Abstract
Bradykinin and interleukin-1β (IL-1β) induce cyclooxygenase 2 (COX-2) in human airway smooth muscle cells. Here we extended our study to explore the gene transcriptional regulation. By transfection with various COX-2 promoter reporter constructs, we found that the bp −327-to-+59 promoter region was essential for COX-2 gene transcription by bradykinin and IL-1β and that the cyclic AMP response element (CRE) was critical in bradykinin-induced transcription, whereas nuclear factor IL-6 and CRE and, to a lesser extent, nuclear factor-κB (NF-κB) were involved in IL-1β-induced transcription. An electrophoretic mobility shift assay revealed that both bradykinin and IL-1β elicited CRE-binding protein-1 (CREB-1) binding, and IL-1β also elicited CCAAT/enhancer-binding protein β and NF-κB binding to their respective elements in the COX-2 promoter. These transcription factors were associated with the COX-2 promoter, which was dynamically linked to different patterns of histone H4 acetylation by bradykinin and IL-1β, as demonstrated by chromatin immunoprecipitation. We also revealed that endogenous prostaglandin E2 was critical in bradykinin-induced COX-2 transcription initiation and involved in IL-1β-induced COX-2 transcription at a latter stage. Our result provide the first evidence that COX-2 transcriptional regulation by different stimuli involves different promoter elements and transcription factors and is associated with chromatin remodeling after selective histone H4 acetylation in a stimulus-specific manner.
Cyclooxygenase (COX) is the rate-limiting enzyme for the conversion of arachidonic acid to prostanoids and exists in two isoforms. COX-1 is constitutively expressed and homeostatic in function as the housekeeping form (54). In contrast, COX-2 is associated with inflammation and is induced in response to mitogenic (32) and proinflammatory (4, 38, 39) stimuli. The expression of COX-2 is a key element in various pathophysiological processes, including inflammation (53), cardiovascular disease (13, 19), tissue remodeling (9), and cancer (10, 16).
Human COX-2 is encoded by a 7.5-kb genomic DNA with 10 exons (58). The 5′-flanking promoter region of human COX-2 contains a canonical TATA-box and multiple regulatory elements, including two putative nuclear factor κB (NF-κB)-binding sites, one nuclear factor interleukin-6 (NF-IL-6)/CCAAT/enhancer-binding protein (C/EBP)-binding site, and one cyclic AMP-response element (CRE) (52). Recent studies of the human COX-2 promoter have demonstrated that COX-2 expression is critically governed by different transcription factors, including CRE-binding protein (CREB) (56), C/EBP (20), activating protein-1 (AP-1) (51), and NF-κB (24), in a highly cell type-specific and stimulus-specific manner. For example, the knockout of C/EBPβ in macrophages blocks COX-2 expression but has no effect on COX-2 expression in fibroblasts (20). In addition, both IL-1β and tumor necrosis factor alpha (TNF-α) induce COX-2 transcription via NF-κB transactivation in a variety of cells, such as lung epithelial cells (37) and monocytes (28). In contrast, only IL-1β and not TNF-α causes COX-2 protein expression in human airway smooth muscle (ASM) cells (4, 38).
We and others have demonstrated that human ASM cells exert important synthetic functions. In addition to the synthesis of cytokines/chemokines (15, 40, 41) and growth factors (30, 33), human ASM cells express COX-2 upon stimulation by a variety of proinflammatory cytokines and other mediators (4, 18, 38, 39) and release large quantities of prostanoids (mainly prostaglandin E2 [PGE2]), which in turn, in an autocrine manner, modulates the cell functions such as proliferation (5), relaxation (43, 44), and the synthesis of chemokines and growth factors (30, 40). We have shown previously that proinflammatory mediators bradykinin (BK) (39) and IL-1β (38) induce COX-2 protein expression in human ASM cells, but the patterns of COX-2 protein expression are different. BK-induced COX-2 protein appears after 1 h and disappears after 16 to 24 h of treatment (39), whereas IL-1β-induced COX-2 protein appears after 2 h and sustains beyond 24 h treatment (38). It is also interesting that the patterns of PGE2 production by BK and IL-1β are also different. BK induces PGE2 production minutes after stimulation and before COX-2 protein expression (39), whereas IL-1β induces PGE2 production after 2 h treatment as a consequence of COX-2 protein expression (38). These results suggest that the molecular mechanisms by which BK and IL-1β regulate COX-2 expression in human ASM cells are different, which have not been clearly elucidated so far. Since BK-induced IL-8 production from human ASM cells is largely dependent upon the early release of PGE2 (40), whether its effect on COX-2 expression is also PGE2 dependent remains to be investigated.
In the quiescent cells, DNA packaging in condensed chromatin is tightly compacted to prevent transcription factor accessibility. Upon stimulation by inflammatory mediators such as IL-1β, the condensed chromatin becomes loose (chromatin remodeling), which makes the cis elements in gene-specific promoters available for transcription factors to access, resulting in the protein-DNA binding and regulation of inflammatory gene transcription (27). Chromatin is mainly composed of four core histones, an H3-H4 tetramer and two H2A-H2B dimers (2, 3). When the cells are stimulated with inflammatory mediators, the histones in the chromatin undergo an array of posttranscriptional modifications on N-terminal tail domains, including acetylation, phosphorylation, and methylation (49). These modifications, as well as the primary sequence of histone tails, are highly conserved between species and have been closely linked to gene transcription (21). Very recent studies have shown that high levels of acetylation of histone H4 have been correlated with the upregulation of proinflammatory gene transcription (27).
Although transcription regulation of human COX-2 by IL-1β has been studied in other cell systems, it is not clear how the COX-2 gene is regulated transcriptionally by external stimuli such as IL-1β and BK in human ASM cells, particularly in terms of the transcription factors acting on the promoter sequences and the involvement of chromatin remodeling through histone acetylation. In addition, because of its cell-specific and stimulus-specific nature, human COX-2 transcriptional regulation in human ASM cells is likely to be different from other cells and from IL-1β to BK. In the present study we proposed to investigate the molecular mechanisms by which BK and IL-1β regulate COX-2 transcription in human ASM cells. By using promoter reporter transfection, Western blotting, electrophoretic mobility shift assay (EMSA) and chromatin immunoprecipitation (ChIP) assay, we examined the involvement of different cis elements in the COX-2 promoter and the roles of different transcription factors in BK- and IL-1β-induced COX-2 transcription, explored the possibility that PGE2 mediated BK-induced COX-2 transcription, and assessed the association of chromatin remodeling with COX-2 promoter after histone acetylation. We demonstrate that COX-2 transcription by BK and IL-1β involves different regulatory elements, transcription factors, and histone H4 acetylation.
MATERIALS AND METHODS
Culture of human ASM cells.
Primary human ASM cells were prepared from explants of human ASM as previously reported (38) and cultured in Dulbecco modified Eagle medium (Sigma, Poole, United Kingdom) containing 10% fetal calf serum (Seralab, Loughborough, United Kingdom), 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2.5 μg of amphotericin B/ml, and 4 mM l-glutamine (Sigma) with humidified 5% CO2-95% air at 37°C. This protocol was approved by the Nottingham City Hospital Research Ethics Committee. Cells at passages 5 to 6 have previously been demonstrated to depict the immunohistochemical and light microscopic characteristics of typical human ASM cells (38) and were contributed to all experiments in the present study.
DNA constructs.
The COX-2 firefly luciferase reporter constructs containing different human COX-2 promoter fragments (from −1423 to +59 and −327/+59) and those containing 327-bp promoter fragment (from −327 to +59) with mutations of the NF-κB (mNF-κB), NF-IL-6 (mNF-IL-6), or CRE (mCRE) site or both NF-IL-6 and CRE sites [m(CRE+NF-IL-6)] have been previously described in detail (24-26).The COX-2 firefly luciferase reporter constructs containing 7,200- and 3,920-bp promoter fragments (i.e., from positions −7200 to +1 and from positions −3920 to +1) with the putative peroxisome proliferator response element (PPRE) were kindly provided by Thomas McIntyre (University of Utah, Salt Lake City) and have been previously described in detail (45). The internal control Renilla luciferase reporter construct, RL-SV40, was obtained from Promega (Southampton, United Kingdom).
Transient transfection.
