Abstract
Heteromerization of opioid receptors has been shown to alter opioid receptor pharmacology. However, how receptor heteromerization affects the processes of endocytosis and postendocytic sorting has not been closely examined. This question is of particular relevance for heteromers of the μ-opioid receptor (MOR) and δ-opioid receptor (DOR), because the MOR is recycled primarily after endocytosis and the DOR is degraded in the lysosome. Here, we examined the endocytic and postendocytic fate of MORs, DORs, and DOR/MOR heteromers in human embryonic kidney 293 cells stably expressing each receptor alone or coexpressing both receptors. We found that the clinically relevant MOR agonist methadone promotes endocytosis of MOR but also the DOR/MOR heteromer. Furthermore, we show that DOR/MOR heteromers that are endocytosed in response to methadone are targeted for degradation, whereas MORs in the same cell are significantly more stable. It is noteworthy that we found that the DOR-selective antagonist naltriben mesylate could block both methadone- and [d-Ala2,NMe-Phe4,Gly-ol5]-enkephalin-induced endocytosis of the DOR/MOR heteromers but did not block signaling from this heteromer. Together, our results suggest that the MOR adopts novel trafficking properties in the context of the DOR/MOR heteromer. In addition, they suggest that the heteromer shows “biased antagonism,” whereby DOR antagonist can inhibit trafficking but not signaling of the DOR/MOR heteromer.
Introduction
δ-Opioid receptors (DORs) and μ-opioid receptors (MORs) belong to the G protein-coupled receptor (GPCR) superfamily, and upon activation they regulate a variety of physiological functions including pain processing, anxiety, and reward (for review see Bodnar, 2010). After activation, opioid receptors, like most GPCRs, can be rapidly phosphorylated by GPCR kinases, bind arrestin proteins (Ferguson et al., 1998), and be endocytosed. After endocytosis receptors are then either targeted to degradation (for the DOR) (Whistler et al., 2002) or recycled back to the cell surface (for the MOR) (Law et al., 2000; Whistler et al., 2002).
Many GPCRs, including opioid receptors, are believed to function as dimers or higher-order oligomers (Rozenfeld and Devi, 2010). There is substantial evidence that the MOR and DOR form heteromers in vitro (Cvejic and Devi, 1997; George et al., 2000; Gomes et al., 2000, 2004; Fan et al., 2005; Hasbi et al., 2007) and mounting evidence that they form functional heteromers in vivo as well (Gupta et al., 2010; Wang et al., 2010; He et al., 2011). Coexpression of opioid receptors has been shown to alter opioid ligand properties and affect receptor signaling in cell culture model systems (Jordan and Devi, 1999; George et al., 2000; Gomes et al., 2004; Rozenfeld and Devi, 2007; Kabli et al., 2010), and these differences are hypothesized to occur as a consequence of receptor heteromerization. In addition, the DOR/MOR heteromer is reported to couple preferentially with the inhibitory pertussis toxin-insensitive Gαz subunit instead of pertussis toxin-sensitive Gαi (Fan et al., 2005; Hasbi et al., 2007). Furthermore, DOR/MOR heteromerization seems to also influence receptor maturation (Décaillot et al., 2008) and arrestin-mediated signaling (Rozenfeld and Devi, 2007). In addition, some MOR- and DOR-selective agonists have been shown to promote endocytosis when both receptors are coexpressed, although this phenomenon seems to be ligand-dependent (Hasbi et al., 2007; Kabli et al., 2010), occurring with some but not all agonists. However, those prior studies did not examine endocytosis or postendocytic trafficking of DOR/MOR heteromers in response to many of the clinically relevant opioid drugs. In particular, there has been no exploration of the postendocytic fate of the DOR/MOR heteromer after activation by MOR agonists. This is particularly important for heteromers containing MOR and DOR because these two receptors have dramatically different postendocytic fates (Law et al., 2000; Tsao and von Zastrow, 2000; Whistler et al., 2002). Specifically, after endocytosis MORs are reported to be recycled (Law et al., 2000; Whistler et al., 2002; Liang et al., 2008) and show rapid functional resensitization (Alvarez et al., 2002). In contrast, the DOR binds the GPCR-associated sorting protein and is targeted to the lysosomal degradation pathway after endocytosis (Tsao and von Zastrow, 2000; Whistler et al., 2002), although the rate and extent of degradation are reported to be agonist-dependent (Zhang et al., 1999; Lecoq et al., 2004; Binyaminy et al., 2008; Archer-Lahlou et al., 2009).
Here, we examined the endocytic and postendocytic trafficking properties of the DOR/MOR heteromers and examined whether the heteromer showed changes in “biased agonism” for trafficking compared with the receptor homomers.
Materials and Methods
Reagents.
[d-Ala2,NMe-Phe4,Gly-ol5]-enkephalin (DAMGO), naltriben mesylate (NTB), [d-Pen2,d-Pen5]-enkephalin (DPDPE), and 7-benzylidene naltrexone maleate (BNTX) were purchased from Tocris Bioscience (Ellisville, MO). Naltrindole hydrochloride (NTI) and methadone hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). All compounds were dissolved in water, with the exception of BNTX and NTB, which were dissolved in 5% dimethyl sulfoxide. Mouse M1 and M2 monoclonal antibody, anti-FLAG M2 affinity matrix, albumin from bovine serum, l-glutathione, iodoacetamide, gelatin from bovine skin type B, Triton X-100, and Tween 20 were purchased from Sigma-Aldrich. Anti-HA.11 beads were from Covance Research Products (Princeton, NJ).
Cell Culture.
HEK293 cells (American Type Culture Collection, Manassas, VA) were grown in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT). N-terminal signal sequence and either HA- or FLAG-tagged c-DNA murine opioid receptor constructs were stably expressed in HEK293 cells. For generation of clonal stable cell lines, single colonies were chosen and propagated in the presence of selection-containing medium. Cell lines were carefully matched for expression (see Waldhoer et al., 2005).
Immunofluorescence Confocal Microscopy.
