Abstract
Sed5p is the only syntaxin family member required for protein transport through the yeast Golgi and it is known to bind up to nine other soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) proteins in vivo. We describe in vitro binding experiments in which we identify ternary and quaternary Sed5p-containing SNARE complexes. The formation of SNARE complexes among these endoplasmic reticulum- and Golgi-localized proteins requires Sed5p and is syntaxin-selective. In addition, Sed5p-containing SNARE complexes form selectively and this selectivity is mediated by Sed5p-containing intermediates that discriminate among subsequent binding partners. Although many of these SNAREs have overlapping distributions in vivo, the SNAREs that form complexes with Sed5p in vitro reflect their functionally distinct locales. Although SNARE–SNARE interactions are promiscuous and a single SNARE protein is often found in more than one complex, both the biochemical as well as genetic analyses reported here suggest that this is not a result of nonselective direct substitution of one SNARE for another. Rather our data are consistent with the existence of multiple (perhaps parallel) trafficking pathways where Sed5p-containing SNARE complexes play overlapping and/or distinct functional roles.
INTRODUCTION
It is generally accepted that proteins are transported through the secretory pathway in membrane-bound vesicles that bud from one compartment and fuse with another (Rothman, 1994). For the integrity of the endomembrane system to be maintained it is clear that a significant degree of fidelity is required in the specificity of vesicle-organelle targeting and fusion events. Although the precise molecular requirements for such events are not fully understood, the components required for vesicle fusion specificity in yeast likely include the Ypt family of GTPases, vesicle tethering protein complexes, and members of a protein superfamily known as soluble NSF (N-ethylmaleimide-senstive factor) attachment protein receptors or SNAREs (Waters and Hughson, 2000). SNAREs have been considered strong candidates for the molecules that direct vesicle-organelle targeting specify for a variety of reasons. SNAREs are cytoplasmically orientated membrane proteins that have distinct (but in some instances overlapping) intracellular distributions throughout the endomembrane system. And every known fusion step requires a member of the SNARE superfamily called a syntaxin (Nichols and Pelham, 1998). However, there have been a number of recent reports that suggest SNARE–SNARE interactions alone are not sufficient to account for the specificity of vesicle-targeting reactions. For example, a single SNARE can participate in more than one transport step (Fischer von Mollard and Stevens, 1999) and noncognate mammalian SNARE complexes form in vitro (Fasshauer et al., 1999; Yang et al., 1999).
SNARE proteins form complexes that are reversibly disassembled by the ATPase NSF (N-ethylmaleimide-sensitive factor, the product of the SEC18 gene in yeast) in the presence of a group soluble cofactors known as SNAPs (soluble NSF-attachment proteins) (Söllner et al., 1993). Neuronal SNARE proteins form a stable complex consisting of a parallel four α-helix bundle comprised of the C-terminal core domains of syntaxin 1A, synaptobrevin II, and two α helices contributed by one molecule of SNAP-25B (Poirier et al., 1998; Sutton et al., 1998). A similar structure has also been suggested for the yeast exocytic SNARE complex (Katz et al., 1998).
Budding yeast have eight syntaxins only one of which (Sed5p) is essential for traffic through the Golgi (Holthuis et al., 1998; Pelham, 1998, 1999). Sed5p is known to bind to at least nine SNARE proteins and thus yeast Golgi SNARE complexes are likely to contain Sed5p and as yet undefined combinations of the SNAREs Sec22p, Bos1p, Bet1p, Ykt6p, Vti1p, Gos1p, Tlg1p, Snc2p, and Sft1p (Søgaard et al., 1994; McNew et al., 1997; Nichols and Pelham, 1998; Grote and Novick, 1999). Among this group of SNARE proteins there are no structural equivalents of SNAP-25 and therefore the composition of Sed5p-containing SNARE complexes may differ from their yeast and mammalian cell exocytic counterparts (Weimbs et al., 1998; Fasshauer et al., 1999; Yang et al., 1999). Although it is unlikely that Sed5p forms a single complex with these SNAREs, biochemical and genetic studies suggest that a single SNARE is likely to be present in more than one complex. Indeed, examination of binary interactions among these proteins have revealed that such interactions are promiscuous in vitro and genetic analyses also suggest that some of these SNAREs may be able to directly substitute for one another in vivo (Tsui and Banfield, 2000).
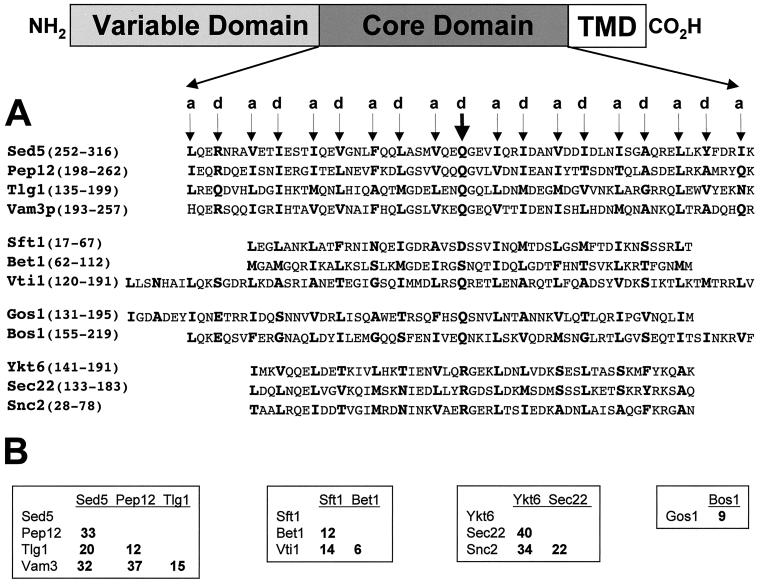
The fact that the core domains of SNARE proteins that interact with Sed5p are significantly divergent among their respective families (Figure 1), taken together with the observation that many of these SNAREs have distinct functional locales, but share overlapping distributions in vivo, led us to address the question of whether Sed5p can discriminate among this group of proteins and form selective complexes with these SNAREs.
Figure 1.
(A) Amino acid sequence alignments of the heptad repeat/core domains of the SNAREs used in these studies. The a and d positions of the predicted hydrophobic layers (heptad repeats) are indicated above the arrows and the amino acids at these positions are highlighted in bold. The thick arrow indicates the position of the d amino acid, which has been used to define proteins as either R- or Q-SNAREs (Fasshauer et al., 1998). This position also corresponds to the zero ionic layer in the structure of the neuronal SNARE complex (Sutton et al., 1998). SNARE proteins are aligned with their putative family members: the syntaxins (Sed5p, Pep12p, Vam3p, and Tlg1p), the Bet1 family (Sft1p, Bet1p, and Vti1p), the Gos1p family (Gos1p and Bos1p), and the synaptobrevin family (Ykt6p, Sec22p, and Snc2p). The position of the amino acids in each of the proteins used in the alignments, is indicated in parentheses. The assignment of SNAREs to a particular family is based in part on amino acid sequence similarity as well as on the behavior of individual SNARE proteins in in vitro mixing assays (see text for details). (B) Pairwise percentage of amino acid identities among the aligned SNARE family proteins as indicated in A.
In this study we identify a number of ternary and quaternary Sed5p-containing SNARE complexes. Our in vitro biochemical studies reveal that multimeric SNARE–SNARE interactions facilitate the selective formation Sed5p-containing complexes. In addition, although a single SNARE protein is present in more than one complex, genetic analyses described here suggest that the apparent functional redundancy of some SNAREs is not the result of indiscriminant direct substitution of one SNARE for another.
MATERIALS AND METHODS
Strains and Media
All yeast strains were grown at 25 or 37°C in either yeast extract-peptone-dextrose medium (YEPD), synthetic dextrose medium lacking the appropriate amino acids or on plates containing 5-fluoro-orotic acid (5-FOA) as appropriate. Standard yeast molecular genetic techniques were carried out as described by (Guthrie and Fink, 1991). Yeast transformations were performed using lithium acetate as described by (Elble, 1992). A list of yeast strains used in this study can be found in Table 1.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| SEY6210 | MATα ura3-52 leu2-3, −112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | S. Emr |
| SARY160 | MATα ura3-52 leu2-3, −112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 sft1Δ∷LEU2+ pSFT1 [2μ URA3 TPI-SFT1] | Banfield lab |
| SARY245 | MATα/a his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 MET15/met15Δ0 LYS2/lys2Δ0 ura3Δ0/ura3Δ0 BET1/betΔ1∷KAN | Research Genetics |
| SARY244 | MATα/a his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 MET15/met15Δ0 LYS2/lys2Δ0 ura3Δ0/ura 3Δ0 BOS1/bos1Δ∷KAN | Research Genetics |
| SARY242 | MATα/a his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 MET15/met15Δ0 LYS2/lys2Δ0 ura3Δ0/ura3Δ0 VTI1/vti1Δ∷KAN | Research Genetics |
| SARY270 | MATα ura3Δ0 his3Δ1 leu2Δ0 LYS2 met15Δ0 bet1Δ∷KAN, pBET1 [2μ URA3 BET1] (derived from SARY245) | This study |
| SARY260 | MATa ura3Δ0 his3Δ1 leu2Δ0 LYS2 bos 1Δ∷KAN, pBOS1 [2μ URA3 BOS1] (derived from SARY244) | This study |
| SARY296 | MATα his3Δ1 leu2Δ/ura3Δ0 MET15 LYS2 vti1Δ∷KAN pVTI1 [2μ URA3 VTI1] (derived from SARY242) | This study |
| SARY158 | MATα ura3-52 leu2-3, −112 his3Δ200 trp1-Δ901 lys2-801 suc2-Δ9 ykt6Δ∷LEU2 pYKT6 [2μ URA3 YKT6] | Banfield lab |
| Y26 | MATa gos1Δ∷URA3 trp1-Δ1 can1-100 his3-11,15 leu2-3,112 ade2-1 | W. Hong |
| RSY279 | MATα ura3-52 sec22-3 his4-619 | C. Kaiser |
| SARY327 | MATα/a GOS1/gos1Δ∷URA3 SEC22/sec22-3 ura3-52/ura3-52 (Y26/RSY279 diploid) | This study |
| SARY342 | MATa gos1Δ∷URA3 sec22-3 (derived from SARY327) | This study |
| SARY316 | MATα his3Δ1 leu2Δ0 MET15 lys2Δ ura3Δ0 snc2Δ∷KAN | Research Genetics |
| SARY325 | MATα his3Δ1 leu2Δ0 MET15 lys2Δ ura3Δ0 snc2Δ∷KAN sft1Δ∷LEU2 pSFT1 [2μ URA3 SFT1] | This study |
| SARY323 | MATα trp1-Δ901 lys2 MET15 ura3 his3 sec22Δ∷KAN | This study |
| SARY324 | MATα trp1-Δ901 lys2 MET15 ura3 his3 sec22Δ∷KAN sft1Δ∷LEU2 pSFT1 [2μ URA3 SFT1] | This study |
| SARY181 | MATα ura3-52 leu2-3, −112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 sft1Δ∷LEU2+ pBET1 [2μ URA3 BET1] | Tsui and Banfield, 2000 |
| SARY208 | MATα ura3-52 leu2-3, −112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 sft1Δ∷LEU2+ pSNC2 [2μ URA3 SNC2] | Tsui and Banfield, 2000 |
The bacterial strains BL21 and Top 10 (Invitrogen, San Diego, CA) were grown at 30°C or 37°C in either 2 X YT or LB supplemented with the appropriate antibiotic (amplicilin at 100 μg/ml or kanamycin at 100 μg/ml).
