Abstract
The objective of this study was to investigate how a measure of educational and occupational attainment, a component of cognitive reserve, modifies the relationship between biomarkers of pathology and cognition in Alzheimer's disease. The biomarkers evaluated quantified neurodegeneration via atrophy on magnetic resonance images, neuronal injury via cerebral spinal fluid t-tau, brain amyloid-β load via cerebral spinal fluid amyloid-β1–42 and vascular disease via white matter hyperintensities on T2/proton density magnetic resonance images. We included 109 cognitively normal subjects, 192 amnestic patients with mild cognitive impairment and 98 patients with Alzheimer's disease, from the Alzheimer's Disease Neuroimaging Initiative study, who had undergone baseline lumbar puncture and magnetic resonance imaging. We combined patients with mild cognitive impairment and Alzheimer's disease in a group labelled ‘cognitively impaired’ subjects. Structural Abnormality Index scores, which reflect the degree of Alzheimer's disease-like anatomic features on magnetic resonance images, were computed for each subject. We assessed Alzheimer's Disease Assessment Scale (cognitive behaviour section) and mini-mental state examination scores as measures of general cognition and Auditory–Verbal Learning Test delayed recall, Boston naming and Trails B scores as measures of specific domains in both groups of subjects. The number of errors on the American National Adult Reading Test was used as a measure of environmental enrichment provided by educational and occupational attainment, a component of cognitive reserve. We found that in cognitively normal subjects, none of the biomarkers correlated with the measures of cognition, whereas American National Adult Reading Test scores were significantly correlated with Boston naming and mini-mental state examination results. In cognitively impaired subjects, the American National Adult Reading Test and all biomarkers of neuronal pathology and amyloid load were independently correlated with all cognitive measures. Exceptions to this general conclusion were absence of correlation between cerebral spinal fluid amyloid-β1–42 and Boston naming and Trails B. In contrast, white matter hyperintensities were only correlated with Boston naming and Trails B results in the cognitively impaired. When all subjects were included in a flexible ordinal regression model that allowed for non-linear effects and interactions, we found that the American National Adult Reading Test had an independent additive association such that better performance was associated with better cognitive performance across the biomarker distribution. Our main conclusions included: (i) that in cognitively normal subjects, the variability in cognitive performance is explained partly by the American National Adult Reading Test and not by biomarkers of Alzheimer's disease pathology; (ii) in cognitively impaired subjects, the American National Adult Reading Test, biomarkers of neuronal pathology (structural magnetic resonance imaging and cerebral spinal fluid t-tau) and amyloid load (cerebral spinal fluid amyloid-β1–42) all independently explain variability in general cognitive performance; and (iii) that the association between cognition and the American National Adult Reading Test was found to be additive rather than to interact with biomarkers of Alzheimer's disease pathology.
Keywords: Alzheimer's disease, mild cognitive impairment, CSF biomarkers, MRI, cognitive reserve
Introduction
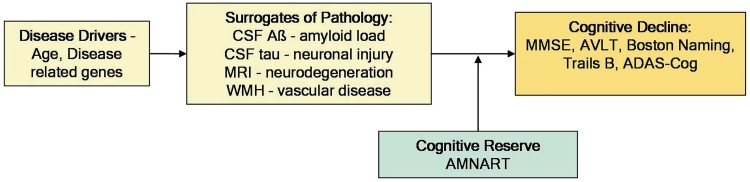
Subjects with Alzheimer's disease at autopsy typically were demented in life. However, careful autopsy studies consistently reveal an important clinical–pathological discordance. Up to 30% of subjects who were cognitively normal in life are found at autopsy to have a pathological profile, typically dominated by amyloid plaques, consistent with a diagnosis of Alzheimer's disease (Katzman et al., 1988; Crystal et al., 1993; Hulette et al., 1998; Price and Morris, 1999; Schmitt et al., 2000; Morris and Price, 2001; Riley et al., 2002; Knopman et al., 2003). Understanding this disassociation between cognition and pathology is important for the success of preventive strategies targeted at neuropathology in the non-demented elderly as well as relevant for improving our understanding of the disease. The concept of cognitive reserve (Stern, 2009) is increasingly employed to explain this disassociation. This theory posits that subjects with higher cognitive reserve have a greater capacity to cope with pathological insults than those with low cognitive reserve, and these individual differences in reserve mechanisms help explain why cognitive decline may be initiated at different times in relation to the onset of pathology. This concept can also be expressed as the difference between the predicted and the observed cognitive performance of an individual for a given level of brain pathology (Reed et al., 2010). Figure 1 illustrates the question we are addressing—how does cognitive reserve modify the impact of pathology on cognition? In this article, we investigate one aspect of cognitive reserve as measured by the number of errors on the American National Adult Reading Test (AMNART), an indicator of pre-morbid verbal intelligence. While AMNART does not capture all the components that contribute to cognitive reserve, it is a measure of the level of environmental enrichment provided by educational and occupational attainment and has been shown to correlate with IQ (Ryan and Paolo, 1992).
Figure 1.
Illustration of where cognitive reserve acts along the pathology–cognitive decline cascade. Aβ = amyloid-β; AVLT = Auditory–Verbal Learning Test; WMH = white matter hyperintensity volume.
Dementia is a multi-factorial disease wherein cumulative pathological brain insults (usually more than one pathology) result in progressive cognitive decline, which ultimately leads to dementia. Amyloid plaques and neurofibrillary tangles are the well-established pathological hallmarks of Alzheimer's disease. The other main pathological processes in Alzheimer's disease are inflammation and neurodegeneration, which is due to the loss of neurons, synapses and dendritic de-arborization that occurs on a microscopic level. In this article, we restrict ourselves to the primary pathologies of Alzheimer's disease as well as to ischaemic cerebrovascular disease since it is the most common secondary pathological process seen among demented subjects with Alzheimer's disease pathology at autopsy (Snowdon et al., 1997; White et al., 2005; Schneider et al., 2009). We use MRI and CSF biomarkers to quantify each of these pathological changes. Low CSF amyloid-β1–42 reflects deposition of amyloid-β in plaques (Strozyk et al., 2003; Tapiola et al., 2009). High CSF t-tau levels reflect active axonal and neuronal damage (Blennow et al., 1995). Atrophy seen on MRI is the macroscopic result of loss of neurons, synapses and dendritic arborization (Bobinski et al., 2000). We use the Structural Abnormality Index score as an indicator of the severity of the Alzheimer's disease-like pattern of atrophy on structural MRI. Structural Abnormality Index scores were developed to condense the severity and location of Alzheimer's disease-related atrophy on the 3D MRI scan into a single number (Vemuri et al., 2008). White matter hyperintensity (or leukoaraiosis) on fluid-attenuation inversion recovery MRI in elderly subjects is suspected to be a direct manifestation of microvascular ischaemic injury. We use white matter hyperintensity load as an indicator of severity of small vessel disease.
Several recent studies have used biomarkers to understand the effect of cognitive reserve on the relationship between pathology and cognition (Stern, 2009). These studies have examined the effect of cognitive reserve on cross-sectional measures of amyloid load (Kemppainen et al., 2008; Roe et al., 2008a; Rentz et al., 2010; Dumurgier et al., 2010), neurodegeneration (Querbes et al., 2009; Piras et al., 2010), fibre tract integrity (Teipel et al., 2009), white matter hyperintensity (Brickman et al., 2009b), cerebral metabolism (Alexander et al., 1997; Scarmeas et al., 2003; Perneczky et al., 2007; Garibotto et al., 2008; Hanyu et al., 2008; Cohen et al., 2009) and perfusion (Liao et al., 2005) independently. Autopsy studies have shown the association of plaques and tangles with cognitive reserve (Bennett et al., 2003; Boyle et al., 2008; Koepsell et al., 2008; Roe et al., 2008b). However, we know of no in vivo studies that have investigated the effect of cognitive reserve on all the primary pathologies seen in Alzheimer's disease including neurodegeneration and the most common secondary pathology (i.e. vascular disease) in the same cohort of subjects that spans the cognitive spectrum from normal to mild Alzheimer's disease.
The main aim of our article is to investigate how the component of cognitive reserve measured by AMNART modifies the relationship between biomarkers of pathology and cognition in Alzheimer's disease by answering these two questions: (i) does AMNART explain variability in cognitive measures, even after adjusting for biomarkers of pathology within cognitively normal and impaired subjects?; and (ii) does AMNART have an additive association with cognition or does it interact with biomarkers of pathology?
Materials and methods
The data used in this study are from the Alzheimer's Disease Neuroimaging Initiative, a longitudinal multicentre observational study of elderly individuals with normal cognition, amnestic mild cognitive impairment and Alzheimer's disease collected from 59 participating institutes (Jack et al., 2008). Written informed consent was obtained for participation in these studies, as approved by the Institutional Review Board at each of the participating centres. The details of the Alzheimer's Disease Neuroimaging Initiative can be found at http://www.ADNI-info.org.
