Abstract
Hippocampal sclerosis is a relatively common neuropathological finding (∼10% of individuals over the age of 85 years) characterized by cell loss and gliosis in the hippocampus that is not explained by Alzheimer’s disease. Hippocampal sclerosis pathology can be associated with different underlying causes, and we refer to hippocampal sclerosis in the aged brain as hippocampal sclerosis associated with ageing. Much remains unknown about hippocampal sclerosis associated with ageing. We combined three different large autopsy cohorts: University of Kentucky Alzheimer’s Disease Centre, the Nun Study and the Georgia Centenarian Study to obtain a pool of 1110 patients, all of whom were evaluated neuropathologically at the University of Kentucky. We focused on the subset of cases with neuropathology-confirmed hippocampal sclerosis (n = 106). For individuals aged ≥95 years at death (n = 179 in our sample), each year of life beyond the age of 95 years correlated with increased prevalence of hippocampal sclerosis pathology and decreased prevalence of ‘definite’ Alzheimer’s disease pathology. Aberrant TAR DNA protein 43 immunohistochemistry was seen in 89.9% of hippocampal sclerosis positive patients compared with 9.7% of hippocampal sclerosis negative patients. TAR DNA protein 43 immunohistochemistry can be used to demonstrate that the disease is usually bilateral even when hippocampal sclerosis pathology is not obvious by haematoxylin and eosin stains. TAR DNA protein 43 immunohistochemistry was negative on brain sections from younger individuals (n = 10) after hippocampectomy due to seizures, who had pathologically confirmed hippocampal sclerosis. There was no association between cases with hippocampal sclerosis associated with ageing and apolipoprotein E genotype. Age of death and clinical features of hippocampal sclerosis associated with ageing (with or without aberrant TAR DNA protein 43) were distinct from previously published cases of frontotemporal lobar degeneration TAR DNA protein 43. To help sharpen our ability to discriminate patients with hippocampal sclerosis associated with ageing clinically, the longitudinal cognitive profile of 43 patients with hippocampal sclerosis associated with ageing was compared with the profiles of 75 controls matched for age, gender, education level and apolipoprotein E genotype. These individuals were followed from intake assessment, with 8.2 (average) longitudinal cognitive assessments. A neuropsychological profile with relatively high-verbal fluency but low word list recall distinguished the hippocampal sclerosis associated with ageing group at intake (P < 0.015) and also 5.5–6.5 years before death (P < 0.005). This may provide a first step in clinical differentiation of hippocampal sclerosis associated with ageing versus pure Alzheimer’s disease in their earliest stages. In summary, in the largest series of autopsy-verified patients with hippocampal sclerosis to date, we characterized the clinical and pathological features associated with hippocampal sclerosis associated with ageing.
Keywords: biomarkers, PGRN, epilepsy, FTLD, cerebrovascular, stroke
Introduction
Hippocampal sclerosis refers to neuronal cell loss and astrocytosis in subiculum and cornu ammonis subfields of the hippocampal formation unrelated to Alzheimer’s disease pathology. In contrast to the disease also referred to as ‘hippocampal sclerosis’ that affects younger adults (Thom, 2009), hippocampal sclerosis in older individuals is associated with significant ante-mortem cognitive dysfunction (Corey-Bloom et al., 1997; Nelson et al., 2008) but not with epilepsy. There is no universally applied specific nosology for these cases and we use the term ‘HS-Ageing’ to refer to the disease with hippocampal sclerosis pathology in ageing individuals.