Materials
Restriction and modifying enzymes were purchased from New England Biolabs (Beverly, MA). Pfu polymerase was purchased from Stratagene (La Jolla, CA). Anti-(His)6 antibody was purchased from Boerhinger-Mannhiem (Mannheim, Germany). Nitrocellulose membranes, chemiluminescent reagents, enhanced chemiluminescence (ECL) Hyperfilm, and glutathione Sepharose 4B were purchased from Amersham (Little Chalfont, England) and reduced glutathione was purchased from Sigma (St. Louis, MO). All other chemicals were purchased from Sigma (Poole, United Kingdom), Bio-Rad (Richmond, CA), or BDH. Nucleic acids were purified with reagents purchased from Qiagen (Chatsworth, CA). Deoxyribonucleotide primers were purchased from Genosys (Pampisford, United Kingdom) and GENSET (Singapore). Spurr's resin was purchased from Sigma, uranyl acetate from Electron Microscopy Sciences (Fort Washington, PA), and lead citrate from Acros Organics (Geel, Belgium).
Recombinant DNA Procedures
Yeast SNARE proteins were expressed as either glutathione S-transferase (GST) fusion proteins in pGEX2T (Pharmacia, Uppsala, Sweden)) or as N-terminal (His)6 fusions in pET24b(+) (Novagen, Madison, WI). The open reading frames encoding various SNARE proteins were amplified from either genomic DNA or from plasmid DNAs harboring the gene. DNA fragments were amplified with Pfu DNA polymerase (Stratagene) and the polymerase chain reaction by placing a BamHI site upstream of the initiator methionine and an EcoRI (or Mfe1) site just before the sequence encoding the transmembrane domain for the GST-fusion (pGEX2T) constructs. (His)6-tagged SNARE fusion constructs in pET24b(+) were generated by placing a BamHI site upstream of the initiator methionine and a HindIII site just before the sequence encoding the transmembrane domain. All SNARE expression constructs were verified by DNA sequence determination. The SNARE expression plasmids used in this study are given in Table 2.
Table 2.
Plasmids used for the expression of recombinant SNARE proteins
| Plasmid | Description | Source |
|---|---|---|
| pGST-Sft1 | GST-Sft1 (aa 17–78) in pGEX2T | Tsui and Banfield, 2000 |
| pGST-Bet1 | GST-Bet1 (aa 1–123) in pGEX2T | Tsui and Banfield, 2000 |
| pGST-Sed5 | GST-Sed5 (aa 1–319) in pGEX2T | Banfield lab |
| pGST-Vti1 | GST-Vti1 (aa 1–194) in pGEX2T | Tsui and Banfield, 2000 |
| pGST-Snc2 | GST-Snc2 (aa 1–93) in pGEX2T | This study |
| pGST-Vam7 | GST-Snc2 (aa 1–316) in pGEX2T | This study |
| pSed5-HIS24b | Sed5-(His)6 (aa 1–319) in pET24b(+) | Tsui and Banfield, 2000 |
| pYkt6-HIS24b | Ykt6-(His)6 (aa 2–190) in pET24b(+) | Tsui and Banfield, 2000 |
| pGos1-HIS24b | Gos1-(His)6 (aa 2–201) in pET24b(+) | Tsui and Banfield, 2000 |
| pVti1-HIS24b | Vti1-(His)6 (aa 1–194) in pET24b(+) | Tsui and Banfield, 2000 |
| pSec22-HIS24b | Sec22-(His)6 (aa 1–180) in pET24b(+) | Tsui and Banfield, 2000 |
| pBos1-HIS24b | Bos1-(His)6 (aa 1–216) in pET24b(+) | Tsui and Banfield, 2000 |
| pTIg1-HIS24b | Tlg1-(His)6 (aa 1–204) in pET24b(+) | This study |
| pPep12-HIS24b | Pep12-(His)6 (aa 1–266) in pET24b(+) | This study |
| pVam3-HIS24b | Vam3-(His)6 (aa 1–263) in pET24b(+) | This study |
| pNyv1-HIS24b | Nyv1-(His)6 (aa 1–231) in pET24b(+) | This study |
Yeast Molecular Genetics
Yeast transformants, selected on synthetic dextrose medium plates lacking the appropriate amino acid, were patched onto plates containing 5-fluroorotic acid (5-FOA, 100 μg/ml) and incubated at 25°C for 2–3 d. Sporulation was carried out at either 25 or 30°C on minimal sporulation plates (1% wt/vol potassium acetate) for 3–5 d and tetrads were dissected using a Singer MSM micromanipulator. The SNARE deletion strains SARY270 (bet1Δ), SARY260 (bos1Δ), and SARY296 (vti1Δ) were isolated following dissection of the sporulated yeast strains SARY 245, 244, and 242, respectively (Table 1). Disruptants were verified by plasmid shuffling with counter selection and 5-FOA. The SNARE double deletion strains SARY324 (sft1Δ sec22Δ) and SARY325 (sft1Δ snc2Δ) were prepared by direct disruption of the SFT1 gene with LEU2 by using a knockout cassette prepared by polymerase chain reaction-mediated amplification of genomic DNA flanking the SFT1 open reading frame and cotransformation with the plasmid pTPISFT1 (2 μ, URA3) in the strains SARY323 (sec22Δ) and SARY316 (snc2Δ) (Table 1). SFT1 disruptants were identified by plasmid shuffling and counter selection on plates containing 5-FOA.
Preparation of Recombinant SNARE Proteins
Bacterial expression and purification of soluble recombinant SNARE proteins was performed essentially as described previously (Tsui and Banfield, 2000) with the following modifications. Bacterial (Escherichia coli BL21) cultures (400 ml) were grown in LB broth containing either 100 μg/ml ampicillin or 100 μg/ml kanamycin (depending on the expression plasmid) to an OD600 of 0.6. Expression of recombinant SNARE proteins was induced by the addition of isopropyl-thio-β-galactoside to a final concentration of 0.5 mM and incubation at 30°C for an additional 4 h. Bacterial cells were harvested by centrifugation at 4°C, resuspended in 4 ml of ice cold lysis buffer (20 mM HEPES, pH 7.4; 150 mM potassium acetate; 0.05% [vol/vol] Tween 20) containing a protease inhibitor cocktail (Complete; Boehringer-Mannhiem) and lysed by sonication. Following sonication, lysates were clarified by centrifugation (at 4°C) and the supernatants used directly for in vitro binding assays.
In Vitro Binding Assays
Binding assays were carried out by mixing the soluble fraction of bacterial lysates from cells expressing individual recombinant SNARE proteins (as described above). Assays were performed by mixing 300 μl of each SNARE-containing bacterial lysate in a final volume of 1.2 ml. Where the total volume of recombinant SNARE-containing lysates added to the assay was less than 1.2 ml (i.e., when three or fewer were mixed together) the final volume was adjusted to 1.2 ml by using lysate buffer. SNARE protein mixtures were incubated overnight at 4°C with constant gentle mixing. Following incubation, samples were centrifuged (5 min at 14,000 × g, at 4°C) and the supernatants incubated with a 50-μl slurry of glutathione Sepharose 4B beads (equilibrated in lysis buffer) at 4°C for 2 h. Following incubation, beads were washed three times with ∼20-bead volumes of lysis buffer, resuspended in 50 μl of sample buffer (80 mM Tris-HCl pH 6.8, 2% SDS, 10% glycerol, 5% 2-mercaptoethanol, 0.025 mg/ml bromophenol blue), and heated to 95°C for 5 min (unless otherwise indicated). Proteins were resolved by SDS-PAGE on 10% glycine gels. For mixing assays in which the effect of increasing the relative concentration of a single SNARE in a mixture was addressed, progressively increasing volumes of the particular SNARE-containing bacterial lysate (0–800 μl) was mixed with 150 μl of the other three SNARE proteins in a final volume of 1.2 ml, all other procedures remained unchanged (see above).
Immunostaining
A mouse monoclonal antibody that recognizes the (His)6-epitope (BMG-His-1; Boehringer-Mannheim) was used to detect recombinant (His)6-tagged SNARE proteins. All antibody incubations were carried out in phosphate-buffered saline containing 3% dried milk powder and 0.1% Tween 20. After incubation with the anti-(His)6 antibody and peroxidase-conjugated secondary antibody (Amersham), detection was performed using ECL (Amersham) and autoradiography (ECL Hyperfilm; Amersham).
Electron Microscopy
Yeast cells were grown in YEPD for 4 h at 25°C or until an OD660 of ∼0.3. Cells were fixed with potassium permanganate and stained with uranyl acetate and lead citrate as described previously (Banfield et al., 1995). Ultrathin sections (∼50 nm) were obtained from Spurr's resin-impregnated samples by using a Leica Wild M3Z ultramicrotome and sections viewed (at 100 kV) and photographed using a Philips CM20.
RESULTS
To identify Sed5p-containing complexes we prepared recombinant, soluble, but otherwise full-length forms of a number of SNAREs with which Sed5p is known to bind in vivo (reviewed in Nichols and Pelham, 1998; Grote and Novick, 1999). SNARE proteins were expressed in E. coli as either GST- or (His)6-fusion proteins (Table 2) and total soluble protein fractions from bacteria expressing recombinant SNARE proteins were used in binding experiments throughout this study.
Sed5p Forms Select Ternary and Quaternary Complexes with SNAREs Involved in Traffic to and through the Yeast Golgi
Binary interactions among ER and Golgi SNAREs are promiscuous, although not exclusively nonselective (Tsui and Banfield, 2000). Because Sed5p-containing SNARE complexes are likely comprised of two or more SNAREs we wished to determine whether multimeric SNARE interactions displayed the same degree of promiscuity or were more selective.
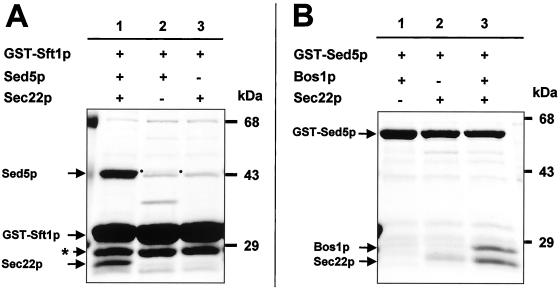
Figure 2 shows the results of mixing GST-Sft1p or GST-Sed5p with various combinations of the (His)6-SNAREs: Sec22p, Bos1p, and Sed5p. Sft1p bound to [Sed5p + Sec22p] (Figure 2A, lane 1) weakly to [Sed5p] (Figure 2A, lane 2), and Sft1p binding to [Sec22p] was not detectable (Figure 2A, lane 3). GST-Sed5p bound to [Sec22p + Bos1p] (Figure 2B, lane 3) and [Sec22p] (Figure 2B, lane 2) but binding to [Bos1p] was not detectable (Figure 2B, lane 1).
Figure 2.
Formation of binary and ternary SNARE complexes with GST-Sft1p and GST-Sed5p. Soluble lysates from bacterial cells expressing recombinant GST- or (His)6-tagged SNARE proteins (lacking their membrane anchor sequences) were mixed together and protein complexes isolated by affinity chromatography with glutathione Sepharose beads. Proteins were resolved by SDS-PAGE and stained with Coomassie brilliant blue. (A) GST-Sft1p binds to [Sed5p + Sec22p] (lane 1) and to [Sed5p] (lane 2), but binding to [Sec22p] is not detectable (lane 3). (B) GST-Sed5p binds [Sec2p + Bos1p] (lane 3) and [Sec22p] albeit weakly (lane 2) but not to [Bos1p] (lane 1). The star symbol (★) in Figure 2A denotes GST derived from proteolysis of GST-Sft1p. The dots either side of lane 2 in A highlight the presence of Sed5p.
