Abstract
Schizophrenia would result from a defective connectivity between several integrative regions as a consequence of neurodevelopmental failure. Various anomalies reminiscent of early brain development disturbances have been observed in patients' left ventral subiculum of the hippocampus (SUB). Numerous data support the hypothesis of a functional dopaminergic dysregulation in schizophrenia. The common target structure for the action of antipsychotics appears to be a subregion of the ventral striatum, the dorsomedial shell part of the nucleus accumbens. Latent inhibition, a cognitive marker of interest for schizophrenia, has been found to be disrupted in acute patients. The present study set out to investigate the consequences of a neonatal functional inactivation of the left SUB by tetrodotoxin (TTX) in 8-day-old rats for the latent inhibition-related dopaminergic responses, as monitored by in vivo voltammetry in freely moving adult animals (11 weeks) in the left core and dorsomedial shell parts of the nucleus accumbens in an olfactory aversion procedure. Results obtained during the retention session of a three-stage latent inhibition protocol showed that the postnatal unilateral functional blockade of the SUB was followed in pre-exposed TTX-conditioned adult rats by a disruption of the behavioral expression of latent inhibition and induced a total and a partial reversal of the latent inhibition-related dopaminergic responses in the dorsomedial shell and core parts of the nucleus accumbens, respectively. The present data suggest that neonatal inactivation of the SUB has more marked consequences for the dopaminergic responses recorded in the dorsomedial shell part, than in the core part of the nucleus accumbens. These findings may provide new insight into the pathophysiology of schizophrenia.
Keywords: neonatal functional inactivation, neurodevelopment, conditioned olfactory aversion, nucleus accumbens, in vivo voltammetry, schizophrenia
INTRODUCTION
Schizophrenia is a very complex and disabling neuropsychiatric disease, the pathophysiology of which is not yet fully understood. According to several recent proposals, the psychic disintegration characteristic of this disease would result from a defective connectivity between various integrative regions having a neurodevelopmental origin (Weinberger and Lipska, 1995; Bullmore et al, 1997; Friston, 1998; Lewis and Levitt, 2002; Sawa and Snyder, 2002; Arnold et al, 2005; Foucher et al, 2005). Thus, the ventral subiculum of the hippocampus (SUB) seems to be subjected to neurodevelopmental abnormalities in schizophrenia, as suggested by the cytoarchitectural and cortical morphometric aberrations reported by several authors, particularly in the left hemisphere (Arnold et al, 1995; Arnold, 2000; Rosoklija et al, 2000; Law et al, 2004). Data obtained over the past three decades also support the hypothesis of a functional dysregulation of the mesostriatal dopaminergic neurons in schizophrenia (Swerdlow and Koob, 1987; Harrison, 1999; Carlsson et al, 2001), consistent with a cortico-subcortical disconnectivity (Kegeles et al, 2000). Moreover, it would appear that the common target structure for the action of antipsychotics is a subregion of the ventral striatum, the dorsomedial shell part of the nucleus accumbens (Deutch and Cameron, 1992; Robertson and Fibiger, 1992; Jennings et al, 2006).
Latent inhibition, a cognitive marker of interest for schizophrenia, has been found to be disrupted in non-treated patients (Baruch et al, 1988; Gray et al, 1992, 1995; Lubow et al, 2000; Rascle et al, 2001). Latent inhibition is a behavioral phenomenon characterized by a decrease or even disappearance of the conditioned response (CR) normally observed during the retention session when the conditional stimulus (CS) is first pre-exposed, by itself, before the conditioning session (Lubow and Moore, 1959). Many studies aimed to identify the neurobiological substrates underlying normal and disrupted expression of latent inhibition (see Lubow, 1989; Weiner, 2003). Differential involvement of the dopaminergic neurons innervating the core and dorsomedial shell parts of the nucleus accumbens in latent inhibition has been highlighted by in vivo methods in animal studies (Young et al, 1993; Murphy et al, 2000; Jeanblanc et al, 2002). The core and dorsomedial shell dopaminergic responses seem to be regulated differently in adult animals by the entorhinal cortex (ENT) and the SUB (Jeanblanc et al, 2004; Peterschmitt et al, 2008). Moreover, the functional inactivation of the two left parahippocampal regions (ENT, SUB) appears sufficient to impair the latent inhibition-related dopaminergic responses in the left hemisphere (Jeanblanc et al, 2004; Peterschmitt et al, 2005, 2008). These findings are consistent with previous anatomofunctional (Louilot and Le Moal, 1994; Louilot and Choulli, 1997) and behavioral data (Besson and Louilot, 1995) showing a left predominance for temporo-accumbal dopaminergic regulation and aversive dopaminergic responses.
Recently, we found that the neonatal functional blockade of the left ENT abolished the latent inhibition-related dopaminergic responses in the left core and left dorsomedial shell parts of the nucleus accumbens (Peterschmitt et al, 2007). Given all the aforementioned data, this raises the question of the consequences of neonatal transient inactivation of the left SUB for latent inhibition-related dopaminergic responses in the two subregions of the nucleus accumbens. During development, impulse electrical activity appears to be crucial for shaping connections, once developing fibers reach the target structure (Stryker and Harris, 1986; Katz and Shatz, 1996; Frotscher et al, 2000; Hutchins and Kalil, 2008). Tetrodotoxin (TTX) is a potent and specific voltage-gated Na+ channel blocker that interrupts neuronal activity (Mosher, 1986). In the present study, the transient neonatal blockade of the SUB was achieved by local microinjection of TTX in 8-day-old rats. This particular time point is critical for the neurodevelopment of rats, comparable in humans to the middle of the second trimester of gestation (Clancy et al, 2001), which is considered to be a time window of great vulnerability for developing schizophrenia (see eg Weinberger and Lipska, 1995; Bullmore et al, 1997; Arnold, 2000; Falkai et al, 2000; Lewis and Levitt, 2002). Latent inhibition-related dopaminergic changes in the left core and left dorsomedial shell subregions of the nucleus accumbens were monitored in freely moving adult animals (11 weeks) using in vivo voltammetry in a three-stage latent inhibition paradigm with an aversive conditioned olfactory procedure (Jeanblanc et al, 2002).
MATERIALS AND METHODS
Animals and Surgery
Subjects. Female rats (Sprague–Dawley, Janvier, Le Genest, France) obtained at 14 days gestation were housed individually at 22±2°C on a 12-h light/12-h dark cycle (lights on at 8 am) and fed ad libitum. At birth, the size of the litters was limited to 12 animals by killing off supernumerary pups with a lethal i.p. injection of pentobarbital. The day of birth was defined as postnatal day 0 (PND0). On PND8, rat pups underwent a microinjection of either phosphate-buffered saline (PBS) in the left SUB (control animals) or of TTX in the left SUB (experimental animals). On PND21, the neonatal rats were weaned. On PND56, the male animals were individually housed in plexiglas cages under a reversed lighting cycle (lights on between 11 pm and 11 am) at 22±2°C, with free access to food and water. A total of 131 Sprague–Dawley male rats were used. On PND70, the male rats were implanted with a microsystem for monitoring behavior and dopaminergic responses, in parallel. All experimental procedures were conducted in accordance with European Community guidelines for the care and use of experimental animals (Council Directive 86/609/EEC) and authorized by the French Ministry of Agriculture (Authorization 67-244).
