Abstract
Heterotrimeric G proteins (αβγ) and Ras proteins are activated by cell-surface receptors that sense extracellular signals. Both sets of proteins were traditionally thought to be constrained to the plasma membrane and some intracellular membranes. Live-cell-imaging experiments have now shown that these proteins are mobile inside a cell, shuttling continually between the plasma membrane and intracellular membranes in the basal state, maintaining these proteins in dynamic equilibrium in different membrane compartments. Furthermore, on receptor activation, a family of G protein βγ subunits translocates rapidly and reversibly to the Golgi and endoplasmic reticulum enabling direct communication between the extracellular signal and intracellular membranes. A member of the Ras family has similarly been shown to translocate on activation. Although the impact of this unexpected intracellular movement of signaling proteins on cell physiology is likely to be distinct, there are striking similarities in the properties of these two families of signal-transducing proteins.
Introduction
Classically, the two important families of signal transducing proteins, heterotrimeric G proteins (αβγ subunits) and Ras, were thought to be localized to the plasma membrane. This localization was consistent with their role as intermediates in the signaling pathway that is activated by receptors embedded in the plasma membrane: G-protein-coupled receptors (GPCRs) in the case of G proteins and receptor tyrosine kinases in the case of Ras proteins. G protein subunits and Ras proteins were later discovered to be present in intracellular membranes and there were suggestions that the proteins were active in these membranes. Early reports showed that G protein subunits were present in the Golgi [1–3] (see Glossary). More recently, evidence has been obtained for G protein activity in endosomes [4–6], the nucleus [7, 8] and mitochondria [9]. Similarly, Ras proteins have been shown to be active in different intracellular membranes, Golgi, endoplasmic reticulum (ER), endosomes and mitochondria [10–12]. However, it was not clear how G proteins or Ras proteins reached these membranes. In the case of Golgi- and ER-localized G protein subunits it was also not clear whether they were residents of these membranes or transient molecules on their way to the plasma membrane. Live-cell-imaging methods have been used in the last few years to directly address these issues, and the potential for spatial and temporal changes in the localization of G proteins and Ras has been scrutinized. Fluorescent protein (FP)-tagged signaling proteins and methods such as FRET (fluorescence resonance energy transfer), FRAP (fluorescence recovery after photobleaching), FLIP (fluorescence loss in photobleaching) and photoactivation have shown that the traditional diagrammatic representation of G proteins and Ras on the cytosolic face of the plasma membrane provides only a partial picture. In this review, we discuss these unexpected findings, the possible mechanisms underlying these events and the physiological implications of these events on downstream cellular signaling events.
Shuttling of G proteins and Ras proteins
Initial evidence from imaging G protein subunits in living mammalian cells using FP-tagged subunits showed them to be present on the plasma membrane and intracellular membranes such as the Golgi and ER in the basal state [13, 14]. When the FRAP of FP-tagged G protein α, β and γ subunits in the Golgi membranes was measured, movement of these subunits was detected in the retrograde direction from the plasma membrane to the Golgi [14] (Figure 1). The t1/2 for recovery was less than 1 min, suggesting that the movement is rapid. Photoactivation of G protein subunits tagged with a photoswitchable FP, Dronpa, showed that the G protein subunits are also capable of equally rapid movement in the anterograde direction. In FLIP experiments in which a region of the cytosol was photobleached repeatedly, a loss in FP-tagged G protein subunits from the proximal regions of the plasma membrane and Golgi was observed. The results suggested that the G protein subunits traverse the cytosol while continually shuttling between the plasma membrane and Golgi membranes in the basal state of the cell. This continual movement of G protein subunits in the basal state in the absence of receptor stimulation was termed as ‘shuttling’, as in the dictionary definition ‘moving or travelling back and forth frequently’. This term has been used to describe similar protein movement across various parts of a cell [15–17].
Figure 1.
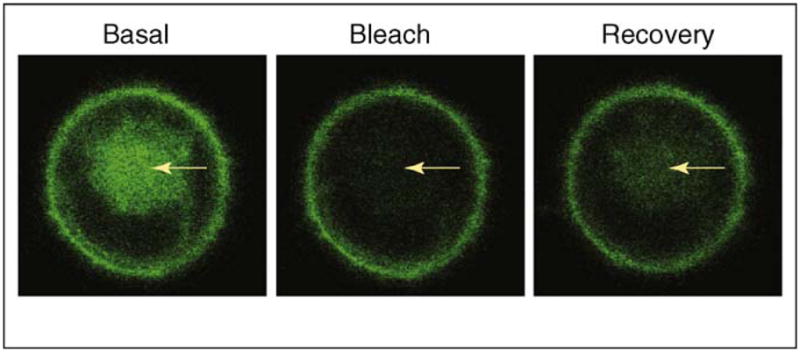
Shuttling of the G protein γ2 subunit from the plasma membrane to the Golgi. Chinese hamster ovary (CHO) cells transiently expressing yellow fluorescent protein (YFP)-γ2 were used. Arrows indicate the Golgi region, which was photobleached in the basal cell by laser and was monitored for fluorescence recovery (FRAP). The recovery shown in the right panel reflects the retrograde movement of YFP-γ2 from the plasma membrane to the Golgi, corresponding to the shuttling movement of this protein. Note that the γ2 subunit types are not capable of translocation on receptor activation, indicating that the ability to shuttle between plasma membrane and intracellular membranes in the basal state does not necessarily confer the ability to translocate on receptor activation.
Consistent with the rapidity of the movement, it was determined to be independent of vesicle-mediated trafficking and is most likely to be diffusive. The movement that is part of shuttling was not inhibited by vesicular trafficking inhibitors such as nocodazole, monensin or low temperature [14]. Inhibition of protein synthesis did not alter the distribution of G proteins observed on the plasma membrane and the Golgi. This result indicated that G protein subunits are not present in the Golgi as molecules in transit, but they are resident [14]. This result also suggested that the anterograde movement of G protein subunits might occur in the absence of protein synthesis. Recent experiments show directly that inhibition of protein synthesis does not prevent the anterograde movement of a G protein α subunit, Gαq [18]. These findings show that the anterograde movement of G proteins is likely to be distinct from the conventional Golgi-to-plasma-membrane biosynthetic trafficking route that has been extensively studied [19, 20]. However, the anterograde movement in shuttling is diffusive similar to the observed anterograde trafficking of Gs and Gq, which were also not vesicle mediated [20].