All transient transfections were conducted by using FuGene 6 according to the recommended protocol of the manufacturer (Roche Molecular Biochemicals, East Sussex, United Kingdom). A total of 3.5 × 104 human ASM cells were seeded in each well of 12-well plates. After 24 h growth, the cells were ca. 50 to 60% confluent and were incubated with 400 μl of Dulbecco modified Eagle medium containing complexes of DNA (0.4 μg/ml)/FuGene 6 (1.2 μl), together with 4 ng of Renilla luciferase reporter/well as an internal control. After 5 h of incubation, the transfected cells were pretreated with or without indomethacin (Indo; Sigma) at 1 μM and its vehicle 0.2% dimethyl sulfoxide (Sigma) for 30 min and then incubated with 10 μM BK, IL-1β at 1 ng/ml, or 2.5 μM PGE2 (all from Sigma) for another 16 h. The cells were then washed with phosphate-buffered saline (PBS) and lysed in 100 μl of reporter lysis buffer (Promega). Firefly luciferase activity from the testing DNA construct reporters and Renilla luciferase activity from the internal control reporter were measured by using the dual-luciferase reporter assay system (Promega) with the MicroLumatPlus LB96V Automatic Microplate Luminometer (Berthold Technologies, Herts, United Kingdom). Relative luciferase activity was obtained by normalizing the firefly luciferase activity against the internal control Renilla luciferase activity. The fold increase was obtained by comparing relative luciferase activity from experiment groups against that from their respective controls. The transfection rate was between 25 and 30% as measured by transfection with a green fluorescent protein expression vector.
Extraction of cytosolic and nuclear fraction proteins.
Nuclear and cytosolic extracts from the cells were prepared as described before (14, 50) with some modifications. After treatment with or without 10 μM BK or IL-1β (1 ng/ml), human ASM cells in 90-mm dishes were washed with cold PBS and harvested in 500 μl of low salt buffer (20 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM NaVO4, 1 mM EDTA, 1 mM EGTA, 0.2% IGEPAL CA-630 (Sigma),10% glycerol, 1 mM phenylmethylsulfonyl fluoride, and 0.01% leupeptin). After 30 min of incubation on ice, the samples were centrifuged at 10,000 × g at 4°C for 30 min, and the supernatants were collected as cytosolic extracts. The cell pellet containing nuclei was then resuspended in 200 μl of high-salt buffer (as low-salt buffer but with 420 mM NaCl and 20% glycerol) and incubated by shaking on ice for a further 30 min. Samples were then centrifuged at 10,000 × g at 4°C for 30 min, and the supernatants were taken as nuclear extracts. After the determination of protein concentrations by using the Bio-Rad protein assay reagent (Bio-Rad Laboratories, Herts, United Kingdom), the samples were stored at −80°C for further EMSA and Western blot analysis.
EMSA.
To assess in vitro protein-DNA binding, EMSA was performed as described previously (50) with some modifications. The wild-type human COX-2 promoter oligonucleotides containing NF-κB (5′-GAGTGGGGACTACCCCCTCTG-3′), NF-IL-6 (C/EBP) (5′-CCCACCGGGCTTACGCAATTTTTTTAAGGG-3′), and CRE (5′-AAAGAAACAGTCATTTCGTCACATGGGCTT-3′) (underlined) were double stranded. The double-stranded consensus CRE oligonucleotides were obtained from Promega. Double-stranded oligonucleotide probes were end-labeled with [γ-32P]ATP (Amersham Pharmacia Biotech, Bucks, United Kingdom) by incubation with T4 polynucleotide kinase (Promega) according to the manufacturer's instructions. Aliquots of 5 μg of nuclear extracts were incubated with the labeled probe (hot probes) in reaction buffer [10 mM Tris-HCl (pH 8.0), 1 mM dithiothreitol, 1 mM EDTA, 15% glycerol, 0.1% Triton X-100, 25 μg of poly(dI-dC)/ml, 0.1 mg of bovine serum albumin/ml, and 50 mM NaCl] in a total volume of 25 μl for 15 min. For the supershift experiments, the reactions were then incubated with specific antibodies for another 15 min. For the competitive experiments, nuclear extracts were first incubated with a 25-fold excess of unlabeled probes (cold probes) for 30 min and then incubated with labeled probes (hot probes) for another 15 min. The resulting reaction products were resolved by 6% polyacrylamide nondenatured gel electrophoresis. The gels were dried and exposed to an X-ray film (Kodak) at −80°C.
Western blot.
Western blot was performed as described before (38) to assess the translocation of the transcription factors from cytosol to the nucleus in response to BK and IL-1β. Briefly, 30 μg of either cytosolic or nuclear proteins were mixed 1:1 (vol/vol) with sample buffer and boiled for 5 min. The protein samples were then separated by 7.5% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. The resolved proteins in gel were electrotransferred to nitrocellulose membrane (Bio-Rad). The blot was blocked with 8% fat-free milk powder in PBS-T (PBS [pH 7.4] with 0.3% Tween 20) at room temperature for 2 h and then incubated with primary antibodies against p65, CREB-1, and C/EBPβ separately (1:1,000 dilution; Santa Cruz Biotechnology, Calif.) for 2 h at room temperature. The blot was subsequently incubated with horseradish peroxidase-conjugated secondary antibody (1:2,000 dilution; Santa Cruz) for 1 h. The signal was detected by incubation with the ECL Western blotting detection reagent (Amersham) for 1 min and finally exposed to Hyperfilm-ECL (Amersham).
ChIP assay.
To detect the in vivo association of nuclear proteins with human COX-2 promoter, ChIP assay was conducted as described previously (23, 31, 55) with some modifications. Human ASM cells in 90-mm dishes were grown to confluence and serum starved for 24 h. After treatment, protein-DNA complexes were fixed by 1% formaldehyde in PBS. The fixed cells were washed and lysed in SDS-lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl [pH 8.0], Complete protease inhibitor cocktail σ) and sonicated on ice. The samples were centrifuged, and the soluble chromatin was precleared by incubation with sheared salmon sperm DNA-protein A slurry (a 50:50 mix; Sigma) for 0.5 h at 4°C with rotation. After centrifugation at 500 × g for 1 min, one portion of the precleared supernatant was used as DNA input control, and the remains were subdivided into aliquots and then incubated with a nonimmune rabbit immunoglobulin G (IgG; Santa Cruz) or IgG antibodies to NF-κB p65 (New England Biolabs, Herts, United Kingdom), C/EBPβ (Santa Cruz), CREB-1 (Santa Cruz), and histone H4 AcK5, AcK8, AcK-12, AcK16 (Serotec, Oxford, United Kingdom) separately, overnight at 4°C. The immunoprecipitated complexes of antibody-protein-DNA were collected by using the above protein A slurry, washed successively with low-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl [pH 8.1], 150 mM NaCl), high-salt buffer (same as the low-salt buffer but with 500 mM NaCl), LiCl buffer (0.25 M LiCl, 1% NP-40, 1% deoxycholate, 1 mM EDTA, 10 mM Tris-HCl [pH 8.1]), and Tris-EDTA (pH 8.0) and then eluted with elution buffer (1% SDS, 100 mM NaHCO3). The cross-linking of protein-DNA complexes was reversed by incubation with 5 M NaCl at 65°C for 4 h, and DNA was digested with 10 mg of proteinase K (Sigma)/ml for 1 h at 45°C. The DNA was then extracted with phenol-chloroform, and the purified DNA pellet was resuspended in H2O and subjected to PCR amplification with the forward primer 5′-AAGACATCTGGCGGAAACC-3′ and the reverse primer 5′-ACAATTGGTCGCTAACCGAG-3′, which were specifically designed from the COX-2 promoter. The 305-bp PCR products were resolved by 3.5% agarose-ethidium bromide gel electrophoresis, visualized by UV, and analyzed with the GeneGenius gel documentation and analysis system (Syngene, Cambridge, United Kingdom).
Statistical analysis.
Data were expressed as mean ± the standard error of the mean (SEM) of n determinations. Statistical analysis was performed by using GraphPad Prism software (version 3.03). Unpaired two-tailed Student t test was used to determine the significant differences between the means; P values of <0.05 were accepted as statistically significant.
RESULTS
Essential elements in the human COX-2 promoter region in response to BK and IL-1β.
To determine the promoter region essentially required for BK- and IL-1β-induced COX-2 transcription, we transfected human ASM cells with reporter constructs containing various lengths of the human COX-2 promoter. Compared to the control, both BK and IL-1β induced COX-2 promoter activity after the transfection of the 7200-bp COX-2 promoter-driven constructs. The effect of both BK and IL-1β was not significantly altered by the transfection of constructs driven by smaller COX-2 promoter fragments, such as the 3,920-, 1,423-, and 327-bp fragments, compared to the 7,200-bp COX-2 promoter-driven constructs (Fig. 1). The results indicate that the PPREs located between positions −3721 and −3707 and the second NF-κB element located between positions −448 to −439 are not essentially involved in BK- and IL-1β-induced COX-2 transcription in the cells, whereas the promoter region from bp −327 to +59 confers the responsiveness of the human COX-2 promoter and is therefore the essential promoter region responsible for BK- and IL-1β-induced COX-2 transcription in these cells.
FIG. 1.
