Abstract
Skeletal adaptations to reduced function are an important source of skeletal variation and may be indicative of environmental pressures that lead to evolutionary changes. Humans serve as a model animal to investigate the effects of loss of craniofacial function through edentulation. In the human maxilla, it is known that edentulation leads to significant changes in skeletal structure such as residual ridge resorption and loss of cortical thickness. However, little is known about changes in bone tissue structure and material properties, which are also important for understanding skeletal mechanics but are often ignored. The aims of this study were to determine cortical material properties in edentulous crania and to evaluate differences with dentate crania and thus examine the effects of loss of function on craniofacial structure. Cortical bone samples from fifteen edentulous human skulls were measured for thickness and density. Elastic properties and directions of maximum stiffness were determined by using ultrasonic techniques. These data were compared to those from dentate crania reported in a previous investigation. Cortical bone from all regions of the facial skeleton of edentulous individuals is thinner than in dentate skulls. Elastic and shear moduli, and density are similar or greater in the zygoma and cranial vault of edentulous individuals, while these properties are less in the maxilla. Most cortical bone, especially in edentulous maxillae, has reduced directional orientation. The loss of significant occlusal loads following edentulation may contribute to the change in material properties and the loss of orientation over time during the normal process of bone remodeling. These results suggest that area-specific cortical microstructural changes accompany bone resorption following edentulation. They also suggest that functional forces are important for maintaining bone mass throughout the craniofacial skeleton, even in areas such as the browridges, which have been thought to be little affected by function, because of low in vivo strains found there in several primate studies.
Keywords: cortical bone, biomechanics, function, adaptation, ultrasonic
INTRODUCTION
Bone structure in vertebrates reflects both the evolutionary history of the species and physiological and behavioral adaptations to environmental change within an organism. While it is notoriously difficult to sort the former from the latter, much work has documented some effects of physiological adaptation on craniofacial skeletal structure. Such studies help establish the limits of physiological, ecological, and functional effects on the bone structure of an organism and are equally as important as studies of phylogenetic structural variation in attempting to understand variations in fossil forms. Here craniofacial cortical bone structure and material properties are examined in a natural experiment, which relates the loss of craniofacial function in humans with the loss of the dentition during aging. The findings of this research are important for studies of primate craniofacial evolutionary change, especially in animals closely related to humans, because of the potential mechanistic implications for understanding the limits of functional effects on craniofacial form.
The relationship between bone structure and function in the craniofacial skeleton of primates can be explored by evaluation of changes in craniofacial structural loss following tooth loss. Experiments involving tooth extraction in primates as experimental animals would be unethical; however, because edentulation in humans is quite common, humans can be used as a model primate to examine the structural and material effects accompanying loss of function.
Gross changes in human alveolar bone with edentulation are well known (Jaul et al., 1980), and radiographic and SEM techniques have been developed to evaluate bone loss (Kingsmill and Boyde, 1998, 1999). In the human maxilla, residual ridge resorption, due to disuse atrophy, leads to significant changes in facial structure, although not overall facial height (Tallgren, 1972), and the rate of resorption is slower and four times less than that found in the mandible (Klemmetti and Vainio, 1994). The ultimate effects of tooth loss on human bone structure may be greater in the maxilla than in the mandible, especially in women afflicted with osteoporosis (Jeffcoat, 1993). In both jaws, residual alveolar ridge loss leads to poor prosthesis fit, and may lead to pathologic fractures in the supporting skeleton.
Studies of craniofacial structural changes following tooth loss have focused primarily on alveolar structures because of the importance of these changes for restoring partial function with the fitting of removable dental prostheses. Little attention has been paid to changes in the skeleton of the upper face or cranial vault because of its lesser importance in human functional dental restoration, and because of the apparent lack of change in gross craniofacial dimensions.
The exploration of corresponding changes within cortical bone structures of the human alveolar region or other parts of the craniofacial skeleton has been limited. Osteopenic effects with functional loss and aging have been reported (Bras et al., 1983), and in the mandible these effects often contribute to a larger amount of malunion or nonunion of fractures in edentulous human patients (Buchbinder, 1993). Likewise, these effects suggest that changes in craniofacial form, elastic properties, strength, and density are often concurrent.
The relationship between function and structure of bone is important for understanding the development, growth, and adaptation of primate skeletons. Bone remodeling, which is responsible for the maintenance of the cortical bone structure and physical properties, is altered by mechanical stimuli (Ascenzi, 1988; Burr et al., 1989, Smit and Burger, 2000). Observations suggest that the direction in which the Haversian systems are oriented corresponds to the direction of the bone’s greatest stiffness (Ascenzi, 1988; Lanyon and Rubin, 1985, Petrtyl et al., 1996, Dechow et al., 2008). However, there is little information on changes in microstructure or elastic properties of bone where loading has been substantially reduced or redirected, such as in the human maxilla following tooth loss.
Previous work shows differences in material properties between cortical bone from human dentate and edentulous mandibles (Schwartz-Dabney and Dechow, 2002). Although no differences exist in cortical bone density, significant differences are found in cortical thickness, elastic anisotropy and shear moduli. There are also differences in the orientation of maximum stiffness in some mandibular regions. It is speculated that these differences reflect changes in function. For instance, the mandibular ramus may become thinner as a secondary response to a decrease in muscular activity and thus loading of the bone (Veyrune and Mioche, 2000).
The aim of this research is to document the material properties of the edentulous craniofacial complex and compare these to data previously reported from dentate individuals (Peterson and Dechow, 2003; Peterson et al., 2006). Of particular interest are differences in the cortical bone of edentulous and dentate individuals in the maxilla, zygoma, and specific areas of the frontal and temporal bones that are important in primate orofacial function. We hypothesize that edentulous craniofacial skeletons, like dentate mandibles, differ regionally in cortical material properties. Studies in primates including humans suggest that bone structure in the upper face (browridges and other portions of the frontal bone and cranial vault) are likely not much influenced by masticatory function because of the low bone strains found in these regions during orofacial function (Hylander et al., 1991; Ravosa, 1991; Ravosa et al., 2000a,b; Kupczik et al., 2009), and because other aspects of local and systemic growth and calcium metabolism, spacial structural relationships, and allometry provide adequate explanations of variation in the craniofacial form of this region (see Lieberman, 2000 for a summary of arguments and related literature). Thus, it is hypothesized that tooth loss will have a marked effect, similar to that observed in the mandible, on the material properties of cortical bone in high-strain areas of the face (e.g., the alveolar and zygomatic regions). In contrast, little effect in the low-strain regions of the upper face and cranial vault is predicted.
MATERIALS and METHODS
Fifteen edentulous human crania (11 females aged 57 to 100 years of age, and four males aged 58 to 78 years of age) and fifteen dentate crania (seven females aged 48 to 95 years of age, and eight males aged 50 to 89 years of age) comprised this study population. Medical status was available for all specimens and no specimen was collected from cadavers known to have died from primary bone diseases. Edentulous human cadavers typically arrived in the willed body facility with dentures included. But it was not possible to know the dental history of these individuals; nor was it possible to assess denture wear in all of those crania, where we obtained the head only, because the dentures are often removed at the time of specimen preparation. It is reasonable to assume that the majority if not all edentulous individuals were denture wearers, because the State of Texas does not allow the use of indigent individuals in willed body programs. Body donation is a practice that involves active planning, which usually occurs years before death.
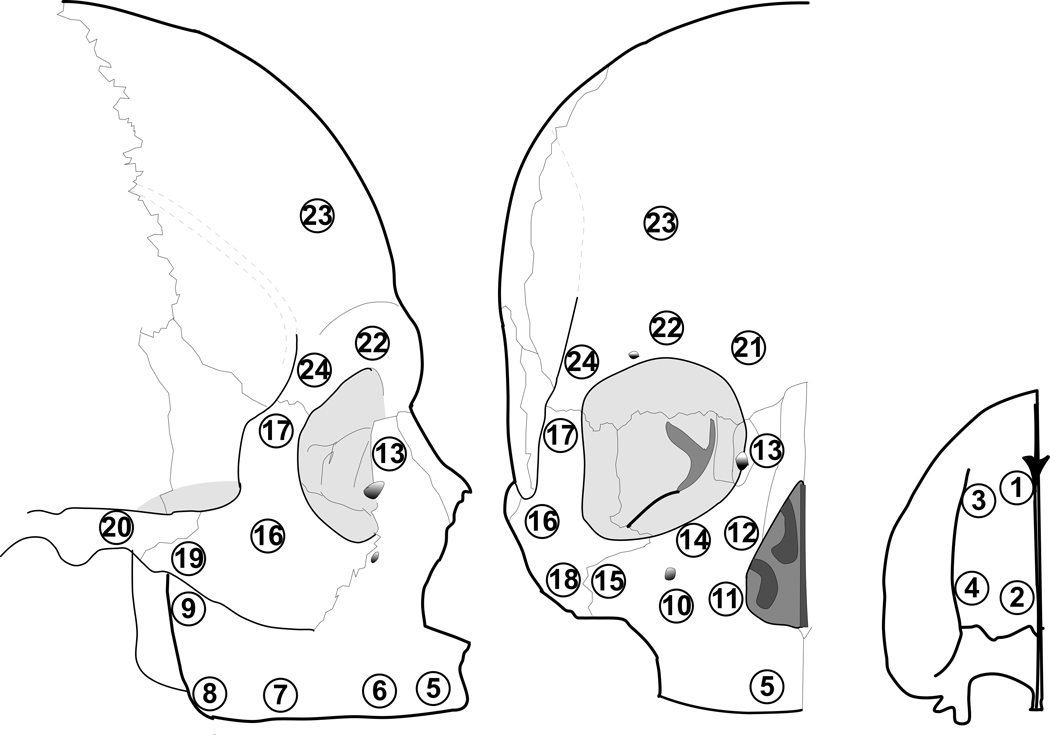
Data for the dentate crania were reported previously (Peterson and Dechow, 2003; Peterson et al., 2006). These data were collected in the same laboratory using the same methods as in the currently described study. All crania were frozen shortly after death and maintained in a fresh (unembalmed) condition. Crania were stored in freezers at −10°C prior to removal of bone specimens. The freezing process has a minimal effect on the elastic properties of the bone (Zioupos and Curry, 2000). Bone cylinders were harvested from 4 frontal sites, 1 temporal site, 15 maxillary sites and 4 zygomatic sites as illustrated in Fig. 1. The distribution of specimens across the cortical surface of bone provided a representative sample of the elastic properties of each bone dependent on functional regions in the mid- and upper face (Peterson and Dechow, 2003; Peterson et al., 2006).