Neonatal TTX inactivation. Neonatal surgery was performed on PND8 (weight 19.1±0.4 g). Pups were anaesthetized by gas anesthesia by vaporizing isoflurane (Forene, ABBOTT, Rungis, France) with an anesthesia vaporizer (Univentor 400, Univentor, Zetjun, Malta) connected to an air pump (Dymax 30, Charles Austen Pumps, Byfleet, UK) and released through a specially adapted gas anesthesia mask. Induction was obtained with a concentration of ∼3.8–4% isoflurane in air. The pups were then immediately placed on a specially adapted stereotaxic apparatus (Unimécanique, Epinay/Seine, France) (incisor bar set at 2.8 mm below the interaural line). Anesthesia was maintained throughout surgery with ∼2% isoflurane in air (Flecknell, 1987). Neonatal microinjection of either PBS or TTX was performed by means of a stainless steel guide cannula (30 gauge, 12.5 mm length, Small Parts, Miami) implanted in the SUB at coordinates 1.1 mm anterior to the interaural line (AP), 4.25 mm lateral to the midline (L), and 4.35 mm below the cortical surface (H). TTX (100 μM) was dissolved in PBS (NaCl 8 g/l, KCl 0.2 g/l, MgCl2, 6H2O 0.1 g/l, KH2PO4 0.2 g/l, Na2 HPO4, 2H2O 1.15 g/l, pH: 7.4). PBS and TTX were infused locally in a total volume of 0.3 μl over a period of 2 min 15 s by means of an infusion pump (Razel, Stamford, CT). Following the microinjection, the cannula was left in place for 4 min to allow PBS and TTX to diffuse in the SUB. It is worth noting that the amount of TTX microinjected in the SUB (100 μM × 0.3 μl), about 10 ng, is similar to that reported in the literature (Zhuravin and Bures, 1991; Ambrogi Lorenzini et al, 1997), and that different authors (Rothfeld et al, 1986; Zhuravin and Bures, 1991) showed that the blockade effects of a local microinjection of 10 ng TTX last for 24 or 48 h. To identify the microinjection site in the SUB, the PBS and TTX solutions were both tinted with Evans Blue (Sigma, St Quentin, Fallavier, France), a vital dye described as remaining visible in the cerebral tissue several weeks after injection (Martin and Ghez, 1993; Peterschmitt et al, 2007; Meyer et al, 2009). Each rat pup was identified by means of small three-digit ear tags (Ref 52-4716, Harvard Apparatus, Les Ulis, France). After the surgical intervention, the neonates were returned to their mothers. Male rat pups from the same litter, microinjected with either PBS or TTX, were placed in the same cage.
Microsystem implantation. Animals microinjected neonatally with either PBS or TTX were implanted in adulthood at PND70 (weight 400±25 g) with a specially designed microsystem (Unimécanique, Epinay/Seine, France) (Louilot et al, 1987) following anaesthetization by i.p. injection of chloral hydrate (400 mg/kg) and after being placed in a stereotaxic frame (incisor bar set at 3.3 mm below the interaural line) (Unimécamique, Epinay/Seine, France). Male rats were implanted either in the left core part of the nucleus accumbens at coordinates 10.2 mm (AP), 1.8 mm (L), and 6.9 mm (H), or in the left dorsomedial shell part of the nucleus accumbens at coordinates 10.2 mm (AP), 1.0 mm (L), and 6.2 mm (H) (Paxinos and Watson, 2009). After surgery, animals were given 1 week to recover.
Behavioral Analysis
Behavioral procedure. The behavioral procedure begins 7 days after the microsystem implantation in the adult animals, so at PND77. The behavioral and voltammetric analyses were performed in parallel. All experiments were carried out during the dark phase of the light/dark cycle. Latent inhibition was measured in a conditioned olfactory aversion procedure in a three-stage latent inhibition paradigm, with banana odor (amyl acetate, Prolabo, Strasbourg, France) as the conditional olfactory stimulus (CS), as previously reported (Jeanblanc et al, 2002; Peterschmitt et al, 2007; Meyer et al, 2009; Louilot et al, 2010). The latent inhibition paradigm consists of three sessions conducted at 72 h intervals. During the first (pre-exposure) session, animals were placed in the experimental cage (24 cm wide × 27 cm long × 44 cm high) for 1 h before being exposed for 2 h to banana odor, a novel olfactory stimulus. During the second (conditioning) session, 3 days later, the animals were placed in the test cage for 1 h before being exposed to banana odor for a further hour, at the end of which they received either an i.p. injection of 2% of body weight of NaCl (0.9%) (control animals) or an i.p. injection of 2% of body weight of a toxic substance inducing nausea (LiCl 0.15 M) (conditioned animals) (Garcia et al, 1985). They were then kept in the test cage for a further hour. During the last (test) session, 3 days later, the animals were again exposed to the conditional olfactory stimulus (banana odor) for one hour. Non-pre-exposed animals, on the other hand, were only subjected to the last two sessions (conditioning and test sessions). All pre-exposed and non-pre-exposed animals were microinjected at PND8 with either PBS or TTX in the SUB. After the neonatal intervention, they were randomized to two pre-exposed control groups (pre-exposed PBS-NaCl; pre-exposed TTX-NaCl), two pre-exposed conditioned groups (pre-exposed PBS-LiCl; pre-exposed TTX-LiCl), two non-pre-exposed control groups (non-pre-exposed PBS-NaCl; non-pre-exposed TTX-NaCl) and two non-pre-exposed conditioned groups (non-pre-exposed PBS-LiCl; non-pre-exposed TTX-LiCl).
Behavioral data analysis. The animals' position in the experimental cage was monitored using a small infrared camera (Ref. 51.8050, CA-H34C, Selectronic, Lille, France) inserted into the ceiling of the cage and connected to a video monitor and video tape. The olfactory stimulus was fed into the cage through a hole in the wall adjacent to the cage door. Attraction or aversion to the banana odor was evaluated by measuring the amount of time spent near the olfactive source. The floor of the cage was divided empirically into two virtual zones. One zone, containing the hole, was a semicircle covering 35% of the total surface area. The rest of the floor made up the second zone. Behavioral analysis was performed over 10-min periods. It was assumed that an animal moving randomly in the cage should spend 35% (210 s) of the 10-min period in the area containing the hole. Results are expressed as mean±SEM of the time spent near the hole.
Voltammetric Analysis
Voltammetric procedure. The electrochemical procedures were those previously described (Gonzalez-Mora et al, 1991; Louilot et al, 1991). A standard three-electrode potentiostatic setting was used with working, reference, and auxiliary electrodes. The working electrodes were pyrolytic carbon fiber microelectrodes (12 μm diameter, 500 μm length, ref AGT 8000, SEROFIM, Gennevilliers, France) that were pretreated electrochemically, according to Gonon et al (1984). Differential normal pulse voltammetry (DNPV) (see O'Neill et al, 1998 for review), combined with the computerized waveform analysis of the catechol peak, was used to establish the selective detection of the extracellular dopamine levels in the core and dorsomedial shell parts of the nucleus accumbens (see Gonzalez-Mora et al, 1991). DNP voltammograms were recorded every minute. The parameters of the voltammetric apparatus (Biopulse, Tacussel, Villeurbanne, France) were as follows: scan range −240, +200 mV; scan rate 10 mV/s; potential step 4 mV; pulse period 400 ms; pre-pulse duration +120 ms; pulse duration 40 ms; pulse amplitude 40 mV.
Voltammetric data. The average amplitude of the last 10 peaks (last 10 min) of dopamine obtained during the control period (variation of voltammetric signal less than 10%) was calculated for each animal and set at 100%. Voltammetric variations, recorded minute by minute, are expressed as percentages (mean±SEM) of the mean values determined before exposure to the olfactory stimulus. Only variations obtained every 2 min are shown on the graphs.
Statistics
Statistical analysis was performed using a multifactorial ANOVA analysis with repeated measurements on the factor time. Only between-subject ANOVAs are shown. Between-subject grouping factors were conditioning with two levels (NaCl, LiCl), microinjection with two levels (PBS, TTX), and pre-exposure with two levels (pre-exposed, non-pre-exposed). The dependent variables were the time spent near the hole for the behavioral study and the dopaminergic variations for the voltammetric study. Contrast analysis of the ANOVA was used to test specific hypotheses (see Rosenthal and Rosnow, 1985; Rosenthal et al, 2000). Statistical significance for all analyses was set at p<0.05.
Histology
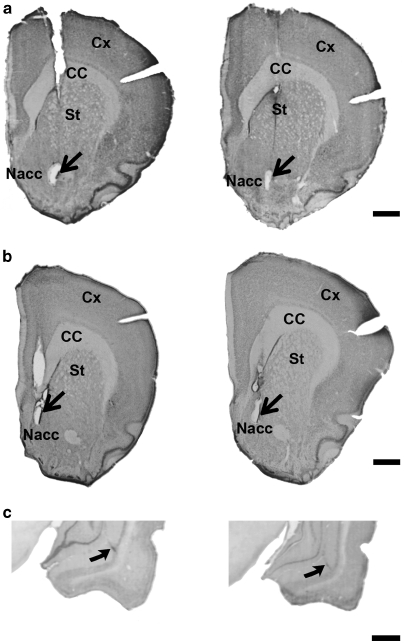
At the end of each experiment, the rats underwent electrocoagulation (Louilot et al, 1985). They then received a lethal i.p. injection of pentobarbital and were intracardially perfused, first with NaCl 0.9 % and then with a 4% paraformaldehyde solution. Their brains were quickly removed from their skull. The brain sections were stained with Thionin Blue so that the position of the recording sites in the left core and the left dorsomedial shell parts of the nucleus accumbens (Figure 1, upper sections) could be verified. The neonatal microinjection site in the left SUB was histologically checked with the vital dye Evans Blue and Neutral Red staining of the SUB sections (Figure 1, lower sections). Paxinos and Watson's atlas (2009) was used as a reference.