Clinical and cognitive assessment
The total sample in this article consists of 399 subjects (109 cognitively normal, 192 amnestic mild cognitive impairment, 98 Alzheimer's disease) who had CSF biomarker data at baseline (CSF was obtained at baseline in ∼51% of the Alzheimer's Disease Neuroimaging Initiative cohort). Baseline clinical diagnosis and cognitive assessments of all three clinical groups and clinical/cognitive assessment scores were considered in this study. AMNART was obtained in each of the individuals (Ryan and Paolo, 1992) and the number of errors on AMNART was used as a measure of cognitive reserve in this study. The AMNART evaluates pre-morbid verbal IQ in individuals and is determined by the number of errors made on a pronunciation list of 50 irregularly spelled words such as ‘ache’, ‘debt’ and ‘bouquet’. Alternative proxies of cognitive reserve exist, such as education, but we chose AMNART since it is more sensitive and shows greater variation than education (Rentz et al., 2010). While the Alzheimer's Disease Neuroimaging Initiative generally classifies subjects as cognitively normal, having mild cognitive impairment, or Alzheimer's disease, for this study, we combined subjects who had mild cognitive impairment and Alzheimer's disease at baseline into a single impaired group since the distribution of AMNART was very similar in amnestic-mild cognitive impairment and Alzheimer's disease. In this way, the impaired group represents a broad spectrum of cognitive impairment and also shows a greater dynamic range of values on standard cognitive tests.
We used Alzheimer's Disease Assessment Scale—Cognitive Behaviour Section (ADAS-Cog; Rosen et al., 1984) and Mini-Mental State Examination (MMSE; Folstein et al., 1975) as overall indices of general cognitive performance. In this study, we used the modified ADAS-Cog score (ADAS-Cog-13) from the Alzheimer's Disease Neuroimaging Initiative, which has two additional components (delayed recall task and a number cancellation task). Additionally, we used domain-specific scores—Auditory–Verbal Learning Test-delayed recall (Rey, 1964) as a measure of memory encoding, Boston naming (Kaplan et al., 1983) to test language functioning and Trails B (US War Department, 1944) as a measure of executive function. We analysed all tests in all subjects.
Cerebral spinal fluid methods and processing
CSF was collected at each site, transferred into polypropylene transfer tubes, frozen on dry ice within 1 h after collection and shipped overnight to the Alzheimer's Disease Neuroimaging Initiative Biomarker Core laboratory at the University of Pennsylvania Medical Centre on dry ice. When samples were received in the Laboratory, they were thawed and aliquots were stored in bar coded polypropylene vials at −80°C. A standardized protocol was implemented to quantify biomarker concentrations in each of the CSF Alzheimer's Disease Neuroimaging Initiative baseline aliquots using a multiplex xMAP Luminex platform (Luminex Corp) with Innogenetics (INNO-BIA AlzBio3, for research use only reagents) immunoassay kit-based reagents, a protocol which has been tested and validated (Vanderstichele, 2008; Shaw et al., 2009). Quality control values obtained during the analyses of Alzheimer's Disease Neuroimaging Initiative baseline CSF aliquots were: inter-day reproducibilities (%CV) for an Alzheimer's disease CSF pool and a routine clinic patient CSF pool of 4.5% and 6.4% for t-tau and 3.3% and 6.9% for amyloid-β1–42; r2 values for comparison of retested samples were 0.98 and 0.90. Baseline CSF values were considered in this study.
Magnetic resonance imaging methods and preprocessing
The 1.5T MRI scans from the Alzheimer's Disease Neuroimaging Initiative were used for this study. The nominal parameters of the morphometric T1-weighted magnetization prepared rapid acquisition gradient echo imaging sequence and T2-weighted MRI scans can be found in Jack et al. (2008). The T1 MRI images are corrected for gradient non-linearity and intensity inhomogeneity (Jack et al., 2008) using a centralized MRI-processing pipeline at the Mayo Clinic, Rochester. Structural Abnormality Index-scores were estimated on these preprocessed images and are described in the online Supplementary Material. The T2/PD and T1-weighted images were used to estimate the white matter hyperintensity load based on the method presented in (Schwarz et al., 2009) (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2864489/).
Statistical analysis
Correlations between American National Adult Reading Test, biomarkers and cognition
We estimated the association between variables using partial Spearman rank-order correlation, which we denote by ‘partial rs’. All correlations adjust for, or partial out, the effects of age and gender. We also report partial correlations after further adjusting for biomarker or AMNART. Rank-order correlations were used because of skewness and otherwise non-normal distributions resulting from floor or ceiling effects. Partial correlations were calculated using SAS version 9.1.3.
An important goal of this study was to characterize how mean cognition changes with AMNART and biomarker levels and to assess whether the possibly protective effect of cognitive reserve is additive, whereby it is relatively constant across levels of neuropathology, or whether there is an interaction in which the protective effect of cognitive reserve depends on the level of the biomarker as would be the case, for example, if cognitive reserve offered protection only at mild levels of neuropathology. To address these questions, we used proportional odds ordinal logistic regression models (Harrell, 2001) treating the cognitive test as an ordinal response. These models generalize binary logistic regression to the case where there are more than two categories but they have a natural ordering. One interpretation of these models is that they treat the observed score on a cognitive test as a discrete or ‘coarsened’ estimate of an unobservable, or latent, cognitive quantity that falls along a true continuum that is assumed to have an underlying continuous logistic distribution. As binary logistic regression can provide the estimated probability of an event or non-event as a function of covariates, proportional odds logistic regression models can be used to estimate the probability of each level of the ordinal response, e.g. MMSE = 30, 29, 28, etc. We then calculate the estimated mean value for the cognitive test as a function of covariates by summing up the scores multiplied by their probabilities (Hannah and Quigley, 1996). For example, if a test took on values 1, 2 or 3, and the estimated probabilities were 0.20, 0.30 and 0.50, the mean would be 1(0.20) + 2(0.30) + 3(0.50) = 2.3. With this approach to estimating the mean in an ordinal logistic regression model, we avoid the problems of using linear regression when there are strong floor or ceiling effects or a large number of ties.
To reduce skewness, we transformed AMNART by taking the square root and took the base 2 logarithm of amyloid-β1–42, t-tau, and white matter hyperintensity volume. So as not to assume strictly linear relationships, in all models we fit the biomarker and AMNART predictors as restricted cubic splines with knots at the 10th, 50th and 90th percentiles (Harrell, 2001). To assess the extent to which the relationship between AMNART and cognition varied by pathology level, we included a linear interaction, or product term, whereby the AMNART score was multiplied by the pathology measure (Harrell, 2001). To adjust for possible age effects and sex effects in the MMSE models we included age, sex, an age by sex interaction, and allowed age and sex to interact with the linear components of the biomarker and AMNART terms.
In all, the full models for MMSE had 18 degrees of freedom. While models of this complexity are reasonable given the large sample size, in order to obtain more stable and smoother estimates, we used a ridge-regression penalization approach with the penalty factor chosen to optimize the ‘corrected Akaike Information Criterion’ (Harrell, 2001). For the MMSE, the penalization reduced the effective degrees of freedom for the amyloid-β1–42 model to 6.3 degrees of freedom, the Structural Abnormality Index model to 9.2 degrees of freedom, the t-tau model to 7.3 degrees of freedom and the white matter hyperintensity model to 6.6 degrees of freedom. We used large-sample Wald tests on the full model to assess: (i) the interaction between biomarker and AMNART; (ii) the biomarker effect controlling for age, sex and AMNART; and (iii) the AMNART effect controlling for age, sex and biomarker. Ordinal regression modelling was performed using Harrell's Design package and R version 2.8.1 (R Development Core Team, 2008).
To explore the effects of measurement error in AMNART and biomarkers on our ordinal regression model estimates, we performed a sensitivity analysis using the simulation-extrapolation method (Cook and Stefanski, 1994). The idea behind this approach is to add measurement error to the predictors and then re-estimate the regression coefficients. By adding increasing amounts of measurement error, for a given regression coefficient, one can plot the resulting estimate on the y-axis and the amount of measurement error on the x-axis and then fit a trend line to these observations. One then extrapolates from this trend line the value of the regression coefficient assuming zero measurement error. We performed the simulation-extrapolation method five times by assuming measurement error was such that the intra-class correlation coefficient for AMNART and the biomarkers was 0.50, 0.60, 0.70, 0.80 and 0.90.