Identification of the essential elements in the human COX-2 promoter region in response to BK and IL-1β. The diagram on the left shows the firefly luciferase reporter constructs driven by various human COX-2 promoter fragments of 7,200, 3,920, 1,423, and 327 bp containing wild-type PPRE (−3721/−3707), distal NF-κB (−448/−439), and proximal NF-κB (−223/−214), NF-IL-6 (−132/−124), and CRE (−59/−53) cis-acting elements. Fifty to 60% confluent human ASM cells in 12-well plates were cotransfected with Renilla luciferase internal control reporter (4 ng/well) and 7,200-, 3,920-, 1,423-, or 327-bp human COX-2 promoter firefly luciferase reporter constructs (0.4 μg/well) by using FuGene 6 transfection reagent as described in Materials and Methods and stimulated with or without BK (10 μM) or IL-1β (1 ng/ml) for a further 16 h. The luciferase activities from firefly and Renilla reporters were assayed by using the dual-luciferase reporter assay system, and the relative luciferase activity was obtained by normalizing the firefly luciferase activity against the internal control Renilla luciferase activity. The results are expressed as mean ± the SEM of three separate experiments performed in duplicate or triplicate.
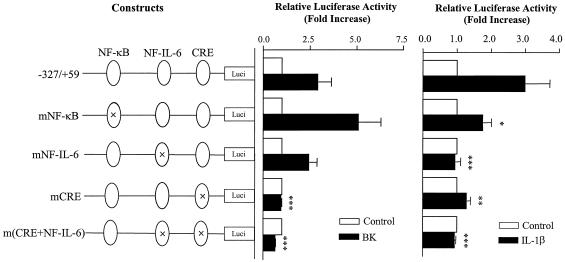
Identification of cis-acting elements at the human COX-2 promoter responsible for BK- and IL-1β-induced COX-2 transcription.
The promoter at bp −327 to +59 contains CRE (positions −59 to −53 [−59/−53]), NF-IL-6 (−132/−124), and NF-κB (−223/−214) cis-acting elements. To identify the elements responsible for BK- and IL-1β-induced COX-2 transcription, the promoter activity of the wild-type fragment (−327 bp/+59 bp) was compared to that of site-directed mutants. BK caused elevation of the transcriptional activity with the wild-type promoter, and mutations on the NF-κB and NF-IL-6 elements, respectively, did not reduce the activity; in contrast, mutation on the CRE site and double mutations on both NF-IL-6 and CRE sites abolished the activity (P < 0.001, Fig. 2). IL-1β also increased the transcriptional activity of the wild-type promoter. When the NF-κB element alone was mutated, IL-1β still markedly stimulated the activity although the effect was reduced compared to the wild-type promoter (P < 0.05, Fig. 2). In contrast, when either NF-IL-6 or CRE element was mutated, the promoter transcription activity was markedly reduced or inhibited (P < 0.001 and P < 0.01, respectively) and double mutations of both elements did not additionally further reduced the promoter activity compared to NF-IL-6 mutation alone (Fig. 2). The results suggest that different regulatory elements are required for human COX-2 transcription by BK and IL-1β in human ASM cells: CRE is essential for BK-induced COX-2 transcription, whereas NF-IL-6 and CRE elements and, to a lesser extent the NF-κB element, are critically involved in COX-2 transcription by IL-1β.
FIG. 2.
Identification of cis-acting elements in BK- and IL-1β-induced human COX-2 promoter activity. The diagram on the left shows the human COX-2 promoter (−327/+59) firefly luciferase reporter constructs containing wild-type CRE (−59/−53), NF-IL-6 (−132/−124), and NF-κB (−223/−214) cis-acting elements or their respective site-directed mutants. Fifty to 60% confluent human ASM cells in 12-well plates were cotransfected with Renilla luciferase internal control reporter (4 ng/well) and either of the human COX-2 constructs (0.4 μg/well) by using FuGene 6 transfection reagent as described in Materials and Methods and then stimulated with or without BK (10 μM) (A) or IL-1β (1 ng/ml) (B) for a further 16 h. The luciferase activities from firefly and Renilla reporters were assayed by using the dual-luciferase reporter assay system, and the relative luciferase activity was obtained by normalizing the firefly luciferase activity against the internal control Renilla luciferase activity. The results are expressed as mean ± the SEM of three separate experiments performed in duplicate or triplicate. ✽, P < 0.05; ✽✽, P < 0.01; ✽✽✽, P < 0.001 (compared to cells transfected with the wild-type human COX-2 promoter [−327/+59] reporter plasmids and stimulated with IL-1β or BK alone).
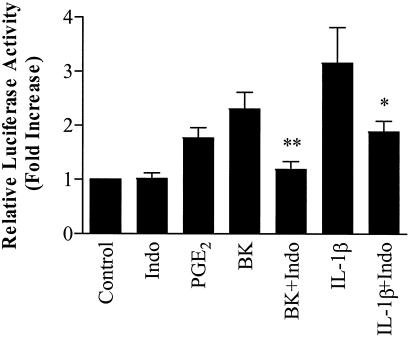
Involvement of PGE2 in BK- and IL-1β-induced COX-2 promoter transactivation.
We have previously reported that PGE2 stimulates COX-2 protein expression on its own and enhances IL-1β-stimulated COX-2 protein expression in human ASM cells (42) and that BK-induced IL-8 production from human ASM cells is largely dependent upon the early endogenous PGE2 generation (40). To clarify whether PGE2 is involved in BK- and IL-1β-induced COX-2 promoter transactivation, the essential promoter (−327/+59) luciferase reporter construct was transfected into the cells. As shown in Fig. 3 BK (10 μM), after 16 h of stimulation, caused a marked increase in COX-2 promoter activity compared to the control and exogenous PGE2 (2.5 μM) mimicked the effect of BK. Furthermore, IL-1β also caused a marked increase in COX-2 promoter activity, and the nonselective COX inhibitor Indo significantly inhibited BK- and IL-1β-induced COX-2 promoter activity (P < 0.01 and P < 0.05, respectively), although it had no effect on its own (Fig. 3). These results strongly suggest that PGE2 is involved in the induction of the COX-2 promoter transactivation by both BK and IL-1β in human ASM cells.
FIG. 3.
Involvement of PGE2 in BK- and IL-1β-induced transcriptional activity of the human COX-2 promoter (−327/+59). Fifty to 60% confluent human ASM cells in 12-well plates were cotransfected with Renilla luciferase internal control reporter (4 ng/well) and the human COX-2 promoter (−327/+59) firefly luciferase reporter constructs (0.4 μg/well) by using FuGene 6 transfection reagent as described in Materials and Methods. The transfected cells were pretreated with or without Indo for 30 min and then incubated with or without BK (10 μM), IL-1β (1 ng/ml), or PGE2 (2.5 μM) for a further 16 h. The luciferase activities from firefly and Renilla reporters were assayed by using the dual-luciferase reporter assay system, and the relative luciferase activity was obtained by normalizing the firefly luciferase activity against the internal control Renilla luciferase activity. The results are expressed as mean ± the SEM of six separate experiments performed in duplicate or triplicate. ✽, P < 0.05; ✽✽, P < 0.01 (compared to IL-1β or BK alone, respectively).
Transcription factor nuclear translocation.
Transcription factors such as NF-κB exist in cytoplasm in quiescent cells but are translocated to the nucleus to bind to their cis-acting elements upon stimulation by proinflammatory mediators. To further clarify the involvement of NF-κB, the CRE-binding protein CREB-1, and the NF-IL-6-binding protein C/EBPβ in BK- and IL-1β-induced COX-2 transcription, Western blotting was used to examine whether these transcription factors were translocated to the nucleus by BK and IL-1β. As shown in Fig. 4, under resting conditions, NF-κB subunit p65 only existed in the cytosol of human ASM cells, but the amount was quickly reduced after 0.5 h treatment with IL-1β and then recovered after 1 h. Simultaneously, a marked movement of p65 to the nucleus was swiftly induced after 0.5 h of treatment with IL-1β and sustained for up to 2 h. In contrast, BK had no effect. CREB-1 existed in both cytosol and nucleus, and no nuclear translocation was observed after treatment with IL-1β and BK. C/EBPβ existed mainly in the cytosol, was markedly reduced after treatment with IL-1β for 0.5 h, and recovered after 1 h. Although a small amount of C/EBPβ existed in the nucleus, a further increase was evidently observed after 0.5 h treatment with IL-1β and sustained for up to 2 h. These results demonstrate that IL-1β, but not BK, causes p65 and C/EBPβ translocation, which may be involved in COX-2 transcription by IL-1β, and that CREB-1 are constitutively present in the nucleus, which may be involved in COX-2 transcription by both IL-1β and BK.
FIG. 4.
