Abstract
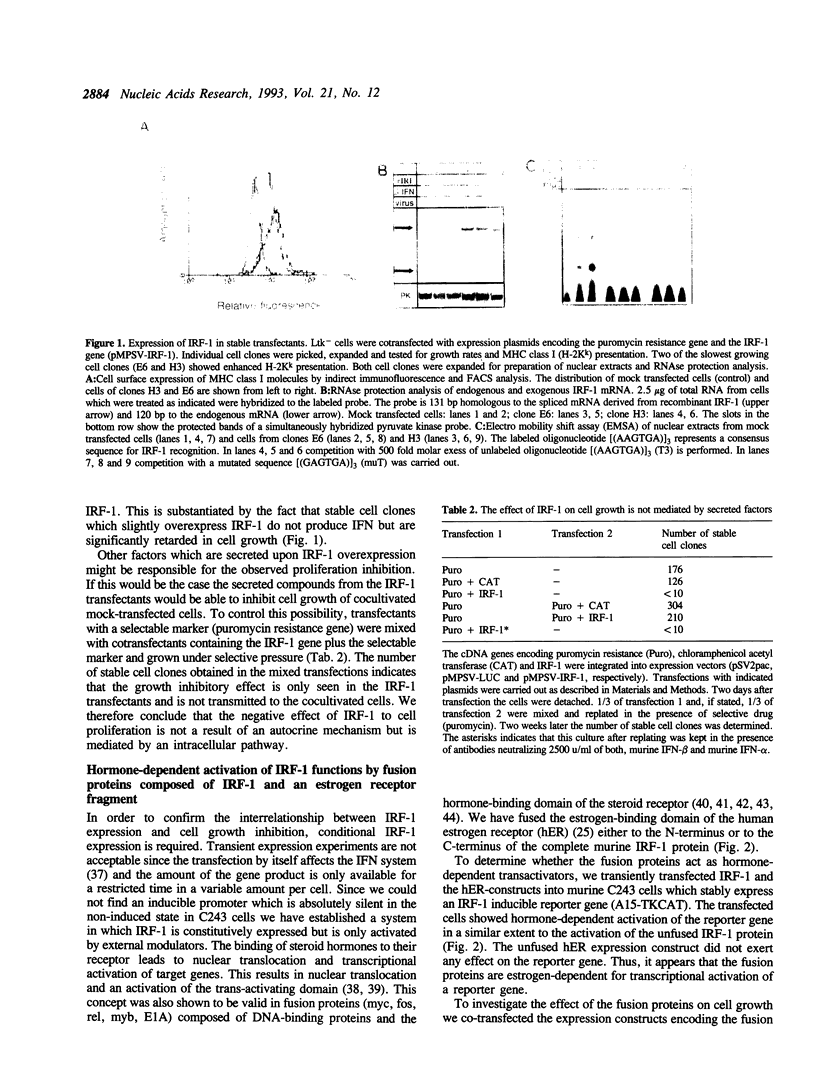
Interferon regulatory factor 1 (IRF-1) is a DNA-binding factor which recognizes regulatory elements in the promoters of interferon (IFN)-beta and some IFN-inducible genes. We observed that expression of transfected murine IRF-1 in different mammalian cell lines leads to down-regulation or stop of proliferation depending on the extent of expression. Expression of fusion proteins composed of IRF-1 and the hormone binding domain of the human estrogen receptor does not exhibit IRF-1 activity in the absence of estrogen. However, after estrogen treatment of the cells IFN-beta promoters are activated and the cells stop growing. As shown by expression of IRF-1 mutants both functions of the IRF-1-protein require DNA-binding and transcriptional activation. Since secreted factors including IFNs are not responsible for the anti-proliferative effect of IRF-1 we suggest that IRF-1 may be regarded as a negative regulator of cell growth which acts by activation of down-stream effector genes.