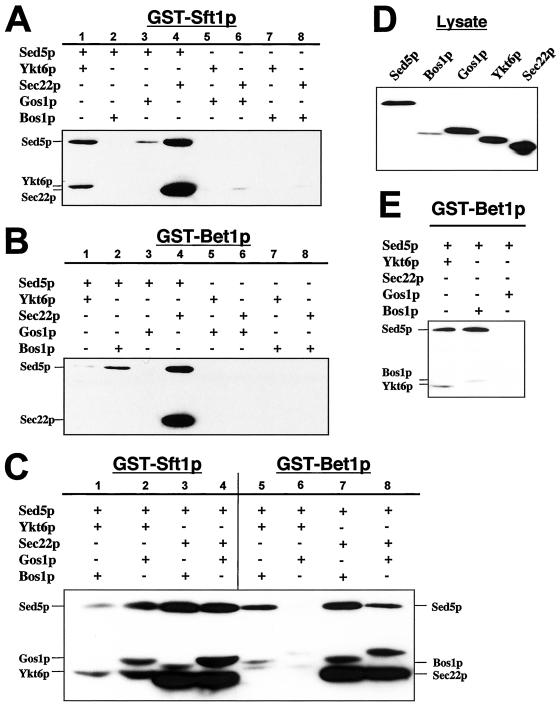
Figure 3, A and B, show the results of mixing experiments with GST-Sft1p and GST-Bet1p, respectively, with various combinations of two other (His)6-SNARE proteins (ternary complex formation). GST-Sft1p forms a ternary complex with [Sed5p + Ykt6p] (lane 1) and [Sed5p + Sec22p] (Figure 3A, lane 4) and binary complex with [Sed5p] but only in the presence of Gos1p (Figure 3A, lane 3). GST-Bet1p forms a ternary complex with [Sed5p + Sec22p] (Figure 3B, lane 4) and with [Sed5p + Ykt6p] (Figure 3, B and E, lane 1) and [Sed5p + Bos1p] (Figure 3, B and E, lane 2). However, less Ykt6p is incorporated into [Bet1p + Sed5p + Ykt6p] (Figure 3B, lane 1) than in the case of [Sft1p + Sed5p + Ykt6p] (Figure 3A, lane 2).
Figure 3.
Sed5p selectively forms ternary and quaternary complexes in vitro with the SNAREs Sft1p, Bet1p, Gos1p, Bos1p, Ykt6p, and Sec22p. Proteins were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and recombinant (His)6-tagged SNAREs detected by immunostaining with the anti-(His)6 antibody BMG-His-1. Ternary complexes: GST-Sft1p was mixed with various combinations of two different (His)6-tagged SNARE proteins as indicated (A). GST-Bet1p was mixed with various combinations of two different (His)6-tagged SNARE proteins as indicated (B). Quaternary complexes: Either GST-Bet1p or GST-Sft1p was mixed with (His)6-Sed5p and various combinations of two other (His)6-tagged SNARE proteins as indicated (C). Immunoblot analysis of aliquots of soluble lysates from bacteria expressing the (His)6-SNAREs used in this experiment (corresponding to ∼5% of input) (D). (E) Longer exposure of a subsection of B reveals some association of Ykt6p with [Bet1p + Sed5p] and of Bos1p with [Bet1p + Sed5p]. Note that in the absence of Sed5p no detectable binding is observed between either GST-Sft1p or GST-Bet1p and other SNAREs (A and B, lanes 5–8).
Two striking observations are that in the absence of Sed5p, ternary complexes comprised of GST-Sft1p or GST-Bet1p and binary combinations of Ykt6p, Sec22p, Gos1p, or Bos1p did not form (Figure 2, A and B, lanes 5–8). And apparent differences in the affinity of (His)6-Sed5p for GST-Sft1p and GST-Bet1p were observed (e.g., compare lane 1 with lane 4 in Figure 3, A and B). These differences may result from the ability of particular SNAREs to selectively facilitate the association of Sed5p with either Sft1p or Bet1p. For example, Ykt6p facilitates the association of Sed5p with Sft1p (Figure 3A, lane 1) but is much less effective in facilitating the association of Sed5p with Bet1p (Figure 3B, lane 1).
Perhaps more striking is the ability of Gos1p and Bos1p to discriminate between [Sed5p + Sft1p] and [Sed5p + Bet1p]. Although Gos1p can facilitate the association of Sed5p with Sft1p (Figure 3A, lane 3) Gos1p does not facilitate the association of Sed5p with Bet1p (Figure 3B, lane 3). The converse is true for Bos1p, Sed5p, and Sft1p (Figure 3A, lane 2) and Bos1p, Sed5p, and Bet1p (Figure 3B, lane 2).
In contrast, Sec22p appears equally effective in its ability to facilitate the association of Sed5p with either Sft1p or Bet1p (Figure 3A, lane 4, and B, lane 4, respectively). And Sec22p is itself incorporated into the ternary complexes [Sft1p + Sed5p + Sec22p] and [Bet1p + Sed5p + Sec22p] (Figure 3, A and B, lane 4). Similarly, although Ykt6p is incorporated into [Sft1p + Sed5p + Ykt6] complex (Figure 3A, lane 1) in contrast to Sec22p, relatively less Ykt6p binds to [Bet1p + Sed5p] (Figure 3B, lane 1, and E, lane 1). A similar scenario is also observed for Bos1p incorporation into [Sed5p + Bet1p + Bos1p]. However, as the level of expression for Bos1p is lower than that of other SNAREs used in this experiment (Figure 3D) the apparent under-incorporation of Bos1p into [Sed5p + Bet1p + Bos1p] may simply reflect a decrease in the relative concentration of biologically active Bos1p in the mixing reaction. None-the-less, these data are consistent with a previous study that demonstrated that Bet1p could facilitate the binding of Bos1p to Sed5p (Stone et al., 1997).
Six ternary Sed5p-containing SNARE complexes were identified of nine possible combinations: [Sed5p + Bos1p + Sec22p], [Sed5p + Sft1p + Sec22p], [Sed5p + Bet1p + Sec22p], [Sed5p + Sft1p + Ykt6p], [Sed5p + Bet1p + Ykt6p], and [Sed5p + Bet1p + Bos1p], two of which ([Sed5p + Bet1p + Ykt6p] and [Sed5p + Bet1p + Bos1p]) form far more less efficiently than the others (Figures 2 and 3, A and B). Recent studies have shown that mammalian SNARE proteins interact promiscuously and nonselectively in vitro (Fasshauer et al., 1999; Yang et al., 1999) we were therefore somewhat surprised that the same was not true for the ternary Golgi SNARE complexes observed in these experiments. The only known SNARE complex structure described to date consists of a four α helical bundle comprised of three different SNAREs (Sutton et al., 1998), one of which (SNAP-25) contributes two of the four helices to the structure. Sed5p-containing SNARE complexes may therefore be comprised of four rather three different SNARE proteins where each SNARE protein contributes a single α helical domain to the structure (Weimbs et al., 1998). However, because the method we used to identify SNARE complexes is semiquantitative and the GST-fusion proteins are present in excess, it is not possible to determine the precise stoichiometry of the complexes identified here. Therefore, these complexes, although comprised of three different SNARE proteins, may be tetrameric/quaternary in composition, because we cannot exclude the possibility that one of the three proteins may be present in a 2:1:1 ratio, with respect to the other SNARE components, rather than in a 1:1:1 ratio. Accordingly, the ternary complexes identified here may represent intermediates in the formation of quaternary SNARE complexes.
We therefore examined SNARE complex formation by using either GST-Sft1p or GST-Bet1p together with (His)6-Sed5p and various combinations of two other (His)6-SNAREs (quaternary complex formation, Figure 3C). We identified five quaternary Sed5p-containing SNARE complexes of eight possible combinations. Sed5p formed three quaternary complexes with Sft1p: [Sft1p + Sed5p + Ykt6p + Gos1p] (Figure 3C, lane 2), [Sft1p + Sed5p + Bos1p + Sec22p] (Figure 3C, lane 3), and [Sft1p + Sed5p + Gos1p + Sec22p] (Figure 3C, lane 4); and two quaternary complexes with Bet1p: [Bet1p + Sed5p + Bos1p + Sec22p] (Figure 3C, lane 7) and [Bet1p + Sed5p + Bos1p + Gos1p] (Figure 3C, lane 8). Ykt6p facilitates the association of Gos1p with the complex [Sft1p + Sed5p + Ykt6p] but does not facilitate the association of Bos1p with [Sft1p + Sed5p + Ykt6p] (compare lanes 1 and 2, Figure 3C). Similarly Sec22p facilitates the association of either Gos1p or Bos1p with the complex [Sft1p + Sed5p + Sec22p]. Neither Gos1p nor Bos1p associates with [Sft1p + Sed5p] in the absence of Ykt6p or Sec22p (Figure 3A, lanes 2 and 3). Evidence of selective binding interactions between SNARE proteins is also observed for [Bet1p + Sed5p]. For example, neither [Ykt6p or Gos1p] nor [Ykt6p or Bos1p] appears to bind [Bet1p + Sed5p] to the same extent as in corresponding experiments where [Sec22p + Bos1p] or [Sec22p + Gos1p] are used or where Bet1p is replaced with Sft1p (e.g., compare Figure 3C, lanes 2 and 6 and 3 and 7). In the case of Ykt6p and Bos1p, the binding pattern appears to be more consistent with two ternary complexes ([Bet1p + Sed5p + Ykt6p] and [Bet1p + Sed5p + Bos1p]), rather than with a single quaternary complex (Figure 3E).
Formation of Quaternary SNARE Complexes Occurs Sequentially and Only in the Presence of Sed5p
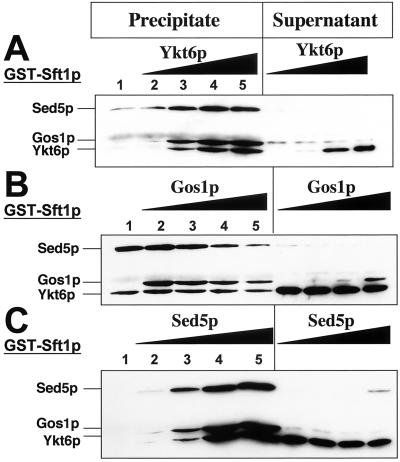
The observation that some SNARE–SNARE interactions may be facilitated by SNARE-specific activation events, in which the “activating SNARE” was underrepresented in the purified complex (Figure 3, B and E, lanes 1 and 2), led us to investigate whether complex assembly proceeded via an ordered series of SNARE–SNARE binding interactions. To address this question we performed a series of mixing experiments in which the relative amount of one of four SNARE proteins was successively increased (Figure 4, A–C). From these studies it is apparent that Ykt6p is required for formation of the quaternary complex [Sft1p + Sed5p + Gos1p + Ykt6p] and that increasing the relative amount of Ykt6p results in a corresponding increase in the amount of quaternary complex purified with GST-Sft1p. In addition, it also appears that Ykt6p can stimulate (albeit weakly) the association of Gos1p with [Sft1p + Sed5p] (Figure 4A). Although the absence of Gos1p does not prevent the formation of [Sft1p + Sed5p + Ykt6p] (Figure 3A), surprisingly increasing the amount of Gos1p in the SNARE mixture results in an eventual and striking decrease in the amount of the quaternary complex formed (Figure 4B, lanes 2–5). In the absence of Sft1p, the ternary complex [Sed5p + Ykt6p + Gos1p] forms readily and at least under the conditions used in this experiment, increasing the amount of Sft1p in the mixture did not result in a decrease in the formation of the quaternary complex (data not shown). Although the two ternary complexes ([Sft1p + Sed5p + Ykt6p] and [Gos1p + Sed5p + Ykt6p]) form readily we cannot exclude the possibility that they represent intermediates in the formation of the quaternary complex [Sft1p + Sed5p + Gos1p + Ykt6p].