Figure 1.
Typical recording sites in the (a) core or (b) dorsomedial shell part of the left nucleus accumbens (Nacc) (upper sections), and (c) typical injection sites in the left SUB (lower sections) of adult animals neonatally microinjected at PND8 with PBS (left column) or TTX (right column). The recording sites were visualized by means of electrocoagulation at the end of the experiment (arrows); sections were stained using Thionin Blue coloration. The center of the microinjection site in the left SUB (lower sections) was identified by means of Evans Blue track (arrows) and the sections were stained using Neutral Red coloration. Scale bar=1 mm. Cx: cortex, CC: corpus callosum; St.: striatum.
RESULTS
Histology
The qualitative macroscopic observations of brain sections at the level of the SUB or the nucleus accumbens reveal no gliosis or anatomical differences between the animals microinjected at PND8 with PBS or TTX in the SUB (Figure 1).
Retention Session: Behavioral Study
The overall ANOVA performed on the 131 animals for the 10-min period preceding the presentation of the banana odor, revealed no significant effect in the time spent near the hole for the conditioning factor (NaCl/LiCl), microinjection factor (PBS/TTX), pre-exposure factor (non-pre-exposed/pre-exposed), or different interactions (conditioning × microinjection, microinjection × pre-exposure, pre-exposure × conditioning, conditioning × microinjection × pre-exposure) (Figure 2).
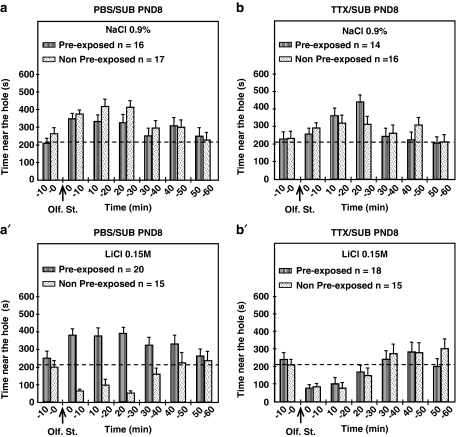
Figure 2.
Time spent near the hole during the test session in pre-exposed (hatched bars) and non-pre-exposed (spotted bars) adult animals microinjected in the left SUB with either PBS (a and a′) or TTX (b and b′) at PND8 of the neonatal period. The arrows indicate when the olfactory stimulus (banana odor) was presented. During the conditioning session, banana odor was associated in control animals with an i.p. injection of NaCl (0.9%) and in conditioned animals with an i.p. injection of the same volume of LiCl (0.15 M). The dashed line represents 35% of one 10-min period (210 s), corresponding to neutral distribution of the rats in the cage. n represents the number of animals per group.
With regard to the pre-exposed PBS-control (n=16) and non-pre-exposed PBS-control (n=17) groups (Figure 2a), the time spent in the area near the hole exceeded the limit of 210 s for the whole hour, following exposure to the olfactory stimulus. For pre-exposed TTX-control (n=14) and non-pre-exposed TTX-control (n=16) animals (Figure 2b), the time spent in the semicircle also exceeded the limit of 210 s for the first 50 min after presentation of the banana odor. With regard to the two PBS-conditioned groups, the profiles of the pre-exposed and non-pre-exposed animals differ (Figure 2a). For the pre-exposed PBS-conditioned group (n=20), the time spent in the area near the olfactory stimulus exceeded the limit of 210 s for the whole hour. By contrast, the time the non-pre-exposed PBS-conditioned group (n=15) spent in the semicircle was well below 210 s for the first half hour. Regarding the pre-exposed TTX-conditioned (n=18) and non-pre-exposed TTX-conditioned groups (n=15) (Figure 2b), the profiles of the amount of time spent in the semicircle in the first half hour were comparable, with values below the limit of 210 s.
The ANOVA for the full hour after the animals' exposure to the olfactory stimulus yielded significant main effects of conditioning (NaCl/LiCl) (F(1,123)=18.87; p<0.0001) and injection (PBS/TTX) (F(1,123)=4.59; p<0.05), as well as significant interactions of pre-exposure × conditioning (F(1,123)=7.05; p<0.01), of injection × pre-exposure (F(1,123)=4.68; p<0.05), and of conditioning × injection × pre-exposure (F(1,123)=9.81; p<0.01), whereas no significant effects were found for the pre-exposure factor (non-pre-exposed/pre-exposed) and the injection × conditioning interaction.
Contrast analysis of ANOVA was used to test the following specific hypotheses: (1) conditioning (NaCl/LiCl) was followed by statistically different responses in non-pre-exposed and pre-exposed groups; (2) Behavioral responses differed significantly in pre-exposed PBS-conditioned and non-pre-exposed PBS-conditioned animals on the one hand, and pre-exposed PBS-conditioned and pre-exposed TTX-conditioned animals on the other hand.
ANOVA contrast analysis for the time spent near the hole for the 60 min following stimulus presentation highlighted a significant conditioning effect for the non-pre-exposed PBS groups (non-pre-exposed PBS-control/non-pre-exposed PBS-conditioned) (F(1,123)=23.11; p<0.00001), non-pre-exposed TTX groups (non-pre-exposed TTX-control/non-pre-exposed TTX-conditioned) (F(1,123)=4.44; p<0.05), and pre-exposed TTX groups (pre-exposed TTX-control/pre-exposed TTX-conditioned) (F(1,123)=6.73; p<0.05), whereas it revealed no significant conditioning effect (NaCl/LiCl) for the pre-exposed PBS groups (pre-exposed PBS-control/pre-exposed PBS-conditioned). A clear statistical pre-exposure effect was found for the pre-exposed PBS-conditioned group (pre-exposed PBS-conditioned/non-pre-exposed PBS-conditioned) (F(1,123)=25.75; p<0.00001), and a significant effect of the neonatal injection was obtained for the pre-exposed TTX-conditioned group (pre-exposed PBS-conditioned/pre-exposed TTX-conditioned) (F(1,123)=18.18; p<0.0001).
The ANOVA for the first 30 min after the animals' exposure to the olfactory stimulus showed significant main effects of conditioning (NaCl/LiCl) (F(1,123)=76.74; p<0.000001), injection (PBS/TTX) (F(1,123)=13.99; p<0.001), and pre-exposure (non-pre-exposed/pre-exposed) (F(1,123)=13.14; p<0.001), as well as significant interactions of pre-exposure × conditioning (F(1,123)=17.1; p<0.0001), of injection × pre-exposure (F(1,123)=5.01; p<0.05), and of conditioning × injection × pre-exposure (F(1,123)=24.18; p<0.00001), whereas no significant effects were found for the injection × conditioning interaction. No statistical results were obtained for the last 30 min of the hour following presentation of the olfactory stimulus.
Contrast analysis of ANOVA was used to test the following specific hypotheses: (1) conditioning (NaCl/LiCl) was followed by statistically different responses in non-pre-exposed and pre-exposed groups; (2) Behavioral responses differed significantly in pre-exposed PBS-conditioned and non-pre-exposed PBS-conditioned animals, on the one hand, and pre-exposed PBS-conditioned and pre-exposed TTX-conditioned animals on the other hand.
ANOVA contrast analysis for the time spent near the hole in the 1st 30 min following presentation of the stimulus revealed a significant conditioning effect for the non-pre-exposed PBS groups (non-pre-exposed PBS-control/non-pre-exposed PBS-conditioned) (F(1,123)=62.91; p<0.000001), non-pre-exposed TTX groups (non-pre-exposed TTX-control/non-pre-exposed TTX-conditioned) (F(1,123)=22.98; p<0.000001), and pre-exposed TTX groups (pre-exposed TTX-control/pre-exposed TTX-conditioned) (F(1,123)=31.43; p<0.000001), but no significant conditioning effect (NaCl/LiCl) for the pre-exposed PBS groups (pre-exposed PBS-control/pre-exposed PBS-conditioned). A clear statistical pre-exposure effect was found for the pre-exposed PBS-conditioned group (pre-exposed PBS-conditioned/non-pre-exposed PBS-conditioned) (F(1,123)=58.91; p<0.000001), and a significant effect of the neonatal injection was obtained for the pre-exposed TTX-conditioned group (pre-exposed PBS-conditioned/pre-exposed TTX-conditioned) (F(1,123)=47.36; p<0.000001).