Despite recent progress from many centres, the specific clinical and pathological features related to HS-Ageing have not been definitively characterized. The disease was described decades ago (Clark et al., 1986; Dickson et al., 1994), yet researchers and clinicians only recently recognized the high prevalence of hippocampal sclerosis pathology in aged populations (Chui et al., 2006; Nelson et al., 2008; Zarow et al., 2008). Many brains from older individuals with hippocampal sclerosis pathology also show aberrant hippocampal TAR-DNA binding protein 43 (TDP-43) immunostaining (Amador-Ortiz et al., 2007; Josephs et al., 2008). However, the relationship between HS-Ageing pathogenesis and TDP-43 proteinopathy is still unclear. HS-Ageing and Alzheimer’s disease have overlapping clinical and radiographical features related to hippocampal atrophy, so improved clinical identification of patients with HS-Ageing would enable more specific management of both patients with HS-Ageing and patients with Alzheimer’s disease.
Autopsy is required for diagnosis of HS-Ageing. We hypothesize that prior studies have under-appreciated the importance of HS-Ageing because of the advanced age of affected patients (see below) and the complex neuropathology in that context. Prevalent non-hippocampal sclerosis brain pathologies include Alzheimer’s disease, cerebrovascular disease, α-synucleinopathies, frontotemporal lobar degeneration (FTLD) and neurofibrillary tangles without amyloid plaques (Jellinger and Attems, 2007; Sonnen et al., 2007; Nelson et al., 2009a, b; Schneider et al., 2009). Because of the age-related clinical and neuropathological ‘noise’, three elements are required for a systematic clinical–pathological study of HS-Ageing, namely excellent ante-mortem documentation, detailed neuropathology and adequate statistical power.
Here, we describe analyses based on 1110 individuals including 106 neuropathologically confirmed hippocampal sclerosis cases from three large autopsy series with extensive ante-mortem longitudinal data: the University of Kentucky Alzheimer’s Disease Centre, the Nun Study and the Georgia Centenarian Study. All neuropathological assessments were performed at the University of Kentucky. The goals were to identify clinical features that help distinguish aged individuals with autopsy-proven hippocampal sclerosis pathology, to refine our understanding of hippocampal sclerosis pathogenesis, and to help define HS-Ageing as a distinct disease entity.
Materials and methods
Clinical cohorts and neuropathological assessments
All protocols were performed with institutional review board approval from the respective institutions. Patients who came to autopsy from the UK-Alzheimer’s Disease Centre, Nun Study (Wolf et al., 1999) and Georgia Centenarian Study (Poon et al., 2007) cohorts were the basis for the study. Samples from 10 additional surgical pathological hippocampus resections were also assessed. These latter cases all had radiographical and pathologically confirmed features of mesial temporal/hippocampal sclerosis and clinical histories of intractable epilepsy (Supplementary Table 1).
Details of UK-Alzheimer’s Disease Centre, Nun Study and Georgia Centenarian Study recruitment have been described elsewhere (Poon et al., 1992; Schmitt et al., 2001; Gosche et al., 2002; Riley et al., 2002; Nelson et al., 2007; Hensley et al., 2010). Mental status testing of UK-Alzheimer’s Disease Centre subjects (Schmitt et al., 2000) employed cognitive instruments that included the mini-mental state examination (MMSE; Folstein et al., 1975) and the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological battery (Morris et al., 1989; Welsh et al., 1992). These measures included 1-min verbal category fluency for animal names and the CERAD word list delayed recall task. Similar measures were used in the cognitive assessments in the Nun Study. Evaluating clinicians and study staff had no knowledge regarding the pathology, thus minimizing case selection bias that would have affected main outcome measures in the current study.
Pathological assessments were performed at the University of Kentucky on all the cases, and the methodology has been described in detail (Davis et al., 1999; Wolf et al., 1999; Riley et al., 2002; Nelson et al., 2007, 2009). The neuropathological criterion for hippocampal sclerosis was selective neuronal loss and gliosis of CA1 and the subiculum of the hippocampus (Amador-Ortiz et al., 2007), not readily ascribable to another pathology such as neurofibrillary tangles or localizable infarction. Alzheimer’s disease-positive pathology referred to cases with Braak stage V or VI and with moderate or severely dense neuritic amyloid plaques according to CERAD criteria (Mirra et al., 1991). Patients with an early-onset (younger than 70 years of age) frontotemporal disease syndrome, known FTLD-type tauopathy, or other rare dementia syndrome (prions, trinucleotide repeat diseases) were excluded up-front from both cases and controls; this involved 31 cases including two relatively early-onset cases with hippocampal sclerosis and progressive supranuclear palsy pathologies.