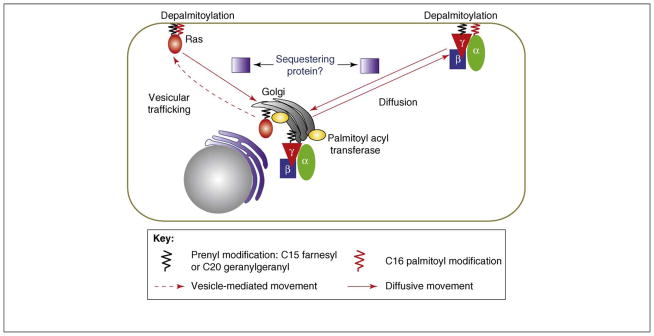
The proteins probably shuttle as heterotrimers and, thus, maintain a pool of activation-capable G proteins in both the plasma and intracellular membranes. A G protein FRET sensor indicated that the G proteins were indeed heterotrimeric in constitution in both the plasma membrane and the Golgi [14]. The sensor also showed that the plasma-membrane-bound species was entirely amenable to receptor activation [21]. An inhibitor of palmitoylation, 2 bromopalmitate (2BP), inhibits the shuttling of the G protein subunits [14], suggesting that it is mediated by a palmitoylation cycle. Recent evidence from examining Gq in live cells [18] is consistent with the results above obtained with the Gαo subtype. Through photoactivation experiments with Gαq–Dendra2 fusion protein, rapid shuttling of Gαq was observed between the plasma membrane and the Golgi complex in a palmitoylation-dependent manner [18]. The shuttling was observed in cells without GPCR activation and in the presence of coexpressed β1γ2, indicating that heterotrimeric species shuttle as observed for Gαo. The kinetics of the movement was comparable with that for Gαo (see earlier). Furthermore, the evidence suggested that the palmitoylation of Gαq is regulated by two palmitoyl acyl transferases present in the Golgi, DHHC3 and DHHC7. Together, the results from analyzing Gαo and Gαq provide strong evidence for a model that involves G protein shuttling occurring through a cycle in which depalmitoylation occurs on the plasma membrane resulting in the movement of the G protein to the Golgi where it is repalmitoylated and moves back to the plasma membrane (Figure 2).
Figure 2.
Palmitoylation-dependent shuttling of signaling proteins. Shuttling of G protein heterotrimers occurs with equal rapidity in both retrograde and anterograde directions through simple diffusion (solid arrows). Shuttling of the Ras proteins involves retrograde movement from the plasma membrane to the Golgi by simple diffusion, whereas the anterograde movement is through vesicle-mediated trafficking and is distinctly slower. Palmitoyl (red) attached to the α subunit and Ras is removed resulting in reduced affinity for the plasma membrane even though the γ subunit and Ras contain a prenyl moiety (black). Palmitoyl transferase in the Golgi acylates the depalmitoylated α subunit and is thought to acylate Ras. Thus, a common mechanism – deacylation at the plasma membrane and palmitoylation at the Golgi – is thought to mediate the shuttling of these two signaling proteins.
The observation of palmitoylation-dependent G protein shuttling between the plasma membrane and the Golgi is reminiscent of a similar movement seen in the case of two Ras proteins, N-Ras and H-Ras. Results from live-cell-imaging experiments showed that N-Ras and H-Ras are continually cycling between the plasma membrane and the Golgi [22, 23]. The movement that occurred from the plasma membrane to the Golgi was inhibited by 2BP and by the attachment of a depalmitoylation-resistant analog to N-Ras suggesting that it was palmitoylation dependent. Consistent with this acylation dependency, N-Ras, which contains one palmitoyl group, moved more rapidly to the Golgi (t1/2 = 1–2 min) compared with H-Ras, which contains two palmitoyl groups (t1/2 = 6–11 min). Thus, pools of H-Ras and N-Ras proteins are maintained in the plasma membrane and Golgi. Unlike G protein shuttling, Ras shuttling between the plasma membrane and Golgi follows two different routes. The retrograde movement from the plasma membrane to the Golgi is a diffusion-driven process regulated by depalmitoylation of Ras on the plasma membrane; by contrast, the anterograde movement from the Golgi to the plasma membrane is likely to be vesicle mediated [22, 23] (Figure 2). Although the palmitoyl attachment occurs on the Gα subunit and the prenyl group is attached to the Gγ subunit [24], in H-Ras and N-Ras both lipids are attached to the same protein [25] (Figure 2). The impact of lipid modifications of Ras and G proteins on their intracellular movement is discussed later. In both Ras and G proteins, shuttling between the plasma membrane and intracellular membranes enables pools of proteins to be maintained in intracellular membranes in which they can execute signal-transducing roles. As discussed later, the shuttling also potentially enables G protein subunits to act as direct and specific intermediates in communicating extracellular signals to intracellular membranes.
Translocation of G proteins
On activation by a GPCR, heterotrimeric Gα and Gβγ subunits have been thought to modulate effector activity at the plasma membrane that change the levels of cAMP, diacylglycerol (DAG), inositol trisphosphate (IP3) and ion conduction [26]. Intracellular regulation by GPCRs has, thus, been thought to be mediated through diffusible small-molecule second messengers. The presence of G protein subunits in intracellular membranes [27] has suggested that G proteins might have a direct role in these membranes and has raised questions about the mechanism that enables G protein subunits to reach these membranes. The shuttling of G proteins between plasma membrane and intracellular membranes is one mechanism that will enable such dynamic populations of membrane-associated G protein populations to coexist. However, the shuttling of the α subunit and the βγ complex suggests that this movement occurs in the inactive heterotrimeric state. In this state, the active components of the G protein (the GTP-bound α subunit or the free βγ complex) will not be accessible to effector molecules.
Evidence that activation of GPCRs leads to the relocalization of a family of G protein βγ complexes from the plasma membrane to the Golgi and ER [13, 28] provides a potential mechanism that makes an active component of the G protein available for effector regulation inside the cell in a signal-dependent manner. Because the observed relocalization of specific G protein βγ complexes occurs in response to receptor activation, the movement was termed as ‘translocation’, as in the dictionary definition ‘changing position from one place to another’. Translocation is distinctly different from the basal shuttling movement described earlier. Translocation of βγ is selective, the α subunit does not translocate and it occurs with all the important subtypes of α subunits [29]. The selective translocation occurs through dissociation of the βγ complex from the palmitoylated α subunit on receptor activation. Although free Gβγ generated after heterotrimer dissociation translocates to the cytosol and then the endomembranes, the free Gα is retained on the plasma membrane probably owing to the affinity of the palmitoyl for the plasma membrane. The depalmitoylation–palmitoylation cycle that regulates Gα shuttling does not, therefore, regulate translocation.
