---|---|
| IQ reduction | Infants | Maternal hair | None | Linear relationship between 1 μg/g increase in maternal hair, mercury concentration, and 0.18 point decrease in IQ133 |
The burden of disease for many settings (including industry, mining, fishing) was estimated. The highest incidence rate for mild mental retardation was calculated for a fishing population in the Amazon (17.37 per 1000 infants) born among a subsistence fishing population in the Amazon, resulting in a loss of 202.8 DALYs per 1000 infants (Table 5). Because no exposure harmonized data are available on a global level, it is extremely difficult to calculate the global burden of disease for mercury.175
TABLE 5.
Methylmercury exposure, mild mental retardation incidence, and DALYs for selected populations175
| Population (reference) | Mean (SD) hair mercury levels (μg/g) |
% of infants losing ≥2 IQ points |
Incidence of mild mental retardation per 1000 infants |
DALYs per 1000 infants |
|---|---|---|---|---|
| Brazilian subsistence fishing population near the Tapajós River in a gold mining region of the Amazon249 |
16.0 (18.92) | 62.44 | 17.37 | 202.8 |
| Chinese fish consumers in Wujiazhan, downstream of a methylmercury-polluted river250 |
2.92 (11.8) | 27.43 | 5.16 | 60.6 |
| Columbian fishing village in the San Jorge River basin near local gold mining activities251 |
5.78 (1.21) | 0.02 | 3.89 | 45.7 |
| Canadian subsistence fishing Nunavik Inuit people in the Arctic207 |
4.5 (1.9) | 0.19 | 3.09 | 36.8 |
| Greenland subsistence fishing Inuit people in the Disko Bay206 | 3.2 (3.4) | 2.28 | 2.52 | 29.9 |
| Canadian fish consumers of Asian-Canadian descent in the Great Lakes “Area of Concern”252 |
2.35 (0.55) | 0.00 | 1.76 | 20.9 |
| Japanese fish consumers in the Akita Prefecture253 | 2.10 (0.98) | 0.00 | 1.45 | 17.3 |
| Canadian sport fishers in the lake St. Pierre region of Quebec254 |
0.68 (0.85) | 0.00 | 0.60 | 7.2 |
Case Management
Case management depends clearly on the severity of symptoms, the source of exposure, the susceptibility of the patient, and the availability of capacities and personal expertise. The severity has to be taken into account (eg, acute, or acute on chronic, or chronic event).
Diagnosis of Mercury Intoxication
A medical history, including an environmental history, a complete physical examination, plus results of mercury measurement in human tissue can exclude or substantiate the diagnosis of mercury intoxication. It is important to handle the collection and analysis of urine and blood mercury tests carefully.67
Human Biomonitoring
Elemental Mercury
Dental amalgam as source of clinical symptoms is very controversial.122,124,125,141,156,176,177 Dental amalgam raises the body burden of mercury, but may not to lead to clinically observable symptoms in children.
Under the high exposure situation in gold mining areas, mercury can cause clinical symptoms in children, which can be diagnosed.61,177-182
Urine levels reflect the acute exposure situation better than blood and much better than hair levels.
Inorganic Mercury
Inorganic mercury exposure is measured in urine if possible using a 24-hour urine sample.67 If the levels are above 10-20 μg/L, it indicates excessive exposure. Neurological signs are very likely if the concentration is above 100 μg/L, but can occur at much lower levels, down to 5-10 μg/L. Mercury blood concentration can be analyzed, but values tend to return to normal (below 5 μg/L) within days after the end of the exposure.67
Organic Mercury
Methylmercury should be measured in blood or hair. In the general population usually the mercury level in hair is 1 part per million or less.67
Clinical Signs and Symptoms—Acute Intoxication
Acute intoxication causes symptoms, depending on the exposure pathway, such as bronchitis, pneumonia, gastroenteritis with blood in the feces, leading to disorders of kidney function. If the history including the environmental history, clinical picture, and mercury levels in urine are concordant, the diagnosis of acute mercury intoxication can be made.183
The symptoms of chronic mercury intoxication in childhood are as follows:
Cerebellar and psychological, vegetative signs: Muscular hypotonia followed by refusing to walk, stand, or sit, disturbed, negative behavior, apathy, loss of appetite, weight loss, nightly sleeping disorders, sleepiness during the day, tremor, ataxia, coordination problems, excessive salivation, metallic taste, increased sweating, severe itchiness, increased blood pressure, tachycardia, light sensitivity, slowly increasing process over weeks.
Skin symptoms: Symmetrical erythema of the nose, hand, and feet, mainly distal, volar and plantar (acrodynia), in cold surroundings more cyanotic and wet, transient, urticaria-like, morbiliform or rubeoliform exanthema, urticaria rubra (scarlatiniform, little pustules), lamellar desquamation of hands and feet.
More neurological symptoms of teenagers: Tremor, dysarthria, paresthesia, ataxia, change of personality, erethism, loss of memory, depression, loss of ability to see colors, concentric narrowing of visual field, unspecific symptoms, such as lack of energy, tiredness, loss of appetite, weight loss, dizziness, headache, concentration problems, sleep disorders.
Measurement of Mercury in Human Specimens
The assessment of mercury toxicity usually begins with an assessment of signs and symptoms. However, most symptoms, particularly at low levels of exposure, may not be specific for mercury exposure. Therefore, diagnosis should include an assessment of mercury exposure.
To assess the exposure to mercury, the source of exposure and the mercury species should be determined to be able to choose the appropriate sample material, the optimal sampling procedure, and sample storage to avoid contamination or losses in mercury concentration during sampling and transport. It is very important to ensure that hypodermic needles and sampling systems are mercury-free. Therefore, specific sampling and test tubes for the analysis of metals and trace elements must be used. Other sampling tubes can be used only if contamination with mercury can be excluded.4
The main method in analytical practice is the analysis of the total amount of inorganic and organic mercury with cold vapor atomic absorption spectrometry after enrichment on a gold–platinum net.4 Speciation of mercury species is more difficult to handle, but possible when preparing the samples adequately. Basic information on analytical methods has been described.184,185 Analytical methods have been summarized.143,186
Urine
Under normal conditions and kidney function, mercury concentration in urine reflects the burden with inorganic mercury, including inorganic mercury salts, mercury vapor from occupational exposure, or amalgam fillings. Urine samples, spot or 24-hour, should be collected in mercury-free polypropylene tubes. For preservation, the sample should be acidified with concentrated acetic acid (1 mL per 50 mL of urine). A 24-hour urine sample is recommended. However, this may not be possible in pediatric cases. Mercury concentrations in urine are ideally adjusted to creatinine concentrations, to account for renal function and differences in hydration.143,187
Blood
Blood mercury concentration is determined using whole blood. Therefore, it is important to avoid blood sample tubes with coagulant additives; the use of K-EDTA tubes is recommended. The mercury concentration in whole blood reflects alimental organic mercury exposure and short-term mercury vapor exposure. Organic mercury is especially found in erythrocytes. Therefore, the separate analysis of whole blood, erythrocytes, and plasma indicates the species of mercury. Normally, the quotient of mercury content in erythrocytes and in plasma is 2:1.143,187
Hair
Hair mercury concentration is assumed to show the concentration of mercury in blood at the time point of hair growth.188 Inorganic as well as organic mercury is incorporated in hair structure and therefore gives information on the duration and kind of exposure depending on the extent of demethylation and length of the hair strand.189 Mainly methylmercury exposure is reflected in mercury hair levels. Ideally, hair samples should be taken from the occipital region near the scalp with a pair of scissors made of stainless steel. The samples can be stored in polypropylene bags or envelopes at room temperature. Initial washing steps should be performed before analysis to remove external contaminants.190 However, metals permeate into the hair structure,191 resulting in difficulties in distinguishing between endogenous and exogenous burden.192
Measurement of Mercury in Other Body Fluids
Under specific circumstances, it may be important to collect samples other than urine, blood, or hair.
Breast Milk
Methylmercury and inorganic mercury are present in human breast milk. About 50% of mercury in breast milk is the inorganic form.193 Breastfed infants are thereby exposed to both forms of mercury.78,101,143,194,195 The benefits of breastfeeding outweigh the potential exposure to mercury from breast milk.196 Women who are breastfeeding should follow local and national advisories for fish consumption. Several methods exist for measurement of mercury in breast milk. Before sampling, the hands and the breast should be washed thoroughly with mercury-free tap water.197 About 10 mL of breast milk should be collected in acid-washed polypropylene tubes and stored deep-frozen at −20°C until analysis. Another possibility to preserve the samples is the lyophilization of liquid breast milk,198 which is an expensive method.197
Feces
Feces are rarely used to establish the diagnosis of pediatric mercury exposures.117,189,199-201 Methylmercury is mainly excreted in feces and therefore this measurement reflects the burden of methylmercury.
Nails
In most epidemiologic and exposure studies, mercury exposure is assessed by analysis of hair, blood, or urine. However, nail analyses have been extensively used to assess body burdens of metals, often in the context of nutritional epidemiology.202 The methodology involves instrumental neutron activation analysis.203 Toenail mercury has also been used in studies of mercury exposures related to cardiovascular endpoints.204 The advantages of nail mercury as a biomarker are ability to measure multiple elements in 1 sample, ease of collection, stability in storage, and relevance to chronic exposure. Toenail mercury concentrations are associated with fish consumption202 and these values are well correlated with exposure predicted from dietary data.205
Umbilical Cord Blood
In epidemiologic and exposure studies, mercury exposure can be assessed by analysis of umbilical cord tissue or umbilical cord blood.95,101,135,206-210 Both are appropriate for measurement to assess prenatal methylmercury exposure.
Analytical Methods and Quality Assurance
For the assessment of mercury in specimens, it is essential to ensure the quality of the analysis.211 Reference material should be as close in chemical composition to that of the sample and should also contain the analyte at about the same concentration as is present in the sample. More information on reference material can be found under http://www.VIRM. net or http://www.rt-corp.com/products. Certified reference material is available.
Speciation might be necessary for proper risk assessment. Speciation is difficult, and it is essential to use reference material and certified reference material for quality control and quality assessment.
The German External Quality Assessment Scheme is a reliable tool for external quality assessment scheme and certification for environmental-medical and occupational-medical toxicologic analyses in biological specimens (http://www.g-equas.de/). This scheme is based on the guidelines of the German Federal Medical Council. Mercury and other parameters in blood, plasma/serum, and urine samples have to be assessed within common environmental concentration ranges. Over 350 laboratories have joined these comparative programs. Twenty-four International Laboratories are commissioned to determine the assigned values. The data evaluated from the results of the comparison programs give a good overview of the current quality of the determination of analyzed samples.212
Surveys, Including Human Exposure Measurements
There are several surveys that included measurements of exposure to mercury. These surveys are important to identify trends in exposure, exposure patterns, vulnerable subgroups, and exposure hot spots.211 Some examples will be given. Other surveys are available at the regional level.
National Health and Nutrition Examination Survey
The US undertakes national periodic surveys of the health and nutritional status of the population, the National Health and Nutrition Examination Survey (NHANES) (http://www.cdc.gov/nchs/nhanes.htm). Data are released and reported in 2-year cycles. Each participant undergoes a household interview and a physical examination. Mercury has been measured in blood and hair of children.213-218
During 1999-2002, the geometric median for total blood mercury concentrations for all childbearing-aged women was 0.92 μg/L, and for children aged 1-5 years was 0.33 μg/L. The 95th percentiles of blood mercury for women were 6.04 μg/L and for children were 2.21 μg/L. Over 5% of US women aged 16-49 years had mercury levels above the US Environmental Protection Agency reference dose of >5.8 μg/L.
NHANES results verify that blood mercury levels in children and women are regularly low (http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5343a5.htm).