Figure 1.
Location of the 24 sites from which cortical bone specimens were removed from the edentulous crania. The small figure on the right shows the sites on the hard palate (inferior view, anterior at top).
Methods for cortical specimen preparation and storage, measurements for thickness and density, and ultrasonic methods for assessing material properties, including elastic moduli, shear moduli, and Poisson’s ratios, are described in great detail in previous publications (Ashman et al., 1984; Dechow and Hylander, 2000, Schwartz-Dabney and Dechow, 2002; Peterson et al., 2006; Wang and Dechow, 2006, Wang et al., 2006, in press). Briefly, sites were marked with a graphite line parallel to the lower border of the mandibular corpus (the mandibular plane). Bone cylinders with an inner diameter of 4 mm were harvested by means of a slow-speed dental handpiece and Nobelpharma trephine burs. Following specimen cutting, endosteal cancellous bone was removed with a Tormek water-cooled fine grinding wheel until there were no visible porosities on the endosteal surface. All cortical samples were measured with a digital caliper to the nearest 0.01 mm to verify diameter (4 mm) and determine cortical thickness, defined as the distance from the periosteum to the cortical-trabecular interface. Apparent density was calculated (mg/cm3) based on dry and wet weight measurements to the nearest 0.001 g with a Mettler-PM460 analytical balance and densitometry apparatus. Cortical bone samples after preparation were kept hydrated in equal proportions of 95% ethanol and isotonic saline, which maintains the elastic properties of bone over time (Ashman et al., 1984; Zioupus et al., 2000). Thickness, density, and a set of longitudinal and transverse ultrasonic velocities were measured on each specimen to allow calculation of the elastic properties in three dimensions. In this technique, longitudinal ultrasonic waves were generated by Panametrics V312-N-SU transducers resonating at 10 MHz. The transducers were powered with a Hewlett Packard Model8100A pulse generator. Pulse delays induced by passage of ultrasonic waves through the bone were read on a Tektronix TD3012B digitizing oscilloscope. The bone cylinder was mounted on a 4-in Sherline Rotary Table (P/N 3700), which allowed accurate rotation to 0.1 degree. Pulse delays for each specimen were measured at 22.5-degree angular intervals up to 180 degrees. Ultrasonic velocities were calculated by dividing the specimen thickness or diameter by the recorded time delay minus the standard system delay. To minimize error, all measurements were repeated twice, and the mean value of the two measurements was used for analysis, unless they showed large differences. In this case, measurements were repeated until consistent results were obtained. Samples which gave readings with inconsistencies greater than 5% were discarded.
To minimize the angular errors incurred by the direct method described previously, we used a routine in Mathcad to fit the data for each bone specimen to a sine function [Y=a*sin(X+b)+c] following Wang and Dechow (2006). The coefficients a, b, and c corresponded to the orientation of the axes of maximum stiffness, the average velocity, and the maximum deviation of the curve from the average velocity. For consistency in comparison, angles for the dentate specimens were recalculated, although differences from previously reported values were minimal. A second minor difference was in the calculation of the final reported values of the Poisson’s ratios (ν). The equations used to calculate the technical constants assume that the material is orthotropic, that is the elastic moduli (E) are different between three mutually perpendicular directions, but are similar within each of these directions. Thus, νjiEi= νijEj, and Poisson’s ratios within the same plane, for example, ν12 and ν21, are identical. Thus, only one of each of the three pairs of Poisson’s ratios, ν12, ν31, and ν23, are reported.
Data were stored in Microsoft EXCEL spreadsheets and analyzed using the Minitab statistical analysis program 14.1 (Minitab Inc., State College, PA). Descriptive statistics were calculated for all measurements. We used a balanced, unrestricted analysis of variance (ANOVA) with a repeated measures design and subject as the repeating factor to test for overall differences among sites in edentulous skulls.
Statistical differences within dentate skulls are not reported, because they have been described previously (Peterson and Dechow, 2003; Peterson et al., 2006). Rather dentate data were used for statistical comparison with data from the edentulous group. ANOVA indicated that the dentate and edentulous samples were not significantly different in age. The proportion of males to females in the edentulous sample (4 to 11) differed from the dentate sample (8 to 7), but tests of differences in material properties by sex within each group showed no significant differences. Given that sex did not have a significant effect on material properties, males and females were pooled within each sample.
For statistical analysis of angular measurements, Oriana Software for Windows Version 2 was used (Kovach Computing Services, Anglesey, Wales, UK). Raleigh’s uniformity test (Zar, 1999) was used to determine whether the means of maximum stiffness were significant (directions of means are oriented and not random). If angular means were significant, differences between them were tested with a generalization of the Watson-Williams test adapted for circular distributions.
RESULTS
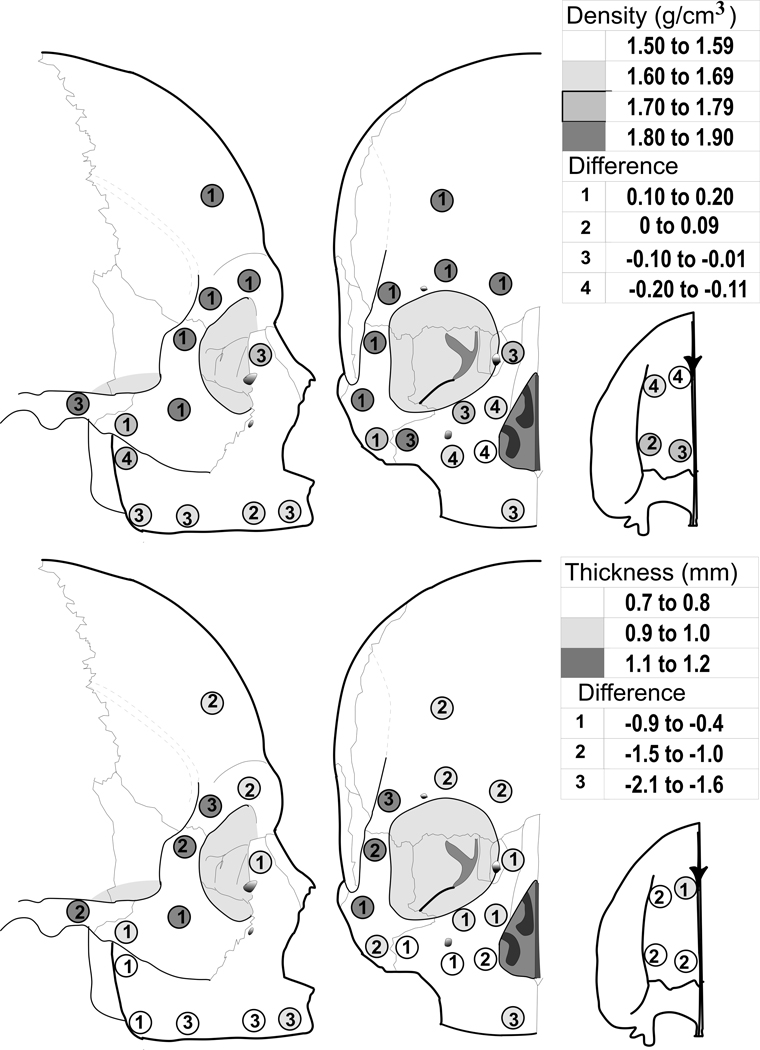
Cortical thickness in edentulous skulls, unlike dentate skulls (Peterson and Dechow, 2003; Peterson et al., 2006) was not significantly different among sites (Fig. 2 and Table 1) with means ranging between 0.7mm and 1.2mm. Cortical bone was significantly thinner in edentulous crania compared to dentate skulls throughout all sites (p=.001), with differences in mean values ranging from 0.4mm to 2.1mm less thick. Regions with the least differences in thickness were those with thinnest cortical bone in the dentate skulls.
Figure 2.
Top: Average cortical plate density in the edentulous crania (shading) and difference compared with dentate crania (numbers). Bottom: Average cortical plate thickness in the edentulous crania (shading) and difference compared with dentate crania (numbers).
Table 1.
