Abstract
Obesity has become an epidemic and its prevalence is increasing exponentially. A great deal of focus has been given to understanding the molecular processes that regulate obesity. The characterization of phospholipase A2s, especially adipose-specific PLA2, have lead to a proposed role of their downstream products in the progression of obesity and obesity related disorders. This review summarizes recent developments in the role of PLA2 and their downstream effects in the development of metabolic disorders.
INTRODUCTION
Obesity is a serious epidemic in our modern society leading to the development of multiple detrimental pathologies. The progression of metabolic pathologies, resulting from obesity, is a complex process with many regulators. Over 30% of the population is considered to be obese in the United States (1). Obesity related disorders are responsible for the development of a multitude of disease processes including type 2 diabetes, cardiovascular diseases, and certain cancers (2). It is clear that behavioral modifications in diet and energy expenditure can result in weight loss (3). However, these effects are often short lived and obesity persists (4). Therefore, much focus has been given to potential pharmaceutical treatments against obesity (5). Especially with the exponential growth of obesity in recent decades, emphasis has been given to the causes and possible therapeutic molecular targets for the prevention and/or treatment of obesity (1).
ADIPOSE TISSUE AND THE DEVELOPMENT OF OBESITY
Although multiple molecular processes are involved in increasing white adipose tissue (WAT) mass, obesity can accompany 1) increase in adipocyte size as a consequence of increased triacylglycerol (TAG) content within the fat cell, as well as 2) increased number of adipocytes resulting from differentiation of precursor cells. Much focus has been given to the molecular regulators of these two processes in the WAT, as control of these processes may prove beneficial in the treatment of obesity. Furthermore, obesity is associated with inflammation of adipose tissue. Adipocytes have the ability to secrete various inflammatory molecules, including tumor necrosis factor α (TNFα) and interleukin-6 (IL-6), and adipose tissue becomes infiltrated with macrophages that are a major source of pro-inflammatory cytokines. Thus, both increased adipokine secretion and macrophage infiltration may contribute to the development of pathologies that accompany obesity (6).
In addition to WAT, brown adipose tissue (BAT), the second type of adipose tissue whose presence in human adults has recently been firmly established, may also play a role in the progression of obesity (7). Unlike WAT, BAT contains a large number of mitochondria and is responsible for heat production through uncoupled respiration for non-shivering thermogenesis. It has been proposed that an increase in the uncoupling protein 1 (UCP-1) expression in the BAT is associated with resistance to obesity, at least in rodents (8;9). In addition, plasticity between WAT and BAT has also been proposed (10;11). In essence, controlling the development of obesity may occur by decreasing WAT mass and its contribution to inflammation as well as by increasing BAT mass or its ability to dissipate heat. Elucidating the molecular mechanisms underlying regulation of these processes in adipose tissue may provide insight into treating obesity.
Recent characterizations of phospholipase A2s (PLA2) have led to a possible link between their activity and downstream effectors, such as prostaglandins and leukotrienes, in the development of metabolic disorders, obesity, and inflammation (12–14). This review summarizes recent developments in the potential role of PLA2, particularly in adipose tissue, and their downstream effects in the progression of obesity and metabolic disorders.
PHOSPHOLIPASE A2 SUPERFAMILY
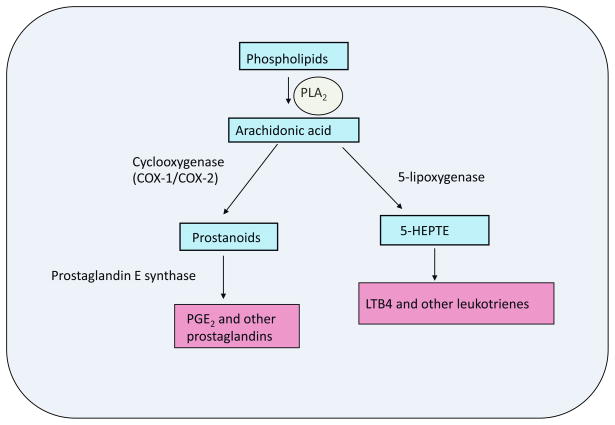
Phospholipases were first identified and examined in snake and bee venom and in mammalian pancreatic juice (15). However, over recent decades the discovery of numerous PLA2 has led to the notion that they play roles in a multitude of physiological functions (16). PLA2 hydrolyzes fatty acids (FA) from the sn-2 position of phospholipids to generate FA and lysophospholipids (15;17). These products can serve as ligands, substrates for bioactive molecule synthesis, or as signaling molecules themselves. Arachidonic acid (AA) is an important FA that is produced by PLA2, because it can be converted into bioactive molecules such as eicosanoids and leukotrienes (Fig. 1) (17;17–19). Thus, it is important to understand the regulatory role of PLA2 as the products of their hydrolytic activity have been implicated in the regulation of metabolic disorders (14;20–22).