Full text
PDF


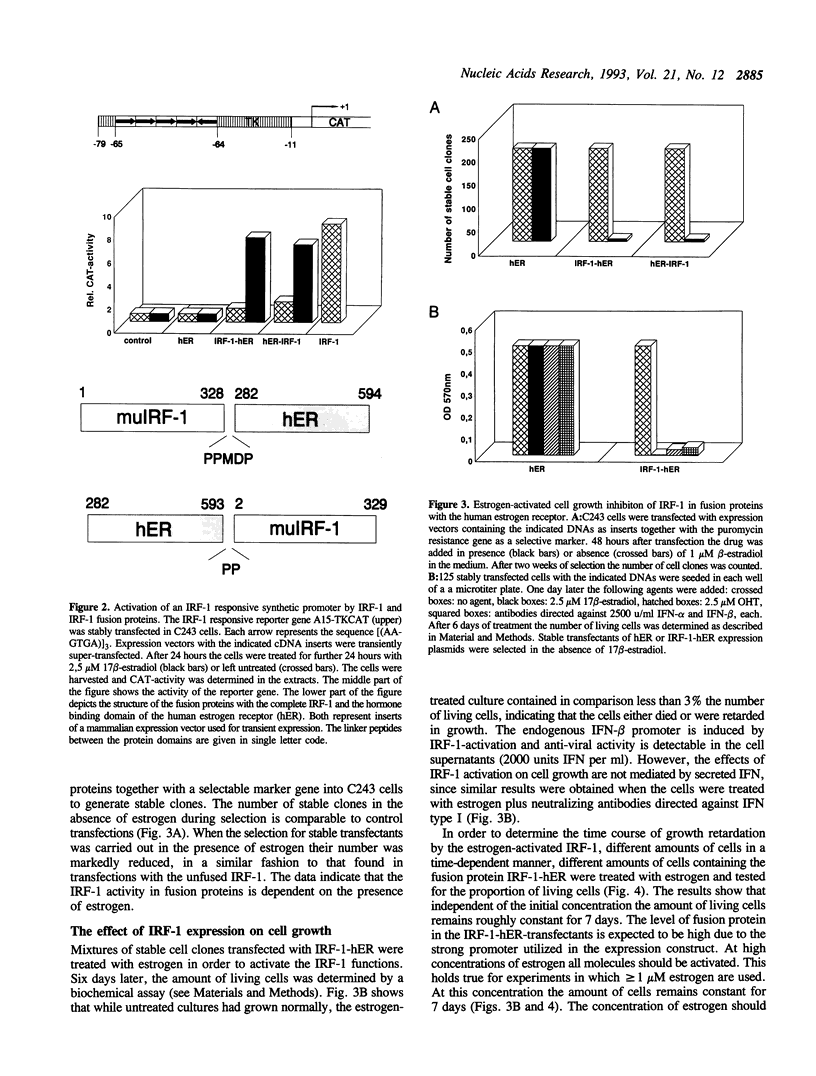


Images in this article
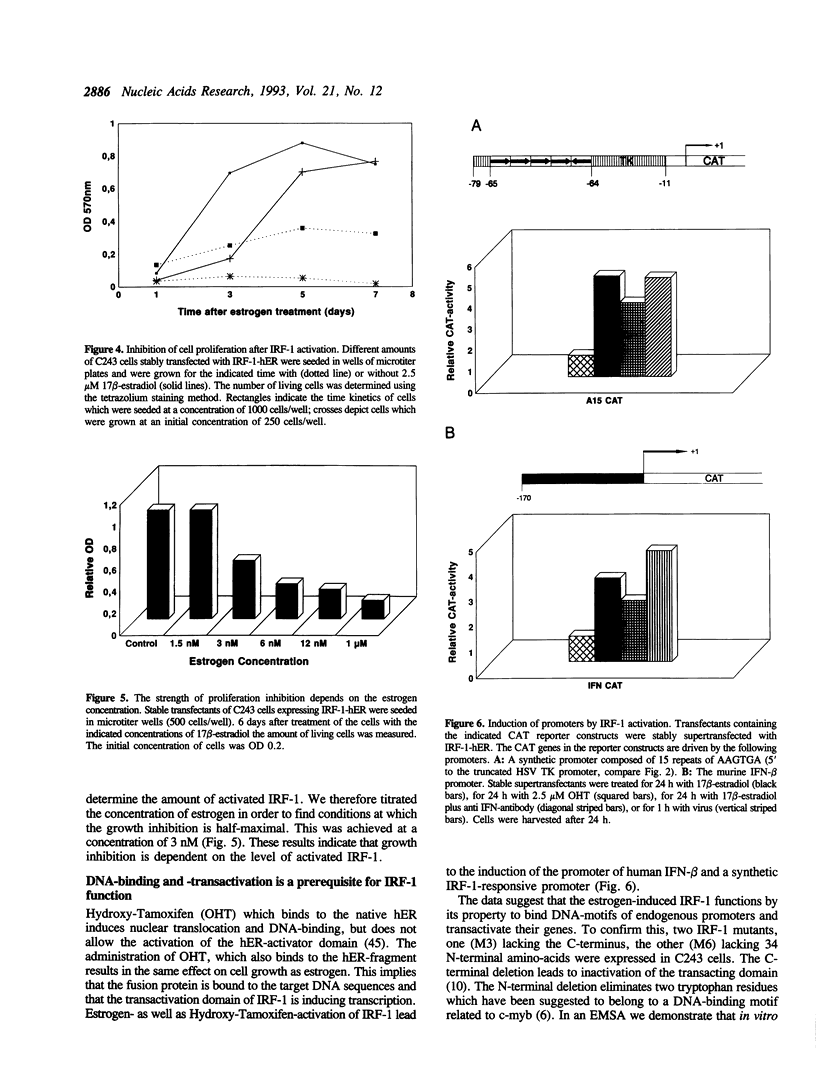
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
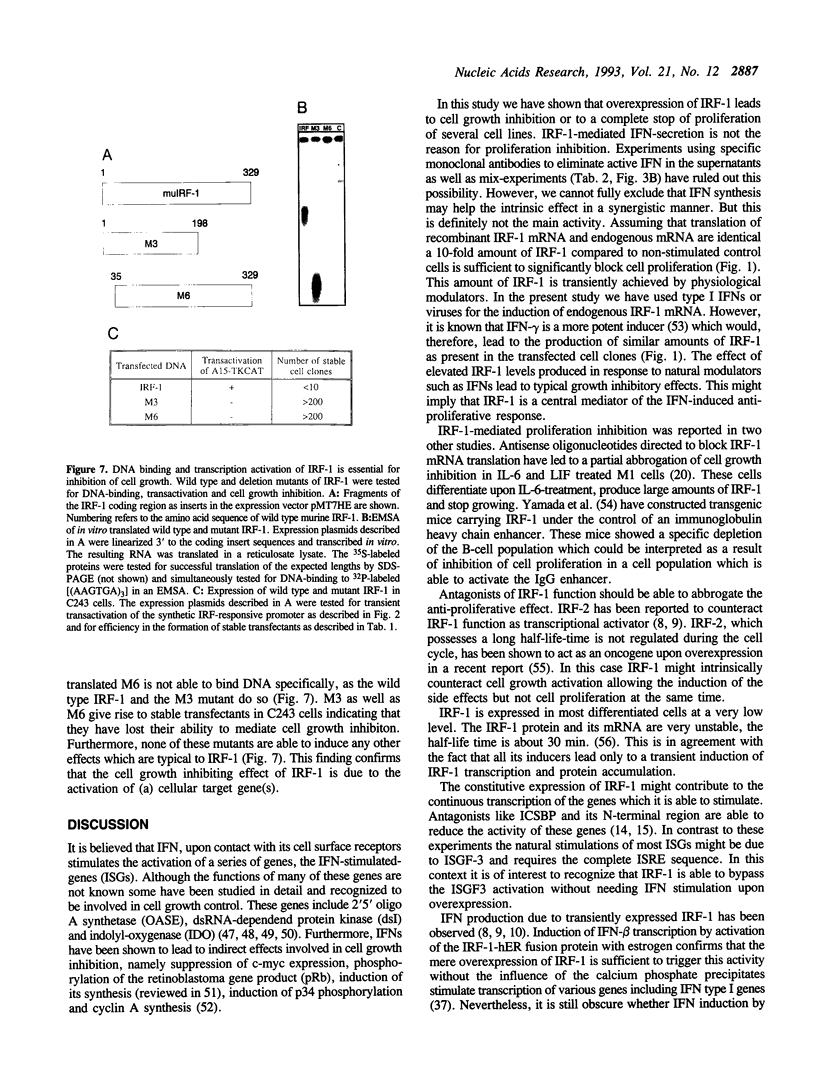
- Abdollahi A., Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Interferon regulatory factor 1 is a myeloid differentiation primary response gene induced by interleukin 6 and leukemia inhibitory factor: role in growth inhibition. Cell Growth Differ. 1991 Aug;2(8):401–407. [PubMed] [Google Scholar]
- Artelt P., Morelle C., Ausmeier M., Fitzek M., Hauser H. Vectors for efficient expression in mammalian fibroblastoid, myeloid and lymphoid cells via transfection or infection. Gene. 1988 Sep 7;68(2):213–219. doi: 10.1016/0378-1119(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Au W. C., Raj N. B., Pine R., Pitha P. M. Distinct activation of murine interferon-alpha promoter region by IRF-1/ISFG-2 and virus infection. Nucleic Acids Res. 1992 Jun 11;20(11):2877–2884. doi: 10.1093/nar/20.11.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Boehmelt G., Walker A., Kabrun N., Mellitzer G., Beug H., Zenke M., Enrietto P. J. Hormone-regulated v-rel estrogen receptor fusion protein: reversible induction of cell transformation and cellular gene expression. EMBO J. 1992 Dec;11(12):4641–4652. doi: 10.1002/j.1460-2075.1992.tb05566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk O., Klempnauer K. H. Estrogen-dependent alterations in differentiation state of myeloid cells caused by a v-myb/estrogen receptor fusion protein. EMBO J. 1991 Dec;10(12):3713–3719. doi: 10.1002/j.1460-2075.1991.tb04939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bybee A., Thomas N. S. The synthesis of p58cyclin A and the phosphorylation of p34cdc2 are inhibited in human lymphoid cells arrested in G1 by alpha-interferon. Biochim Biophys Acta. 1992 Oct 6;1137(1):73–76. doi: 10.1016/0167-4889(92)90102-h. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Hammer J., Loh J. E., Fodor W. L., Flavell R. A. The activation of major histocompatibility complex class I genes by interferon regulatory factor-1 (IRF-1). Immunogenetics. 1992;35(6):378–384. doi: 10.1007/BF00179793. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Dinter H., Hauser H. Superinduction of the human interferon-beta promoter. EMBO J. 1987 Mar;6(3):599–604. doi: 10.1002/j.1460-2075.1987.tb04796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M., Picard D., Yamamoto K. R., Bishop J. M. Chimaeras of myc oncoprotein and steroid receptors cause hormone-dependent transformation of cells. Nature. 1989 Jul 6;340(6228):66–68. doi: 10.1038/340066a0. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Kimura Y., Miyamoto M., Barsoumian E. L., Taniguchi T. Induction of endogenous IFN-alpha and IFN-beta genes by a regulatory transcription factor, IRF-1. Nature. 1989 Jan 19;337(6204):270–272. doi: 10.1038/337270a0. [DOI] [PubMed] [Google Scholar]
