Abstract
Previously it has been reported that caveolin-1 (cav-1) has antiapoptotic activities in prostate cancer cells and functions downstream of androgenic stimulation. In this study, we demonstrate that cav-1 overexpression significantly reduced thapsigargin (Tg)-stimulated apoptosis. Examination of the phosphatidylinositol 3-kinase (PI3-K)/Akt signaling cascade revealed higher activities of PDK1 and Akt but not PI3-K in cav-1-stimulated cells compared to control cells. We subsequently found that cav-1 interacts with and inhibits serine/threonine protein phosphatases PP1 and PP2A through scaffolding domain binding site interactions. Deletion of the cav-1 scaffolding domain significantly reduces phosphorylated Akt and cell viability compared with wild-type cav-1. Analysis of potential substrates for PP1 and PP2A revealed that cav-1-mediated inhibition of PP1 and PP2A leads to increased PDK1, Akt, and ERK1/2 activities. We demonstrate that increased Akt activities are largely responsible for cav-1-mediated cell survival using dominant-negative Akt mutants and specific inhibitors to MEK1/MEK and show that cav-1 increases the half-life of phosphorylated PDK1 and Akt after inhibition of PI3-K by LY294002. We further demonstrate that cav-1-stimulated Akt activities lead to increased phosphorylation of multiple Akt substrates, including GSK3, FKHR, and MDM2. In addition, overexpression of cav-1 significantly increases translocation of phosphorylated androgen receptor to nucleus. Our studies therefore reveal a novel mechanism of Akt activation in prostate cancer and potentially other malignancies.
Prostate cancer remains the second leading cause of cancer mortality among American males. The predominant reason for such high and persistent mortality is the lack of curative therapies for androgen-resistant metastatic disease. It is critical to elucidate the molecular mechanisms that underlie the ultimate androgen-resistant state of prostate cancer and to develop effective therapies for this condition.
Previously, Yang et al. reported that caveolin-1 (cav-1) levels were elevated in metastatic mouse and human prostate cancer (85). cav-1 is a major component of caveolae, flask-shaped membrane invaginations which are involved in multiple cellular processes, including the regulation and transportation of cellular cholesterol and lipids, clathrin-independent endocytosis, and signal transduction (24, 27, 60, 62, 66). The participation of cav-1 in these critical pathways involves the interaction of cav-1 with a relatively large number of molecules in either a scaffolding binding-dependent or -independent manner (41, 63). The wide spectrum of molecular interactions involving cav-1 is consistent with important, context-dependent roles for cav-1 in signal transduction, molecular transport, and other regulatory activities.
The biological functions of cav-1 in cancer are complex, multifaceted, and somewhat controversial (42, 55, 72, 73). Numerous experimental results indicate that cav-1 is a growth suppressor (14, 17, 35). Some investigators have asserted that cav-1 also has tumor suppressor activities (55). Although there is clear evidence for negative growth regulation in specific cell types, in our opinion the biological and genetic evidence for a tumor suppressor function for cav-1 is lacking at this time. However, the available data are consistent with a role for negative growth regulation in specific cell lines and lineages under specific conditions (reviewed by Mouraviev et al. [44]). Interestingly, there is also substantial evidence that cav-1 is overexpressed in metastatic cells and promotes cell survival in prostate cancer and other malignancies. Since the first report that elevated expression of cav-1 is associated with prostate and breast cancer in 1998 (85), we and others have extended this initial observation in prostate cancer (20, 76, 84, 86), and there have been numerous reports of cav-1 overexpression in aggressive stages of other malignacies, including colon cancer (16), bladder cancer (54), esophageal squamous cell cancer (26, 32), papillary carcinoma of the thyroid (28), ovarian cancers (9), myeloma (53), pancreatic ductal adenocarcinoma (67), and lung cancer (25). Overall, an impressive accumulation of data indicates that cav-1 is overexpressed in aggressive forms of specific malignancies and likely contributes to cancer progression.
Recent studies indicate that protein kinase B (PKB)/Akt activities are central to the development and maintenance of specific malignancies (reviewed in references 2, 4, 51, 71, and 80). Akt is constitutively active in many human cancers due to amplification of the Akt gene or as a result of amplification or mutations in components of the signaling pathway that regulate Akt activities (51, 80). In healthy cells, the tumor suppressor PTEN functions as a major negative regulator of the phosphatidylinositol 3-kinase (PI3-K)/Akt pathway through dephosphorylation of PI-3,4-P2 or PI-3,4,5-P3 (6, 12, 36). On the other hand, the phosphorylation state of Akt can also be controlled by serine/threonine protein phosphatases (7, 30, 43, 59, 83). PP1 and PP2A are two major classes of serine/threonine protein phosphatases that are involved in many different cellular processes, including glycogen metabolism, cell cycle regulation, protein synthesis and intracellular transport, RNA splicing, and signal transduction. Specifically, many important signal transduction molecules, including PKA, PKB/Akt, PKC, CREB, glycogen synthase kinase 3 (GSK3), Wee1, adenomatous poliposis coli protein (APC), axin, and mitogen-activated protein (MAP) kinases, are substrates of PP1 and PP2A (7, 30, 43, 83). Through dephosphorylation of these signal transduction regulators, PP1 and PP2A positively or negatively regulate multiple cellular signaling pathways. Recent discoveries of mutations of PP2A in human lung, colon, breast, and colorectal cancers and melanomas support the notion that PP2A may function as a tumor suppressor (5, 11, 57, 58, 64, 81).
To determine the mechanisms through which cav-1 promotes cancer cell survival, we examined the possible involvement of cav-1-mediated regulation of PP1 and PP2A after expression of cav-1 in low-passage LNCaP (cav-1-negative) cells and treatment with thapsigargin (Tg), an apoptosis inducer, or with LY294002, an inhibitor of the PI3-K pathway. Our studies reveal that cav-1 inhibits serine/threonine protein phosphatases PP1 and PP2A through scaffolding domain binding site interactions leading to increased phosphorylation of specific PP1/PP2A substrates. Importantly, cav-1-mediated increased Akt activities are largely responsible for enhanced cell survival of prostate cancer cells.
MATERIALS AND METHODS
Cell lines, antibodies, and reagents.
The human prostate cancer cell line LNCaP was obtained from the American Type Culture Collection, grown in RPMI 1640 medium with 10% fetal calf serum (FCS), and used at passage 30 to 50. Monoclonal antibodies against PI3-K (clone 4), PKBα/Akt (clone 55), caspase 3 (clone 19), caspase 7 (clone B94-1), PP1 catalytic subunit (PP1-C) (clone 24), PP2A catalytic subunit (PP2A-Cα) (clone 46), and androgen receptor (AR) (clone G122-77) were purchased from BD Biosciences. Monoclonal anti-β-actin (clone AC-15) and protease inhibitor cocktail were from Sigma. Rabbit polyclonal antibodies against cav-1 (N-20), PP1-C (FL-18), PKB kinase/PDK1 (H-328), and epidermal growth factor receptor (EGFR) (1005), mouse monoclonal antibodies GSK3β (0011-A) and MDM2 (SMP14), and goat polyclonal antibodies FKHRL1 (N-16), PP2A-Aα subunit (C-20) were obtained from Santa Cruz Biotechnology. Rabbit polyclonal antibodies against IKKα, IKKβ, phospho-Akt (Ser473), phospho-Akt (Thr308), phospho-IKKα (Ser180)/IKKβ (Ser181), phospho-MDM2 (Ser166), phospho-FKHR (Thr24)/FKHRL1 (Thr32), phospho-tyrosine (P-Tyr-100), monoclonal antibody against phospho-CREB (Ser133), and Akt kinase assay kit were from Cell Signaling. Purified rabbit PP1 catalytic subunit was from New England BioLabs. Anti-PI3-K p85 rabbit antiserum, rabbit polyclonal anti-PP2A-C subunit, mouse monoclonal anti-PP2A-B (PR55) subunit, purified human PP2A core enzyme, PDK1 immunoprecipitation kinase assay kit, Ser/Thr phosphatase assay kit 1, and BAD (Ser112/136) phosphorylation detection kit were from Upstate Biotechnology. Antibodies recognizing cleaved caspases 3 and 7 were purchased from Oncogene. Horseradish peroxidase (HRP)-conjugated goat anti-mouse antibodies and HRP-conjugated goat anti-rabbit antibodies were from ICN/Cappel. Thapsigargin (Tg), LY294002, U0126, and PD98059 were from Calbiochem. CellTiter 96 aqueous one solution reagent was from Promega. [γ-32P]ATP was purchased from ICN. Flexible plates for thin-layer chromatography (TLC) was obtained from Whatman. His-V5-tagged human cav-1 plasmid vector was constructed, and its protein was produced and purified as described previously (67a).
Transfection and viral infection.
The plasmid vector expressing wild-type cav-1 (pcav-1) was constructed by inserting the human cav-1 cDNA into pcDNA3.1 (75). Mutant cav-1 with the scaffolding domain deleted (cav-1Δ82-101) was generated by PCR mutagenesis using pcav-1 as a template. In the first step, intermediate PCR product A was produced using T7 promoter primer 5′CTGAGTGATATCCC3′ and primer A carrying an adjacent DNA sequence from both sides of the region deleted (5′CAGACAGCAAAAAACTGTGT3′), and intermediate PCR product B was produced using primer B which also carries a DNA sequence adjacent to both sides of the region deleted (5′TGTGTCAAAAAACGACAGAC3′) and pcDNA3.1/BGH reverse primer (5′TAGAAGGCACAGTCGAGG3′). These two intermediate PCR products which carry 20-mer overlapped DNA sequence were annealed and amplified using T7 promoter primer and pcDNA3.1/BGH reverse primer. The resulting PCR product was digested with EcoRI and inserted into pcDNA3.1 (+) to generate pcav-1Δ82-101. The full sequence was confirmed by sequencing using an automatic sequencer (ABI Prizm 310). Dominant-negative Akt1 (K179M) cDNA expression kit was from Upstate Biotechnology and dominant-negative Akt1 (T308A, S473A) and PTEN expression vectors were a kind gift from Mien-Chie Hung and Mickey Hu (University of Texas, M. D. Anderson Cancer Center) and were described previously (82). Recombinant adenoviral vectors Adcav-1 and AdRSV were generated as described previously (48, 75). Subconfluent cells were trypsinized, collected by centrifugation, and resuspended in regular medium. A single-cell suspension was then seeded at 5 × 105 cells/well (six-well plates) or 2 × 106 cells/10-cm-diameter plate. Cells were infected or transfected the next day. Typically, cells were infected with Adcav-1 or AdRSV in serum-free medium (SFM) at a multiplicity of infection (MOI) of 10 which was demonstrated previously to produce an optimal level of cav-1 for cell survival (37). The infection medium was removed and replaced with complete medium 3 h after the infection. For transfection, 2 μg of DNA was used for each transfection in the six-well plates using Promega Tfx-50 reagent at the Tfx-50/DNA ratio of 2:1 in 1 ml of SFM, and 12 μg of DNA was used to transfect cells in a 10-cm-diameter plate with 5 ml of SFM. One hour after the transfection, two milliliters of RPMI 1640 medium with 15% FCS was added to each well (six-well plate) or 5 ml of RPMI 1640 medium with 20% FCS was added to each 10-cm-diameter plate. For experiments with both infection and transfection, cells were transfected 16 h (overnight) after the viral infection.
Viability assay and apoptosis analysis.
Cells were infected or transfected in six-well plates. Forty-eight hours after infection or transfection, cells were treated with 1 μM Tg for the time indicated. At the end of the treatments, [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazdium, inner salt (MTS), assays were performed using CellTiter 96 aqueous one solution reagent from Promega by a modified version of the procedure. Briefly, 300 μl of MTS solution was added to each well and incubated for 1 h at 37°C. One milliliter of reaction or medium mix was transferred from each well to an Eppendorf tube and then centrifuged in a microcentrifuge for 5 min at 4,000 rpm (∼3,000 × g). One hundred microliters of supernatant was transferred to each well of a 96-well plate, and the relative viability was determined by the measurement of absorbance at 490 nm in a 96-well plate reader. It worth noting that in viral infection experiments a moderate level of cav-1 was generated at an MOI of 10 and the difference in cell proliferation between Adcav-1- and AdRSV-infected cells was minimal (37); therefore, the difference derived from MTS staining mainly represents cell viability. Apoptotic morphology was analyzed with phase-contrast fluorescence microscopy after incubation with 0.2 μg of 4′6′-diamidino-2-phenylindole (DAPI) per ml. The activities of caspases in the cell lysates were analyzed by Western blotting using specific antibodies recognizing procaspases or cleaved forms of caspases, followed by quantitative intensity analysis using software (GelExpert; NucleoVision) and normalized to the intensity of β-actin in the same sample.