Figure 1. Phospholipase A2 and its downstream products.
Phospholipase A2 (PLA2) cleaves sn-2 fatty acids from phospholipids. Arachidonic acid is an important fatty acid produced by PLA2 activity, since it can be converted into many bioactive molecules, prostaglandins and leukotrienes. Numerous enzymes are involved in the generation of bioactive molecules, and cylclooxygenase-1 and -2, prostaglandin E synthase, and 5-lipoxygenase are key players in the production of prostaglandins and luekotrienes.
There are at least 15 major groups of PLA2s which are subcategorized into five types of enzymes, secretory PLA2 (sPLA2), cytosolic PLA2 (cPLA2), Ca2+ -independent PLA2 (iPLA2), platelet-activating factor (PAF) acetylhydrolases, and lysosomal PLA2 (16). sPLA2s are characterized by Ca2+ -dependence for optimum activation, low molecular weights (<20 kDa), and the requirement of histidine for activity (23). Further, sPLA2 have six conserved disulfide bonds and one to two disulfide bonds that are varied. sPLA2 have been most studied in the regulation of nutrient digestion and inflammation processes and PLA2G1B, a pancreatic sPLA2, has been implicated in the regulation of obesity (23). cPLA2s have been characterized as having higher molecular weights (100 kDa) than the sPLA2 and have few to no disulfide bonds. cPLA2s contain C2 domains which results in a dependence on Ca2+ for activity and it appears that Ca2+ may be required for the translocation of cPLA2 from the cytosol to the intracellular membrane (16). iPLA2s are characterized by containing an active site with a catalytic serine and may be involved in membrane remodeling (24). Like cPLA2s, iPLA2s are localized intracellularly, however, unlike cPLA2, Ca2+ is not required for catalytic activity (16). PAF PLA2s are characterized as acetyl hydrolases and have been shown to be involved in proatherogenic, anti-inflammatory, and brain development processes (16;25). PAF PLA2s hydrolyze the acetyl group from the sn-2 position of PAF. Likewise with the iPLA2s the PAF PLA2s have activation through a catalytic serine site and do not require Ca2+ for activity (16). Importantly PAF PLA2s have domains with an affinity for HDL and LDL binding within the catalytic domain (17). Finally, lysosomal PLA2 contain four cysteine residues and has been shown to acylate cerimides (17). We have recently identified a new PLA2 expressed in adipose tissue that is Ca2+-dependent which may act to regulate obesity (26). A transcript encoding an 18-kDa protein was identified and it appears that this protein is highly and differentially expressed predominantly in adipose tissue (26). We named the newly discovered PLA2 adipose-specific PLA2 (AdPLA) (26). On further examination, sequence analysis showed that this protein had been identified as HRASLS3 (Ha-RAS like suppressor 3 or H-Rev-107-1), a class II tumor suppressor with no known molecular function (27). We found that this protein has phospholipase activity to produce FA and lysophospholipids and a preference for hydrolysis at the sn-2 position of phospholipids (26). We proposed that AdPLA may be the first member of an entirely new group of Ca2+-dependent intracellular PLA2s, group XVI. It is clear that PLA2s play a multitude of biological roles and their affects warrant further examination in regards to the regulation of metabolism and obesity.
PLA2 AND METABOLISM
Arachidonic acid released by phospholipid hydrolysis catalyzed by PLA2 can be converted into bioactive molecules such as prostaglandins (PG) that serve a variety of biological roles, such as temperature regulation, smooth muscle dilation, regulation of inflammation, and pain induction. PLA2 activity is considered to be the first rate-limiting enzyme in the generation of the downstream products of AA. Conversion of AA to PG begins via cyclooxygenase (COX) mediated production of intermediate prostanoids followed by isomerization to prostaglandins, such as prostaglandin E2 (PGE2), PGD2, and PGF2α, catalyzed by prostaglandin E synthase (PGES), (28;29) (Fig. 1). PGE2 and PGF2α have previously been reported to inhibit adipocyte differentiation whereas PGD2 was shown to enhance differentiation (30;31). In mature adipocytes, PGE2, has recently been implicated, by us and others, in an antilipolytic role in WAT by autocrine and paracrine regulation (14;32). Along with an antilipolytic effect, it has also been hypothesized that PGE2 is responsible for increased leptin release from adipose tissue which may result in a decrease in hepatic lipogenic gene expression (33). With its known role in the inflammatory process, PGE2 may also contribute to the progression of obesity and insulin resistance (16).