Figure 4.
The formation of quaternary SNARE complexes occurs sequentially and only in the presence of Sed5p. In A–C, the relative amount of a single SNARE was sequentially increased (lanes 2–5), whereas the amount of the other three SNAREs remained constant. SNARE complex formation was monitored following affinity purification of GST-Sft1p on glutathione Sepharose beads (Precipitate) and immunostaining with an anti-(His)6 antibody. Aliquots of supernatants, removed following incubation of the mixtures with reduced glutathione beads (∼5% of total volume), were also analyzed by immunostaining (Supernatant). (A) Effect of increasing concentrations of Ykt6p. Note that (His)6-Yktp stimulates (weakly) the formation of the ternary complex of [Sed5p + Gos1p + Sft1p] (lane 2). (B) Gos1p. Progressively increasing (His)6-Gos1p results in the eventual reduction of the amount of GST-Sft1p quaternary complex (compare lanes 2–5). (C) Sed5p. Neither binary nor ternary Sft1p-SNARE complexes (with Gos1p or Ykt6p) form in the absence of Sed5p (lane 1).
In the absence of Sed5p, formation of the binary complexes [Sft1p + Ykt6p] and [Sft1p + Gos1p] or the ternary complex [Sft1p + Ykt6p + Gos1p] was undetectable (Figure 4C, lane 1). However, increasing the amount of Sed5p in the mixture resulted in a corresponding increase in the amount of quaternary SNARE complex formed (Figure 4C, lanes 2–5). Thus, as in the case of the ternary complexes, quaternary complex formation requires the presence of Sed5p. We previously reported (Tsui and Banfield, 2000) that Sft1p forms binary complexes with Ykt6p and Gos1p. The apparent discrepancies in the results reported here are most likely directly related to differences in the assays used. In our other study, binary interactions were detected in vivo following coexpression of both SNAREs in bacterial cells rather than following mixing of individual proteins in vitro (as described here). The in vivo assay conditions may therefore be more conducive for the detection of weaker binary interactions, perhaps because the activity of the proteins is higher under these conditions.
The mammalian SNARE complexes described to date are SDS-resistant and denature at temperatures >75°C (Fasshauer et al., 1999; Yang et al., 1999). However, neither the [Sed5p + Ykt6p + Sft1p] nor the [Sed5p + Sft1p + Ykt6p + Gos1p] complexes are stable enough to resist treatment with 1% SDS and heating to 37°C for 5 min (data not shown).
Binding of Vti1p to Other Golgi SNAREs Is Selective
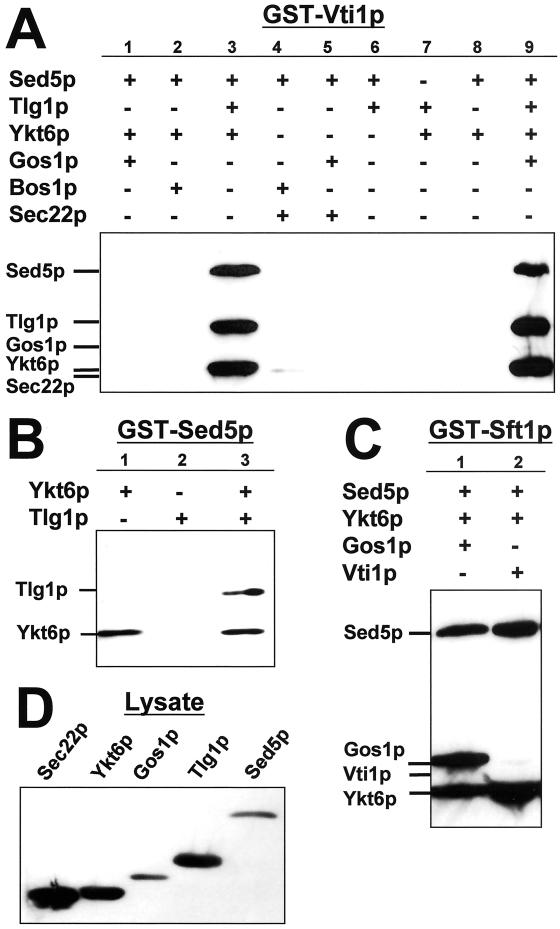
Previous studies have demonstrated a requirement for VTI1 in multiple transport steps, including ER–Golgi traffic (Fischer von Mollard et al., 1997; Lupashin et al., 1997). Vti1p binds to a number of SNARE proteins in vivo, including Ykt6p (Lupashin et al., 1997), five of the eight yeast syntaxin family members (Holthuis et al., 1998), and has been identified as a component of at least two multimeric SNARE complexes (Coe et al., 1999; Ungermann et al., 1999). In addition, the temperature-sensitive growth defects of VTI1 alleles are suppressed by overexpression of Sed5p (Fischer von Mollard et al., 1997) and Tlg1p (Coe et al., 1999) as well as by Ykt6p and Sft1p (Lupashin et al., 1997). And we have shown previously that Vti1p forms a binary complex with either Sed5p or Ykt6p but not Sec22p (Tsui and Banfield, 2000).
Given the data in support of a role for Vti1p in early transport steps (to or from the Golgi) we were somewhat perplexed by the inability of Vti1p to bind SNAREs known to be required for these processes. To address this issue we performed in vitro mixing experiments to identify ternary and or quaternary Vti1p-containing SNARE complexes. GST-Vti1p was mixed with various combinations of SNAREs and the results of these binding experiments are shown in Figure 5A. Vti1p formed a quaternary complex with [Sed5p + Ykt6p + Tlg1p] (Figure 5A, lane 3). However, unlike Sft1p and Bet1p (Figure 3), Vti1p did not form quaternary complexes with either [Sed5p + Ykt6p + Gos1p] (Figure 5A, lane 1), [Sed5p + Ykt6p + Bos1p] (Figure 5A, lane 2), [Sed5p + Sec22p + Gos1p] (Figure 5A, lane 4), or [Sed5p + Sec22p + Bos1p] (Figure 5A, lane 5). The apparent inability of Bos1p to be incorporated into a ternary or quaternary complex with Vti1p may simply reflect a decreased amount of active Bos1p added to the mixing reactions, because the expression level of Bos1p is lower than that of the other SNAREs used (compare Figures 3D and 6B). However, under similar conditions Bos1p is incorporated into quaternary complexes with GST-Sft1p and GST-Bet1p (Figure 3C), suggesting that Bos1p does indeed have a relatively lower binding affinity for Vti1p.
Figure 5.
Vti1p forms a quaternary complex with Sed5p, Tlg1p, and Ykt6p. Mixing assays and immunostaining were performed as described in MATERIALS AND METHODS. (A) GST-Vti1p was mixed with (His)6-Sed5p and various combinations of six other (His)6-tagged SNARE proteins as indicated. (B) GST-Sed5p was mixed with either (His)6-Ykt6p or (His)6-Tlg1p or both (His)6-tagged proteins as indicated. Note that Ykt6p facilitates the association of Tlg1p with Sed5p. (C) GST-Sft1p was mixed in various combinations with four different (His)6-tagged SNARE proteins as indicated. (D) Immunostaining of aliquots of soluble lysates from bacteria expressing the (His)6-SNAREs used in this experiment (∼5% of input). For the relative expression levels of (His)6-Vti1p and (His)6-Bos1p, see Figure 6B.
Figure 6.
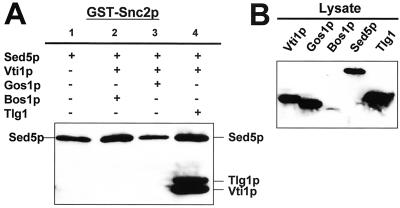
Snc2p forms a quaternary complex with Sed5p, Vti1p, and Tlg1p. Mixing assays and immunostaining were performed as described in the MATERIALS AND METHODS. (A) GST-Snc2p and (His)6-Sed5p were mixed with various combinations of five different (His)6-tagged SNARE proteins as indicated. (B) Immunostaining of aliquots of soluble lysates from bacteria expressing the (His)6-SNAREs used in this experiment (∼5% of input).
Formation of the [Vti1p + Sed5p + Ykt6p + Tlg1p] quaternary complex is most likely mediated by Vti1p binding directly to the [Sed5p + Ykt6p + Tlg1p] intermediate ternary complex (Figure 5B, lane 3) because neither [Vti1p + Sed5p + Tlg1p] (Figure 5A, lane 6) nor [Vti1p + Sed5p + Ykt6p] (Figure 5A, lane 8) form. In the absence of Sed5p the ternary complex [Vti1p + Tlg1p + Ykt6p] did not form (Figure 5A, lane 7).
It is possible that ternary Vti1p-containing complexes do not form efficiently in the presence of [Sed5p, Ykt6p, Gos1p] or [Sed5p, Sec22p, Bos1p]. Perhaps because the [Sed5p + Ykt6p + Gos1p] and [Sed5p + Sec22p + Bos1p] ternary complexes form much more readily (Figures 2 and 3) sequestering these SNAREs and thereby preventing their interaction with Vti1p. However, at least for [Sed5p, Ykt6p, Gos1p] this seems unlikely, because addition of GST-Vti1p and Tlg1p to a mixture of these SNAREs results in the formation of the quaternary Vti1p-containing complex (Figure 5A, lane 9). These data are consistent with the requirement of the [Sed5p + Ykt6p + Tlg1p] intermediate complex to which Vti1p (but not Gos1p) binds directly. Formation of this ternary complex is presumably dependent on the “selective” activation of Sed5p by Ykt6p because Tlg1p fails to bind Sed5p in the absence of Ykt6p (Figure 5B, lane 2).
Because overproduction of Vti1p can partially suppress the sft1-1 temperature-sensitive growth defect (Lupashin et al., 1997) it is possible that Vti1p does not function in an analogous manner to Sft1p (or Bet1p) in SNARE complexes but rather forms ternary or quaternary complexes with Sft1p. We therefore tested whether Vti1p was incorporated into a SNARE complex with Sft1p, Sed5p, and Ykt6p. Vti1p does not form a quaternary complex with [Sed5p + Sft1p + Ykt6p] (Figure 5C, lane 2). Taken together our results suggest that Vti1p displays similar SNARE-binding characteristics to Sft1p and Bet1p, an observation that is consistent with the assignment of these proteins based on amino acid sequence alignments (Lupashin et al., 1997).
Snc2p Forms Select SNARE Complexes with Sed5p
Overexpression of Snc2p (but not Snc1p, Table 4) can partially suppress the temperature-sensitive growth defects of sft1-1 cells as well as support the growth of a sft1Δ strain at 25°C (Tsui and Banfield, 2000; Table 3). In addition, Snc2p has been shown to bind Sed5p in vivo (Grote and Novick, 1999). These data suggest that Snc2p may participate in SNARE complex formation together with Sed5p and other Golgi SNAREs. Because the composition of any such complexes should provide insight into the mechanism by which overexpression of Snc2p can bypass the requirement for Sft1p we carried out protein mixing experiments by using GST-Snc2p and (His)6-Sed5p and various combinations of other (His)6-tagged SNAREs (Figure 6). As was the case for the other synaptobrevin family members (Ykt6p and Sec22p), Snc2p formed a binary complex with Sed5p (Figure 6A, lane 1). In addition, of the three ternary SNARE combinations examined, one quaternary complex, [Snc2p + Sed5p + Vti1p + Tlg1p] was identified (Figure 6A, lane 4). Snc2p does not form a quaternary complex with [Sed5p, Vti1p, Bos1p] (Figure 6A, lane 2) or [Sed5p, Vti1p, Gos1p] (Figure 6A, lane 3).
Table 4.