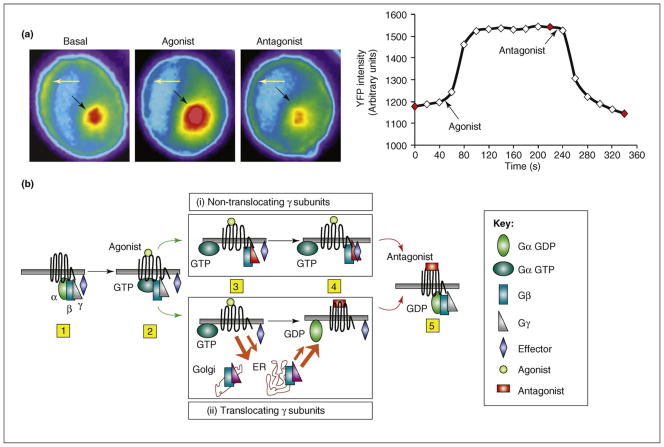
Translocation of Gβγ shows spatially and temporally distinct properties dependent on the γ subunit type associated with the βγ complex. Translocation is targeted predominantly to the Golgi (Figure 3a) or the ER [28]. When the rate of translocation of βγ complexes containing different γ subunits was measured by activating M2 acetylcholine receptors, the t1/2 varied from ~6 to 85s [28]. These results suggest that members of the large and diverse mammalian γ subunit family possess qualitatively different translocation properties. The differential spatiotemporal characteristics of translocation of different γ subtypes suggest that receptor activation in cells expressing the different subtypes might have distinctly different βγ-mediated signaling properties. On deactivating a receptor, equally rapid reversal of translocation occurs. The rapidity of the translocation and the equally rapid ability of the translocated G protein βγ complex to reverse translocate to the plasma membrane on GPCR inactivation suggests that by continually shuttling between plasma membrane and the Golgi, G proteins are constantly sensing the state of the receptor on the plasma membrane. G protein shuttling is, thus, harnessed to translocate the βγ complex to internal membranes (Figure 2). The ability of βγ11 modified with the hydrophobic farnesyl (C15) moiety or βγ13 with the even more hydrophobic geranylgeranyl (C20) moiety to translocate rapidly (t1/2 ~10 s) through the hydrophilic cytosol is surprising. One possibility is that a sequestering protein that binds to Gγ and masks the prenyl moiety of the γ subunit enables it to diffuse through the cytosol (Box 1). The presence of a similar protein has been suggested to facilitate the movement of farnesylated H-Ras and N-Ras through the cytosol [30]. A Rab escort protein is known to sequester the prenyl moiety of Rab, and a RhoGDI (Rho GDP-dissociation inhibitor) acts similarly in the case of Rho to facilitate the movement of these proteins through the cytosol [31, 32].
Figure 3.
Translocation of G protein βγ9 to the Golgi from the plasma membrane in response to M2 cetylcholine receptor activation. CHO cells stably expressing the M2 acetylcholine receptor and transiently transfected with G protein αo, β1 and YFP-γ9 subunits were used. Left hand side: images of the YFP-γ9 subunit from the cells were captured before agonist treatment and followed by agonist and antagonist treatment at 20 s intervals. Cells were exposed to 100 μM carbachol (an agonist) followed by 100 μM atropine (an antagonist) at defined time points. (a) Images showing translocation of YFP-γ9 to the Golgi. Arrows represent the regions in the cell where intensity changes in response to receptor activation and deactivation are observed. Yellow arrows indicate the plasma membrane and black arrows indicate the Golgi region. The decrease in fluorescence intensity over time is due to photobleaching. Right hand side: plot representing YFP intensity changes over time in the Golgi. Arrows indicate the time of addition of agonist and antagonist. Points shown in red represent the images shown in left panel. (b) Model for the mechanistic basis of translocation. After receptor activation by an agonist (steps 1 and 2), the G protein follows pathways (i) or (ii) depending on its γ subunit type. (i) Non-translocating γ subunits enable the βγ complex to stay on the activated receptor after the release of the α subunit (step 3). Competing affinities for an effector (blue diamond) and the receptor determine dissociation of βγ from the receptor (step 4). (ii) Translocation-capable γ subunits have low affinity for the activated receptor compared with a target in the Golgi or ER and translocate as a βγ complex to these membranes (step 3). Orange arrows indicate that translocation rates vary among βγ types depending on their relative affinities for the activated receptor or effectors. Receptor inactivation leads to an increase in affinity for the βγ complex and higher α-GDP concentration (step 4). Both pathways finally lead to increased α-GDP βγ binding to the inactivated receptor (step 5).
Box 1. Mechanism and function of shuttling and translocation.
The unexpected finding that two families of signal-transducing proteins are capable of directed movement between membrane compartments inside a cell raises new questions. How are the G protein βγ complex and Ras proteins that are prenylated capable of diffusion through the cytosol? One of the puzzles about the shuttling of G proteins and Ras proteins between the plasma membrane and the Golgi is that both are modified with hydrophobic prenyl moieties that are expected to impede free movement through the cytosol. The ability of free βγ complexes to translocate from the plasma membrane to the Golgi is also a surprise for the same reason. One possibility is that there is a sequestering protein that masks the hydrophobic prenyl moiety facilitating the diffusion of these proteins through the cytosol.
How does acylation mediate the translocation of βγ complexes? There is evidence for the α subunits of G proteins being palmitoylated and shuttling of the heterotrimer being mediated by a depalmitoylation–palmitoylation cycle. But there is no evidence that the βγ complex is palmitoylated. So, the possibility that translocation is aided by an unidentified protein that undergoes transient palmitoylation is attractive.
What are the physiological roles of the receptor-mediated βγ complex translocation to the Golgi and ER? Does removal of the βγ complex on the plasma membrane and lowered concentration of the heterotrimer lead to signal attenuation? Do the G protein βγ complexes and Ras have specific roles in regulating Golgi function? How and why are the βγ complexes targeted to the Golgi complex or the ER? Are there protein targets in the intracellular membranes that recognize specific βγ complexes?
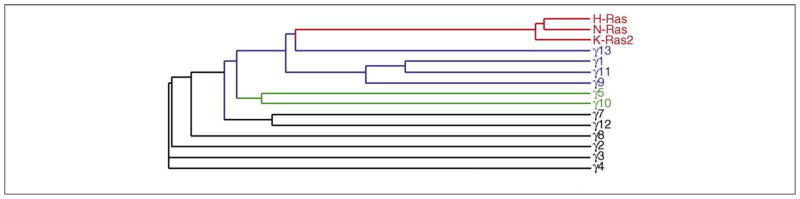
In terms of the mechanistic basis of the βγ complex translocation, it is diffusion mediated, palmitoylation dependent and is independent of whether the subunit type is modified with farnesyl or geranylgeranyl [28]. Translocation is strikingly dependent on the amino acid sequence of the γ subunits. A phylogenetic tree of the γ subunit family shows that subunits with shared translocation properties also are more closely related at the level of their primary structures (Figure 4). Based on extensive evidence for the interaction of the C-terminal domain of the γ subunit with a receptor [33–37], this domain has been mutated and shown to regulate translocation capability [28, 38, 39]. Although the prenyl moiety at the C terminus of γ subunits influences receptor interaction [40, 41], it is clear that it is the specific amino acid residues at the C-terminal domain that dictate the translocation behavior of a γ subunit. For instance, geranylgeranylated βγ13 translocates as rapidly as farnesylated βγ9 or βγ11, and a geranylgeranylated non-translocating γ3 subunit can be mutated at the C terminus to confer translocation capability [28].
Figure 4.
The evolutionary relationship between the primary structures of G protein γ subunits and Ras proteins. Protein sequences of γ subunits and Ras proteins of human origin were used to generate a phylogenetic tree based on sequence alignment at ClustalW website (www.ebi.ac.uk/ClustalW). Color codes are given to proteins based on their intracellular movement observed in living cells: red, Ras proteins; blue, rapidly translocating Gγ subunits; green, slowly translocating Gγ subunits; black, non-translocating Gγ subunits.