HEK293 cells stably expressing N-terminal FLAG-MOR alone, HA-DOR alone, or FLAG-MOR and HA-DOR together were incubated with monoclonal anti-HA.11 antibody (Covance Research Products) and/or M1 anti-FLAG antibody (Sigma-Aldrich) for 30 min to label surface receptors. Cells were treated as indicated. Subsequently, cells were fixed with 3.7% formaldehyde in PBS for 20 min at room temperature and permeabilized with 0.1% Triton X-100, essentially as described (Whistler and von Zastrow, 1998). Cells were then incubated with subtype-selective fluorescent anti-mouse antibody directed against M1 (Alexa488 IgG2b) and HA (Alexa594 IgG1, 1:1000, 30 min) (Invitrogen) to label MOR and DORs with different colors. After staining, cells were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and analyzed using a Zeiss LSM 510 META Axioplan 2 confocal microscope (Carl Zeiss Inc., Thornwood, NY).
Biotin Protection Endocytosis and Endocytosis-Degradation Assays.
HEK293 cells stably expressing N-terminal FLAG-MOR alone or FLAG-MOR and HA-DOR together were grown to 90% confluence in 10-cm plates. Cells were washed twice in PBS and biotinylated with 0.3 mg/ml disulfide-cleavable biotin (Thermo Fisher Scientific, Waltham, MA) at 4°C for 30 min to selectively label a pool of receptors at the cell surface as described (Finn and Whistler, 2001). For quantification of endocytosis, cells were washed in PBS and placed in prewarmed medium for 15 min before treatment with ligand or no treatment for 30 min. For quantification of stability/degradation, cells were incubated with ligand for prolonged periods of time as indicated. Concurrent with ligand treatment total and strip plates remained at 4°C. After ligand treatment, plates were washed in PBS, and the remaining cell surface-biotinylated receptors were stripped in 50 mM glutathione, 75 mM NaCl, 75 mM NaOH, and 10% fetal bovine serum at 4°C for 60 min (twice 30 min; including strip but not total). Cells were quenched with PBS containing 50 mM iodoacetamide and 10% bovine serum albumin for 30 min (including total). Afterward, all cells were lysed in 0.1% Triton X-100, 150 mM NaCl, 25 mM KCl, and 10 mM Tris· HCl, pH 7.4 with protease inhibitors (Roche Diagnostics, Basel, Switzerland). Lysates were cleared by centrifugation at 10,600g (Eppendorf 5417R; Eppendorf North America, New York, NY) for 10 min at 4°C. In cells expressing only one type of receptor they were immunoprecipitated overnight at 4°C with anti-FLAG M2 or HA.11 affinity matrix (depending on the epitope tag), washed, and resolved by SDS-PAGE. The “protected” pool of endocytosed receptors were visualized by streptavidin overlay. This protected pool shrinks across time for receptors that are degraded, because no new receptors are biotinylated. This pool remains constant for receptors that are endocytosed, recycled, and re-endocytosed. For monitoring homomer versus heteromer trafficking in the same cells, cells were biotinylated, treated with agonist for the indicated time, stripped, quenched, and lysed as above. Lysates were then incubated with anti-FLAG M2 affinity matrix overnight at 4°C, which immunoprecipitated both FLAG-MOR homomers and FLAG-MOR/HA-DOR heteromers. The lysate remaining was separated from the pellet and then immunoprecipitated with HA.11 affinity matrix to isolate HA-DOR homomers. The pellet containing FLAG M2 affinity matrix, and therefore both MOR homomers and DOR/MOR heteromers, was incubated with FLAG peptide to release all receptors to the lysate. This lysate was then incubated with HA.11 affinity matrix to selectively immunoprecipitate HA-DOR/FLAG-MOR heteromers (that had already been immunoprecipitated with M2 matrix). The HA.11 affinity matrix contained the DOR/MOR heteromers, whereas the lysate contained MOR homomers. Finally, the lysate remaining from the immunoprecipitation with HA.11 affinity matrix was incubated with anti-FLAG M2 affinity matrix to specifically isolate FLAG-MOR homomers. All matrix/beads were washed and precipitates were deglycosylated with peptide N-glycosidase F (New England Biolabs, Ipswich, MA) in 10 mM Tris, pH 7.5, for 1 h at 37°C, denatured with SDS sample buffer (no reducing agent), and resolved by SDS/PAGE. Blots were blocked in 5% milk, washed thoroughly, and incubated with Vectastain ABC reagent (Vector Laboratories) for 30 min and washed thoroughly again. Blots were developed with enhanced chemiluminescence reagents (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK), scanned, and quantified using ImageJ software (National Institutes of Health, Bethesda, MD). No previous studies have used serial immunoprecipitation to specifically follow the postendocytic fate of homomer and heteromer species in the same cell line.
Calcium Mobilization Assay.
HEK293 cells stably expressing N-terminal FLAG-MOR alone or FLAG-MOR and HA-DOR together were seeded onto 96-well black clear-bottom plates from Corning Life Sciences (Lowell, MA). Cells were then transiently transfected with chimeric G protein Δ6-Gqi4-myr (100 ng for every 70,000 cells) (Kostenis, 2001). One day after transfection, cells were loaded for 60 min with a Ca2+ fluorophore (Molecular Devices, Sunnyvale, CA) and stimulated with ligand as indicated in the figure legends. Intracellular Ca2+ release was measured immediately after agonist application in a Flex apparatus (Molecular Devices) for 2 min. For experiments with antagonist, cells were preincubated with antagonist at the stated concentrations for 20 min before measurement of Ca2+ release in relative fluorescence units (RFU). Data are represented as percentage of the maximal effect given by the MOR agonist.
Results
MOR Agonists Promote Endocytosis of DORs When Both Opioid Receptors Are Coexpressed.
Epitope-tagged versions of the murine DOR (HA-DOR) and MOR (FLAG-MOR) were stably expressed alone or together in a set of cell lines carefully matched for expression as described previously (Waldhoer et al., 2005). We then examined whether endocytosis of the MOR was affected by the presence of the DOR and vice versa. Cells expressing MOR or DOR alone or coexpressing MOR and DOR were treated with 1 μM of the MOR agonists methadone and DAMGO (Fig. 1). MORs were endocytosed upon activation with both DAMGO and methadone (Fig. 1A). The MOR agonist morphine did not promote endocytosis of either MOR or DOR (Supplemental Fig. 1). DORs were not endocytosed after application of the MOR agonists DAMGO or methadone when DOR was expressed alone (Fig. 1B), consistent with the low affinity of these drugs for the DOR (Raynor et al., 1994; Schmidt et al., 2002). However, DORs were readily endocytosed in response to the DOR agonist DPDPE (Supplemental Fig. 1).