Trimeric and tetrameric Sed5p-containing SNARE complexes identified in this study
| Trimeric complexes | Tetrameric complexes | ||||
|---|---|---|---|---|---|
| Sed5p | Sed5p | Sed5p | Sed5p | Sed5p | Sed5pa |
| Ykt6p | Sec22p | Sec22p | Ykt6p | Sec22p | Ykt6p |
| Gos1p | Sft1p | Bos1p | Gos1p | Bos1p | Tlg1p |
| Sft1p | Sftp1 | Vti1p | |||
| Sed5p | Sed5p | Sed5pab | Sed5p | Sed5p | Sed5p |
| Ykt6p | Sec22p | Ykt6p | Sec22p | Sec22p | Sec22p |
| Sft1p | Bet1p | Bet1p | Gos1p | Gos1p | Bos1p |
| Sft1p | Bet1p | Bet1p | |||
| Sed5pab | Sed5p | Sed5pa | |||
| Bos1p | Tlg1p | Snc2p | |||
| Bet1p | Ykt6p | Tlg1p | |||
| Vti1p | |||||
SNARE complexes in bold font represent those predicted to remain in a gos1Δ sec22Δ strain.
These complexes formed inefficiently and or nonstoichiometrically (see text for details).
Table 3.
Multicopy suppression profiles of SNARE deletion strains
| Multicopy plasmidb | Yeast deletion
strainsa
|
||||||
|---|---|---|---|---|---|---|---|
| sft1Δ | bet1Δ | bos1Δ | ykt6Δ | vti1Δ | sft1Δ sec22Δ | sft1Δ snc2Δ | |
| pSFT1 | ++++ | − | − | −c | − | ++++ | ++++ |
| pBET1 | +++c | ++++ | − | − | − | +++ | ++ |
| pBOS1 | − | − | ++++ | − | − | n.d. | n.d. |
| pYKT6 | − | − | − | ++++ | − | n.d. | n.d. |
| pVTI1 | − | − | − | − | ++++ | n.d. | n.d. |
| pSEC22 | − | − | − | − | − | n.d. | n.d. |
| pSED5 | +c | − | − | − | − | n.d. | n.d. |
| pGOS1 | − | − | − | − | − | n.d. | n.d. |
| pSNC2d | ++c | − | − | −c | − | ++ | ++ |
| pTLG1 | − | − | − | − | − | n.d. | n.d. |
n.d. not determined; +/− denotes relative growth rates at 25°C on plates containing 5-FOA where ++++ is wild-type and − is no growth detected.
sft1Δ (SARY160), bet1Δ (SARY270), bos1Δ (SARY260), ykt6Δ (SARY158), vti1Δ (SARY296), sft1Δ (SARY160), sec22Δ (SARY323), sft1Δ, snc2Δ (SARY324); see Table 1.
Containing genomic DNA fragments encoding the various SNARE genes.
Described previously (Tsui and Banfield, 2000).
2μ SNC1 does not support the growth of sft1Δ cells.
The quaternary Snc2p-containing SNARE complex [Snc2p + Sed5p + Vti1p + Tlg1p] could be considered the result of replacement of one SNARE family member with another (i.e., the result of nonselective SNARE associations). For example, a quaternary complex where another member of the yeast synaptobrevin family, Ykt6p, replaces Snc2p, does form ([Ykt6p + Sed5p + Vti1p + Tlg1p]); see Figure 5A, lane 3. However, formation of the Snc2p-containing intermediate ternary complex differs from that of its Ykt6p-containing counterpart, because the [Snc2p + Sed5p + Tlg1p] complex did not form (data not shown), whereas the [Ykt6p + Sed5p + Tlg1p] complex formed (Figure 5B, lane 3). This result is intriguing because it suggests that the order and/or spatial context in which SNAREs interact with one another to form a particular complex may be SNARE–SNARE and/or complex-specific. If such a process exists in cells there could very well be a mechanism by which SNARE complex assembly is proofread, facilitating and or inhibiting their formation.
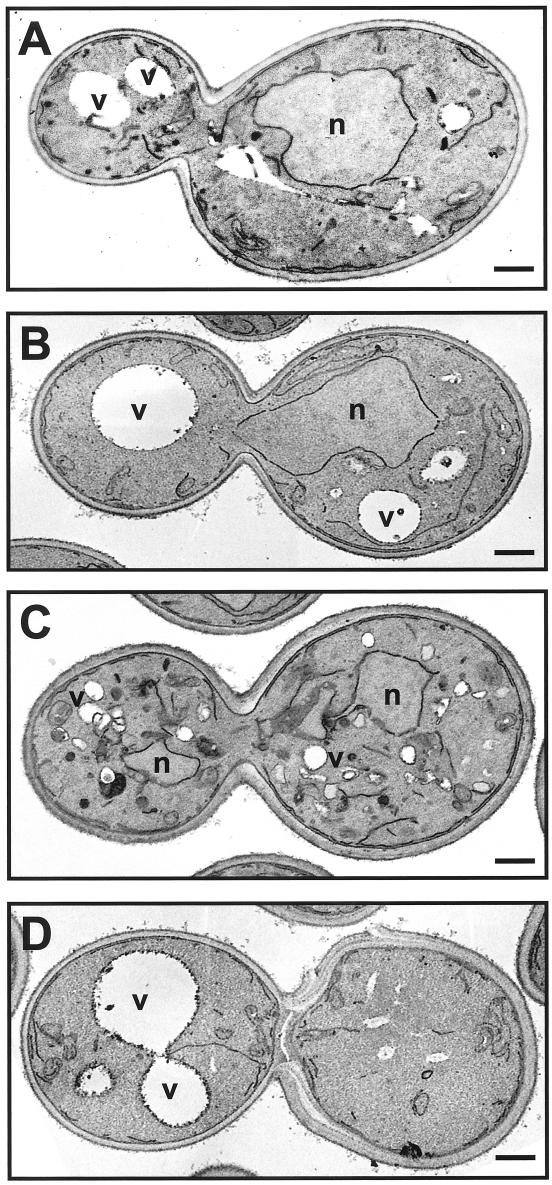
The presence of Snc2p together with Vti1p and Tlg1p in a Sed5p-containing complex suggests that these SNAREs may be involved in traffic from the trans-Golgi network and or endosomes to early Golgi compartments (Holthuis et al., 1998; Coe et al., 1999). This may provide a potential mechanism by which overexpression of Snc2p may compensate for the absence of Sft1p. Overexpression of Snc2p is unlikely to be sufficient to restore normal traffic, however, because sft1Δ cells overexpressing Snc2p grow slowly and display anomalies in intracellular membrane morphology consistent with persistent defects in membrane traffic (Figure 8C).
Figure 8.
Electron micrographs of sft1Δ cells containing either the 2 μ SFT1 (SARY160) 2 μ BET1 (SARY181), or 2 μ SNC2 plasmids (SARY208). Yeast strains were grown at 25°C for 4 h and permanganate fixed to accentuate membranes. (A) sft1Δ 2 μ SFT1 (SARY160). Overexpression of Sft1p results in some vacuolar fragmentation as well as the accumulation of some dark membranous structures. (B) sft1Δ 2 μ BET1 (SARY181). Cells are indistinguishable from wild-type (D). (C) sft1Δ 2 μ SNC2 cells (SARY208). Note no significant differences in amount of ER membranes (compare with A, B, and D) but fragmentation of the vacuoles and the appearance of numerous dark membranous structures. (D) Wild-type parental strain (SEY6210, Table 1). The bar in the bottom right-hand side of each micrograph corresponds to 0.5 μm. N, nucleus; V, vacuole. The doubling times for each of the strains at 25°C were pSFT1 sft1Δ (SARY160), 2.0 h; pBET1 sft1Δ (SARY181), 2.3 h; pSNC2 sft1Δ (SARY 208), 6.7 h; and wild-type (SEY6210), 1.9 h (Table 1).
Yeast Golgi SNARE Complexes Display Syntaxin Selectivity
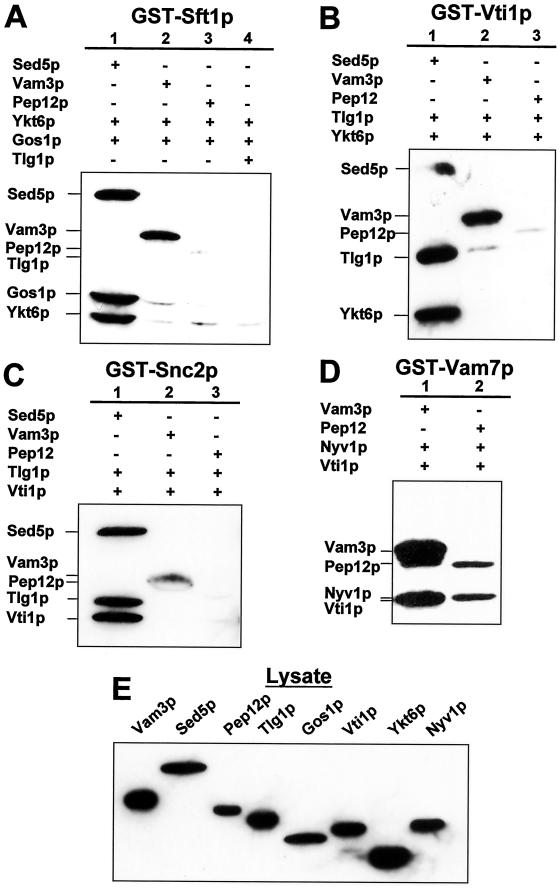
We have demonstrated that the formation of Sed5p-containing SNARE complexes exhibit a significant degree of selectivity in vitro. Our results suggest that the basis for these selective binding interactions is the result of SNARE-specific interactions involving the selective activation of Sed5p. In vitro mixing studies, with mammalian SNARE proteins (Fasshauer et al., 1999; Yang et al., 1999), demonstrated that noncognate SNARE complexes formed with syntaxin family members (although syntaxin 5 was not used). We wished to determine whether any of the Sed5p-containing SNARE complexes identified here would form if another member of the yeast syntaxin family replaced Sed5p. For these studies we chose three ternary SNARE combinations that form a quaternary complex with Sed5p: [Snc2p + Tlg1p + Vti1p], [Sft1p + Ykt6p + Gos1p], and [Vti1p + Tlg1p + Ykt6p]. Vti1p is known to bind to five of the eight yeast syntaxin members, including Pep12p, Tlg1p, Tlg2p, and Vam3p. Snc2p binds the syntaxins Sso1 and 2, and Tlg2p and Sft1p binds to Sed5p. We carried out mixing experiments in which Sed5p was replaced by either Vam3p or by Pep12p (GST-Snc2p, GST-Vti1p, and GST-Sft1p, Figure 7, A–C, respectively) as well as by Tlg1p (GST-Sft1p, Figure 7A, lane 4). In all three of these experimental series quaternary complex formation was only observed in the presence of Sed5p. Vam3p bound to GST-Vti1p in the presence of Tlg1p and Ykt6p (Figure 7B, lane 2), to GST-Snc2p in the presence of Tlg1p and Vti1p (Figure 7C, lane 2) as well as to GST-Sft1p in the presence of Ykt6p and Gos1p (Figure 7A, lane 2). In contrast, significantly less Pep12p bound to GST-Sft1p, GST-Vti1p, and GST-Snc2p (lane 3 of Figure 7, A–C, respectively).
Figure 7.