To summarize, pre-exposed PBS-conditioned animals microinjected at PND8 in the left SUB differ significantly from non-pre-exposed PBS-conditioned animals. For pre-exposed TTX-conditioned animals and non-pre-exposed TTX-conditioned animals, however, similar results were obtained.
Retention Session: Voltammetric Results
Only animals with implantation sites clearly located in the core part (Figure 1a) or dorsomedial shell part (Figure 1b) of the nucleus accumbens were considered for the voltammetric analysis (Figure 3).
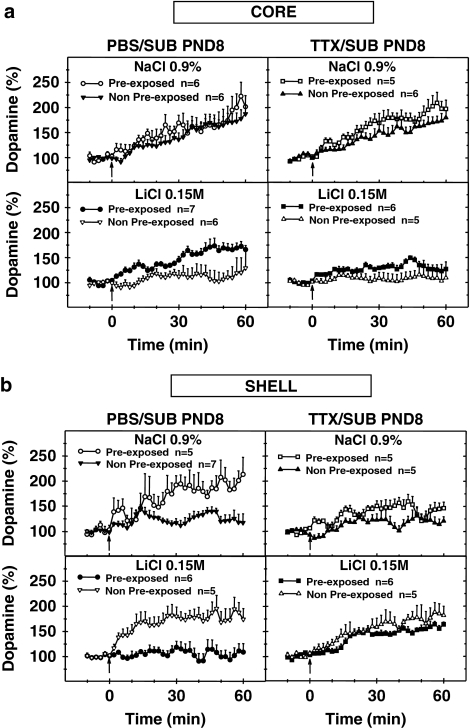
Figure 3.
Dopaminergic variations in the (a) core and (b) dorsomedial shell parts of the nucleus accumbens recorded during the test session in adult pre-exposed and adult non-pre-exposed animals after transitory blockade of the left SUB by TTX at PND8 of the neonatal period. Extracellular dopaminergic levels were assessed using DNPV and computer-assisted numerical analysis in freely moving rats. Voltammograms were recorded every minute. Only mean values and SEM corresponding to two scans are presented. Where no SEM is indicated, the size is less than the radius of the symbol. The arrows indicate presentation of the olfactory stimulus (banana odor). n represents the number of rats per group. Results were analyzed by factorial ANOVA.
Dopaminergic variations recorded in the core part of the nucleus accumbens. With respect to the PBS groups (Figure 3a), after the banana odor was presented, there was a rapid increase in the dopaminergic responses of the pre-exposed PBS-control animals during the first half hour of the retention session. The dopamine signal then continued to rise until the end of the hour (58th min). In the case of the non-pre-exposed PBS-control animals, the dopaminergic responses recorded in the core part of the nucleus accumbens increased gradually after the olfactory stimulus was presented and until the end of the hour. With the pre-exposed PBS-conditioned animals, the dopaminergic responses rose fairly sharply following presentation of the stimulus, before peaking in the 46th minute. With the non-pre-exposed PBS-conditioned animals, there were small dopaminergic variations that oscillated around the baseline and peaked in the 60th min.
With regard to the TTX groups (Figure 3a), presentation of the olfactory stimulus in the case of the pre-exposed TTX-control animals was followed by a rise in the dopamine levels, which peaked towards the end of the hour, in the 56th min. In the case of the non-pre-exposed TTX-control animals, dopaminergic variations were characterized by a steady increase following exposure to the banana odor, reaching a maximum at the end of the hour. The dopaminergic changes with respect to the two TTX-conditioned groups differed. With the pre-exposed TTX-conditioned animals, the dopamine signal increased gradually, peaking first in the 10th min and then oscillating above the baseline for the rest of the hour. A maximal value was obtained in the 44th min. With the non-pre-exposed TTX-conditioned animals, the dopamine signal fluctuated around the basal level, peaking in the 48th min.
The overall ANOVA performed for the dopaminergic variations in the core subregion (n=47) with respect to the full hour following exposure to the olfactory stimulus highlighted significant differences with the dopaminergic variations for the conditioning factor (NaCl/LiCl) (F(1,39)=14.20; p<0.001), as well as the pre-exposure factor (non-pre-exposed/pre-exposed) (F(1,39)=9.04; p<0.005), but revealed no significant effect for the microinjection factor (PBS/TTX). The various interactions (conditioning × microinjection, microinjection × pre-exposure, pre-exposure × conditioning or conditioning × microinjection × pre-exposure) were not found to be statistically different.
Contrast analysis of ANOVA was used to test the ensuing specific hypotheses: (1) a significant effect of conditioning (NaCl/LiCl) is observed for the pre-exposed groups; (2) a significant effect of pre-exposure (non-pre-exposed/pre-exposed) is observed for the PBS-conditioned and the TTX-conditioned groups. Contrast analysis of ANOVA for dopaminergic variations recorded in the core part of the nucleus accumbens for the whole hour following presentation of the olfactory stimulus revealed significant effects of the conditioning factor (NaCl/LiCl) for pre-exposed TTX groups (pre-exposed TTX-control/pre-exposed TTX-conditioned) (F(1,39)=5.41; p<0.05). Conversely, no significant effect of the conditioning factor was observed for the pre-exposed PBS animals (pre-exposed PBS-control/pre-exposed PBS-conditioned) (F(1,39)=0.27; ns). The contrast analysis showed a significant effect of the pre-exposure factor for the PBS-conditioned animals (pre-exposed PBS-conditioned/non-pre-exposed PBS-conditioned) (F(1,39)=7.06; p<0.05), but no statistical effect for the TTX-conditioned animals (pre-exposed TTX-conditioned/non-pre-exposed TTX-conditioned) (F(1,39)=1.72; ns).
In summary, the dopaminergic variations recorded in the core part of the nucleus accumbens with respect to the pre-exposed PBS-control animals, and pre-exposed PBS-conditioned animals increased in a similar way following the CS presentation (banana odor). Increases in the pre-exposed PBS-conditioned animals were significantly different from the dopaminergic variations recorded in the non-pre-exposed PBS-conditioned animals. Regarding the pre-exposed TTX-conditioned animals, the dopaminergic responses were less pronounced and significantly different from the increases observed in the pre-exposed TTX-control animals, but not statistically different from those observed in the non-pre-exposed TTX-conditioned animals.
Dopaminergic variations recorded in the dorsomedial shell part of the nucleus accumbens. With respect to the PBS groups (Figure 3b), exposure to the olfactory stimulus in the case of the pre-exposed PBS-control group was followed during the retention session by a rapid increase in the dopaminergic signal until the end of the hour, peaking in the 60th min, whereas in the case of the non-pre-exposed PBS-control animals, variations of dopamine increased moderately throughout the hour following presentation of the banana odor, peaking in the 14th min. With respect to the pre-exposed PBS-conditioned animals, dopaminergic levels remained fairly stable and oscillated around the basal level. The non-pre-exposed PBS-conditioned animals were characterized by a marked dopaminergic increase, which peaked in the 48th min.
With regard to the TTX groups (Figure 3b), a moderate and steady increase was observed in the case of the pre-exposed TTX-control group for the whole hour following presentation of the olfactory stimulus, with a maximal value attained in the 44th min. With the non-pre-exposed TTX-control animals, the extracellular dopaminergic levels increased only slightly after the olfactory stimulus presentation, reaching a maximal value in the 48th min. With the pre-exposed TTX-conditioned animals, the dopaminergic responses remained close to the baseline for the first 15 min. Then, the dopamine signal rose sharply and remained fairly stable, peaking in the 56th min. The non-pre-exposed TTX-conditioned animals displayed a marked increase, peaking in the 56th min.
The overall ANOVA carried out for the full hour after the animals' exposure to the olfactory stimulus showed no significant difference in the dopaminergic variations in the dorsomedial shell subregion (n=44) for the conditioning factor (NaCl/LiCl), pre-exposure factor (non-pre-exposed/pre-exposed), microinjection factor (PBS/TTX), or microinjection × pre-exposure interaction and the microinjection × conditioning interaction. This analysis highlighted a significant difference for the pre-exposure × conditioning interaction (F(1,36)=19.16; p<0.0001) and the conditioning × microinjection × pre-exposure interaction (F(1,36)=4.59; p<0.05).
The following specific hypotheses were then tested with ANOVA contrast analysis: (1) a significant effect of the conditioning (NaCl/LiCl) is observed for the non-pre-exposed and pre-exposed groups; (2) the dopaminergic variations recorded in the dorsomedial shell part of the nucleus accumbens for pre-exposed PBS-conditioned animals are significantly different from, on one hand, those recorded for non-pre-exposed PBS-conditioned animals and, on the other hand, those observed for pre-exposed TTX-conditioned animals.