The simplest model underlying receptor-mediated βγ translocation is shown in Figure 3b. In the basal state, the γ subunit has a high affinity for the inactive receptor, and the βγ complex present as inactive heterotrimer with the α subunit is retained on the plasma membrane. Translocating γ subunits have a lower affinity for the activated state of a receptor and, when the receptor is activated, βγ containing these γ subunits dissociate from the Gα subunit and move to the Golgi or ER where a protein target with a relatively high affinity for the complex is present. Receptor deactivation results in reversal of the translocation because the inactive receptor has a high affinity for the βγ complex. The ability to rapidly reverse suggests that the βγ complex is continually sensing the cytosolic surfaces of the intracellular and plasma membranes. γ Subunits, which are translocation incompetent, do not have lowered affinity for the activated receptor, and the βγ complexes containing these subunits are retained on the plasma membrane. In this model, the translocation of βγ complexes is controlled by the relative affinities of activated and deactivated GPCRs for βγ subunits.
Although a G protein heterotrimer is inactive, activation leads to the generation of two active species: the α subunit and the βγ complex. Thus, the translocation of Gβγ potentially enables it to directly modulate the function of effectors in the Golgi. It has been shown that free Gβγ introduced into cells through permeabilization or overexpression breaks down the Golgi into vesicles [42]. The formation of these vesicles is mediated by protein kinase D (PKD) suggesting that these vesicles transport cargo to the plasma membrane [43, 44]. However, the stimulus for G protein action on the Golgi and the source of any βγ that might act on the Golgi complex in intact cells has not been identified. GPCR-activated βγ translocation to the Golgi provides a mechanism that enables Gβγ to reach the Golgi from the plasma membrane and regulate secretory-vesicle formation in an extracellular-stimulus-dependent manner. Consistent with such a possibility, the M3 acetylcholine receptor regulation of insulin secretion has been demonstrated to be mediated by PKD recently [45]. The translocation of Gβγ away from the plasma membrane can also modulate G-protein-mediated signaling responses downstream because it reduces the concentration of heterotrimer available to the receptor and also the βγ available for acting on plasma membrane effectors. Consistent with this expectation, there are suggestions that light adaptation in rod photoreceptors is mediated by the translocation from the rod outer to the inner segment of the subunits of Gt, the G protein specific to these cells [46, 47]. Gt subunit translocation occurs in response to rhodopsin activation by light [46]. This translocation is consistent with the ability of the rod γ subunit to translocate rapidly in mammalian cells in response to GPCR activation [28]. Furthermore, light-activated translocation seems to be mediated by simple diffusion as in the case of Gβγs described earlier, although reversal is dependent on the cytoskeleton [48, 49]. In contrast to the translocation of Gβγ complexes discussed earlier, both the Gtα and βγ subunits translocate and seem to do so independently (t1/2: αt ~5 min and βγt ~12 min) [46]. Also, reversal of translocation on dark adaptation is a very slow process lasting 200 min for αt [50]. It is not clear whether under basal conditions Gt shuttles between the rod outer and inner segments.
The selective receptor-stimulated translocation of βγ is also a distinctly different process compared with the internalization of the receptor [51], which does not involve the Golgi and is temporally separated from βγ translocation (D.K.S. and N.G., unpublished,). The receptor-induced βγ translocation is also distinctly different from Gs internalization that was observed first several years back [52, 53] and again more recently [54, 55]. The internalized Gs localizes to cytosolic vesicles [52, 54, 55] on receptor activation. The rate of this internalization is relatively slow, ranging in minutes [55], compared with the selective translocation of Gβγ, which takes place in the order of seconds [13, 28, 29]. Furthermore, the internalization of Gαs and Gβγ occur together reminiscent of Gt translocation on rhodopsin activation discussed later. Finally, it has been shown that receptor-stimulated Gs internalization occurs as a result of enhanced depalmitoylation of Gαs [52, 53]. Reports from other laboratories have, however, been unable to detect internalization of Gs from the plasma membrane to the cytosol on activation or depalmitoylation [56, 57]. The relationship between the translocation of Gt subunits on rhodopsin activation or Gs internalization on β2-adrenoceptor activation and the rapid, directed and selective translocation of the βγ complex on receptor activation to the Golgi or ER remains unclear.
Translocation of Ras proteins
In the case of Golgi-localized Ras, direct activation by a plasma-membrane-bound epidermal growth factor receptor has been observed in live cells using a sensor that detects Ras activity [12, 58]. Activation on the plasma membrane has been shown to be rapid, whereas on the Golgi it is slower and sustained. Ras shuttling is thought to distribute Ras subtypes differentially in intracellular membranes leading to the activation of different effectors enriched specifically in these compartments [10, 12]. These temporal and spatial distinctions in activity enable Ras activated pathways to provide different responses. A more refined picture of the physiological consequences of these differential responses is likely to emerge in the future. As in the case of G proteins, it is unclear whether Ras has a direct role in regulating Golgi function.
Similar to the G protein subunits, translocation of a Ras protein, K-Ras, from the plasma membrane to the Golgi and endosomes has been observed in hippocampal neurons in response to stimulation that induces Ca2+ influx in neurons [59]. The translocation is slower than that of the rapidly translocating Gβγ complexes (t1/2 ~4.5 min compared with ~10 s for Gβγ). This translocation is consistent with the dynamic association of K-Ras with membranes [60]. It occurs through cytosolic diffusion aided by binding to calmodulin, which probably helps to mask the hydrophobic prenyl moiety [59]. In contrast to H-Ras and N-Ras, which contain palmitoyl moieties in addition to the prenyl modification, K-Ras contains only the prenyl moiety. It is thought to interact with membranes through the prenyl moiety and a C-terminal polybasic domain [25]. Other evidence suggests that K-Ras can translocate from the plasma membrane to the mitochondria through phosphorylation, which counteracts the affinity of the polybasic domain for membranes [61]. Similar to the G protein βγ complex, reverse translocation of K-Ras probably occurs through a non-vesicular pathway involving simple diffusion.