Besides the production of PGs, AA produced by PLA2 activity can be used in the production of leukotrienes via 5-lipoxygenase pathway (Fig. 1) (19;34). Although there has been no clear evidence that leukotrienes are involved in the progression of obesity, the need of further examination is suggested. Leukotrienes have long been associated with inflammation that results in asthma and drugs that act to inhibit leukotrienes have proven to be an effective treatment of asthma (35). Although not strong, there is indirect evidence that the production of leukotrienes may be associated with obesity (12;34). Recently studies on obese asthmatic patients suggested an association between obesity and urinary levels of leukotriene E4 (12). Further linking leukotrienes to obesity, macrophage leukotriene content has been reported to be regulated by leptin (34). Future studies on the relationship between leukotriene induced inflammation and obesity may provide insight into the development and/or treatment of obesity.
Finally, PLA2 may also play a role in the synthesis of endocannabinoids that function through binding to G-coupled type 1 and type 2 cannabinoid receptors. Endocannabinoids, such as N-arachidonoylethanolamine (anandamide) and 2-arachodoyl glycerol, can control metabolism at both central and peripheral levels (36). The major synthetic pathway for the synthesis of N-acylethanolamine is through N-acylation of phosphatidylethanolamine by transferring an acyl group from the sn-1 position to the amino group of phosphatidylethanolamine (PE) to generate N-acyl PE, followed by the phospholipase D catalyzed hydrolysis to generate N-acylethanolamine. However, one of the alternate pathways is conversion of N-acyl PE to N-acyl lysoPE catalyzed by soluble PLA2 and N-acyl lysoPE can then be used for generation of N-acylethanolamine (37). It has been shown that endocannabinoids are produced in adipose tissue and their levels appear to be increased in obese states, possibly due to decreased expression of endocannabinoid metabolizing enzymes (38). In this regard, endocannabinoids have been reported to stimulate lipogenesis and inhibit lipolysis, and may be linked to inflammation in adipose tissue (37). Furthermore, metabolically related N-oleoylethanolamine and N-palmitoylethanolamine have been reported to be potent endogenous ligands of PPARα which is well established to increase fatty acid oxidation and to be a target for the treatment of dyslipidemia (37;38).
AN ADIPOSE SPECIFIC PLA2: AdPLA
PLA2s have been given attention recently as possible therapeutic targets for obesity and the development of type 2 diabetes. Both genetic and pharmacological inhibition of particular PLA2s has resulted in obesity resistant mouse models, suggesting a potential target for anti-obesity drugs (14;39;40). Additionally, endoplasmic reticulum stress in the pancreatic beta cell has been linked to increasing Group VIA PLA2 (iPLA2β) expression and activity resulting in an increase in ceramides in the cytosol and thus a promotion of apoptosis in the beta cells, which ultimately may increase metabolic disorders (41). Conversely iPLA2β has also been implicated in a protective role in mitochondrial membrane repair in beta cells (42). While group X sPLA2 have been shown to be involved in regulating gene expression in macrophages, resulting in alterations in cholesterol efflux, and negative regulation of adipogenesis through activation of liver X receptor (LXR) (43). These recent data suggest that the downstream effects of PLA2s may play important roles in regulating metabolism in various tissues.