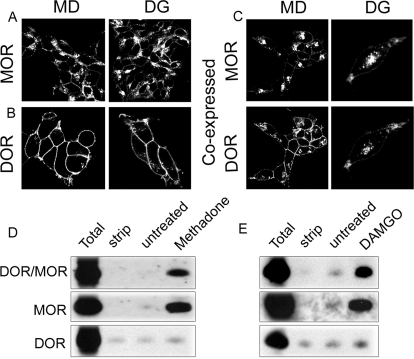
Fig. 1.
Agonist-occupied MORs promote coendocytosis of DORs. A to C, HEK293 cells stably expressing FLAG-MOR (A), HA-DOR (B), or FLAG-MOR and HA-DOR (C) were incubated with antibody recognizing the N-terminal epitope tags for 30 min to label surface receptors. Cells were then treated with 1 μM MOR agonist methadone (MD) or MOR agonist DAMGO (DG) for 30 min at 37°C. Cells were fixed and stained as in Materials and Methods. In cells coexpressing DOR and MOR, images were captured consecutively from dual color channels (green and red fluorescence). Images are representative examples of multiple independent experiments. D and E, endocytosis of DOR/MOR heteromers, MOR homomers, and DOR homomers was analyzed by a biotin protection endocytosis assay in cells coexpressing FLAG-MOR and HA-DOR. Cells were biotinylated then treated with 1 μM methadone (MD) or DAMGO (DG) for 30 min. Endocytosed-protected homomeric and heteromeric receptors were separated by serial immunoprecipitation and resolved by SDS-PAGE as three populations: DOR homomers, MOR homomers, and DOR/MOR heteromers (see Materials and Methods). Total refers to the biotinylated receptor signal present in cells after initial labeling and without further manipulation; strip refers to biotinylated cells that were reacted with glutathione without other manipulations, demonstrating the efficiency with which biotin was cleaved from receptors and represents the background. Both total and strip serve as internal controls within each experiment. A representative immunoblot is shown for DOR/MOR heteromers (top), MOR homomers (middle), and DOR homomers (bottom).
We next examined whether coexpression of MOR and DOR altered endocytosis in response to the small-molecule agonist methadone. Indeed, in the presence of MOR, DORs were endocytosed in response to methadone (Fig. 1C). Likewise, the peptide agonist DAMGO also promoted endocytosis of the DOR when it was coexpressed with MOR (Fig. 1C). Morphine did not promote endocytosis of MOR and DOR even when they were coexpressed (Supplemental Fig. 1).
The effects of methadone and DAMGO on endocytosis of MOR and DOR homomers and DOR/MOR heteromers were then quantified by biotin protection assay and serial immunoprecipitation as described under Materials and Methods. In brief, cells coexpressing DORs and MORs were biotinylated with thio-cleavable biotin to label all the receptors present at the surface. Cells were then pretreated with the MOR agonist methadone or DAMGO (1 μM, 30 min; Fig. 1, D and E). Residual surface biotin was stripped leaving only the protected endocytosed pool. We then selectively immunoprecipitated DOR/MOR heteromers and MOR homomers in the DOR/MOR coexpressing cell line by serial immunoprecipitation (see Materials and Methods). Both methadone and DAMGO promoted endocytosis of MOR homomers (Fig. 1, D and E middle) and DOR/MOR heteromers (Fig. 1, D and E top), but not DOR homomers in the same cells (Fig. 1, D and E bottom).
MOR Endocytosis Is Inhibited in the Presence of DOR by DOR Antagonist.
Based on these results, we hypothesized that the DORs were being coendocytosed with the MORs in response to methadone (or DAMGO) as a heteromeric complex. If this were the case, we expected that occupying the DOR with an antagonist might selectively influence the trafficking of the DOR/MOR heteromer but not MOR homomers in response to MOR agonist. To examine this hypothesis, cells expressing MOR or DOR alone or coexpressing MOR and DOR were preincubated with the DOR-selective antagonist NTB for 20 min, then challenged with 1 μM of methadone or DAMGO for another 30 min still in the presence of antagonist. Under these conditions, NTB seemed to inhibit the endocytosis of MORs in the cells expressing MOR and DOR (Fig. 2C). This was unlikely to be a nonspecific effect of NTB on the MOR homomers, because NTB did not block endocytosis of the MOR in cells expressing only MOR (Fig. 2A), whereas it did block endocytosis of the DOR in response to the DOR agonist DPDPE in cells expressing DOR alone (Supplemental Fig. 1C). As expected, pretreatment with a MOR-specific antagonist, CTAP (D-Phe-Cys-Trp-Arg-Thr-Pen-Thr-NH2), blocked endocytosis of MOR homomers in response to methadone in a dose-dependent manner (Supplemental Fig. 2A), as well as DOR/MOR heteromers (Supplemental Fig. 2B).
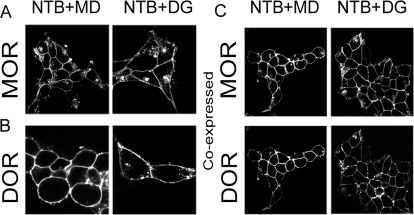
Fig. 2.
DOR antagonist NTB inhibits endocytosis in the DOR/MOR cell line. HEK293 cells stably expressing FLAG-MOR (A), HA-DOR (B), or FLAG-MOR and HA-DOR (C) were incubated with antibody recognizing the N-terminal epitope tags for 30 min at 37°C to label surface receptors. The DOR antagonist NTB (1 μM) was then added, followed 20 min later by the MOR agonist methadone (MD; 1 μM) or DAMGO (DG; 1 μM). After 30 min at 37°C, cells were fixed and stained as in Materials and Methods. In cells coexpressing DOR and MOR, images were captured consecutively from dual color channels (green and red fluorescence). Images are representative examples of multiple independent experiments.