German Environmental Survey
The German Environmental Survey, originated in 1985,218 measured mercury in children and others. Age, socioeconomic status, migrant status, size of the community, and frequency of fish consumption were found to be significant predictors of mean levels of mercury in blood. The percentage of quantifiable mercury levels in urine was found to increase with an increasing number of teeth with amalgam fillings. Quantifiable levels of mercury in urine were more often detected in boys and migrants than in girls and nonmigrants, respectively (for details, http://www.umweltdaten.de/publikationen/fpdf-l/3355.pdf).219
Czech Republic
In the Czech Republic, the Environmental Health Monitoring System generated children’s data in the period 2001-2003: mercury in blood (n = 333), and mercury in urine (n = 619). The median mercury levels in blood were 0.42 μg/L and in urine 0.37 μg/g creatinine. No differences were observed in blood mercury levels in boys and girls.220
Mercury Levels in Populations
Data from epidemiologic surveys have been used to estimate mercury levels in populations. Data from NHANES and data from the German Environmental Survey IV are shown in Table 6. In both countries mercury has been recognized as an important pollutant and precautionary measures have already been taken. Thus, the data might not reflect the average values in other developed and developing countries.
TABLE 6.
Summary of mercury concentrations in urine and blood
| Age Blood [μg/L] | Children Environment Survey2552003-06, Germany2193-14 |
NHANES Survey 1999-2002, USA2561-5 |
||
|---|---|---|---|---|
| All | Fish consumption ≤3 times per month |
Fish consumption >3 times per month |
||
| N | 1552 | 891 | 660 | 1577 |
| Min | <0.2* | <0.2a | <0.2a | — |
| Max | 6.3 | 6.3 | 2.4 | — |
| Median (95% CI) | 0.2 | <0.2a | 0.3 | 0.26 (0.23-0.29) |
| 95% percentile | 1.0 | 0.8 | 1.2 | 2.21 (1.80-3.66) |
| Arithmetic mean | 0.33 | 0.27 | 0.41 | — |
| Geometric mean (95% CI) | 0.23 (0.22-0.24) | <0.2a | 0.29 (0.27-0.31) | 0.33 (0.30-0.37) |
| Urine [μg/L] | All | No amalgam fillings | 1-2 teeth | >2 teeth | Not performed |
|---|---|---|---|---|---|
| N | 1734 | 1612 | 68 | 39 | — |
| Min | <0.1b | <0.1b | <0.1b | <0.1b | — |
| Max | 52.0 | 52.0 | 4.7 | 3.4 | — |
| Median | <0.1b | 0.3 | 1.2 | 1.7 | — |
| 95% percentile | 0.5 | 0.5 | 1.5 | 3.1 | — |
| Arithmetic mean | 0.19 | 0.18 | 0.35 | 0.51 | — |
| Geometric mean (CI) | <0.1b | <0.1b | 0.16 (0.12-0.21) | 0.21 (0.14-0.32) | — |
LOD = limit of detection in blood 0.2 μg/L.
LOD = limit of detection in urine 0.1 μg/L.
In 1992, the German Human Biomonitoring Commission was established as a joint activity of the Federal Health Office and the Federal Environment Agency. The goal is to clarify fundamental and practical issues related to human biomonitoring. The Human Biomonitoring Commission’s mandate is to support the Federal Environment Agency in its work by providing expert advice. Up to now, the commission has derived several human biomonitoring reference values, such as for lead, cadmium, mercury, pentachlorophenol bis(2-ethylhexyl) phthalate (PCP) and di-(2-ethylhexyl)-phthalate (DEHP) in body liquids (blood and urine).221 The reference values are defined as 95th percentile values selected from a representative cohort. Levels of lead, mercury, cadmium, and arsenic in blood and urine of children were determined in the German Environmental Survey 2003/2006 (GerES IV).219 Based on the obtained data, reference values for the population and the subgroups were established. The calculation of reference values is performed in analogy to the International Union of Pure and Applied Chemistry guidelines.222 Roughly speaking, the reference value is determined using the 95% percentile of the collected survey data. In Germany, the recent published reference value for mercury in urine and blood of children aged 6-12 years is 0.7 and 1.0 μg/L respectively.187,223
In 1999, the German Environmental Agency published human biomonitoring (HBM) threshold values for mercury in urine and blood.224 Two HBM values (HBM I and HBM II) were defined.221 The HBM I value was set as a check value. Mercury concentrations below this limit were not expected to cause adverse health effects and no action is needed. At a mercury concentration level between HBM I and HBM II, adverse health effects cannot be excluded with sufficient certainty. Therefore, possible sources of mercury burden should be eliminated and the mercury concentrations in blood and urine of the patient should be monitored. The HBM II value was set as an action or intervention value. When the mercury concentration in blood or urine exceeds this limit, adverse health effects are possible and, consequently, individual medical intervention and reduction of exposure are urgently needed. Drasch et al. raised concern that a more complex ranking, which includes some medical parameters in addition to the blood and urine values, would be more appropriate.225
Hence, the essential exposure pathways and predictors, such as fish consumption or the number of teeth with amalgam fillings, has been known and discussed.187
Derived from Czech Republic human biomonitoring data, the following reference values were developed for the period 2001-2003220:
| Czech reference value for mercury in urine |
Girls | 5.5 μg/g creatinine |
| Czech reference value for mercury in urine |
Boys | 3.7 μg/g creatinine |
| Czech reference value for mercury in blood |
Children | 1.5 μg/L |
Hair is a useful and widely accepted indicator medium for the assessment of populations exposed to methylmercury.
Environmental Monitoring
Monitoring the environment for mercury indicates the extent of external mercury exposure for children. Media used for environmental monitoring include the following4,5,226:
Air: Mercury can be analyzed in air, either with personal mercury vapor samplers, which are analyzed latterly using atomic absorption spectrometry, or with passive diffuse samplers. Mobile analyzers, such as, eg, the Lumex, can measure elemental mercury in air continuously.
Food: Mercury can be analyzed in food and other biota. The analysis of mercury in fish is common. Speciation is essential to determine the amount of methylmercury.
Soil and sediments: Mercury can be monitored in soil and sediments. It is important to ensure a proper sampling protocol. Total mercury and methylmercury can be determined.
Water: Mercury can be monitored in water. It is important that the sample is representative and that sample containers are free of mercury contamination.
Environmental Guidelines
Guidelines for water, air, and soil have been set nationally and internationally. International guidelines for air, water, soil and food are as follows:
Air
The World Health Organization guideline value for inorganic mercury vapor is 1 μg/m3 as an annual average.227 A tolerable concentration is 0.2 μg/m3 for long-term inhalation exposure to elemental mercury vapor, and a tolerable intake of total mercury is 2 μg/kg body weight per day.70
Fish
Food and Agriculture Organization/World Health Organization Codex Alimentarius—Commission guideline levels for methylmercury in fish 0.5 mg/kg for predatory fish (such as shark, swordfish, tuna, pike and others) is 1 mg/kg.228
Food
For methylmercury, the Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives (JECFA) set in 2004 a tolerable weekly intake of 1.6 μg/kg body weight per week to protect the developing fetus from neurotoxic effects.229 JEFCA230 confirmed this provisional tolerable weekly intake level, taking into account that adults might not be as sensitive as the developing fetus, in 2003 (JECFA/61/SC http://www.who.int/ipcs/food/jecfa/summaries/en/summary_61.pdf) and 2006 (JECFA/67/SC http://www.who.int/ipcs/food/jecfa/summaries/summary67.pdf).231,232
Soil
United Nations Environment Programme Global Mercury Assessment quotes for soil, preliminary critical limits to prevent ecological effects due to mercury in organic soils with 0.07-0.3 mg/kg for the total mercury content in soil.4
Water
The WHO guideline value is 1 μg/L for total mercury.233
Preventing Mercury-Related Human Health Effects
Because mercury is hazardous to children’s health, attention needs to be drawn to prevention.
Medical Treatment
Treatment begins with the elimination of exposure.67,234
There is no indication for chelation of low-level, chronic methylmercury poisoning. When confronted with a child who has suspected symptomatic mercury intoxication, it is critical to consult your local poison center or clinical toxicologist for guidance on whether chelation treatment is advised.
Preventing Mercury Exposure
Food Advisory
Children ingest mercury mainly due to the consumption of methylmercury in carnivorous fish. A tolerable intake of 1.6 μg/kg body weight per week for methylmercury was established by the Joint Food and Agriculture Organization/WHO Expert Committee on Food Additives to protect the developing fetus from neurotoxic effects.229 In 2006, this Committee clarified that life stages other than the embryo and fetus may be less sensitive to the adverse effects of methylmercury.231 For adults, up to about twice the tolerable intake per week would probably not pose any risk of neurotoxicity. However, available data did not allow firm conclusions to be drawn for children (up to about 17 years), as they may be more sensitive than adults. Hence the tolerable intake established in 2004 applies also to children.
The US Environmental Protection Agency calculated a benchmark reference dose of 0.1 μg methylmercury per kilogram body weight and a benchmark biomarker concentration in maternal hair of 1 μg mercury per gram of maternal hair, a level at which people are unlikely to develop adverse effects.235 The US Environmental Protection Agency used clinical endpoints in modeling a benchmark dose and included the results of neurocognitive and neuropsychological testing of children.235
This analysis supported a range of benchmark estimates, which were all consistent with a reference dose (RF) of 0.1 μg/kg/d (for pregnant women). The issues involved in this estimate include the following: (1) toxicokinetic conversion from biomarkers of mercury exposure (mercury in cord blood; mercury in hair) to an intake value; (2) consideration of maternal and fetal toxicokinetics; (3) integration of results of multiple tests from several studies; and (4) choices for uncertainty factors. In addition, the current US Environmental Protection Agency benchmark does not take into account other effects of mercury, such as cardiovascular effects reported in adults and children or immunotoxic effects reported in adults.134,204
In a critique of this approach, Stern applied probabilistic models to the toxicokinetic issues and suggested a revised RfD of 0.03 μg/kg/d (for pregnant women) following the same benchmark criteria.237
Based on epidemiologic and toxicologic studies and population surveys, several guidance levels have been set to indicate levels of exposure that are associated with risks of adverse health effects. These levels can be helpful to guide decisions concerning the need for medical interventions or exposure reductions. Table 7 gives an overview of these published guidance levels for mercury in blood, urine, and hair.
TABLE 7.
Guidance levels for mercury concentrations in blood, urine, and hair
This benchmark reference dose is currently under discussion.236,238 Axelrad estimated that there is a linear relationship between 1 μg/g increase in maternal hair mercury concentration and 0.18 point decrease in IQ (Table 4).133 The reference concentration for mercury vapor is as well under discussion.239
Information material on mercury in fish is widely published by US Environmental Protection Agency, the European Commission, and other agencies.240,241
Hygiene and Behaviors
Children should not live close to mercury emissions, such as gold mining areas. Separation of housing and mining is essential. Children should not work as miners or in any other way with immediate contact to mercury. Children should not play with liquid mercury.
Cultural Practices
Children should not use skin lightening creams because they contain mercury. Traditional medicine, such as Ayuvedic medicine, that contains mercury and other toxic metals should not be given to children.
In the Medical Domain
Mercury should be removed from medical devices if possible. Because electronic thermometers may be available and are easier and safer to use, the unnecessary risk of mercury-containing thermometers should be avoided. In dentistry, non-mercury-containing composite is preferable.
Broken Bulbs
There are some suggested ways to prevent exposure to mercury through broken bulbs. The US Environmental Protection Agency web site lists actions to reduce exposure when a compact fluorescent light bulb breaks, including the following: opening a window, leaving the room for 15 minutes, and methods for the physical clean, including sealing the bulb in a plastic bag.43 Similar instructions are provided by the Australian Government.242 These guidelines are being discussed and updated in the US. Part of the reason for the update is that recent findings indicate that peak exposure from a broken compact fluorescent light bulb occurs hours after the breakage, and that plastic bags do not prevent exposure from broken compact fluorescent light bulbs. A recent study has concluded that 1 of the best measures for reducing mercury exposure after a compact fluorescent light bulb breaks is to sprinkle the area with nanoselenium powder or to cover the broken bulb with a cloth infused with nanoselenium powder to absorb the mercury vapors, while the study also cautions about the unknown health implications of nanotechnology.40 There is potential danger of exposure to individuals who work in waste sites. Recent findings indicate that once in waste sites, broken compact fluorescent light bulbs continue to be a source of mercury exposure for several days.243
World Health Organization Recommendations
National, regional, and global actions, both immediate and long term, are needed to reduce or eliminate releases of mercury and its compounds to the environment. The WHO has committed to work with the health sector and national, regional, and global health partners in these efforts.1
World Health Organization Recommendations to Reduce Mercury Exposure
Reduce mercury exposure
—Eliminate the use of mercury wherever possible
—Promote the development of alternatives to the use of mercury
Elimination of mercury-related diseases requires strategic action to
—Conduct national assessments of mercury usage and disposal and implement educational activities for the health, environment, and other sectors.