Contrast analysis of ANOVA for the dopaminergic variations recorded in the dorsomedial shell part of the nucleus accumbens for the whole hour following exposure to the banana odor revealed significant effects of the conditioning factor for the non-pre-exposed PBS groups (non-pre-exposed PBS-control/non-pre-exposed PBS-conditioned) (F(1,36)=7.75; p<0.01), and non-pre-exposed TTX groups (non-pre-exposed TTX-control/non-pre-exposed TTX-conditioned) (F(1,36)=5.15; P<0.05) and a significant effect of the conditioning factor for pre-exposed PBS animals (pre-exposed PBS-control/pre-exposed PBS-conditioned) (F(1,36)=14.81; p<0.0005), but not for pre-exposed TTX animals (pre-exposed TTX-control/pre-exposed TTX-conditioned). The contrast analysis also revealed a significant effect of the pre-exposure factor for PBS-conditioned animals (pre-exposed PBS-conditioned/non-pre-exposed PBS-conditioned) (F(1,36)=14.22; p<0.001) and of the microinjection factor for the pre-exposed-conditioned groups (pre-exposed PBS-conditioned/pre-exposed TTX-conditioned) (F(1,36)=4.51; p<0.05).
To sum up, the dopaminergic variations recorded during the retention session in the dorsomedial shell part of the nucleus accumbens following intervention in the left SUB at PND8 amounted to moderate increases for the non-pre-exposed PBS-control and non-pre-exposed TTX-control groups, and marked increases for the non-pre-exposed PBS-conditioned and non-pre-exposed TTX-conditioned groups. Unlike in non-pre-exposed PBS-conditioned animals, in pre-exposed PBS-conditioned animals, the dopamine signal oscillated around the baseline. For the pre-exposed TTX-conditioned animals, the dopamine increase is not significantly different from that observed in the non-pre-exposed TTX-conditioned group.
DISCUSSION
The present study was designed to investigate the consequences of neonatal functional inactivation of the left ventral SUB by TTX in 8-day-old rats for latent inhibition-related dopaminergic responses monitored in grown-up rats in the left core and left dorsomedial shell parts of the nucleus accumbens in an olfactory aversion procedure. The postnatal unilateral (left) functional blockade of the SUB was followed in pre-exposed TTX-conditioned adult rats by a disruption of latent inhibition behavior and induced a partial and total reversal of the latent inhibition-related dopaminergic responses in the core and the dorsomedial shell parts of the nucleus accumbens, respectively.
The neonatal TTX inactivation of the left SUB induced no significant behavioral and dopaminergic differences in adulthood between non-pre-exposed PBS and non-pre-exposed TTX animals. With respect to the two postnatal microinjections (PBS or TTX), the non-pre-exposed control (NaCl) and non-pre-exposed-conditioned (LiCl) groups displayed attraction or aversion towards the olfactory stimulus. These responses were similar to those previously obtained using the same conditioning procedure in adult animals free of neonatal intervention (Jeanblanc et al, 2002, 2004; Peterschmitt et al, 2008) and suggest that olfactory perception is not impaired following neonatal TTX inactivation of the SUB. Regarding pre-exposed PBS animals, the behavioral and dopaminergic responses for pre-exposed PBS-control and pre-exposed PBS-conditioned animals are consistent with the latent inhibition responses reported in the same paradigm in adult animals without neonatal PBS microinjection (Jeanblanc et al, 2002, 2004; Peterschmitt et al, 2008). Thus, it appears that the neonatal intervention in the SUB does not, by itself, induce any significant effects on the different responses observed in adult animals.
Pre-exposed TTX-conditioned adult rats displayed typical aversive responses corresponding to a complete disappearance of the latent inhibition behavior. This is in keeping with recent observations (Meyer et al, 2009). The present results show that TTX inactivation of the left SUB at PND8 induces a differential reversal of the latent inhibition-related dopaminergic responses in the two subregions of the nucleus accumbens. In the core part of the nucleus accumbens, dopaminergic responses in pre-exposed TTX-conditioned animals were in between those obtained in non-pre-exposed TTX-conditioned animals and pre-exposed PBS-conditioned animals. By contrast, in the dorsomedial shell part of the nucleus accumbens, dopaminergic changes were similar in pre-exposed TTX-conditioned animals and non-pre-exposed TTX-conditioned rats and characteristic of aversion. Contrary to the SUB neonatal inactivation, our previous data showed that the neonatal inactivation at PND8 of the left ENT, which is closely interconnected with the SUB (van Groen and Lopes da Silva, 1986; Naber et al, 2000; van Groen et al, 2003; O'Mara, 2005), induced a total disappearance of the typical latent inhibition-related dopaminergic responses in both the core and dorsomedial shell parts of the nucleus accumbens (Peterschmitt et al, 2007). These data argue in favor of an anatomical specificity of the neonatal TTX inactivation in the SUB as compared with the same inactivation of the ENT (Peterschmitt et al, 2007; Meyer et al, 2009).
No macroscopic anatomical changes are observed in adult animals subjected to postnatal TTX microinjection in the left SUB. The neonatal blockade at PND8 may affect subicular developing fibers inasmuch as in the second week after birth the afferent and efferent connections of the SUB are still maturing (Schlessinger et al, 1975; Singh, 1977a, 1977b; Fricke and Cowan, 1978). The establishment of neural networks depends in part on electrical activity involved in neuronal survival (Farber and Olney, 2003), synaptic proliferation (Marty et al, 2000), axons' myelination (Barres and Raff, 1993; Demerens et al, 1996; Zalc and Fields, 2000), the rearrangement of synaptic connections in the target structures (Stryker and Harris, 1986; Katz and Shatz, 1996; Hutchins and Kalil, 2008), and the maturation of dendritic spines (Drakew et al, 1999; Frotscher et al, 2000). The transient functional disconnection of the SUB in the critical 2-week period after birth (Clancy et al, 2001) may thus, by a combination of the afore mentioned mechanisms, permanently affect communication with structures directly connected to the SUB, in particular the nucleus accumbens (Brog et al, 1993; Groenewegen et al, 1987, 1999; Mulder et al, 1998). Indeed, the dopaminergic changes in pre-exposed TTX-conditioned animals in both the core and dorsomedial shell parts of the nucleus accumbens after SUB inactivation at PND8 are different from those observed after SUB blockade in adult animals before the pre-exposure session in previous experiments in our laboratory (Peterschmitt et al, 2008). There is no direct demonstration in the present paper of the specificity of the developmental aspect of SUB neonatal inactivation, but the differences in dopaminergic variations obtained for different ages of TTX microinjection (present study, Peterschmitt et al, 2008) are compatible with neurodevelopmental consequences of SUB postnatal inactivation.
The disappearance of the behavioral expression of latent inhibition after neonatal inactivation of the SUB is comparable with that observed after transient TTX blockade of the SUB at pre-exposure in adult animals (Peterschmitt et al, 2008). Those results have been interpreted as a consequence of the failure of the SUB to encode the CS-related information during the first session (see Peterschmitt et al, 2008, for discussion). The present results could have a similar explanation. A permanent dysfunction of the SUB following neonatal inactivation would prevent the learning and memorization of the information about the CS in the pre-exposure stage. Thus, after neonatal SUB inactivation, it is only the association between the CS and the aversion acquired during the conditioning stage that would be retrieved during the retention stage. This may explain the aversion behavior in pre-exposed TTX-conditioned animals during the retention phase.
The present data suggest neonatal inactivation of the SUB has more serious consequences for the dopaminergic responses recorded in the dorsomedial shell part of the nucleus accumbens than those recorded in the core part of the nucleus accumbens. Projections from the SUB to the two subregions of the nucleus accumbens have been described, although the SUB appears to reach predominantly the dorsomedial shell subregion (Brog et al, 1993; Groenewegen et al, 1987, 1999; Mulder et al, 1998). Regulation of dopamine release and metabolism in the nucleus accumbens by SUB projections has been reported for both the core and dorsomedial shell parts in adult animals (Louilot and Le Moal, 1994; Blaha et al, 1997; Legault and Wise, 1999; Legault et al, 2000; Taepavarapruk et al, 2000; Floresco et al, 2001; Cano-Cebrian et al, 2003). Previous data showed that unlike the SUB, neonatal blockade of the ENT induced a complete disruption of the typical latent inhibition-related dopaminergic responses in both the core and in the dorsomedial shell parts of the nucleus accumbens (Peterschmitt et al, 2007). The ENT sends projections both to the core and dorsomedial shell parts of the nucleus accumbens (Totterdell and Meredith, 1997). Therefore, a tentative explanation for the present dopaminergic changes recorded in the core could be that the control exerted by the SUB on dopamine release in the core part of the nucleus accumbens following neonatal inactivation is defective, but partially offset by the intact ENT projections. Such a compensatory mechanism would not exist for the dorsomedial shell subregion, which receives equivalent innervation from the SUB and the ENT (Brog et al, 1993; Groenewegen et al, 1987, 1999; Mulder et al, 1998; Totterdell and Meredith, 1997).