- Fujita T., Reis L. F., Watanabe N., Kimura Y., Taniguchi T., Vilcek J. Induction of the transcription factor IRF-1 and interferon-beta mRNAs by cytokines and activators of second-messenger pathways. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9936–9940. doi: 10.1073/pnas.86.24.9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen O. S., Sentenac A., Fromageot P. Specific DNA binding by c-Myb: evidence for a double helix-turn-helix-related motif. Science. 1991 Sep 6;253(5024):1140–1143. doi: 10.1126/science.1887237. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet. 1991;25:89–123. doi: 10.1146/annurev.ge.25.120191.000513. [DOI] [PubMed] [Google Scholar]
- Harada H., Fujita T., Miyamoto M., Kimura Y., Maruyama M., Furia A., Miyata T., Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989 Aug 25;58(4):729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- Harada H., Kitagawa M., Tanaka N., Yamamoto H., Harada K., Ishihara M., Taniguchi T. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science. 1993 Feb 12;259(5097):971–974. doi: 10.1126/science.8438157. [DOI] [PubMed] [Google Scholar]
- Harada H., Willison K., Sakakibara J., Miyamoto M., Fujita T., Taniguchi T. Absence of the type I IFN system in EC cells: transcriptional activator (IRF-1) and repressor (IRF-2) genes are developmentally regulated. Cell. 1990 Oct 19;63(2):303–312. doi: 10.1016/0092-8674(90)90163-9. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kanei-Ishii C., Sarai A., Sawazaki T., Nakagoshi H., He D. N., Ogata K., Nishimura Y., Ishii S. The tryptophan cluster: a hypothetical structure of the DNA-binding domain of the myb protooncogene product. J Biol Chem. 1990 Nov 15;265(32):19990–19995. [PubMed] [Google Scholar]
- Kessler D. S., Levy D. E., Darnell J. E., Jr Two interferon-induced nuclear factors bind a single promoter element in interferon-stimulated genes. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8521–8525. doi: 10.1073/pnas.85.22.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi A. Cytokine triggered molecular pathways that control cell cycle arrest. J Cell Biochem. 1992 Sep;50(1):1–9. doi: 10.1002/jcb.240500102. [DOI] [PubMed] [Google Scholar]
- Koromilas A. E., Roy S., Barber G. N., Katze M. G., Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science. 1992 Sep 18;257(5077):1685–1689. doi: 10.1126/science.1382315. [DOI] [PubMed] [Google Scholar]
- Kumar R., Mendelsohn J. Role of 2'-5'-oligoadenylate synthetase in gamma-interferon-mediated growth inhibition of A431 cells. Cancer Res. 1989 Sep 15;49(18):5180–5184. [PubMed] [Google Scholar]