—Promote the use of mercury-free alternatives, eg, for manometers and thermometers, and ensure that mercury-containing devices are taken back by the manufacturer or properly disposed of.
—Develop mercury cleanup and waste-handling, storage, and safe-handling procedures; promote environmentally sound management of health-related waste-containing mercury (as set out in the UN, Basel convention on the control of trans-boundary movements of hazardous wastes and their disposal).
—Encourage countries to develop and implement policies and legislation on mercury; highlight the role of the health sector in dealing with mercury-containing material, health-care waste, and emission reduction; and promote effective ways to control mercury emissions from cremation.
—Encourage international agencies to work with manufacturers, wholesalers, and retailers to develop and make widely available inexpensive mercury-free products and facilitate their procurement.
—Assist countries in preparing advice for pregnant and lactating women and children, about the risks and benefits of fish consumption, indicating the type of fish that may be eaten and how often. WHO strongly recommends breastfeeding because the presence of methylmercury in breast milk is not sufficient to outweigh its benefits.
—Identify traditional practices, folk medicines, and cosmetics involving mercury and disseminate information on mercury hazards, exposure prevention, and how to clean up spillages.
—Promote long-term monitoring (including biological measurements of exposure) and programs to reduce occupational exposure.
Education
The protection of children’s health depends on members of the family and community, as well as on local, regional, national, and international bodies. Childhood exposure to elemental mercury often accrues due to inappropriate handling and cleanup. Health education and policy initiatives are needed as primary prevention.62 The WHO has provided good examples of how to protect children.244
Health care plays an important role as 1 source of mercury. For instance, the United Nations Environment Programme lists various health care-related products and activities as “important sources of anthropogenic releases” of mercury. These include fluorescent lamps, manometers, thermometers, and other instruments; dental amalgam fillings; waste treatment and incineration of products containing mercury (http://www.noharm.org/globalsoutheng/mercury/issue).178,245
The Agency for Toxic Substances and Disease Registry provides environmental public health training courses (http://app2.erg.com/registration/index.htm accessed June 29, 2009). These courses provide instruction on conducting public health assessments, health consultations, exposure investigations, community involvement, health studies, and health education. There are specific trainings on mercury. On-line training material is available (http://www.atsdr.cdc.gov/ accessed June 29, 2009).
The WHO has developed training material to train health care providers that includes a module on mercury. “Children”s Health and the Environment—WHO Training Package for the Health Sector” is available at http://atwww.who.int/ceh.
Public Health Initiatives
There are several nongovernmental organizations that are involved in the global initiatives to reduce mercury as a global pollutant, such as the Zero Mercury Campaign (http://www.zeromercury.org/), the European Environmental Bureau (http://www.eeb.org/), Health Care without Harm (http://www.noharm.org/us/mercury/resources), and the Health and Environment Alliance (http://www.env-health.org/r/81). Nurses can play a critical role in preventive strategies, as well as in the national debate on energy production and dependence on fossil fuels.246
World Health Organization Strategic Steps—Mercury in Health Care247
Short Term
Develop mercury clean up and waste handling and storage procedures. Until countries in transition and developing countries have access to mercury-free alternatives, it is imperative that safe handling procedures be instituted that minimize and eliminate patient, occupational, and community exposures. Proper procedures should include spill clean-up response, educational programs, protective gear, appropriate waste storage containment, staff training, and engineered storage facilities. Countries that have access to affordable alternatives should develop and implement plans to reduce the use of mercury equipment and replace them with mercury-free alternatives. Before final replacement has taken place, and to ensure that new devices conform with recommended validation protocols, health-care facilities will need to keep mercury as the “gold” standard to ensure proper calibration of mercury sphygmomanometers.
Medium Term
Increase efforts to reduce the number of unnecessary uses of mercury equipment. Hospitals should inventory their use of mercury. This inventory should be categorized into immediately replaceable and gradually replaceable. Replaced devices should be taken back by the manufacturer or by the alternative equipment provider. Progressively discourage the import and sale of mercury-containing health care devices and mercury use in health care settings, also using global multilateral environmental agreements to this end. Provide support to countries to ensure that the recovered mercury equipment is not pushed back in the supply chain.
Long Term
Support a ban for use of mercury-containing devices and effectively promote the use of mercury-free alternatives. Support countries in developing a national guidance manual for sound management of health care mercury waste. Support countries in the development and implementation of a national plan, policies, and legislation on mercury health care waste. Promote the principles of environmentally sound management of health care waste containing mercury, as set out in the United Nations, Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and their Disposal. Support the allocation of human and financial resources to ensure procurement of mercury-free alternatives and a sound management of health care waste-containing mercury.
FIG 5.
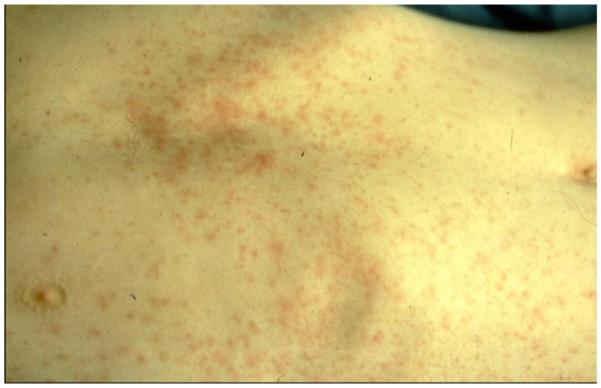
Acrodynia: Exanthema due to mercury intoxication from a mercury thermometer broken in the children’s room 4 months previously.158 (Color version of figure is available online.)
Acknowledgments
The authors are grateful to Drs Alessandro Alimonti, Nida Bisbelli, Marcello Conti, Paul Dargan, Jules de Kom, Gustav Drasch, Jenny Pronczuk, and Ellen Silbergeld for their contributions to this work.
References
- 1.World Health Organization . Exposure to mercury: A major public health concern. WHO, Public Health and Environment; Geneva: [cited 2010 July]. 2007. Available at: http://www.who.int/phe/news/Mercury-flyer.pdf. [Google Scholar]
- 2.Clarkson TW, Magos L, Myers GJ. The toxicology of mercury—current exposures and clinical manifestations. N Engl J Med. 2003;349:1731–7. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- 3.Mergler D, Anderson HA, Chan LH, Mahaffey KR, Murray M, Sakamoto M, et al. Methylmercury exposure and health effects in humans: a worldwide concern. Ambio. 2007;36:3–11. doi: 10.1579/0044-7447(2007)36[3:meahei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Drasch G, Horvat M, Stoeppler M. Mercury. In: Merian E, Anke M, Ihnat M, Stoeppler M, editors. Elements and their compounds in the environment. Wiley-VHC Verlag; Weinheim, Germany: 2004. pp. 931–1005. [Google Scholar]
- 5.United Nations Environment Programme-Chemicals . Global Mercury Assessment. Geneva: 2002. Available at: http://www.unep.org/gc/gc22/Document/UNEP-GC22-INF3.pdf. [Google Scholar]
- 6.Commission on the European Communities . Community Strategy Concerning Mercury—communication from the Commission to the Council and the European Parliament. Commission on the European Communities; 2005. (Com (2005)20 final) [Google Scholar]
- 7.European Parliament . Community Strategy Concerning Mercury—European Parliament Resolution on the Community Strategy Concerning Mercury. European Parliament; Strasbourg: 2006. (2005/2050(INI)) P6_TA(2006)0078. [Google Scholar]
- 8.Secretariat for the Strategic Approach to International Chemicals Management . Strategic approach to International chemicals management—Comprising The Dubai Declaration on International chemicals management, the Overarching Policy strategy and the Global Plan of action. Secretariat Strateg Approach Int Chem Manag-UNEP/SAICM; [cited 2007 October]. 2006. Available at: http://www.chem.unep.ch/saicm/SAICM%20texts/standalone_txt.pdf. [Google Scholar]
- 9.United Nations Environment Programme Report of The International Conference on Chemicals Management on the Work of Its First Session; [accessed October 2007]. 2006. http://www.chem.unep.ch/saicm/SAICM%20texts/Final%20ICCM%20report%20Eng.pdf. [Google Scholar]
- 10.World Health Organization Final report. Forum:V—Fifth Session of the Intergovernmental Forum on Chemical Safety (IFCS); Budapest. 2006; Geneva: WHO; 2006. [Google Scholar]
- 11.Landrigan P, Nordberg M, Lucchini R, Nordberg G, Grandjean P, Iregren A, et al. The Declaration of Brescia on Prevention of the Neurotoxicity of Metals. Am J Ind Med. 2006;50:709–11. doi: 10.1002/ajim.20404. [DOI] [PubMed] [Google Scholar]
- 12.Schuster PF, Krabbenhoft DP, Naftz DL, Cecil LD, Olson ML, Dewild JF, et al. Atmospheric mercury deposition during the last 270 years: a glacial ice core record of natural and anthropogenic sources. Environ Sci Technol. 2002;36:2303–10. doi: 10.1021/es0157503. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel SJ, Yassi A, Spiegel JM, Veiga MM. Reducing mercury and responding to the global gold rush. Lancet. 2005;366:2070–2. doi: 10.1016/S0140-6736(05)67868-3. [DOI] [PubMed] [Google Scholar]
- 14.Bose-O’Reilly S, Drasch G, Beinhoff C, Rodrigues-Filho S, Roider G, Lettmeier B, et al. Health assessment of artisanal gold miners in Indonesia. Sci Total Environ. 2010;408:713–25. doi: 10.1016/j.scitotenv.2009.10.070. [DOI] [PubMed] [Google Scholar]
