Abstract
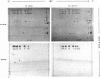
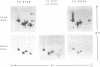
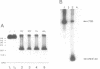
The U1 snRNP-specific 70K protein is one of the few snRNP proteins from higher eukaryotic cells that is phosphorylated in vivo (1,2). Immunoaffinity purified spliceosomal snRNPs (U1, U2, U5, and U4/U6) were tested for their ability to phosphorylate in vitro the U1-specific 70K protein. An snRNP-associated kinase activity which phosphorylates all U1-70K isoelectric variants was identified. Like its in vivo counterpart, this snRNP-associated enzyme phosphorylates solely serine residues of the 70K protein, preferentially utilizing ATP as a phosphodonor. Tryptic phosphopeptide analysis revealed an overlapping set of at least four radiolabeled peptides in the in vivo and in vitro phosphorylated protein, suggesting that the snRNP-associated serine kinase is responsible, at least in part, for the 70K protein phosphorylation observed in vivo. Chymotryptic digestion of in vitro, 32P-labeled 70K protein and in vitro phosphorylation studies with a synthetic peptide, indicated that the multiple 70K phosphorylation sites are limited to a highly charged, C-terminal domain of the protein. In vitro phosphorylation studies with the splicing factor ASF/SF2 and several deletion mutants demonstrated that, similar to the U1-70K protein, the snRNP-associated serine kinase phosphorylates the carboxy terminal RS-rich domain of ASF/SF2. A potential general role for this enzyme in the phosphorylation of splicing factors and its consequences for pre-mRNA splicing regulation are discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M., Bringmann P., Lührmann R. Purification of small nuclear ribonucleoprotein particles with antibodies against modified nucleosides of small nuclear RNAs. Methods Enzymol. 1990;181:232–257. doi: 10.1016/0076-6879(90)81125-e. [DOI] [PubMed] [Google Scholar]
- Bingham P. M., Chou T. B., Mims I., Zachar Z. On/off regulation of gene expression at the level of splicing. Trends Genet. 1988 May;4(5):134–138. doi: 10.1016/0168-9525(88)90136-9. [DOI] [PubMed] [Google Scholar]
- Bister K., Trachmann C., Jansen H. W., Schroeer B., Patschinsky T. Structure of mutant and wild-type MC29 v-myc alleles and biochemical properties of their protein products. Oncogene. 1987 May;1(2):97–109. [PubMed] [Google Scholar]
- Bringmann P., Rinke J., Appel B., Reuter R., Lührmann R. Purification of snRNPs U1, U2, U4, U5 and U6 with 2,2,7-trimethylguanosine-specific antibody and definition of their constituent proteins reacting with anti-Sm and anti-(U1)RNP antisera. EMBO J. 1983;2(7):1129–1135. doi: 10.1002/j.1460-2075.1983.tb01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caizergues-Ferrer M., Belenguer P., Lapeyre B., Amalric F., Wallace M. O., Olson M. O. Phosphorylation of nucleolin by a nucleolar type NII protein kinase. Biochemistry. 1987 Dec 1;26(24):7876–7883. doi: 10.1021/bi00398a051. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. D., Maniatis T. Isolation of a complementary DNA that encodes the mammalian splicing factor SC35. Science. 1992 Apr 24;256(5056):535–538. doi: 10.1126/science.1373910. [DOI] [PubMed] [Google Scholar]
- Ge H., Manley J. L. A protein factor, ASF, controls cell-specific alternative splicing of SV40 early pre-mRNA in vitro. Cell. 1990 Jul 13;62(1):25–34. doi: 10.1016/0092-8674(90)90236-8. [DOI] [PubMed] [Google Scholar]
- Ge H., Zuo P., Manley J. L. Primary structure of the human splicing factor ASF reveals similarities with Drosophila regulators. Cell. 1991 Jul 26;66(2):373–382. doi: 10.1016/0092-8674(91)90626-a. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Conway G. C., Kozak D. Purification and characterization of pre-mRNA splicing factor SF2 from HeLa cells. Genes Dev. 1990 Jul;4(7):1158–1171. doi: 10.1101/gad.4.7.1158. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Mayeda A., Kozak D., Binns G. Functional expression of cloned human splicing factor SF2: homology to RNA-binding proteins, U1 70K, and Drosophila splicing regulators. Cell. 1991 Jul 26;66(2):383–394. doi: 10.1016/0092-8674(91)90627-b. [DOI] [PubMed] [Google Scholar]