IP and immunoblotting.
Unless specifically indicated, immunoprecipitation (IP) was performed as follows. Cells were washed with ice-cold phosphate-buffered saline (PBS), lysed on the plates by incubation for 10 min on ice with IP lysis buffer, which consists of 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1 mM β-glycerophosphate, 60 mM octylglucoside, and protease inhibitor cocktail. Cells were then scraped off the dish, transferred to microcentrifuge tubes, placed on ice for 20 min, and centrifuged for 10 min at high speed in a microcentrifuge at 4°C. The supernatant (cell lysate) was transferred to a fresh tube and frozen at −80°C until use. Typically, 500 μg of cell lysate proteins were diluted to 500 μl with IP lysis buffer and precleared by incubation with 3 μg of the corresponding immunoglobulin G (IgG) from healthy cells for 30 min at 4°C on a rocker, followed by the addition of 50 μl of IP lysis buffer-prewashed protein A or G plus agarose and incubation for another 1 h. The cleared cell lysate was incubated with 3 μg of specific antibody or the corresponding IgG overnight at 4°C on a rocker. The immunocomplex was captured by the addition of 50 μl of IP lysis buffer-prewashed protein A or G plus agarose and incubation for another 2 h. The agarose beads were washed three times with IP lysis buffer and resuspended in 40 μl of 2× sample buffer. Ten microliters of each sample containing immunocomplex was separated by electrophoresis on a sodium dodecyl sulfate (SDS)-12% polyacrylamide gel, transferred to a nitrocellulose membrane, and blotted with specific antibodies. For regular Western blot analysis, protein concentration in cell lysates was determined by the Bio-Rad protein assay, and 30 μg of protein from each sample was loaded on the gel. β-Actin served as a loading control. For quantitative analyses, the intensity of protein bands was determined by densitometry (GelExpert; NucleoVision) and normalized to the intensity of β-actin in the same sample.
Phosphatase activity assay.
Cells were lysed on the plates with phosphatase lysis buffer containing 20 mM HEPES (pH 7.4), 10% glycerol, 0.1% Nonidet P-40 (NP-40), 1 mM EGTA, 30 mM β-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 μg of leupeptin per ml, and aprotinin (29). PP1-C or PP2A-C IP complexes were prepared as described above, except that different lysis buffers and a shorter incubation (1 h) of the lysate and antibody were used. The agarose beads were washed twice with phosphatase lysis buffer and once with phosphatase assay buffer (50 mM Tri-HCl [pH 7.0], 100 mM CaCl2). Activities of PP1 and PP2A were determined by using a malachite green phosphatase assay protocol with a phosphopeptide (K-R-pT-I-R-R) as the substrate (Upstate Biotechnology), followed by the measurement of absorbance at 620 nm.
Akt activity assay.
The cells were washed with ice-cold PBS and lysed on the plates with Akt kinase lysis buffer (Cell Signaling). The Akt activity assay was performed according to the manufacturer's suggested protocol (Cell Signaling). Ten microliters of each resulting reaction product in SDS sample buffer was loaded on a SDS-12% polyacrylamide gel. Phosphorylation of GSK3 was determined by Western blotting with anti-phospho-GSK3α/β (Ser9/21) antibody.
PI3-K activity assay.
Cell lysates were prepared as described above for the Akt activity assay. PI3-K activity assays were performed using a protocol modified from the method of Bonnefoy-Berard et al. (3). Briefly, 500 μg of cell lysate proteins were immunoprecipitated with monoclonal antibody against phospho-tyrosine for 3 h at 4°C with gentle rocking, and the beads were washed twice with lysis buffer and twice with PI3-K assay buffer (20 mM Tris [pH 7.5], 100 mM NaCl, 0.5 mM EGTA, 20 mM MgCl2). PI was dissolved in chloroform (5 mg/ml) and diluted to 0.5 mg/ml with PI3-K assay buffer, followed by sonication. The washed beads were resuspended in 20 μl of PI3-K assay buffer, 20 μl of sonicated PI was added, and the reaction was initiated by adding 10 μl of ATP solution (50 μM ATP and 10 μCi of [γ-32P]ATP in PI3-K assay buffer). After 15-min incubation at room temperature in a shaking incubator, the reaction was terminated by adding 150 μl of chloroform-methanol-concentrated HCl (50:100:1), and the lipid was extracted after addition of 100 μl of chloroform. The chloroform phase was washed with 200 μl of methanol-1 N HCl (1:1). Five microliters of washed chloroform phase from each sample was loaded on a TLC plate and analyzed by ascending chromatography in chloroform-methanol-29.5% ammonium hydroxide-water (90:90:8:19), followed by autoradiography.
PDK1 activity assay.
Cell lysates were prepared as described above. PDK1 activity assay was performed according to the manufacturer's protocol (Upstate Biotechnology). Radioactivity that remained on the P81 paper was read with TopCount (Packard).
IP using purified cav-1, PP1, and PP2A proteins.
Five hundred nanograms of purified cav-1 protein was incubated with 500 ng of purified PP1 (catalytic subunit) or PP2A (core enzyme) in 100 μl of TNES lysis buffer (50 mM Tris [pH 7.5], 100 mM NaCl, 2 mM EDTA, 1% NP-40, protease inhibitor cocktail) for 1 h at 4°C on a rocker. One microgram of normal rabbit IgG or rabbit polyclonal antibodies specific for cav-1 or the PP1-C or PP2A-Cα subunit was added, and incubation was continued overnight at 4°C on a rocker. The immunocomplexes were captured by the addition of 20 μl of prewashed protein A or G plus agarose and incubation for another 2 h.
Effects of cav-1 on PP1 and PP2A activities.
PP1 (catalytic subunit) (0.02 U) or PP2A (core enzyme) (0.01 U) was incubated with or without 100 ng of purified cav-1 protein for 1 h at 4°C. Phosphatase assay buffer and a phosphopeptide (K-R-pT-I-R-R) were added to a final volume of 50 μl. Reactions were initiated by the addition of 200 μM phosphopeptide, and reaction mixtures were incubated at room temperature for 10 min. Reactions were terminated by the addition of 100 μl of malachite green solution. Activities of PP1 and PP2A were determined by the measurement of absorbance at 620 nm.
Subcellular distribution of cav-1, PP1, and PP2A in cav-1-expressing LNCaP cells.
Adcav-1-infected LNCaP cells were washed once with ice-cold PBS, scraped from plates in ice-cold homogenization buffer (10 mm HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.2 mM EGTA, 1 mM β-glycerophosphate, 0.5 mM dithiothreitol [DTT], 1 mM NaVO4, protease inhibitor cocktail), and incubated on ice for 15 min. Cells were disrupted mechanically by being passed 20 times through a 26 3/8 gauge needle. Nuclei, mitochondria, and unbroken cells were removed after centrifugation at 10,000 × g for 10 min at 4°C. The microsomal fraction was obtained after centrifugation of 10,000 × g supernatant at 100,000 × g for 1 h at 4°C. The 100,000 × g supernatant was designated the cytosolic fraction. The distributions of cav-1, PP1, and PP2A in the microsomal fraction and cytosol were examined by Western blotting using specific antibodies against cav-1, PP1-C, or PP2A-Cα. EGFR was used as a marker for the plasma membrane.
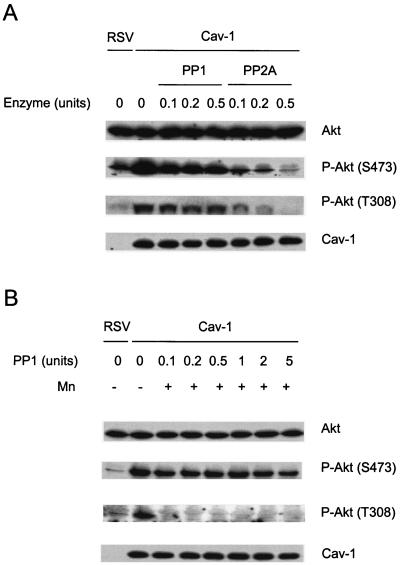
Effects of PP1 or PP2A on cav-1-mediated regulation of Akt phosphorylation.
Fifty micrograms of cav-1-infected LNCaP cell lysate proteins were incubated for 30 min at 30°C with purified PP1 or PP2A at the concentrations indicated in a protein phosphatase assay buffer (Upstate Biotechnology) or in a PP1-specific assay buffer (with 1 mM MnCl2; New England BioLabs) in a final volume of 20 μl. The reactions were terminated by adding 4 μl of 6× SDS sample buffer and heating for 5 min in a boiling water bath. The effects of PP1 or PP2A on cav-1-mediated regulation of Akt phosphorylation were analyzed by Western blotting using phospho-specific antibodies for Akt at both sites S473 and T308. Total Akt was also evaluated as a loading control.
Time course analyses for phospho-Akt and phospho-PDK1.
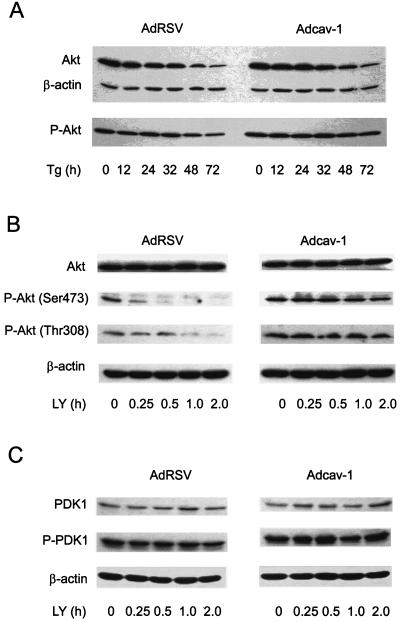
Forty-eight hours after infection, Adcav-1-infected cells or AdRSV-infected cells were treated with 1 μM Tg or 20 μM LY294002 for the times indicated. The cells were washed with ice-cold PBS and lysed on the plates with Akt kinase lysis buffer. Western blotting was performed as described above. For quantitative analyses, the intensity of interested protein bands was determined by using GelExpert software (NucleoVision) and normalized to the intensity of β-actin in the same sample. Half-life was obtained from equations generated by fitting a line to the data points.
In vivo phosphorylation of AR.
LNCaP cells were infected with Adcav-1 or AdRSV at an MOI of 10. Forty-eight hours after infection, cells were treated for 30 min with 20 μM PI3-K inhibitor LY294002 in complete RPMI 1640 medium. Cells were washed once with phosphate-free, serum-free RPMI 1640 medium and incubated in the same medium for 30 min. The cells were then incubated for 6 h in phosphate-free serum-free RPMI 1640 medium containing 5 pM or 5 nM dehydrotestosterone (DHT) and 200 μCi of 32Pi per ml. Radioactive medium was removed, and cells were washed three times with ice-cold PBS, lysed on the plate with a modified version of the ice-cold homogenization buffer of Ren et al. (56) (10 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.2 mM EGTA, 0.5 mM DTT, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM PMSF, 1 μg of leupeptin per ml), and incubated on ice for 15 min. The cells were then mechanically disrupted by being passed 20 times through a 26 3/8-gauge needle, and the lysate was centrifuged for 5 min at 800 × g. The resulting soluble fraction was saved as the cytosolic fraction, and the resulting nuclear pellet was resuspended in a modified version of the ice-cold nuclear extract buffer of Ren et al. (56) (20 mM HEPES [pH 7.9], 0.4 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.2 mM EGTA, 0.5 mM DTT, 25% glycerol, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM PMSF, 1 μg of leupeptin per ml), incubated on ice for 30 min, and centrifuged for 10 min at high speed to remove insoluble materials. The resulting nuclear extract and the cytosolic fraction were precleared by incubation with normal mouse IgG and protein A or G plus agarose for 2 h. The cleared nuclear extract and cytosolic fraction were incubated overnight at 4°C with a mouse monoclonal antibody specific for AR, followed by an incubation with protein A or G plus agarose for 2 h. The immunocomplexes were washed three times with IP lysis buffer and then resuspended in 30 μl of SDS sample buffer. After the separation on an SDS-10% polyacrylamide gel, total AR was analyzed by Western blotting with a polyclonal antibody specific for AR, and the phosphorylated AR was analyzed by autoradiography.