Role of lipid modifications in the intracellular movement of G proteins and Ras
Although the shuttling of both G proteins and Ras is controlled by a depalmitoylation–palmitoylation cycle, both families of proteins are also modified with other lipids that could potentially influence the intracellular movement between hydrophobic membranes. Although G protein γ subunits and Ras are modified with a prenylfarnesyl or geranylgeranyl at the C terminus, some Gα types such as Gαo are modified with an additional myristoyl group at the N terminus. However, no distinct impact of type of prenyl moiety on Gγ subunit shuttling or receptor-activated translocation has been observed. Gβγ2, which is geranylgeranylated, and Gβγ11, which is farnesylated, show similar shuttling properties [14]. Gγ11, which is farnesylated, and Gγ13, which is geranylgeranylated, translocate equally rapidly on receptor activation [28]. Evidence from the observation of Gαo and Gαq suggest that there are no substantial differences in the shuttling kinetics of these subunits despite Gαo being modified by an additional myristoyl moiety [14, 18]. Experiments with H-Ras have revealed that the C-terminal prenyl moiety plays no part in Ras shuttling [23]. A geranylgeranylated H-Ras shuttles with equal efficiency as a farnesylated H-Ras [23]. The Ras proteins, H-Ras, N-Ras and K-Ras, are modified with farnesyl at the C terminus but only H-Ras and N-Ras are palmitoylated, whereas K-Ras contains a polybasic domain with affinity for negatively charged groups of phospholipids, which provides the membrane-binding capability [62]. Similarly Rap1a and Rap2b are geranylgeranylated but only the latter is palmitoylated [59]. When C-terminal domains of K-Ras, H-Ras, Rap1a and Rap2b were tagged with FPs and examined, only the protein fusions with non-palmitoylated tails translocated on stimulating neuronal activity [59]. However, both farnesylated and geranylgeranylated domains translocated, showing overall that the prenyl moiety similar to the G protein γ subunits has no role in the translocation process but the palmitoylation hinders it.
These strikingly similar observations with G proteins and Ras proteins suggest a model encompassing the roles of different lipid modifications for the movement of these proteins between cellular membranes. Both palmitoyl and a prenyl group enable retention of these proteins on the plasma membrane. G protein heterotrimers are retained on the membrane because of the palmitoyl present on the α subunit and the prenyl moiety on the βγ complex. H-Ras and N-Ras are retained on the membrane by the palmitoyl and prenyl moieties, whereas K-Ras binding is dependent on the prenyl moiety and the polybasic domain. Most members of the Gα family are palmitoylated, and depalmitoylation will reduce their affinity for the plasma membrane similar to depalmitoylated H-Ras and N-Ras. It is possible that the affinity of the prenyl moiety alone is insufficient for membrane binding. Alternatively, a masking sequestering protein might prevent membrane interaction. Thus, shuttling of proteins between the plasma membrane and endomembranes is entirely dictated by a palmitoylation cycle. Although K-Ras does not shuttle between membranes regulated by a depalmitoylation–palmitoylation cycle, it is nevertheless rapidly and continually associating and dissociating from membranes [63, 64], which enables it to be potentially redistributed to specific endomembranes where it might have specific functions. It is possible that, similar to K-Ras, the non-palmitoylated but prenylated Gγ subunits enable βγ complexes to continually sense different membranes through a similar rapid process of dissociation and reassociation. The ability of the translocated Gβγs to rapidly reverse translocation from the Golgi to the plasma membrane on receptor inactivation [13, 28] suggests that they might be continually sensing these two membrane surfaces.
In contrast to basal level shuttling, only the non-palmitoylated proteins are capable of translocation on receptor activation. Weakened association between the palmitoylated Gα and Gβγ subunits results in βγ translocation. Again, it has to be considered that a sequestering protein might mask the hydrophobic prenyl moiety during translocation of Gβγ preventing retention on the plasma membrane and facilitating diffusion through the hydrophilic cytosol. For K-Ras, Ca2+/calmodulin has been identified as such a sequestering protein [59, 65, 66].
When a phylogenetic tree is generated using amino acid sequences of Gγ subunits and Ras, an unexpected finding emerges (Figure 4). The translocation-capable K-Ras protein is more related to the rapidly translocating Gγ subunits (Figure 4). This relationship suggests that the translocation capabilities of these proteins lie in their specific amino acid sequences.
Conclusions
The movement of signal-transducing intermediates is a requirement for intracellular communication of biological messages emanating from outside a cell. Small molecules enable this communication to occur rapidly through cytosolic diffusion. Classically, it has been thought that when GPCRs sense an extracellular signal, second messengers such as cAMP and IP3 diffuse away from the plasma membrane to regulate intracellular effectors. The unexpected movement of G proteins and their active subunits provides an alternative mechanism for the direct transfer of extracellular input to the interior of a cell. Because diffusion of molecules through the cytosol is much more rapid compared with movement along a membrane surface, this mechanism enables speedy communication between extracellular signal and intracellular membranes. The ability to target activated Gβγ to specific membrane components inside a cell such as the Golgi or ER provides a more specific mode of regulation compared with the diffusion of second messengers. Classically, Gβγ effectors such as adenyl cyclases [67], phospholipase Cβ [68, 69], potassium channels [70], PI3K [71] and mitogen-activated protein kinases [72, 73] are plasmamembrane bound [74]. But several effectors are also present at various intracellular locations: PKD [43] in the Golgi; glucocorticoid receptors [8] and histone deacetylases [75] in the nucleus; phosducin [76], phosducin-like protein [77, 78], nucleotide exchange factor P-Rex1 [79] and cool-2 [80] in the cytosol; and tubulin in the cytoskeleton [81]. Extracellular- signal-stimulated translocation of Gβγ to these intracellular effectors can modulate important physiological processes such as secretion. Temporal differences in the translocation of different βγ subtypes enable variations in the time of induction and duration of activity. Spatial differences in the target of the translocated βγ subtypes also enable different effectors to be activated in different cell compartments. The variety in the rate and spatial target of the translocation of the βγ subunit type complexes thus enables more complex encoding of the signaling output so that physiological responses can be distinct.
It is possible that receptor-stimulated translocation of Gβγ will be observed to be widespread and seen to occur between the plasma membrane and other intracellular membranes such as endosomes, the nucleus and mitochondria. But many questions still remain unanswered regarding the mechanistic basis of the intracellular mobility of G protein subunits in addition to Ras proteins (Box 1). Their identification, apart from providing a template for understanding protein movement, is also likely to be of pharmacological value because GPCRs are the single most important target for commercially available therapeutic drugs [82, 83] (Box 2). The regulation of G protein shuttling, translocation and Ras shuttling by a depalmitoylation–palmitoylation cycle points to a central role for palmitoylation in the regulation of signaling activity. Palmitoylation of G protein α subunits and the Ras proteins was identified many years back [84] but the role was unclear. Palmitoylation-dependent mechanisms are now known to be important mediators of the intracellular movement of G protein subunits and Ras. But this is not the only mechanism because the mobility of Gt subunits in rod photoreceptors and that of K-Ras in different cell types is likely to be regulated by mechanisms that do not involve palmitoylation. Overall, various mechanisms seem to have been recruited to induce and control the movement of these signal-transducing proteins inside cells in an evolutionarily convergent manner.
Box 2. Potential therapeutics.
The discovery of both constitutive and activation-dependent movement of G proteins and Ras in mammalian cells raises the possibility of using this knowledge pharmacologically. Heterotrimeric G proteins mediate the vast majority of physiological responses to signals from outside the cell. These signals include light, neurotransmitters, hormones and odorant molecules. The Ras family is an important mediator of cell proliferation and differentiation that is triggered by growth factor receptors. Unraveling the mechanistic bases of the basal level shuttling or the activity-dependent translocation of these two crucial families of proteins can provide novel targets for directing newly designed therapeutic drugs. Such drugs can potentially regulate the activity-dependent translocation of Gβγ. If the receptor-mediated translocation of the βγ complex is involved in regulating a Golgi function such as secretion, GPCR regulation of important secretory hormones such as insulin can be regulated by using small molecules or by suitably engineering the translocation properties of G protein subunits in cells. Similarly, if βγ translocation leads to lowered cellular responses to stimuli, inhibiting βγ translocation would result in heightened responses in cells. Such an intervention would be of help in cells that display weakened responses in the diseased state.