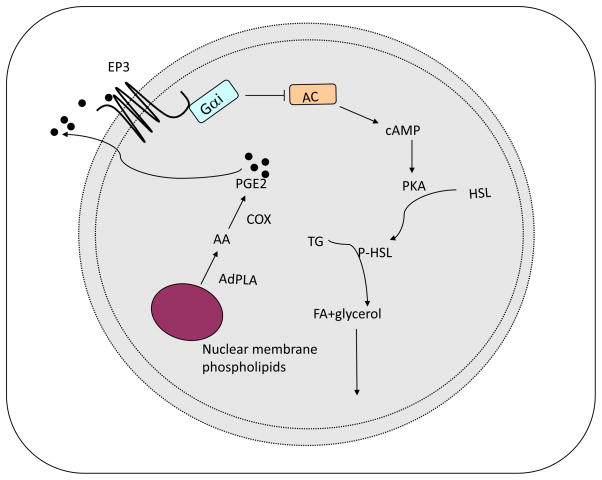
We recently identified a calcium-dependent PLA2, that we named AdPLA, which is mainly expressed in adipose tissue and induced in high fat diet-induced and genetic obesity (14;26). Through generation of an AdPLA knockout mouse model, it was established that AdPLA is a predominant PLA2 in adipose tissue (14). Furthermore, the AdPLA null mice exhibited resistance to high fat diet-induced obesity and displayed an increase in lipolytic activity in white adipose tissue (14). We also detected PGE2 to be present at the highest level in normal adipose tissue over other prostaglandins that are present below the concentrations for binding to their cognate receptors (14). In addition, among the receptors for PGE2, the prostaglandin E receptor 3 (EP3) is the most highly expressed in adipocytes. Other PGE2 receptors, EP1, EP2 and EP4, as well as the prostacyclin (IP) and DP receptors are expressed at very low levels in adipocytes (14). Therefore, we hypothesized that, in adipose tissue, PGE2 produced upon release of AA catalyzed by AdPLA may act through the Giα coupled- EP3 to suppress lipolysis in a cAMP dependent manner (Fig. 2) (14). This proposed relationship, AdPLA-PGE2-EP3, provides an autocrine-paracrine regulation of lipolysis in the adipose tissue, which has not been appreciated previously (Fig. 2). We found in our AdPLA knockout mice a decrease in PGE2 production along with increased lipolysis resulting in a lean phenotype even when on a high fat diet. The antilipolytic effect of PGE2 was prevented in the presence of an EP3 antagonist, L826266, in adipocytes, further solidifying the involvement of EP3 in the regulation of lipolysis by AdPLA mediated PGE2 production (14). To further examine the role of AdPLA in obesity, AdPLA knockout mice were bred with the leptin deficient ob/ob mice, a genetically obese mouse model that is used extensively to examine the development and treatment of obesity. By 6 weeks of age, the double knockout mice displayed a leaner phenotype when compared to the ob/ob mice and these decreases in body weight were accompanied by an increase in energy expenditure, adipose tissue lipolysis, and FA oxidation within this tissue (14). Despite the lean phenotype, both the AdPLA knockout and double knockout mice were insulin resistant due to ectopic fat storage. It may be possible that partial knocking down of AdPLA may provide benefits to be lean but without ectopic fat storage, under high fat feeding or leptin deficient-resistant conditions. Regardless, our results show for the first time a mechanistic role of PLA2 activity in the regulation of adipose tissue lipolysis and further examination of AdPLA activity is necessary.
Figure 2. The role of AdPLA-PGE2-EP3 signaling in the regulation of lipolysis.
AdPLA is an adipose specific PLA2 that can produce arachidonic acid (AA) from the sn-2 position of membrane phospholipids. Subsequently, prostaglandin E2 (PGE2) is produced via cyclooxygenase (COX) pathway and acts to suppress lipolysis through Giα-coupled prostaglandin E3 (EP3) to inhibit adenylate cyclase (AC). This model suggests an autocrine-paracrine role of PGE2 in the regulation of lipolysis in adipose tissue.
CYCLOOXYGENASE IN ADIPOSE TISSUE
The role of two COXs, COX-1 and COX-2, in PG production is well established in various tissues (44). COX-2 has been proposed to be involved in adipose tissue metabolism and may play a role in the development of obesity (8;45;46). However, there are conflicting data as to the importance of COX-2 in the regulation of metabolic disorders and obesity (46–48). COX-2 has been implicated in playing a role in the inflammatory process as a result of obesity or high fat feeding (47). Selective COX-2 inhibitors, celecoxib and nimesulide, used in combination with high fat diet in rats, yielded increases in insulin sensitivity and decreases in TNFα mRNA. Studies revealed a possible link between monocyte chemattractant protein-1 (MCP-1) and COX-2 (49). This link was further verified with decreases in macrophage infiltration into the adipose tissue, suggesting that COX-2 is an important regulator in the inflammatory process under high fat feeding conditions (49). These results taken with our results on AdPLA knockout mice suggest that inhibition of the COX-2 pathway in adipose tissue may prove beneficial in the treatment of obesity and in the prevention of inflammation that occurs with obesity.