RESULTS
cav-1 protects cells from Tg-mediated cell death.
To extend our observations of cav-1-mediated protection against androgen ablation,c-myc overexpression, and growth or survival factor withdrawal-induced apoptosis in prostate cancer cells (37, 48, 75), we tested cav-1-mediated protection against another apoptosis inducer, Tg, which can cause calcium efflux and induce apoptosis and which to some extent mimics the calcium effects generated by androgen withdrawal. Low-passage LNCaP cells (cav-1 negative) were infected with a cav-1-expressing adenoviral vector (Adcav-1) or a control adenoviral vector carrying only the respiratory syncytial virus (RSV) promoter (AdRSV) at a MOI of 10, which had previously been demonstrated to produce an optimal level of cav-1 for cell survival (37). Forty-eight hours after infection, cells were treated with 1 μM Tg or not treated with Tg for 24, 32, or 48 h. Cell viability of Adcav-1 or AdRSV-infected cells with or without Tg treatment was evaluated by MTS staining (Fig. 1A). Forty-eight hours after Tg treatment, cav-1-infected cells maintained ∼62% viability relative to Tg-untreated cells, whereas control RSV-infected cells were ∼30% viable. The results show that the expression of cav-1 increased cell viability approximately twofold compared with control AdRSV-infected cells.
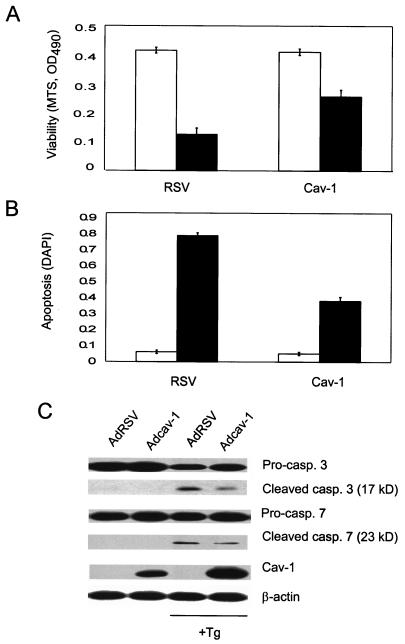
FIG. 1.
cav-1 inhibits Tg-mediated apoptosis in LNCaP prostate cancer cells. Forty-eight hours after infection with Adcav-1 or control vector AdRSV, cells were treated with 1 μM Tg for 48 h (▪) or not treated with Tg (□). (A) MTS assay. Relative viability was determined by measuring the absorbance or optical density at a wavelength of 490 nm (OD490) in an MTS staining assay. (B) Apoptosis was analyzed by DAPI staining and expressed as the ratio of apoptotic nuclei to total nuclei. (C) The activities of caspases in the cell lysates were analyzed by Western blotting using specific antibodies recognizing procaspases (Pro-casp.) (inactive) or cleaved caspases (casp.) (active).
To verify cell protection by cav-1 against Tg-induced apoptosis, we evaluated apoptosis in cav-1-expressing cells and AdRSV-infected control cells by quantitative analyses of DAPI-stained apoptotic and nonapoptotic nuclei. In addition, the activities of two major executor caspases, caspases 3 and 7, which have been widely used as markers for apoptosis, were also analyzed. Tg-induced apoptosis initiated 24 h after the treatment became obvious at 32 h and more extensive at 48 h. Apoptotic cells or nuclei were significantly reduced in cav-1-expressing cells. Figure 1B documents that 48 h after the treatment with Tg, the expression of cav-1 suppressed Tg-induced apoptosis from 76 to 37%. In untreated control cells, caspases 3 and 7 were present in procaspase forms. Forty-eight hours after Tg treatment, procaspases were reduced, and cleaved active caspases 3 and 7 were increased in the cells (Fig. 1C). As determined by the levels of cleaved active caspases, quantitative analysis demonstrated decreases of ∼40 to 50% in caspase 3 and caspase 7 activities in cav-1-expressing cells relative to RSV-infected cells. These data show that cav-1 is capable of protecting cells from Tg-induced apoptosis.
cav-1 increases PDK1 and Akt kinase activity.
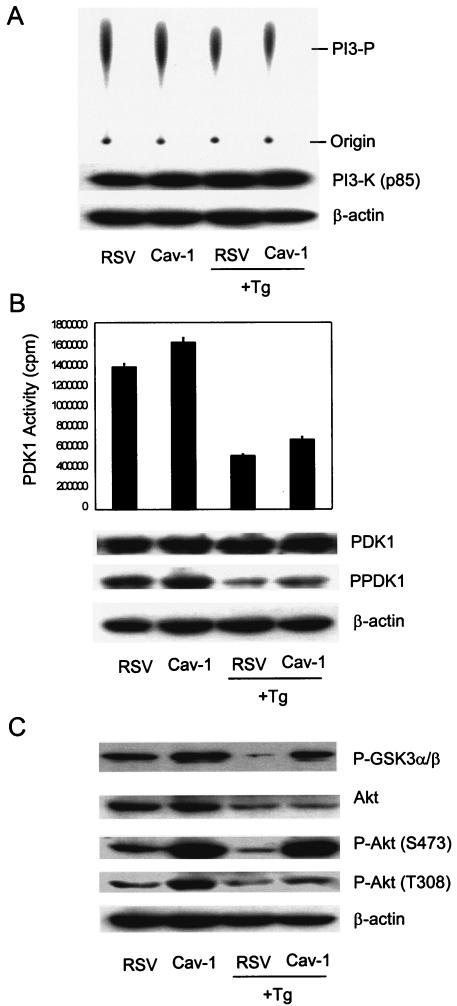
Since gene amplification and/or aberrant regulation in the PI3-K/Akt cascade is common in multiple types of cancer (reviewed in reference 51) and is also associated with androgen refractory properties of prostate cancer cells (22, 45, 46, 82), we examined both protein expression and kinase activities for major components of the PI3-K/Akt cascade. There was no difference in protein expression levels for PI3-K in cav-1-expressing cells compared to control vector-infected cells (Fig. 2A). Although PI3-K activity was reduced by Tg treatment, no difference was found between cav-1-expressing and control cells (Fig. 2A). Tg treatment also led to reduced PDK1 activity; however, compared with control RSV-infected cells, PDK1 activity was approximately 17% higher in cav-1-expressing cells and approximately 30% higher in Tg-treated cav-1-expressing cells (Fig. 2B). In the same lysates, there were no differences in PDK1 protein expression (Fig. 2B). After Tg treatment, total Akt protein levels showed significant reductions, presumably due to proapoptotic activities of Tg that can involve reduction of protein synthesis and protein degradation; however, significantly higher Akt kinase activity (determined by phosphorylation of its substrate GSK3) was observed in cav-1-expressing cells. Akt kinase activity was approximately twofold higher in cav-1-expressing cells and was about eightfold higher in Tg-treated cav-1-expressing cells compared with their corresponding control cells (Fig. 2C). Thus, the results suggest that cav-1-mediated survival activities were not a result of higher PI3-K activities but were derived from increased PDK1 and Akt kinase activities.
FIG. 2.
cav-1 increases activities of PDK1 and Akt but not PI3-K. Protein expression levels and kinase activities of PI3-K, PDK1, and Akt were determined in cav-1-expressing or vector control LNCaP cells before and after treatment with 1 μM Tg for 48 h (+Tg). (A) PI3-K kinase assay by TLC (top gel). (B) PDK1 IP kinase assay. (C) Akt IP kinase assay using recombinant GSK3 as a substrate. Total protein and phosphorylated protein (indicated by P- prefix before protein name) of each kinase were analyzed and presented in each blot. β-Actin served as a loading control.
cav-1 interacts with and inhibits PP1 and PP2A in vivo and in vitro.
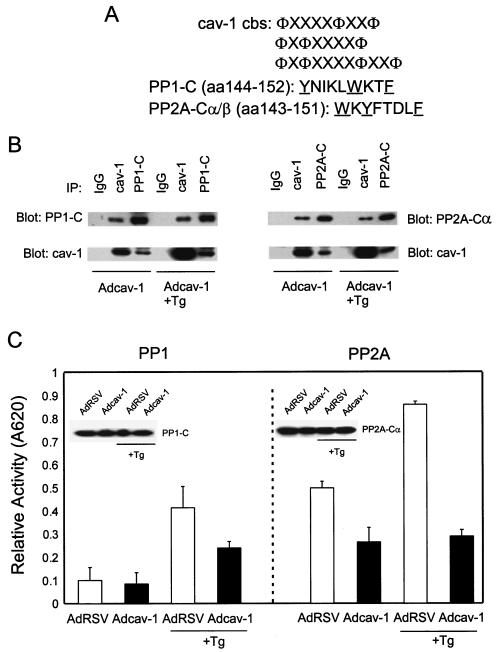
It was of interest that PDK1 and Akt are substrates for serine/threonine protein phosphatases PP1 and PP2A. PP1 and PP2A are two major classes of serine/threonine protein phosphatases that dephosphorylate a broad spectrum of protein kinases, including Akt and MAP kinase (7, 43). Interestingly, both catalytic subunits of PP1 and PP2A possess a previously described consensus caveolin binding site (Fig. 3A) (also see reference 8). IP experiments demonstrated that cav-1 is present in PP1-C or PP2A-Cα IP complexes and that PP1-C or PP2A-Cα is also found in the cav-1 IP complex (Fig. 3B), suggesting that cav-1 can bind to PP1 or PP2A. Importantly, serine/threonine protein phosphatase activity assays using PP1-C or PP2A-Cα IP complexes and a synthetic phosphopeptide substrate indicated that interaction with cav-1 leads to the inhibition of PP1 and PP2A activities (Fig. 3C). Reduced overall activities of PP1 and PP2A favor the maintenance of Akt phosphorylation and increased Akt activity. Western blots in Fig. 3C (inserts) show expression of PP1-C and PP2A-Cα in the same cell lysates.
FIG. 3.