- Kumar V., Green S., Staub A., Chambon P. Localisation of the oestradiol-binding and putative DNA-binding domains of the human oestrogen receptor. EMBO J. 1986 Sep;5(9):2231–2236. doi: 10.1002/j.1460-2075.1986.tb04489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. J. Tumor suppressor genes. Cell. 1991 Jan 25;64(2):313–326. doi: 10.1016/0092-8674(91)90641-b. [DOI] [PubMed] [Google Scholar]
- Maruyama M., Fujita T., Taniguchi T. Sequence of a cDNA coding for human IRF-1. Nucleic Acids Res. 1989 Apr 25;17(8):3292–3292. doi: 10.1093/nar/17.8.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeall J., Sánchez A., Gray P. P., Chesterman C. N., Sleigh M. J. Hyperinducible gene expression from a metallothionein promoter containing additional metal-responsive elements. Gene. 1989 Mar 15;76(1):81–88. doi: 10.1016/0378-1119(89)90010-3. [DOI] [PubMed] [Google Scholar]
- Meurs E. F., Galabru J., Barber G. N., Katze M. G., Hovanessian A. G. Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):232–236. doi: 10.1073/pnas.90.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M., Fujita T., Kimura Y., Maruyama M., Harada H., Sudo Y., Miyata T., Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988 Sep 9;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- Nelson N., Marks M. S., Driggers P. H., Ozato K. Interferon consensus sequence-binding protein, a member of the interferon regulatory factor family, suppresses interferon-induced gene transcription. Mol Cell Biol. 1993 Jan;13(1):588–599. doi: 10.1128/mcb.13.1.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourbakhsh M., Hoffmann K., Hauser H. Interferon-beta promoters contain a DNA element that acts as a position-independent silencer on the NF-kappa B site. EMBO J. 1993 Feb;12(2):451–459. doi: 10.1002/j.1460-2075.1993.tb05677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oie H. K., Gazdar A. F., Buckler C. E., Baron S. High interferon producing line of transformed murine cells. J Gen Virol. 1972 Oct;17(1):107–109. doi: 10.1099/0022-1317-17-1-107. [DOI] [PubMed] [Google Scholar]
- Pine R. Constitutive expression of an ISGF2/IRF1 transgene leads to interferon-independent activation of interferon-inducible genes and resistance to virus infection. J Virol. 1992 Jul;66(7):4470–4478. doi: 10.1128/jvi.66.7.4470-4478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine R., Decker T., Kessler D. S., Levy D. E., Darnell J. E., Jr Purification and cloning of interferon-stimulated gene factor 2 (ISGF2): ISGF2 (IRF-1) can bind to the promoters of both beta interferon- and interferon-stimulated genes but is not a primary transcriptional activator of either. Mol Cell Biol. 1990 Jun;10(6):2448–2457. doi: 10.1128/mcb.10.