Effect of IL-1β and BK on the nuclear translocation of transcription factors. Confluent and serum-starved human ASM cells in 90-mm dishes were incubated with or without IL-1β (1 ng/ml) or BK (10 μM) for the times indicated. Cytosolic and nuclear proteins were prepared, and the presence of NF-κB p65, CREB-1, and C/EBPβ in both fractions was analyzed by Western blotting with a specific antibody as described in Materials and Methods. These blots are representative of two independent experiments, with similar results.
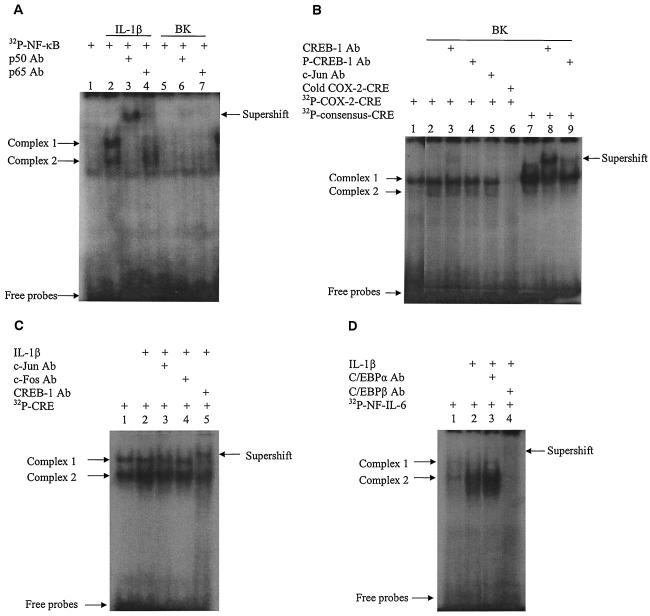
In vitro binding of transcription factors to their cis-acting elements in the COX-2 promoter.
We then analyzed the in vitro binding of NF-κB, CREB-1, and C/EBPβ to their regulatory elements at the COX-2 promoter by EMSA. As shown in Fig. 5A, IL-1β induced two nuclear protein-DNA complexes with a probe containing the COX-2 NF-κB cis-acting element compared to the control, and both were supershifted by p50 antibody, whereas only complex 1 was supershifted by p65 antibody, suggesting that p50 was involved in complexes 1 and 2 and that p65 was mainly involved in complex 1. In contrast, BK-stimulated nuclear protein did not generate visible complexes with the NF-κB probe (Fig. 5A). Since CREB and AP-1 (c-Jun/c-Fos) have been reported to bind to the CRE cis-acting element in the human COX-2 promoter (51), their binding to the CRE probes was investigated. Untreated cells produced one nuclear protein-DNA complex with the COX-2 CRE (Fig. 5B, lane 1). Treatment with BK (at 10 μM for 1 h) modestly induced another protein-DNA complex (complex 2, lane 2). A light supershift was observed with the CREB-1 antibody (lane 3), but preincubation with antibodies against phosphorylated CREB (p-CREB, lane 4) and c-Jun (lane 5) did not generate any supershifted bands. Competition assay with excessive unlabeled COX-2 CRE probe (cold COX-2-CRE, lane 6) completely abolished the complex formed with labeled probe, suggesting that the complex is specific to the COX-2 CRE. Compared to the complex with COX-2 CRE, BK-induced nuclear proteins formed a major complex with the consensus CRE probe (lane 7), which was markedly decreased by preincubation with the CREB-1 antibody, accompanied by a major supershift (lane 8). A reduction of the major complex and a weak supershift were also observed with the phosphorylated CREB-1 antibody (p-CREB; lane 9). The results suggest that BK induces a modest in vitro CREB-1 binding to the CRE in the human COX-2 promoter. Compared to the control (lane 1, Fig. 5C), IL-1β (at 1 ng/ml for 1 h) enhanced the protein-DNA complexes 1 and 2 with the COX-2 CRE probe (lane 2), and complex 1 was supershifted by CREB-1 antibody, whereas complex 2 was reduced (lane 5), suggesting that IL-1β also induces CREB-1 binding to the COX-2 CRE. However, neither c-Jun nor c-Fos antibody could generate supershift (lanes 3 and 4), suggesting that the AP-1 dimers c-Jun and c-Fos do not bind to the COX-2 CRE. Both C/EBPα and C/EBPβ have been shown to bind to the COX-2 NF-IL-6 element (29). In our cell system, treatment with IL-1β (1 ng/ml for 1 h) induced the nuclear protein-DNA complexes (Fig. 5D, lane 2) with the COX-2 NF-IL-6 probe compared to the control (lane 1), which were abolished by C/EBPβ antibody accompanied by a retarded band (lane 4). In contrast, C/EBPα antibody did not alter the complexes (lane 3), demonstrating that IL-1β induces C/EBPβ binding to the COX-2 NF-IL-6 element.
FIG. 5.
Effect of BK and IL-1β on the in vitro binding of transcription factors to their respective cis-acting elements in the human COX-2 promoter. Confluent and serum-starved human ASM cells in 90-mm dishes were incubated without (lane 1) or with BK (10 μM, A and B) or IL-1β (1 ng/ml, C and D) for 1 h. Nuclear proteins were extracted, and the in vitro nuclear protein-DNA binding was analyzed by EMSA with 32P-labeled DNA probes containing either the human COX-2 promoter NF-κB cis element (A), the human COX-2 promoter CRE cis element (B and C), the consensus CRE cis element (B), or the human COX-2 promoter NF-IL-6 cis element (D) as described in Materials and Methods. Supershifts were performed to determine which transcription factors were present in the protein-DNA complexes by using specific antibodies against individual transcription factor. A competition assay was conducted by incubating the nuclear extracts with a 25-fold excess of unlabeled (cold) COX-2 CRE probe for 30 min prior to the 32P-labeled probe (see panel B). The results are representative of three independent experiments with similar results.
In vivo association of transcription factors with the COX-2 promoter in BK- and IL-1β-stimulated human ASM cells.
By using EMSA to detect the in vitro binding of transcription factors to the COX-2 promoter, we showed BK- and IL-1β-induced binding of CREB-1 to CRE element, IL-1β-induced binding of C/EBPβ to NF-IL-6 element and p65/p50 to NF-κB element in the COX-2 promoter. To further understand the roles of transcription factors and the COX-2 promoter regulatory elements in BK- and IL-1β-induced COX-2 transcription, in vivo association of these transcription factors with the COX-2 promoter was evaluated by the semiquantitative ChIP assay. To determine whether the transcription factors could specifically associate with the COX-2 promoter, PCR amplifications were conducted on a fixed amount of immunoprecipitated DNA, followed by 30 cycles of PCR with the specific primer pairs encompassing position −299 to +6 region of the human COX-2 promoter (Fig. 6A). After BK treatment, CREB-1 immunoprecipitates showed a marked enrichment of the COX-2 promoter DNA compared to the nonimmune IgG immunoprecipitate control (Fig. 6A), indicating that the COX-2 promoter DNA precipitated by the CREB-1 antibody was specifically associated with CREB-1. Immunoprecipitates with an antibody to p65, CREB-1, or C/EBPβ resulted in different patterns of enrichment of the DNA segments encompassing the COX-2 promoter after BK and IL-1β treatment (Fig. 6B). BK (10 μM) quickly induced an enrichment of CREB-1-associated COX-2 promoter DNA at 0.5 h compared to control (0 h); this level then decreased over 1 to 5 h but remained at a level higher than that of the control. In contrast, BK had a modest or no effect on p65 and C/EBPβ association with the COX-2 promoter DNA (Fig. 6B). The results suggest that CREB-1 is the key transactivator in BK-induced COX-2 transcription. After IL-1β (1 ng/ml) treatment, an enrichment of p65-, C/EBPβ-, and CREB-1-associated COX-2 promoter DNA appeared at 0.5 h and sustained up to 2.5 h (p65 and C/EBPβ) and 5 h (CREB-1), respectively (Fig. 6B). These data suggest that p65, C/EBPβ, and CREB-1 are all involved in IL-1β-induced COX-2 transcription in human ASM cells.
FIG. 6.
Effect of BK and IL-1β on the in vivo DNA association of transcription factors and histone H4 acetylation at the COX-2 promoter. (A) The sequence of the human COX-2 promoter region (−299/+6) amplified by PCR primer pairs indicated by the arrows. The CRE, NF-IL-6, and NF-κB cis-acting elements in the promoter are in boldface. An enrichment of the COX-2 promoter DNA is shown after PCR amplification of immunoprecipitates of CREB-1-associated DNA from cells treated with BK for 1 h. (B and C) Confluent and serum-starved human ASM cells in 90-mm dishes were incubated with BK (10 μM) or IL-1β (1 ng/ml) for the times indicated. The in vivo protein-DNA complexes were cross-linked by formaldehyde treatment and chromatin pellets were extracted and sonicated. p65, CREB-1, and C/EBPβ (B) and acetylated histone H4 lysine residues (i.e., AcK5, AcK8, AcK12, and AcK16) (C) were immunoprecipitated with specific antibodies, and the associated COX-2 promoter DNA was amplified by PCR as described in Materials and Methods. The input represents PCR products from chromatin pellets prior to immunoprecipitation. The results are representatives of two independent experiments with similar results.