- 15.Bose-O’Reilly S, Drasch G, Beinhoff C, Tesha A, Drasch K, Roider G, et al. Health assessment of artisanal gold miners in Tanzania. Sci Total Environ. 2010;408:796–805. doi: 10.1016/j.scitotenv.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 16.OSPAR Commission . Mercury losses from the chlor-alkali industry in 2005. Vol. 207. 2007. pp. 1–39. (Hazard Substances Series 2007). Publication 317/2007. [Google Scholar]
- 17.United Nations Environment Programme (UNEP) UNEP Balkans Technical Report—analytical Results of UNEP Field Samples from Industrial Hot Spots in Albania. Geneva: [cited 2007 December]. 2000. Available at: http://enrin.grida.no/htmls/albania/reports/tech/docs/tec_anal.pdf. [Google Scholar]
- 18.Kariyanna H, Sitaram GS. Endemic diseases of south India—a medical geology perspective. J Appl Geochem. 2007;9:142–9. [Google Scholar]
- 19.Zheng N, Wang Q, Zheng D. Mercury contamination and health risk to crops around the zinc smelting plant in Huludao city, northeastern China. Environ Geochem Health. 2007;29:385–93. doi: 10.1007/s10653-007-9083-3. [DOI] [PubMed] [Google Scholar]
- 20.Blacksmith Institute . World’s Worst polluted places—The top ten 2007. Blacksmith Institute; New York, NY: Available at: www.blacksmithinstitute.org. [Google Scholar]
- 21.Cheng J, Yuan T, Wang W, Jia J, Lin X, Qu L, et al. Mercury pollution in two typical areas in Guizhou province, China and its neurotoxic effects in the brains of rats fed with local polluted rice. Environ Geochem Health. 2006;28:499–507. doi: 10.1007/s10653-005-7570-y. [DOI] [PubMed] [Google Scholar]
- 22.Feng X, Li P, Qiu G, Wang S, Li G, Shang L, et al. Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou province, China. Environ Sci Technol. 2008;42:326–32. doi: 10.1021/es071948x. [DOI] [PubMed] [Google Scholar]
- 23.Horvat M, Nolde N, Fajon V, Jereb V, Logar M, Lojen S, et al. Total mercury, methylmercury and selenium in mercury polluted areas in the province Guizhou, China. Sci Total Environ. 2003;304:231–56. doi: 10.1016/S0048-9697(02)00572-7. [DOI] [PubMed] [Google Scholar]
- 24.Jie XL, Jin GW, Cheng JP, Wang WH, Lu J, Qu LY. Consumption of mercury-contaminated rice induces oxidative stress and free radical aggravation in rats. Biomed Environ Sci. 2007;20:84–9. [PubMed] [Google Scholar]
- 25.Cooke CA, Balcom PH, Biester H, Wolfe AP. Over three millennia of mercury pollution in the Peruvian Andes. Proc Natl Acad Sci USA. 2009;106:8830–4. doi: 10.1073/pnas.0900517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conti ME. In: Biological monitoring: theory and applications. Bioindicators and biomarkers for environmental quality and human exposure assessment. The Sustainable World, editor. WIT Press; Southampton: 2008. p. 256. [Google Scholar]
- 27.Hospitals for a Healthy Environment . Making Medicine Mercury Free. Hospitals for a Healthy Environment; Lyme, NH: [cited 2007 August]. 2005. Available at: http://www.h2e-online.org/pubs/mercuryreport.pdf. [Google Scholar]
- 28.Higueras P, Oyarzun R, Lillo J, Sanchez-Hernandez JC, Molina JA, Esbri JM, et al. The almaden district (Spain): anatomy of one of the world’s largest Hg-contaminated sites. Sci Total Environ. 2006;356:112–24. doi: 10.1016/j.scitotenv.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 29.Hylander LD, Meili M. 500 Years of mercury production: global annual inventory by region until 2000 and associated emissions. Sci Total Environ. 2003;304:13–27. doi: 10.1016/S0048-9697(02)00553-3. [DOI] [PubMed] [Google Scholar]
- 30.Zagar D, Knap A, Warwick JJ, Rajar R, Horvat M, Cetina M. Modelling of mercury transport and transformation processes in the Idrijca and Soca river system. Sci Total Environ. 2006;368:149–63. doi: 10.1016/j.scitotenv.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 31.The Madison declaration on mercury pollution. Ambio. 2007;36:62–5. [PubMed] [Google Scholar]
- 32.Via CS, Nguyen P, Niculescu F, Papadimitriou J, Hoover D, Silbergeld EK. Low-dose exposure to inorganic mercury accelerates disease and mortality in acquired murine lupus. Environ Health Perspect. 2003;111:1273–7. doi: 10.1289/ehp.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Counter SA, Buchanan LH. Mercury exposure in children: a review. Toxicol Appl Pharmacol. 2004;198:209–30. doi: 10.1016/j.taap.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 34.Ullrich SM, Tanton TW, Abdrashitova SA. Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol. 2001;31:241–93. [Google Scholar]
- 35.Al-Saleh IA. Health implications of mercury exposure in children. Int J Environ Healthc. 2009;3:22–57. [Google Scholar]
- 36.International Programme on Chemical Safety; World Health Organization, editor. Environmental Health Criteria 101—Methylmercury. World Health Organization; Geneva: 1990. [Google Scholar]
- 37.Dufault R, LeBlanc B, Schnoll R, Cornett C, Schweitzer L, Wallinga D, et al. Mercury from chlor-alkali plants: measured concentrations in food product sugar. Environ Health. 2009;8:2. doi: 10.1186/1476-069X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Saleh I, Shinwari N. Report on the levels of cadmium, lead, and mercury in imported rice grain samples. Biol Trace Elem Res. 2001;83:91–6. doi: 10.1385/BTER:83:1:91. [DOI] [PubMed] [Google Scholar]
- 39.Stemp-Morlock G. Mercury: cleanup for broken CFLs. Environ Health Perspect. 2008;116:A378. doi: 10.1289/ehp.116-a378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson NC, Manchester S, Sarin L, Gao Y, Kulaots I, Hurt RH. Mercury vapor release from broken compact fluorescent lamps and in situ capture by new nanomaterial sorbents. Environ Sci Technol. 2008;42:5772–8. doi: 10.1021/es8004392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eckelman MJ, Anastas PT, Zimmerman JB. Spatial assessment of net mercury emissions from the use of fluorescent bulbs. Environ Sci Technol. 2008;42:8564–70. doi: 10.1021/es800117h. [DOI] [PubMed] [Google Scholar]
- 42.Kondro W. Mercury disposal sole health concern with fluorescent lights. CMAJ. 2007;177:136–7. doi: 10.1503/cmaj.070816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.U.S. Environmental Protection Agency . Mercury - Spills, Disposal and Site Cleanup. U.S. Environmental Protection Agency; [cited 2009 19th of June]. 2009. Available from: http://www.epa.gov/mercury/spills/#fluorescent. [Google Scholar]
- 44.Singhvi R, Mehra Y, Patel J, Miller D, Kalnicky D. Simulation: A Preliminary Investigation of Metallic Mercury Vapor. Fate and Transport in a Trailer. US Environmental Protection Agency; Edison, NJ: 2005. Ritualistic use of Mercury. [Google Scholar]
- 45.Dargan PI, Gawarammana IB, Archer JRH, House IM, Shaw D, Wood DM. Heavy metal poisoning from ayurvedic traditional medicines: an emerging problem? Int J Environ Healthc. 2008;2:463–74. [Google Scholar]
- 46.U.S. Environmental Protection Agency . Task force on ritualistic uses of mercury-EPA/540-R-01-005. Washington, DC: 2002. [Google Scholar]
- 47.Al-Saleh I, Al-Doush I. Mercury content in skin-lightening creams and potential hazards to the health of Saudi women. J Toxicol Environ Health. 1997;51:123–30. doi: 10.1080/00984109708984016. [DOI] [PubMed] [Google Scholar]
- 48.Hursh JB, Clarkson TW, Miles EF, Goldsmith LA. Percutaneous absorption of mercury vapor by man. Arch Environ Health. 1989;44:120–7. doi: 10.1080/00039896.1989.9934385. [DOI] [PubMed] [Google Scholar]
- 49.Palmer RB, Godwin DA, McKinney PE. Transdermal kinetics of a mercurous chloride beauty cream: an in vitro human skin analysis. J Toxicol Clin Toxicol. 2000;38:701–7. doi: 10.1081/clt-100102383. [DOI] [PubMed] [Google Scholar]
- 50.Jarosinska D, Gee D. Children’s environmental health and the precautionary principle. Int J Hyg Environ Health. 2007;210:541–6. doi: 10.1016/j.ijheh.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 51.Selevan SG, Kimmel CA, Mendola P, Pronczuk-Garbino J. Children’s health and the environment - a global perspective. WHO press; Geneva: 2005. Windows of susceptibility to environmental exposures in children; pp. 17–26. [Google Scholar]
- 52.Weiss B. Vulnerability of children and the developing brain to neurotoxic hazards. Environ Health Perspect. 2000;108(Suppl 3):375–81. doi: 10.1289/ehp.00108s3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sly PD, Pronczuk J. Guest editorial: susceptibility of children to pollutants. Paediatr Respir Rev. 2007;8:273–4. doi: 10.1016/j.prrv.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization, editor. Principles for Evaluating Health Risks in Children associated with Exposure to Chemicals-Environmental Health Criteria. Vol. 237. Geneva: 2006. pp. 1–329. [Google Scholar]
- 55.Rice D, Barone S., Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(suppl 3):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grandjean P. Methylmercury toxicity and functional programming. Reprod Toxicol. 2007;23:414–20. doi: 10.1016/j.reprotox.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Murata K, Weihe P, Budtz-Jorgensen E, Jorgensen PJ, Grandjean P. Delayed brainstem auditory evoked potential latencies in 14-year-old children exposed to methylmercury. J Pediatr. 2004;144:177–83. doi: 10.1016/j.jpeds.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 58.Grandjean P, White R. Neurodevelopmental disorders. In: Tamburlini G, von Ehrenstein OS, Bertollini R, editors. Children’s health and environment: a review of evidence. WHO-EEA; Kopenhagen: 2002. pp. 66–78. [Google Scholar]
- 59.Eisler R. Mercury hazards from gold mining to humans, plants, and animals. Rev Environ Contam Toxicol. 2004;181:139–98. doi: 10.1007/0-387-21733-9_4. [DOI] [PubMed] [Google Scholar]
- 60.International Labour Organization (ILO) International Programme on the Elimination of Child Labour (IPEC) http://www.ilo.org/ipecinfo/product/download.do?type=document&id=4146.