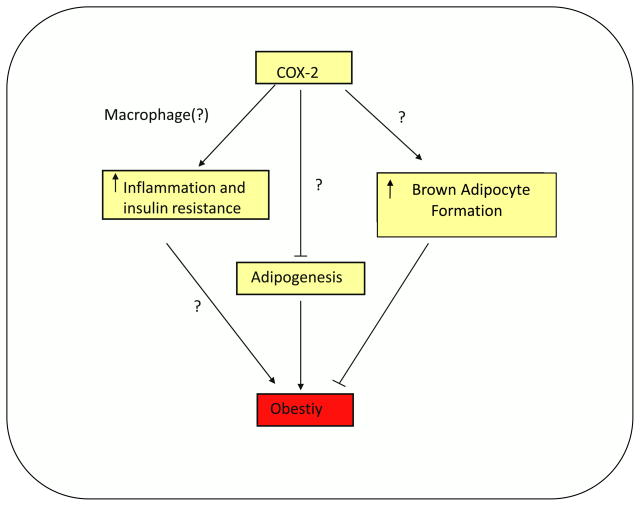
However, COX-2 has also been implicated in the so called “transdifferentiation” of WAT into BAT or “browning” of WAT (8;48). Chronic cold exposure have been shown to result in a two fold increase in COX-2 mRNA levels in mice in WAT. Additionally, COX-2 mRNA was increased upon treatment with β3 -adrenergic receptor agonist, CL316243, or norepinephrine in intra-abdominal adipose tissue as well as in adipocytes. The increase in COX-2 levels was accompanied by an increase in UCP-1 expression. In fact, treatment with CL316243 produced a “BAT-like phenotype” in WAT of mice on a standard chow diet which was ameliorated with celecoxib treatment and in COX-2 −/− mice (46). Additional studies revealed that inhibition of COX-2 with indomethacin or ablation of COX-2 resulted in a repression of cold-induced UCP-1 expression (8). It has also been proposed that PGE 2, through EP4 signaling with a lesser contribution through EP3, may induce UCP-1 expression in WAT upon cold exposure, possibly via recruitment of brown adipocytes in WAT depots (8). In contrast, we found an increase in UCP-1 mRNA in white adipose tissue from our AdPLA KO mice which had lower PGE2 content (14). Furthermore, these mice had an increase in FA oxidation within adipocytes, further suggesting that the WAT may take on more of a “BAT-like phenotype” with increased lipolysis (14). It is possible that effects of COX-2 in inducing BAT-like cells in WAT depots and in promoting BAT-like phenotype by affecting PGE2 mediated regulation of lipolysis in mature adipocytes may be through different mechanisms. Furthermore, recent studies suggest a link between COX-2 deficiency and adipocyte differentiation (45). Stable transfection of antisense COX-2 was shown to increase 3T3-L1 adipocyte differentiation, accompanying a decrease in PGE2 and PGF2α levels (50). In COX-2 knockout mice, the authors reported a decrease in body weight and reduced adiposity with an accompanied increase in oxygen consumption. Interestingly, there were no changes in PGE2 production in the COX-2 deficient mice and it was hypothesized that another prostaglandin, PGJ2, that can act as a ligand for PPARγ which is a critical transcription factor for adipogenesis, may have accounted for the decreased adiposity (45). However, the physiological role of PGJ2 in adipogenesis needs further studies, since it does not appear to be present at a significant level in this tissue (14). Taken together, COX-2 may be responsible for inducing adipogenesis and increasing the inflammatory process under high fat feeding conditions, and may also play a role in promoting a “BAT-like phenotype”. The role of COX-2 in the regulation of these processes requires further studies. Some of the proposed effects of COX-2 are somewhat in discordance with each other since increasing inflammation is not a beneficial process but, increasing brown adipose tissue mass may be advantageous in the treatment of obesity (Fig. 3). Thus, in order to determine the feasibility of COX-2 as a potential drug target in the treatment and prevention of obesity, the mechanism by which these intracellular events occur must be elucidated.
Figure 3. Potential role of cyclooxygenase-2s in obesity.
Cyclooxyganse-2 (COX-2) may contribute to increased inflammation and insulin resistance associated with obesity, possibly through increasing cytokine secretion or by increasing prostaglandin production from the macrophage. On the other hand, COX-2 may also act to increase brown adipocyte generation in white adipose tissue thus ameliorating obesity.