In vivo association of acetylated histone H4 lysine residues with the COX-2 promoter in BK- and IL-1β-stimulated human ASM cells.
Proinflammatory genes can be modulated by chromatin remodeling via stimulus-dependent acetylation of histone H4 (27). We then analyzed the histone H4 acetylation events involved in COX-2 transactivation by ChIP assay. Equal amount nuclear samples were immunoprecipitated with an antibody against acetylated histone H4 lysine residue AcK5, AcK8, AcK12, or AcK16, and the immunoprecipitated DNA was amplified by PCR with the specific human COX-2 primer pairs (Fig. 6A). Different patterns of immunoprecipitate-associated COX-2 promoter enrichment were observed after BK and IL-1β treatment (Fig. 6C). BK quickly caused a marked enrichment of AcK16-associated COX-2 promoter DNA at 0.5 h compared to control (0 h), which soon returned to baseline (1 h). BK also caused an enrichment of AcK5- and AcK8-associated COX-2 promoter DNA at a later time point (1 h) but had no effect on AcK12 association with the COX-2 promoter (Fig. 6C). In contrast, IL-1β treatment only resulted in a marked enrichment of AcK8-associated COX-2 promoter DNA at 0.5 h, and this enrichment then decreased gradually over 1 to 5 h; IL-1β treatment, however, had no effect on AcK5, AcK12, and AcK16 association with the COX-2 promoter (Fig. 6C). These data suggest that BK- and IL-1β-induced COX-2 transcription requires chromatin remodeling via acetylation of distinct histone H4 lysine residues.
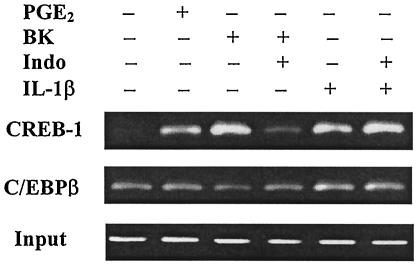
Involvement of PGE2 in BK- and IL-1β-induced association of transcription factors with the COX-2 promoter.
We showed (Fig. 3) that PGE2-stimulated, whereas the COX inhibitor Indo-inhibited, BK- and IL-1β-induced COX-2 promoter activity (16 h), which suggests that PGE2 may mediate the effect of both BK and IL-1β on COX-2 transcription. To explore whether PGE2 was involved in BK- and IL-1β-induced transcription factor association with the COX-2 promoter and therefore the initiation of transcription, a ChIP assay was performed. PGE2 treatment (2.5 μM, 1 h) caused a marked enrichment of CREB-1-associated COX-2 promoter DNA but had no effect on C/EBPβ association with the COX-2 promoter (Fig. 7). Both BK (10 μM) and IL-1β (1 ng/ml) treatment (1 h) stimulated robust enrichment of CREB-1-associated COX-2 promoter DNA, and IL-1β (but not BK) also enhanced C/EBPβ-associated COX-2 promoter DNA (Fig. 7). Pretreatment with Indo (1 μM, 30 min) selectively reduced BK-induced CREB-1 association with the COX-2 promoter and had no effect on IL-1β-induced CREB-1 and C/EBPβ association with the COX-2 promoter (Fig. 7). The results indicate that BK-induced the early association of CREB-1 with the COX-2 promoter and the consequent initiation of COX-2 gene transcription are largely dependent upon endogenous PGE2 production; in contrast, IL-1β-induced effect is PGE2 independent.
FIG. 7.
Effect of BK and IL-1β on the association of transcription factors with the human COX-2 promoter and the involvement of PGE2 in the process. Confluent and serum-starved human ASM cells in 90-mm dishes were pretreated with or without Indo (1 μM) for 30 min and then incubated with BK (10 μM) or IL-1β (1 ng/ml) or PGE2 (2.5 μM) for 1 h. The in vivo protein-DNA complexes were cross-linked by formaldehyde treatment, and chromatin pellets were extracted and sonicated. CREB-1 and C/EBPβ were immunoprecipitated with specific antibodies, and the associated COX-2 promoter DNA was amplified by PCR as described in Materials and Methods. The specific COX-2 primers were described in Fig. 6A. The input represents PCR products from chromatin pellets prior to immunoprecipitation. The results are representatives of two independent experiments with similar results.
DISCUSSION
The major findings of our current study are that the transcriptional regulation of COX-2 by BK and IL-1β in human ASM cells involves different promoter elements and transcription factors and is associated with chromatin remodeling after selective acetylation of histone H4 lysine residues in a stimulus-specific manner. We have also demonstrated that endogenous PGE2 is critical in the initiation of BK-induced COX-2 transcription and also takes part in IL-1β-induced COX-2 transcription in an autocrine manner at a latter stage. The results provide important information about the molecular mechanisms by which COX-2 gene transcription is regulated.
Accumulating evidence has demonstrated that a number of transcription factors, such as CREB, c-Jun, c-Fos, and ATF-2, can bind to CRE in the COX-2 promoter and initiate the transcription (51). We showed in the current study by EMSA that both BK and IL-1β induced modest increases of the in vitro binding of CREB-1, but not c-Jun, to the COX-2 CRE compared to the high intensity of CREB-1-DNA complex in control cells. Because the accessibility of the binding sites is not controlled by chromatin remodeling in EMSA, transcription factors that are present in the nucleus can bind to the probes containing their binding sites. This is supported by our unpublished observation that TNF-α induces p65 nuclear translocation in our cell system and causes in vitro binding to COX-2 NF-κB probe as detected by EMSA, even though it does not induce COX-2 expression. Since CREB-1 constantly existed in the nucleus of unstimulated human ASM cells (Fig. 4), a high level of basal binding of CREB-1 with the COX-2-CRE probe, and modest increases after BK and IL-1β treatment were consequently observed. We therefore used the semiquantitative ChIP assay to detect the in vivo association of transcription factors with the COX-2 promoter. We demonstrated that after 0.5 h BK and IL-1β treatment there was a marked induction of CREB-1 association with the COX-2 promoter compared to the control. The association appears earlier than BK-induced COX-2 protein expression (1 h) (39). Because BK did not cause the association of p65 and C/EBPβ with the COX-2 promoter, the results are consistent with those from the reporter gene assay and clearly indicate that CREB-1 is the key factor that binds to CRE and initiates BK-induced COX-2 transcription. Furthermore, IL-1β-induced CREB-1 association with the COX-2 promoter is in accordance with the requirement of CRE for IL-1β-induced COX-2 promoter activity, suggesting that CREB-1 is a major transcription factor in IL-1β-induced COX-2 transcription. It has been reported that both CREB and phosphorylated CREB are involved in the regulation of CRE-dependent genes (17, 34). However, we did not detect phosphorylated CREB-1 in the nuclear protein-COX-2 CRE complexes, although the phosphorylated CREB-1 existed in the complexes with consensus CRE probe. This suggests that CREB-1 rather than phosphorylated CREB is involved in the transcription regulation of COX-2 by both BK and IL-1β.
In addition to CREB-1, other transcription factors are also involved in IL-1β-induced COX-2 transcription. NF-κB has been regarded as a key transcription factor regulating COX-2 gene expression in a number of cell systems (28, 37, 57). In the present study, we found that deletion of the distal NF-κB element (−448/−214) in the COX-2 promoter did not alter BK- and IL-1β-induced COX-2 promoter activity and that mutation of the proximal NF-κB element (−223/−214) only slightly reduced IL-1β-induced COX-2 promoter activity but had no effect on BK-induced COX-2 promoter activity. These results indicate that in human ASM cells NF-κB is not involved in BK-induced COX-2 transcription and that NF-κB does not play a key role in IL-1β-induced COX-2 transcription but may be required for optimized effect. The latter concept is further supported by the following facts. (i) BK does not induce NF-κB translocation but still induces COX-2 transcription. (ii) TNF-α, which stimulates NF-κB p65 and p50 nuclear translocation in human ASM cells (unpublished observations), has no effect on COX-2 expression (38). (iii) The NF-κB inhibitor SN50 inhibits COX-2 protein expression in human amnion mesenchymal cells (57), whereas the NF-κB inhibitor pyrrolidinedithiocarbamate has no effect on IL-1β-induced COX-2 expression in human ASM cells (unpublished observation).