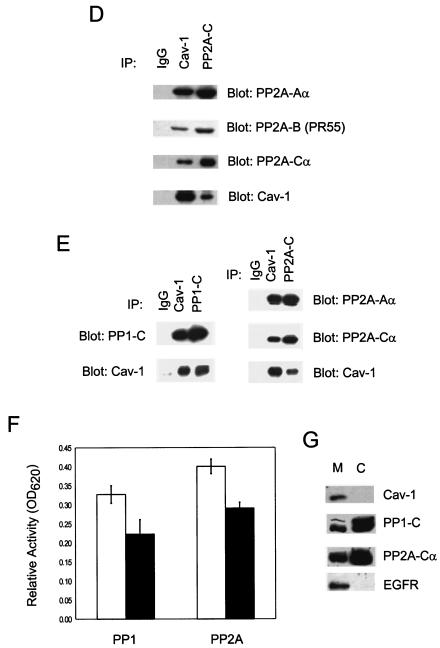
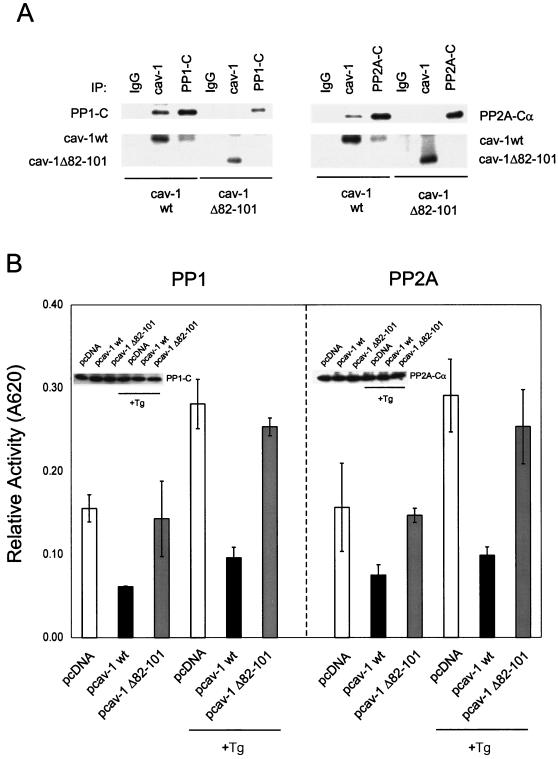
cav-1 interacts with and inhibits PP1 and PP2A. (A) cav-1 consensus binding sites (cbs) and putative cav-1 binding sites in catalytic (C) subunits of PP1 and PP2A. Aromatic residues (Φ) and any amino acid (X) in the cav-1 consensus binding sites are indicated. The aromatic residues in PP1-C and PP2A-C are underlined. aa, amino acids. (B) Analysis of IP complexes of cav-1, PP1-C, and PP2A-Cα in Adcav-1-infected LNCaP cell lysates showing that cav-1 coprecipitates with PP1-C or PP2A-Cα. (C) Serine/threonine protein phosphatase assay using PP1-C or PP2A-Cα IP complexes, indicating that cav-1 inhibits PP1 and PP2A activities. Relative activity was determined by measuring the absorbance at 620 nm (A620). The protein expression levels of PP1-C and PP2A-Cα in the same cell lysates are shown in the blot inserts. (D) Detection of two other subunits of PP2A (PP2A-Aα and PP2A-B) in cav-1 or PP2A-Cα IP complexes. (E) Analysis of cav-1 and PP1-C IP complexes after the interaction of purified cav-1 protein with the catalytic subunit of PP1 in vitro (left), and analysis of cav-1 or PP2A-Cα IP complex after the interaction of purified cav-1 protein with PP2A core enzyme in vitro (right). Note that cav-1 interacts with the C subunit of PP1 outside the context of holoenzyme and cav-1 interacts with PP2A in its core enzyme form. (F) Purified cav-1 protein inhibits PP1 and PP2A activities in vitro. Cells were treated with purified cav-1 (▪) or not treated with purified cav-1 (□). Relative activity was determined by measuring the optical density at 620 nm (OD620). (G) Distribution of cav-1, PP1, and PP2A in the microsomal fraction (M) (100,000 × g pellet) and cytosol fraction (C) (100,000 × g supernatant). EGFR was used as a marker for the plasma membrane.
Both PP1 and PP2A consist of multiple subunits and may exist in different forms. For example, PP2A can exist as free catalytic subunit (C subunit), core enzyme (A and C units), or holoenzyme (A, B, and C subunits). Although the amino acid sequences of PP1 and PP2A showed that both their catalytic subunits possess a previously described consensus caveolin-1 binding site, indicating that cav-1 may interact with C subunits of PP1 and PP2A, and coimmunoprecipitation of cav-1 and PP1-C or PP2A-Cα has been demonstrated above, the form(s) of PP1 and PP2A with which cav-1 interacts has not been fully demonstrated in an experimental model. To address this question for PP2A, we also probed for A and B subunits of PP2A using antibodies specific for the α isoform of the A subunit of PP2A (PP2A-Aα) and for the B subunit (PR55) of PP2A [PP2A-B (PR55)] in cav-1 or PP2A-Cα IP complexes. The results show that PP2A-Aα and PP2A-B (PR55) are present in cav-1 or PP2A-Cα IP complexes (Fig. 3D), implying that cav-1 may associate with PP2A holoenzyme. In our in vitro IP experiments using purified PP2A core enzyme (Fig. 3E, discussed below), we demonstrated that cav-1 can interact with PP2A core enzyme and that PP2A-Aα is present in cav-1 or PP2A-C IP complexes. Although we did not have the opportunity to test free PP2A-C directly, in our in vitro IP experiments using free PP1-C, we demonstrated that cav-1 can interact with PP1-C outside the context of holoenzyme (Fig. 3E). Overall, our data suggest that cav-1 binds directly to catalytic subunit (C subunit) of PP2A within the context of holoenzyme; however, our data do not exclude the possibility that cav-1 interacts with free catalytic subunit.
To determine whether the effects of cav-1 on PP1 and PP2A are direct and whether cav-1 interacts with and inhibits PP1 and PP2A in vitro, we performed in vitro IP experiments using purified His-V5-tagged human cav-1, PP1 (C subunit), and PP2A (core enzyme). The data presented in Fig. 3E show that cav-1 binds effectively to PP1-C or PP2A core enzyme in vitro, suggesting that the effects of cav-1 on PP1 and PP2A are direct. The results of in vitro phosphatase assays indicate that the addition of cav-1 reduced PP1 and PP2A activities by approximately 30% under these basic conditions (Fig. 3F).
To analyze the compartmentalization of cav-1-PP1 and cav-1-PP2A interactions, we determined subcellular distribution of cav-1, PP1, and PP2A. Our results reveal that cav-1 is present exclusively in the membrane, while PP1 and PP2A are present in both the cytosol and membrane (Fig. 3G). Therefore, cav-1-bound phosphatases are likely membrane bound, and the interactions of cav-1 with PP1 or PP2A most likely occur in the membrane.
cav-1 interacts with and inhibits PP1 and PP2A through scaffolding domain binding site interactions with catalytic subunits of PP1 and PP2A.
To test the interaction of cav-1 with PP1 and PP2A through a scaffold domain binding site-mediated mechanism, a vector expressing mutated cav-1 with deletion of the scaffolding domain (pcav-1Δ82-101) was constructed and used in IP experiments and serine/threonine protein phosphatase assays. The IP experiments demonstrate that wild-type cav-1 coimmunoprecipitated with PP1 or PP2A, but cav-1 Δ82-101 failed to coimmunoprecipitate with either PP1 or PP2A (Fig. 4A). The results of serine/threonine protein phosphatase assays showed that the deletion of scaffolding domain in cav-1 largely abolished the inhibition of PP1 and PP2A by cav-1 (Fig. 4B). Western blots in Fig. 4B (inserts) show expression of PP1 and PP2A in the same cell lysates. These results indicate that the scaffolding domain in cav-1 is required for its interaction with and inhibition of PP1 and PP2A.
FIG. 4.
The scaffolding domain in cav-1 is required for interaction with and inhibition of PP1 and PP2A and is required for cav-1-mediated survival activities against Tg-induced apoptosis. (A) Analysis of IP complexes of cav-1, PP1-C, and PP2A-Cα, showing that transfected wild-type cav-1 (cav-1wt) but not cav-1Δ82-101 coprecipitates with PP1-C or PP2A-Cα. (B) Deletion of the scaffolding domain from cav-1 diminished the inhibition of PP1 and PP2A by cav-1. Inserted blots indicate protein expression levels of PP1 and PP2A in the same cell lysates. Relative activity was determined by measuring the absorbance at 620 nm (A620). (C) Analysis of the phosphorylation state of Akt in wild-type cav-1- or cav-1Δ82-101-transfected LNCaP cells. P-Akt, phosphorylated Akt. (D) Viability assay for wild-type cav-1- or cav-1Δ82-101-transfected LNCaP cells after treatment with Tg for 48 h. Viability was determined in an MTS assay measuring the optical density at 490 nm (OD490). Empty vector pcDNA was used as a control.
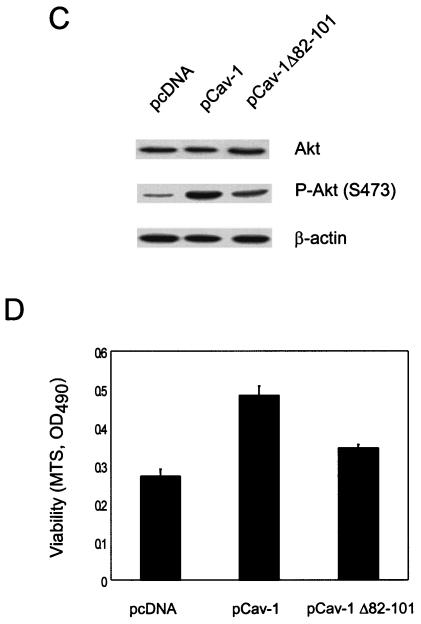
To determine whether deletion of the cav-1 scaffolding domain has an effect on Akt phosphorylation and cav-1-mediated cell protection against Tg-induced apoptosis, we performed Akt phosphorylation analysis and viability assays in LNCaP cells expressing wild-type cav-1 or cav-1Δ82-101 deletion mutant. Our results show that deletion of the cav-1 scaffolding domain significantly reduced the level of phosphorylated Akt compared with wild-type cav-1 (Fig. 4C). The results of viability assays also suggest that the scaffolding domain plays an important role in the cav-1-mediated cell protection against Tg-mediated apoptosis (Fig. 4D).
Akt activity is largely responsible for cav-1-mediated survival activities.
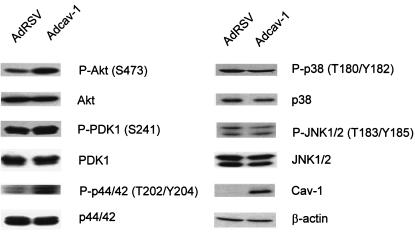
Since PP1 and PP2A have a broad range of substrates and may independently target different serine/threonine protein kinases, we examined a panel of substrate kinases that are involved in survival pathways, including PDK1, Akt, ERK1/2, p38 MAP kinase, and JNK1/2, in Tg-treated cav-1-expressing cells or vector control cells. The results showed that the expression of cav-1 resulted in higher levels of phosphorylated Akt, PDK1, and ERK1/2; a slightly lower level of phosphorylated p38 MAP kinase; and an unchanged level of phosphorylated JNK1/2 (Fig. 5). Since PDK1 and Akt are in the same pathway, the effect of cav-1 expression on the activity of p38 was minimal, and there was no effect of cav-1 expression on JNK1/2 detected, we then decided to focus on functional analysis of the Akt and ERK1/2 pathways.
FIG. 5.
cav-1 selectively increases activities of Akt, PDK1, and p42/44 MAP kinase. Phosphorylation states of a panel of PP1 and PP2A substrate kinases in Tg-treated cav-1-expressing or vector control LnCaP cells. The results showed that the expression of cav-1 resulted in increased phosphorylation of Akt, PDK1, and ERK1/2, slightly decreased phosphorylation of p38 MAP kinase, and unchanged phosphorylation of JNK1/2. P, phosphorylated protein.
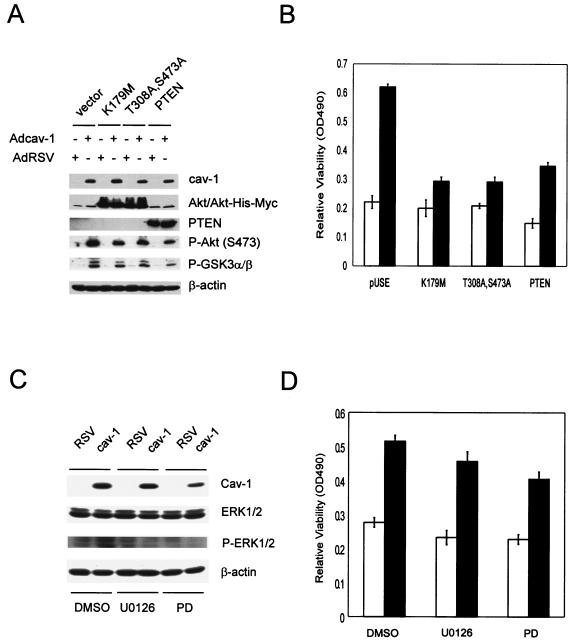
To determine the contribution of Akt pathway to cav-1-mediated cell survival, we transfected cav-1-expressing cells (Adcav-1) and control cells (AdRSV) with the following constructs: (i) a control plasmid vector; (ii) a dominant-negative Akt construct carrying a point mutation in its ATP binding pocket (DN-Akt K179M); (iii) a dominant-negative Akt construct bearing defective phosphorylation sites at both T308 and S473 (DN-Akt T308A S473A); (iv) a plasmid vector expressing wild-type PTEN. Our data showed that DN-Akt and PTEN proteins were highly expressed in transfected cells, which led to moderate reduction of endogenous active Akt (phosphorylated Akt) and Akt activity (indicated by phosphorylation of downstream target GSK3α/β) (Fig. 6A). The results of viability assays (Fig. 6B) showed that in the vector-transfected group (pUSE) the cell viability in cav-1-expressing cells was more than twofold higher in AdRSV-infected cells 48 h after treatment with Tg. Notably, this cav-1-mediated cell protection was largely eliminated in DN-Akt-transfected cells. Interestingly, although cell viability was lower in PTEN-transfected cells relative to the vector-transfected group, the pattern of cell protection by cav-1 remained. These data indicate that Akt activity is largely responsible for cav-1-mediated survival activities and that the action of cav-1 is likely downstream of PTEN.