Additionally, the ability to image FP-tagged Gβγ translocation in live cells rapidly enables novel direct assays that screen for GPCR directed drugs. This translocation-dependent assay is a direct readout of GPCR activation without involving monitoring classical secondary messengers such as Ca2+, IP3 or cAMP. The assay measures the first step in the activation of a GPCR signaling pathway. It eliminates the non-specific molecules that act at downstream steps in this pathway. The rapid reversibility of the βγ translocation on GPCR deactivation further enables this assay to be used for both GPCR activator (agonist) and inhibitor (antagonist) screens. Because translocation and reversal occurs with t1/2 s of ~10 s and a cell can be used repeatedly, the assay provides a rapid way to screen for multiple drug compounds using same cell. The translocation-based assay thus has advantages over existing technologies used for identifying compounds that act on GPCRs, which still form the single largest target family for the drug discovery process.
Acknowledgments
Supported by National Institutes of Health grants GM69027 and GM080558 (www.nih.gov) and American Heart Association postdoctoral fellowships to D.K.S. and M.C. (www.americanheart.org). We thank Vani Kalyanaraman for valuable discussions.
Glossary
- Diffusion
the process that leads to movement of molecules inside the cell from a region of high concentration to a region of low concentration by energy-independent random molecular motion
- Fluorescent protein (FP)
a protein that fluoresces in living cells when excited with light of a specific wavelength and can be genetically tagged to any protein of interest to study their behavior by fluorescence imaging. Availability of spectrally distinct proteins for tagging enables simultaneous imaging of many cellular proteins at the same time
- FRAP
fluorescence recovery after photobleaching; a technique in which the mobility of a FP-tagged protein of interest is studied inside a cell. It is performed by bleaching the FP in a selected part of a cell and then monitoring the recovery in the bleached region
- Golgi
an intracellular membrane organelle localized to the perinuclear region and composed of membrane stacks. It is involved in the post-translational processing of proteins and serves as a site of packaging and trafficking of secretory and plasma-membrane-bound molecules inside a cell
- Live-cell-imaging
techniques to image dynamics of protein movement and localization in living cells. It is performed by genetically tagging proteins under study with FPs such as green FP (GFP) and then studying them inside a living cell in their actual milieu
- Palmitoylation cycle
depalmitoylation–palmitoylation events that lead to the removal and reattachment of a 16-carbon palmitoyl modification to various proteins
- Photoactivation
an optical process in which a bleached FP can be reactivated using light of a specific wavelength. Photoactivation of a protein of interest tagged with a photoactivatable FP such as Dronpa can enable migration of the protein to be studied
- Prenyl modification
post-translational addition of an isoprenoid lipid group such as the 15C farnesyl or the 20C geranylgeranyl at the C-terminal end of a protein. Presence of a -CAAX motif at the C-terminal sequence determines prenylation. The ‘X’ amino acid determines whether the cysteine within the CAAX box is farnesylated or geranylgeranylated. If X is serine, methionine or glutamine it leads to farnesylation of proteins and if it is leucine or phenylalanine the protein is modified with geranylgeranyl
- Shuttling
bidirectional movement of proteins between two target sites inside a living cell
- Translocation
relocalization of proteins from one part of the cell to another part, usually as a result of a stimulation or perturbation
- Vesicular trafficking
movement of molecules from the Golgi to the plasma membrane or cell exterior through small lipid carriers called vesicles. These lipid vesicles require the cytoskeleton for their movement
References
- 1.Denker SP, et al. Differential distribution of α subunits and βγ subunits of heterotrimeric G proteins on Golgi membranes of the exocrine pancreas. J Cell Biol. 1996;133:1027–1040. doi: 10.1083/jcb.133.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pimplikar SW, Simons K. Regulation of apical transport in epithelial cells by a Gs class of heterotrimeric G protein. Nature. 1993;362:456–458. doi: 10.1038/362456a0. [DOI] [PubMed] [Google Scholar]
- 3.Stow JL, et al. A heterotrimeric G protein, Gαi–3, on Golgi membranes regulates the secretion of a heparan sulfate proteoglycan in LLC-PK1 epithelial cells. J Cell Biol. 1991;114:1113–1124. doi: 10.1083/jcb.114.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slessareva JE, et al. Activation of the phosphatidylinositol 3- kinase Vps34 by a G protein α subunit at the endosome. Cell. 2006;126:191–203. doi: 10.1016/j.cell.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Regalado A, et al. G protein-coupled receptor-promoted trafficking of Gβ1γ2 leads to AKT activation at endosomes via a mechanism mediated by Gβ1γ2–Rab11a interaction. Mol Biol Cell. 2008;19:4188–4200. doi: 10.1091/mbc.E07-10-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Dyke RW. Heterotrimeric G protein subunits are located on rat liver endosomes. BMC Physiol. 2004;4:1. doi: 10.1186/1472-6793-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar V, et al. Activated nuclear metabotropic glutamate receptor mGlu5 couples to nuclear Gq/11 proteins to generate inositol 1,4,5-trisphosphate-mediated nuclear Ca2+ release. J Biol Chem. 2008;283:14072–14083. doi: 10.1074/jbc.M708551200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kino T, et al. G protein β interacts with the glucocorticoid receptor and suppresses its transcriptional activity in the nucleus. J Cell Biol. 2005;169:885–896. doi: 10.1083/jcb.200409150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreeva AV, et al. Gα12 is targeted to the mitochondria and affects mitochondrial morphology and motility. FASEB J. 2008;22:2821–2831. doi: 10.1096/fj.07-104224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocks O, et al. Spatio-temporal segregation of Ras signals: one ship, three anchors, many harbors. Curr Opin Cell Biol. 2006;18:351–357. doi: 10.1016/j.ceb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Plowman SJ, Hancock JF. Ras signaling from plasma membrane and endomembrane microdomains. Biochim Biophys Acta. 2005;1746:274–283. doi: 10.1016/j.bbamcr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Quatela SE, Philips MR. Ras signaling on the Golgi. Curr Opin Cell Biol. 2006;18:162–167. doi: 10.1016/j.ceb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Akgoz M, et al. Receptor-mediated reversible translocation of the G protein βγ complex from the plasma membrane to the Golgi complex. J Biol Chem. 2004;279:51541–51544. doi: 10.1074/jbc.M410639200. [DOI] [PubMed] [Google Scholar]