Cortical Thickness (mm) and Density (g/cm3)
| Cortical Thickness | Density | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Site | Dentate | Edentate | Dif | Dentate | Edentate | Dif | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Maxilla | 1 | 1.8 | 0.5 | 1.0 | 0.3 | −0.8 | 1.67 | 0.10 | 1.54 | 0.20 | −0.13 |
| 2 | 2.0 | 0.9 | 0.8 | 0.2 | −1.2 | 1.74 | 0.13 | 1.73 | 0.14 | −0.01 | |
| 3 | 2.7 | 1.0 | 0.8 | 0.3 | −1.9 | 1.81 | 0.15 | 1.67 | 0.16 | −0.14 | |
| 4 | 2.3 | 0.8 | 0.8 | 0.2 | −1.5 | 1.65 | 0.15 | 1.74 | 0.17 | 0.09 | |
| 5 | 2.9 | 1.7 | 1.0 | 0.3 | −1.9 | 1.75 | 0.17 | 1.69 | 0.10 | −0.06 | |
| 6 | 2.8 | 1.5 | 0.7 | 0.2 | −2.1 | 1.59 | 0.16 | 1.66 | 0.15 | 0.07 | |
| 7 | 2.3 | 0.9 | 0.7 | 0.2 | −1.6 | 1.72 | 0.21 | 1.68 | 0.14 | −0.04 | |
| 8 | 1.5 | 0.7 | 0.7 | 0.2 | −0.8 | 1.65 | 0.09 | 1.63 | 0.18 | −0.02 | |
| 9 | 1.1 | 0.2 | 0.7 | 0.3 | −0.4 | 1.82 | 0.13 | 1.71 | 0.18 | −0.11 | |
| 10 | 1.3 | 0.5 | 0.7 | 0.2 | −0.6 | 1.78 | 0.16 | 1.65 | 0.22 | −0.13 | |
| 11 | 1.8 | 0.7 | 0.8 | 0.2 | −1.0 | 1.70 | 0.15 | 1.52 | 0.20 | −0.18 | |
| 12 | 1.5 | 0.4 | 1.0 | 0.3 | −0.5 | 1.80 | 0.11 | 1.70 | 0.18 | −0.10 | |
| 13 | 1.4 | 0.4 | 1.0 | 0.3 | −0.4 | 1.82 | 0.18 | 1.74 | 0.13 | −0.08 | |
| 14 | 1.8 | 0.5 | 0.9 | 0.2 | −0.9 | 1.80 | 0.10 | 1.77 | 0.13 | −0.03 | |
| 15 | 1.2 | 0.3 | 0.7 | 0.3 | −0.5 | 1.89 | 0.13 | 1.81 | 0.18 | −0.08 | |
| Zygomatic | 16 | 2.0 | 0.5 | 1.2 | 0.5 | −0.8 | 1.69 | 0.21 | 1.83 | 0.07 | 0.14 |
| 17 | 2.3 | 0.5 | 1.2 | 0.3 | −2.0 | 1.75 | 0.19 | 1.85 | 0.05 | 0.10 | |
| 18 | 2.2 | 0.4 | 0.9 | 0.4 | −1.3 | 1.59 | 0.16 | 1.80 | 0.14 | 0.21 | |
| 19 | 2.0 | 0.6 | 1.0 | 0.2 | −1.0 | 1.64 | 0.19 | 1.75 | 0.14 | 0.11 | |
| Temporal | 20 | 2.2 | 0.5 | 1.1 | 0.4 | −1.1 | 1.88 | 0.11 | 1.85 | 0.10 | −0.03 |
| Frontal | 21 | 2.5 | 0.4 | 1.0 | 0.3 | −1.5 | 1.76 | 0.11 | 1.89 | 0.06 | 0.13 |
| 22 | 2.4 | 0.5 | 1.0 | 0.3 | −1.4 | 1.76 | 0.15 | 1.87 | 0.10 | 0.12 | |
| 23 | 2.7 | 0.5 | 1.0 | 0.3 | −1.7 | 1.75 | 0.15 | 1.91 | 0.06 | 0.16 | |
| 24 | 2.2 | 0.5 | 1.1 | 0.4 | −1.1 | 1.75 | 0.12 | 1.85 | 0.07 | 0.10 | |
| ANOVA | F | P | F | P | |||||||
| Sites | 3.09 | NS | 3.1 | 0.05 | |||||||
| Edent. vs. Dent | 18.4 | 0.001 | 127.6 | 0.001 | |||||||
Cortical density in edentulous skulls was significantly different among sites (p=0.05) (Fig. 2 and Table 1). Cortical bone from the alveolar sites (#5–8), the anterior palate (#1 and 3), and the inferior part of the body of the maxilla (#10–12) was the least dense while cortical bone from the supraorbit (#21–24), lateral orbital portion of the zygoma (#16 and 17), and posterior zygomatic arch (#20) was the most dense. The densest sites (>1800 mg/cm3) were also the thickest sites, such as those located at the lateral orbit (#17 and 24) and temporal bone (#20). Cortical bone from the posterior palate (#2 and 4), and portions of the zygoma (#18 and 19) and the body of the maxilla (#9, 13, and 14) were intermediate in density. There were significant differences in density between cortical samples from dentate and edentulous skulls (p=0.001). Overall, cortical bone from the maxilla and posterior zygomatic arch from the edentulous group were up to 200mg/cm3 or 13.2% less dense (site #18). This result contrasted with the upper and mid-facial sites (frontal and zygomatic bones) in the edentulous group, which were more dense than similar bone from the dentate group.
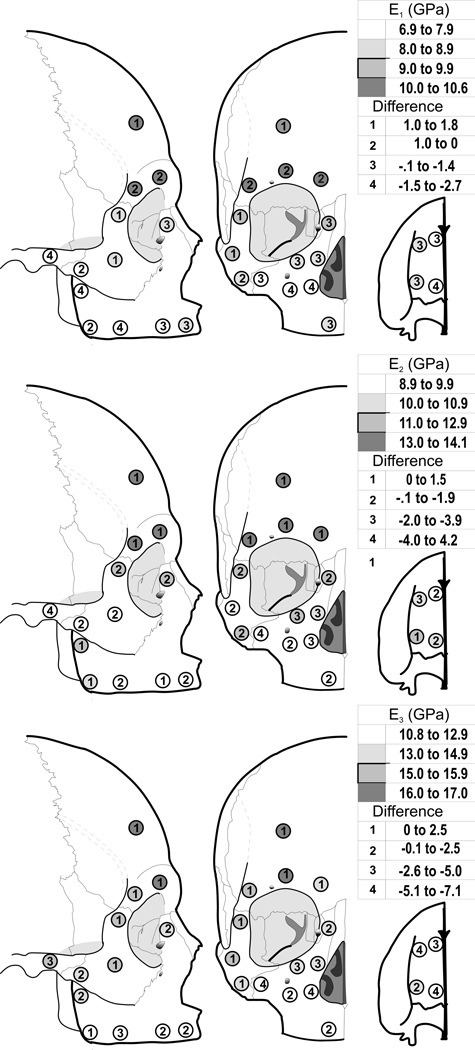
Patterns of statistically significant regional variation in elastic moduli (E1, E2 and E3) in edentulous crania (Fig. 3, and Table 2) were similar to those in cortical density. Stiffest bone was found in the supraorbital and cranial vault region, and least stiff bone was found in the alveolar region. Cortical bone on the palate, zygoma, and lateral, medial, and inferior to the orbit had intermediate amounts of stiffness. Differences between edentulous and dentate skulls were also significant. Cortical bone superior to the orbit tended to be on average slightly more stiff than that in dentate individuals, while all other sites varied from being slightly less stiff to much less stiff (up to 38%), especially in some regions of the maxilla.
Figure 3.
Average cortical bone elastic modulus E1 (Top), E2 (Middle), and E3 (Bottom) in the edentulous crania (shading) and difference compared with dentate crania (numbers).
Table 2.