FIG. 6.
Akt activities are largely responsible for cav-1-mediated cell survival activities. (A) Protein expression of two dominant-negative Akt mutants (K179M and T308A S473A) and wild-type PTEN in LNCaP cells and their effects on endogenous Akt and Akt activity. P, phosphorylated protein. (B) Relative viability was determined by MTS staining by measuring the optical density at 490 nm (OD490). Cells were infected with AdRSV (□) or Adcav-1 (▪) followed by transfection with control vector pUSE, DN-Akt, or PTEN and treatment with Tg. (C) MEK-specific inhibitors U0126 and PD98059 (PD) remarkably reduced phosphorylation of ERK1/2. DMSO, dimethyl sulfoxide; P-ERK1/2, phosphorylated ERK1/2. (D) U0126 and PD98059 (PD) reduced cell viability by ∼15 to 30% in both cav-1-expressing and control cells. Relative viability was determined by measuring the optical density at 490 nm (OD490).
To evaluate the contribution of ERK1/2 pathway, we treated Adcav-1- or AdRSV-infected cells with U0126, an inhibitor specific to MEK1/2 (direct upstream kinase of ERK1/2) or with PD989059, an inhibitor specific to MEK (MAP kinase kinase) (15). The data in Fig. 6C show that both U0126 and PD98059 led to reduction of phosphorylated ERK1/2. The results of the viability assay showed that cell viability was reduced by treatment with U0126 and PD98059 about 15 and 20%, respectively, but the reduction was observed in both Adcav-1- and AdRSV-infected cells (Fig. 6D). These data suggest that the ERK1/2 pathway may contribute to general cell survival activities, but its contribution to cav-1-mediated cell survival activities appears to be minimal.
PP1 and PP2A dephosphorylate Akt in vitro.
The results presented above demonstrate that cav-1 maintains phosphorylated Akt through interaction with and inhibition of serine/threonine protein phosphatases PP1 and PP2A and that elevated Akt activities are largely responsible for cav-1-mediated survival activities. However, the roles of PP1 and PP2A in the regulation of the Akt pathway in prostate cancer are poorly understood. To address this question, we incubated lysates from cav-1-expressing, LY294002-treated cells with different concentrations of purified PP1 or PP2A enzyme in a protein phosphatase assay buffer (Upstate Biotechnology) or a PP1-specific assay buffer (with 1 mM MnCl2; New England BioLabs) for 30 min at 30°C. The phosphorylation status of Akt in PP1- or PP2A-supplemented cell lysates was compared with control cell lysates from cav-1-expressing or RSV-infected cells. The results presented in Fig. 7 reveal that PP2A effectively dephosphorylates Akt at both S473 and T308 in cav-1-expressing cells in phosphatase assay buffers (without MnCl2), while PP1 is significantly less effective (Fig. 7A). Interestingly, in the presence of MnCl2, PP1 efficiently dephosphorylates Akt at T308 (Fig. 7B). Our data suggest that both PP1 and PP2A may regulate the phosphorylation status of Akt. However, PP2A appears to be predominantly Akt phosphatase, since it dephosphorylates Akt at both S473 and T308 efficiently and the activity of PP1 is limited to only T308 and is strictly dependent on Mn2+.
FIG. 7.
Dephosphorylation of Akt in cav-1-expressing, LY294002-treated LNCaP cell lysate in vitro using purified PP1 and PP2A enzymes. (A) The cell lysates were incubated with purified PP1 or PP2A at the indicated concentrations for 30 min at 30°C in a phosphatase assay buffer (without Mn2+; Upstate Biotechnology). P-Akt, phosphorylated Akt. (B) The cell lysates were incubated with purified PP1 at the indicated concentrations for 30 min at 30°C in an PP1-specific assay buffer (with 1 mM MnCl2 [+ Mn]; New England Biolabs). Note that PP2A efficiently dephosphorylates Akt at both S473 and T308, while PP1 is significantly less effective in dephosphorylation of S473, and dephosphorylation of T308 by PP1 is Mn2+ dependent. The phosphorylation state of Akt in RSV-infected LY294002-treated LNCaP cell lysate is also shown as a control.
cav-1 maintains PDK1 and Akt in a phosphorylated state.
The viability studies described above demonstrated that Akt activity was important in cav-1-mediated cell survival and that enhanced Akt activity was not derived from increased PI3-K activity but instead was most likely derived from the inhibition of serine/threonine protein phosphatases PP1 and PP2A. We then hypothesized further that cav-1 affects Akt protein stability or alters the steady state of phosphorylated Akt in Tg-treated cells. To examine this hypothesis, we performed a time course experiment for the analysis of total Akt and phospho-Akt after treating cav-1-expressing or RSV control LNCaP cells (48 h after infection) with Tg. Our experimental results demonstrated that although expression of cav-1 had only a small effect on Akt total protein degradation, it maintained phosphorylated Akt at higher levels over a period of time in Tg-treated cells (Fig. 8A). According to the data analysis by fitting a line to the data points, the half-life of phosphorylated Akt under these experimental conditions in control cell lysates is 65.2 h, whereas in cav-1-expressing cells, it is 224.7 h (Table 1).
FIG. 8.
cav-1 increases the half-lives of phosphorylated Akt (P-Akt) and PDK1 (P-PDK1) in LY294002- or Tg-treated LNCaP cells. (A) Time course analysis of P-Akt (S473) in Tg-treated cav-1-expressing (Adcav-1) and control (AdRSV) LNCaP cells. (B and C) Time course analyses of P-Akt (S473) and P-Akt (T308) and P-PDK1 (S241) in cav-1-expressing (Adcav-1) and control (AdRSV) LNCaP cells treated with PI3-K inhibitor LY294002 (LY). The half-lives of phosphorylated Akt and PDK1 are summarized in Table 1.
TABLE 1.
Half-lives of phosphorylation of PDK1 and Akt after the treatment with PI3-K inhibitor LY294002 or Tglegend
| Adenoviral vector | Half-life (h) of phosphorylated protein after treatment with:
|
|||
|---|---|---|---|---|
| 1 μM Tg | 20 μM LY294002
|
|||
| P-Akt (S473) | P-PDK1 (S241) | P-Akt (S473) | P-Akt (T308) | |
| Adcav-1 | 224.7 | 13.98 | 2.99 | 2.75 |
| AdRSV | 65.2 | 7.43 | 0.46 | 0.99 |
aThe levels of phosphorylated protein (phosphorylated Akt [P-Akt] or PDK1 [P-PDK1]) in Fig. 7 were determined by densitometry (GelExpert; NucleoTech) and normalized to the level of β-actin in the same sample. The half-life was derived from equations generated by fitting a line to the data points.
To confirm the ability of cav-1 to maintain phosphorylated Akt, we analyzed Akt phosphorylation after treating cav-1-expressing or vector control LNCaP cells (48 h after infection) with PI3-K-specific inhibitor LY294002. The results presented in Fig. 8B and Table 1 show that the half-life of phosphorylated Akt at S473 in vector control cells is 0.46 h after treatment with inhibitor, whereas the half-life in cav-1-expressing cells was 2.99 h, representing an increase in the half-life of approximately sixfold. The levels of phosphorylation of Akt at T308 were initially higher (approximately twofold) in cav-1-expressing cells relative to that in vector control cells, and its half-life was 2.75 h compared with a half-life of 0.99 h in vector control cells, indicating an approximately threefold increase in the half-life in cav-1-expressing cells.
To evaluate the contribution of upstream kinase PDK1 to the phosphorylation of Akt, the same time course experiment for phosphorylated PDK1 was performed using the same cell lysates (Fig. 8C). Initially, the levels of phosphorylation of PDK1 at S241 were slightly higher (∼17%) in cav-1-expressing cells than in vector control cells. The half-life of phosphorylation of PDK1 in cav-1-expressing cells was 13.98 h, while that in vector control cells was 7.43 h, suggesting an approximately twofold increase in the half-life in cav-1-expressing cells (Fig. 8C and Table 1). These data demonstrate that cav-1 is capable of maintaining phosphorylated Akt after apoptotic challenge or experimental inhibition of Akt phosphorylation. Higher PDK1 activity derived from both a higher initial level and a prolonged half-life of phosphorylated PDK1 may contribute in part to the increase in the half-life of Akt in cav-1-expressing cells.
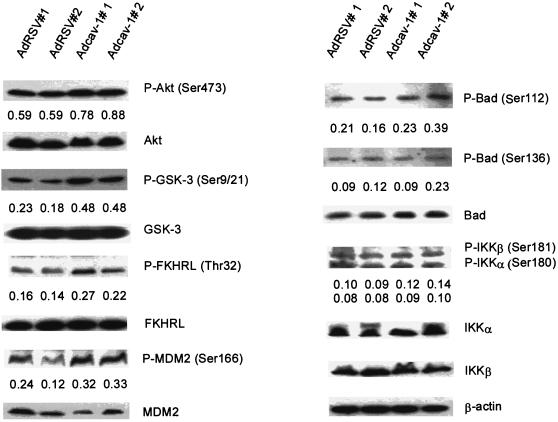
Expression of cav-1 increases phosphorylation of multiple substrates of Akt.
Since the Akt pathway is largely responsible for cav-1-mediated cell survival activities in this model system, we evaluated the phosphorylation state of a panel of Akt substrates after the expression of cav-1 and treatment with Tg. The results in Fig. 9 demonstrated that the expression of cav-1 significantly increased the phosphorylation of GSK3 (Ser9/21) (134%), FKHRL1 (Thr32) (47 to 80%), and MDM2 (Ser166) (78 to 81%) and marginally increased phosphorylation of BAD (Ser112) and IκB kinase α (IKKα) (Ser180)/IKKβ (Ser181) but did not significantly alter phosphorylation of CREB (Ser133) and AFX (Ser193) under these conditions (data not shown). These data clearly demonstrate that the expression of cav-1 leads to significantly higher levels of phosphorylation of specific Akt substrates, which in turn can promote cell survival.
FIG. 9.
The expression of cav-1 leads to increased phosphorylation of multiple Akt substrates. The phosphorylation state of Akt substrates was analyzed using Tg-treated cav-1-expressing and control cells. Data used were from two independent viral infection experiments (indicated as #1 and #2). The numbers under each gel band are the ratios of phosphorylated protein (indicated by P- prefix before the protein name) to β-actin in the same sample. The protein expression of corresponding molecules in the same cell lysates are also shown. Note significantly increased phosphorylation levels of GSK3α/β, FKHR, and MDM2 in cav-1-expressing cells. Marginally increased phosphorylation of BAD and IKKα/β were also observed.
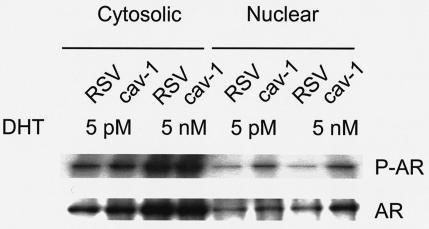
cav-1 expression leads to increased nuclear translocation of AR.