- 61.Bose-O’Reilly S, Lettmeier B, Gothe RM, Beinhoff C, Siebert U, Drasch G. Mercury as a serious health hazard for children in gold mining areas. Environ Res. 2008;107:89–97. doi: 10.1016/j.envres.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Lee R, Middleton D, Caldwell K, Dearwent S, Jones S, Lewis B, et al. A review of events that expose children to elemental mercury in the United States. Environ Health Perspect. 2009;117:871–8. doi: 10.1289/ehp.0800337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blumenthal I. Should we ban the mercury thermometer? Discussion paper. J R Soc Med. 1992;85:553–5. doi: 10.1177/014107689208500915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Otto M, Ahlemeyer C, Tasche H, von Muhlendahl KE. Mercury exposure. Nature. 1994;367:110. doi: 10.1038/367110b0. [DOI] [PubMed] [Google Scholar]
- 65.Agocs MM, Etzel RA, Parrish RG, Paschal DC, Campagna PR, Cohen DS, et al. Mercury exposure from interior latex paint. N Engl J Med. 1990;323:1096–101. doi: 10.1056/NEJM199010183231603. [DOI] [PubMed] [Google Scholar]
- 66.Beusterien KM, Etzel RA, Agocs MM, Egeland GM, Socie EM, Rouse MA, et al. Indoor air mercury concentrations following application of interior latex paint. Arch Environ Contam Toxicol. 1991;21:62–4. doi: 10.1007/BF01055557. [DOI] [PubMed] [Google Scholar]
- 67.Goldman LR, Shannon MW. Committee on Environmental Health, American Academy of Pediatrics. Technical report: mercury in the environment: implications for pediatricians. Pediatrics. 2001;108:197–205. doi: 10.1542/peds.108.1.197. [DOI] [PubMed] [Google Scholar]
- 68.Gordon AT. Short-term elemental mercury exposures at three Arizona schools: public health lessons learned. J Toxicol Clin Toxicol. 2004;42:179–87. doi: 10.1081/clt-120030944. [DOI] [PubMed] [Google Scholar]
- 69.MacLehose R, Pitt G, Will S, Jones A, Duane L, Flaherty S, et al. Mercury contamination incident. J Public Health Med. 2001;23:18–22. doi: 10.1093/pubmed/23.1.18. [DOI] [PubMed] [Google Scholar]
- 70.Risher JF. The Concise International Chemical Assessment Document Series. World Health Organization; Geneva: 2003. Elemental Mercury and inorganic compounds: human health aspects. Available at: http://www.who.int/ipcs/publications/cicad/en/cicad50.pdf. [Google Scholar]
- 71.Zeitz P, Orr MF, Kaye WE. Public health consequences of mercury spills: hazardous substances emergency events surveillance system, 1993-98. Environ Health Perspect. 2002;110:129–32. doi: 10.1289/ehp.02110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weldon MM, Smolinski MS, Maroufi A, Hasty BW, Gilliss DL, Boulanger LL, et al. Mercury poisoning associated with a Mexican beauty cream. West J Med. 2000;173:15–8. doi: 10.1136/ewjm.173.1.15. [Discussion, 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rai V, Kakkar P, Singh J, Misra C, Kumar S, Mehrotra S. Toxic metals and organochlorine pesticides residue in single herbal drugs used in important ayurvedic formulation—“Dashmoola”. Environ Monit Assess. 2007;143(1-3):273–7. doi: 10.1007/s10661-007-9976-8. [DOI] [PubMed] [Google Scholar]
- 74.Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, Davis RB, et al. Heavy metal content of ayurvedic herbal medicine products. JAMA. 2004;292:2868–73. doi: 10.1001/jama.292.23.2868. [DOI] [PubMed] [Google Scholar]
- 75.Crump K, Viren J, Silvers A, Clewell H, 3rd, Gearhart J, Shipp A. Reanalysis of dose–response data from the Iraqi methylmercury poisoning episode. Risk Anal. 1995;15:523–32. doi: 10.1111/j.1539-6924.1995.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 76.Marsh DO, Clarkson TW, Cox C, Myers GJ, Amin-Zaki L, Al-Tikriti S. Fetal methylmercury poisoning. Relationship between concentration in single strands of maternal hair and child effects. Arch Neurol. 1987;44:1017–22. doi: 10.1001/archneur.1987.00520220023010. [DOI] [PubMed] [Google Scholar]
- 77.Amin-Zaki L, Majeed MA, Clarkson TW, Greenwood MR. Methylmercury poisoning in Iraqi children: clinical observations over two years. BMJ. 1978;1:613–6. doi: 10.1136/bmj.1.6113.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amin-Zaki L, Majeed MA, Greenwood MR, Elhassani SB, Clarkson TW, Doherty RA. Methylmercury poisoning in the Iraqi suckling infant: a longitudinal study over five years. J Appl Toxicol. 1981;1:210–4. doi: 10.1002/jat.2550010405. [DOI] [PubMed] [Google Scholar]
- 79.Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, Al-Rawi NY, et al. Methylmercury poisoning in Iraq. Science. 1973;181:230–41. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- 80.Centers for Disease Control and Prevention . CDC’s third national report on human exposure to environmental chemicals—spotlight on mercury. Department of Health and Human Services Centers for Disease Control and Prevention; Atlanta, Georgia: 2005. NCEH Pub. No. 05-0570. [Google Scholar]
- 81.Aberg B, Ekman L, Falk R, Greitz U, Persson G, Snihs JO. Metabolism of methyl mercury (203Hg) compounds in man. Arch Environ Health. 1969;19:478–84. doi: 10.1080/00039896.1969.10666872. [DOI] [PubMed] [Google Scholar]
- 82.Miettinen JK, Rahola T, Hattula T, Rissanen K, Tillander M. Elimination of 203Hg-methylmercury in man. Ann Clin Res. 1971;3:116–22. [PubMed] [Google Scholar]
- 83.Hightower JM, Moore D. Mercury levels in high-end consumers of fish. Environ Health Perspect. 2003;111:604–8. doi: 10.1289/ehp.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burger J, Johnson BB, Shukla S, Gochfeld M. Perceptions of recreational fishing boat captains: knowledge and effects of fish consumption advisories. Risk Anal. 2003;23:369–78. doi: 10.1111/1539-6924.00315. [DOI] [PubMed] [Google Scholar]
- 85.Burger J, Stern AH, Gochfeld M. Mercury in commercial fish: optimizing individual choices to reduce risk. Environ Health Perspect. 2005;113:266–71. doi: 10.1289/ehp.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.U.S. Environmental Protection Agency . What You Need to Know about Mercury in Fish and Shellfish. U.S. Environmental Protection Agency; [19th of June 2009]. 2004. Available at: http://www.epa.gov/waterscience/fish/advice/ [Google Scholar]
- 87.Grandjean P, Jorgensen PJ, Weihe P. Human milk as a source of methylmercury exposure in infants. Environ Health Perspect. 1994;102:74–7. doi: 10.1289/ehp.9410274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vimy MJ, Hooper DE, King WW, Lorscheider FL. Mercury from maternal “silver” tooth fillings in sheep and human breast milk. A source of neonatal exposure. Biol Trace Elem Res. 1997;56:143–52. doi: 10.1007/BF02785388. [DOI] [PubMed] [Google Scholar]
- 89.Yoshida M, Satoh H, Kishimoto T, Yamamura Y. Exposure to mercury via breast milk in suckling offspring of maternal Guinea pigs exposed to mercury vapor after parturition. J Toxicol Environ Health. 1992;35:135–9. doi: 10.1080/15287399209531602. [DOI] [PubMed] [Google Scholar]
- 90.Breastfeeding and the use of human milk. American Academy of Pediatrics. Work Group on Breastfeeding. Pediatrics. 1997;100:1035–9. doi: 10.1542/peds.100.6.1035. [DOI] [PubMed] [Google Scholar]
- 91.Sandborgh-Englund G, Elinder CG, Johanson G, Lind B, Skare I, Ekstrand J. The absorption, blood levels, and excretion of mercury after a single dose of mercury vapor in humans. Toxicol Appl Pharmacol. 1998;150:146–53. doi: 10.1006/taap.1998.8400. [DOI] [PubMed] [Google Scholar]
- 92.Henriksson J, Tjalve H. Uptake of inorganic mercury in the olfactory bulbs via olfactory pathways in rats. Environ Res. 1998;77:130–40. doi: 10.1006/enrs.1997.3817. [DOI] [PubMed] [Google Scholar]
- 93.Fang SC. Comparative study of uptake and tissue distribution of methylmercury in female rats by inhalation and oral routes of administration. Bull Environ Contam Toxicol. 1980;24:65–72. doi: 10.1007/BF01608077. [DOI] [PubMed] [Google Scholar]
- 94.Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- 95.Akagi H, Grandjean P, Takizawa Y, Weihe P. Methylmercury dose estimation from umbilical cord concentrations in patients with Minamata disease. Environ Res. 1998;77:98–103. doi: 10.1006/enrs.1997.3822. [DOI] [PubMed] [Google Scholar]
- 96.Deleu D, Hanssens Y, Al-Salmy HS, Hastie I. Peripheral polyneuropathy due to chronic use of topical ammoniated mercury. J Toxicol Clin Toxicol. 1998;36:233–7. doi: 10.3109/15563659809028945. [DOI] [PubMed] [Google Scholar]
- 97.Gotelli CA, Astolfi E, Cox C, Cernichiari E, Clarkson TW. Early biochemical effects of an organic mercury fungicide on infants: “dose makes the poison. Science. 1985;227:638–40. doi: 10.1126/science.2857500. [DOI] [PubMed] [Google Scholar]
- 98.Kjellstrom T, Kennedy P, Wallis S, Mantell C. Physical and Mental Development of Children with Prenatal Exposure to Mercury from Fish. Stage I: Preliminary Tests at Age 4. National Swedish Environmental Protection Board; Solna, Sweden: 1986. [Google Scholar]
- 99.Kjellstrom T, Kennedy P, Wallis S, Stewart A, Friberg L, Lind B. Physical and Mental Development of Children with Prenatal Exposure to Mercury from Fish. Stage II: Interviews and Psychological Tests at Age 4. National Swedish Environmental Protection Board; Solna, Sweden: 1989. [Google Scholar]
- 100.Grandjean P, Weihe P, Jorgensen PJ, Clarkson T, Cernichiari E, Videro T. Impact of maternal seafood diet on fetal exposure to mercury, selenium, and lead. Arch Environ Health. 1992;47:185–95. doi: 10.1080/00039896.1992.9938348. [DOI] [PubMed] [Google Scholar]
- 101.Grandjean P, Weihe P, White RF. Milestone development in infants exposed to methylmercury from human milk. Neurotoxicology. 1995;16:27–33. [PubMed] [Google Scholar]
- 102.Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–28. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 103.Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA. 1998;280:701–7. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- 104.Davidson PW, Myers GJ, Cox C, Shamlaye CF, Marsh DO, Tanner MA, et al. Longitudinal neurodevelopmental study of Seychellois children following in utero exposure to methylmercury from maternal fish ingestion: outcomes at 19 and 29 months. Neurotoxicology. 1995;16:677–88. [PubMed] [Google Scholar]
- 105.Marsh DO, Clarkson TW, Myers GJ, Davidson PW, Cox C, Cernichiari E, et al. The Seychelles study of fetal methylmercury exposure and child development: introduction. Neurotoxicology. 1995;16:583–96. [PubMed] [Google Scholar]
- 106.Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, et al. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet. 2003;361:1686–92. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- 107.Myers GJ, Davidson PW, Cox C, Shamlaye CF, Tanner MA, Choisy O, et al. Neurodevelopmental outcomes of Seychellois children sixty-six months after in utero exposure to methylmercury from a maternal fish diet: pilot study. Neurotoxicology. 1995;16:639–52. [PubMed] [Google Scholar]
- 108.Myers GJ, Thurston SW, Pearson AT, Davidson PW, Cox C, Shamlaye CF, et al. Postnatal exposure to methyl mercury from fish consumption: a review and new data from the Seychelles Child Development Study. Neurotoxicology. 2009;30:338–49. doi: 10.1016/j.neuro.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Wijngaarden E, Beck C, Shamlaye CF, Cernichiari E, Davidson PW, Myers GJ, et al. Benchmark concentrations for methyl mercury obtained from the 9-year follow-up of the Seychelles Child Development Study. Neurotoxicology. 2006;27:702–9. doi: 10.1016/j.neuro.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 110.World Health Organization [accessed August 2007];Thiomersal and vaccines: questions and answers. 2006 http://www.who.int/vaccine_safety/topics/thiomersal/questions/en/print.html.