To further examine and quantify whether the effects of the DOR antagonist on MOR endocytosis were selective to the heteromer, we used serial immunoprecipitation and the modified version of the biotin protection assay to independently monitor the extent of endocytosis of DOR/MOR heteromers and MOR homomers. Cells coexpressing DORs and MORs were biotinylated with thio-cleavable biotin then pretreated with the DOR antagonist NTB (from 0.05 to 1 μM, 20 min) or vehicle, followed by methadone or DAMGO (1 μM, 30 min; Fig. 3). Residual surface biotin was then stripped, leaving only the protected endocytosed pool. We then selectively immunoprecipitated DOR/MOR heteromers and MOR homomers in the DOR/MOR-coexpressing cell line by serial immunoprecipitation. We found that both MOR homomers and DOR/MOR heteromers were endocytosed by both methadone and DAMGO (Fig. 3A, compare with untreated). The DOR antagonist NTB significantly inhibited DOR/MOR endocytosis in response to both methadone and DAMGO in a concentration-dependent manner compared with its effect on MOR homomer endocytosis in the same cell line (Figs. 2A, top compared with bottom and 3B). NTB has a >100-fold higher affinity for DOR over MOR (Ki = 0.013 nM for DOR and Ki = 12 nM for MOR) (Raynor et al., 1994; Kim et al., 2001). Nevertheless, at high concentrations, selectivity can be lost (Kim et al., 2001). Consistent with this, endocytosis of MOR homomers was also inhibited by the highest concentration of the DOR antagonist NTB, albeit to a significantly smaller degree than DOR/MOR heteromer endocytosis (Fig. 3, A, top compared with bottom, and B). These effects were not unique to NTB. Pretreatment of cells coexpressing MOR and DOR with two other DOR antagonists, either BNTX (Ki = 0.66 nM for DOR and Ki = 18 nM for MOR) or NTI (Ki = 0.02 nM for DOR and Ki = 64 nM for MOR) (1 μM, 20 min), also significantly and selectively inhibited methadone- and DAMGO-induced endocytosis of the DOR/MOR heteromer compared with the MOR homomers present in the same cells (Fig. 4).
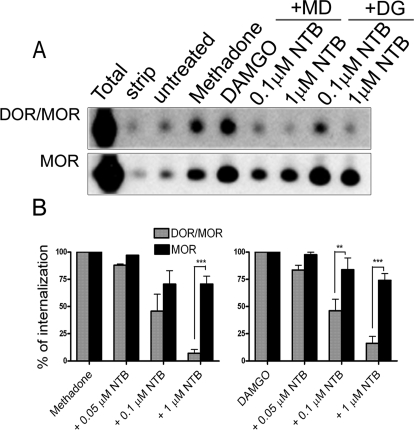
Fig. 3.
Endocytosis of the DOR/MOR heteromer can be inhibited by NTB in a dose-dependent manner. A, endocytosis of DOR/MOR heteromers and MOR homomers was analyzed by biotin protection endocytosis assay in cells coexpressing FLAG-MOR and HA-DOR. Cells were biotinylated then pretreated with different concentrations of NTB (or left untreated) for 20 min as indicated. Next, cells were treated with 1 μM methadone (MD) or DAMGO (DG) for an additional 30 min. Endocytosed protected receptors were separately resolved by SDS-PAGE as two populations, MOR homomers and DOR/MOR heteromers, after serial immunoprecipitation (see Materials and Methods). Total refers to the biotinylated receptor signal present in cells after initial labeling and without further manipulation; strip refers to biotinylated cells that were reacted with glutathione without other manipulations, demonstrating the efficiency with which biotin was cleaved from receptors and represents the background. Both total and strip serve as internal controls within each experiment. A representative immunoblot is shown for DOR/MOR heteromers (top) and MOR homomers (bottom). B, quantification of multiple experiments performed as in A. Histogram shows the endocytosis produced in the presence of antagonist as a percentage of that produced with methadone or DAMGO alone. Shown are the mean ± S.E.M. of n = three to five independent experiments (two-way ANOVA, Bonferroni post-test: **, p < 0.01; ***, p < 0.001).
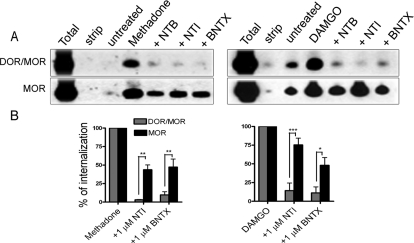
Fig. 4.
Endocytosis of the DOR/MOR heteromer can be inhibited by several DOR antagonists. A, endocytosis was assessed as in Fig. 3A but in the presence of the DOR-selective antagonists, NTI and BNTX (both at 1 μM). B, quantification of A was performed as in Fig. 3B. Shown are the mean ± S.E.M. of n = three to seven independent experiments (one-way ANOVA, Dunnet post-test compared with agonist treatment without any pretreatment: *, p < 0.05; **, p < 0.01; ***, p < 0.001).
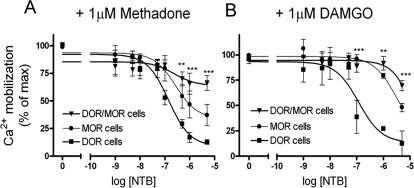
NTB Inhibits Endocytosis of DOR/MOR Heteromers Without Inhibiting Signaling.
We next examined whether the DOR antagonist NTB affected signaling of DOR/MOR heteromers in response to MOR agonist. Cells expressing MOR or DOR alone or coexpressing DOR and MOR were pretreated with increasing concentrations of NTB followed by treatment with methadone (1 μM; Fig. 5A) or DAMGO (1 μM; Fig. 5B). Drug-mediated signaling was assessed by measuring Ca2+ release from intracellular stores (see Materials and Methods and Kostenis, 2001). Both methadone and DAMGO showed equivalent potency and efficacy in MOR and DOR/MOR cell lines and significantly reduced potency and efficacy in cells with only DOR (see Table 1). Although NTB inhibited endocytosis of the DOR/MOR heteromer (Fig. 3), we found that, even at the highest doses, NTB inhibited only 34 ± 7% of methadone-mediated signaling (Fig. 5A) or 31 ± 5% of DAMGO-mediated signaling (Fig. 5B) in the DOR/MOR cells. NTB was actually more effective at inhibiting methadone- and DAMGO-mediated signaling in cells expressing only MOR (Fig. 5), where NTB inhibited 63 ± 10% of the methadone- and 52 ± 4% of the DAMGO-mediated signaling. As expected, NTB was very effective at inhibiting both methadone-mediated (87 ± 2%) (Fig. 5A) and DAMGO-mediated (87 ± 12%) (Fig. 5B) signaling in cells expressing only DOR (Fig. 5A). NTB also effectively blocked signaling from the DOR-selective agonist DPDPE in cells expressing DOR and cells expressing DOR/MOR (Supplemental Fig. 3).