Our results also showed a different involvement of C/EBPβ in COX-2 transcription by IL-1β compared to BK. Several C/EBP family members, including C/EBPα and C/EBPβ, have been shown to bind to the COX-2 NF-IL-6 element and are functionally involved in the transcriptional regulation of COX-2 (29). EMSA analysis showed that IL-1β induced in vitro binding of C/EBPβ to NF-IL-6, in accordance with the involvement of its respective element in IL-1β-induced COX-2 transcription. By applying ChIP assay we also demonstrated that C/EBPβ was associated with the COX-2 promoter. C/EBPβ is therefore actively involved in the transcription regulation of COX-2 by IL-1β. Although a modest increase in C/EBPβ association with the COX-2 promoter was also observed, it occurred at a much later time than that of CREB-1, and because NF-IL-6 element was not involved in BK-induced COX-2 promoter activity, it is unlikely that C/EBPβ is involved in the effect of BK.
Chromatin remodeling after CREB-binding protein (CBP)-associated histone acetylation is believed to be a prerequisite for active transcription of genes upon stimulation by inflammatory mediators (27). Proinflammatory genes that are expressed in a stimulus-specific manner have been shown to be embedded in stimulus-specific local chromatin regions characterized by hyperacetylation of histone (27, 36, 59). The direct link between histone acetylation and transcriptionally active chromatin became apparent with the use of ChIP procedure for the fraction of chromatin by applying an antibody which specifically recognizes hyperacetylated histones (22, 31, 36). With the use of ChIP in the current study, we not only demonstrated that transcription activators were in their native setting associated with the COX-2 promoter in vivo but also found that these associations were dynamically linked to the stimulus-specific histone H4 acetylation of different lysine residues by BK and IL-1β, respectively. These finds suggest that the stimulus-specific chromatin remodeling may control COX-2 transcription by BK and IL-1β by allowing selective transcription factors to bind to their respective elements in the promoter. This is further supported by the lack of effect of TNF-α on the acetylation of histone H4 lysines 8 and 16 at the COX-2 promoter (unpublished observation), which are the main sites IL-1β and BK acetylates. Although a direct link between the acetylation of individual histone H4 lysine residues and the association of individual transcription factors to the COX-2 promoter cannot be established from the current results, our findings strongly suggest that different patterns of histone acetylation may result in different conformational change of the chromatin structure and are therefore associated with different patterns of transcription factor association with the COX-2 promoter and probably different kinetics of COX-2 expression by BK and IL-1β. Since CREB-1 (7), C/EBPβ (35), c-Jun (1), and p65 (47) have all been shown to interact with the universal activator CBP, which exerts intrinsic histone acetyltransferase (HAT) activity (8), it is likely that the activity of HAT from CBP may be responsible for the acetylation of histone involved in COX-2 transcription by BK and IL-1β. However, the involvement of other histone modifications, i.e., methylation and phosphorylation, in both BK and IL-1β induced COX-2 expression cannot be excluded, and further studies will be needed to establish the direct link.
Since both BK- and IL-1β-induced COX-2 promoter activity (−327 bp, 16 h) was inhibited by the COX inhibitor Indo, the results suggest that prostanoids and, in particular, PGE2, since it is the predominant form of prostanoid from human ASM cells (38), is a mediator for both. This is supported by previous reports that PGE2 causes COX-2 protein expression on its own and enhances IL-1β-induced COX-2 protein expression in human ASM cells (6, 42). However, we also found in the present study that PGE2, BK, and IL-1β all stimulated CREB-1 association with the COX-2 promoter (1 h) and Indo inhibited the effect of BK but not IL-1β. This discrepancy of the effect of Indo on COX-2 promoter activity and CREB-1 association with the COX-2 promoter by BK and IL-1β can be explained by the time course of PGE2 production and COX-2 protein expression. PGE2 is released from the cells immediately after BK stimulation and before COX-2 induction (39); in contrast, PGE2 is only produced after COX-2 protein expression 2 h after IL-1β treatment (38). The inhibition on BK-induced CREB-1 association with the COX-2 promoter (1 h) indicates the involvement of endogenous PGE2 from COX-1 in this process, whereas the lack of effect on IL-1β-induced CREB-1 association with the COX-2 promoter implies that PGE2 is not involved in this process. This is because endogenous PGE2 is produced only after IL-1β-induced COX-2 protein expression (38), and there is no PGE2 increase at the time when IL-1β induces CREB-1 association with the COX-2 promoter. Hence, a mediator(s) other than PGE2 is responsible for IL-1β effect at the particular time. However, after 16 h of incubation with BK and IL-1β, the accumulation of PGE2 must have, to a certain extent, contributed to the accumulation of luciferase. The blockade of PGE2 accumulation by Indo then results in a reduction of COX-2 promoter activity. Taken together, our results demonstrate clearly that PGE2 plays a different role in BK- and IL-1β-induced COX-2 expression because of the time course difference in PGE2 production. PGE2 is critical in the initiation of BK-induced, but not IL-1β-induced COX-2 expression; however, the inability of Indo to abolish BK-induced CREB-1 association with the COX-2 promoter and the promoter activity suggests that BK, to a much lesser extent, may also utilize another pathway other than PGE2 to induce CREB-1 association with the COX-2 promoter and COX-2 gene transcription. PGE2 is likely to be involved in IL-1β-induced COX-2 expression at a later stage by inducing or enhancing the association of CREB-1 with the COX-2 promoter after it is produced as a consequence of COX-2 protein expression.
Since PGE2 stimulates cyclic AMP generation via either EP2 or EP4 adenylyl cyclase-coupled receptors in human ASM cells (6), it is likely that PGE2 upregulates COX-2 expression by a cyclic-AMP-dependent positive feedback mechanism that eventually leads to CREB binding to CRE. This is in agreement with the findings that the β2-adrenoceptor agonists salbutamol and salmeterol and the direct adenylyl cyclase activator forskolin also induce COX-2 (unpublished observation).
The finding that Indo reduced BK- and IL-1β-induced COX-2 promoter activity in the present study appears to contradict our recent finding that Indo enhances IL-1β-induced COX-2 protein expression and causes COX-2 induction on its own, albeit inhibiting COX-2 activity and consequently blocking the enhancing effect of endogenous PGE2 in human ASM cells (42). This discrepancy can be explained by the fact that Indo is also a peroxisome proliferator-activated receptor γ (PPARγ) activator. PPARs are members of the nuclear receptor superfamily of ligand-activated transcription factors, exist in three subtypes [α, β(δ), and γ (11)], and have complex regulatory effects on the expression of a broad array of genes, including those involved in immune and inflammatory responses (46, 48). We have demonstrated recently that Indo enhances IL-1β-induced COX-2 transcription and the process is prostanoid independent, involves PPARγ activation, and requires a PPRE sequence located between positions −3721 and −3707 in the COX-2 promoter (42). The reason that BK- and IL-1β-induced COX-2 promoter activity was inhibited, rather than enhanced, by Indo in the present study was likely due to the lack of PPRE sequence in the COX-2 promoter fragment (−327/+59) that drove the reporter construct. If fact, Indo has been shown to increase the promoter activity of a COX-2 reporter construct (3.9 kb) containing the PPRE but has no effect on another COX-2 reporter construct containing no PPRE (3.5kb) (42). Consequently, under this particular condition (no PPRE in COX-2 promoter) Indo is most likely to act as a COX inhibitor. However, since PPARs may modulate gene transcription in a PPRE binding-independent manner by interfering negatively with other transcription factors such as NF-κB and AP-1 (12), such an effect, although unlikely, cannot be excluded from the context of current study.
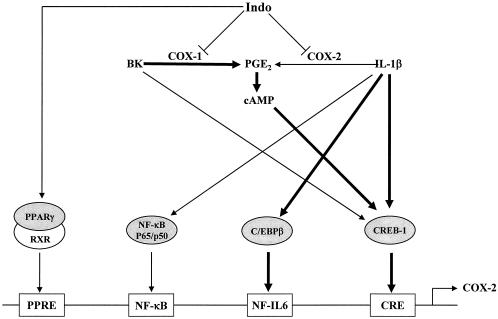
In summary, we have demonstrated that the transcriptional regulation of COX-2 expression in human ASM cells by BK and IL-1β requires different regulatory elements, transcription factors, and histone H4 acetylation. BK upregulates COX-2 transcription mainly through CREB-1 binding to the CRE in the COX-2 promoter via a PGE2-cyclic-AMP-dependent mechanism. IL-1β-initiated COX-2 transcription requires CREB-1 binding to the CRE and C/EBPβ binding to the NF-IL-6 cis element, largely via a PGE2-independent mechanism, although late endogenous PGE2 also enhances IL-1β-induced COX-2 transcription. NF-κB transactivation does not play a key role but optimizes IL-1β-induced COX-2 transcription. The COX inhibitor Indo inhibits both BK- and IL-1β-induced PGE2 release thus prevents its activation on COX-2 promoter; however, it also induces COX-2 transcription by activating PPARγ, which forms a heterodimer with retinoid X receptor and binds to PPRE of COX-2 promoter (Fig. 8). Different patterns of transcription factor association with the COX-2 promoter are likely linked with chromatin remodeling according to different patterns of histone H4 acetylation in a stimulus-specific manner.