Results
Patient characteristics
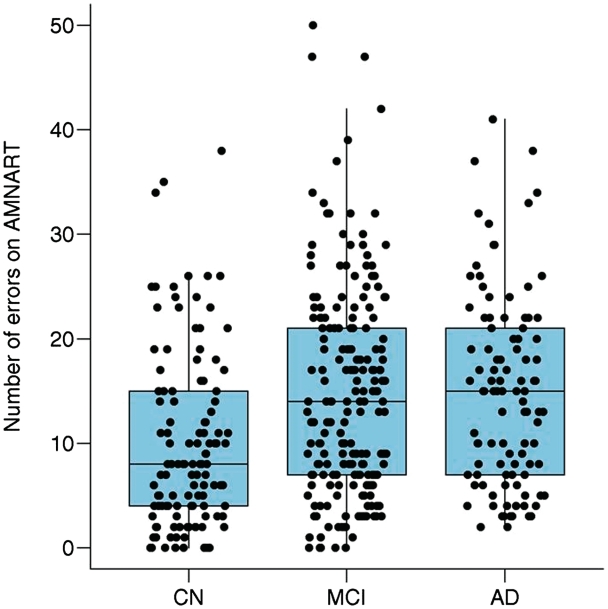
The demographics, clinical summary, biomarker characteristics of cognitively normal, cognitively impaired subjects (amnestic mild cognitive impairment and Alzheimer's disease) are shown in Table 1. Figure 2 shows the distribution of AMNART scores separately for cognitively normal, amnestic mild cognitive impairment and Alzheimer's disease. Based on the Wilcoxon rank-sum/Mann–Whitney U-test, the number of errors was significantly lower for the cognitively normal group than amnestic mild cognitive impairment (area under receiver operating characteristic curve = 0.64, P < 0.001) or Alzheimer's disease (area under receiver operating characteristic curve = 0.67, P < 0.001) while amnestic mild cognitive impairment and Alzheimer's disease were not different (area under receiver operating characteristic curve = 0.52, P = 0.53).
Table 1.
Patient characteristics at baseline
| Cognitively normal (n = 109) | Cognitively impaired (n = 290) | |
|---|---|---|
| Gender: female, n (%) | 52 (48) | 105 (36) |
| Apolipoprotein E ε4 carriers, n (%) | 27 (25) | 172 (59) |
| Age, median (IQR), years | 76 (72–78) | 75 (70–80) |
| Education, median (IQR), years | 16 (14–18) | 16 (13–18) |
| Amyloid-β1–42, median (IQR), pg/ml | 221 (154–248) | 143 (127–169) |
| t-tau, median (IQR), pg/ml | 63 (47–87) | 96 (68–135) |
| Structural Abnormality Index, median (IQR) | −0.9 (−1.5 to −0.4) | 0.2 (−0.5 to 0.9) |
| White matter hyperintensity volume, median (IQR), cm3 | 0.2 (0.1–0.5) | 0.3 (0.1–0.8) |
| AMNART, median (IQR) | 8 (4–15) | 14 (7–21) |
| MMSE, median (IQR) | 29 (29–30) | 26 (24–28) |
| ADAS-Cog, median (IQR) | 9.7 (6.3–13.0) | 21.7 (16.7–27.3) |
| CDR-SB, median (IQR) | 0.0 (0.0–0.0) | 2.0 (1.0–3.5) |
| Auditory–Verbal Learning Test delayed recall, median (IQR) | 7 (5–10) | 1 (0–3) |
| Boston Naming, median (IQR) | 28 (26–30) | 26 (23–28) |
CDR-SB = Clinical Dementia Rating ‘sum of boxes’; IQR = interquartile range defined as (25th percentile, 75th percentile).
Figure 2.
Box plots of the distribution of AMNART errors by clinical group. The horizontal lines within each box represent the 25, 50 and 75th percentiles. The vertical lines extend out to the furthest point within 1.5 interquartile ranges of the box, where an interquartile range is the 75th minus the 25th percentiles. AD = Alzheimer's disease; CN = cognitively normal; MCI = mild cognitive impairment.
Correlations between American National Adult Reading Test, biomarkers and cognition
Cognitively normal subjects
Among cognitively normal subjects, we found that none of the biomarkers (CSF amyloid-β1–42, CSF t-tau, Structural Abnormality Index or white matter hyperintensity volume) correlated with the measures of cognition we assessed with all correlations <0.17 in absolute value (P > 0.05). In contrast AMNART correlated with MMSE (age and gender adjusted partial rs = −0.37, P < 0.01) and Boston naming (age and gender adjusted partial rs = −0.31, P < 0.01) but did not correlate with ADAS-Cog, Auditory–Verbal Learning Test or Trails B (P > 0.05). The magnitude of the correlation between MMSE and AMNART was similar (partial rs = −0.37 to −0.36, P < 0.01) after adjusting for each of the CSF and MRI biomarkers individually, as well as when all biomarkers of pathology where combined (partial rs = −0.36, P < 0.01). Similarly the correlation between Boston naming and AMNART were similar before and after adjusting for each of the CSF and MRI biomarkers individually as well as when all were combined. These correlations are illustrated in Table 2.
Table 2.
Partial Spearman rank correlations for the cognitively normal patients
| Predictor | Adjustment variablesa | Cognitive measurement |
||||
|---|---|---|---|---|---|---|
| ADAS-Cog | MMSE | Auditory–Verbal Learning Test | Boston Naming | Trails B | ||
| Predictor variable | ||||||
| Amyloid-β1–42 | −0.14 | −0.14 | 0.04 | 0.12 | −0.11 | |
| STAND | 0.07 | 0.01 | −0.04 | −0.16 | 0.04 | |
| t-tau | 0.06 | 0.05 | −0.01 | −0.03 | 0.14 | |
| WMH | 0.17 | 0.14 | 0.04 | 0.04 | −0.04 | |
| AMNART | 0.16 | −0.37** | −0.05 | −0.31** | 0.07 | |
| AMNART adjusted for biomarkers | ||||||
| AMNART | Amyloid-β1–42 | 0.17 | −0.36** | −0.06 | −0.32** | 0.08 |
| AMNART | STAND | 0.16 | −0.37** | −0.05 | −0.31** | 0.07 |
| AMNART | t-tau | 0.16 | −0.37** | −0.05 | −0.31** | 0.06 |
| AMNART | WMH | 0.17 | −0.36** | −0.05 | −0.32** | 0.07 |
| AMNART | Amyloid-β1–42, t-tau, STAND and WMH | 0.18 | −0.36** | −0.06 | −0.34** | 0.07 |
| Biomarkers adjusted for AMNART | ||||||
| Amyloid-β1–42 | −0.15 | −0.13 | 0.05 | 0.15 | −0.11 | |
| STAND | 0.07 | 0.00 | −0.04 | −0.17 | 0.04 | |
| t-tau | 0.05 | 0.06 | −0.00 | −0.02 | 0.14 | |
| WMH | 0.18 | 0.12 | 0.04 | 0.03 | −0.03 | |
a Age and gender are included.
STAND = Structural Abnormality Index; WMH = white matter hyperintensity.
*P-value between 0.01 and 0.05.
**P-value < 0.01.
Cognitively impaired subjects
Among impaired subjects, the biomarkers of Alzheimer's disease (CSF amyloid-β1–42, CSF t-tau and MRI) correlated with the measures of general cognitive performance (ADAS-Cog, MMSE; age- and gender-adjusted absolute partial rs = 0.19–0.51, P < 0.01). These correlations were little changed after further adjusting for AMNART (absolute partial rs = 0.17–0.51, P < 0.01). Most of the Alzheimer's disease biomarkers correlated with the domain scores (Auditory–Verbal Learning Test, Boston naming, Trails B). The exception was absence of correlation between CSF amyloid-β1–42 with Boston naming and Trails B. These correlations were little changed as well after adjusting for AMNART. White matter hyperintensity did not correlate with the ADAS-Cog, MMSE or Auditory–Verbal Learning Test, but did correlate with domains measures for language (Boston naming) and executive function (Trails B).
AMNART correlated with ADAS-Cog (age and gender adjusted partial rs = 0.14, P = 0.02), MMSE (age and gender adjusted partial rs = −0.21, P < 0.01), Boston naming (age and gender adjusted partial rs = −0.37, P < 0.01) and Trails B (age and gender adjusted partial rs = 0.22, P < 0.01). After further adjusting for each of the CSF and MRI biomarkers, the magnitude of correlation of AMNART with cognitive measures was similar irrespective of the biomarker adjusted for. This correlation was also similar after adjusting for all the biomarkers. These correlations are illustrated in Table 3. As can be observed from the table, the correlation of AMNART with the cognitive measures does not change after adjustment by any of the biomarkers. Also, there was no apparent relationship between AMNART and biomarker among impaired subjects, suggesting AMNART, amyloid-β1–42, t-tau and Structural Abnormality Index are independent predictors of cognitive performance.
Table 3.