Fig. 5.
NTB shows significantly reduced antagonism of signaling on DOR/MOR heteromers. Cells coexpressing DOR/MOR (▴), DOR only (■), or MOR only (●) were pretreated with increasing concentrations of the DOR antagonist NTB for 20 min. Calcium release caused by chimeric G protein Δ6-Gqi4-myr activation (see Materials and Methods) was measured in a Flex apparatus upon stimulation with methadone (A) or DAMGO (B) (1 μM). Maximal effects for methadone (RFU) were: MOR (1038 ± 88), DOR (565 ± 32), and DOR/MOR (843 ± 76). Maximal effects for DAMGO (RFU) were: MOR (735 ± 51), DOR (582 ± 55), and DOR/MOR (956 ± 56). Data represent means ± S.E.M. of n = three to five experiments carried out in triplicate (two-way ANOVA and Bonferroni post-test: **, p < 0.01; ***, p < 0.001 DOR/MOR compared with DOR-only cells).
TABLE 1.
G protein Δ6-Gqi4-myr-mediated Ca2+ release of HEK293 cells stably expressing opioid receptors
Data are mean ± S.E.M. (n = 3–6): one-way ANOVA and Tukey post-test.
| Log EC50 |
Emax |
|||||||
|---|---|---|---|---|---|---|---|---|
| Methadone | DAMGO | Morphine | DPDPE | Methadone | DAMGO | Morphine | DPDPE | |
| RFU | ||||||||
| DOR | −5.2 ± 0.5* | −5.7 ± 0.1*** | −6.0 ± 0.3 | −7.8 ± 0.2 | 565 ± 32*** | 582 ± 55*** | 472 ± 40 | 741 ± 95 |
| MOR | −6.7 ± 0.3 | −7.5 ± 0.1 | −7.4 ± 0.3 | −4.6 ± 0.6## | 1038 ± 88 | 735 ± 51 | 598 ± 36 | N.D. |
| DOR/MOR | −6.9 ± 0.2 | −7.7 ± 0.2 | −7.2 ± 0.5 | −7.8 ± 0.1 | 843 ± 76 | 956 ± 56 | 512 ± 45 | 734 ± 92 |
N.D., not determined.
P < 0.05 and
P < 0.001 DOR compared with DOR/MOR or MOR;
P < 0.05 MOR compared with DOR or DOR/MOR.
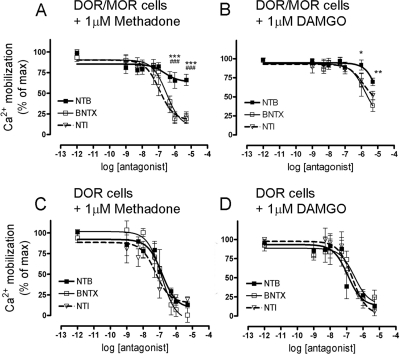
Therefore, NTB shows biased antagonism on the DOR/MOR heteromer by antagonizing only endocytosis but not DAMGO- or methadone-mediated signaling of the heteromeric complex (Figs. 3 and 5). It is noteworthy that this biased antagonism seems to be ligand-selective. Specifically, both BNTX and NTI not only blocked endocytosis of the DOR/MOR heteromer (Fig. 3), but also blocked methadone-mediated signaling on DOR/MOR cells (Fig. 6A; 81 ± 6 and 79 ± 6% of inhibition for BNTX and NTI, respectively). BNTX and NTI both also substantially blocked DAMGO-mediated signaling on DOR/MOR cells (Fig. 6B; 62 ± 8 and 48 ± 6% of inhibition for BNTX and NTI, respectively). As expected, all three DOR antagonists antagonized methadone- and DAMGO-mediated signaling in cells expressing only DOR (Fig. 6, C and D) (see Table 2).
Fig. 6.
Reduced antagonism of DOR/MOR heteromer signaling is NTB specific. A and B, cells coexpressing DOR/MOR were pretreated with increasing concentrations of NTB (■), BNTX (□), or NTI (▵) for 20 min and signaling in response to methadone (A) or DAMGO (B) (both 1 μM) was measured as in Fig. 5. C and D, cells expressing only DOR are shown as a control for each antagonist upon methadone (C) or DAMGO (D) (both 1 μM) stimulation. Data represent means ± S.E.M. of n = four to five experiments carried out in triplicate (two-way ANOVA and Bonferroni post-test: *, p < 0.05, **, p < 0.01, ***, p < 0.001 NTB compared with BNTX; ###, p < 0.001 NTB compared with NTI). See Table 2.
TABLE 2.
Antagonism of DOR ligands on 1 μM methadone or DAMGO
Data are mean ± S.E.M. (n = 4–5): one-way ANOVA and Tukey post-test.
| Log IC50 |
Maximal Inhibition |
|||||||
|---|---|---|---|---|---|---|---|---|
| DOR |
DOR/MOR |
DOR |
DOR/MOR |
|||||
| Methadone | DAMGO | Methadone | DAMGO | Methadone | DAMGO | Methadone | DAMGO | |
| % | ||||||||
| NTB | −6.8 ± 0.2 | −6.9 ± 0.3 | −6.6 ± 0.5 | −4.2 ± 7 | 87 ± 2 | 87 ± 12 | 34 ± 7***### | 31 ± 5** |
| BNTX | −6.9 ± 0.2 | −6.5 ± 0.3 | −6.5 ± 0.1 | −5.8 ± 0.3 | 90 ± 9 | 75 ± 9 | 81 ± 6 | 62 ± 8 |
| NTI | −7.2 ± 0.3 | −6.6 ± 0.2 | −6.9 ± 0.1 | −6.9 ± 0.1 | 81 ± 3 | 94 ± 1 | 79 ± 6 | 48 ± 6 |
P < 0.01 and
P < 0.001 NTB compared with BNTX;
P < 0.001 NTB compared with NTI.