- 111.World Health Organization - Global Advisory Committee on Vaccine Safety . Statement on Thiomersal. World Health Organization; Geneva: [cited 2010 August]. 2006. Available at: http://www.who.int/vaccine_safety/topics/thiomersal/statement200308/en/index.html. [Google Scholar]
- 112.World Health Organization - Global Advisory Committee on Vaccine Safety . Thiomersal. World Health Organization; Geneva: [cited 2010 August]. 2005. Available at: http://www.who.int/vaccine_safety/topics/thiomersal/June_2005/en/index.html. [Google Scholar]
- 113.Baker JP. Mercury, vaccines, and autism: one controversy, three histories. Am J Public Health. 2008;98:244–53. doi: 10.2105/AJPH.2007.113159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Silbergeld EK. Mercury, vaccines, and autism, revisited. Am J Public Health. 2008;98:1350. doi: 10.2105/AJPH.2008.138776. [Author reply: 1350-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Redwood L, Bernard S, Brown D. Predicted mercury concentrations in hair from infant immunizations: cause for concern. Neurotoxicology. 2001;22:691–7. doi: 10.1016/s0161-813x(01)00067-5. [DOI] [PubMed] [Google Scholar]
- 116.Burbacher TM, Shen DD, Liberato N, Grant KS, Cernichiari E, Clarkson T. Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal. Environ Health Perspect. 2005;113:1015–21. doi: 10.1289/ehp.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pichichero ME, Gentile A, Giglio N, Umido V, Clarkson T, Cernichiari E, et al. Mercury levels in newborns and infants after receipt of thimerosal-containing vaccines. Pediatrics. 2008;121:e208–14. doi: 10.1542/peds.2006-3363. [DOI] [PubMed] [Google Scholar]
- 118.Marques RC, Dorea JG, Fonseca MF, Bastos WR, Malm O. Hair mercury in breast-fed infants exposed to thimerosal-preserved vaccines. Eur J Pediatr. 2007;166:935–41. doi: 10.1007/s00431-006-0362-2. [DOI] [PubMed] [Google Scholar]
- 119.Thompson WW, Price C, Goodson B, Shay DK, Benson P, Hinrichsen VL, et al. Early thimerosal exposure and neuropsychological outcomes at 7 to 10 years. N Engl J Med. 2007;357:1281–92. doi: 10.1056/NEJMoa071434. [DOI] [PubMed] [Google Scholar]
- 120.Thimerosal in Vaccines—an Interim Report to Clinicians. American Academy of Pediatrics. Committee on Infectious Diseases and Committee on Environmental Health. Pediatrics. 1999;104:570–4. [PubMed] [Google Scholar]
- 121.Berman RF, Pessah IN, Mouton PR, Mav D, Harry J. Low-level neonatal thimerosal exposure: further evaluation of altered neurotoxic potential in SJL mice. Toxicol Sci. 2008;101:294–309. doi: 10.1093/toxsci/kfm265. [DOI] [PubMed] [Google Scholar]
- 122.Drasch G, Aigner S, Roider G, Staiger F, Lipowsky G. Mercury in human colostrum and early breast milk. Its dependence on dental amalgam and other factors. J Trace Elem Med Biol. 1998;12:23–7. doi: 10.1016/s0946-672x(98)80017-5. [DOI] [PubMed] [Google Scholar]
- 123.Dye BA, Schober SE, Dillon CF, Jones RL, Fryar C, McDowell M, et al. Urinary mercury concentrations associated with dental restorations in adult women aged 16-49 years: United States, 1999-2000. Occup Environ Med. 2005;62:368–75. doi: 10.1136/oem.2004.016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bellinger DC, Daniel D, Trachtenberg F, Tavares M, McKinlay S. Dental amalgam restorations and children’s neuropsychological function: the New England Children’s Amalgam Trial. Environ Health Perspect. 2007;115:440–6. doi: 10.1289/ehp.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bellinger DC, Trachtenberg F, Barregard L, Tavares M, Cernichiari E, Daniel D, et al. Neuropsychological and renal effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006;295:1775–83. doi: 10.1001/jama.295.15.1775. [DOI] [PubMed] [Google Scholar]
- 126.Committee Methods and Quality Assurance in Environmental Medicine Amalgam: orientation from the environmental medicine viewpoint. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2007 Oct;50(10):1304–7. doi: 10.1007/s00103-007-0338-z. [DOI] [PubMed] [Google Scholar]
- 127.Trasande L, Landrigan PJ, Schechter C. Public health and economic consequences of methyl mercury toxicity to the developing brain. Environ Health Perspect. 2005;113:590–6. doi: 10.1289/ehp.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Trasande L, Schechter C, Haynes KA, Landrigan PJ. Applying cost analyses to drive policy that protects children: mercury as a case study. Ann NY Acad Sci. 2006;1076:911–23. doi: 10.1196/annals.1371.034. [DOI] [PubMed] [Google Scholar]
- 129.Sundseth K, Pacny JM, Pacyna EG, Munthe J, Belhaj M. Economic benefits from decreased mercury emissions: projections for 2020. J Cleaner Product. 2010;18:386–94. [Google Scholar]
- 130.Myers GJ, Marsh DO, Davidson PW, Cox C, Shamlaye CF, Tanner M, et al. Main neurodevelopmental study of Seychellois children following in utero exposure to methylmercury from a maternal fish diet: outcome at six months. Neurotoxicology. 1995;16:653–64. [PubMed] [Google Scholar]
- 131.Myers GJ, Davidson PW, Shamlaye CF, Axtell CD, Cernichiari E, Choisy O, et al. Effects of prenatal methylmercury exposure from a high fish diet on developmental milestones in the Seychelles Child Development Study. Neurotoxicology. 1997;18:819–29. [PubMed] [Google Scholar]
- 132.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–62. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 133.Axelrad DA, Bellinger DC, Ryan LM, Woodruff TJ. Dose–response relationship of prenatal mercury exposure and IQ: an integrative analysis of epidemiologic data. Environ Health Perspect. 2007;115:609–15. doi: 10.1289/ehp.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grandjean P, Murata K, Budtz-Jorgensen E, Weihe P. Cardiac autonomic activity in methylmercury neurotoxicity: 14-year follow-up of a Faroese birth cohort. J Pediatr. 2004;144:169–76. doi: 10.1016/j.jpeds.2003.10.058. [DOI] [PubMed] [Google Scholar]
- 135.Lederman SA, Jones RL, Caldwell KL, Rauh V, Sheets SE, Tang D, et al. Relation between cord blood mercury levels and early child development in a World Trade Center cohort. Environ Health Perspect. 2008;116:1085–91. doi: 10.1289/ehp.10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chevrier C, Sullivan K, White RF, Comtois C, Cordier S, Grandjean P. Qualitative assessment of visuospatial errors in mercury-exposed Amazonian children. Neurotoxicology. 2008;30(1):37–46. doi: 10.1016/j.neuro.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 137.Marques RC, Garrofe Dorea J, Bastos W Rodrigues, de Freitas Rebelo M, de Freitas Fonseca M, Malm O. Maternal mercury exposure and neuro-motor development in breastfed infants from Porto Velho (Amazon), Brazil. Int J Hyg Environ Health. 2007;210:51–60. doi: 10.1016/j.ijheh.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 138.Freire C, Ramos R, Lopez-Espinosa MJ, Diez S, Vioque J, Ballester F, et al. Hair mercury levels, fish consumption, and cognitive development in preschool children from Granada, Spain. Environ Res. 2010;110:96–104. doi: 10.1016/j.envres.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 139.Plusquellec P, Muckle G, Dewailly E, Ayotte P, Begin G, Desrosiers C, et al. The relation of environmental contaminants exposure to behavioral indicators in Inuit preschoolers in Arctic Quebec. Neurotoxicology. 2010;31:17–25. doi: 10.1016/j.neuro.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 140.Ye X, Qian H, Xu P, Zhu L, Longnecker MP, Fu H. Nephrotoxicity, neurotoxicity, and mercury exposure among children with and without dental amalgam fillings. Int J Hyg Environ Health. 2008;212(4):378–86. doi: 10.1016/j.ijheh.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barregard L, Trachtenberg F, McKinlay S. Renal effects of dental amalgam in children: the New England children’s amalgam trial. Environ Health Perspect. 2008;116:394–9. doi: 10.1289/ehp.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Drasch G, Bose-O’Reilly S, Beinhoff C, Roider G, Maydl S. The Mt. Diwata study on the Philippines, 1999—assessing mercury intoxication of the population by small scale gold mining. Sci Total Environ. 2001;267:151–68. doi: 10.1016/s0048-9697(00)00806-8. [DOI] [PubMed] [Google Scholar]
- 143.Agency for Toxic Substances and Disease Registry . Toxicological Profile for Mercury. U.S. Department of Health and Human Service - Public Health Service - Agency for Toxic Substances and Disease Registry; Atlanta, Georgia: [cited 2009 August]. 1999. Available at: http://www.atsdr.cdc.gov/toxprofiles/tp46.pdf. [Google Scholar]
- 144.Kim DS, Lee EH, Yu SD, Cha JH, Ahn SC. Heavy metal as risk factor of cardiovascular disease—an analysis of blood lead and urinary mercury. J Prev Med Public Health. 2005;38:401–7. [PubMed] [Google Scholar]
- 145.Lim S, Chung HU, Paek D. Low dose mercury and heart rate variability among community residents nearby to an industrial complex in Korea. Neurotoxicology. 2010;31:10–6. doi: 10.1016/j.neuro.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 146.Thurston SW, Bovet P, Myers GJ, Davidson PW, Georger LA, Shamlaye C, et al. Does prenatal methylmercury exposure from fish consumption affect blood pressure in childhood? Neurotoxicology. 2007;28:924–30. doi: 10.1016/j.neuro.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aronow R, Cubbage C, Wiener R, Johnson B, Hesse J, Bedford J. Mercury exposure from interior latex paint—Michigan. Morb Mortal Wkly Rep. 1990;39:125–6. [PubMed] [Google Scholar]
- 148.Fillion M, Mergler D, Passos CJ Sousa, Larribe F, Lemire M, Guimaraes JR. A preliminary study of mercury exposure and blood pressure in the Brazilian Amazon. Environ Health. 2006;5:29. doi: 10.1186/1476-069X-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yorifuji T, Tsuda T, Kawakami N. Age standardized cancer mortality ratios in areas heavily exposed to methyl mercury. Int Arch Occup Environ Health. 2007;80:679–88. doi: 10.1007/s00420-007-0179-y. [DOI] [PubMed] [Google Scholar]
- 150.International Agency for Research on Cancer [cited 2008 March];Mercury and mercury compounds. International Agency for Research on Cancer (IARC) - Summaries & Evaluations. 1993 Vol 58:239. Available at: http://www.inchem.org/documents/iarc/vol58/mono58-3.html.