Elastic moduli (GPa)
| E1 | E2 | E3 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Sit e |
Dentate | Edentate | Dif | Dentate | Edentate | Dif | Dentate | Edentate | Dif | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Maxilla | 1 | 8.3 | 1.9 | 7.8 | 1.3 | 0.5 | 11.3 | 2.7 | 9.9 | 1.5 | 1.4 | 14.1 | 2.9 | 11.5 | 2.2 | 2.6 |
| 2 | 8.9 | 1.9 | 7.3 | 0.9 | 1.6 | 11.9 | 2.3 | 10.8 | 1.5 | 1.1 | 16.5 | 4.0 | 11.4 | 2.5 | 5.1 | |
| 3 | 10.3 | 2.0 | 8.9 | 2.5 | 1.4 | 13.6 | 2.1 | 10.5 | 1.6 | 3.1 | 17.3 | 3.4 | 11.7 | 1.9 | 5.6 | |
| 4 | 8.9 | 2.9 | 8.6 | 1.5 | 0.3 | 10.9 | 2.7 | 11.1 | 1.4 | −0.2 | 15.6 | 3.7 | 14.5 | 2.4 | 1.1 | |
| 5 | 10.0 | 3.3 | 8.7 | 1.8 | 1.3 | 11.0 | 2.7 | 9.9 | 1.8 | 1.1 | 14.3 | 3.8 | 11.9 | 2.1 | 2.4 | |
| 6 | 7.2 | 1.5 | 6.9 | 1.2 | 0.3 | 8.7 | 2.3 | 9.5 | 2.0 | −0.8 | 12.2 | 1.9 | 11.1 | 2.1 | 1.1 | |
| 7 | 9.8 | 2.4 | 7.2 | 1.0 | 2.6 | 11.3 | 3.0 | 9.8 | 1.7 | 1.5 | 16.0 | 4.3 | 11.5 | 2.5 | 4.5 | |
| 8 | 6.9 | 1.1 | 7.1 | 1.3 | −0.2 | 8.8 | 1.0 | 10.3 | 3.4 | −1.5 | 10.5 | 1.3 | 11.8 | 2.5 | −1.3 | |
| 9 | 9.8 | 2.3 | 7.6 | 2.7 | 2.2 | 11.7 | 1.4 | 11.9 | 2.6 | −0.2 | 15.6 | 2.8 | 13.9 | 3.2 | 1.7 | |
| 10 | 7.6 | 2.3 | 7.4 | 1.3 | 0.2 | 10.7 | 3.3 | 10.5 | 1.8 | 0.2 | 14.2 | 4.2 | 12.3 | 2.8 | 1.9 | |
| 11 | 9.0 | 1.9 | 7.2 | 1.5 | 1.8 | 11.2 | 2.2 | 8.9 | 1.8 | 2.3 | 16.4 | 3.6 | 10.8 | 2.4 | 5.6 | |
| 12 | 10.0 | 1.7 | 8.7 | 1.2 | 1.3 | 13.5 | 1.6 | 10.4 | 2.4 | 3.1 | 17.6 | 3.4 | 13.1 | 3.3 | 4.5 | |
| 13 | 9.9 | 3.0 | 9.1 | 1.0 | 0.8 | 12.8 | 2.8 | 11.4 | 1.5 | 1.4 | 17.0 | 3.3 | 14.5 | 2.2 | 2.5 | |
| 14 | 9.4 | 1.6 | 8.8 | 1.2 | 0.6 | 13.3 | 2.1 | 11.4 | 2.2 | 1.9 | 17.8 | 2.3 | 14.8 | 2.3 | 3.0 | |
| 15 | 9.2 | 1.5 | 7.8 | 1.3 | 1.4 | 14.0 | 1.7 | 9.8 | 1.3 | 4.2 | 18.7 | 3.4 | 11.6 | 1.7 | 7.1 | |
| Zygomati c |
16 | 7.7 | 1.6 | 9.5 | 1.9 | −1.8 | 12.9 | 2.6 | 12.4 | 1.5 | 0.5 | 15.4 | 3.4 | 15.4 | 3.5 | 0 |
| 17 | 8.2 | 1.8 | 9.8 | 1.4 | −1.6 | 11.9 | 2.3 | 11.6 | 1.8 | 0.3 | 15.2 | 2.0 | 15.9 | 2.2 | −0.7 | |
| 18 | 7.8 | 2.4 | 8.7 | 0.9 | −0.9 | 11.8 | 2.0 | 11.4 | 1.2 | 0.4 | 14.1 | 3.1 | 14.7 | 2.2 | −0.6 | |
| 19 | 8.0 | 2.9 | 8.3 | 1.3 | −0.3 | 10.7 | 2.8 | 10.8 | 1.8 | −0.1 | 13.8 | 3.6 | 13.6 | 2.9 | 0.2 | |
| Temporal | 20 | 10.2 | 1.8 | 7.5 | 1.6 | 2.7 | 13.5 | 1.5 | 9.5 | 1.5 | 4.0 | 18.9 | 3.1 | 15.4 | 3.2 | 3.5 |
| Frontal | 21 | 9.5 | 1.5 | 10.2 | 1.0 | −0.7 | 12.4 | 2.5 | 13.2 | 2.1 | −0.8 | 14.5 | 2.4 | 14.7 | 2.4 | −0.2 |
| 22 | 9.5 | 1.2 | 10.0 | 1.5 | −0.5 | 13.3 | 1.1 | 14.1 | 1.8 | −0.8 | 16.0 | 2.4 | 16.4 | 2.8 | −0.4 | |
| 23 | 9.3 | 2.0 | 10.6 | 0.6 | −1.3 | 12.5 | 2.5 | 13.6 | 1.5 | −1.1 | 14.5 | 3.1 | 17.0 | 1.5 | −2.5 | |
| 24 | 9.4 | 1.8 | 10.0 | 2.2 | −0.6 | 12.8 | 3.3 | 13.5 | 2.4 | −0.7 | 15.4 | 4.1 | 15.4 | 1.4 | 0 | |
| ANOVA | F | P | F | P | F | P | ||||||||||
| Sites | 2.26 | 0.001 | 3.41 | 0.001 | 3.55 | 0.001 | ||||||||||
| Edent. vs. Dent | 18.01 | 0.001 | 38.71 | 0.001 | 44.10 | 0.001 | ||||||||||
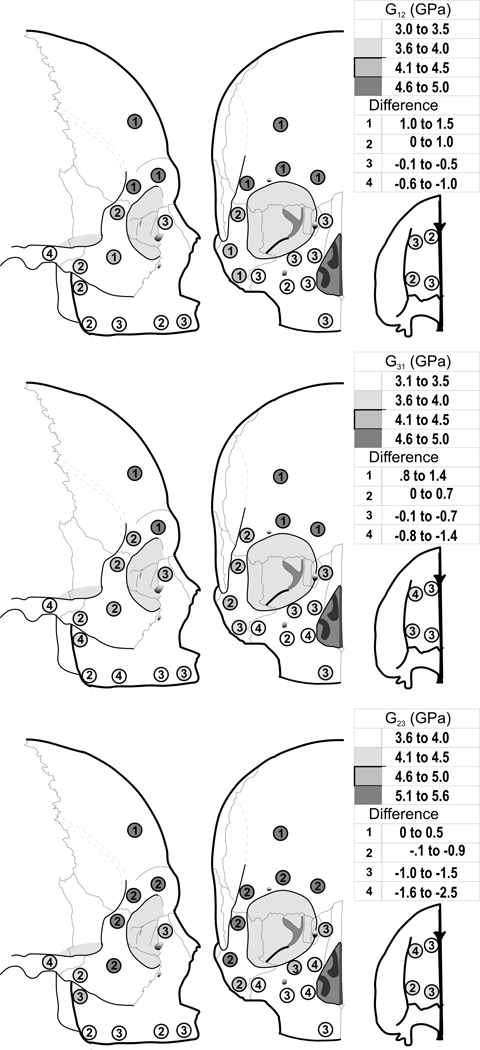
Shear moduli, G12, G31, and G23, showed statically significant differences among sites in the edentulous crania, and statistically significant differences between edentulous and dentate crania (Fig. 4, Table 3). These patterns of differences were similar to those found for elastic moduli.
Figure 4.
Average cortical bone shear modulus G12 (Top), G31 (Middle), and G23 (Bottom) in the edentulous crania (shading) and difference compared with dentate crania (numbers).
Table 3.
Shear moduli (GPa)
| G12 | Dif | G31 | Dif | G23 | Dif | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Site | Dentate | Edentate | Dentate | Edentate | Dentate | Edentate | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Maxilla | 1 | 3.4 | 0.9 | 3.4 | 0.4 | 0 | 3.7 | 0.7 | 3.4 | 0.4 | 0.3 | 4.9 | 1.2 | 3.8 | 0.7 | 1.1 |
| 2 | 3.6 | 0.8 | 3.5 | 0.6 | 0.1 | 4.1 | 0.8 | 3.5 | 0.5 | 0.6 | 5.4 | 1.0 | 4.2 | 0.6 | 1.2 | |
| 3 | 4.1 | 0.6 | 3.6 | 0.7 | 0.5 | 4.5 | 1.0 | 3.6 | 0.5 | 0.9 | 5.9 | 1.1 | 4.1 | 0.7 | 1.8 | |
| 4 | 3.5 | 1.2 | 3.9 | 0.8 | −0.4 | 4.0 | 1.0 | 3.9 | 0.5 | 0.1 | 5.1 | 1.1 | 4.5 | 0.6 | 0.6 | |
| 5 | 3.8 | 1.2 | 3.6 | 0.6 | 0.2 | 4.2 | 1.3 | 3.7 | 0.7 | 0.5 | 4.9 | 1.4 | 3.8 | 0.7 | 1.1 | |
| 6 | 2.8 | 0.8 | 3.1 | 0.4 | −0.3 | 3.5 | 0.4 | 3.1 | 0.5 | 0.4 | 4.2 | 0.9 | 3.7 | 0.8 | 0.5 | |
| 7 | 3.7 | 1.0 | 3.2 | 0.7 | 0.5 | 4.5 | 1.1 | 3.2 | 0.3 | 1.3 | 5.2 | 1.2 | 3.8 | 0.7 | 1.4 | |
| 8 | 2.8 | 0.3 | 3.3 | 0.8 | −0.5 | 2.9 | 0.3 | 3.3 | 0.7 | −0.4 | 4.0 | 0.7 | 3.8 | 1.0 | 0.2 | |
| 9 | 3.6 | 0.7 | 3.6 | 1.1 | 0 | 4.4 | 1.0 | 3.6 | 1.0 | 0.8 | 5.6 | 1.0 | 4.6 | 1.1 | 1.0 | |
| 10 | 3.1 | 0.8 | 3.4 | 0.6 | −0.3 | 3.5 | 1.0 | 3.5 | 0.6 | 0 | 5.3 | 1.5 | 4.0 | 0.8 | 1.3 | |
| 11 | 3.5 | 0.7 | 3.1 | 0.6 | 0.4 | 4.1 | 0.8 | 3.1 | 0.6 | 1.0 | 5.3 | 0.9 | 3.6 | 0.8 | 1.7 | |
| 12 | 4.0 | 0.6 | 3.7 | 0.7 | 0.3 | 4.5 | 0.7 | 3.9 | 0.8 | 0.6 | 6.2 | 0.7 | 4.3 | 1.0 | 1.9 | |
| 13 | 4.0 | 1.1 | 3.9 | 0.4 | 0.1 | 4.4 | 1.2 | 4.1 | 0.5 | 0.3 | 5.9 | 1.1 | 4.7 | 0.6 | 1.2 | |