Endocytosis of DOR/MOR Heteromers Leads to Degradation.
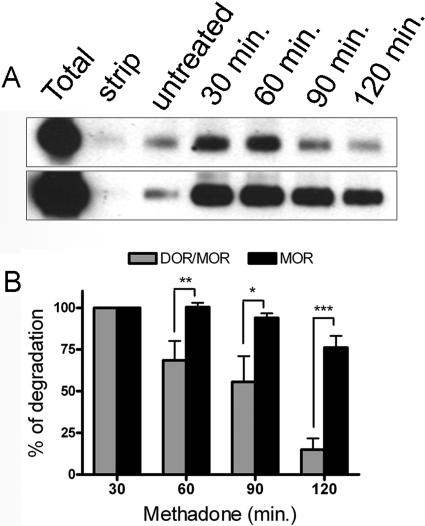
Several groups have reported that the MOR and DOR have different fates after endocytosis. However, the postendocytic fate of the DOR/MOR heteromer after endocytosis in response to MOR agonist is unknown. Consequently, we next assessed what postendocytic fate these heteromeric complexes followed, that of the MOR (recycling) or that of the DOR (degradation). Using a modified version of the biotin protection assay and serial immunoprecipitation, we assessed the postendocytic fate of MOR homomers and DOR/MOR heteromers in the DOR/MOR cell line (see Materials and Methods). As reported previously, endocytosed homomeric MORs were relatively stable even under constant agonist pressure for 2 h (Fig. 7, A bottom and B). In contrast, the endocytosed pool of DOR/MOR heteromers in the same cells were significantly more degraded after 2 h of agonist treatment (Fig. 7, A top and B; compared with MOR at the same time points). Thus, it seems that the DOR/MOR heteromer adopts the fate of the DOR after endocytosis in response to MOR agonist.
Fig. 7.
Endocytosis of the DOR/MOR heteromer leads to the degradation of the receptor complex. Postendocytic stability of DOR/MOR heteromers and MOR homomers from the same cell line were analyzed by biotin protection-degradation assay. Cells coexpressing FLAG-MOR and HA-DOR were biotinylated, then left untreated or treated with 1 μM methadone for 30, 60, 90, and 120 min before stripping. Total refers to the biotinylated receptor signal present in cells after initial labeling and without further manipulation; strip refers to biotinylated cells that were reacted with glutathione without other manipulations, demonstrating the efficiency with which biotin can be cleaved from surface receptors and represents the background. The stability of the protected endocytosed DOR/MOR heteromers (top) and MOR homomers (bottom) was assessed by serial immunoprecipitation followed by SDS-PAGE and streptavidin overlay (see Materials and Methods) at the time points stated. B, quantification of experiments in A is shown for DOR/MOR heteromers versus MOR homomers. Histogram shows the mean stability of the biotinylated endocytosed receptors relative to the endocytosed pool seen after 30 min of stimulation. Shown are the mean ± S.E.M. n = 4 to 10 independent experiments (two-way ANOVA, Bonferroni post-test: *, p < 0.05; **, p < 0.01; ***, p < 0.001).
Discussion
Here, we show that the DOR/MOR heteromer is unique in its trafficking properties in several ways. First, the MOR agonists methadone and DAMGO can promote endocytosis of DORs when the receptors are coexpressed. Second, DOR-selective antagonists can selectively inhibit endocytosis of the DOR/MOR heteromer but not the MOR homomer in the same cell in response to activation by MOR agonists. Third, and especially intriguing, is our observation that the DOR-selective antagonist NTB inhibits DOR/MOR heteromer endocytosis in a dose-dependent manner, without antagonizing G protein-mediated signaling from this receptor. Fourth, we found that the DOR/MOR heteromer adopts the postendocytic fate of the DOR receptor after endocytosis in response to MOR agonist, and is, therefore, degraded rather than recycled. Consequently, NTB can selectively block down-regulation of DOR/MOR heteromers by inhibiting their endocytosis without blocking signaling from the heteromer.
Although opioid receptor heteromers are widely accepted to exist in heterologous expression systems, the existence, and functional significance, of receptor heteromers in native tissues is still a matter of controversy. It has been reported that MORs and DORs are not colocalized in the spinal cord of mice expressing a green fluorescent protein-tagged DOR (Scherrer et al., 2009). In opposition to these findings, two studies demonstrated that endogenous MORs and DORs can indeed colocalize in small dorsal root ganglia neurons of mice (Wang et al., 2010; He et al., 2011). It is noteworthy that several groups have reported increased expression of functional DORs after varying physiological stimuli, including chronic morphine treatment, stress, chronic inflammatory pain, and ethanol consumption (for review see Bie and Pan, 2007; Cahill et al., 2007), suggesting that the prevalence of DOR/MOR heteromers could change under these conditions. Indeed, chronic morphine treatment has been shown to promote up-regulation of an opioid receptor complex that is recognized by an antibody selective for DOR/MOR heteromers (Gupta et al., 2010). Taken together, we believe there is increasing evidence that the DOR/MOR heteromer is a functional receptor unit in vivo. Thus, understanding the unique properties of this receptor heteromer could help reveal its functional role.
Once endocytosed, GPCRs can take distinct trafficking routes that further shape the signaling response. GPCRs are either 1) rapidly targeted to the lysosomes for its degradation, resulting in complete termination of receptor signal activity, 2) rapidly recycled back to the plasma membrane, resulting in resensitization and signal recovery, or 3) are retained in endosomes, traversing the degradative and/or recycling pathways at a much slower rate (for review see Marchese et al., 2008). Several groups have demonstrated that the MOR and DOR have different postendocytic fates. For example, we have seen that DORs heterologously expressed in HEK293 cells exhibit pronounced down-regulation within 2 h of exposure to agonist, whereas MORs expressed at similar levels are primarily recycled and significantly more stable (Whistler et al., 2002). The MOR has also been shown to resensitize in rat brain slices (Alvarez et al., 2002) and apparently in humans as well (Szeto et al., 2001). Thus, there remained the intriguing question as to the postendocytic fate of the DOR/MOR heteromer. We envisioned at least three possible outcomes for the DOR/MOR heteromers: 1) MORs within the heteromeric complex would take DORs back to the plasma membrane, converting DOR into a recycling receptor, 2) DORs would drag MORs in the heteromer to the lysosome, converting MOR into a degrading receptor, or 3) the receptors would separate after endocytosis and travel on their own. Here, we found that MORs that were heteromerized and coendocytosed with DORs were degraded more rapidly and to a further and greater extent that homomeric MORs in the same cells after endocytosis in response to MOR agonist. Thus, our data suggest either that the heteromer is a stable unit after endocytosis and that degradation “wins” in the battle for the fate of the heteromer, or coendocytosis of the MOR and DOR as a heteromer somehow marks the MORs in this complex for degradation. This codegradation of MOR and DOR would not be expected to occur under conditions where the MOR (and/or DOR) is not significantly endocytosed, such as after activation by morphine (Keith et al., 1998; Arttamangkul et al., 2008) (Supplemental Fig. 1). Thus, it is unlikely that the codegradation of MOR together with DOR is responsible for tolerance to the antinociceptive effects of chronic morphine as has been proposed (He et al., 2011).