CONCLUSIONS
PLA2 activity plays a major role in regulating metabolic processes in various tissues. It has been demonstrated that the products downstream of PLA2 can act as important biological mediators in adipose tissue. It would be important to delineate the roles and underlying mechanisms of these products as they may affect development of obesity which is an ever growing problem in our society. Anti-obesity research is an expanding field and the discovery of drug targets for the treatment of obesity is essential to prevent further disease progress such as type 2 diabetes, cardiovascular diseases, and certain cancers. The downstream products of PLA2, such as prostaglandins and leukotrienes, and the enzymes involved in their synthesis, such as COX-2, may prove to be an area of focus for possible anti-obesity therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and Trends in Obesity Among US Adults, 1999–2008. JAMA: The Journal of the American Medical Association. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Bray GA. Medical Consequences of Obesity. J Clin Endocrinol Metab. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 3.Foreyt JP, Goodrick GK. Evidence for Success of Behavior Modification in Weight Loss and Control. Annals of Internal Medicine. 1993;119:698–701. doi: 10.7326/0003-4819-119-7_part_2-199310011-00014. [DOI] [PubMed] [Google Scholar]
- 4.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson Br, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H. Lifestyle, Diabetes, and Cardiovascular Risk Factors 10 Years after Bariatric Surgery. New England Journal of Medicine. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 5.Elangbam CS. Review Paper: Current Strategies in the Development of Anti-obesity Drugs and Their Safety Concerns. Veterinary Pathology Online. 2009;46:10–24. doi: 10.1354/vp.46-1-10. [DOI] [PubMed] [Google Scholar]
- 6.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. The Journal of Clinical Investigation. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and Importance of Brown Adipose Tissue in Adult Humans. New England Journal of Medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madsen L, Pedersen LM, Lillefosse HH, Fjaere E, Bronstad I, Hao Q, Petersen RK, Hallenborg P, Ma T, De Matteis R, Araujo P, Mercader J, Bonet ML, Hansen JB, Cannon B, Nedergaard J, Wang J, Cinti S, Voshol P, Doskeland SO, Kristiansen K. UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0011391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricquier D, Bouillaud F, Toumelin P, Mory G, Bazin R, Arch J, Penicaud L. Expression of uncoupling protein mRNA in thermogenic or weakly thermogenic brown adipose tissue. Evidence for a rapid beta-adrenoreceptor-mediated and transcriptionally regulated step during activation of thermogenesis. J Biol Chem. 1998;261:13905–13910. [PubMed] [Google Scholar]
- 10.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor γ (PPARγ) of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1 containing adipocytes molecularly distinc from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cinti S. Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab. 2009;297:E977–E986. doi: 10.1152/ajpendo.00183.2009. [DOI] [PubMed] [Google Scholar]
- 12.Giouleka P, Papatheodorou G, Lyberopoulos P, Karakatsani A, Alchanatis M, Roussos C, Papiris S, Loukides S. Body mass index is associated with leukotriene inflammation in asthmatics. Eur J Clin Invest. 2010 doi: 10.1111/j.1365-2362.2010.02371.x. [DOI] [PubMed] [Google Scholar]
- 13.Kunikata T, Yamane H, Segi E, Matsuoka T, Sugimoto Y, Tanaka S, Tanaka H, Nagai H, Ichikawa A, Narumiya S. Suppression of allergic inflammation by the prostaglandin E receptor subtype EP3. Nature Immunol. 2005;5:524–531. doi: 10.1038/ni1188. [DOI] [PubMed] [Google Scholar]
- 14.Jaworski K, Ahmadian M, Duncan RE, Sarkadi-Nagy E, Varady KA, Hellerstein MK, Lee H-Y, Samuel VT, Shulman GI, Kim K-H, de Val S, Kang C, Sul HS. AdPLA ablation increases lipolysis and prevents obesity induced high-fat feeding or leptin deficiency. Nat Med. 2009;15:159–168. doi: 10.1038/nm.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 16.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Burke JE, Dennis EA. Phospholipase A2 biochemistry. Cardiovasc Drugs Ther. 2009;23:49–59. doi: 10.1007/s10557-008-6132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han SK, Kim KP, Koduri R, Bittova L, Munoz NM, Leff AR, Wilton DC, Gelb MH, Cho W. Roles of Trp31 in High Membrane Binding and Proinflammatory Activity of Human Group V Phospholipase A2. J Biol Chem. 1999;274:11881–11888. doi: 10.1074/jbc.274.17.11881. [DOI] [PubMed] [Google Scholar]