- Kuo H. C., Nasim F. H., Grabowski P. J. Control of alternative splicing by the differential binding of U1 small nuclear ribonucleoprotein particle. Science. 1991 Mar 1;251(4997):1045–1050. doi: 10.1126/science.1825520. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehmeier T., Foulaki K., Lührmann R. Evidence for three distinct D proteins, which react differentially with anti-Sm autoantibodies, in the cores of the major snRNPs U1, U2, U4/U6 and U5. Nucleic Acids Res. 1990 Nov 25;18(22):6475–6484. doi: 10.1093/nar/18.22.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lührmann R., Kastner B., Bach M. Structure of spliceosomal snRNPs and their role in pre-mRNA splicing. Biochim Biophys Acta. 1990 Nov 30;1087(3):265–292. doi: 10.1016/0167-4781(90)90001-i. [DOI] [PubMed] [Google Scholar]
- Mattox W., Ryner L., Baker B. S. Autoregulation and multifunctionality among trans-acting factors that regulate alternative pre-mRNA processing. J Biol Chem. 1992 Sep 25;267(27):19023–19026. [PubMed] [Google Scholar]
- Mayeda A., Zahler A. M., Krainer A. R., Roth M. B. Two members of a conserved family of nuclear phosphoproteins are involved in pre-mRNA splicing. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1301–1304. doi: 10.1073/pnas.89.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermoud J. E., Cohen P., Lamond A. I. Ser/Thr-specific protein phosphatases are required for both catalytic steps of pre-mRNA splicing. Nucleic Acids Res. 1992 Oct 25;20(20):5263–5269. doi: 10.1093/nar/20.20.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Query C. C., Keene J. D. A human autoimmune protein associated with U1 RNA contains a region of homology that is cross-reactive with retroviral p30gag antigen. Cell. 1987 Oct 23;51(2):211–220. doi: 10.1016/0092-8674(87)90148-6. [DOI] [PubMed] [Google Scholar]
- Roth M. B., Murphy C., Gall J. G. A monoclonal antibody that recognizes a phosphorylated epitope stains lampbrush chromosome loops and small granules in the amphibian germinal vesicle. J Cell Biol. 1990 Dec;111(6 Pt 1):2217–2223. doi: 10.1083/jcb.111.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. B., Zahler A. M., Stolk J. A. A conserved family of nuclear phosphoproteins localized to sites of polymerase II transcription. J Cell Biol. 1991 Nov;115(3):587–596. doi: 10.1083/jcb.115.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritz R. A., Strunk K., Surowy C. S., Hoch S. O., Barton D. E., Francke U. The human U1-70K snRNP protein: cDNA cloning, chromosomal localization, expression, alternative splicing and RNA-binding. Nucleic Acids Res. 1987 Dec 23;15(24):10373–10391. doi: 10.1093/nar/15.24.10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter V., Kahrs A., Fischer U., Kornstädt U., Lührmann R. In vitro reconstitution of U1 and U2 snRNPs from isolated proteins and snRNA. Mol Biol Rep. 1992 Sep;16(4):229–240. doi: 10.1007/BF00419662. [DOI] [PubMed] [Google Scholar]
- Tazi J., Daugeron M. C., Cathala G., Brunel C., Jeanteur P. Adenosine phosphorothioates (ATP alpha S and ATP tau S) differentially affect the two steps of mammalian pre-mRNA splicing. J Biol Chem. 1992 Mar 5;267(7):4322–4326. [PubMed] [Google Scholar]
- Theissen H., Etzerodt M., Reuter R., Schneider C., Lottspeich F., Argos P., Lührmann R., Philipson L. Cloning of the human cDNA for the U1 RNA-associated 70K protein. EMBO J. 1986 Dec 1;5(12):3209–3217. doi: 10.1002/j.1460-2075.1986.tb04631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley J. C., Cone R. D., Tartof D., Chung S. Y. Small nuclear ribonucleoprotein complexes of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6762–6766. doi: 10.1073/pnas.79.22.6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woppmann A., Patschinsky T., Bringmann P., Godt F., Lührmann R. Characterisation of human and murine snRNP proteins by two-dimensional gel electrophoresis and phosphopeptide analysis of U1-specific 70K protein variants. Nucleic Acids Res. 1990 Aug 11;18(15):4427–4438. doi: 10.1093/nar/18.15.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore P. D., Patton J. G., Green M. R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992 Feb 13;355(6361):609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]