FIG. 8.
Schematic of proposed mechanisms by which BK and IL-1β regulate COX-2 gene transcription in human ASM cells. Regulatory elements are not drawn to their positions in the COX-2 promoter. Thicker lines represent major pathways.
Acknowledgments
This study was supported the National Asthma Campaign (grant 01/029).
We thank Colin Clelland for providing us with specimens of human trachea. We also thank Thomas McIntyre of the University of Utah for kindly providing the COX-2 constructs.
REFERENCES
- 1.Bannister, A. J., T. Oehler, D. Whilhelm, P. Angel, and T. Kouzarides. 1995. Stimulation of c-Jun activity by CBP:c-Jun residues Ser 63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene 11:2509-2514. [PubMed] [Google Scholar]
- 2.Beato, M. 1996. Chromatin structure and the regulation of gene expression: remodeling at the MMTV promoter. J. Mol. Med. 74:711-724. [DOI] [PubMed] [Google Scholar]
- 3.Beato, M., and K. Eisfeld. 1997. Transcription factor access to chromatin. Nucleic Acids Res. 25:3559-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belvisi, M. G., M. A. Saunders, E. B. Haddad, S. J. Hirst, M. H. Yacoub, P. J. Barnes, and J. A. Mitchell. 1997. Induction of cyclo-oxygenase-2 by cytokines in human cultured airway smooth muscle cells: novel inflammatory role of this cell type. Br. J. Pharmacol. 120:910-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belvisi, M. G., M. Saunders, M. Yacoub, and J. A. Mitchell. 1998. Expression of cyclo-oxygenase-2 in human airway smooth muscle is associated with profound reductions in cell growth. Br. J. Pharmacol. 125:1102-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonazzi, A., M. Bolla, C. Buccellati, A. Hernandez, S. Zarini, T. Vigano, F. Fumagalli, S. Viappiani, S. Ravasi, P. Zannini, G. Chiesa, G. Folco, and A. Sala. 2000. Effect of endogenous and exogenous prostaglandin E2 on interleukin-1β-induced cyclooxygenase-2 expression in human airway smooth-muscle cells. Am. J. Respir. Crit. Care Med. 162:2272-2284. [DOI] [PubMed] [Google Scholar]
- 7.Cardinaux, J.-R., J. C. Notis, Q. Zhang, N. Vo, J. C. Craig, D. M. Fass, R. G. Brennan, and R. H. Goodman. 2000. Recruitment of CREB binding protein is sufficient for CREB-mediated gene activation. Mol. Cell. Biol. 20:1546-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, C.-J., Z. Deng, A. Y. Kim, G. A. Blobel, and P. M. Lieberman. 2001. Stimulation of CREB binding protein nucleosomal histone acetyltransferase activity by a class of transcriptional activators. Mol. Cell. Biol. 21:476-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cipollone, F., C. Prontera, B. Pini, M. Marini, M. Fazia, D. De Cesare, A. Iezzi, S. Ucchino, G. Boccoli, V. Saba, F. Chiarelli, F. Cuccurullo, and A. Mezzetti. 2001. Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E2-dependent plaque instability. Circulation 104:921-927. [DOI] [PubMed] [Google Scholar]
- 10.Coffey, R. J., C. J. Hawkey, L. Damstrup, R. Graves-Deal, V. C. Daniel, P. J. Dempsey, R Chinery, S. C. Kirkland, R. N. DuBois, T. L. Jetton, and J. D. Morrow. 1997. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc. Natl. Acad. Sci. USA 94:657-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corton, J. C., S. P. Anderson, and A. Stauber. 2000. Central role of peroxisome proliferator-activated receptors in the actions of peroxisome proliferators. Annu. Rev. Pharmacol. Toxicol. 40:491-518. [DOI] [PubMed] [Google Scholar]
- 12.Delerive, P., K. De Bosscher, S. Besnard, W. V. Berghe, J. M. Peters, F. J. Gonzalez, J.-C. Fruchart, A. Tedgui, G. Haegeman, and B. Staels. 1999. Peroxisome proliferator-activated receptor α negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. J. Biol. Chem. 274:32048-32054. [DOI] [PubMed] [Google Scholar]
- 13.Dubois, R. N., S. B. Abramson, L. Crofford, R. A. Grupta, L. S. Simon, L. B. Van De Putte, and P. E. Lipsky. 1998. Cyclooxygenase in biology and disease. FASEB J. 12:1063-1073. [PubMed] [Google Scholar]
- 14.Eickelberg, O., M. Roth, R. Lörx, V. Bruce, J. Rüdiger, M. Johnson, and L.-H. Block. 1999. Ligand-independent activation of the glucocorticoid receptor by β2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J. Biol. Chem. 274:1005-1010. [DOI] [PubMed] [Google Scholar]
- 15.Elias, J. A., Y. Wu, T. Zheng, and R. Panettieri. 1997. Cytokine- and virus-stimulated airway smooth muscle cells produce IL-11 and other IL-6-type cytokines. Am. J. Physiol. Lung Cell Mol. Physiol. 273:L648-L655. [DOI] [PubMed] [Google Scholar]
- 16.Fang, H. Y., T.-S. Lin, J.-P. Lin, Y. C. Wu, K.-C. Chow, and L.-S. Wang. 2003. Cyclooxygnase-2 in human non-small cell lung cancer. Eur. J. Surg. Oncol. 29:171-177. [DOI] [PubMed] [Google Scholar]
- 17.Felinski, E. A., J. Kim, J. Lu, and P. G. Quinn. 2001. Recruitment of an RNA polymerase II complex is mediated by the constitutive activation domain in CREB, independently of CREB phosphorylation. Mol. Cell. Biol. 21:1001-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong, C. Y., L. Pang, E. Holland, and A. J. Knox. 2000. TGFβ1 stimulates PGE2 release, cyclooxygenase-2 induction and IL-8 release from human airway smooth muscle cells. Am. J. Physiol. 279:L201-L207. [DOI] [PubMed] [Google Scholar]
- 19.Goetzl, E. J., S. An, and W. L. Smith. 1995. Specificity of expression and effects of eicosanoid mediators in normal physiology and human diseases. FASEB J. 9:1051-1058. [DOI] [PubMed] [Google Scholar]
- 20.Gorgoni, B., M. Caivano, C. Arizmend, and V. Poli. 2001. The transcription factor C/EBPβ is essential for inducible expression of the cox-2 gene in macrophages but not in fibroblasts. J. Biol. Chem. 276:40769-40777. [DOI] [PubMed] [Google Scholar]
- 21.Hebbes, T. A., A. Clayton, A. Thome, and C. Crane-Robinson. 1994. Core histone hyperacetylation co-maps with generalized DNase I sensitivity in the chicken β-globin chromosomal domain. EMBO J. 13:1823-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hebbes, T. R., A. W. Thorne, and C. Crane-Robinson. 1989. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 7:1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hecht, A., and M. Grunstein. 1999. Mapping DNA interaction sites of chromosomal proteins using immunoprecipitation and polymerase chain reaction. Methods Enzymol. 304:399-414. [DOI] [PubMed] [Google Scholar]
- 24.Inoue, H., and T. Tanabe. 1998. The transcriptional role of the nuclear factor κB site in induction by lipopolysaccharide and suppression by dexamethasone of cyclooxygenase-2 in U937 cells. Biochem. Biophysiol. Res. Commun. 244:143-148. [DOI] [PubMed] [Google Scholar]
- 25.Inoue, H., K. Umesono, T. Nishimori, Y. Hirata, and T. Tanabe. 1999. Glucocorticoid-mediated suppression of the promoter activity of the cyclooxygenase-2 gene is modulated by expression of its receptor in vascular endothelial cells. Biochem. Biophysiol. Res. Commun. 254:292-298. [DOI] [PubMed] [Google Scholar]
- 26.Inoue, H., T. Nanayama, S. Hara, C. Yokoyama, and T. Tanabe. 1994. The cyclic AMP response element plays an essential role in the expression of the human prostaglandin-endoperoxide synthase 2 gene in differentiated U937 monocytic cells. FEBS Lett. 350:51-54. [DOI] [PubMed] [Google Scholar]
- 27.Ito, K., P. J. Barnes, and I. M. Adcock. 2000. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1β-induced histone H4 acetylation on lysines 8 and 12. Mol. Cell. Biol. 20:6891-6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jobin, C., O. Morteau, D. S. Han, and S. R. Balfour. 1998. Specific NF-κB blockade selectively inhibits tumor necrosis factor-α-induced COX-2 but not constitutive COX-1 gene expression in HT-29 cells. Immunology 95:537-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, Y., and S. M. Fischer. 1998. Transcriptional regulation of cyclooxygenase-2 in mouse skin carcinoma cells: regulatory role of CCAAT/enhancer-binding proteins in the differential expression of cyclooxygenase-2 in normal and neoplastic tissues. J. Biol. Chem. 273:27686-27694. [DOI] [PubMed] [Google Scholar]