AR has been one of the major focuses in studies of normal and abnormal prostate growth (18, 49). Although the general molecular mechanism of androgen action has been established, the discovery of specific molecular pathways which lead to precise regulation of AR in either androgen-dependent or -independent manners remains an area of intensive investigation for prostate and prostate malignancies. Because of the potential link of cav-1 with AR activities (37), we examined AR phosphorylation and its nuclear translocation in cav-1-expressing LNCaP cells. The results from the in vivo phosphorylation of AR demonstrated that the expression of cav-1 in cav-1-negative LNCaP cells led to significantly increased nuclear translocation of phosphorylated AR at both ablation (5 pM) and physiological (5 nM) androgen concentrations (Fig. 10), suggesting a potential synergetic role for cav-1 in the regulation of AR nuclear translocation. These data together with the data showing increased phosphorylation of Akt substrates GSK3α/β, FKHRL1, and MDM2 strongly demonstrate that cav-1 is able to generate a broad range of cell survival activities.
FIG. 10.
The expression of cav-1 in cav-1-negative LNCaP cells leads to increased nuclear translocation of phosphorylated AR (P-AR) in vivo. Forty-eight hours after infection with Adcav-1 or AdRSV, cells were incubated for 6 h in phosphate-free serum-free RPMI 1640 medium containing 5 pM or 5 nM DHT and 200 μCi of 32Pi per ml, followed by fractionation of cytosol and nuclear fractions. The top blot shows the effect of cav-1 expression on phosphorylation of AR and nuclear translocation of P-AR in vivo by autoradiography, and the bottom blot shows Western blot analysis of total AR in the same sample.
DISCUSSION
We and others have identified cav-1 as being an important metastasis-related gene in multiple malignancies (25, 32, 37, 50, 67, 68, 76, 85, 86). Our previous work clearly indicated an association of cav-1 with antiapoptotic activities in specific relevant biological contexts, including serum deprivation (37), androgen withdrawal (48), and in response to c-myc overexpression (75). However, prior to this report an underlying molecular mechanism for cav-1-stimulated cancer cell survival had not been demonstrated. In this study we demonstrate in vivo and in vitro that cav-1 maintains the phosphorylated state of Akt through scaffolding binding site interactions with and inhibition of two major serine/threonine protein phosphatases, PP1 and PP2A.
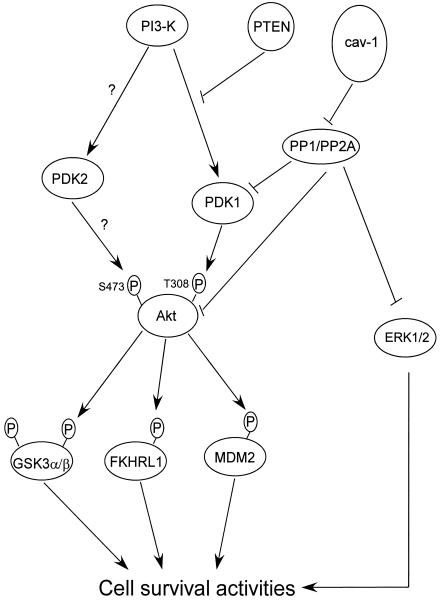
The phosphorylation and dephosphorylation of structural and regulatory proteins are major intracellular control mechanisms in eukaryotes. While protein kinases play important roles in intracellular signal transduction, the dynamics of protein phosphorylation is also controlled by protein phosphatases. During normal embryonic development and throughout adult life, intracellular signaling needs to be precisely coordinated and integrated for normal cell functions. Oncogenic perturbation can arise not only as a result of overactivation of oncogenic kinases but also as a consequence of obstruction of normal autoinhibitory and regulatory constraints (2). Our study indicates that in prostate cancer cells cav-1 plays a role as a positive regulator in the Akt signaling pathway through inhibition of negative regulators PP1 and PP2A. Both PP1 and PP2A carry a consensus cav-1 binding motif in their catalytic subunits. cav-1 binding to the catalytic subunits of PP1 and PP2A through the cav-1 scaffolding domain presumably disrupts the normal catalytic functions of the enzymes and therefore leads to inhibition of PP1 and PP2A. As a consequence, the reduced activities of PP1 and PP2A lead to higher phosphorylation levels of their specific substrates, including PDK1, Akt, and ERK1/2. Our data also demonstrate that an activated Akt pathway is mainly responsible for cav-1-mediated cell survival activities and indicate that cav-1-mediated MAP kinase pathway activation may contribute to a lesser extent to specific cav-1-stimulated cell survival. Our data further showed that by maintaining higher Akt activity, the expression of cav-1 leads to increased phosphorylation of multiple Akt downstream targets, including GSK3α/β, FKHR, and MDM2. All of these proteins affect cell survival through diverse mechanisms (1, 23, 51). Previous studies have indicated that specific substrates of GSK3α/β, including β-catenin and c-myc are overexpressed in prostate cancer (10, 21, 31, 77). Inactivation of GSK3α/β by phosphorylation would favor increased β-catenin and c-myc protein levels, since GSK3α/β-mediated phosphorylation leads to ubiquitin-mediated protein degradation in both cases. Phosphorylation of FKHR by Akt disrupts FKHR-mediated transcription of specific proapoptotic genes by promoting export of FKHR from the nucleus to the cytoplasm, resulting in its sequestration by the 14-3-3 protein (51). On the basis of our results, the activities of known FKHR target genes should be analyzed in prostate cancer tissues. Interestingly, members of the forkhead family modulate the transcriptional regulation of p27Kip1 (13, 47, 65), which has been shown to be downregulated in advanced prostate cancer (34, 40, 79, 87). Akt-mediated phosphorylation of MDM2 promotes its translocation into the nucleus where it inhibits p53 activities. Although mutations in p53 are relatively common in advanced prostate cancer (74), further studies that focus on MDM2-p53 interactions may reveal additional mechanisms for altered p53 activities in prostate cancer. Interestingly, we also show that expression of cav-1 leads to increased nuclear translocation of phosphorylated AR, a critical activity in AR action. (18, 49). Although the question of whether AR is a substrate for Akt remains controversial (19, 38, 82), our results showing increased nuclear translocation of phosphorylated AR by cav-1 provides new insight into the role of cav-1 in hormone-resistant and progressive prostate malignancies. As summarized in Fig. 11, overall, our results indicate that cav-1 overexpression contributes to molecular imbalances that favor malignant progression through its inhibition of PP1 and PP2A.
FIG. 11.
Summary of the role of cav-1 in prostate cancer cells. P, phosphate group.
In addition to this study, previous reports have linked cav-1 to the PI3-K/Akt signaling pathway. A recent report indicated that cav-1 is associated with ceramide-mediated inhibition of PI3-K activity and is able to sensitize Rat-1 fibroblast cells to ceramide-induced cell death in a sphingomyelinase-dependent manner (88). Another report showed that Akt activity was suppressed by cav-1 overexpression in a stable clone of T24 cells. Stably expressed antisense cav-1 diminished staurosporine-induced apoptosis in NIH 3T3 and T24 cells, and treatment with PI3-K inhibitor LY294002 restored the sensitivity of these cells to staurosporine (39). A recent paper reported that cav-1 activates the PI3-K/Akt pathway and sensitizes 293 cells and HeLa cells to arsenite cytotoxicity (61). Although the effects of cav-1 on the PI3-K/Akt pathway are unique and context dependent, these three reports are in line with a concept that cav-1 can play a proapoptotic role in specific model systems.
Alternatively, several other studies demonstrated that cav-1 or caveolae play an important role in PI3-K/Akt-mediated cell survival activities. In myeloma cells, inhibition of cav-1 phosphorylation or disruption of caveolae blocked interaction of cav-1 with interleukin-6 or insulin-like growth factor 1 and activation of downstream PI3-K/Akt pathway (53). Similarly, in 3T3-L1 adipocyte cells, disruption of caveolae obstructed propagation of insulin receptor signal to downstream targets (52). In vascular smooth muscle cells, disruption of caveolae inhibited tyrosine phosphorylation of the EGFR and subsequent activation of Akt by angiotensin II (78). Our studies also suggest a positive role for cav-1 in Akt signaling. However, unlike other cell models, expression of cav-1 in cav-1-negative LNCaP cells did not stimulate PI3-K activity but maintained higher activities of PDK1 and Akt, two major downstream components of PI3-K, through interaction with and inhibition of two major serine/threonine protein kinases, PP1 and PP2A. Thus, all available information points to a cell type-dependent and context-dependent function and biological consequences of cav-1 in PI3-K/Akt signaling.
It is interesting that in LNCaP cells Akt is constitutively active due to PTEN mutation. This would provide significant advantages for cell survival; however, overexpression of cav-1 imparts additional protection. Specifically, when these cells are subjected to certain types of experimental stresses, such as Tg or LY294002, which can lead to suppression of Akt activities, cav-1-mediated inhibition of PP1 and PP2A provides a unique advantage by further increasing Akt activities and establishing a greater protective barrier. We speculate that overexpression of cav-1 specifically provides prostate cancer cells selection advantages after androgen withdrawal therapy and may provide other malignant cells contextual survival advantages (reviewed by Mouraviev et al. [44]).
It is of interest to note that in our model cav-1 and PP1 or PP2A have opposing functions regarding the regulation of Akt phosphorylation. Many observations support a role for PP2A in the suppression of tumorigenesis. Several PP2A inhibitors, such as okadaic acid and calyculins, are tumor promoters, and deregulation of PP2A leads to increased cell motility and invasiveness (reviewed in reference 64). The PP2R1B gene, which encodes the β isoform of the A subunit of PP2A, is located in a region showing high-frequency loss heterozygosity(81). Mutations in the gene encoding the A subunit of PP2A have been found in human breast, lung, colon, and colorectal carcinomas and melanomas (5, 81). These mutations displayed defective or decreased binding to the C or B subunit of PP2A and therefore impair the normal functions of PP2A (57, 58, 81). In addition, expression of the PP2A subunit Bγ gene was found to be suppressed in human melanoma (11). All these findings support the notion that PP2A may function as a tumor suppressor protein.
Although the role of PP1 in tumorigenesis was not as evident as the role of PP2A, it has been reported that PP1 interacts with and dephosphorylates the retinoblastoma (Rb) tumor suppressor protein, returning Rb to its growth suppressive state (reviewed in reference 70), and that genetic alterations of PP1 were found in human cancers (33, 69). In summary, the results from our study add new information regarding the functional significance of regulation of the Akt pathway by PP1 and PP2A in prostate cancer. In LNCaP cells, PP2A effectively dephosphorylates Akt at both S473 and T308 in vitro, suggesting that it may function as a primary Akt phosphatase in vivo. In our model, PP1 is less efficient in dephosphorylation of Akt at S473, and its activity to site T308 is Mn2+ dependent. Interestingly, through its binding to and inhibition of PP1 and PP2A, cav-1 is able to maintain activated Akt, leading to increased survival following experimental induction of apoptosis. Our elucidation of opposing functional roles of cav-1 and PP1 or PP2A in regulation of the Akt pathway support the concept of PP1 and/or PP2A as a tumor suppressor protein and further support our notion that cav-1 is an important metastasis-related gene.
Acknowledgments
This work was supported in part by National Cancer Institute grants CA50588, CA68814, and SPORE P50-58204.
We thank Jianxiang Wang for construction and preparation of viral vectors AdRSV and Adcav-1, Mien-Chie Hung and Mickey Hu (University of Texas, M. D. Anderson Cancer Center) for DN-Akt (T308A, S473A) and PTEN expression vectors, and Terry L. Timme for critically reading the manuscript.