- 14.Chisari M, et al. Shuttling of G protein subunits between the plasma membrane and intracellular membranes. J Biol Chem. 2007;282:24092–24098. doi: 10.1074/jbc.M704246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ando R, et al. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science. 2004;306:1370–1373. doi: 10.1126/science.1102506. [DOI] [PubMed] [Google Scholar]
- 16.Shrum CK, et al. Stimulated nuclear translocation of NF-κB and shuttling differentially depend on dynein and the dynactin complex. Proc Natl Acad Sci U S A. 2009;106:2647–2652. doi: 10.1073/pnas.0806677106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nix DA, Beckerle MC. Nuclear-cytoplasmic shuttling of the focal contact protein, zyxin: a potential mechanism for communication between sites of cell adhesion and the nucleus. J Cell Biol. 1997;138:1139–1147. doi: 10.1083/jcb.138.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsutsumi R, et al. Identification of G protein α subunit-palmitoylating enzyme. Mol Cell Biol. 2009;29:435–447. doi: 10.1128/MCB.01144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaelson D, et al. Membrane trafficking of heterotrimeric G proteins via the endoplasmic reticulum and Golgi. Mol Biol Cell. 2002;13:3294–3302. doi: 10.1091/mbc.E02-02-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takida S, Wedegaertner PB. Exocytic pathway-independent plasma membrane targeting of heterotrimeric G proteins. FEBS Lett. 2004;567:209–213. doi: 10.1016/j.febslet.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 21.Azpiazu I, Gautam N. A fluorescence resonance energy transfer-based sensor indicates that receptor access to a G protein is unrestricted in a living mammalian cell. J Biol Chem. 2004;279:27709–27718. doi: 10.1074/jbc.M403712200. [DOI] [PubMed] [Google Scholar]
- 22.Goodwin JS, et al. Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J Cell Biol. 2005;170:261–272. doi: 10.1083/jcb.200502063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocks O, et al. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–1752. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- 24.Marrari Y, et al. Assembly and trafficking of heterotrimeric G proteins. Biochemistry. 2007;46:7665–7677. doi: 10.1021/bi700338m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pechlivanis M, Kuhlmann J. Hydrophobic modifications of Ras proteins by isoprenoid groups and fatty acids – more than just membrane anchoring. Biochim Biophys Acta. 2006;1764:1914–1931. doi: 10.1016/j.bbapap.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- 27.Dupre DJ, et al. The role of Gβγ subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol. 2009;49:31–56. doi: 10.1146/annurev-pharmtox-061008-103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saini DK, et al. A family of G protein βγ subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation. J Biol Chem. 2007;282:24099–24108. doi: 10.1074/jbc.M701191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azpiazu I, et al. G protein βγ11 complex translocation is induced by Gi, Gq and Gs coupling receptors and is regulated by the α subunit type. Cell Signal. 2006;18:1190–1200. doi: 10.1016/j.cellsig.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meder D, Simons K. Cell biology. Ras on the roundabout. Science. 2005;307:1731–1733. doi: 10.1126/science.1110551. [DOI] [PubMed] [Google Scholar]
- 31.Alexandrov K, et al. Rab escort protein-1 is a multifunctional protein that accompanies newly prenylated rab proteins to their target membranes. EMBO J. 1994;13:5262–5273. doi: 10.1002/j.1460-2075.1994.tb06860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dransart E, et al. RhoGDI-3, a promising system to investigate the regulatory function of rhoGDIs: uncoupling of inhibitory and shuttling functions of rhoGDIs. Biochem Soc Trans. 2005;33:623–626. doi: 10.1042/BST0330623. [DOI] [PubMed] [Google Scholar]
- 33.Azpiazu I, Gautam N. G protein γ subunit interaction with a receptor regulates receptor-stimulated nucleotide exchange. J Biol Chem. 2001;276:41742–41747. doi: 10.1074/jbc.M104034200. [DOI] [PubMed] [Google Scholar]
- 34.Hou Y, et al. Selective role of G protein γ subunits in receptor interaction. J Biol Chem. 2000;275:38961–38964. doi: 10.1074/jbc.C000604200. [DOI] [PubMed] [Google Scholar]
- 35.Kisselev O, Gautam N. Specific interaction with rhodopsin is dependent on the γ subunit type in a G protein. J Biol Chem. 1993;268:24519–24522. [PubMed] [Google Scholar]
- 36.Kisselev O, et al. Receptor-G protein coupling is established by a potential conformational switch in the βγ complex. Proc Natl Acad Sci U S A. 1995;92:9102–9106. doi: 10.1073/pnas.92.20.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kisselev OG, Downs MA. Rhodopsin controls a conformational switch on the transducin γ subunit. Structure. 2003;11:367–373. doi: 10.1016/s0969-2126(03)00045-5. [DOI] [PubMed] [Google Scholar]
- 38.Gautam N. A conformational switch regulates receptor–G protein interaction. Structure. 2003;11:359–360. doi: 10.1016/s0969-2126(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 39.Akgoz M, et al. G protein βγ complex translocation from plasma membrane to Golgi complex is influenced by receptor γ subunit interaction. Cell Signal. 2006;18:1758–1768. doi: 10.1016/j.cellsig.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kisselev O, et al. Efficient interaction with a receptor requires a specific type of prenyl group on the G protein γ subunit. J Biol Chem. 1995;270:25356–25358. doi: 10.1074/jbc.270.43.25356. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda H, et al. Role of the prenyl group on the G protein γ subunit in coupling trimeric G proteins to A1 adenosine receptors. J Biol Chem. 1996;271:18588–18595. doi: 10.1074/jbc.271.31.18588. [DOI] [PubMed] [Google Scholar]
- 42.Jamora C, et al. Regulation of Golgi structure through heterotrimeric G proteins. Cell. 1997;91:617–626. doi: 10.1016/s0092-8674(00)80449-3. [DOI] [PubMed] [Google Scholar]
- 43.Jamora C, et al. Gβγ-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell. 1999;98:59–68. doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- 44.Diaz Anel AM, Malhotra V. PKCη is required for β1γ2/β3γ2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J Cell Biol. 2005;169:83–91. doi: 10.1083/jcb.200412089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sumara G, et al. Regulation of PKD by the MAPK p38δ in insulin secretion and glucose homeostasis. Cell. 2009;136:235–248. doi: 10.1016/j.cell.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calvert PD, et al. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16:560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Kassai H, et al. Farnesylation of retinal transducin underlies its translocation during light adaptation. Neuron. 2005;47:529–539. doi: 10.1016/j.neuron.2005.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenzweig DH, et al. Subunit dissociation and diffusion determine the subcellular localization of rod and cone transducins. J Neurosci. 2007;27:5484–5494. doi: 10.1523/JNEUROSCI.1421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reidel B, et al. The translocation of signaling molecules in dark adapting mammalian rod photoreceptor cells is dependent on the cytoskeleton. Cell Motil Cytoskeleton. 2008;65:785–800. doi: 10.1002/cm.20300. [DOI] [PubMed] [Google Scholar]