- 151.Eke D, Celik A. Genotoxicity of thimerosal in cultured human lymphocytes with and without metabolic activation sister chromatid exchange analysis proliferation index and mitotic index. Toxicol Vitro. 2008;22:927–34. doi: 10.1016/j.tiv.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 152.Sakamoto M, Nakano A, Akagi H. Declining Minamata male birth ratio associated with increased male fetal death due to heavy methylmercury pollution. Environ Res. 2001;87:92–8. doi: 10.1006/enrs.2001.4293. [DOI] [PubMed] [Google Scholar]
- 153.Sikorski R, Juszkiewicz T, Paszkowski T, Szprengier-Juszkiewicz T. Women in dental surgeries: reproductive hazards in occupational exposure to metallic mercury. Int Arch Occup Environ Health. 1987;59:551–7. doi: 10.1007/BF00377918. [DOI] [PubMed] [Google Scholar]
- 154.World Health Organization Principles and methods for assessing autoimmunity associated with exposure to chemicals. Environmental Health Criteria. 2006;236:1–333. [Google Scholar]
- 155.Silbergeld EK, Silva IA, Nyland JF. Mercury and autoimmunity: implications for occupational and environmental health. Toxicol Appl Pharmacol. 2005;207:282–92. doi: 10.1016/j.taap.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 156.Shenker BJ, Maserejian NN, Zhang A, McKinlay S. Immune function effects of dental amalgam in children: a randomized clinical trial. J Am Dent Assoc. 2008;139:1496–505. doi: 10.14219/jada.archive.2008.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.von Mühlendahl KE. Feer’s disease (acrodynia)—a strange disease. Umweltmed Forsch Prax. 2008;13:73–9. [Google Scholar]
- 158.von Mühlendahl KE, Schulter-Wissermann H, Grips M, Feer M. Skin changes. Pädiatr Prax. 1995;49:647–52. [Google Scholar]
- 159.Foulds DM, Copeland KC, Franks RC. Mercury poisoning and acrodynia. Am J Dis Child. 1987;141:124–5. doi: 10.1001/archpedi.1987.04460020014006. [DOI] [PubMed] [Google Scholar]
- 160.Gattineni J, Weiser S, Becker AM, Baum M. Mercury intoxication: lack of correlation between symptoms and levels. Clin Pediatr. 2007;46:844–6. doi: 10.1177/0009922807303893. [DOI] [PubMed] [Google Scholar]
- 161.Bose S, Drasch GA, Eife R, Laub MC. Chronic metal intoxication as cause of neuropaediatric diseases. Pädiatrische Praxis. 1993;45:183–97. [Google Scholar]
- 162.von Muhlendahl KE. Intoxication from mercury spilled on carpets. Lancet. 1990;336:1578. doi: 10.1016/0140-6736(90)93352-p. [DOI] [PubMed] [Google Scholar]
- 163.Castoldi AF, Coccini T, Manzo L. Neurotoxic and molecular effects of methylmercury in humans. Rev Environ Health. 2003;18:19–31. doi: 10.1515/reveh.2003.18.1.19. [DOI] [PubMed] [Google Scholar]
- 164.Castoldi AF, Onishchenko N, Johansson C, Coccini T, Roda E, Vahter M, et al. Neurodevelopmental toxicity of methylmercury: Laboratory animal data and their contribution to human risk assessment. Regul Toxicol Pharmacol. 2008;51:215–29. doi: 10.1016/j.yrtph.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 165.Coccini T, Randine G, Candura SM, Nappi RE, Prockop LD, Manzo L. Low-level exposure to methylmercury modifies muscarinic cholinergic receptor binding characteristics in rat brain and lymphocytes: physiologic implications and new opportunities in biologic monitoring. Environ Health Perspect. 2000;108:29–33. doi: 10.1289/ehp.0010829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gundacker C, Komarnicki G, Jagiello P, Gencikova A, Dahmen N, Wittmann KJ, et al. Glutathione-S-transferase polymorphism, metallothionein expression, and mercury levels among students in Austria. Sci Total Environ. 2007;385:37–47. doi: 10.1016/j.scitotenv.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 167.Drasch G, Schupp I, Hofl H, Reinke R, Roider G. Mercury burden of human fetal and infant tissues. Eur J Pediatr. 1994;153:607–10. doi: 10.1007/BF02190671. [DOI] [PubMed] [Google Scholar]
- 168.Sims L, Indukuri A, Walsh J, Spiller HA, Kaelin C, Thoroughman DA. Mercury exposure—Kentucky. MMWR Morb Mortal Wkly Rep. 2004;54:797–9. [Google Scholar]
- 169.Azziz-Baumgartner E, Luber G, Schurz-Rogers H, Backer L, Belson M, Kieszak S, et al. Exposure assessment of a mercury spill in a Nevada School—2004. Clin Toxicol. 2007;45:391–5. doi: 10.1080/15563650601031569. [DOI] [PubMed] [Google Scholar]
- 170.Pike-Paris A. Mercury 101. Pediatr Nurs. 2004;30:150–3. [PubMed] [Google Scholar]
- 171.Koyun M, Akman S, Guven AG. Mercury intoxication resulting from school barometers in three unrelated adolescents. Eur J Pediatr. 2004;163:131–4. doi: 10.1007/s00431-003-1389-2. [DOI] [PubMed] [Google Scholar]
- 172.Balk SJ. The clinical environmental history: experience in the USA. In: Pronczuk-Garbino J, editor. Children’s health and the environment - a global perspective. WHO press; Geneva: 2005. pp. 240–9. [Google Scholar]
- 173.Pronczuk J. Paediatric environmental history-taking in developing countries. In: Pronczuk-Garbino J, editor. Children’s health and the environment - a global perspective. WHO press; Geneva: 2005. pp. 227–39. [Google Scholar]
- 174.American Academy of Pediatrics, Committee on Environmental Health . In: Pediatric Environmental Health. 2nd ed Etzel RA, editor. American Academy of Pediatrics; Elk Grove Village, IL: 2003. [Google Scholar]
- 175.World Health Organization . In: Mercury: assessing the environmental burden of disease at national and local levels. Poulin J, Gibb H, editors. WHO; Geneva: 2008. [Google Scholar]
- 176.Lauterbach M, Martins IP, Castro-Caldas A, Bernardo M, Luis H, Amaral H, et al. Neurological outcomes in children with and without amalgam-related mercury exposure: seven years of longitudinal observations in a randomized trial. J Am Dent Assoc. 2008;139:138–45. doi: 10.14219/jada.archive.2008.0128. [DOI] [PubMed] [Google Scholar]
- 177.Dunn JE, Trachtenberg FL, Barregard L, Bellinger D, McKinlay S. Scalp hair and urine mercury content of children in the Northeast United States: the New England children’s Amalgam Trial. Environ Res. 2008;107:79–88. doi: 10.1016/j.envres.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Harari R, Forastiere F, Axelson O. Unacceptable “occupational” exposure to toxic agents among children in Ecuador. Am J Ind Med. 1997;32:185–9. doi: 10.1002/(sici)1097-0274(199709)32:3<185::aid-ajim1>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 179.Counter SA, Buchanan LH, Ortega F. Mercury levels in urine and hair of children in an Andean gold-mining settlement. Int J Occup Environ Health. 2005;11:132–7. doi: 10.1179/oeh.2005.11.2.132. [DOI] [PubMed] [Google Scholar]
- 180.Pinheiro MC, Crespo-Lopez ME, Vieira JL, Oikawa T, Guimaraes GA, Araujo CC, et al. Mercury pollution and childhood in Amazon Riverside villages. Environ Int. 2007;33:56–61. doi: 10.1016/j.envint.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 181.Grandjean P, White RF, Nielsen A, Cleary D, de Oliveira SEC. Methylmercury neurotoxicity in Amazonian children downstream from gold mining. Environ Health Perspect. 1999;107:587–91. doi: 10.1289/ehp.99107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Akagi H, Castillo ES, Cortes-Maramba N, Francisco-Rivera AT, Timbang TD. Health assessment for mercury exposure among schoolchildren residing near a gold processing and refining plant in Apokon, Tagum, Davao del Norte, Philippines. Sci Total Environ. 2000;259:31–43. doi: 10.1016/s0048-9697(00)00547-7. [DOI] [PubMed] [Google Scholar]
- 183.Von Muhlendahl KE. [cited 2010 12th of May];Paediatric guideline mercury. 2004 Available at: http://www.gpaev.de/typo/fileadmin/user_upload/GPA/dateien_indiziert/Leitlinien/umw_Leitlinie_Quecksilber.pdf.
- 184.Clevenger WL, Smith BW, Winefordner JD. Trace determination of Mercury: a review. Crit Rev Anal Chem. 1997;27:1–26. [Google Scholar]
- 185.Morita M, Yoshinaga J, Edmondst JS. The determination of mercury species in environmental and biological samples. Pure Appl Chem. 1998;70:1585–615. [Google Scholar]
- 186.Risher JF, De Rosa CT. Inorganic: the other mercury. J Environ Health. 2007;70:9–16. [Discussion, 40] [PubMed] [Google Scholar]
- 187.Wilhelm M, Schulz C, Schwenk M. Revised and new reference values for arsenic, cadmium, lead, and mercury in blood or urine of children: basis for validation of human biomonitoring data in environmental medicine. Int J Hyg Environ Health. 2006;209:301–5. doi: 10.1016/j.ijheh.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 188.Phelps RW, Clarkson TW, Kershaw TG, Wheatley B. Interrelationships of blood and hair mercury concentrations in a north American population exposed to methylmercury. Arch Environ Health. 1980;35:161–8. doi: 10.1080/00039896.1980.10667486. [DOI] [PubMed] [Google Scholar]
- 189.U.S. Environmental Protection Agency Mercury Study. Report to Congress. 1997;Volume IV: An Assessment of Exposure to Mercury in the United States
- 190.United Nations Environment Programme. World Health Organization. International Atomic Energy Agency The determination of methylmercury, total mercury and total selenium in human hair. Reference Methods for Marine Pollution Studies. 1987
- 191.Bos AJ, van der Stap CC, Valkovic V, Vis RD, Verheul H. Incorporation routes of elements into human hair; implications for hair analysis used for monitoring. Sci Total Environ. 1985;42:157–69. doi: 10.1016/0048-9697(85)90015-4. [DOI] [PubMed] [Google Scholar]
- 192.Kijewski H, editor. The Forensic Impact of the Concentration of Mineral Nutrients in Human Scalp Hair. Verlag Schmidt-Römhild; Lübeck: 1993. [Google Scholar]
- 193.Yang J, Jiang Z, Wang Y, Qureshi IA, Wu XD. Maternal-fetal transfer of metallic mercury via the placenta and milk. Ann Clin Lab Sci. 1997;27:135–41. [PubMed] [Google Scholar]
- 194.Bjornberg KA, Vahter M, Berglund B, Niklasson B, Blennow M, Sandborgh-Englund G. Transport of methylmercury and inorganic mercury to the fetus and breast-fed infant. Environ Health Perspect. 2005;113:1381–5. doi: 10.1289/ehp.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bose-O’Reilly S, Lettmeier B, Roider G, Siebert U, Drasch G. Mercury in breast milk—A health hazard for infants in gold mining areas? Int J Hyg Environ Health. 2008;211:615–23. doi: 10.1016/j.ijheh.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 196.LaKind JS, Brent RL, Dourson ML, Kacew S, Koren G, Sonawane B, et al. Human milk biomonitoring data: interpretation and risk assessment issues. J Toxicol Environ Health A. 2005;68:1713–69. doi: 10.1080/15287390500225724. [DOI] [PubMed] [Google Scholar]
- 197.Oskarsson A, Schultz A, Skerfving S, Hallen IP, Ohlin B, Lagerkvist BJ. Total and inorganic mercury in breast milk in relation to fish consumption and amalgam in lactating women. Arch Environ Health. 1996;51:234–41. doi: 10.1080/00039896.1996.9936021. [DOI] [PubMed] [Google Scholar]
- 198.Gundacker C, Pietschnig B, Wittmann KJ, Lischka A, Salzer H, Hohenauer L, et al. Lead and mercury in breast milk. Pediatrics. 2002;110:873–8. doi: 10.1542/peds.110.5.873. [DOI] [PubMed] [Google Scholar]
- 199.Bjorkman L, Sandborgh-Englund G, Ekstrand J. Mercury in saliva and feces after removal of amalgam fillings. Toxicol Appl Pharmacol. 1997;144:156–62. doi: 10.1006/taap.1997.8128. [DOI] [PubMed] [Google Scholar]
- 200.Engqvist A, Colmsjo A, Skare I. Speciation of mercury excreted in feces from individuals with amalgam fillings. Arch Environ Health. 1998;53:205–13. doi: 10.1080/00039899809605697. [DOI] [PubMed] [Google Scholar]
- 201.Ishihara N. Excretion of methyl mercury in human feces. Arch Environ Health. 2000;55:44–7. doi: 10.1080/00039890009603384. [DOI] [PubMed] [Google Scholar]
- 202.Rees JR, Sturup S, Chen C, Folt C, Karagas MR. Toenail mercury and dietary fish consumption. J Expo Sci Environ Epidemiol. 2007;17:25–30. doi: 10.1038/sj.jes.7500516. [DOI] [PubMed] [Google Scholar]
- 203.Bode P, de Kok J. Trends in trace element determinations in blood, serum, and urine of the Dutch population, and the role of neutron activation analysis. Biol Trace Elem Res. 1999;71-72:15–20. doi: 10.1007/BF02784186. [DOI] [PubMed] [Google Scholar]
- 204.Guallar E, Sanz-Gallardo MI, Van’T Veer P, Bode P, Aro A, Gomez-Aracena J, et al. Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med. 2002;347:1747–54. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- 205.MacIntosh DL, Williams PL, Hunter DJ, Sampson LA, Morris SC, Willett WC, et al. Evaluation of a food frequency questionnaire-food composition approach for estimating dietary intake of inorganic arsenic and methylmercury. Cancer Epidemiol Biomarkers Prev. 1997;6:1043–50. [PubMed] [Google Scholar]