Partial Spearman rank correlations for the cognitively impaired patients
| Predictor | Adjustment variablesa | Cognitive measurement |
||||
|---|---|---|---|---|---|---|
| ADAS-Cog | MMSE | Auditory–Verbal Learning Test | Boston Naming | Trails B | ||
| Predictor variable | ||||||
| Amyloid-β1-42 | −0.27** | 0.19** | 0.21** | 0.00 | −0.09 | |
| STAND | 0.51** | −0.35** | −0.27** | −0.29** | 0.33** | |
| t-tau | 0.22** | −0.19** | −0.23** | −0.12* | 0.14* | |
| WMH | 0.11 | −0.06 | −0.05 | −0.15* | 0.23** | |
| AMNART | 0.14* | −0.21** | −0.03 | −0.37** | 0.22** | |
| AMNART adjusted for biomarkers | ||||||
| AMNART | Amyloid-β1-42 | 0.16** | −0.22** | −0.04 | −0.37** | 0.23** |
| AMNART | STAND | 0.14* | −0.20** | −0.02 | −0.37** | 0.22** |
| AMNART | t-tau | 0.14* | −0.20** | −0.02 | −0.37** | 0.22** |
| AMNART | WMH | 0.15* | −0.21** | −0.04 | −0.37** | 0.22** |
| AMNART | Amyloid-β1-42, t-tau, STAND and WMH | 0.16** | −0.21** | −0.02 | −0.37** | 0.21** |
| Biomarkers adjusted for AMNART | ||||||
| Amyloid-β1-42 | −0.28** | 0.20** | 0.21** | 0.02 | −0.10 | |
| STAND | 0.51** | −0.35** | −0.26** | −0.29** | 0.33** | |
| t-tau | 0.22** | −0.17** | −0.22** | −0.12 | 0.14* | |
| WMH | 0.10 | −0.04 | −0.05 | −0.13* | 0.21** | |
a Age and gender are included.
STAND = Structural Abnormality Index; WMH = white matter hyperintensity.
*P value between 0.01 and 0.05.
**P value < 0.01.
Effect of American National Adult Reading Test on the relationship between biomarkers and cognition
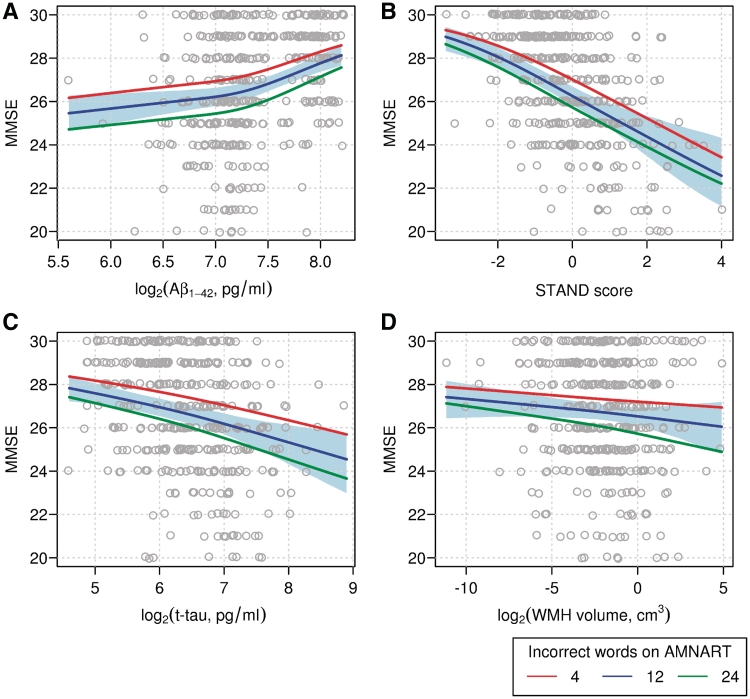
We illustrate the interrelationship between MMSE, biomarker and AMNART in Fig. 3. In each panel, we show MMSE as a function of the biomarker level and show the estimated mean MMSE for three levels of AMNART. We found that MMSE depended significantly on amyloid-β1–42 (P < 0.001), Structural Abnormality Index (P < 0.001) and t-tau (P < 0.001) but not white matter hyperintensity volume (P = 0.88). In all models, the effect of AMNART on the relationship between MMSE and biomarker level was highly significant (P < 0.001). In other words, for a given level of cognition, fewer errors on AMNART, i.e. higher cognitive reserve, is associated with higher levels of t-tau, lower levels of CSF amyloid-β1–42 and greater cerebral atrophy when compared with subjects with low cognitive reserve.
Figure 3.
Scatter plots of MMSE versus neuropathology markers: (A) CSF Amyloid-β1–42 (B) STAND-score (C) t-tau and (D) WMH. Superimposed lines represent estimated mean MMSE as a function of the neuropathology marker for varying levels of AMNART. The red line represents the 15th percentile of four errors on AMNART indicating a ‘good’ score, the blue line represents the median of 12 errors indicating an ‘average’ score, and the green line represents the 85th percentile of 24 errors indicating a ‘bad’ score. The shaded region about the blue line indicates a 95% bootstrap confidence interval. These estimates come from penalized ordinal logistic regression models as described in the methods. STAND = Structural Abnormality Index; WMH = white matter hyperintensity.
For log CSF amyloid-β1–42 (Fig. 3A) there is an upward, but possibly non-linear, trend indicating higher levels of amyloid-β1–42 are associated with better performance on MMSE. Figure 3B shows a clear, approximately linear, downward trend in MMSE as Structural Abnormality Index increases while for any given level of Structural Abnormality Index worse AMNART results in lower average MMSE. For log t-tau, the association with MMSE appears approximately linear. For log white matter hyperintensity, no significant association with MMSE was observed, while the additive effect of AMNART was pronounced. We found no evidence of an interaction between AMNART and the marker (P > 0.59 for each marker) and infer that there is an approximate ‘additive’ association such that for a given level of the biomarker, better performance on AMNART corresponds with an upward shift in average MMSE. In contrast, an interaction between AMNART and biomarker would have resulted in significantly different slopes for the different AMNART groups in the regression of MMSE on biomarkers (Fig. 3), which was not found.
In our sensitivity analysis evaluating the effects of measurement error in AMNART or the biomarkers, we found that increasing amounts of measurement error tended to attenuate observed associations but that our estimates, as summarized graphically in Fig. 3, were little changed after correction using the simulation-extrapolation method.
Discussion
The concept of cognitive reserve is being employed increasingly to explain the difference between observed and expected cognitive performance for a given degree of Alzheimer's disease pathology. We investigated the contribution of cognitive reserve on this disassociation using biomarkers that capture two major aspects of Alzheimer's disease pathology (amyloid and neuronal) as well as vascular pathology, which is very common among the elderly population. The major findings from this study are: (i) that in cognitively normal subjects, the variability in cognition is explained partly by AMNART but not by biomarkers of Alzheimer's disease pathology; (ii) in cognitively impaired subjects, AMNART, biomarkers of neuronal pathology (structural MRI and CSF t-tau) and amyloid load (CSF amyloid-β1–42) were all independently associated with cognitive and functional performance; and (iii) that the association between cognition and AMNART was found to be additive rather than to depend on biomarkers of Alzheimer's disease pathology such that better performance on AMNART corresponded with better cognition across the Alzheimer's disease continuum.
Cognitive reserve and cognition
The concept of reserve stemmed from the observation that there is often poor one-to-one correspondence between the presence of pathology at autopsy and cognition in life (Katzman et al., 1988). Cognitive reserve is an inclusive term that has been loosely used to explain the inter-subject variability in cognitive performance in the face of brain pathology. There are several processes that constitute cognitive reserve including genetic and environmental influences, number of neurons and synapses, the sensitivity of neurons and glia to pathology, neuroplasticity etc. Stern (2006) formally categorized cognitive reserve mechanisms into two parts: active and passive. Passive cognitive reserve is created by pre-existing networks that are more efficient or have greater capacity, and thus may be less susceptible to disruption by pathology. Active cognitive reserve is created by alternate networks that compensate for pathological disruption of pre-existing networks. AMNART, which measures pre-morbid verbal intelligence, may possibly capture components from both of these cognitive reserve mechanisms.
In cognitively normal subjects we found that AMNART correlated moderately with Boston naming (partial rs = −0.31, P < 0.01) and MMSE (partial rs = −0.37, P < 0.01). This might be expected since AMNART and Boston naming are both verbal semantic knowledge tasks and MMSE has been shown to be correlated with education (Schmand et al., 1995). In cognitively normal subjects, AMNART acts as a measure of verbal ability and was found to be correlated with education. In cognitively impaired subjects, we found that AMNART explained variability in MMSE (P < 0.01), ADAS-Cog (P = 0.02), Boston naming (P < 0.05) and Trails B (P < 0.05). These results support the notion that AMNART explains inter-subject variation in the cognitive response to brain pathology in cognitively impaired.