REFERENCES
- 1.Alessi, D. R., F. B. Caudwell, M. Andjelkovic, B. A. Hemmings, and P. Cohen. 1996. Molecular basis for the substrate specificity of protein kinase B: comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 399:333-338. [DOI] [PubMed] [Google Scholar]
- 2.Blume-Jensen, P., and T. Hunter. 2001. Oncogenic kinase signalling. Nature 411:355-365. [DOI] [PubMed] [Google Scholar]
- 3.Bonnefoy-Berard, N., Y. C. Liu, M. von Willebrand, A. Sung, C. Elly, T. Mustelin, H. Yoshida, K. Ishizaka, and A. Altman. 1995. Inhibition of phosphatidylinositol 3-kinase activity by association with 14-3-3 proteins in T cells. Proc. Natl. Acad. Sci. USA 92:10142-10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brazil, D. P., and B. A. Hemmings. 2001. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 26:657-664. [DOI] [PubMed] [Google Scholar]
- 5.Calin, G. A., M. G. di Iasio, E. Caprini, I. Vorechovsky, P. G. Natali, G. Sozzi, C. M. Croce, G. Barbanti-Brodano, G. Russo, and M. Negrini. 2000. Low frequency of alterations of the alpha (PPP2R1A) and beta (PPP2R1B) isoforms of the subunit A of the serine-threonine phosphatase 2A in human neoplasms. Oncogene 19:1191-1195. [DOI] [PubMed] [Google Scholar]
- 6.Cantley, L. C., and B. G. Neel. 1999. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA 96:4240-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, P. T. 2002. Protein phosphatase 1—targeted in many directions. J. Cell Sci. 115:241-256. [DOI] [PubMed] [Google Scholar]
- 8.Couet, J., S. Li, T. Okamoto, T. Ikezu, and M. P. Lisanti. 1997. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem. 272:6525-6533. [DOI] [PubMed] [Google Scholar]
- 9.Davidson, B., J. M. Nesland, I. Goldberg, J. Kopolovic, W. H. Gotlieb, M. Bryne, G. Ben-Baruch, A. Berner, and R. Reich. 2001. Caveolin-1 expression in advanced-stage ovarian carcinoma—a clinicopathologic study. Gynecol. Oncol. 81:166-171. [DOI] [PubMed] [Google Scholar]
- 10.Degeorges, A., F. Hoffschir, O. Cussenot, C. Gauville, A. Le Duc, B. Dutrillaux, and F. Calvo. 1995. Recurrent cytogenetic alterations of prostate carcinoma and amplification of c-myc or epidermal growth factor receptor in subclones of immortalized PNT1 human prostate epithelial cell line. Int. J. Cancer 62:724-731. [DOI] [PubMed] [Google Scholar]
- 11.Deichmann, M., M. Polychronidis, J. Wacker, M. Thome, and H. Naher. 2001. The protein phosphatase 2A subunit Bγ gene is identified to be differentially expressed in malignant melanomas by subtractive suppression hybridization. Melanoma Res. 11:577-585. [DOI] [PubMed] [Google Scholar]
- 12.Di Cristofano, A., and P. P. Pandolfi. 2000. The multiple roles of PTEN in tumor suppression. Cell 100:387-390. [DOI] [PubMed] [Google Scholar]
- 13.Dijkers, P. F., R. H. Medema, C. Pals, L. Banerji, N. S. Thomas, E. W. Lam, B. M. Burgering, J. A. Raaijmakers, J. W. Lammers, L. Koenderman, and P. J. Coffer. 2000. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27KIP1. Mol. Cell. Biol. 20:9138-9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelman, J. A., C. C. Wykoff, S. Yasuhara, K. S. Song, T. Okamoto, and M. P. Lisanti. 1997. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J. Biol. Chem. 272:16374-16381. [DOI] [PubMed] [Google Scholar]
- 15.Erhardt, P., E. J. Schremser, and G. M. Cooper. 1999. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol. Cell. Biol. 19:5308-5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine, S. W., M. P. Lisanti, F. Galbiati, and M. Li. 2001. Elevated expression of caveolin-1 in adenocarcinoma of the colon. Am. J. Clin. Pathol. 115:719-724. [DOI] [PubMed] [Google Scholar]
- 17.Galbiati, F., D. Volonte, J. Liu, F. Capozza, P. G. Frank, L. Zhu, R. G. Pestell, and M. P. Lisanti. 2001. Caveolin-1 expression negatively regulates cell cycle progression by inducing G0/G1 arrest via a p53/p21WAF1/Cip1-dependent mechanism. Mol. Biol. Cell 12:2229-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelmann, E. P. 2002. Molecular biology of the androgen receptor. J. Clin. Oncol. 20:3001-3015. [DOI] [PubMed] [Google Scholar]
- 19.Gioeli, D., S. B. Ficarro, J. J. Kwiek, D. Aaronson, M. Hancock, A. D. Catling, F. M. White, R. E. Christian, R. E. Settlage, J. Shabanowitz, D. F. Hunt, and M. J. Weber. 2002. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J. Biol. Chem. 277:29304-29314. [DOI] [PubMed] [Google Scholar]
- 20.Goh, M., F. Chen, M. T. Paulsen, A. M. Yeager, E. S. Dyer, and M. Ljungman. 2001. Phenylbutyrate attenuates the expression of Bcl-XL, DNA-PK, caveolin-1, and VEGF in prostate cancer cells. Neoplasia 3:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gounari, F., S. Signoretti, R. Bronson, L. Klein, W. R. Sellers, J. Kum, A. Siermann, M. M. Taketo, H. von Boehmer, and K. Khazaie. 2002. Stabilization of beta-catenin induces lesions reminiscent of prostatic intraepithelial neoplasia, but terminal squamous transdifferentiation of other secretory epithelia. Oncogene 21:4099-4107. [DOI] [PubMed] [Google Scholar]
- 22.Graff, J. R., B. W. Konicek, A. M. McNulty, Z. Wang, K. Houck, S. Allen, J. D. Paul, A. Hbaiu, R. G. Goode, G. E. Sandusky, R. L. Vessella, and B. L. Neubauer. 2000. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J. Biol. Chem. 275:24500-24505. [DOI] [PubMed] [Google Scholar]
- 23.Grimes, C. A., and R. S. Jope. 2001. The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Prog. Neurobiol. 65:391-426. [DOI] [PubMed] [Google Scholar]
- 24.Harris, J., D. Werling, J. C. Hope, G. Taylor, and C. J. Howard. 2002. Caveolae and caveolin in immune cells: distribution and functions. Trends Immunol. 23:158-164. [DOI] [PubMed] [Google Scholar]
- 25.Ho, C. C., P. H. Huang, H. Y. Huang, Y. H. Chen, P. C. Yang, and S. M. Hsu. 2002. Up-regulated caveolin-1 accentuates the metastasis capability of lung adenocarcinoma by inducing filopodia formation. Am. J. Pathol. 161:1647-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu, Y. C., K. Y. Lam, S. Law, J. Wong, and G. Srivastava. 2001. Profiling of differentially expressed cancer-related genes in esophageal squamous cell carcinoma (ESCC) using human cancer cDNA arrays: overexpression of oncogene MET correlates with tumor differentiation in ESCC. Clin Cancer Res. 7:3519-3525. [PubMed] [Google Scholar]
- 27.Ikonen, E., and R. G. Parton. 2000. Caveolins and cellular cholesterol balance. Traffic 1:212-217. [DOI] [PubMed] [Google Scholar]
- 28.Ito, Y., H. Yoshida, K. Nakano, K. Kobayashi, T. Yokozawa, K. Hirai, F. Matsuzuka, N. Matsuura, K. Kakudo, K. Kuma, and A. Miyauchi. 2002. Caveolin-1 overexpression is an early event in the progression of papillary carcinoma of the thyroid. Br. J. Cancer 86:912-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivaska, J., L. Nissinen, N. Immonen, J. E. Eriksson, V. M. Kahari, and J. Heino. 2002. Integrin α2β1 promotes activation of protein phosphatase 2A and dephosphorylation of Akt and glycogen synthase kinase 3β. Mol. Cell. Biol. 22:1352-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janssens, V., and J. Goris. 2001. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353:417-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkins, R. B., J. Qian, M. M. Lieber, and D. G. Bostwick. 1997. Detection of c-myc oncogene amplification and chromosomal anomalies in metastatic prostatic carcinoma by fluorescence in situ hybridization. Cancer Res. 57:524-531. [PubMed] [Google Scholar]
- 32.Kato, K., Y. Hida, M. Miyamoto, H. Hashida, T. Shinohara, T. Itoh, S. Okushiba, S. Kondo, and H. Katoh. 2002. Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer 94:929-933. [PubMed] [Google Scholar]
- 33.Kohno, T., S. Takakura, T. Yamada, A. Okamoto, T. Tanaka, and J. Yokota. 1999. Alterations of the PPP1R3 gene in human cancer. Cancer Res. 59:4170-4174. [PubMed] [Google Scholar]
- 34.Kuczyk, M. A., C. Bokemeyer, J. Hartmann, J. Schubach, C. Walter, S. Machtens, R. Knuchel, C. Kollmannsberger, U. Jonas, and J. Serth. 2001. Predictive value of altered p27Kip1 and p21WAF/Cip1 protein expression for the clinical prognosis of patients with localized prostate cancer. Oncol. Rep. 8:1401-1407. [DOI] [PubMed] [Google Scholar]
- 35.Lee, S. W., C. L. Reimer, P. Oh, D. B. Campbell, and J. E. Schnitzer. 1998. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene 16:1391-1397. [DOI] [PubMed] [Google Scholar]
- 36.Leslie, N. R., and C. P. Downes. 2002. PTEN: the down side of PI 3-kinase signalling. Cell. Signal. 14:285-295. [DOI] [PubMed] [Google Scholar]
- 37.Li, L., G. Yang, S. Ebara, T. Satoh, Y. Nasu, T. L. Timme, C. Ren, J. Wang, S. A. Tahir, and T. C. Thompson. 2001. Caveolin-1 mediates testosterone-stimulated survival/clonal growth and promotes metastatic activities in prostate cancer cells. Cancer Res. 61:4386-4392. [PubMed] [Google Scholar]
- 38.Lin, H. K., S. Yeh, H. Y. Kang, and C. Chang. 2001. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc. Natl. Acad. Sci. USA 98:7200-7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu, J., P. Lee, F. Galbiati, R. N. Kitsis, and M. P. Lisanti. 2001. Caveolin-1 expression sensitizes fibroblastic and epithelial cells to apoptotic stimulation. Am. J. Physiol. Cell Physiol. 280:C823-C835. [DOI] [PubMed] [Google Scholar]
- 40.Loda, M. 2000. p27KIP1: androgen regulation and prognostic significance in prostate cancer. Adv. Clin. Pathol. 4:226-232. [PubMed] [Google Scholar]
- 41.Lu, M. L., M. C. Schneider, Y. Zheng, X. Zhang, and J. P. Richie. 2001. Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. J. Biol. Chem. 276:13442-13451. [DOI] [PubMed] [Google Scholar]
- 42.Massimino, M. L., C. Griffoni, E. Spisni, M. Toni, and V. Tomasi. 2002. Involvement of caveolae and caveolae-like domains in signalling, cell survival and angiogenesis. Cell. Signal. 14:93-98. [DOI] [PubMed] [Google Scholar]
- 43.Millward, T. A., S. Zolnierowicz, and B. A. Hemmings. 1999. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 24:186-191. [DOI] [PubMed] [Google Scholar]
- 44.Mouraviev, V., L. Li, S. A. Tahir, G. Yang, T. M. Timme, A. Goltsov, C. Ren, T. Satoh, T. M. Wheeler, M. M. Ittmann, B. J. Miles, R. J. Amato, D. Kadmon, and T. C. Thompson. 2002. The role of caveolin-1 in androgen insensitive prostate cancer. J. Urol. 168:1589-1596. [DOI] [PubMed] [Google Scholar]
- 45.Mousses, S., U. Wagner, Y. Chen, J. W. Kim, L. Bubendorf, M. Bittner, T. Pretlow, A. G. Elkahloun, J. B. Trepel, and O. P. Kallioniemi. 2001. Failure of hormone therapy in prostate cancer involves systematic restoration of androgen responsive genes and activation of rapamycin sensitive signaling. Oncogene 20:6718-6723. [DOI] [PubMed] [Google Scholar]