| 14 | 3.9 | 0.7 | 3.8 | 0.6 | 0.1 | 4.4 | 0.8 | 4.0 | 0.7 | 0.4 | 6.1 | 0.9 | 4.8 | 0.7 | 1.3 | |
| 15 | 3.8 | 0.8 | 3.4 | 0.4 | 0.4 | 4.3 | 0.5 | 3.4 | 0.6 | 0.9 | 6.5 | 0.7 | 4.0 | 0.6 | 2.5 | |
| Zygomatic | 16 | 3.0 | 0.7 | 4.5 | 0.7 | −1.5 | 3.6 | 0.8 | 4.1 | 0.9 | −0.5 | 5.6 | 1.2 | 5.1 | 0.7 | 0.5 |
| 17 | 3.2 | 0.7 | 4.2 | 0.7 | −1.0 | 4.0 | 0.9 | 4.3 | 0.7 | −0.3 | 5.7 | 1.0 | 5.1 | 1.0 | 0.6 | |
| 18 | 2.8 | 0.7 | 4.1 | 0.4 | −1.3 | 3.4 | 1.0 | 3.9 | 0.5 | −0.5 | 5.0 | 1.1 | 4.8 | 0.7 | 0.2 | |
| 19 | 3.0 | 1.2 | 3.7 | 0.6 | −0.7 | 3.4 | 1.2 | 3.7 | 0.6 | −0.3 | 5.0 | 1.4 | 4.4 | 0.9 | 0.6 | |
| Temporal | 20 | 4.0 | 0.9 | 3.1 | 0.7 | 0.9 | 4.8 | 0.7 | 3.4 | 0.7 | 1.4 | 6.3 | 0.7 | 4.4 | 0.6 | 1.9 |
| Frontal | 21 | 3.5 | 0.8 | 4.7 | 0.7 | −1.2 | 4.0 | 0.9 | 4.7 | 0.6 | −0.7 | 5.3 | 0.8 | 5.1 | 0.8 | 0.2 |
| 22 | 3.6 | 0.5 | 4.7 | 0.6 | −1.1 | 3.9 | 0.7 | 4.8 | 0.7 | −0.9 | 5.7 | 0.6 | 5.6 | 0.8 | 0.1 | |
| 23 | 3.4 | 1.0 | 4.8 | 0.4 | −1.4 | 3.7 | 1.1 | 5.0 | 0.3 | −1.3 | 5.3 | 1.2 | 5.5 | 0.5 | −0.2 | |
| 24 | 3.4 | 0.7 | 4.7 | 0.8 | −1.3 | 4.0 | 0.9 | 4.4 | 0.8 | −0.4 | 5.6 | 1.6 | 5.4 | 0.8 | 0.2 | |
| ANOVA | F | P | F | P | F | P | ||||||||||
| Sites | 1.95 | 0.007 | 2.25 | 0.001 | 3.57 | 0.001 | ||||||||||
| Edent. vs. Dent | 4.58 | 0.043 | 14.41 | 0.001 | 96.67 | 0.001 | ||||||||||
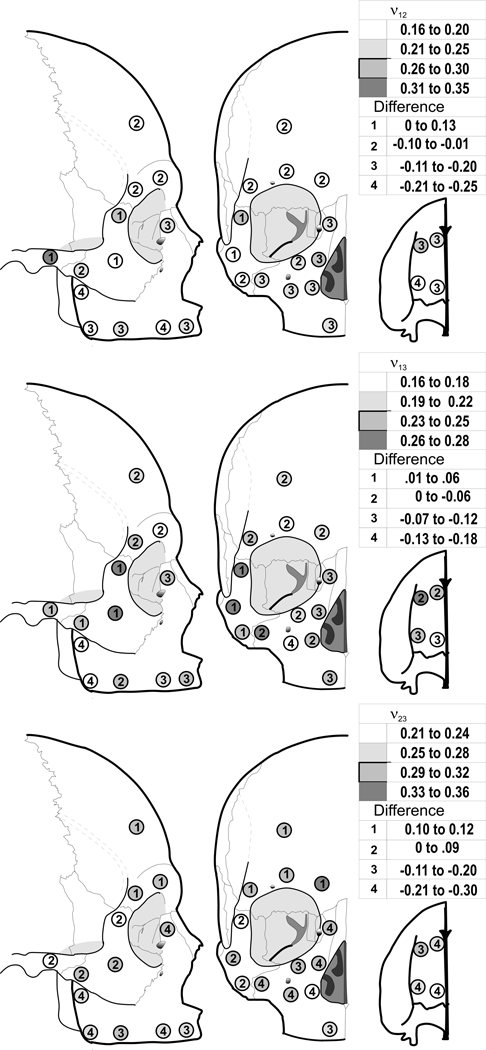
Poisson’s ratios, ν12, ν31, and ν23, showed statistically significant differences among sites in edentulous skulls (Fig. 5, and Table 4). All three ratios showed significant differences between edentulous and dentate crania. For ν12, Poisson’s ratios were larger in the zygomatic and supraorbital areas and smaller elsewhere. For ν31 and ν23, the ratio was slightly larger in the zygomatic region, but smaller elsewhere in the cranium.
Figure 5.
Average cortical bone Poisson’s ratio ν12 (Top), ν31 (Middle), and ν23 (Bottom) in the edentulous crania (shading) and difference compared with dentate crania (numbers).
Table 4.
Poisson’s Ratios
| ν12 | Dif | ν31 | Dif | ν23 | Dif | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Site | Dentate | Edentate | Dentate | Edentate | Dentate | Edentate | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Maxilla | 1 | 0.34 | 0.08 | 0.21 | 0.07 | .13 | 0.29 | 0.07 | 0.24 | 0.14 | 0.05 | 0.47 | 0.13 | 0.26 | 0.09 | 0.21 |
| 2 | 0.37 | 0.06 | 0.17 | 0.08 | .2 | 0.28 | 0.07 | 0.16 | 0.07 | 0.12 | 0.51 | 0.11 | 0.24 | 0.10 | 0.27 | |
| 3 | 0.37 | 0.08 | 0.26 | 0.09 | .11 | 0.30 | 0.09 | 0.28 | 0.15 | 0.02 | 0.48 | 0.07 | 0.30 | 0.07 | 0.18 | |
| 4 | 0.41 | 0.09 | 0.20 | 0.07 | .21 | 0.30 | 0.07 | 0.21 | 0.09 | 0.09 | 0.51 | 0.14 | 0.26 | 0.10 | 0.25 | |
| 5 | 0.37 | 0.10 | 0.23 | 0.05 | .14 | 0.33 | 0.09 | 0.25 | 0.08 | 0.08 | 0.41 | 0.10 | 0.27 | 0.05 | 0.14 | |
| 6 | 0.42 | 0.18 | 0.20 | 0.07 | .22 | 0.29 | 0.10 | 0.20 | 0.11 | 0.09 | 0.50 | 0.22 | 0.28 | 0.08 | 0.22 | |
| 7 | 0.42 | 0.13 | 0.22 | 0.07 | .20 | 0.28 | 0.08 | 0.23 | 0.07 | 0.05 | 0.48 | 0.14 | 0.30 | 0.09 | 0.18 | |
| 8 | 0.38 | 0.07 | 0.18 | 0.07 | .20 | 0.36 | 0.12 | 0.18 | 0.05 | 0.18 | 0.50 | 0.15 | 0.25 | 0.10 | 0.25 | |
| 9 | 0.43 | 0.15 | 0.18 | 0.08 | .25 | 0.30 | 0.04 | 0.16 | 0.09 | 0.14 | 0.53 | 0.17 | 0.28 | 0.12 | 0.25 | |
| 10 | 0.38 | 0.11 | 0.21 | 0.06 | .17 | 0.34 | 0.07 | 0.17 | 0.09 | 0.17 | 0.56 | 0.21 | 0.29 | 0.07 | 0.27 | |
| 11 | 0.40 | 0.09 | 0.23 | 0.09 | .17 | 0.29 | 0.13 | 0.25 | 0.06 | 0.04 | 0.49 | 0.10 | 0.27 | 0.08 | 0.22 | |
| 12 | 0.38 | 0.02 | 0.26 | 0.06 | .12 | 0.32 | 0.07 | 0.25 | 0.09 | 0.07 | 0.52 | 0.08 | 0.31 | 0.08 | 0.21 | |
| 13 | 0.38 | 0.09 | 0.25 | 0.07 | .13 | 0.31 | 0.04 | 0.24 | 0.06 | 0.07 | 0.51 | 0.16 | 0.30 | 0.07 | 0.21 | |
| 14 | 0.35 | 0.10 | 0.25 | 0.05 | .10 | 0.27 | 0.10 | 0.22 | 0.09 | 0.05 | 0.51 | 0.16 | 0.31 | 0.04 | 0.20 | |
| 15 | 0.38 | 0.08 | 0.24 | 0.09 | .14 | 0.28 | 0.07 | 0.26 | 0.05 | 0.02 | 0.58 | 0.15 | 0.29 | 0.09 | 0.29 | |
| Zygomatic | 16 | 0.15 | 0.05 | 0.18 | 0.06 | −.03 | 0.23 | 0.07 | 0.27 | 0.11 | −0.04 | 0.21 | 0.05 | 0.30 | 0.07 | −0.09 |
| 17 | 0.20 | 0.07 | 0.26 | 0.06 | −.06 | 0.26 | 0.05 | 0.27 | 0.06 | −0.01 | 0.23 | 0.05 | 0.24 | 0.07 | −0.01 | |
| 18 | 0.19 | 0.07 | 0.18 | 0.08 | .01 | 0.26 | 0.05 | 0.24 | 0.07 | 0.02 | 0.20 | 0.04 | 0.27 | 0.09 | −0.07 | |
| 19 | 0.25 | 0.11 | 0.21 | 0.08 | .04 | 0.25 | 0.09 | 0.23 | 0.07 | 0.02 | 0.22 | 0.08 | 0.31 | 0.06 | −0.09 | |
| Temporal | 20 | 0.20 | 0.07 | 0.33 | 0.08 | −.13 | 0.19 | 0.08 | 0.25 | 0.09 | −0.06 | 0.17 | 0.08 | 0.22 | 0.07 | −0.05 |
| Frontal | 21 | 0.25 | 0.09 | 0.19 | 0.09 | .06 | 0.23 | 0.07 | 0.21 | 0.06 | 0.02 | 0.23 | 0.07 | 0.34 | 0.04 | −0.11 |
| 22 | 0.21 | 0.06 | 0.19 | 0.06 | .02 | 0.22 | 0.06 | 0.18 | 0.07 | 0.04 | 0.20 | 0.05 | 0.32 | 0.04 | −0.12 | |
| 23 | 0.25 | 0.08 | 0.19 | 0.07 | .06 | 0.24 | 0.06 | 0.19 | 0.06 | 0.05 | 0.21 | 0.06 | 0.32 | 0.05 | −0.11 | |
| 24 | 0.26 | 0.11 | 0.17 | 0.07 | .09 | 0.23 | 0.06 | 0.23 | 0.09 | 0 | 0.21 | 0.07 | 0.31 | 0.05 | −0.10 | |
| ANOVA | F | P | F | P | F | P | ||||||||||