Biomarkers and cognition
In cognitively normal subjects, we did not find significant correlations between any of the biomarkers and the cognitive measures we assessed (Auditory–Verbal Learning Test delayed recall and Boston Naming). Approximately one-third of elderly cognitively normal subjects have amyloid pathology (Katzman et al., 1988; Crystal et al., 1993; Hulette et al., 1998; Price and Morris, 1999; Schmitt et al., 2000; Morris and Price, 2001; Riley et al., 2002; Knopman et al., 2003). Amyloid deposition is believed to occur early in the disease process and does not directly cause clinical symptoms (Jack et al., 2009; Mormino et al., 2009). We, therefore, did not expect to find a strong correlation between CSF amyloid-β1–42 and cognition. On the other hand, neurofibrillary tangles and neurodegeneration are believed to be downstream pathological events that progressively worsen in the presence of a relatively static total load of amyloid and which lead directly to cognitive impairment (Ingelsson et al., 2004; Jack et al., 2010). The absence of substantial neurodegenerative pathology in the cognitively normal subjects explains the absence of a strong correlation between biomarkers of neuronal pathology and cognition in these subjects. The literature on the lack of correlation between biomarkers of amyloid load and cognition in the cognitively normal is, however, not consistently unanimous. While some studies have found a poor correlation between amyloid load and cognition in cognitively normal subjects (Aizenstein et al., 2008; Jack et al., 2009; Vemuri et al., 2010) others have found significant correlations between amyloid load and cognition (Pike et al., 2007; Villemagne et al., 2008; Storandt et al., 2009). The most logical explanation for these conflicting results is that different studies include different blends of three different groups of cognitively normal subjects: (i) normal cognition in the absence of amyloid load and neurodegeneration; (ii) normal cognition in the presence of some amyloid load and absence of neurodegeneration; and (iii) early cognitive decline in the presence of amyloid load and neurodegeneration; thus leading to different conclusions.
In cognitively impaired subjects, both biomarkers of neuronal pathology (CSF t-tau and structural MRI) and amyloid-β amyloid load (CSF amyloid-β1–42) explained variability in general cognitive performance (ADAS-Cog and MMSE). Most of the biomarkers of Alzheimer's disease correlated with the domain-specific scores as well (Auditory–Verbal Learning Test, Boston naming, Trails B) except for the lack of correlation between CSF amyloid-β1–42 with Boston naming and Trails B. Our finding of stronger correlations between structural MRI and cognitive performance than between CSF measures and cognitive measures is consistent with several recent studies (Vemuri et al., 2009; Fjell et al., 2010; Walhovd et al., 2010). This is also consistent with a recent pathology study that found that the effect of processing resources (cognitive reserve) is slightly greater on the association between neuronal pathology and cognition than plaques and cognition (Boyle et al., 2008).
In this study we found that white matter hyperintensity did not correlate with measures of general cognitive performance (ADAS-Cog and MMSE) and memory domain scores (Auditory–Verbal Learning Test) in clinically impaired subjects. However, white matter hyperintensity correlated with domain scores for language (Boston naming) and executive functioning (Trails B). Some literature has shown that the degree of white matter hyperintensity does not greatly impact cognitive performance in Alzheimer's disease (Wahlund et al., 1994; Hirono et al., 2000; Kono et al., 2004) while others have found that the degree of white matter hyperintensity does significantly impact cognitive performance in Alzheimer's disease (DeCarli et al., 1995; Fazekas et al., 1996), specifically deficits in executive function and speed of cognitive processing (Brickman et al. 2009a; Venkatraman et al. 2010). These inconsistent results regarding correlations between white matter hyperintensity and general cognition measures could be because the effect of white matter hyperintensity on general cognition (measured by MMSE and ADAS-Cog) might be small and therefore differences in the population recruitment mechanisms and patient numbers may have led to different conclusions.
Cognitive reserve and biomarkers of Alzheimer's disease pathology are independent predictors of cognitive performance
In cognitively normal subjects, since biomarkers of pathology do not explain a significant amount of variability in cognition, it is not surprising that adjusting the correlation of AMNART with Boston naming by the biomarkers does not appreciably affect the strength of this association. In cognitively impaired subjects, both biomarkers of Alzheimer's disease pathology and AMNART explained significant amount of variability in the measures of cognitive and functional performance. The strength of the partial correlation between AMNART and the cognitive measures after adjusting for each one of the biomarkers was similar to the strength of the correlation before adjustment. In the reverse analysis shown in Table 3, the strength of correlation between Alzheimer's disease biomarkers and cognitive measures was also similar before and after adjustment for AMNART. These two results taken together indicate that AMNART and biomarkers are independent predictors of cognitive performance in Alzheimer's disease.
The notion of an independent effect of AMNART on the relationship between cognition and biomarkers was further strengthened by our ordinal logistic regression findings. We found strong evidence (P < 0.001) that AMNART and each biomarker variable were additively associated with cognition as measured by MMSE and found no evidence for interactions between AMNART and the biomarkers (P > 0.59). We did not find that the effect of AMNART diminished with higher levels of pathology indicating that an additive-protective effect of AMNART is constant across the observed range of pathological severity. Though the models summarized in Fig. 3 allow for an interaction between AMNART and biomarkers, there was very little evidence of such. This, along with the lack of rank correlation between AMNART and biomarkers, suggest that it is less an issue of being underpowered to detect the interaction than that the data are consistent with a process by which AMNART and pathology operate as largely independent but additive predictors. While the additive model is supported by our data, at some point the aggregate effects of neuropathology can be expected to dominate any neuroprotective effects afforded by cognitive reserve. Although education has been well accepted as a measure of cognitive reserve, in our preliminary analysis we found that education did not correlate with cognition after adjusting for AMNART. This suggests that AMNART may be a more robust marker of the environmental enrichment aspect of cognitive reserve than education. Rentz et al. (2010) also found that education does not add any (significant) information in a model of amyloid and cognition that included AMNART. This may be due to the fact that education levels do not as effectively capture the environmental enrichment afforded by life-long learning as effectively as AMNART. While other markers of cognitive reserve exist, we have only presented AMNART in this study. We also specifically tested MRI measures of total intracranial volume as an independent measure of reserve and found few associations with cognition and when present they were very weak. The ‘Methods’ and ‘Results’ are presented in the online Supplementary Material.