- 30.Knox, A. J., L. Corbett, J. Stocks, E. Holland, Y. M. Zhu, and L. Pang. 2001. Human airway smooth muscle cells secrete vascular endothelial growth factor: up-regulation by bradykinin via a protein kinase C and prostanoid-dependent mechanism. FASEB J. 15:2480-2488. [DOI] [PubMed] [Google Scholar]
- 31.Kou, M.-H., and C. D. Allis. 1999. In vivo cross-linking and immunoprecipitation for studying dynamic protein-DNA associations in a chromatin environment. Methods 19:425-433. [DOI] [PubMed] [Google Scholar]
- 32.Lasa, M., K. R. Mathani, A. Finch, G. Brewer, J. Saklatvala, and A. R Clark. 2000. Regulation cyclooxygnase-2 mRNA stability by mitogen-activated protein kinase p38 signalling cascade. Mol. Cell. Biol. 20:4265-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKay, S., J. C. de Jongste, P. R. Saxena, and H. S. Sharma. 1998. Angiotensin II induces hypertrophy of human airway smooth muscle cells: expression of transcription factors and transforming growth factor-β1. Am. J. Respir. Cell. Mol. Biol. 18:823-833. [DOI] [PubMed] [Google Scholar]
- 34.Michael, L. F., H. Asahara, A. I. Shulman, W. L. Kraus, and M. Montminy. 2000. The phosphorylation status of a cyclic AMP-responsive activator is modulated via a chromatin-dependent mechanism. Mol. Cell. Biol. 20:1596-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mink, S., B. Haenig, and K. H. Klempnauer. 1997. Interaction and functional collaboration of p300 and C/EBPβ. Mol. Cell. Biol. 17:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mutskov, V., D. Gerber, D. Angelov, J. Ausio, J. Workman, and S. Dimitrov. 1998. Persistent interaction of core histone tails with nucleosomal DNA following acetylation and transcription factor binding. Mol. Cell. Biol. 18:6293-6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton, R., L. M. Kuitert, M. Bergmann, I. M. Adcock, and P. J. Barnes. 1997. Evidence for involvement of NF-κB in the transcriptional control of COX-2 gene expression by IL-1β. Biochem. Biophys. Res. Commun. 237:28-32. [DOI] [PubMed] [Google Scholar]
- 38.Pang, L., and A. J. Knox. 1997. Effect of interleukin-1β, tumour necrosis factor-α and interferon-γ on the induction of cyclo-oxygenase-2 in cultured human airway smooth muscle cells. Br. J. Pharmacol. 121:579-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pang, L., and A. J. Knox. 1997. PGE2 release by bradykinin in human airway smooth muscle cells: involvement of cyclooxygenase-2 induction. Am. J. Physiol. Lung Cell. Mol. Physiol. 273:L1132-L1140. [DOI] [PubMed] [Google Scholar]
- 40.Pang, L., and A. J. Knox. 1998. Bradykinin stimulates IL-8 production in cultured human airway smooth muscle cells: role of cyclooxygenase products. J. Immunol. 161:2509-2515. [PubMed] [Google Scholar]
- 41.Pang, L., and A. J. Knox. 2001. Regulation of TNF-α-induced eotaxin release from cultured human airway smooth muscle cells by β2-agonists and corticosteroids. FASEB J. 15:261-269. [DOI] [PubMed] [Google Scholar]
- 42.Pang, L., M. Nie, L. Corbett, and A. J. Knox. 2003. Cyclooxygenase-2 expression by non-steroidal anti-inflammatory drugs in human airway smooth muscle cells: role of peroxisome proliferator-activated receptors. J. Immunol. 170:1043-1051. [DOI] [PubMed] [Google Scholar]
- 43.Pang, L., E. Holland, and A. J. Knox. 1998. Impaired cAMP production in human airway smooth muscle cells by bradykinin: role of cyclooxygenase products. Am. J. Physiol. 275:L322-L329. [DOI] [PubMed] [Google Scholar]
- 44.Pang, L., E. Holland, and A. J. Knox. 1998. Role of cyclo-oxygenase-2 induction in interleukin-1β induced attenuation of cultured human airway smooth muscle cell cyclic AMP generation in response to isoprenaline. Br. J. Pharmacol. 125:1320-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pontsler, A. V., A. S. Hilaire, G. K. Marathe, G. A. Zimmerman, and T. M. McIntyre. 2002. Cyclooxygenase-2 is induced in monocytes by peroxisome proliferator activated receptor γ and oxidized alkyl phospholipids from oxidized low density lipoprotein. J. Biol. Chem. 277:13029-13036. [DOI] [PubMed] [Google Scholar]
- 46.Ricote, M., A. C. Li, T. M. Willson, C. J. Kelly, and C. K. Glass. 1998. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391:79-82. [DOI] [PubMed] [Google Scholar]
- 47.Sheppard, K.-A., D. W. Rose, Z. K. Haque, R. Kurokawa, E. McInerney, S. Westin, D. Thanos, M. G. Rosenfeld, C. K. Glass, and T. Collins. 1999. Transcriptional activation by NF-κB requires multiple coactivators. Mol. Cell. Biol. 19:6367-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staels, B., W. Koenig, A. Habib, R. Merval, M. Lebret, I. P. Torra, P. Delerive, A. Fadel, G. Chinetti, J.-C. Fruchart, J. Najib, J. Maclouf, and A. Tedgui. 1998. Activation of human aortic smooth-muscle cells is inhibited by PPARγ activators. Nature 393:790-793. [DOI] [PubMed] [Google Scholar]
- 49.Strahl, B. D., and C. D. Allis. 2000. The language of covalent histone modifications. Nature 403:41-45. [DOI] [PubMed] [Google Scholar]
- 50.Stylianou, E., M. Nie, A. Ueda, and L. Zhao. 1999. c-Rel and p65 transactivate the monocyte chemoattractant protein-1 gene in interleukin-1 stimulated mesangial cells. Kidney Int. 56:873-882. [DOI] [PubMed] [Google Scholar]
- 51.Subbaramaiah, K., D. T. Lin, J. C Hart, and A. J. Dannenberg. 2001. Peroxisome proliferator-activated receptor γ ligands suppress the transcriptional activation of cyclooxygnase-2: evidence for involvement of activator protein-1 and CREB-binding protein/p300. J. Biol. Chem. 276:12440-12448. [DOI] [PubMed] [Google Scholar]
- 52.Tazawa, R., X. M. Xu, K. K. Wu, and L. H. Wang. 1994. Characterization of genomic structure, chromosome location and promoter of human prostaglandin H synthase-2 gene. Biochem. Biophys. Res. Commun. 203:190-199. [DOI] [PubMed] [Google Scholar]
- 53.Vane, J. R., J. A. Mitchell, I. Appleton, A. Tomlinson, D. Bishop-Bailey, J. Croxtall, and D. A. Willoughby. 1994. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc. Natl. Acad. Sci. USA 91:2046-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, L. H., A. Hajibeigi, X. M. Xu, D. Loose-Mitchell, and K. K. Wu. 1993. Characterization of the promoter of human prostaglandin H synthase-1 gene. Biochem. Biophys. Res. Commun. 190:406-411. [DOI] [PubMed] [Google Scholar]
- 55.Weinmann, A. S., S. M. Bartley, T. Zhang, M. Q. Zhang, and P. J. Farnham. 2001. Use of chromatin immunoprecipitation to clone novel E2F target promoters. Mol. Cell. Biol. 21:6820-6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie, W., B. S. Fletcher, R. D. Andersen, and H. R. Herschman. 1994. v-src induction of the TIS10/PGS2 prostaglandin synthase gene is mediated by an ATF/CRE transcription response element. Mol. Cell. Biol. 14:6531-6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan, X., X. C. Wu, M. Sun, B. K. Tsang, and W. Gibb. 2000. Nuclear factor kappa B activation and regulation of cyclooxygenase type-2 expression in human amnion mesenchymal cells by interleukin-1β. Biol. Reprod. 66:1667-1671. [DOI] [PubMed] [Google Scholar]
- 58.Yokoyama, C., and T. Tanabe. 1989. Cloning of human gene encoding prostaglandin endoperoxide synthase and primary structure of the enzyme. Biochem. Biophys. Res. Commun. 165:888-894. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, W.-H., R. Srihari, R. N. Day, and F. Schaufele. 2001. CCAAT/enhancer-binding protein α alters histone H3 acetylation at large subnuclear domains. J. Biol. Chem. 276:40373-49376. [DOI] [PubMed] [Google Scholar]