Vam3p and Pep12p do not form quaternary complexes with [Ykt6p + Gos1p + Sft1p], [Ykt6p + Tlg1p + Vti1p] or [Snc2p + Tlg1p + Vti1p]. Mixing assays and immunostaining were preformed as described in MATERIALS AND METHODS. (A) GST-Sft1p was mixed with either the syntaxin (His)6-Sed5p, (His)6-Vam3p, (His)6-Pep12p or (His)6-Tlg1p together with the (His)6-SNAREs Ykt6p and Gos1p. (B) GST-Vti1p was mixed with either (His)6-Sed5p, (His)6-Vam3p or (His)6-Pep12p along with the (His)6-SNAREs Tlg1p and Ykt6p. (C) GST-Snc2p was mixed with (His)6-Sed5p, (His)6-Vam3p, or (His)6-Pep12p together with (His)6-Vti1p and (His)6-Tlg1p. (D) GST-Vam7p was mixed with either (His)6-Vam3p or (His)6-Pep12p together with (His)6-Vti1p and (His)6-Tlg1p. (E) Immunostaining of aliquots of soluble lysates from bacteria expressing the (His)6-SNAREs used in this experiment (∼5% of input).
Vam3p has recently been shown to form a quaternary complex with [Vam7p + Nyv1p + Vti1p] (Fukuda et al., 2000). To further demonstrate biological activity for Vam3p (and Pep12p) we carried out mixing experiments with GST-Vam7p together with Vam3p, Nyv1p, and Vti1p. Vam3p forms a quaternary complex with [Vam7p + Nyv1p + Vti1p] (Figure 7D, lane 1). Although not evident on the immunoblot shown, both Nyv1p and Vti1p can be detected following protein staining with Coomassie brilliant blue. Pep12p also binds to GST-Vam7p and may form either a quaternary complex or ternary complex with [Vam7p, Nyv1p and Vti1p] (Figure 7D, lane 2).
In contrast to previous reports we conclude, at least for the proteins used in these experiments, that the quaternary Golgi SNARE complexes identified here form as a result of selective SNARE–SNARE interactions and only with the syntaxin Sed5p.
Evidence for Selective SNARE Complex Assembly In Vivo
A number of the ternary as well as quaternary complexes identified in this study may form as a result of direct substitution of one SNARE family member by another. For example, in the ternary complexes [Sed5p + Sec22p + Sft1p] and [Sed5p + Sec22p + Bet1p] Bet1p could be considered to substitute directly for Sft1p and vice versa. Similarly in the quaternary complexes [Sed5p + Sec22p + Sft1p + Gos1p] and [Sed5p + Sec22p + Sft1p + Bos1p] Gos1p could be considered to substitute directly for Bos1p and vice versa. If SNAREs can simply substitute for one another by direct, nonselective substitution of one SNARE by another, overexpression of these proteins, which may result in alterations to their intracellular distributions, should allow some SNAREs to compensate for the absence of others. If this were the case one might expect to identify BOS1 and GOS1 and/or SFT1 and BET1 as reciprocal multicopy suppressors of the lethality that results from deletion of these genes. Indeed, there are a number of examples in which overexpression of a particular SNARE can suppress the temperature-sensitive growth defects or lethality associated with mutations in another SNARE gene. For example, overexpression of Ykt6p can partially suppress the temperature-sensitive growth defect in bos1-1 and sec22 cells (Banfield et al., 1995; McNew et al., 1997) and overexpression of either BET1 or SNC2 can support the growth of yeast cells in the absence of SFT1 (Tsui and Banfield, 2000).
To address this issue we transformed haploid yeast strains, in which either the SFT1, BET1, BOS1, YKT6, or VTI1 genes had been deleted, with 2 μ plasmids harboring various SNARE genes (see MATERIALS AND METHODS; Tables 1 and 3). With the exception of the sft1Δ strain, the lethality associated with deletion of the other SNARE genes could be not be overcome by any of the 2 μ plasmids tested (Table 3). Of the 11 SNARE genes examined only 2 μ BET1 (but not CEN BET1), 2 μ SNC2 (but not SNC1) and 2 μ SED5 could support the growth of sft1Δ cells. SARY181 cells (sft1Δ, 2 μ BET1) grow at wild-type rates and at least by electron microscopy, appear indistinguishable from their wild-type counterparts (Figure 8B). SARY208 (sft1Δ, 2 μ SNC2) cells on the other hand exhibit defects in intracellular membrane organization (reminiscent of sft1 mutants) that are consistent with persistent defects in retrograde Golgi traffic (Figure 8C; Banfield et al., 1995).
The BET1 result could be explained by the direct substitution of Bet1p for Sft1p in the [Sed5p + Ykt6p + Sft1p] ternary complex, although the [Sed5p + Ykt6p + Bet1p] complex forms relatively less efficiently in vitro than the [Sed5p + Ykt6p + Sft1p] ternary complex (Figure 3B, lane 1). The other Sft1p-containing complexes described here ([Sed5p + Sec22p + Sft1p], [Sed5p + Sec22p + Gos1p + Sft1p] and [Sed5p + Sec22p + Bos1p + Sft1p]) can be excluded because the presence of the SEC22 (or SNC2) gene is not required for overexpression of Bet1p to bypass the requirement for SFT1 (Table 3). Applying similar reasoning one might have expected that overexpression of Sec22p or Snc2p should support the growth of ykt6Δ cells and that overexpression of Gos1p (or Tlg1p) should support the growth of bos1Δ cells, however this is not the case (Table 3).
In context of these results it is difficult to envision the precise mechanism by which overexpression of SNC2 or BET1 supports the growth of sft1Δ cells. Our results suggest that it may not be directly related to ability of these SNAREs to form Sed5p-containing complexes. Rather these SNAREs (with the exception of SFT1) may have other essential activities that are SNARE-specific and therefore cannot be substituted for.
Some Sed5p-containing SNARE Complexes Are Functionally Redundant
With the identification of the Sed5p-containing Golgi SNARE complexes described here it is apparent that a significant degree of functional redundancy exists among them. For example, Gos1p and Sec22p are found in 9 of the 15 complexes identified (Table 4). Yeast strains in which either GOS1 or SEC22 have been disrupted are viable at 25°C (Sacher et al., 1997; McNew et al., 1998). gos1Δ cells exhibit modest growth and secretory defects that are consistent with a role for Gos1p in multiple transport steps (McNew et al., 1998), whereas sec22Δ cells are temperature-sensitive for growth (Sacher et al., 1997). Because neither GOS1 nor SEC22 is required for growth at 25°C clearly the Gos1p- and Sec22p-containing complexes (ternary as well as quaternary) must be functionally redundant under these circumstances.
If functional Sed5p-containing complexes are exclusively quaternary, we reasoned that a yeast strain with either deletions or mutations in both GOS1 and SEC22 may not be viable, because this would result in elimination (or defects in the formation) of all of the Sed5p-containing quaternary complexes containing the essential SNAREs Bet1p, Sft1p, and Bos1p (Table 4). However, we found that gos1Δ sec22Δ as well as the gos1Δ sec22-3 strains are viable at 25°C (Figure 9), although the growth rate of the double mutant is somewhat slower than the single mutant strains. The doubling times for each of the strains at 25°C were Y26 (gos1Δ), 2.2 h; RSY279 (sec22-3), 2.4 h; and SARY 342 (gos1Δ sec22-3), 2.8 h. In addition, examination of gos1Δ sec22-3 cells by electron microscopy revealed that the double mutant exhibits defects in membrane morphology that are only slightly more profound than either of the single mutants combined. The gos1Δ sec22-3 cells exhibit moderate accumulation of ER membranes, vesicles, and structures typical of mutants with defects in traffic within the Golgi (data not shown).
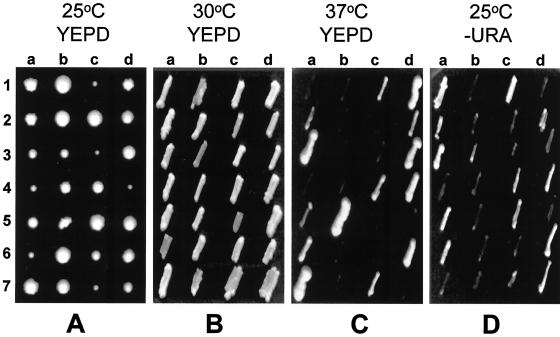
Figure 9.
gos1Δ and sec22-3 do not display synthetic lethal interactions. Following sporulation of the heterozygous double mutant diploid (SARY327, Table 1), tetrads were dissected on YEPD plates containing 1 M sorbitol and incubated at 25°C for 5 d. (A) Plates shown in B–D were incubated at the indicated temperatures after replica plating (A) for 2 d. Tetrads 2, 4, and 6 are the parental ditype and tetrads 1, 3, 5, and 7 are tetratype. Spores 1a, 3b, 5c, and 7d correspond to the gos1Δ sec22-3 double mutant progeny. The same result was also obtained for the gos1Δ and sec22Δ mutations (data not shown).
Although it is likely that we have not identified all of the Sed5p-containing Golgi SNARE complexes (mixing experiments with GST-Sft1p and GST-Bet1p together with Sed5p and combinations of Snc2p and Gos1p, Bos1p, or Tlg1p were not described) our results suggest that at least five quaternary complexes may be functionally redundant. However, the observation that gos1Δ sec22-3 cells display defects in membrane traffic suggests that some of the missing complexes, although not required for growth at 25°C, may play a role in maintaining the integrity of the endomembrane system. The requirement for SEC22 at elevated temperatures reflects a need for at least some of the Sec22p-containing complexes under these conditions. However, the Gos1p-, Snc2p-, and under some circumstances the Tlg1p-containing complexes (Table 4) are presumably dispensable, at least in their corresponding single gene deletion strains.
We predicted that only 6 of the 15 Sed5p-containing complexes identified here would remain in a gos1Δ sec22Δ strain, four ternary complexes and two quaternary complexes (Table 4). However, it is possible that the presence of each of the essential SNARE proteins (Sft1p, Bet1p, Vti1p, Bos1p) in at least one or more of the remaining complexes could explain why the gos1Δ sec22Δ/s22-3 double mutants are viable. If the ternary complexes represent intermediates in the formation of functional quaternary complexes, perhaps Bet1p, Sft1p, and Bos1p have an essential function that is not directly related to SNARE complex formation as prelude to membrane fusion. For example, Bet1p and Bos1p bind to Sec23p and Sec24p, an interaction that is presumably required for nucleation of COPII-coated vesicles (Springer and Schekman, 1998). Alternatively, yeast may use both ternary as well as quaternary Golgi SNARE complexes.
The SNARE-complexes predicted to remain in gos1Δ sec22Δ cells (based on our results) could be viewed, as the minimum number required for traffic to and through the yeast Golgi. Whether they represent the only possible complexes that can fulfill this requirement is questionable as deletion of either SNC2 and under some circumstances TLG1 (Holthuis et al., 1998 and Coe et al., 1999) is also tolerated. None-the-less these results suggest that Sed5p-containing SNARE complexes can be conditionally nonessential and thus a degree of functional redundancy may exist in vivo for Golgi SNARE-complexes.
DISCUSSION
We have described recombinant SNARE protein mixing experiments in which many of the possible combinations of the SNAREs Sft1p, Bet1p, Vti1p, Bos1p, Gos1, Tlg1p, Sec22p, Ykt6p, Snc2p, and Sed5p were assayed for their ability to form ternary or quaternary complexes with one another as well as with Sed5p. In total eight ternary Sed5p-containing SNARE complexes and seven quaternary Sed5p-containing SNARE complexes were identified (Table 4). With the exception of [Sed5p + Bos1p + Bet1p], SNARE complex formation required the presence of a member of the synaptobrevin family (Ykt6p, Sec22p, or Snc2p). Vti1p was found only in quaternary complexes and with the exception of those complexes containing Tlg1p and Sed5p (both currently classified as yeast syntaxin family members) all of the other Sed5p-containing SNARE complexes identified here contained a single member of their representative SNARE family (Figure 1 and Table 4). Of the eight Sed5p-containing ternary SNARE complexes identified in this study (stoichiometry aside) all could be considered to be composed of one R- and two Q-SNAREs except [Sed5p + Bos1p + Bet1p], which is comprised of three Q-SNAREs (Fasshauer et al., 1998). All of the quaternary complexes are comprised of one R- and three Q-SNAREs, a composition reminiscent of the neuronal SNARE complex (Sutton et al., 1998).