- 50.Elias RV, et al. Temporal kinetics of the light/dark translocation and compartmentation of arrestin and α-transducin in mouse photoreceptor cells. Mol Vis. 2004;10:672–681. [PubMed] [Google Scholar]
- 51.Gainetdinov RR, et al. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 52.Wedegaertner PB, et al. Activation-induced subcellular redistribution of Gsα. Mol Biol Cell. 1996;7:1225–1233. doi: 10.1091/mbc.7.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wedegaertner PB, Bourne HR. Activation and depalmitoylation of Gsα. Cell. 1994;77:1063–1070. doi: 10.1016/0092-8674(94)90445-6. [DOI] [PubMed] [Google Scholar]
- 54.Allen JA, et al. β-adrenergic receptor stimulation promotes Gαs internalization through lipid rafts: a study in living cells. Mol Pharmacol. 2005;67:1493–1504. doi: 10.1124/mol.104.008342. [DOI] [PubMed] [Google Scholar]
- 55.Hynes TR, et al. Live cell imaging of Gs and the β2-adrenergic receptor demonstrates that both αs and β1γ7 internalize upon stimulation and exhibit similar trafficking patterns that differ from that of the β2-adrenergic receptor. J Biol Chem. 2004;279:44101–44112. doi: 10.1074/jbc.M405151200. [DOI] [PubMed] [Google Scholar]
- 56.Huang C, et al. Persistent membrane association of activated and depalmitoylated G protein α subunits. Proc Natl Acad Sci U S A. 1999;96:412–417. doi: 10.1073/pnas.96.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones TL, et al. The stoichiometry of Gαs palmitoylation in its basal and activated states. Biochemistry. 1997;36:7185–7191. doi: 10.1021/bi9628376. [DOI] [PubMed] [Google Scholar]
- 58.Bivona TG, et al. Phospholipase Cγ activates Ras on the Golgi apparatus by means of RasGRP1. Nature. 2003;424:694–698. doi: 10.1038/nature01806. [DOI] [PubMed] [Google Scholar]
- 59.Fivaz M, Meyer T. Reversible intracellular translocation of KRas but not HRas in hippocampal neurons regulated by Ca2+/calmodulin. J Cell Biol. 2005;170:429–441. doi: 10.1083/jcb.200409157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silvius JR, et al. K-ras4B and prenylated proteins lacking ‘second signals’ associate dynamically with cellular membranes. Mol Biol Cell. 2006;17:192–202. doi: 10.1091/mbc.E05-05-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bivona TG, et al. PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol Cell. 2006;21:481–493. doi: 10.1016/j.molcel.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 62.Hancock JF, et al. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- 63.Leventis R, Silvius JR. Lipid-binding characteristics of the polybasic carboxy-terminal sequence of K-ras4B. Biochemistry. 1998;37:7640–7648. doi: 10.1021/bi973077h. [DOI] [PubMed] [Google Scholar]
- 64.Yokoe H, Meyer T. Spatial dynamics of GFP-tagged proteins investigated by local fluorescence enhancement. Nat Biotechnol. 1996;14:1252–1256. doi: 10.1038/nbt1096-1252. [DOI] [PubMed] [Google Scholar]
- 65.Villalonga P, et al. Calmodulin binds to K-Ras, but not to H- or N-Ras, and modulates its downstream signaling. Mol Cell Biol. 2001;21:7345–7354. doi: 10.1128/MCB.21.21.7345-7354.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sidhu RS, et al. Ca2+/calmodulin binds and dissociates K-RasB from membrane. Biochem Biophys Res Commun. 2003;304:655–660. doi: 10.1016/s0006-291x(03)00635-1. [DOI] [PubMed] [Google Scholar]
- 67.Taussig R, et al. Regulation of purified type I and type II adenylylcyclases by G protein βγ subunits. J Biol Chem. 1993;268:9–12. [PubMed] [Google Scholar]
- 68.Akgoz M, et al. Role of the G protein γ subunit in βγ complex modulation of phospholipase Cβ function. J Biol Chem. 2002;277:19573–19578. doi: 10.1074/jbc.M201546200. [DOI] [PubMed] [Google Scholar]
- 69.Fogg VC, et al. Role of the γ subunit prenyl moiety in G protein βγ complex interaction with phospholipase Cβ. J Biol Chem. 2001;276:41797–41802. doi: 10.1074/jbc.M107661200. [DOI] [PubMed] [Google Scholar]
- 70.Lei Q, et al. Activation and inhibition of G protein-coupled inwardly rectifying potassium (Kir3) channels by G protein βγ subunits. Proc Natl Acad Sci U S A. 2000;97:9771–9776. doi: 10.1073/pnas.97.17.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Viard P, et al. Gβγ dimers stimulate vascular L-type Ca2+ channels via phosphoinositide 3-kinase. FASEB J. 1999;13:685–694. doi: 10.1096/fasebj.13.6.685. [DOI] [PubMed] [Google Scholar]
- 72.Coso OA, et al. Signaling from G protein-coupled receptors to c-Jun kinase involves βγ subunits of heterotrimeric G proteins acting on a Ras and Rac1-dependent pathway. J Biol Chem. 1996;271:3963–3966. doi: 10.1074/jbc.271.8.3963. [DOI] [PubMed] [Google Scholar]
- 73.Luttrell LM, et al. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gβγ subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- 74.Clapham DE, Neer EJ. G protein βγ subunits. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 75.Spiegelberg BD, Hamm HE. Gβγ binds histone deacetylase 5 (HDAC5) and inhibits its transcriptional co-repression activity. J Biol Chem. 2005;280:41769–41776. doi: 10.1074/jbc.M504066200. [DOI] [PubMed] [Google Scholar]
- 76.Schulz R. The pharmacology of phosducin. Pharmacol Res. 2001;43:1–10. doi: 10.1006/phrs.2000.0757. [DOI] [PubMed] [Google Scholar]
- 77.Lukov GL, et al. Role of the isoprenyl pocket of the G protein βγ subunit complex in the binding of phosducin and phosducin-like protein. Biochemistry. 2004;43:5651–5660. doi: 10.1021/bi035903u. [DOI] [PubMed] [Google Scholar]
- 78.Thibault C, et al. Interaction of phosducin-like protein with G protein βγ subunits. J Biol Chem. 1997;272:12253–12256. doi: 10.1074/jbc.272.19.12253. [DOI] [PubMed] [Google Scholar]
- 79.Barber MA, et al. Membrane translocation of P-Rex1 is mediated by G protein βγ subunits and phosphoinositide 3-kinase. J Biol Chem. 2007;282:29967–29976. doi: 10.1074/jbc.M701877200. [DOI] [PubMed] [Google Scholar]
- 80.Feng Q, et al. Novel regulatory mechanisms for the Dbl family guanine nucleotide exchange factor Cool-2/α-Pix. EMBO J. 2004;23:3492–3504. doi: 10.1038/sj.emboj.7600331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Montoya V, et al. G protein βγ subunits interact with αβ- and γ-tubulin and play a role in microtubule assembly in PC12 cells. Cell Motil Cytoskeleton. 2007;64:936–950. doi: 10.1002/cm.20234. [DOI] [PubMed] [Google Scholar]
- 82.Overington JP, et al. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 83.Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 84.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]