| Sites | 6.12 | 0.001 | 1.10 | NS | 3.62 | 0.001 | ||||||||||
| Edent. vs. Dent | 57.68 | 0.001 | 11.15 | 0.001 | 22.76 | 0.001 | ||||||||||
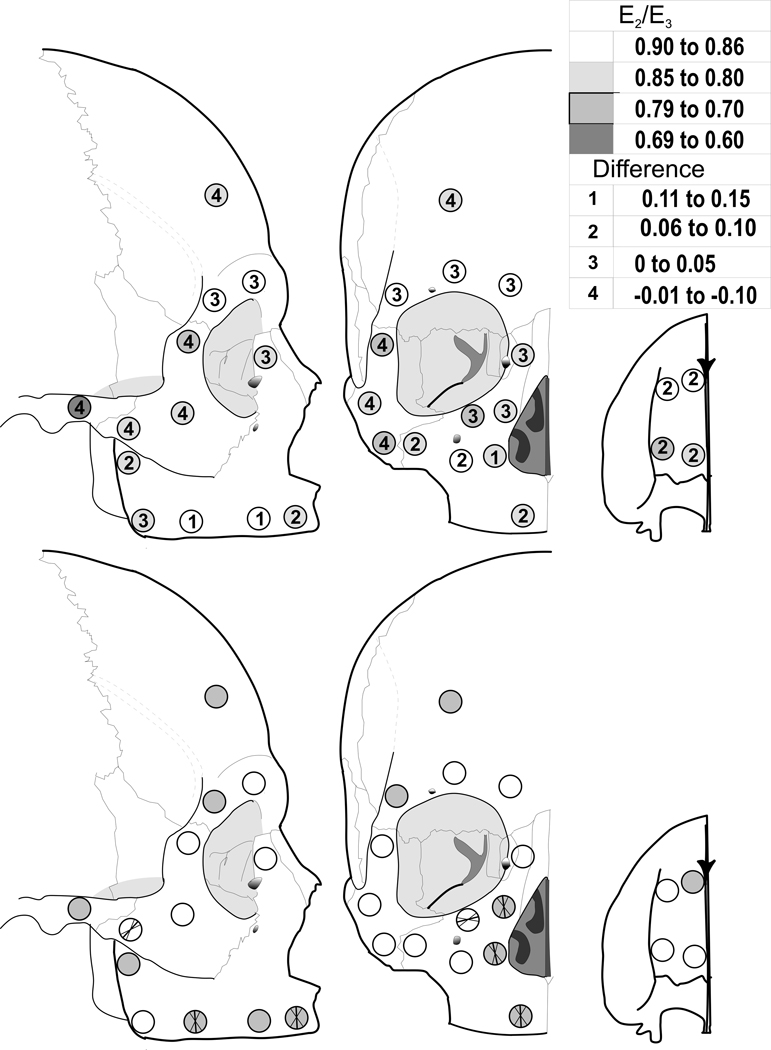
The anisotropy ratio (E2/E3) differed statistically among sites in edentulous crania, and between edentulous and dentate crania (Fig. 6 and Table 5). In the edentulous crania, the greatest differences in stiffness between the 2 and 3 directions (i.e. the two orthogonal axes of minimum and maximum stiffness within the plane of the cortical plate) were in the zygomatic region and at several other scattered sites. These differences were not as consistent among sites as those found for other elastic properties. In differences between edentulous and dentate crania, only three zygomatic sites (#16, 17, and 18), one temporal site (#20) and one frontal site (#23) had greater anisotropy (smaller ratio) in the edentulous crania, while all other sites had lower (or equal) anisotropy (larger ratio), relative to the dentate crania.
Figure 6.
Top: Average cortical bone anisotropy (E2 /E3) in the edentulous crania (shading) and difference compared with dentate crania (numbers). Bottom: Six sites show significant mean orientations of maximum stiffness (E3) among the edentulous crania. These sites are indicated by the bold intersecting lines that illustrate the mean orientations and the non-bolded lines that are the 95% confidence intervals. The shaded sites are those with significant average orientations in the dentate comparison sample (see Table 6).
Table 5.
Anisotropy E2/E3
| Region | Site | Dentate | Edentate | Dif | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Maxilla | 1 | 0.81 | 0.11 | 0.87 | 0.06 | −0.06 |
| 2 | 0.74 | 0.11 | 0.81 | 0.06 | −0.07 | |
| 3 | 0.80 | 0.10 | 0.90 | 0.05 | −0.10 | |
| 4 | 0.70 | 0.09 | 0.78 | 0.10 | −0.08 | |
| 5 | 0.78 | 0.09 | 0.84 | 0.12 | −0.06 | |
| 6 | 0.72 | 0.17 | 0.86 | 0.04 | −0.14 | |
| 7 | 0.72 | 0.13 | 0.86 | 0.07 | −0.14 | |
| 8 | 0.85 | 0.07 | 0.85 | 0.12 | 0 | |
| 9 | 0.77 | 0.11 | 0.85 | 0.07 | −0.08 | |
| 10 | 0.77 | 0.12 | 0.86 | 0.09 | −0.09 | |
| 11 | 0.69 | 0.08 | 0.83 | 0.08 | −0.14 | |
| 12 | 0.78 | 0.10 | 0.81 | 0.07 | −0.03 | |
| 13 | 0.75 | 0.08 | 0.80 | 0.12 | −0.05 | |
| 14 | 0.75 | 0.11 | 0.78 | 0.12 | −0.03 | |
| 15 | 0.77 | 0.15 | 0.85 | 0.08 | −0.08 | |
| Zygomatic | 16 | 0.85 | 0.09 | 0.83 | 0.12 | 0.02 |
| 17 | 0.78 | 0.08 | 0.73 | 0.10 | 0.05 | |
| 18 | 0.85 | 0.11 | 0.79 | 0.10 | 0.06 | |
| 19 | 0.77 | 0.10 | 0.81 | 0.11 | −0.04 | |
| Temporal | 20 | 0.73 | 0.11 | 0.63 | 0.12 | 0.10 |
| Frontal | 21 | 0.86 | 0.11 | 0.90 | 0.06 | −0.04 |
| 22 | 0.84 | 0.09 | 0.87 | 0.09 | −0.03 | |
| 23 | 0.87 | 0.08 | 0.80 | 0.10 | 0.07 | |
| 24 | 0.84 | 0.09 | 0.87 | 0.09 | −0.03 | |
| ANOVA | F | P | ||||
| Sites | 2.55 | 0.001 | ||||
| Edent. vs. Dent | 2.58 | 0.001 | ||||
Rayleigh's test for uniformity demonstrated significant mean directions of maximum stiffness among individuals at 6 of 24 sites in the edentulous crania (Fig. 6 and Table 6), compared with 12 of 24 sites in the dentate crania, indicating an overall decrease in consistent orientation of orthotropic axes in the 2–3 plane in the edentulous crania. In the edentulous group, five significant sites were located in the maxilla (compared with seven in the dentate group), and one was in the zygoma (similar to the dentate group). In the posterior zygomatic arch (temporal bone), the edentulous crania did not show a consistent orientation, while the dentate crania did. In the frontal bone, including the browridges, none of the four sites showed a consistent orientation in edentulous crania, while one did among dentate crania.
Table 6.
Direction of greatest stiffness by degrees. Sites that did not show a significant mean vector orientation as determined with Rayleigh’s test of uniformity are excluded. At sites where mean vector orientations were significant for both dentate and edentulous groups, none showed a significant difference in orientation at P<0.05.
| Mean Vector |
Circular CI |
Mean Vector |
Circular CI | Difference | ||
|---|---|---|---|---|---|---|
| Dentate | Edentate | |||||
| Site | Mean ° | 95% CI | Mean ° | 95% CI | ||
| Maxilla | 1 | −11 | ±34 | NS | ||
| 5 | 7 | ±24 | −3 | ±32 | 10 | |
| 6 | −1 | ±33 | NS | |||
| 7 | 2 | ±34 | −4 | ±30 | 6 | |
| 9 | −11 | ±27 | NS | |||
| 11 | 5 | ±16 | −12 | ±26 | 17 | |
| 12 | −14 | ±28 | −7 | ±31 | 7 | |
| 14 | NS | 86 | ±24 | |||
| Zygomatic | 19 | NS | 43 | ±21 | ||
| 20 | 23 | ±8 | NS | |||
| Temporal | 23 | 6 | ±20 | NS | ||
| Frontal | 24 | 23 | ±23 | NS | ||
In the edentulous crania, the direction of maximum stiffness in the alveolar region (#5, 7) and in the area lateral to the piriform aperture (#11, 12) was oriented roughly superoinferiorly, and at the inferior orbital rim (#14), it paralleled the lower border of the orbit. The inferior zygoma (#19) showed a significant direction of maximum stiffness that paralleled the zygotemporal suture and the direction of pull of the superficial masseter.