We failed to identify a single ternary or quaternary SNARE complex that formed in the absence of Sed5p. Because we have previously shown that binary interactions can be detected among the SNAREs used here, following their coexpression in bacterial cells (Tsui and Banfield, 2000), it seems likely that SNAREs have a lower affinity for one another in the absence of Sed5p. Or alternatively, the specific activity of SNARE proteins in vivo is relatively higher than that of the isolated proteins.
The selectivity of Sed5p-containing SNARE complex formation appears to a property of the individual proteins themselves and of particular SNARE–SNARE interactions. For example, Sft1p binds to [Sed5p + Ykt6p + Gos1p] but not to [Vam3p + Ykt6p + Gos1p] or [Pep12p + Ykt6p + Gos1p], suggesting that formation of the quaternary complex with Sft1p specifically requires Sed5p and is thus syntaxin-selective (Figure 7A). In addition, Sft1p binds to [Sed5p + Ykt6p + Gos1p] but not to [Sed5p + Ykt6p + Bos1p] (Figure 4). In both the [Sed5p + Sec22p + Sft1p] and [Sed5p + Sec22p + Bet1p] ternary complexes as well as the [Sed5p + Sec22p + Gos1p/Bos1p + Sft1p/Bet1p] quaternary complexes, SNARE complex formation appears to be mediated by [Sed5p + Sec22p], suggesting that this binary complex can bind other SNAREs indiscriminately. However, Vti1p does not form a complex with either [Sed5p + Sec22p + Gos1p] or [Sed5p + Sec22p + Bos1p] thus [Sed5p + Sec22p] can discriminate between Vti1p and Sft1p or Bet1p in vitro (Figure 5).
The composition of some of the Sed5p-containing complexes identified here suggests that they may form as a result of nonselective or promiscuous SNARE–SNARE associations (Table 4). For example, Sed5p binds to at least 15 different binary and or ternary combinations of nine different SNARE proteins. Although some complexes may represent intermediates in the formation of quaternary complexes, a given SNARE protein is represented in anywhere from one (Snc2p) to seven (Sec22p) different complexes (Table 4). However, despite the appearance of promiscuous SNARE–SNARE interactions, a number of SNARE combinations do not form with Sed5p under our assay conditions (Figures 3, 5, 6, and 7), a result that is not consistent with noncognate SNARE–SNARE associations. In addition, at least for [Sed5p + Vti1p + Tlg1p + Ykt6] and [Sed5p + Vti1p + Tlg1p + Snc2p], quaternary complex formation occurs via different ternary intermediates (Figures 5 and 6), suggesting that SNARE complex formation is sequential (Figure 4) and selective.
It therefore appears that the formation of Sed5p-containing complexes in vitro is the result of direct binding interactions between particular combinations of SNAREs, the intermediates of which can discriminate between subsequent or additional binding partners. The results of multicopy suppressor analyses (in yeast strains in which essential SNARE genes have been deleted) also support the view that direct substitution of one SNARE family member by another in vivo is unlikely (Table 3). Taken together our results are consistent with either a distinct physiological requirement for these Sed5p-containing complexes and/or other essential roles for the individual proteins not directly related to SNARE complex formation as a prelude to membrane fusion. Indeed, if SNARE–SNARE interactions are nonselective in vivo it is rather difficult to reconcile the requirement for six of the nine Sed5p-binding SNARE proteins, three of which (Bet1p, Sft1p, and Vti1p) albeit spatially distinct, appear to be functionally analogous. However, because deletion of GOS1 and SEC22 is not lethal at least some of the Sed5p-containing complexes identified here may be functionally redundant (Table 4). This apparent functional redundancy does not however appear to be a result of indiscriminant, nonselective SNARE–SNARE interactions during complex formation but rather a reflection of the involvement of more than one SNARE complex in a given transport step.
In general, the formation of Sed5p-containing complexes involved interactions in which one SNARE (e.g., Gos1p) facilitated the association of two other SNAREs (e.g., Sed5p and Sft1p) with one another (Figure 3, A and B). We have described the events leading to SNARE complex formation as involving or requiring the selective activation of Sed5p and suggest that this is a putative mechanism whereby intermediate Sed5p-containing SNARE complexes discriminate between their respective binding partners. The mechanism by which this process occurs in vitro must therefore be a property of the proteins themselves. Indeed, the amino acid sequence similarity among the core domains of these SNARE families is low (between 6 and 14% among Sft1p, Bet1p, and Vti1p and 9% for Gos1p and Bos1p). In addition, Vti1p has a longer core domain than either Sft1p or Bet1p, a feature that may be directly related to Vti1p's ability to bind distinct combinations of SNAREs (Figure 1). It is therefore possible that the primary amino acid sequences of these proteins are significantly divergent as to play a part in facilitating or in the prevention of associations among these SNAREs.
A variety of plausible mechanisms can be envisioned whereby selective SNARE complex formation between Sed5p and its numerous binding partners could be accomplished in vitro. Because we used the full-length soluble domains of SNAREs (Table 2) it is possible that the specificity of SNARE complex formation may have been influenced by the presence of the so-called variable N-terminal domains of these proteins. Although reportedly this is not the case for the mammalian SNAREs (Fasshauer et al., 1999; Yang et al., 1999). Indeed, the N-terminal domains of syntaxin 1a and Sso1p (a yeast syntaxin family member) adopt a 3 α helical structure that apparently binds directly to the respective core domains of these proteins (Nicholson et al., 1998; Dulubova et al., 1999; Fiebig et al., 1999; Misura et al., 2000). This self-association is likely important for homomulterization of syntaxins, a process that may be related to the formation of complexes with other SNARE proteins (Lerman et al., 2000). However, in such a “closed” conformation syntaxin is thought to be inaccessible to interactions with other SNAREs. Because the N-terminal domain of Sed5p can bind to its core domain in vitro (Kosodo et al., 1998), it is possible that Sed5p also adopts a closed conformation. Thus, in addition to differences in the amino acid sequence composition of the core domains of SNAREs directly influencing their interactions with one another, some SNAREs may be able to selectively activate Sed5p via displacement or alteration of its N-terminal domain. Such a mechanism would not be unprecedented, because displacement of the N-terminal domain of Sso1p is involved in complex formation with the SNAREs Sec9p and Snc1p (Nicholson et al., 1998; Fiebig et al., 1999). It is also possible that some nonsyntaxin SNAREs also adopt a closed conformation that functions in an analogous manner to that of the syntaxins. Or that SNAREs, which exist primarily in a random coil conformation in their uncomplexed (homomeric) states (Rice et al., 1997; Tishgarten et al., 1999), undergo specific structural alterations upon binding particular SNAREs, facilitating or preventing their interactions with others.
Our results on selective formation of Sed5p-containing complexes are in contrast with previous reports with mammalian SNAREs, which demonstrated that SNARE complex formation is nonselective (Fasshauer et al., 1999; Yang et al., 1999). Our study differs in a number of ways. We examined interactions between Sed5p and SNAREs with which it is known to bind in vivo. Neither Yang et al. (1999) nor Fasshauer et al. (1999) used syntaxin 5 in their experiments and with the exception of rSec22b (Yang et al., 1999), no other ER or Golgi SNAREs were used. Also the absence of any structural equivalents of SNAP-25 among the yeast ER-Golgi SNAREs may very well be significant. Lastly, we used recombinant proteins in our mixing assays where one SNARE was expressed as a GST-fusion protein. We cannot therefore rule out the possibility that the presence of the GST moiety somehow reduced the efficiency of SNARE–SNARE interactions, such that only the most stable complexes were detected. Although we did not directly examine the thermostability of SNARE complexes, neither the quaternary complex [Sed5p + Ykt6p + Gos1p + GST-Sft1p] nor the ternary complex [Sed5p + Ykt6p + Gos1p] was resistant to temperature or SDS (data not shown). The discrepancy between the apparent stabilities of yeast Golgi SNARE and mammalian SNARE complexes may therefore be a reflection of the structure of the nonexocytic yeast complexes themselves (Weimbs et al., 1998; Ungermann et al., 1999) and thus a significant factor in the differences observed in the selectivity of complex formation in vitro.
Although analysis of SNARE proteins has helped elucidate trafficking steps in yeast, SNAREs have fallen out of favor as the sole candidate molecules imparting specificity to membrane fusion reactions. This is chiefly due to the observation that a single SNARE can participate in multiple transport steps (e.g., Vti1p, Fischer von Mollard et al., 1997, Lupashin et al., 1997; Fischer von Mollard and Stevens, 1999), the identification of an increasing number of additional factors (Waters and Hughson, 2000), and also because previous in vitro binding studies have not revealed the degree of specificity required for SNAREs to be the primary targeting molecules in vivo (Fasshauser et al., 1999; Yang et al., 1999; Tsui and Banfield, 2000). The results of our study demonstrate (in particular for Vti1p and Sed5p) that SNAREs that participate in multiple trafficking steps can discriminate among their multiple SNARE binding partners in vitro. The ability of Sed5p to form select complexes among the SNARE proteins with which it interacts suggests that these complexes may play overlapping or even distinct roles in traffic to and through the Golgi.
Although it is possible that SNAREs are sorted into transport vesicles individually (Springer and Schekman, 1998; Peng et al., 1999), we only detected SNARE complex formation in the presence of Sed5p. Sed5p has been shown to cycle between the Golgi and ER (Wooding and Pelham, 1998). It is therefore possible that Sed5p recycles in a complex with other SNARE proteins. This would be consistent with the presence of Sed5p (and its mammalian counterpart syntaxin 5) on isolated transport intermediates together with some of their SNARE-binding partners (Allan et al., 2000; Cao and Barlowe, 2000). In addition, the asymmetric requirement for SNAREs (Bet1p and Bos1p) on vesicles and on Golgi membranes (Sed5p) could also be consistent with spatially distinct structural states for SNAREs (Cao et al., 2000). If Sed5p recycles in a complex with other SNARE proteins presumably negative regulators of Sed5p function (SNARE masters such as Sly1p; Gerst, 1999) would not be required. This prediction is consistent with the requirement for functional Sed5p (along with Ypt1p and Sly1p) on Golgi membranes but not on transport vesicles (Cao et al., 2000) as well as with the existence of two distinct populations of Sed5p, one bound to Sly1p (Lupashin and Waters, 1997). Recycling SNAREs in complexes, if coupled to vesicle formation, could provide a means by which to ensure that only fusion-competent transport vesicles were generated.
The multicopy suppressor analyses we describe suggest that it is unlikely that straightforward, direct substitution of one SNARE by another accounts for the apparent redundancy of some SNARE complexes in vivo. How can we reconcile this observation with fact that some SNAREs (GOS1, SEC22, SNC2) are nonessential. Perhaps the simplest explanation is that cells have more than one trafficking pathway for a particular step or alternatively that more than one SNARE complex defines a single trafficking step. Suppression of the lethality of sft1Δ cells by overexpression of Bet1p or Snc2p is consistent with the use of either less efficient parallel pathways or by the alteration of normal trafficking pathways, as is the appearance of these cells upon examination by electron microscopy (Figure 8).