Implications for the relationship between cognitive reserve, biomarkers and cognition
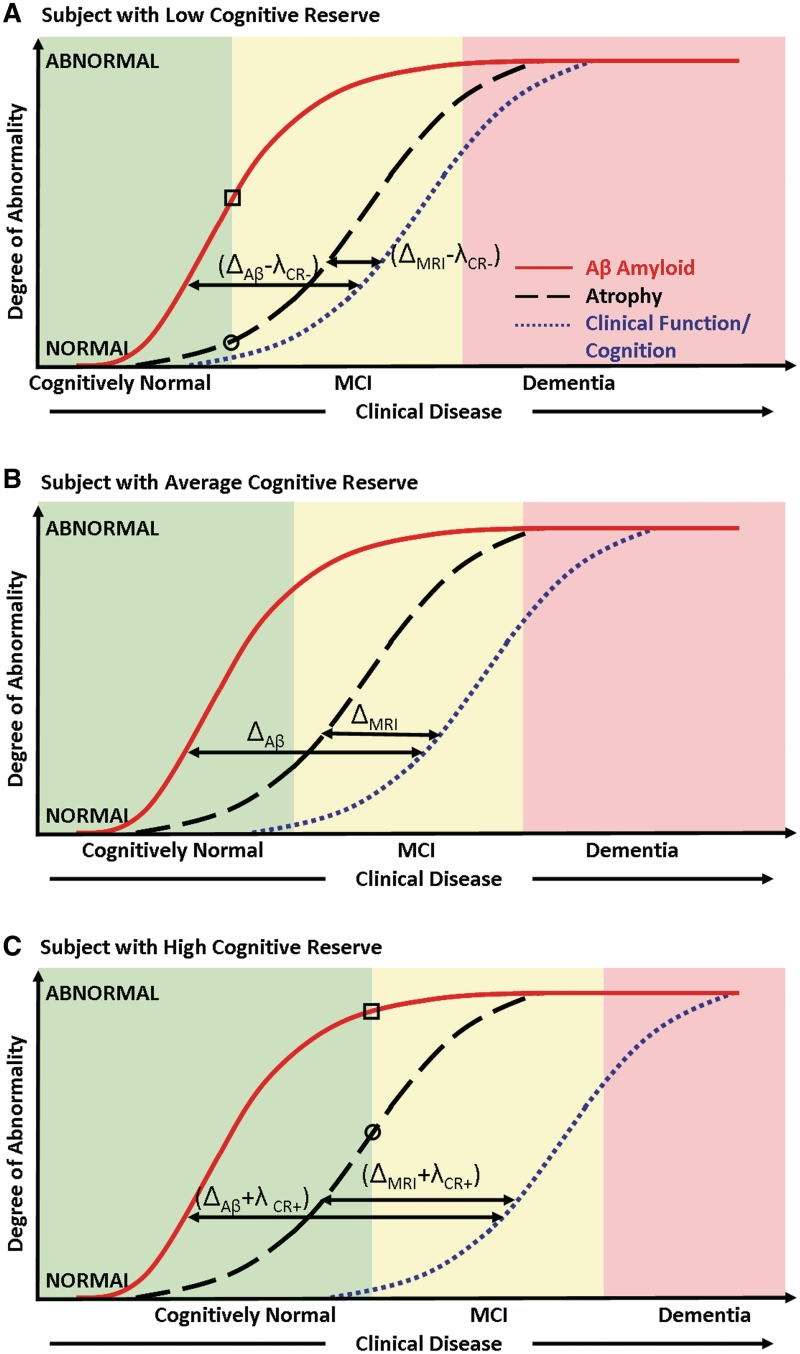
The key observations in this study can be summarized using Fig. 4. The cognitive decline or clinical function in mild cognitive impairment and Alzheimer's disease can be viewed as a downstream process caused by an increasing neurodegenerative pathological burden. If we plot the degree of abnormality in biomarkers and clinical function/cognition as a function of disease stage, the effect of cognitive reserve can be graphically conceptualized as moving the cognition curves (Fig. 4B, indicates a ‘reference’ level of cognitive reserve) relative to the biomarker curves which are located upstream. Movement of cognition relative to biomarkers due to the effect of cognitive reserve is to the left (Fig. 4A, less cognitive reserve) or right (Fig. 4C, greater cognitive reserve). If Δi indicates the distance between the biomarker curves and cognition for a subject with average cognitive reserve at a fixed point of cognition where i denotes the different biomarkers that measure different aspects of Alzheimer's disease pathology (i denotes amyloid-β load in the brain, t-tau or MRI); in this study we found evidence that the distance between both the biomarker and cognition curve is increased from Δi to (Δi + λCR+) for subjects with high cognitive reserve and decreased from Δi to (Δi − λCR−) in subjects with lower cognitive reserve, where λCR+ denotes the shift of the curves in subjects with high cognitive reserve and λCR− denotes the shift in subjects with low cognitive reserve. In particular we found evidence that λCR+ and λCR− are constants independent of the biomarker variable, i.e. the effect of cognitive reserve is additive; therefore, the distance moved by the cognition curve relative to the biomarker curves would be the same irrespective of the biomarker type. The evidence for this also comes from the fact that the strength of correlation between cognition and AMNART did not change much despite adjusting for each of the biomarkers.
Figure 4.
Model illustrating the independent effect of cognitive reserve on the relationship between biomarkers of pathology and cognition in subjects with (A) low, (B) average and (C) high cognitive reserve. Clinical disease stage is indicated on the horizontal axis and the magnitude of biomarker abnormalities (from normal to maximally abnormal) on the vertical axis. The biomarker curve labels are indicated in A. In A and C, the levels of amyloid-β are indicated by a square and the levels of atrophy are indicated by a circle at the point where cognitively normal subjects progress to mild cognitive impairment. This illustrates that an equivalent clinical diagnostic threshold, subjects with high cognitive reserve have greater biomarker abnormalities than low cognitive reserve subjects. MCI = mild cognitive impairment.
A plausible model for the development of Alzheimer's disease posits that amyloid deposition occurs early in the process but by itself does not directly cause clinical symptoms (Jack et al., 2009; Mormino et al., 2009). On the other hand, impaired cognitive performance is largely driven by neurodegeneration that may be mediated by tau pathology. Based on this evidence, it has been hypothesized that the Alzheimer's disease pathological cascade is a roughly two-stage process where amyloidosis and neuronal pathology (tauopathy, neuronal injury and neurodegeneration) are largely sequential rather than concurrent processes (Ingelsson et al., 2004; Jack et al., 2009, 2010). In this analysis we also found support for this model since the correlation between MRI and cognition was stronger than the correlation between CSF amyloid-β1–42 indicating that amyloid-β levels in the brain are saturating and MRI atrophy levels evolve simultaneously with declining cognitive performance leading to a stronger correlation. This directly translates into the fact that Δamyloid-β > ΔMRI. The primary observed effect of cognitive reserve was not to alter Δamyloid-β − ΔMRI, but to reduce or increase the distance between the biomarker curves and cognitive performance from Δi to (Δi + λCR+) or (Δi − λCR−) based on the subject's cognitive reserve.
Evidence for the suggested model in the literature
Two different approaches have been taken to study the effects of cognitive reserve—studies that have investigated the effect of cognitive reserve on the relationship between biomarkers and cognition or the effect of cognitive reserve on declining cognition. One class of articles provides strong evidence that biomarkers are more abnormal at a given level of cognitive performance in subjects with higher cognitive reserve when compared with subjects with lower cognitive reserve. This has been shown to be true for biomarkers of amyloid load (Kemppainen et al., 2008; Roe et al., 2008a; Rentz et al., 2010), neurodegeneration and fibre tract integrity (Querbes et al., 2009; Teipel et al., 2009; Piras et al., 2010), cerebral metabolism and perfusion (Alexander et al., 1997; Scarmeas et al., 2003; Liao et al., 2005; Perneczky et al., 2007; Garibotto et al., 2008; Hanyu et al., 2008; Cohen et al., 2009) and white matter hyperintensity load (Brickman et al., 2009b). Other reports support the cognitive reserve hypothesis by showing that high cognitive reserve delays the onset of Alzheimer's disease in the elderly (Hall et al., 2007; Ngandu et al., 2007). There was evidence for this hypothesis in our analysis where we found that better cognitive performance in both cognitively normal and cognitively impaired subjects modestly correlated with the AMNART errors after adjusting for the biomarker levels. This directly translates into the fact that at a given level of cognitive performance the degree of biomarker abnormality is generally higher in subjects with greater cognitive reserve. This can be illustrated as follows in Fig. 4: at the conceptual point where a subject progresses from cognitively normal to mild cognitive impairment in Fig. 4A and C, the levels of biomarker abnormality (square marker for amyloid-β levels and circle for atrophy levels) are higher in Fig. 4C (high cognitive reserve) when compared to Fig. 4A (low cognitive reserve) at the same level of cognitive performance.
A second group of articles has investigated how cognitive reserve affects the rate of cognitive decline in Alzheimer's disease. The studies that have investigated the effect of cognitive reserve on the rate of decline in Alzheimer's disease (i.e. after onset of dementia) support the hypothesis that although cognitive reserve may delay the onset of dementia, after the onset of dementia the rate of cognitive decline differs based on the subjects’ cognitive reserve. Some authors have found that subjects with higher cognitive reserve decline much faster (Unverzagt et al., 1998; Stern et al., 1999; Andel et al., 2006; Hall et al., 2007; Bruandet et al., 2008; Roselli et al., 2009) while others have found that subjects with higher cognitive reserve decline more slowly (Fritsch et al., 2001, 2002; Bennett et al., 2003; Manly et al., 2003; Le Carret et al., 2005) when compared to subjects with low cognitive reserve. In addition, there was also evidence that the rate of decline is the same in both the groups (Del Ser et al., 1999; Paradise et al., 2009). We speculate that these inconsistent results may be due to the fact that subjects were sampled at different levels of biomarker abnormality along the cognition curve or at different cognitive performance at baseline resulting in differences in the observed rates. In response to increasing biomarker abnormality, there is an early increase in the slope of cognitive decline as well as an early saturation of the slope in subjects with low cognitive reserve when compared to high cognitive reserve simply due to the shift of the curves relative to the biomarker curves. This observation suggests the importance of measuring and adjusting for the degree of biomarker abnormality in order to more accurately understand the effect of cognitive reserve.
Limitations of this study
There were several limitations of this study. First, we used AMNART which only measures one aspect of cognitive reserve, namely the pre-morbid verbal intelligence. While AMNART might not accurately capture all aspects of cognitive reserve, we believe that it provides a reasonable approximation of the beneficial effect of education and life-long learning. A related issue is that the particular aspect of cognitive reserve that AMNART is sensitive to cannot be measured perfectly. Although AMNART has been described as among the most reliable instruments in clinical use (Strauss et al., 2006), and for that matter the biomarkers, are subject to various degrees of measurement error that can affect statistical inferences. Usually measurement error will attenuate associations although that is not always the case. Using the simulation-extrapolation method, our findings were largely unaffected by measurement error.