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine R., Levy D. E., Reich N., Darnell J. E., Jr Transcriptional stimulation by CaPO4-DNA precipitates. Nucleic Acids Res. 1988 Feb 25;16(4):1371–1378. doi: 10.1093/nar/16.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N. C., Darnell J. E., Jr Differential binding of interferon-induced factors to an oligonucleotide that mediates transcriptional activation. Nucleic Acids Res. 1989 May 11;17(9):3415–3424. doi: 10.1093/nar/17.9.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann E., Schwarz H., Deiner E. M., Leitner I., Eilers M., Berger J., Busslinger M., Beug H. Activation of an inducible c-FosER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid cell conversion. Cell. 1992 Dec 24;71(7):1103–1116. doi: 10.1016/s0092-8674(05)80060-1. [DOI] [PubMed] [Google Scholar]
- Reis L. F., Harada H., Wolchok J. D., Taniguchi T., Vilcek J. Critical role of a common transcription factor, IRF-1, in the regulation of IFN-beta and IFN-inducible genes. EMBO J. 1992 Jan;11(1):185–193. doi: 10.1002/j.1460-2075.1992.tb05041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rysiecki G., Gewert D. R., Williams B. R. Constitutive expression of a 2',5'-oligoadenylate synthetase cDNA results in increased antiviral activity and growth suppression. J Interferon Res. 1989 Dec;9(6):649–657. doi: 10.1089/jir.1989.9.649. [DOI] [PubMed] [Google Scholar]
- Schwarz L. A., Stevens A. M., Hrachovy J. A., Yu-Lee L. Y. Interferon regulatory factor-1 is inducible by prolactin, interleukin-2 and concanavalin A in T cells. Mol Cell Endocrinol. 1992 Jul;86(1-2):103–110. doi: 10.1016/0303-7207(92)90180-e. [DOI] [PubMed] [Google Scholar]
- Sims S. H., Cha Y., Romine M. F., Gao P. Q., Gottlieb K., Deisseroth A. B. A novel interferon-inducible domain: structural and functional analysis of the human interferon regulatory factor 1 gene promoter. Mol Cell Biol. 1993 Jan;13(1):690–702. doi: 10.1128/mcb.13.1.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superti-Furga G., Bergers G., Picard D., Busslinger M. Hormone-dependent transcriptional regulation and cellular transformation by Fos-steroid receptor fusion proteins. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5114–5118. doi: 10.1073/pnas.88.12.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N., Taniguchi T. Cytokine gene regulation: regulatory cis-elements and DNA binding factors involved in the interferon system. Adv Immunol. 1992;52:263–281. doi: 10.1016/s0065-2776(08)60877-9. [DOI] [PubMed] [Google Scholar]
- Taylor M. W., Feng G. S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991 Aug;5(11):2516–2522. [PubMed] [Google Scholar]