The relationships between the behavioral responses and the dopaminergic variations in the two parts of the nucleus accumbens could be considered in the context of the switching model of latent inhibition proposed by Weiner (2003), which was developed initially on the basis of lesion results, which showed that latent inhibition expression was spared after core lesions, but disrupted after shell lesions (Tai et al, 1995; Weiner et al, 1996, 1999; Jongen-Rêlo et al, 2002; Gal et al, 2005; Pothuizen et al, 2005; but see Pothuizen et al, 2006). According to this model, the core part of the nucleus accumbens is involved in a switching mechanism between responding according to the CS-reinforcement associations acquired during conditioning and responding to the CS-no event associations acquired during pre-exposure; the shell inhibiting this mechanism. Further, the two behavioral responses (CR or latent inhibition response) in this model are associated with no initial increase in dopamine and a rapid increase in dopamine release, respectively, in the core subregion (Weiner, 2003, 2010; see also Cassaday and Moran, 2010). This hypothesis would be consistent with the dopaminergic variations observed in the non-pre-exposed PBS-conditioned or non-pre-exposed TTX-conditioned animals, which showed an aversion towards the CS and dopaminergic changes in pre-exposed PBS-conditioned animals that displayed a behavioral preference towards the CS. However, the present results obtained in pre-exposed TTX-conditioned animals, which showed intermediate dopaminergic changes between the two latter groups but which showed a withdrawal behavior, are not easily interpreted with the proposed switching model alone. One interpretation would be that a given increase of the dopamine level in the core part would be necessary to switch the response according to the CS–no event associations acquired during pre-exposure. The present data could also be tentatively interpreted as reflecting a functional complementarity between the core and anterior dorsal striatal subregions (see Voorn et al, 2004). After neonatal blockade of the SUB, an absence of dopaminergic changes in the anterior part of the dorsal striatum in pre-exposed TTX-conditioned animals has recently been reported (Meyer et al, 2009).
Regarding the dorsomedial shell part of the nucleus accumbens, no increase in dopamine was obtained in the present study in the pre-exposed PBS-conditioned group displaying latent inhibition, whereas dopamine levels were increased for the three other conditioned groups displaying aversion towards the CS. These variations in conditioned animals seem fairly compatible with the switching model proposed by Weiner (see Weiner, 2003, 2010), according to which, inhibition of the switching leading to latent inhibition manifestation is thought to correspond to a decrease in dopamine levels in the dorsomedial shell, whereas disinhibition of the switching mechanism and disruption of latent inhibition would correspond to a rise in dopamine levels in this shell subregion. However, the possibility that the dopaminergic changes in the dorsomedial shell may partly reflect the salience of the stimulus acquired in a given situation (for reviews, see Horvitz, 2000; Young et al, 1998, 2005) cannot be ruled out, and this suggestion would be consistent with the increases in the dopamine signal obtained in (1) the four control groups showing an approach towards the stimulus and (2) the non-pre-exposed PBS-conditioned, non-pre-exposed TTX-conditioned, and pre-exposed TTX-conditioned groups that exhibit an aversive behavior.
To conclude, the present experimental data support the view that early functional impairment of the SUB results in a disappearance of the normal expression of the latent inhibition phenomenon and latent inhibition-related dopaminergic responses in the core and dorsomedial shell subregions of the nucleus accumbens. They could open up new prospects for modeling the pathophysiology of schizophrenia. Not only have SUB anomalies reminiscent of neurodevelopmental failures been described in schizophrenia (Arnold et al, 1995; Arnold, 2000; Rosoklija et al, 2000; Law et al, 2004), but also a reduction in latent inhibition has been reported in neuroleptic-naive patients with schizophrenia (Baruch et al, 1988; Gray et al, 1992, 1995; Lubow et al, 2000 Rascle et al, 2001). Moreover, the dorsomedial shell part of the nucleus accumbens seems to be the target structure for the common action of neuroleptics (Deutch and Cameron, 1992; Robertson and Fibiger, 1992; Jennings et al, 2006), this being consistent with enhanced dopaminergic transmission in the dorsomedial shell in acute patients. Thus, it could be tentatively suggested that an increased release of dopamine in this accumbal subregion may result in a disinhibition of the switching mechanism and the reduction of latent inhibition observed in patients with schizophrenia.
Acknowledgments
This research was supported by INSERM & Région Alsace, and Fondation de France (FM) and Electricité de France (EDF) (AL). The authors thank Séverine Eybrard for her technical assistance.
The authors declare no conflict of interest.
References
- Ambrogi Lorenzini CG, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Analysis of mnemonic processing by means of totally reversible neural inactivations. Brain Res Protoc. 1997;1:391–398. doi: 10.1016/s1385-299x(97)00017-2. [DOI] [PubMed] [Google Scholar]
- Arnold SE. Cellular and molecular neuropathology of the parahippocampal region in schizophrenia. Ann NY Acad Sci. 2000;911:275–292. doi: 10.1111/j.1749-6632.2000.tb06732.x. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Franz BR, Gur RC, Gur RE, Shapiro RM, Moberg PJ, et al. Smaller neuron size in schizophrenia in hippocampal subfields that mediate cortical-hippocampal interactions. Am J Psychiatry. 1995;152:738–748. doi: 10.1176/ajp.152.5.738. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Talbot K, Hahn CG. Neurodevelopment, neuroplasticity,and new genes for schizophrenia. Prog Brain Res. 2005;147:319–345. doi: 10.1016/S0079-6123(04)47023-X. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Baruch I, Hemsley DR, Gray JA. Differential performance of acute and chronic schizophrenics in a latent inhibition task. J Nerv Ment Dis. 1988;176:598–606. doi: 10.1097/00005053-198810000-00004. [DOI] [PubMed] [Google Scholar]
- Besson C, Louilot A. Asymmetrical involvement of mesolimbic dopaminergic neurons in affective perception. Neuroscience. 1995;68:963–968. doi: 10.1016/0306-4522(95)00255-h. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci. 1997;9:902–911. doi: 10.1111/j.1460-9568.1997.tb01441.x. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The pattern of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:225–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Frangou S, Murray RM. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res. 1997;28:143–156. doi: 10.1016/s0920-9964(97)00114-x. [DOI] [PubMed] [Google Scholar]
- Cano-Cebrian MJ, Zornoza-Sabina T, Guerri C, Polache A, Granero L. Acamprosate blocks the increase in dopamine extracellular levels in nucleus accumbens evoked by chemical stimulation of the ventral hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:324–327. doi: 10.1007/s00210-003-0795-3. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–290. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- Cassaday HJ, Moran PM.2010Latent inhibition and other salience modulation effects: same neural substratesIn: Lubow RE, Weiner I (eds).Latent Inhibition: Cognition, Neuroscience and Applications to Schizophrenia1st edn. Cambridge University Press: Cambridge; 342–371. [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, et al. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci USA. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AY, Cameron DS. Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell. Neuroscience. 1992;46:49–56. doi: 10.1016/0306-4522(92)90007-o. [DOI] [PubMed] [Google Scholar]
- Drakew A, Frotscher M, Heimrich B. Blockade of neuronal activity alters spine maturation of dentate granule cells but not their dendritic arborization. Neuroscience. 1999;94:767–774. doi: 10.1016/s0306-4522(99)00378-4. [DOI] [PubMed] [Google Scholar]
- Falkai P, Schneider-Axmann T, Honer WG. Entorhinal cortex pre-alpha cell clusters in schizophrenia: quantitative evidence of a developmental abnormality. Biol Psychiatry. 2000;47:937–943. doi: 10.1016/s0006-3223(99)00250-4. [DOI] [PubMed] [Google Scholar]