A second limitation is that ideally cognitive reserve metrics should be obtained in middle age before any disease-related changes occur. In our data set, fewer AMNART errors were found in cognitively normal subjects than either mild cognitive impairment or Alzheimer's disease, but error rates did not differ between mild cognitive impairment and Alzheimer's disease. We, therefore, analysed cognitively normal subjects alone, and mild cognitive impairment and Alzheimer's disease as a single group. Rentz et al. (2010) went a step further by adjusting AMNART by MMSE to remove the confounding effect of cognitive performance on this variable. However, if we were to apply this adjustment to remove the effect of cognitive decline, we would be unable to observe a relationship between the predictor variable (AMNART adjusted for cognition) and the outcome variable (cognition) due to circularity issues. And, one of our main objectives was to evaluate the effects of AMNART and biomarkers independently on cognitive performance within clinical groups. Third, the Alzheimer's Disease Neuroimaging Initiative selection criteria excluded subjects with significant cerebrovascular disease (Hachinski score had to be <4). Therefore, the lack of a strong relationship between cognition and white matter hyperintensity in this cohort might partly be attributed to the selection criteria. Fourth, the Alzheimer's Disease Neuroimaging Initiative cohort is not population based; control and mild cognitive impairment subjects were subject to selection criteria and demented subjects were limited to mild Alzheimer's disease. The recruitment mechanisms were those used for clinical trials in Alzheimer's disease and included memory clinics, patient registries, public media campaigns and other forms of public advertisements. Consequently, inferences about the diagnostic sensitivity, specificity etc., of biomarkers in the general population cannot be drawn from Alzheimer's Disease Neuroimaging Initiative data. However, we believe that because the Alzheimer's Disease Neuroimaging Initiative incorporates patients across a cognitive spectrum ranging from normal to mild Alzheimer's disease, biologically based conclusions concerning the influence of cognitive reserve on the relationship between Alzheimer's disease biomarkers and cognition are valid. Last, the cohort we used in this study does not have autopsy confirmation, which is a limitation of almost all such observational studies.
Funding
National Institutes of Health (grant AG11378; P50 AG16574, U01 AG06786); Robert H. Smith Family Foundation Research Fellowship; the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation, USA and Opus building NIH (grant C06 RR018898). The Alzheimer's Disease Neuroimaging Initiative data was used for this study. The Foundation for the National Institutes of Health (www.fnih.org) coordinates the private sector participation of the $60 million Alzheimer's Disease Neuroimaging Initiative public–private partnership that was begun by the National Institute on Ageing (NIA) and supported by the National Institutes of Health. To date, more than $27 million has been provided to the Foundation for NIH by Abbott, AstraZeneca AB, Bayer Schering PharmaAG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Merck and Co., Inc., Novartis AG, Pfizer Inc., F. Hoffmann-LaRoche, Schering-Plough, Synarc Inc. and Wyeth, as well as non-profit partners the Alzheimer's Association and the Institute for the Study of Ageing.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative database (http://www.loni.ucla.edu/ADNI). As such, the investigators within the Alzheimer's Disease Neuroimaging Initiative contributed to the design and implementation of the Alzheimer's Disease Neuroimaging Initiative and/or provided data but did not participate in analysis or writing of this report. The Alzheimer's Disease Neuroimaging Initiative investigators include those found in this listing http://www.loni.ucla.edu/ADNI/Collaboration/ADNI_Manuscript_Citations.pdf).
Glossary
Abbreviations
- ADAS-Cog
Alzheimer's disease Assessment Scale (Cognitive Behaviour Section)
- AMNART
American National Adult Reading Test
- MMSE
Mini-Mental State Examination
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–17. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, et al. Association of premorbid intellectual function with cerebral metabolism in Alzheimer's disease: implications for the cognitive reserve hypothesis. Am J Psychiatry. 1997;154:165–72. doi: 10.1176/ajp.154.2.165. [DOI] [PubMed] [Google Scholar]
- Andel R, Vigen C, Mack WJ, Clark LJ, Gatz M. The effect of education and occupational complexity on rate of cognitive decline in Alzheimer's patients. J Intl Neuropsychol Soc. 2006;12:147–52. doi: 10.1017/S1355617706060206. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60:1909–15. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995;26:231–45. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- Bobinski M, de Leon MJ, Wegiel J, Desanti S, Convit A, Saint Louis LA, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer's disease. Neuroscience. 2000;95:721–5. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Schneider JA, Bienias JL, Bennett DA. Processing resources reduce the effect of Alzheimer pathology on other cognitive systems. Neurology. 2008;70:1534–42. doi: 10.1212/01.wnl.0000304345.14212.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Muraskin J, Zimmerman ME. Structural neuroimaging in Altheimer's disease: do white matter hyperintensities matter? Dialogues Clin Neurosci. 2009a;11:181–90. doi: 10.31887/DCNS.2009.11.2/ambrickman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Siedlecki KL, Muraskin J, Manly JJ, Luchsinger JA, Yeung LK, et al. White matter hyperintensities and cognition: Testing the reserve hypothesis. Neruobiol Aging. 2009b doi: 10.1016/j.neurobiolaging.2009.10.013. [Epub ahead of print] advance access date: Nov 19 2009, doi:10.1016/j.neurobiolaging.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruandet A, Richard F, Bombois S, Maurage CA, Masse I, Amouyel P, et al. Cognitive decline and survival in Alzheimer's disease according to education level. Dement Geriatr Cogn Disord. 2008;25:74–80. doi: 10.1159/000111693. [DOI] [PubMed] [Google Scholar]
- Cohen AD, Price JC, Weissfeld LA, James J, Rosario BL, Bi W, et al. Basal cerebral metabolism may modulate the cognitive effects of Abeta in mild cognitive impairment: an example of brain reserve. J Neurosci. 2009;29:14770–8. doi: 10.1523/JNEUROSCI.3669-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JR, Stefanski LA. Simulation-extrapolation estimation in parametric measurement error models. J Am Stat Assoc. 1994;89:1314–28. [Google Scholar]
- Crystal HA, Dickson DW, Sliwinski MJ, Lipton RB, Grober E, Marks-Nelson H, et al. Pathological markers associated with normal aging and dementia in the elderly. Ann Neurol. 1993;34:566–73. doi: 10.1002/ana.410340410. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–84. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- Del Ser T, Hachinski V, Merskey H, Munoz DG. An autopsy-verified study of the effect of education on degenerative dementia. Brain. 1999;122(Pt 12):2309–19. doi: 10.1093/brain/122.12.2309. [DOI] [PubMed] [Google Scholar]
- Dumurgier J, Paquet C, Benisty S, Kiffel C, Lidy C, Mouton-Liger F, et al. Inverse association between CSF Aβ 42 levels and years of education in mild form of Alzheimer's disease: The cognitive reserve theory. Neurobiology of disease. 2010;40:456–9. doi: 10.1016/j.nbd.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Kapeller P, Schmidt R, Offenbacher H, Payer F, Fazekas G. The relation of cerebral magnetic resonance signal hyperintensities to Alzheimer's disease. J Neurol Sci. 1996;142:121–5. doi: 10.1016/0022-510x(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, et al. CSF biomarkers in prediction of cerebral and clinical change in mild cognitive impairment and Alzheimer's disease. J Neurosci. 2010;30:2088–101. doi: 10.1523/JNEUROSCI.3785-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fritsch T, McClendon MJ, Smyth KA, Lerner AJ, Chen CH, Petot GJ, et al. Effects of educational attainment on the clinical expression of Alzheimer's disease: results from a research registry. Am J Alzheimers Dis Other Demen. 2001;16:369–76. doi: 10.1177/153331750101600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch T, McClendon MJ, Smyth KA, Ogrocki PK. Effects of educational attainment and occupational status on cognitive and functional decline in persons with Alzheimer-type dementia. Intl Psychogeriatr. 2002;14:347–63. doi: 10.1017/s1041610202008554. [DOI] [PubMed] [Google Scholar]
- Garibotto V, Borroni B, Kalbe E, Herholz K, Salmon E, Holtoff V, et al. Education and occupation as proxies for reserve in aMCI converters and AD: FDG-PET evidence. Neurology. 2008;71:1342–9. doi: 10.1212/01.wnl.0000327670.62378.c0. [DOI] [PubMed] [Google Scholar]
- Hall CB, Derby C, LeValley A, Katz MJ, Verghese J, Lipton RB. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology. 2007;69:1657–64. doi: 10.1212/01.wnl.0000278163.82636.30. [DOI] [PubMed] [Google Scholar]
- Hannah M, Quigley P. Presentation of ordinal regression analysis on the original scale. Biometrics. 1996;52:771–5. [Google Scholar]
- Hanyu H, Sato T, Shimizu S, Kanetaka H, Iwamoto T, Koizumi K. The effect of education on rCBF changes in Alzheimer's disease: a longitudinal SPECT study. Eur J Nucl Med Mol Imaging. 2008;35:2182–90. doi: 10.1007/s00259-008-0848-4. [DOI] [PubMed] [Google Scholar]
- Harrell FEJ. Regression modeling strategies. New York: Springer; 2001. [Google Scholar]