There were four sites (# 5, 7, 11, and 12) that had significant mean directions of stiffness for both the dentate and edentulous skulls. These orientations were not significantly different between dentate and edentulous groups. Like the dentate maxilla, significant mean directions of stiffness were found at two sites (#11 and 12) within the frontomaxillary pillar of bone. The dentate sample also had significant orientations at frontal sites (#23 and 24), a palatal site (#1), and on the posterior zygomatic arch (#20) that were not found in the edentulous group. The edentulous group had a significant orientation at the inferior zygomatic (#19) that was not significantly oriented in the dentate group.
DISCUSSION
This research compares the material properties of cortical bone within the human edentulous craniofacial complex and between edentulous and dentate skulls. As in the dentate craniofacial skeleton (Peterson and Dechow, 2002, 2003; Peterson, et al., 2006), differences in material properties vary regionally. Cortical bone from the human maxilla was the least dense and stiff, while cortical bone from the supraorbital area, lateral orbital portion of the zygoma, and posterior zygomatic arch was the most dense and stiff.
Contrary to the hypothesis that fewer differences exist in regions of the human crania that have been shown to have low strains in vivo nonhuman primate studies and in in vitro human studies, differences in material properties were evident throughout the craniofacial skeleton between edentulous and dentate crania, and these differences were usually region specific. Cortical bone from the maxilla of edentulous skulls had lower density, elastic and shear moduli, and Poisson’s ratios than those from similar areas in dentate skulls. In contrast, cortical bone from the supraorbital area had slightly greater density and stiffness than cortical bone from dentate crania, and cortical bone in the edentulous zygoma had higher density, E1, E3 , G12,G31 and ν23, but lower E2 and G23. The single temporal site (#20) in the posterior zygomatic arch in edentulous skulls had lower density, elastic and shear moduli, and higher Poisson’s ratios. These differences in material properties suggest differences in bone microstructure that have yet to be investigated.
The results show that there is a remarkable difference in cortical thickness throughout the human middle and upper face. It is well accepted that bone mass is reduced during the process of aging (Jee, 2001). But as we pointed out earlier, the ages of the two groups were not significantly different. Rather, differences in bone mass in the upper face, superior region of the maxilla, the palate, and the zygoma seem to parallel the reduction in structure reported for the maxillary alveolar region in a large number of investigations of edentulation (e.g., Tallgren, 1972; Jaul et al., 1980; Jeffcoat, 1993; Klemmetti and Vainio, 1994; Tanaka et al., 1994), suggesting that these differences are a direct result of bone adaptation following tooth loss.
Another possible consideration is that some studies in humans have shown a moderate association between tooth loss and systemic osteoporosis (Krall et al, 1996; Gur et al, 2003; Tagauchi et al, 2004; Nicopoulou-Karayianne et al, 2009), although other studies and reviews suggest that this evidence is weak and that associations between systemic bone loss and that of the jaws remain unconvincing overall (Dervis, 2005; Famili et al, 2005). All available studies focus on the jaws, and in particular, the alveolar bone, and there are no reports on changes in the upper face and cranium associated with osteoporosis.
Functionally, edentulous humans have lower masticatory loads than dentate individuals. Loss of teeth, or edentulation, decreases the magnitude of the bite force in denture patients by 4 to 5 times compared to those with natural dentitions (Helkimo et al., 1977). Other functional changes, such as changes in the direction of biting, are not well documented, although limited evidence and consensus among Prosthodontists, suggests that bite forces and masticatory cycles in denture patients become much more vertically oriented, as lateral forces are what lead to problems with adequate denture retention during function (Peterson and Dechow, 2003).
Although it is clear that bone loading is necessary to maintain bone mass, it is not at all clear what magnitudes or frequencies of loading and associate strain are needed to maintain bone mass; nor is it clear whether different skeletal organs or regions respond similarly to reductions in function (Ascenzi, 1988; Burr et al., 1989; Smit and Burger, 2000; Jee, 2001). Likewise, little is known about in vivo mechanics of the palate, midface, and upper face during function in humans, although it is possible to make inferences from in vitro and nonhuman primate studies. Most regions of the dentate midface and palate must bear significant occlusal loads during function, and the zygomatic region must bear significant loads from both occlusal loads and masseter function. In contrast, the brow ridges and cranial vault are unlikely to have high strains because of the rigid structure of the cranium and their relative distance from regions of force application. Despite the low strains that are likely in the upper face of dentate individuals during function, the results of this study strongly suggest that reduction of that function still has a pronounced effect on cortical bone structure, especially cortical thickness.
In contrast to cortical thickness, density and the elastic moduli were similar or greater in the zygoma and cranial vault sites compared to the dentate skulls. This might be a secondary adaptation to relatively larger strains due to a reduction of bone mass, similar to the secondary increase of cortical thickness in edentulous mandibular corpora incurred by reduction in alveolar height and corporal cross section (Schwarzt-Dabney and Dechow, 2002). Mechanical function stimulates the maintenance of structure in cortical bone by as yet poorly understood mechanisms (Ascenzi, 1988; Burr et al., 1989; Smit and Burger, 2000; Jee, 2001). Conversely, and perhaps more likely, greater density and stiffness may be related to greater mineralization in the browridges resulting from relatively lesser amount of remodeling. A microstructural explanation of this finding cannot be determined by this investigation, and these phenomena reflect the complexity of possible mechanisms of bone adaptation.
The sites nearest the incisor and the second molar, and lateral to the piriform aperture are significantly directional in both the edentulous and dentate skulls. Endo (1966) indicates that the orientation of strain is in a similar direction during in vitro incisor and canine loading but not during molar loading, as the latter does not result in direct loading along the frontomaxillary pillar. Since an effective way for bone to resist deformation is for the direction of maximum stiffness to be aligned with the direction of load (principal stress) (Dechow and Hylander, 2000), then the less oblique direction of maximum stiffness may be a structural adaptation to resist relatively greater amounts of bending and decreased shear in the those regions. Likewise, the loss of significant orientation in some sites might suggest either the (1) loss of a constant loading direction during function or (2) smaller-than-threshold loads or lower frequencies of loads needed for orienting bone microstructure during bone remodeling.
The differences between dentate and edentulous middle and upper faces have similarities and differences to patterns found in human mandibles. The edentulous mandible is significantly stiffer than the dentate mandible in the retromolar and superior ramus regions (Schwartz-Dabney and Dechow, 2002). Likewise, cortical thickness is greater in the edentulous mandibular corpus, and density is not significantly different between the dentate and edentulous groups. Schwartz-Dabney and Dechow (2002) suggest that the symphysis of the edentulous mandibles may compensate for the thinner cortex with increased density and stiffness.
It is interesting to contemplate a link between the thinning of cortical bone in the upper face and reduced occlusal and muscular forces. As we have previously noted, this possibility is surprising in the upper face because this region has been shown to have low strains during function, suggesting that the skeletal structure is not dependent on occlusal and muscular loads. The low strains in the supraorbital region suggest that functional loads do not contribute to the brow ridge development (Hylander et al., 1991; Picq and Hylander, 1989) thus calling into question the masticatory stress hypothesis. Endo (1966) concluded that brow ridges in primates are bent in frontal plane yet are not stressed more during incision than molar biting. However, Hylander et al. (1991) point out that the strains measured by Endo are very small and they suggest that there is no relationship between browridge morphology and mastication or other oral functions. Rather, they suggest that the form of the browridges is due to spatial orientation of the orbits and brain (reviewed in Leiberman, 2000). Our results do not directly address the issue of the significance of function on the development of variations in the form of the supraorbital region. But they do suggest, despite the low strains, that cortical bone mass in this area is dependent for maintenance on normal masticatory muscular and occlusal loads.
Thus, it cannot be ruled out that, however low the bone strains, the total volume of loading (magnitude X frequency) does have an impact on supraorbital bone maintenance and three-dimensional shape, and likewise may produce an effect during individual development. How bone adaptation varies regionally at a molecular level, and how associated variations in mechanisms of bone formation and maintenance came about during evolution, remain the key questions for understanding the phenomenon described in this study.
In summary, the loss of significant occlusal loads following edentulation may contribute to the change in material properties and the loss of orientation over time during the normal process of bone turnover and remodeling. These results suggest that cortical microstructural changes accompany bone resorption following edentulation. The regional differences in patterns of change in material properties with edentulation in the upper facial skeleton suggest area specific adaptation to loss or reduction of bone function both regionally and globally.
ACKNOWLEDGEMENTS
This project was supported in part by a NIH grant (NIDCR-K08 DE00403) and by the National Science Foundation Physical Anthropology HOMINID program (NSF BCS 0725126, 0725183). We thank Ms. Leslie Smith for help in preparing tables and figures.