- Farber NB, Olney JW. Drugs of abuse that cause developing neurons to commit suicide. Brain Res Dev Brain Res. 2003;147:37–45. doi: 10.1016/j.devbrainres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Flecknell PA. Laboratory Animal Anaesthesia: An Introduction for Research Workers and Technicians. San Diego, CA: Academic Press; 1987. [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucher JR, Vidailhet P, Chanraud S, Gounot D, Grucker D, Pins D, et al. Functional integration in schizophrenia: too little or too much. Preliminary Results fMRI Data Neuroimage. 2005;26:374–388. doi: 10.1016/j.neuroimage.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Fricke R, Cowan WM. An autoradiographic study of the development of the entorhinal and commissural afferents of the dentate gyrus of the rat. J Comp Neurol. 1978;173:231–250. doi: 10.1002/cne.901730203. [DOI] [PubMed] [Google Scholar]
- Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30:115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Drakew A, Heimrich B. Role of afferent innervation and neuronal activity in dendritic development and spine maturation of fascia dentata granule cells. Cereb Cortex. 2000;10:946–951. doi: 10.1093/cercor/10.10.946. [DOI] [PubMed] [Google Scholar]
- Gal G, Schiller D, Weiner I. Latent inhibition is disrupted by nucleus accumbens shell lesion but is abnormally persistent following entire nucleus accumbens lesion: the neural site controlling the expression and disruption of the stimulus preexposure effect. Behav Brain Res. 2005;162:246–255. doi: 10.1016/j.bbr.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Garcia J, Lasiter PS, Bermudez-Rattoni F, Deems DA. A general theory of aversion learning. Ann NY Acad Sci. 1985;443:8–21. doi: 10.1111/j.1749-6632.1985.tb27060.x. [DOI] [PubMed] [Google Scholar]
- Gonon FG, Navarre F, Buda MJ. In vivo monitoring of dopamine release in the rat brain with differential normal pulse voltammetry. Anal Chem. 1984;56:573–575. doi: 10.1021/ac00267a060. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mora JL, Guadalupe T, Fumero B, Mas M. Mathematical resolution of mixed in vivo voltammetry signals. Models, equipment, assessment by simultaneous microdialysis sampling. J Neurosci Methods. 1991;39:231–244. doi: 10.1016/0165-0270(91)90102-6. [DOI] [PubMed] [Google Scholar]
- Gray NS, Hemsley DR, Gray JA. Abolition of latent inhibition in acute, but not chronic, schizophrenics. Neurol Psychiatry Brain Res. 1992;1:83–89. [Google Scholar]
- Gray NS, Pilowsky LS, Gray JA, Kerwin RW. Latent inhibition in drug naive schizophrenics: relationship to duration of illness and dopamine D2 binding using SPET. Schizophr Res. 1995;17:95–107. doi: 10.1016/0920-9964(95)00034-j. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. An NY Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Hutchins BI, Kalil K. Differential outgrowth of axons and their branches is regulated by localized calcium transients. J Neurosci. 2008;28:143–153. doi: 10.1523/JNEUROSCI.4548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, Hoeltzel A, Louilot A. Dissociation in the involvement of dopaminergic neurons innervating the core and shell subregions of the nucleus accumbens in latent inhibition and affective perception. Neuroscience. 2002;111:351–353. doi: 10.1016/s0306-4522(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, Peterschmitt Y, Hoeltzel A, Louilot A. Influence of the entorhinal cortex on accumbal and striatal dopaminergic responses in a latent inhibition paradigm. Neuroscience. 2004;128:187–200. doi: 10.1016/j.neuroscience.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Jennings CA, Cluderay JE, Gartlon J, Cilia J, Lloyd A, Jones DN, et al. The effects of ziprasidone on regional c-Fos expression in the rat forebrain. Psychopharmacology (Berl) 2006;184:13–20. doi: 10.1007/s00213-005-0222-1. [DOI] [PubMed] [Google Scholar]
- Jongen-Rêlo AL, Kaufmann S, Feldon J. A differential involvement of the shell and core subterritories of the nucleus accumbens of rats in attentional processes. Neuroscience. 2002;111:95–109. doi: 10.1016/s0306-4522(01)00521-8. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Zea-Ponce Y, Rodenhiser-Hill J, Mann JJ, Van Heertum RL, et al. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry. 2000;48:627–640. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Hyde TM, Kleinman JE, Harrison PJ. Reduced spinophilin but not microtubule-associated protein 2 expression in the hippocampal formation in schizophrenia and mood disorders: molecular evidence for a pathology of dendritic spines. Am J Psychiatry. 2004;161:1848–1855. doi: 10.1176/ajp.161.10.1848. [DOI] [PubMed] [Google Scholar]
- Legault M, Rompre PP, Wise RA. Chemical stimulation of the ventral hippocampus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. J Neurosci. 2000;20:1635–1642. doi: 10.1523/JNEUROSCI.20-04-01635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault M, Wise RA. Injections of N-methyl--aspartate into the ventral hippocampus increase extracellular dopamine in the ventral tegmental area and nucleus accumbens. Synapse. 1999;31:241–249. doi: 10.1002/(SICI)1098-2396(19990315)31:4<241::AID-SYN1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Louilot A, Choulli MK. Asymmetrical increases in dopamine turn-over in the nucleus accumbens and lack of changes in locomotor responses following unilateral dopaminergic depletions in the entorhinal cortex. Brain Res. 1997;778:150–157. doi: 10.1016/s0006-8993(97)01050-0. [DOI] [PubMed] [Google Scholar]
- Louilot A, Gonon F, Buda M, Simon H, Le Moal M, Pujol JF. Effects of d- and l-amphetamine on DA metabolism and ascorbic acid levels in nucleus accumbens and olfactory tubercle as studied by in vivo differential pulse voltammetry. Brain Res. 1985;336:253–263. doi: 10.1016/0006-8993(85)90652-3. [DOI] [PubMed] [Google Scholar]
- Louilot A, Gonzalez-Mora JL, Guadalupe T, Mas M. Sex-related olfactory stimuli induce a selective increase in DA release in the nucleus accumbens of male rats. A voltammetric study. Brain Res. 1991;553:313–317. doi: 10.1016/0006-8993(91)90841-i. [DOI] [PubMed] [Google Scholar]
- Louilot A, Jeanblanc J, Peterschmitt Y, Meyer F.2010Parahippocampal region-dopaminergic neuron relationships in latent inhibitionIn: Lubow RE, Weiner I (eds).Latent Inhibition: Cognition, Neuroscience and Applications to Schizophrenia Cambridge: Cambridge University Press; 319–341. [Google Scholar]
- Louilot A, Le Moal M. Lateralized interdependence between limbicotemporal and ventrostriatal dopaminergic transmission. Neuroscience. 1994;59:495–500. doi: 10.1016/0306-4522(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Louilot A, Serrano A, D'Angio M. A novel carbon fiber implantation assembly for cerebral voltammetric measurements in freely moving rats. Physiol Behav. 1987;41:227–231. doi: 10.1016/0031-9384(87)90357-x. [DOI] [PubMed] [Google Scholar]
- Lubow RE. Latent Inhibition and Conditioned Attention Theory. Cambridge University Press: New York; 1989. [Google Scholar]
- Lubow RE, Kaplan O, Abramovich P, Rudnick A, Laor N. Visual search in schizophrenia: latent inhibition and novel pop-out effects. Schizophr Res. 2000;45:145–156. doi: 10.1016/s0920-9964(99)00188-7. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: the effect of non-reinforced pre-exposure to the conditioned stimulus. J Comp Physiol Psychol. 1959;52:415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Differential impairments in reaching and grasping produced by local inactivation within the forelimb representation of the motor cortex in the cat. Exp Brain Res. 1993;94:429–443. doi: 10.1007/BF00230201. [DOI] [PubMed] [Google Scholar]
- Marty S, Wehrle R, Sotelo C. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci. 2000;20:8087–8095. doi: 10.1523/JNEUROSCI.20-21-08087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F, Peterschmitt Y, Louilot A. Postnatal functional inactivation of the entorhinal cortex or ventral subiculum has different consequences for latent inhibition-related striatal dopaminergic responses in adult rats. Eur J Neurosci. 2009;29:2035–2048. doi: 10.1111/j.1460-9568.2009.06755.x. [DOI] [PubMed] [Google Scholar]
- Mosher HS. The chemistry of tetrodotoxin. Ann NY Acad Sci. 1986;479:32–43. doi: 10.1111/j.1749-6632.1986.tb15559.x. [DOI] [PubMed] [Google Scholar]