- Hirono N, Kitagaki H, Kazui H, Hashimoto M, Mori E. Impact of white matter changes on clinical manifestation of Alzheimer's disease: A quantitative study. Stroke. 2000;31:2182–8. doi: 10.1161/01.str.31.9.2182. [DOI] [PubMed] [Google Scholar]
- Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in ‘normal’ aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–74. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, Frosch MP, et al. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62:925–31. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–65. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia, PA: Lea and Febiger; 1983. [Google Scholar]
- Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–44. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- Kemppainen NM, Aalto S, Karrasch M, Nagren K, Savisto N, Oikonen V, et al. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer's disease. Ann Neurol. 2008;63:112–8. doi: 10.1002/ana.21212. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–95. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- Koepsell TD, Kurland BF, Harel O, Johnson EA, Zhou XH, Kukull WA. Education, cognitive function, and severity of neuropathology in Alzheimer disease. Neurology. 2008;70:1732–9. doi: 10.1212/01.wnl.0000284603.85621.aa. [DOI] [PubMed] [Google Scholar]
- Kono I, Mori S, Nakajima K, Nakagawa M, Watanabe Y, Kizu O, et al. Do white matter changes have clinical significance in Alzheimer's disease? Gerontology. 2004;50:242–6. doi: 10.1159/000078353. [DOI] [PubMed] [Google Scholar]
- Le Carret N, Auriacombe S, Letenneur L, Bergua V, Dartigues JF, Fabrigoule C. Influence of education on the pattern of cognitive deterioration in AD patients: the cognitive reserve hypothesis. Brain Cogn. 2005;57:120–6. doi: 10.1016/j.bandc.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Liao YC, Liu RS, Teng EL, Lee YC, Wang PN, Lin KN, et al. Cognitive reserve: a SPECT study of 132 Alzheimer's disease patients with an education range of 0–19 years. Dement Geriatr Cogn Disord. 2005;20:8–14. doi: 10.1159/000085068. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Touradji P, Tang MX, Stern Y. Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol. 2003;25:680–90. doi: 10.1076/jcen.25.5.680.14579. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–23. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Price AL. Pathologic correlates of non-demented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J Mol Neurosci. 2001;17:101–18. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- Ngandu T, von Strauss E, Helkala EL, Winblad B, Nissinen A, Tuomilehto J, et al. Education and dementia: what lies behind the association? Neurology. 2007;69:1442–50. doi: 10.1212/01.wnl.0000277456.29440.16. [DOI] [PubMed] [Google Scholar]
- Paradise M, Cooper C, Livingston G. Systematic review of the effect of education on survival in Alzheimer's disease. Int Psychogeriatr. 2009;21:25–32. doi: 10.1017/S1041610208008053. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Drzezga A, Diehl-Schmid J, Li Y, Kurz A. Gender differences in brain reserve: an (18)F-FDG PET study in Alzheimer's disease. J Neurol. 2007;254:1395–400. doi: 10.1007/s00415-007-0558-z. [DOI] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain. 2007;130:2837–44. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Piras F, Cherubini A, Caltagirone C, Spalletta G. Education mediates microstructural changes in bilateral hippocampus. Hum Brain Mapp. 2010 doi: 10.1002/hbm.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in non-demented aging and ‘preclinical’ Alzheimer's disease. Ann Neurol. 1999;45:358–68. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Querbes O, Aubry F, Pariente J, Lotterie JA, Demonet JF, Duret V, et al. Early diagnosis of Alzheimer's disease using cortical thickness: impact of cognitive reserve. Brain. 2009;132:2036–47. doi: 10.1093/brain/awp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for statistical computing; 2008. [Google Scholar]
- Reed BR, Mungas D, Farias ST, Harvey D, Beckett L, Widaman K, et al. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain. 2010 doi: 10.1093/brain/awq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67:353–64. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L'examen Clinique en Psychologie. Paris: Presses Universitaires de Frances; 1964. [Google Scholar]
- Riley KP, Snowdon DA, Markesbery WR. Alzheimer's neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–77. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- Roe CM, Mintun MA, D'Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol. 2008a;65:1467–71. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Xiong C, Miller JP, Cairns NJ, Morris JC. Interaction of neuritic plaques and education predicts dementia. Alzheimer Dis Assoc Disord. 2008b;22:188–93. doi: 10.1097/WAD.0b013e3181610fff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli F, Tartaglione B, Federico F, Lepore V, Defazio G, Livrea P. Rate of MMSE score change in Alzheimer's disease: influence of education and vascular risk factors. Clin Neurol Neurosurg. 2009;111:327–30. doi: 10.1016/j.clineuro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–64. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Paolo AM. A screening procedure for estimating premorbid intelligence in the elderly. Clin Neuropsychol. 1992;6:53–62. [PubMed] [Google Scholar]
- Scarmeas N, Zarahn E, Anderson KE, Habeck CG, Hilton J, Flynn J, et al. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol. 2003;60:359–65. doi: 10.1001/archneur.60.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmand B, Lindeboom J, Hooijer C, Jonker C. Relation between education and dementia: the role of test bias revisited. J Neurol Neurosurg Psychiatry. 1995;59:170–4. doi: 10.1136/jnnp.59.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. ‘Preclinical’ AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–6. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–8. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz CG, Fletcher E, DeCarli C, Carmichael OT. Fully-Automated White Matter Hyperintensity Detection with Anotomical Prior Knowledge and without FLAIR. Williamsburg, VA: Information Processing in Medical Imaging (IPMI); 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–7. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S69–74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–28. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology. 1999;53:1942–7. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–81. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen S. A compendium of neuropsychological tests: administration, norms, and commentary. Oxford: 2006. [Google Scholar]
- Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60:652–6. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–9. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Wagner M, Kohl T, Burger K, Reiser MF, et al. White matter microstructure in relation to education in aging and Alzheimer's disease. J Alzheimers Dis. 2009;17:571–83. doi: 10.3233/JAD-2009-1077. [DOI] [PubMed] [Google Scholar]
- US War Department. Army Individual Test Battery: Manual of Directions and Scoring. Washington DC, USA: US War Department, Adjutant General's Office; 1944. [Google Scholar]
- Unverzagt FW, Hui SL, Farlow MR, Hall KS, Hendrie HC. Cognitive decline and education in mild dementia. Neurology. 1998;50:181–5. doi: 10.1212/wnl.50.1.181. [DOI] [PubMed] [Google Scholar]
- Vanderstichele H, De Meyer G, Shapiro F, Engelborghs B, DeDeyn PP, Shaw LM, et al. Alzheimer's disease biomarkers: from concept to clinical utility. In: Galimberti D, Scarpini E, editors. Biomarkers for Early Diagnosis of Alzheimer's Disease. Hauppauge, NY: Nova Science Publishers, Inc; 2008. pp. 81–122. [Google Scholar]
- Vemuri P, Gunter JL, Senjem ML, Whitwell JL, Kantarci K, Knopman DS, et al. Alzheimer's disease diagnosis in individual subjects using structural MR images: validation studies. Neuroimage. 2008;39:1186–97. doi: 10.1016/j.neuroimage.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Trojanowski JQ, Shaw LM, et al. Serial MRI and CSF Biomarkers in Normal Aging, MCI and AD. Neurology. 2010;75:143–151. doi: 10.1212/WNL.0b013e3181e7ca82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: Diagnostic discrimination and cognitive correlations. Neurology. 2009;73:287–93. doi: 10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman VK, Aizenstein H, Guralnik J, Newman AB, Glynn NW, Taylor C, et al. Executive control function, brain activation and white matter hyperintensities in older adults. Neuroimage. 2010;49:3436–42. doi: 10.1016/j.neuroimage.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Pike KE, Darby D, Maruff P, Savage G, Ng S, et al. Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer's disease. Neuropsychologia. 2008;46:1688–97. doi: 10.1016/j.neuropsychologia.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Wahlund LO, Basun H, Almkvist O, Andersson-Lundman G, Julin P, Saaf J. White matter hyperintensities in dementia: does it matter? Magn Reson Imaging. 1994;12:387–94. doi: 10.1016/0730-725x(94)92531-3. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Brewer J, McEvoy LK, Fennema-Notestine C, Hagler DJ, Jr, et al. Combining MR imaging, positron-emission tomography, and CSF biomarkers in the diagnosis and prognosis of Alzheimer disease. AJNR Am J Neuroradiol. 2010;31:347–54. doi: 10.3174/ajnr.A1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L, Small BJ, Petrovitch H, Ross GW, Masaki K, Abbott RD, et al. Recent clinical-pathologic research on the causes of dementia in late life: update from the Honolulu-Asia Aging Study. J Geriatr Psychiatry Neurol. 2005;18:224–7. doi: 10.1177/0891988705281872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.