Aberrant TDP-43 immunohistochemistry refers to staining that is cytoplasmic, neuritic or tangle like; TDP-43 is normally localized to the nucleus. Severity of hippocampal TDP-43 pathology was graded on a 0–3 semiquantitative scale that is described in detail in Supplementary Fig. 1. TDP-43 immunostaining was performed as follows: 5-µm-thick sections of paraffin-embedded tissue were placed on Plus-slides. Slides were dried overnight at 40°C. Subsequently, sections were unmasked in citrate buffer (pH 6) and heated in a pressure cooker (3 min). Sections were placed in formic acid for 3 min and then 3% hydrogen–methanol solution (30 min) with rinses in distilled water. Sections were then blocked with 5% goat serum in Tris-buffered saline for 1 h at room temperature and then incubated overnight in a 1:500 dilution of anti-TDP-43 (Proteintech) in Tris-buffered saline at 4°C in a humidity chamber. After thorough rinsing in Tris-buffered saline, sections were incubated in secondary antibody (biotinylated anti-goat IgG) for 1 h. Following additional rinsing in Tris-buffered saline, sections were incubated in ABC reagent from the Vector kit (Vector Labs) for 1 h at room temperature. After rinses in Tris-buffered saline, sections were developed in freshly prepared Vector Nova Red chromagen from the Vector kit and counterstained with Mayer’s haematoxylin.
Statistical methods
Data analyses related to age at death were based on combining data on all subjects who came to autopsy in the three research cohorts. Logistic regression models were used to determine the probability of Alzheimer’s disease (CERAD possible or probable, Braak stage V or VI at autopsy) and the probability of hippocampal sclerosis pathology (regardless of laterality or severity) as a function of age at death.
Detailed longitudinal cognitive assessments were analysed for members of the UK-Alzheimer’s Disease Centre and the Nun Study. A subset of these patients (n = 43 HS-Ageing, n = 75 controls) had been followed longitudinally, beginning with an ‘intake’ examination where they were not severely demented based on MMSE scores (>20). Five cases were impaired at intake with word list delayed recall scores of zero despite higher MMSE scores at intake that subsequently declined; these were included due to our a priori criteria. For all the included cases, we selected up to two controls without hippocampal sclerosis pathology, by matching the cases on age at entry, gender, apolipoprotein E (APOE) ε4 allele status, (non-)dementia status and the number of cognitive assessments. These 118 participants had a total of 966 assessments for an average of 8.2 assessments per participant (ranges 1–17) for MMSE, a total of 945 assessments for an average of 8.0 assessments per participant (ranges 1–16) for word list delayed recall and a total of 961 assessments for an average of 8.1 assessments per participant (ranges 1–17) for verbal fluency.
In modelling the change in scores, the following two characteristics had to be accounted for: (i) between and within subject variability; and (ii) floor and ceiling effects for the scores. The floor score was zero while the ceiling score related to the maximum attainable for each test. To account for (i) and (ii), the non-linear mixed effects regression model of Martins et al. (2005) and Nelson et al. (2009) was fitted to the assessment data. This is the three parameter logistic regression model:
where Yit represents the score for the ith participant at year t of study. Parameter a is the asymptote, the highest score for an individual subject; parameter b is a scaling effect representing 75% of the asymptote; and parameter c is the midpoint of the curve or 50% of the asymptote. We assume that parameters b and c depend only on the fixed effects, while parameter a depends on both fixed and random effects.