- Mulder AB, Hodenpijl MG, Lopes da Silva FH. Electrophysiology of the hippocampal and amygdaloid projections to the nucleus accumbens of the rat: convergence, segregation, and interaction of inputs. J Neurosci. 1998;18:5095–5102. doi: 10.1523/JNEUROSCI.18-13-05095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CA, Pezze MA, Feldon J, Heidbreder C. Differential involvement of dopamine in the shell and core of the nucleus accumbens in the expression of latent inhibition to an aversively conditioned stimulus. Neuroscience. 2000;97:469–477. doi: 10.1016/s0306-4522(00)00043-9. [DOI] [PubMed] [Google Scholar]
- Naber PA, Witter MP, Lopes Silva FH. Networks of the hippocampal memory system of the rat: the pivotal role of the subiculum. Ann NY Acad Sci. 2000;911:392–403. doi: 10.1111/j.1749-6632.2000.tb06739.x. [DOI] [PubMed] [Google Scholar]
- O'Mara S. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. J Anat. 2005;207:271–282. doi: 10.1111/j.1469-7580.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill RD, Lowry JP, Mas M. Monitoring brain chemistry in vivo: voltammetric techniques, sensors, and behavioral applications. Crit Rev Neurobiol. 1998;12:69–127. doi: 10.1615/critrevneurobiol.v12.i1-2.40. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.2009The Rat Brain in Stereotaxic CoordinatesCompact 6th edn.Academic Press: New-York [Google Scholar]
- Peterschmitt Y, Hoeltzel A, Louilot A. Striatal dopaminergic responses observed in latent inhibition are dependent on the hippocampal ventral subicular region. Eur J Neurosci. 2005;22:2059–2068. doi: 10.1111/j.1460-9568.2005.04366.x. [DOI] [PubMed] [Google Scholar]
- Peterschmitt Y, Meyer F, Louilot A. Neonatal functional blockade of the entorhinal cortex results in disruption of accumbal dopaminergic responses observed in latent inhibition paradigm in adult rats. Eur J Neurosci. 2007;8:2504–2513. doi: 10.1111/j.1460-9568.2007.05503.x. [DOI] [PubMed] [Google Scholar]
- Peterschmitt Y, Meyer F, Louilot A. Differential influence of the ventral subiculum on dopaminergic responses observed in core and dorsomedial shell subregions of the nucleus accumbens in latent inhibition. Neuroscience. 2008;154:898–910. doi: 10.1016/j.neuroscience.2008.03.073. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Jongen-Rêlo AL, Feldon J, Yee BK. Double dissociation of the effects of selective nucleus accumbens core and shell lesions on impulsive-choice behaviour and salience learning in rats. Eur J Neurosci. 2005;10:2605–2616. doi: 10.1111/j.1460-9568.2005.04388.x. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Jongen-Rêlo AL, Feldon J, Yee BK. Latent inhibition of conditioned taste aversion is not disrupted, but can be enhanced, by selective nucleus accumbens shell lesions in rats. Neuroscience. 2006;137:1119–1130. doi: 10.1016/j.neuroscience.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Rascle C, Mazas O, Vaiva G, Tournant M, Raybois O, Goudemand M, et al. Clinical features of latent inhibition in schizophrenia. Schizophr Res. 2001;51:149–161. doi: 10.1016/s0920-9964(00)00162-6. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Fibiger HC. Neuroleptics increase c-fos expression in the forebrain: contrasting effects of haloperidol and clozapine. Neuroscience. 1992;46:315–328. doi: 10.1016/0306-4522(92)90054-6. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow RL. Contrasts Analysis. Focused comparisons in the Analysis of Variance. Cambridge University Press: New York; 1985. [Google Scholar]
- Rosenthal R, Rosnow RL, Rubin DB. Contrasts and Effect Sizes in Behavioral Research. Cambridge University Press: New York; 2000. [Google Scholar]
- Rosoklija G, Toomayan G, Ellis SP, Keilp J, Mann JJ, Latov N, et al. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- Rothfeld JM, Harlan RE, Shivers BD, Pfaff DW. Reversible disruption of lordosis via midbrain infusions of procaine and tetrodotoxin. Pharmacol Biochem Behav. 1986;25:857–863. doi: 10.1016/0091-3057(86)90398-9. [DOI] [PubMed] [Google Scholar]
- Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296:692–695. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975;159:149–175. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Singh SC. Comparison of electron microscopy and silver staining for the detection of the first entorhinal synapses to develop in the dentate gyrus. Anat Embryol (Berl) 1977a;151:71–79. doi: 10.1007/BF00315299. [DOI] [PubMed] [Google Scholar]
- Singh SC. The development of olfactory and hippocampal pathways in the brain of the rat. Anat Embryol (Berl) 1977b;151:183–199. doi: 10.1007/BF00297480. [DOI] [PubMed] [Google Scholar]
- Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986;6:2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF. DA, schizophrenia, mania, and depression: toward a unified hypothesis of cortico-striato-pallido-thalamic function. Behav Brain Sci. 1987;10:197–245. [Google Scholar]
- Tai CT, Cassaday HJ, Feldon J, Rawlins JN. Both electrolytic, and excitotoxic lesions of nucleus accumbens disrupt latent inhibition of learning in rats. Neurobiol Learn Mem. 1995;64:36–48. doi: 10.1006/nlme.1995.1042. [DOI] [PubMed] [Google Scholar]
- Taepavarapruk P, Floresco SB, Phillips AG. Hyperlocomotion and increased dopamine efflux in the rat nucleus accumbens evoked by electrical stimulation of the ventral subiculum: role of ionotropic glutamate and dopamine D1 receptors. Psychopharmacology (Berl) 2000;151:242–251. doi: 10.1007/s002130000376. [DOI] [PubMed] [Google Scholar]
- Totterdell S, Meredith GE. Topographical organization of projections from the entorhinal cortex to the striatum of the rat. Neuroscience. 1997;78:715–729. doi: 10.1016/s0306-4522(96)00592-1. [DOI] [PubMed] [Google Scholar]
- van Groen T, Lopes da Silva FH. Organization of the reciprocal connections between the subiculum and the entorhinal cortex in the cat: II. An electrophysiological study. J Comp Neurol. 1986;251:111–120. doi: 10.1002/cne.902510108. [DOI] [PubMed] [Google Scholar]
- van Groen T, Miettinen P, Kadish I. The entorhinal cortex of the mouse: organization of the projection to the hippocampal formation. Hippocampus. 2003;13:133–149. doi: 10.1002/hipo.10037. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Lipska BK. Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: a search for common ground. Schizophr Res. 1995;16:87–110. doi: 10.1016/0920-9964(95)00013-c. [DOI] [PubMed] [Google Scholar]
- Weiner I. The “two-headed” latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacology. 2003;169:257–297. doi: 10.1007/s00213-002-1313-x. [DOI] [PubMed] [Google Scholar]
- Weiner I.2010What the brain teaches us about latent inhibition (LI): the neural substrates of the expression and prevention of LIIn: Lubow RE, Weiner I (eds).Latent Inhibition: Cognition, Neuroscience and Applications to Schizophrenia Cambridge University Press: Cambridge; 372–415. [Google Scholar]
- Weiner I, Gal G, Feldon J. Disrupted and undisruptable latent inhibition following shell and core lesions. Ann NY Acad Sci. 1999;877:723–727. doi: 10.1111/j.1749-6632.1999.tb09310.x. [DOI] [PubMed] [Google Scholar]
- Weiner I, Gal G, Rawlins JN, Feldon J. Differential involvement of the shell and core subterritories of the nucleus accumbens in latent inhibition and amphetamine-induced activity. Behav Brain Res. 1996;81:123–133. doi: 10.1016/s0166-4328(96)00051-4. [DOI] [PubMed] [Google Scholar]
- Young AM, Ahier RG, Upton RL, Joseph MH, Gray JA. Increased extracellular dopamine in the nucleus accumbens of the rat during associative learning of neutral stimuli. Neuroscience. 1998;83:1175–1183. doi: 10.1016/s0306-4522(97)00483-1. [DOI] [PubMed] [Google Scholar]
- Young AM, Joseph MH, Gray JA. Latent inhibition of conditioned dopamine release in rat nucleus accumbens. Neuroscience. 1993;54:5–9. doi: 10.1016/0306-4522(93)90378-s. [DOI] [PubMed] [Google Scholar]
- Young AM, Moran PM, Joseph MH. The role of dopamine in conditioning and latent inhibition: what, when, where and how. Neurosci Biobehav Rev. 2005;29:963–976. doi: 10.1016/j.neubiorev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Zalc B, Fields RD. Do action potentials regulate myelination. Neuroscientist. 2000;6:5–13. doi: 10.1177/107385840000600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuravin IA, Bures J. Extent of the tetrodotoxin induced blockade examined by pupillary paralysis elicited by intracerebral injection of the drug. Exp Brain Res. 1991;83:687–690. doi: 10.1007/BF00229849. [DOI] [PubMed] [Google Scholar]