- 19.Kim KP, Rafter JD, Bittova L, Han SK, Snitko Y, Munoz NM, Leff AR, Cho W. Mechanism of Human Group V Phospholipase A2(PLA2)-induced Leukotriene Biosynthesis in Human Neutrophils. J Biol Chem. 2001;276:11126–11134. doi: 10.1074/jbc.M004604200. [DOI] [PubMed] [Google Scholar]
- 20.Ahren B, Magrum LJ, Havel PJ, Greene SF, Phinney SD, Johnson PR, Stern JS. Augmented Insulinotropic Action of Arachidonic Acid through the Lipoxygenase Pathway in the Obese Zucker Rat. Obesity. 2000;8:475–480. doi: 10.1038/oby.2000.59. [DOI] [PubMed] [Google Scholar]
- 21.Escoubet B, Griffaton G, Guesnet P, Lechat P, Lavau M. Prostaglandin synthesis and membrane fatty acid composition in the heart of obese Zucker rats. Biochem Biophys Res Comm. 1987;146:589–595. doi: 10.1016/0006-291x(87)90569-9. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu T, Ohto T, Kita A. Cytosolic phospholipase A2: Biochemical properties and physiological roles. Life. 2006;58:328–333. doi: 10.1080/15216540600702289. [DOI] [PubMed] [Google Scholar]
- 23.Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 24.Balsinde J, Bianco ID, Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of calcium-independent phospholipase A2 prevents arachidonic acid incorporation and phospholipid remodeling in P388D1 macrophages. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8527–8531. doi: 10.1073/pnas.92.18.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karasawa K, Harada A, Satoh N, Inoue K, Setaka M. Plasma platelet activating factor-acetylhydrolase (PAF-AH) Prog Lipid Res. 2003;42:93–114. doi: 10.1016/s0163-7827(02)00049-8. [DOI] [PubMed] [Google Scholar]
- 26.Duncan RE, Sarkadi-Nagy E, Jaworski K, Ahmadian M, Sul HS. Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA) J Biol Chem. 2008;283:25428–25436. doi: 10.1074/jbc.M804146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sers C, Emmenegger U, Husmann K, Bucher K, Andres AC, Schafer R. Growth-inhibitory Activity and Downregulation of the Class II Tumor-suppressor Gene H-rev107 in Tumor Cell Lines and Experimental Tumors. The Journal of Cell Biology. 1997;136:935–944. doi: 10.1083/jcb.136.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panesar M, Papillon J, McTavish AJ, Cybulsky AV. Activation of phospholipase A2 by complement C5b-9 in glomerular epithelial cells. The Journal of Immunology. 1997;159:3584–3594. [PubMed] [Google Scholar]
- 29.Murakami M, Nakatani Y, Tanioka T, Kudo I. Prostaglandin E synthase. Prostaglandins & Other Lipid Mediators. 2002;68–69:383–399. doi: 10.1016/s0090-6980(02)00043-6. [DOI] [PubMed] [Google Scholar]
- 30.Fujimori K, Ueno T, Amano F. Prostaglandin F2α suppresses early phase of adipogenesis, but is not associated with osteoblastogenesis in mouse mesenchymal stem cells. Prostaglandins Other Lipid Mediat. 2010;93:52–59. doi: 10.1016/j.prostaglandins.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Miyoshi H, Nishimura K, Jisaka M, Nagaya T, Yokota K. Gene expression of isoformic enzymes in arachidonate cyclooxygenase pathway and the regulation by tumor necrosis factor [alpha] during life cycle of adipocytes. Prostaglandins & Other Lipid Mediators. 2007;83:213–218. doi: 10.1016/j.prostaglandins.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Wortman P, Miyazaki Y, Kalupahana N, Kim S, Hansen-Petrik M, Saxton A, Claycombe K, Voy B, Whelan J, Moustaid-Moussa N. n3 and n6 polyunsaturated fatty acids differentially modulate prostaglandin E secretion but not markers of lipogenesis in adipocytes. Nutrition & Metabolism. 2009;6:5. doi: 10.1186/1743-7075-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fain JN, Leffler CW, Bahouth SW, Rice AM, Rivkees SA. Regulation of leptin release and lipolysis by PGE2 in rat adipose tissue. Prostaglandins & Other Lipid Mediators. 2000;62:343–350. doi: 10.1016/s0090-6980(00)00088-5. [DOI] [PubMed] [Google Scholar]