- Vara J. A., Portela A., Ortín J., Jiménez A. Expression in mammalian cells of a gene from Streptomyces alboniger conferring puromycin resistance. Nucleic Acids Res. 1986 Jun 11;14(11):4617–4624. doi: 10.1093/nar/14.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veals S. A., Santa Maria T., Levy D. E. Two domains of ISGF3 gamma that mediate protein-DNA and protein-protein interactions during transcription factor assembly contribute to DNA-binding specificity. Mol Cell Biol. 1993 Jan;13(1):196–206. doi: 10.1128/mcb.13.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veals S. A., Schindler C., Leonard D., Fu X. Y., Aebersold R., Darnell J. E., Jr, Levy D. E. Subunit of an alpha-interferon-responsive transcription factor is related to interferon regulatory factor and Myb families of DNA-binding proteins. Mol Cell Biol. 1992 Aug;12(8):3315–3324. doi: 10.1128/mcb.12.8.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Sakakibara J., Hovanessian A. G., Taniguchi T., Fujita T. Activation of IFN-beta element by IRF-1 requires a posttranslational event in addition to IRF-1 synthesis. Nucleic Acids Res. 1991 Aug 25;19(16):4421–4428. doi: 10.1093/nar/19.16.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster N. J., Green S., Jin J. R., Chambon P. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell. 1988 Jul 15;54(2):199–207. doi: 10.1016/0092-8674(88)90552-1. [DOI] [PubMed] [Google Scholar]
- Weisz A., Marx P., Sharf R., Appella E., Driggers P. H., Ozato K., Levi B. Z. Human interferon consensus sequence binding protein is a negative regulator of enhancer elements common to interferon-inducible genes. J Biol Chem. 1992 Dec 15;267(35):25589–25596. [PubMed] [Google Scholar]
- Yamada G., Ogawa M., Akagi K., Miyamoto H., Nakano N., Itoh S., Miyazaki J., Nishikawa S., Yamamura K., Taniguchi T. Specific depletion of the B-cell population induced by aberrant expression of human interferon regulatory factor 1 gene in transgenic mice. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):532–536. doi: 10.1073/pnas.88.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikomi T., Bocquel M. T., Berry M., Gronemeyer H., Chambon P. Cooperation of proto-signals for nuclear accumulation of estrogen and progesterone receptors. EMBO J. 1992 Oct;11(10):3681–3694. doi: 10.1002/j.1460-2075.1992.tb05453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Lee L. Y., Hrachovy J. A., Stevens A. M., Schwarz L. A. Interferon-regulatory factor 1 is an immediate-early gene under transcriptional regulation by prolactin in Nb2 T cells. Mol Cell Biol. 1990 Jun;10(6):3087–3094. doi: 10.1128/mcb.10.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., Helinski D. R., DeLuca M. Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7870–7873. doi: 10.1073/pnas.82.23.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]