Several groups have shown that heteromerization of MOR and DOR changes binding and signaling properties (Gomes et al., 2000, 2004; Kabli et al., 2010; Yekkirala et al., 2010). For example, combining MOR agonist and DOR antagonist can increase both the binding and signaling properties of the MOR (Gomes et al., 2000; Rozenfeld and Devi, 2007). Indeed, some ligands have been reported to have altered affinity for the DOR/MOR as well as for the κ-opioid receptor/DOR heteromer complex than to the respective homomers (Gomes et al., 2000, 2004; Waldhoer et al., 2005; Kabli et al., 2010). Therefore, we cannot rule out the possibility that this may be the case for NTB used in this study as well. Nevertheless, our observation that endocytosis of the DOR/MOR is blocked by NTB indicates that the ligand is engaging the heteromeric target and displaying biased antagonism. This biased antagonism is ligand-selective [BNTX and NTI, for example, block not only trafficking but also signaling (see Figs. 4 and 6)]. DOR antagonists are reported to reduce the development of tolerance to morphine in vivo (for review see Ananthan, 2006). Consequently, there has been much interest in combining DOR antagonists with MOR agonists to delay or reduce the development of opioid tolerance and dependence (Abdelhamid et al., 1991; Fundytus et al., 1995; Hepburn et al., 1997; Gomes et al., 2004; Daniels et al., 2005). It is noteworthy that our results suggest that the efficacy of these combination therapies probably will show ligand bias, and any interpretation of these data must include a consideration of the effects of these drug combinations not only on the signaling, but also on the trafficking of the MOR, DOR, and DOR/MOR heteromers.
Supplementary Material
This study was supported by a Schrödinger fellowship from the Austrian Science Fund [Fellowship J 2967-B09] (to L.M.-L.); the National Institutes of Health National Institute on Drug Abuse [Grant DA015232] (to J.L.W.); and funds provided by the State of California for medical research through the University of California, San Francisco (to J.L.W.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.179093.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- DOR
- δ-opioid receptor
- MOR
- μ-opioid receptor
- GPCR
- G-protein coupled receptor
- HEK
- human embryonic kidney
- PBS
- phosphate-buffered saline
- ANOVA
- analysis of variance
- RFU
- relative fluorescence units
- PAGE
- polyacrylamide gel electrophoresis
- DPDPE
- [d-Pen2,d-Pen5]-enkephalin
- BNTX
- 7-benzylidene naltrexone maleate
- NTB
- naltriben mesylate
- NTI
- naltrindole hydrochloride
- DAMGO
- [d-Ala2,NMe-Phe4,Gly-ol5]-enkephalin
- HA
- hemagglutinin.
Authorship Contributions
Participated in research design: Milan-Lobo and Whistler.
Conducted experiments: Milan-Lobo.
Performed data analysis: Milan-Lobo.
Wrote or contributed to the writing of the manuscript: Milan-Lobo and Whistler.
Other: Milan-Lobo and Whistler acquired funding for the research.
References
- Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. (1991) Selective blockage of δ-opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther 258:299–303 [PubMed] [Google Scholar]
- Alvarez VA, Arttamangkul S, Dang V, Salem A, Whistler JL, Von Zastrow M, Grandy DK, Williams JT. (2002) μ-Opioid receptors: ligand-dependent activation of potassium conductance, desensitization, and internalization. J Neurosci 22:5769–5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthan S. (2006) Opioid ligands with mixed μ/δ opioid receptor interactions: an emerging approach to novel analgesics. AAPS J 8:E118–E125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer-Lahlou E, Audet N, Amraei MG, Huard K, Paquin-Gobeil M, Pineyro G. (2009) Src promotes δ-opioid receptor (DOR) desensitization by interfering with receptor recycling. J Cell Mol Med 13:147–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low MJ, von Zastrow M, Pintar J, Williams JT. (2008) Differential activation and trafficking of μ-opioid receptors in brain slices. Mol Pharmacol 74:972–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bie B, Pan ZZ. (2007) Trafficking of central opioid receptors and descending pain inhibition. Mol Pain 3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binyaminy B, Gafni M, Shapira M, Sarne Y. (2008) Agonist-specific down regulation of μ-opioid receptors: different cellular pathways are activated by different opioid agonists. Life Sci 82:831–839 [DOI] [PubMed] [Google Scholar]
- Bodnar RJ. (2010) Endogenous opiates and behavior: 2009. Peptides 31:2325–2359 [DOI] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Morinville A. (2007) Trafficking of δ-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci 28:23–31 [DOI] [PubMed] [Google Scholar]
- Cvejic S, Devi LA. (1997) Dimerization of the δ-opioid receptor: implication for a role in receptor internalization. J Biol Chem 272:26959–26964 [DOI] [PubMed] [Google Scholar]
- Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. (2005) Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci USA 102:19208–19213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Décaillot FM, Rozenfeld R, Gupta A, Devi LA. (2008) Cell surface targeting of μ-δ opioid receptor heterodimers by RTP4. Proc Natl Acad Sci USA 105:16045–16050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T, Varghese G, Nguyen T, Tse R, O'Dowd BF, George SR. (2005) A role for the distal carboxyl tails in generating the novel pharmacology and G protein activation profile of μ- and δ-opioid receptor hetero-oligomers. J Biol Chem 280:38478–38488 [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Zhang J, Barak LS, Caron MG. (1998) Molecular mechanisms of G protein-coupled receptor desensitization and resensitization. Life Sci 62:1561–1565 [DOI] [PubMed] [Google Scholar]
- Finn AK, Whistler JL. (2001) Endocytosis of the μ-opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron 32:829–839 [DOI] [PubMed] [Google Scholar]