- 34.Mancuso P, Canetti C, Gottschalk A, Tithof PK, Peters-Golden M. Leptin augments alveolar macrophage leukotriene synthesis by increasing phospholipase activity and enhancing group IVC iPLA2 (cPLA2gamma) protein expression. AJP - Lung Cellular and Molecular Physiology. 2004;287:L497–L502. doi: 10.1152/ajplung.00010.2004. [DOI] [PubMed] [Google Scholar]
- 35.Wenzel SE. Antileukotriene Drugs in the Management of Asthma. JAMA: The Journal of the American Medical Association. 1998;280:2068–2069. doi: 10.1001/jama.280.24.2068. [DOI] [PubMed] [Google Scholar]
- 36.Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, Petrosino S, Hoareau L, Festy F, Pasquali R, Roche R, Maj M, Pagotto U, Monteleone P, Di Marzo V. Regulation, Function, and Dysregulation of Endocannabinoids in Models of Adipose and {beta}-Pancreatic Cells and in Obesity and Hyperglycemia. Journal of Clinical Endocrinology Metabolism. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- 37.Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nature Reviews. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- 38.Annuzzi G, Piscitelli F, Di Marino L, Patti L, Giacco R, Costabile G, Bozzetto L, Riccardi G, Verde R, Petrosino S, Rivellese AA, Di Marzo V. Differential alterations of the concentrations of endocannabinoids and related lipids in the subcutnaeous adipose tissue of obese diabteic patients. Lipids in Health and Disease. 2010;9:43. doi: 10.1186/1476-511X-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hui DY, Cope MJ, Labonte ED, Chang HT, Shao J, Goka E, Abousalham A, Charmot D, Buysse J. The phospholipase A2 inhibitor methyl indoxam suppresses diet-induced obesity and glucose intolerance in mice. British Journal of Pharmacology. 2009;157:1263–1269. doi: 10.1111/j.1476-5381.2009.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ii H, Yokoyama N, Yoshida S, Tsutsumi K, Hatakeyama S, Sato T, Ishihara K, Akiba S. Alleviation of high-fat diet-induced fatty liver damage in group IVA phospholipase A2-knockout mice. PLoS ONE. 2009;4:e8089. doi: 10.1371/journal.pone.0008089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lei X, Zhang S, Emani B, Barbour SE, Ramanadham S. A link between endoplasmic reticulum stress-induced B–cell apoptosis and the group VIA Ca2+-independent phospholipase A2 (iPLA2B) Diabetes, Obesity and Metabolism. 2010;12(Sup):93–98. doi: 10.1111/j.1463-1326.2010.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Z, Zhang X, Zhao C, Choi J, Shi J, Song K, Turk J, Ma ZA. Protection of Pancreatic beta-Cells by Group VIA Phospholipase A2-Mediated Repair of Mitochondrial Membrane Peroxidation. Endocrinology. 2010;151:3038–3048. doi: 10.1210/en.2010-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Shridas P, Forrest K, Bailey W, Webb NR. Group X secretory phospholipase A2 negatively regulates adipogenesis in murine models. The FASEB Journal. 2010;24:4313–4324. doi: 10.1096/fj.10-154716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 45.Ghoshal S, Trivedi DB, Graf GA, Loftin CD. Cyclooxygenase-2 deficiency attenuates adipose tissue differentiation and inflammation in mice. J Biol Chem. 2010 doi: 10.1074/jbc.M110.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nusing RM, Meyer CW, Wahli W, Klingenspor M, Herzig S. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- 47.Hsieh PS, Jin JS, Chiang CF, Chan PC, Chen CH, Shih KC. COX-2 mediated inflammation in fat is crucial for obesity-linked insulin resistance and fatty liver. Obesity. 2009;17:1150–1157. doi: 10.1038/oby.2008.674. [DOI] [PubMed] [Google Scholar]
- 48.Yan H, Kermouni A, Abdel-Hafez M, Lau DC. Role of cyclooxygenases COX-1 and COX-2 in modulating adipogenesis in 3T3-L1 cells. J Lipid Res. 2003;44:424–429. doi: 10.1194/jlr.M200357-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Hsieh PS, Lu KC, Chen CH. Suppressive effect of COX2 inhibitor on the progression of adipose inflammation in high-fat-induced obsese rats. Eur J Clin Invest. 2010;40:164–171. doi: 10.1111/j.1365-2362.2009.02239.x. [DOI] [PubMed] [Google Scholar]
- 50.Chu X, Nishimura K, Jisaka M, Nagaya T, Shono F, Yokota K. Up-regulation of adipogenesis in adipocytes expressing stably cyclooxygenase-2 in the antisense direction. Prostaglandins Other Lipid Mediat. 2010;91:1–9. doi: 10.1016/j.prostaglandins.2009.10.002. [DOI] [PubMed] [Google Scholar]