- 46.Murillo, H., H. Huang, L. J. Schmidt, D. I. Smith, and D. J. Tindall. 2001. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology 142:4795-4805. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura, N., S. Ramaswamy, F. Vazquez, S. Signoretti, M. Loda, and W. R. Sellers. 2000. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol. Cell. Biol. 20:8969-8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nasu, Y., T. L. Timme, G. Yang, C. H. Bangma, L. Li, C. Ren, S. H. Park, M. DeLeon, J. Wang, and T. C. Thompson. 1998. Suppression of caveolin expression induces androgen sensitivity in metastatic androgen-insensitive mouse prostate cancer cells. Nat. Med. 4:1062-1064. [DOI] [PubMed] [Google Scholar]
- 49.Navarro, D., O. P. Luzardo, L. Fernandez, N. Chesa, and B. N. Diaz-Chico. 2002. Transition to androgen-independence in prostate cancer. J. Steroid Biochem. Mol. Biol. 81:191-201. [DOI] [PubMed] [Google Scholar]
- 50.Nestl, A., O. D. Von Stein, K. Zatloukal, W. G. Thies, P. Herrlich, M. Hofmann, and J. P. Sleeman. 2001. Gene expression patterns associated with the metastatic phenotype in rodent and human tumors. Cancer Res. 61:1569-1577. [PubMed] [Google Scholar]
- 51.Nicholson, K. M., and N. G. Anderson. 2002. The protein kinase B/Akt signalling pathway in human malignancy. Cell. Signal. 14:381-395. [DOI] [PubMed] [Google Scholar]
- 52.Parpal, S., M. Karlsson, H. Thorn, and P. Stralfors. 2001. Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J. Biol. Chem. 276:9670-9678. [DOI] [PubMed] [Google Scholar]
- 53.Podar, K., Y. T. Tai, C. E. Cole, T. Hideshima, M. Sattler, A. Hamblin, N. Mitsiades, R. L. Schlossman, F. E. Davies, G. J. Morgan, N. C. Munshi, D. Chauhan, and K. C. Anderson. 2003. Essential role of caveolae in interleukin-6- and insulin-like growth factor I-triggered Akt-1-mediated survival of multiple myeloma cells. J. Biol. Chem. 278:5794-5801. [DOI] [PubMed] [Google Scholar]
- 54.Rajjayabun, P. H., S. Garg, G. C. Durkan, R. Charlton, M. C. Robinson, and J. K. Mellon. 2001. Caveolin-1 expression is associated with high-grade bladder cancer. Urology 58:811-814. [DOI] [PubMed] [Google Scholar]
- 55.Razani, B., A. Schlegel, J. Liu, and M. P. Lisanti. 2001. Caveolin-1, a putative tumour suppressor gene. Biochem. Soc. Trans. 29:494-499. [DOI] [PubMed] [Google Scholar]
- 56.Ren, C., L. Li, A. A. Goltsov, T. L. Timme, S. A. Tahir, J. Wang, L. Garza, A. C. Chinault, and T. C. Thompson. 2002. mRTVP-1, a novel p53 target gene with proapoptotic activities. Mol. Cell. Biol. 22:3345-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruediger, R., H. T. Pham, and G. Walter. 2001. Alterations in protein phosphatase 2A subunit interaction in human carcinomas of the lung and colon with mutations in the Aβ subunit gene. Oncogene 20:1892-1899. [DOI] [PubMed] [Google Scholar]
- 58.Ruediger, R., H. T. Pham, and G. Walter. 2001. Disruption of protein phosphatase 2A subunit interaction in human cancers with mutations in the Aα subunit gene. Oncogene 20:10-15. [DOI] [PubMed] [Google Scholar]
- 59.Schonthal, A. H. 2001. Role of serine/threonine protein phosphatase 2A in cancer. Cancer Lett. 170:1-13. [DOI] [PubMed] [Google Scholar]
- 60.Schroeder, F., A. M. Gallegos, B. P. Atshaves, S. M. Storey, A. L. McIntosh, A. D. Petrescu, H. Huang, O. Starodub, H. Chao, H. Yang, A. Frolov, and A. B. Kier. 2001. Recent advances in membrane microdomains: rafts, caveolae, and intracellular cholesterol trafficking. Exp. Biol. Med. (Maywood) 226:873-890. [DOI] [PubMed] [Google Scholar]
- 61.Shack, S., X. T. Wang, G. C. Kokkonen, M. Gorospe, D. L. Longo, and N. J. Holbrook. 2003. Caveolin-induced activation of the phosphatidylinositol 3-kinase/Akt pathway increases arsenite cytotoxicity. Mol. Cell. Biol. 23:2407-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaul, P. W., and R. G. Anderson. 1998. Role of plasmalemmal caveolae in signal transduction. Am. J. Physiol. 275:L843-L851. [DOI] [PubMed] [Google Scholar]
- 63.Smart, E. J., G. A. Graf, M. A. McNiven, W. C. Sessa, J. A. Engelman, P. E. Scherer, T. Okamoto, and M. P. Lisanti. 1999. Caveolins, liquid-ordered domains, and signal transduction. Mol. Cell. Biol. 19:7289-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sontag, E. 2001. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell. Signal. 13:7-16. [DOI] [PubMed] [Google Scholar]
- 65.Stahl, M., P. F. Dijkers, G. J. Kops, S. M. Lens, P. J. Coffer, B. M. Burgering, and R. H. Medema. 2002. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J. Immunol. 168:5024-5031. [DOI] [PubMed] [Google Scholar]
- 66.Sternberg, P. W., and S. L. Schmid. 1999. Caveolin, cholesterol and Ras signalling. Nat. Cell Biol. 1:E35-E37. [DOI] [PubMed] [Google Scholar]
- 67.Suzuoki, M., M. Miyamoto, K. Kato, K. Hiraoka, T. Oshikiri, Y. Nakakubo, A. Fukunaga, T. Shichinohe, T. Shinohara, T. Itoh, S. Kondo, and H. Katoh. 2002. Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br. J. Cancer 87:1140-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67a.Tahir, S. A., C. Ren, T. L. Timme, Y. Gdor, R. Hoogeveen, J. D. Morrisett, A. Frolov, G. Ayala, T. M. Wheeler, and T. C. Thompson. 2003. Development of an immunoassay for serum caveolin-1: a novel biomarker for prostate cancer. Clin Cancer Res. 9:3653-3659. [PubMed] [Google Scholar]
- 68.Tahir, S. A., G. Yang, S. Ebara, T. L. Timme, T. Satoh, L. Li, A. Goltsov, M. Ittmann, J. D. Morrisett, and T. C. Thompson. 2001. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res. 61:3882-3885. [PubMed] [Google Scholar]
- 69.Takakura, S., T. Kohno, R. Manda, A. Okamoto, T. Tanaka, and J. Yokota. 2001. Genetic alterations and expression of the protein phosphatase 1 genes in human cancers. Int. J. Oncol. 18:817-824. [DOI] [PubMed] [Google Scholar]
- 70.Tamrakar, S., E. Rubin, and J. W. Ludlow. 2000. Role of pRB dephosphorylation in cell cycle regulation. Front. Biosci. 5:D121-D137. [DOI] [PubMed] [Google Scholar]
- 71.Testa, J. R., and A. Bellacosa. 2001. AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. USA 98:10983-10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson, T. C., T. L. Timme, L. Li, and A. Goltsov. 1999. Caveolin-1—a metastasis-related gene that promotes cell survival in prostate cancer. Apoptosis 4:233-237. [DOI] [PubMed] [Google Scholar]
- 73.Thompson, T. C., T. L. Timme, L. Li, C. Ren, A. Goltsov, S. Tahir, and G. Yang. 2001. Molecular pathways that underlie prostate cancer progression: the role of caveolin-1. In L. Chung (ed.), Prostate cancer in the 21st century. Humana Press, Totowa, N.J.
- 74.Thompson, T. C., T. L. Timme, and I. Sehgal. 1998. Oncogenes, growth factors, and hormones in prostate cancer, p. 327-359. In R. B. Dickson and D. S. Salomon (ed.), Hormones and growth factors in development and neoplasia. Wiley-Liss, Inc., New York, N.Y.
- 75.Timme, T. L., A. Goltsov, S. Tahir, L. Li, J. Wang, C. Ren, R. N. Johnston, and T. C. Thompson. 2000. Caveolin-1 is regulated by c-myc and suppresses c-myc-induced apoptosis. Oncogene 19:3256-3265. [DOI] [PubMed] [Google Scholar]
- 76.Tso, C. L., W. H. McBride, J. Sun, B. Patel, K. H. Tsui, S. H. Paik, B. Gitlitz, R. Caliliw, A. van Ophoven, L. Wu, J. deKernion, and A. Belldegrun. 2000. Androgen deprivation induces selective outgrowth of aggressive hormone-refractory prostate cancer clones expressing distinct cellular and molecular properties not present in parental androgen-dependent cancer cells. Cancer J. 6:220-233. [PubMed] [Google Scholar]
- 77.Tsuchiya, N., Y. Kondo, A. Takahashi, H. Pawar, J. Qian, K. Sato, M. M. Lieber, and R. B. Jenkins. 2002. Mapping and gene expression profile of the minimally overrepresented 8q24 region in prostate cancer. Am. J. Pathol. 160:1799-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ushio-Fukai, M., L. Hilenski, N. Santanam, P. L. Becker, Y. Ma, K. K. Griendling, and R. W. Alexander. 2001. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J. Biol. Chem. 276:48269-48275. [DOI] [PubMed] [Google Scholar]
- 79.Vis, A. N., B. W. van Rhijn, M. A. Noordzij, F. H. Schroder, and T. H. van der Kwast. 2002. Value of tissue markers p27kip1, MIB-1, and CD44s for the pre-operative prediction of tumour features in screen-detected prostate cancer. J. Pathol. 197:148-154. [DOI] [PubMed] [Google Scholar]
- 80.Vivanco, I., and C. L. Sawyers. 2002. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer 2:489-501. [DOI] [PubMed] [Google Scholar]
- 81.Wang, S. S., E. D. Esplin, J. L. Li, L. Huang, A. Gazdar, J. Minna, and G. A. Evans. 1998. Alterations of the PPP2R1B gene in human lung and colon cancer. Science 282:284-287. [DOI] [PubMed] [Google Scholar]
- 82.Wen, Y., M. C. Hu, K. Makino, B. Spohn, G. Bartholomeusz, D. H. Yan, and M. C. Hung. 2000. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 60:6841-6845. [PubMed] [Google Scholar]
- 83.Wera, S., and B. A. Hemmings. 1995. Serine/threonine protein phosphatases. Biochem. J. 311:17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu, D., T. L. Foreman, C. W. Gregory, M. A. McJilton, G. G. Wescott, O. H. Ford, R. F. Alvey, J. L. Mohler, and D. M. Terrian. 2002. Protein kinase Cɛ has the potential to advance the recurrence of human prostate cancer. Cancer Res. 62:2423-2429. [PubMed] [Google Scholar]
- 85.Yang, G., L. D. Truong, T. L. Timme, C. Ren, T. M. Wheeler, S. H. Park, Y. Nasu, C. H. Bangma, M. W. Kattan, P. T. Scardino, and T. C. Thompson. 1998. Elevated expression of caveolin is associated with prostate and breast cancer. Clin. Cancer Res. 4:1873-1880. [PubMed] [Google Scholar]
- 86.Yang, G., L. D. Truong, T. M. Wheeler, and T. C. Thompson. 1999. Caveolin-1 expression in clinically confined human prostate cancer: a novel prognostic marker. Cancer Res. 59:5719-5723. [PubMed] [Google Scholar]
- 87.Yang, R. M., J. Naitoh, M. Murphy, H. J. Wang, J. Phillipson, J. B. deKernion, M. Loda, and R. E. Reiter. 1998. Low p27 expression predicts poor disease-free survival in patients with prostate cancer. J. Urol. 159:941-945. [PubMed] [Google Scholar]
- 88.Zundel, W., L. M. Swiersz, and A. Giaccia. 2000. Caveolin 1-mediated regulation of receptor tyrosine kinase-associated phosphatidylinositol 3-kinase activity by ceramide. Mol. Cell. Biol. 20:1507-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]