- Fundytus ME, Schiller PW, Shapiro M, Weltrowska G, Coderre TJ. (1995) Attenuation of morphine tolerance and dependence with the highly selective δ-opioid receptor antagonist TIPP[psi]. Eur J Pharmacol 286:105–108 [DOI] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O'Dowd BF. (2000) Oligomerization of μ- and δ-opioid receptors. Generation of novel functional properties. J Biol Chem 275:26128–26135 [DOI] [PubMed] [Google Scholar]
- Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. (2004) A role for heterodimerization of μ and δ opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci USA 101:5135–5139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. (2000) Heterodimerization of μ and δ opioid receptors: a role in opiate synergy. J Neurosci 20:RC110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, Lim M, Maillet E, Junek M, Cahill CM, et al. (2010) Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal 3:ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Nguyen T, Fan T, Cheng R, Rashid A, Alijaniaram M, Rasenick MM, O'Dowd BF, George SR. (2007) Trafficking of preassembled opioid μ-δ heterooligomer-Gz signaling complexes to the plasma membrane: coregulation by agonists. Biochemistry 46:12997–13009 [DOI] [PubMed] [Google Scholar]
- He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, Li Q, Yang H, Luo J, Li ZY, et al. (2011) Facilitation of μ-opioid receptor activity by preventing δ-opioid receptor-mediated codegradation. Neuron 69:120–131 [DOI] [PubMed] [Google Scholar]
- Hepburn MJ, Little PJ, Gingras J, Kuhn CM. (1997) Differential effects of naltrindole on morphine-induced tolerance and physical dependence in rats. J Pharmacol Exp Ther 281:1350–1356 [PubMed] [Google Scholar]
- Jordan BA, Devi LA. (1999) G-protein-coupled receptor heterodimerization modulates receptor function. Nature 399:697–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabli N, Martin N, Fan T, Nguyen T, Hasbi A, Balboni G, O'Dowd BF, George SR. (2010) Agonists at the δ-opioid receptor modify the binding of μ-receptor agonists to the μ-δ receptor hetero-oligomer. Br J Pharmacol 161:1122–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, von Zastrow M. (1998) μ-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol Pharmacol 53:377–384 [PubMed] [Google Scholar]
- Kim KW, Son Y, Shin BS, Cho KP. (2001) Pharmacological effects of naltriben as a ligand for opioid μ and κ receptors in rat cerebral cortex. Life Sci 68:1305–1315 [DOI] [PubMed] [Google Scholar]
- Kostenis E. (2001) Is Galpha16 the optimal tool for fishing ligands of orphan G-protein-coupled receptors? Trends Pharmacol Sci 22:560–564 [DOI] [PubMed] [Google Scholar]
- Law PY, Erickson LJ, El-Kouhen R, Dicker L, Solberg J, Wang W, Miller E, Burd AL, Loh HH. (2000) Receptor density and recycling affect the rate of agonist-induced desensitization of μ-opioid receptor. Mol Pharmacol 58:388–398 [DOI] [PubMed] [Google Scholar]
- Lecoq I, Marie N, Jauzac P, Allouche S. (2004) Different regulation of human δ-opioid receptors by SNC-80 [(+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide] and endogenous enkephalins. J Pharmacol Exp Ther 310:666–677 [DOI] [PubMed] [Google Scholar]
- Liang YJ, Wu DF, Stumm R, Höllt V, Koch T. (2008) Membrane glycoprotein M6A promotes μ-opioid receptor endocytosis and facilitates receptor sorting into the recycling pathway. Cell Res 18:768–779 [DOI] [PubMed] [Google Scholar]
- Marchese A, Paing MM, Temple BR, Trejo J. (2008) G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol 48:601–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. (1994) Pharmacological characterization of the cloned κ-, δ-, and μ-opioid receptors. Mol Pharmacol 45:330–334 [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. (2007) Receptor heterodimerization leads to a switch in signaling: β-arrestin2-mediated ERK activation by μ-δ opioid receptor heterodimers. FASEB J 21:2455–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. (2010) Receptor heteromerization and drug discovery. Trends Pharmacol Sci 31:124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. (2009) Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137:1148–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Vormfelde S, Klinder K, Gundert-Remy U, Gleiter CH, Skopp G, Aderjan R, Fuhr U. (2002) Affinities of dihydrocodeine and its metabolites to opioid receptors. Pharmacol Toxicol 91:57–63 [DOI] [PubMed] [Google Scholar]
- Szeto HH, Soong Y, Wu D, Fasolo J. (2001) Resensitization of blood pressure response to μ-opioid peptide agonists after acute desensitization. Anesth Analg 93:581–586 [DOI] [PubMed] [Google Scholar]
- Tsao P, von Zastrow M. (2000) Downregulation of G protein-coupled receptors. Curr Opin Neurobiol 10:365–369 [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL. (2005) A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci USA 102:9050–9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, et al. (2010) Coexpression of δ- and μ-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci USA 107:13117–13122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, Murray SR, Von Zastrow M. (2002) Modulation of postendocytic sorting of G protein-coupled receptors. Science 297:615–620 [DOI] [PubMed] [Google Scholar]
- Whistler JL, von Zastrow M. (1998) Morphine-activated opioid receptors elude desensitization by β-arrestin. Proc Natl Acad Sci USA 95:9914–9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekkirala AS, Kalyuzhny AE, Portoghese PS. (2010) Standard opioid agonists activate heteromeric opioid receptors: evidence for morphine and [d-Ala2-MePhe4-Glyol5] enkephalin as selective μ-δ agonists. ACS Chem Neurosci 1:146–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Law PY, Barak LS, Caron MG. (1999) Agonist-specific regulation of δ-opioid receptor trafficking by G protein-coupled receptor kinase and β-arrestin. J Recept Signal Transduct Res 19:301–313 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.