Thus, it appears that a significant degree of selectivity in the formation of Sed5p-containing SNARE complexes can be achieved in vitro and that the basis for these selective interactions is entirely a property of the primary amino acid sequences of these proteins. The number and composition of the Sed5p-containing SNARE complexes identified in this study suggests that multimeric SNARE interactions may facilitate the fidelity of vesicle targeting between the ER and Golgi in yeast either directly or by providing a scaffold for the assembly of step-specific accessory factors. In this regard, while this manuscript was under review, three related articles appeared (Fukuda et al. 2000; McNew et al. 2000; Parlati et al. 2000). In so far as our data overlap with these studies our results are mostly in agreement. For example, McNew et al. (2000) report that the SNAREs [Sed5p + Bos1p + Sec22p] + [Bet1p] form a fusion competent quaternary complex but that the SNAREs [Sed5p + Bos1p + Sec22p] + [Vti1p] do not. Similarly, we found that GST-Bet1p forms a quaternary complex with [Sed5p + Bos1p + Sec22p] but that GST-Vti1p does not form a quaternary complex with [Sed5p + Bos1p + Sec22p]. In contrast however, we did identify a quaternary complex comprised of [Sed5p + Bos1p + Sec22p] and GST-Sft1p. This apparent discrepancy could be due to the differences in the assays used. The assay used by McNew et al. (2000) is sensitive to the topological lipid distributions of SNAREs (Parlati et al., 2000); our assay measured SNARE interactions in solution. Thus, it is possible that Sft1p does not act as a vSNARE to [Sed5p + Bos1p + Sec22p] (as in the case of Bet1p) but rather as a tSNARE light chain (Parlati et al., 2000).
ACKNOWLEDGMENTS
We thank Pelly Ng for excellent technical assistance, Wilson Chan for preparing the samples for electron microscopy, and Keith Molding of MPCF, Materials Characterisation and Preparation Facility (HKUST) for the use of the Philips CM20. Thanks also to Chris Rock, Andy Miller, and Mingjie Zhang for improvements to the manuscript. This work was supported by a grant from the Research Grant Council of Hong Kong (HKUST646/96 M) to D.K.B.
Abbreviations used:
- 5-FOA
5-fluoroorotic acid
- GST
glutathione S-transferase
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment receptor protein
REFERENCES
- Allan BB, Moyer BD, Balch WE. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 2000;289:444–448. doi: 10.1126/science.289.5478.444. [DOI] [PubMed] [Google Scholar]
- Banfield DK, Lewis MJ, Pelham HRB. A SNARE-like protein required for traffic through the Golgi complex. Nature. 1995;375:806–809. doi: 10.1038/375806a0. [DOI] [PubMed] [Google Scholar]
- Cao X, Barlowe C. Asymmetric requirements for a Rab GTPase and SNARE proteins in fusion of COPII vesicles with acceptor membranes. J Cell Biol. 2000;149:55–66. doi: 10.1083/jcb.149.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JG, Lim AC, Xu J, Hong W. A role for Tlg1p in the transport of proteins within the Golgi apparatus of Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:2407–2423. doi: 10.1091/mbc.10.7.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Sudhof TC, Rizo J. A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elble R. A simple and efficient procedure for transformation of yeasts. Biotechniques. 1992;13:18–20. [PubMed] [Google Scholar]
- Fasshauer D, Antonin W, Margittai M, Pabst S, Jahn R. Mixed and non-cognate SNARE complexes. Characterization of assembly and biophysical properties. J Biol Chem. 1999;274:15440–15446. doi: 10.1074/jbc.274.22.15440. [DOI] [PubMed] [Google Scholar]
- Fasshauer D, Sutton RB, Brünger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig KM, Rice LM, Pollock E, Brunger AT. Folding intermediates of SNARE complex assembly. Nat Struct Biol. 1999;6:117–23. doi: 10.1038/5803. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G, Nothwehr SF, Stevens TH. The yeast v-SNARE Vti1p mediates two vesicle transport pathways through interactions with the t-SNAREs Sed5p and Pep12p. J Cell Biol. 1997;137:1511–1524. doi: 10.1083/jcb.137.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard GF, Stevens TH. The Saccharomyces cerevisiae v-SNARE Vti1p is required for multiple membrane transport pathways to the vacuole. Mol Biol Cell. 1999;10:1719–1732. doi: 10.1091/mbc.10.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R, McNew JA, Weber T, Parlati F, Engel T, Nickel W, Rothman JE, Sollner TH. Functional architecture of an intracellular membrane t-SNARE. Nature. 2000;407:198–202. doi: 10.1038/35025084. [DOI] [PubMed] [Google Scholar]
- Gerst JE. SNAREs and SNARE regulators in membrane fusion and exocytosis. Cell Mol Life Sci. 1999;55:707–734. doi: 10.1007/s000180050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E, Novick PJ. Promiscuity in rab-SNARE interactions. Mol Biol Cell. 1999;10:4149–4161. doi: 10.1091/mbc.10.12.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, C., and Fink, G.R. (1991). Guide to yeast genetics and molecular biology. Methods Enzymol. 194. [PubMed]
- Holthuis JCM, Nichols BJ, Dhruvakumar S, Pelham HRB. Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 1998;17:113–126. doi: 10.1093/emboj/17.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L, Hanson PI, Heuser JE, Brennwald P. Genetic and morphological analyses reveal a critical interaction between the C-termini of two SNARE proteins and a parallel four helical arrangement for the exocytic SNARE complex. EMBO J. 1998;17:6200–6209. doi: 10.1093/emboj/17.21.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosodo Y, Noda Y, Yoda K. Protein-protein interactions of the yeast Golgi tSNARE Sed5 protein distinct from its neuronal plasma membrane cognate syntaxin1. Biochem Biophys Res Commun. 1998;250:212–216. doi: 10.1006/bbrc.1998.9288. [DOI] [PubMed] [Google Scholar]
- Lerman JC, Robblee J, Fairman R, Hughson FM. Structural. Analysis of the Neuronal SNARE Protein Syntaxin-1A. Biochemistry. 2000;39:8470–8479. doi: 10.1021/bi0003994. [DOI] [PubMed] [Google Scholar]
- Lupashin VV, Pokrovskaya ID, McNew JA, Waters MG. Characterization of a novel yeast SNARE protein implicated in Golgi retrograde traffic. Mol Biol Cell. 1997;8:2659–2676. doi: 10.1091/mbc.8.12.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupashin VV, Waters MG. t-SNARE activation through transient interaction with a rab-like guanosine triphosphatase. Science. 1997;276:1255–1258. doi: 10.1126/science.276.5316.1255. [DOI] [PubMed] [Google Scholar]
- McNew JA, Coe JG, Sogaard M, Zemelman BV, Wimmer C, Hong W, Sollner TH. Gos1p, a Saccharomyces cerevisiae SNARE protein involved in Golgi transport. FEBS Lett. 1998;435:89–95. doi: 10.1016/s0014-5793(98)01044-8. [DOI] [PubMed] [Google Scholar]
- McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, Sollner TH, Rothman JE. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 2000;407:153–159. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- McNew JA, Søgaard M, Lampen NM, Machida S, Ruby Ye R, Lacomis L, Tempst P, Rothman JE, Sollner T. Ykt6p, a prenylated SNARE essential for endoplasmic reticulum-Golgi transport. J Biol Chem. 1997;272:17776–17783. doi: 10.1074/jbc.272.28.17776. [DOI] [PubMed] [Google Scholar]
- Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- Nicholson KL, Munson M, Miller RB, Filip TJ, Fairman R, Hughson FM. Regulation of SNARE complex assembly by an N-terminal domain of the t-SNARE Sso1p. Nat Struct Biol. 1998;5:793–802. doi: 10.1038/1834. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Pelham HR. SNAREs and membrane fusion in the Golgi apparatus. Biochim Biophys Acta. 1998;1404:9–31. doi: 10.1016/s0167-4889(98)00044-5. [DOI] [PubMed] [Google Scholar]
- Parlati F, McNew JA, Fukuda R, Miller R, Sollner TH, Rothman JE. Topological restriction of SNARE-dependent membrane fusion. Nature. 2000;407:194–198. doi: 10.1038/35025076. [DOI] [PubMed] [Google Scholar]
- Peng R, Grabowski R, De Antoni A, Gallwitz D. Specific interaction of the yeast cis-Golgi syntaxin Sed5p and the coat protein complex II component Sec24p of endoplasmic reticulum-derived transport vesicles. Proc Natl Acad Sci USA. 1999;96:3751–3756. doi: 10.1073/pnas.96.7.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham HRB. Getting through the Golgi complex. Trends Cell Biol. 1998;8:45–49. doi: 10.1016/s0962-8924(97)01185-9. [DOI] [PubMed] [Google Scholar]
- Pelham HRB. SNAREs and the secretory pathway-lessons from yeast. Exp Cell Res. 1999;247:1–8. doi: 10.1006/excr.1998.4356. [DOI] [PubMed] [Google Scholar]
- Poirier MA, Xiao W, Macosko JC, Chan C, Shin YK, Bennett MK. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat Struct Biol. 1998;5:765–769. doi: 10.1038/1799. [DOI] [PubMed] [Google Scholar]
- Rice LM, Brennwald P, Brunger AT. Formation of a yeast SNARE complex is accompanied by significant structural changes. FEBS Lett. 1997;415:49–55. doi: 10.1016/s0014-5793(97)01091-0. [DOI] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Sacher M, Stone S, Ferro-Novick S. The synaptobrevin-related domains of Bos1p and Sec22p bind to the syntaxin-like region of Sed5p. J Biol Chem. 1997;272:17134–17138. doi: 10.1074/jbc.272.27.17134. [DOI] [PubMed] [Google Scholar]
- Søgaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Sollner T. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Springer S, Schekman R. Nucleation of COPII vesicular coat complex by endoplasmic reticulum to Golgi vesicle SNAREs. Science. 1998;281:698–700. doi: 10.1126/science.281.5377.698. [DOI] [PubMed] [Google Scholar]
- Stone S, Sacher M, Mao Y, Carr C, Lyons P, Quinn AM, Ferro-Novick S. Bet1p activates the v-SNARE Bos1p. Mol Biol Cell. 1997;7:1175–1181. doi: 10.1091/mbc.8.7.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brünger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Tishgarten T, Yin FF, Faucher KM, Dluhy RA, Grant TR, Fischer von Mollard G, Stevens TH, Lipscomb LA. Structures of yeast vesicle trafficking proteins. Protein Sci. 1999;8:2465–2473. doi: 10.1110/ps.8.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui MMK, Banfield DK. Yeast Golgi SNARE interactions are promiscuous. J Cell Sci. 2000;113:135–144. doi: 10.1242/jcs.113.1.145. [DOI] [PubMed] [Google Scholar]
- Ungermann C, von Mollard GF, Jensen ON, Margolis N, Stevens TH, Wickner W. Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion. J Cell Biol. 1999;145:1435–1442. doi: 10.1083/jcb.145.7.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MG, Hughson FM. Membrane tethering and fusion in the secretory and endocytic pathways. Traffic. 2000;1:588–597. doi: 10.1034/j.1600-0854.2000.010802.x. [DOI] [PubMed] [Google Scholar]
- Weimbs T, Mostov K, Seng HL, Hofmann K. A model for structural similarity between different SNARE complexes based on sequence relationships. Trends Cell Biol. 1998;8:260–262. doi: 10.1016/s0962-8924(98)01285-9. [DOI] [PubMed] [Google Scholar]
- Wooding S, Pelham HRB. The dynamics of Golgi protein traffic visualized in living yeast cells. Mol Biol Cell. 1998;9:2667–2680. doi: 10.1091/mbc.9.9.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Gonzalez L, Jr, Prekeris R, Steegmaier M, Advani RJ, Scheller RH. SNARE interactions are not selective. Implications for membrane fusion specificity. J Biol Chem. 1999;274:5649–5653. doi: 10.1074/jbc.274.9.5649. [DOI] [PubMed] [Google Scholar]