LITERATURE CITED
- Ascenzi A. The micromechanics versus the macromechanics of cortical bone - a comprehensive presentation. J Biomech Eng. 1988;110:357–363. doi: 10.1115/1.3108454. [DOI] [PubMed] [Google Scholar]
- Bras J, van Ooij CP, Duns JY, Wansink HM, Driessen RM, van den Akker HP. Mandibular atrophy and metabolic bone loss: A radiologic analysis of 126 patients. Int J Oral Surg. 1983;12:309–313. doi: 10.1016/s0300-9785(83)80018-0. [DOI] [PubMed] [Google Scholar]
- Buchbinder D. Treatment of fractures of the edentulous mandible, 1943 to 1993: a review of the literature. J Oral Maxillofac Surg. 1993;51:1174–1180. doi: 10.1016/s0278-2391(10)80285-x. [DOI] [PubMed] [Google Scholar]
- Burr DB, Schaffler MB, Yang KH, Wu DD, Lukoschek M, Kandzari D, Sivaneri N, Blaha JD, Radin EL. The effects of altered strain environments on bone tissue kinetics. Bone. 1989;10:215–221. doi: 10.1016/8756-3282(89)90056-2. [DOI] [PubMed] [Google Scholar]
- Dechow PC, Hylander WL. Elastic properties and masticatory bone stress in the macaque mandible. Am J Phys Anthropol. 2000;112:553–574. doi: 10.1002/1096-8644(200008)112:4<553::AID-AJPA9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Dechow PC, Chung DH, Bolouri . Relationship between three dimensional microstructure and elastic properties of cortical bone in the human mandible and femur. In: Vinyard CJ, Ravosa MJ, Wall CE, editors. Primate Craniofacial Function and Biology. New York: Springer; 2008. pp. 265–292. [Google Scholar]
- Dervis E. Oral implications of osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:349–356. doi: 10.1016/j.tripleo.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Endo B. Experimental studies on the mechanical significance of the form of the human facial skeleton. J Faculty of Science. 1966;3:5–102. [Google Scholar]
- Famili P, Cauley J, Suzuki JB, Weyant R. Longitudinal study of periodontal disease and edentulism with rates of bone loss in older women. J Periodontol. 2005;76:11–15. doi: 10.1902/jop.2005.76.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur A, Nas K, Kayhan O, Atay MB, Akyuz G, Sindal D, aksit R, Oncel S, Dilsen G, Cevik R, Gunduz OH, Ersoy Y, Altay Z, Ozturk C, Akkus S, Senocak O, Kavuncu V, Kirnap M, Tekeoglu I, Erdogan F, Sarac AJ, Demiralp L, Demirkesen A, Adam M. The relation between tooth loss and bone mass in postmenopausal osteoporotic women in Turkey: a multicenter study. J Bone Miner Metab. 2003;21:43–47. doi: 10.1007/s007740300007. [DOI] [PubMed] [Google Scholar]
- Helkimo E, Carlsson GE, Helkimo M. Bite force and state of dentition. Acta Odont Scand. 1977;35:297–303. doi: 10.3109/00016357709064128. [DOI] [PubMed] [Google Scholar]
- Hylander WL, Picq PG, Johnson KR. Masticatory-stress hypotheses and the supraorbital region of primates. Am J Phys Anthropol. 1991;86:1–36. doi: 10.1002/ajpa.1330860102. [DOI] [PubMed] [Google Scholar]
- Jaul DH, McNamara JA, Jr, Carlson DS, Upton LG. A cephalometric evaluation of edentulous Rhesus monkeys (Macaca mulatta): A long-term study. J Prosth Dent. 1980;44:453–460. doi: 10.1016/0022-3913(80)90110-9. [DOI] [PubMed] [Google Scholar]
- Jee WSS. Integrated bone tissue physiology: Anatomy and physiology. In: Cowin SC, editor. Bone Mechanics. Boca Raton, Florida: CRC Press, Inc.; 2001. pp. 1–55. [Google Scholar]
- Jeffcoat MK. Bone loss in the oral cavity. J Bone Miner Res. 1993;8 Suppl:467–473. doi: 10.1002/jbmr.5650081307. [DOI] [PubMed] [Google Scholar]
- Kingsmill VJ, Boyde A. Variation in the apparent density of human mandibular bone with age and dental status. J Anat. 1998;192:233–244. doi: 10.1046/j.1469-7580.1998.19220233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsmill VJ, Boyde A. Mineralization density and apparent density of bone in cranial and postcranial sites in the aging human. Osteop Intl. 1999;9:260–268. doi: 10.1007/s001980050146. [DOI] [PubMed] [Google Scholar]
- Klemetti E, Vainio P. Effect of maxillary edentulousness on mandibular residual ridges. Scand J Dent Res. 1994;102:309–312. doi: 10.1111/j.1600-0722.1994.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Krall EA, Garcia Ri, Dawson-Hughes B. Increased risk of tooth loss is related to bone loss at the whole body, hip, and spine. Calcif Tissue Int. 1996;59:433–437. doi: 10.1007/BF00369206. [DOI] [PubMed] [Google Scholar]
- Kupczik K, Dobson CA, Crompton RH, Phillips R, Oxnard CE, Fagan MJ, O'Higgins P. Masticatory loading and bone adaptation in the supraorbital torus of developing macaques. Am J Phys Anthropol. 2009;139:193–203. doi: 10.1002/ajpa.20972. [DOI] [PubMed] [Google Scholar]
- Lanyon LE, Rubin CT. Functional adaptation in skeletal structures. In: Hildebrand M, Bramble DM, Liem KF, Wake DB, editors. Functional Vertebrate Morphology. Cambridge, Massachusetts: Belknap Harvard Press; 1985. pp. 1–25. [Google Scholar]
- Lieberman DE. Ontogeny, homology, and phylogeny in the Hominid craniofacial skeleton: the problem of the browridge. In: O'Higgins P, Cohn M, editors. Development, Growth and Evolution: Implications for the Study of Hominid Skeletal Evolution. London: Academic Press; 2000. pp. 85–122. [Google Scholar]
- Nicopoulou-Karayianni K, Tzoutzoukos P, Mitsea A, Karayiannis A, Tsiklakis K, Jacobs R, Lindh C, van der Stelt P, Allen P, Graham J, Horner K, Devlin H, Pavitt S, Yuan J. Tooth loss and osteoporosis: the OSTEODENT Study. J Clin Periodontol. 2009;36:190–197. doi: 10.1111/j.1600-051X.2008.01365.x. [DOI] [PubMed] [Google Scholar]
- Peterson J, Dechow PC. Material properties of the inner and outer cortical tables of the human parietal bone. Anat Rec. 2002;268:7–15. doi: 10.1002/ar.10131. [DOI] [PubMed] [Google Scholar]
- Peterson J, Dechow PC. Material properties of the human cranial vault and zygoma. Anat Rec. 2003;274A:785–797. doi: 10.1002/ar.a.10096. [DOI] [PubMed] [Google Scholar]
- Peterson J, Wang Q, Dechow PC. Material properties of the dentate maxilla. Anat Rec. 2006;288 A:962–972. doi: 10.1002/ar.a.20358. [DOI] [PubMed] [Google Scholar]
- Petrtyl M, Hert J, Fiala P. Spatial organization of the haversian bone in man. J Biomechanics. 1995;29:161–169. doi: 10.1016/0021-9290(94)00035-2. [DOI] [PubMed] [Google Scholar]
- Picq PG, Hylander WL. Endo's stress analysis of the primate skull and the functional significance of the supraorbital region. Am J Phys Anthropol. 1989;79:393–398. doi: 10.1002/ajpa.1330790315. [DOI] [PubMed] [Google Scholar]
- Ravosa MJ. Interspecific perspective on mechanical and nonmechanical models of primate circumorbital morphology. Am J Phys Anthropol. 1991;86:369–396. doi: 10.1002/ajpa.1330860305. [DOI] [PubMed] [Google Scholar]
- Ravosa MJ, Noble VE, Hylander WL, Johnson KR, Kowalski EM. Masticatory stress, orbital orientation and the evolution of the primate postorbital bar. J Hum Evol. 2000a;38:667–693. doi: 10.1006/jhev.1999.0380. [DOI] [PubMed] [Google Scholar]
- Ravosa MJ, Vinyard CJ, Hylander WL. Stressed out: masticatory forces and primate circumorbital form. Anat Rec. 2000b;261:173–175. doi: 10.1002/1097-0185(20001015)261:5<173::AID-AR6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Schwartz-Dabney CL, Dechow PC. Edentulation alters material properties of cortical bone in the human mandible. J Dent Res. 2002;81:613–617. doi: 10.1177/154405910208100907. [DOI] [PubMed] [Google Scholar]
- Schwartz-Dabney CL, Dechow PC. Variations in cortical material properties throughout the human dentate mandible. Am J Phys Anthropol. 2003;120:252–277. doi: 10.1002/ajpa.10121. [DOI] [PubMed] [Google Scholar]
- Smit T, Burger E. Is BMU-coupling a strain-regulated phenomenon? A finite element analysis. J Bone Miner Res. 2000;15:301–307. doi: 10.1359/jbmr.2000.15.2.301. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Fujiwara S, Masunari N, Suzuki G. Self-reported number of remaining teeth is associated with bone mineral density of the femoral neck, but not of the spine, in Japanese men and women. Osteoporos Int. 2004;15:842–846. doi: 10.1007/s00198-004-1609-2. [DOI] [PubMed] [Google Scholar]
- Tallgren A. The continuing reduction of the residual alveolar ridges in complete denture wearers: a mixed-longitudinal study covering 25 years. J Prosthet Dent. 1972;27:120–132. doi: 10.1016/0022-3913(72)90188-6. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Tomitsuka K, Shionoya K, Andou H, Kimijima Y, Tashiro T, Amagasa T. Aetiology of maxillofacial fracture. Brit J Oral Maxillofac Surg. 1994;32:19–23. doi: 10.1016/0266-4356(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Veyrune J-L, Mioche L. Complete denture wearers: electromyography of mastication and texture perception whilst eating meat. Eur J Oral Sci. 2000;108:83–92. doi: 10.1034/j.1600-0722.2000.90780.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ashley DW, Dechow PC. Regional, ontogenetic, and sex-related variations in elastic properties of cortical bone in baboon mandibles. Am J Phys Anthropol. doi: 10.1002/ajpa.21170. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Dechow PC. Elastic properties of external cortical bone in the craniofacial skeleton of the rhesus macaque. Am J Phys Anthropol. 2006;131:402–415. doi: 10.1002/ajpa.20438. [DOI] [PubMed] [Google Scholar]
- Wang Q, Strait DS, Dechow PC. A comparison of cortical elastic properties in the craniofacial skeletons of three primate species and its relevance to the study of human evolution. J Hum Evol. 2006;51:375–382. doi: 10.1016/j.jhevol.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. 4th edition. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]