- 206.Bjerregaard P, Hansen JC. Organochlorines and heavy metals in pregnant women from the Disko bay area in Greenland. Sci Total Environ. 2000;245:195–202. doi: 10.1016/s0048-9697(99)00444-1. [DOI] [PubMed] [Google Scholar]
- 207.Muckle G, Ayotte P, Dewailly EE, Jacobson SW, Jacobson JL. Prenatal exposure of the northern Quebec Inuit infants to environmental contaminants. Environ Health Perspect. 2001;109:1291–9. doi: 10.1289/ehp.011091291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Grandjean P, Budtz-Jorgensen E, Jorgensen PJ, Weihe P. Umbilical cord mercury concentration as biomarker of prenatal exposure to methylmercury. Environ Health Perspect. 2005;113:905–8. doi: 10.1289/ehp.7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sakamoto M, Kaneoka T, Murata K, Nakai K, Satoh H, Akagi H. Correlations between mercury concentrations in umbilical cord tissue and other biomarkers of fetal exposure to methylmercury in the Japanese population. Environ Res. 2007;103:106–11. doi: 10.1016/j.envres.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 210.Jedrychowski W, Perera F, Jankowski J, Rauh V, Flak E, Caldwell KL, et al. Fish consumption in pregnancy, cord blood mercury level and cognitive and psychomotor development of infants followed over the first three years of life Krakow epidemiologic study. Environ Int. 2007;33:1057–62. doi: 10.1016/j.envint.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 211.Angerer J, Ewers U, Wilhelm M. Human biomonitoring: state of the art. Int J Hyg Environ Health. 2007;210:201–28. doi: 10.1016/j.ijheh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 212.Schaller KH, Angerer J, Drexler H. Quality assurance of biological monitoring in occupational and environmental medicine. J Chromatogr B Anal Technol Biomed Life Sci. 2002;778:403–17. doi: 10.1016/s1570-0232(02)00171-x. [DOI] [PubMed] [Google Scholar]
- 213.National Center for Health Statistics . National Health and Nutrition Examination Survey 2003-2004. Center for Disease Control; Atlanta, GA: [cited 2007 September]. 2007. Available at: http://www.cdc.gov/nchs/about/major/nhanes/nhanes2003-2004/nhanes03_04.htm. [Google Scholar]
- 214.McDowell MA, Dillon CF, Osterloh J, Bolger PM, Pellizzari E, Fernando R, et al. Hair mercury levels in U.S. children and women of childbearing age: reference range data from NHANES 1999-2000. Environ Health Perspect. 2004;112:1165–71. doi: 10.1289/ehp.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Schober SE, Sinks TH, Jones RL, Bolger PM, McDowell M, Osterloh J, et al. Blood mercury levels in US children and women of childbearing age, 1999-2000. JAMA. 2003;289:1667–74. doi: 10.1001/jama.289.13.1667. [DOI] [PubMed] [Google Scholar]
- 216.National Center for Environmental Health . Third National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention; Atlanta, GA: 2005. [Google Scholar]
- 217.Blood mercury levels in young children and childbearing-aged women—United States, 1999-2002. MMWR Morb Mortal Wkly Rep. 2004;53:1018–20. [PubMed] [Google Scholar]
- 218.Schulz C, Conrad A, Becker K, Kolossa-Gehring M, Seiwert M, Seifert B. Twenty Years of the German Environmental Survey (Geres): Human Biomonitoring—Temporal and Spatial Germany: West/East Germany) differences in population exposure. Int J Hyg Environ Health. 2007;210:271–97. doi: 10.1016/j.ijheh.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 219.Becker K, Muessig-Zufika M, Conrad A, Luedecke A, Schulz C, Seiwert M, et al. German Environmental Survey for Children 2003/06 - GerES IV - Human Biomonitoring: Levels of selected substances in blood and urine of children in Germany. Federal Environment Agency - Germany; Berlin: 2008. pp. 1–93. Research Report 202 62 219, UBA-FB 001026. Available at: http://www.umweltdaten.de/publikationen/fpdf-l/3355.pdf. [Google Scholar]
- 220.Batariova A, Spevackova V, Benes B, Cejchanova M, Smid J, Cerna M. Blood and urine levels of Pb, Cd and Hg in the general population of the Czech Republic and proposed reference values. Int J Hyg Environ Health. 2006;209:359–66. doi: 10.1016/j.ijheh.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 221.Schulz C, Angerer J, Ewers U, Kolossa-Gehring M. The German Human Biomonitoring Commission. Int J Hyg Environ Health. 2007;210:373–82. doi: 10.1016/j.ijheh.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 222.Poulsen OM, Holst E, Christensen JM. Calculation and application of coverage intervals for biological reference values. Pure Appl Chem. 1997;69:1601–11. [Google Scholar]
- 223.Commission Human Biomonitoring of the Federal Environmental Agency New and updated reference values of contaminants in blood and urine of children—arsenic, lead, cadmium and Mercury. Bundesgesundheitsblatt. 2005;11:8–16. [Google Scholar]
- 224.Commission Human Biomonitoring of the Federal Environmental Agency Monograph Mercury—reference values and human biomonitoring values. Bundesgesundheitsblatt. 1999;42:522–32. [Google Scholar]
- 225.Drasch G, Bose-O’Reilly S, Maydl S, Roider G. Scientific comment on the German human biological monitoring values (HBM values) for mercury. Int J Hyg Environ Health. 2002;205:509–12. doi: 10.1078/1438-4639-00178. [DOI] [PubMed] [Google Scholar]
- 226.United Nations Environment Programme - Chemicals . Toolkit for Identification and Quantification of Mercury Releases. Geneva: 2005. pp. 1–275. [Google Scholar]
- 227.World Health Organization . Air Quality Guidelines, global update 2005. WHO; Copenhagen: 2006. [Google Scholar]
- 228.General C. Standard for contaminants and toxins in foods—CODEX STAN 1993-1995. FAO; 2007. Rev 3-2007. [Google Scholar]
- 229.Joint FAO/WHO Expert Committee on Food Additives . In: Evaluation of certain food additives and contaminants. World Health Organization, editor. WHO; Geneva: 2004. (Technical Report Series). [PubMed] [Google Scholar]
- 230.Joint FAO/WHO Expert Committee on Food Additives . Evaluation of certain food additives and contaminants. Geneva: 2007. (Technical Report Series). http://whqlibdoc.who.int/trs/ [Google Scholar]
- 231.Joint FAO/WHO Expert Committee on food additives . In: Summary and conclusions. Jecfa, editor. FAO/WHO; 2006. [Google Scholar]
- 232.Joint FAO/WHO Expert Committee on food additives . In: Summary and conclusions. Jecfa, editor. FAO/WHO; 2003. [Google Scholar]
- 233.World Health Organization . In: Guidelines for Drinking-water Quality. 3rd ed. World Health Organization, editor. WHO; Geneva: 2004. pp. 1–515. p. 1-515. [Google Scholar]
- 234.Bose-O’Reilly S, Drasch G, Beinhoff C, Maydl S, Vosko MR, Roider G, et al. The Mt. Diwata study on the Philippines, 2000-treatment of mercury intoxicated inhabitants of a gold mining area with DMPS (2,3-dimercapto-1-propane-sulfonic acid, Dimaval) Sci Total Environ. 2003;307:71–82. doi: 10.1016/s0048-9697(02)00547-8. [DOI] [PubMed] [Google Scholar]
- 235.U.S. Environmental Protection Agency Volume I: Executive Summary1997 Report No.: EPA-452/R-97-003.
- 236.Rice DC. The US EPA. Reference dose for methylmercury: sources of uncertainty. Environ Res. 2004;95:406–13. doi: 10.1016/j.envres.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 237.Stern AH. A revised probabilistic estimate of the maternal methyl mercury intake dose corresponding to a measured cord blood mercury concentration. Environ Health Perspect. 2005;113:155–63. doi: 10.1289/ehp.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rice DC, Schoeny R, Mahaffey K. Methods and rationale for derivation of a reference dose for methylmercury by the U.S. EPA. Risk Anal. 2003;23:107–15. doi: 10.1111/1539-6924.00294. [DOI] [PubMed] [Google Scholar]
- 239.Lettmeier B, Boese-O’Reilly S, Drasch G. Proposal for a revised reference concentration (RfC) for mercury vapour in adults. Sci Total Environ. 2010;408:3530–5. doi: 10.1016/j.scitotenv.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 240.U.S. Food and Drug Administration . What You need to know about Mercury in Fish and Shellfish. U.S. Food and Drug Administration; Silver Spring, MD: [cited 2010 August]. 2004. EPA-823-R-04-005. Available at: http://www.cfsan.fda.gov/%7Edms/admehg3.html. [Google Scholar]
- 241.European Commission Health and Consumer Protection Directorate-General-Directorate D-Food Safety . Methyl mercury in fish and fishery products. European Commission-Health and Consumer Protection DG; Brussels: [cited 2010 August]. 2004. [Google Scholar]
- 242.Australian Government-Department of the Environment-Water-Heritage and the Arts . Safe disposal of mercury-containing lamps. Australian government-Department of the Environment-Water-Heritage and the Arts; Canberra ACT, Australia: 2009. Available at: Safe disposal of mercury-containing lamps. [Google Scholar]
- 243.Southworth GR, Lindberg SE, Bogle MA, Zhang H, Kuiken T, Price J, et al. Airborne emissions of mercury from municipal solid waste. II: Potential losses of airborne mercury before landfill. J Air Waste Manag Assoc. 2005;55:870–7. doi: 10.1080/10473289.2005.10464695. [DOI] [PubMed] [Google Scholar]
- 244.Boese-O’Reilly S, Shimkin MKE. Taking action to protect children from environmental hazards. In: Pronczuk-Garbino J, editor. Children’s health and the environment - a global perspective. WHO press; Geneva: 2005. pp. 253–72. [Google Scholar]
- 245.Karliner J, Harvie J. The global movement for mercury-free health care. Health Care Without Harm Publication; [cited 2010 August]. 2007. Available at: http://www.noharm.org/globalsoutheng/mercury/issue. [Google Scholar]
- 246.C. Electric power plant emissions and public health. Am J Nurs. 2008;108:62–70. doi: 10.1097/01.NAJ.0000310342.98495.b6. [Quiz, 71] [DOI] [PubMed] [Google Scholar]
- 247.World Health Organization . Policy paper: Mercury in Health Care (WHO/SDE/WSH/05.08) World Health Organization; Geneva: [cited 2007 July]. 2005. Available at: http://www.who.int/water_sanitation_health/medicalwaste/mercurypolpap230506.pdf. [Google Scholar]
- 248.FDA, Food and Drug Administration, FDA . National Marine Fisheries Service Survey of Trace Elements in the Fishery Resource, Report 1978; 1990-2004. “The Occurrence of Mercury in the Fishery Resources of the Gulf of Mexico” Report 2000. FDA; 2000. [Google Scholar]
- 249.Santos EC, de Jesus IM, Vde M Camara, Brabo E, Loureiro EC, Mascarenhas A, et al. Mercury exposure in Munduruku Indians from the community of Sai Cinza, state of Para, Brazil. Environ Res. 2002;90:98–103. doi: 10.1006/enrs.2002.4389. [DOI] [PubMed] [Google Scholar]
- 250.Zhang L, Wang Q. Preliminary study on health risk from mercury exposure to residents of Wujiazhan town on the di er Songhua river, Northeast China. Environ Geochem Health. 2006;28:67–71. doi: 10.1007/s10653-005-9013-1. [DOI] [PubMed] [Google Scholar]
- 251.Olivero J, Johnson B, Arguello E. Human exposure to mercury in San Jorge river basin, Colombia (South America) Sci Total Environ. 2002;289:41–7. doi: 10.1016/s0048-9697(01)01018-x. [DOI] [PubMed] [Google Scholar]
- 252.Cole DC, Kearney J, Sanin LH, Leblanc A, Weber JP. Blood mercury levels among Ontario anglers and sport-fish eaters. Environ Res. 2004;95:305–14. doi: 10.1016/j.envres.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 253.Iwasaki Y, Sakamoto M, Nakai K, Oka T, Dakeishi M, Iwata T, et al. Estimation of daily mercury intake from seafood in Japanese women: Akita cross-sectional study. Tohoku J Exp Med. 2003;200:67–73. doi: 10.1620/tjem.200.67. [DOI] [PubMed] [Google Scholar]
- 254.Stamler CJ, Abdelouahab N, Vanier C, Mergler D, Chan HM. Relationship between platelet monoamine oxidase-B (MAO-B) activity and mercury exposure in fish consumers from the lake St. Pierre region of Que., Canada. Neurotoxicology. 2006;27:429–36. doi: 10.1016/j.neuro.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 255.Swain EB, Jakus PM, Rice G, Lupi F, Maxson PA, Pacyna JM, et al. Socioeconomic consequences of mercury use and pollution. Ambio. 2007;36:45–61. doi: 10.1579/0044-7447(2007)36[45:scomua]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 256.Blood M. Levels in young children and childbearing-aged women—United States, 1999-2002. MMWR Morb Mortal Wkly Rep. 2004;53:1018–20. [PubMed] [Google Scholar]
- 257.US Environmental Protection Agency . Results of the Lake Michigan Mass Balance Study: Mercury data report. EPA; Chicago US: 2004. [Google Scholar]