The fixed effects or covariates of interest are APOE allele status (presence/absence of at least one APOE ε4 allele), age at entry (centred at mean of 82.5 years) and their interactions. The random effects (within as well as between) are assumed to follow an independent normal distribution with mean zero and unknown variance. The purpose of the modelling was to determine how each parameter depended on these covariates after accounting for the two sources of variability. Statistical significance for a covariate was determined at the 0.05 level and only significant covariates were retained in the final model. The three parameter logistic models were fitted using PROC NLMIXED. Eventual HS-Ageing pathology and Alzheimer’s disease (‘high likelihood’ with Braak stage V or VI) pathology were the two main factors investigated and the fitted curves were plotted by these two factors. Test scores were modelled starting with the final evaluation and working back for 10 years. This approach was used because starting at ‘baseline’ evaluations and moving forward in time was confounded by variance in the number of years before the advent of cognitive decline.
To assess the ability of verbal fluency and word list delayed recall results to discriminate HS-Ageing, cognitive test results at baseline and 5.6–6.5 years prior to the terminal assessment were compared using a 2 × 2 factorial design analysis of covariance (ANCOVA). The two between-groups factors were HS-Ageing (yes/no) and clinical diagnosis of dementia (yes/no), and covariates included education, presence of at least one APOE ε4 allele, gender, baseline age and an indicator for cohort (Nun Study versus UK-Alzheimer’s disease centre cohort). All statistical analyses were performed using SAS/STAT® 9.2 software.
Results
A total of 106 cases with autopsy-verified HS-Ageing and 1004 controls were included (Table 1). All cases were evaluated bilaterally for hippocampal sclerosis pathology at the UK-Alzheimer’s Disease Centre. Laterality of hippocampal sclerosis in the 106 cases by haematoxylin and eosin stain was 26/106 (24.5%) unilateral left, 16/106 (15.1%) unilateral right and 64/106 (60.4%) bilateral.
Table 1.
Cases with hippocampal sclerosis (n = 106) and controls (n = 1004) by Alzheimer’s disease (Braak V/VI) status; mean age and final MMSE scores (±SD)
| Hippocampal sclerosis positive |
Hippocampal sclerosis negative |
|||||||
|---|---|---|---|---|---|---|---|---|
| Alzheimer’s disease positive | n | Alzheimer’s disease negative | n | Alzheimer’s disease positive | n | Alzheimer’s disease negative | n | |
| UK-Alzheimer’s Disease Centre | ||||||||
| Age at death | 87.2 ± 6.1 | 24 | 88.2 ± 8.2 | 20 | 81.1 ± 8.4 | 262 | 83.0 ± 9.9 | 259 |
| Final MMSE | 9.4 ± 8.1 | 23 | 19.6 ± 8.6 | 16 | 11.7 ± 9.0 | 225 | 24.6 ± 7.5 | 245 |
| Nun Study | ||||||||
| Age at death | 93.1 ± 4.6 | 22 | 93.7 ± 5.8 | 31 | 91.5 ± 4.3 | 90 | 89.5 ± 5.4 | 351 |
| Final MMSE | 6.1 ± 6.8 | 22 | 11.9 ± 9.2 | 31 | 10.1 ± 10.3 | 90 | 21.0 ± 8.9 | 351 |
| Georgia Centenarians | ||||||||
| Age at death | 102.7 ± 2.7 | 4 | 101.4 ± 2.7 | 5 | 102.0 ± 2.7 | 13 | 102.3 ± 2.4 | 29 |
| Final MMSE | 0.0 ± 0.0 | 4 | 14.2 ± 7.8 | 5 | 8.2 ± 7.6 | 12 | 18.3 ± 7.7 | 29 |
| All groups | ||||||||
| Age at death | 91.1 ± 6.9 | 50 | 92.4 ± 7.5 | 56 | 84.4 ± 9.3 | 365 | 87.4 ± 8.8 | 639 |
| Final MMSE | 7.2 ± 7.6 | 49 | 14.5 ± 9.4 | 52 | 11.1 ± 9.4 | 325 | 22.3 ± 8.6 | 625 |
| Total (n) | 106 | 1004 | ||||||
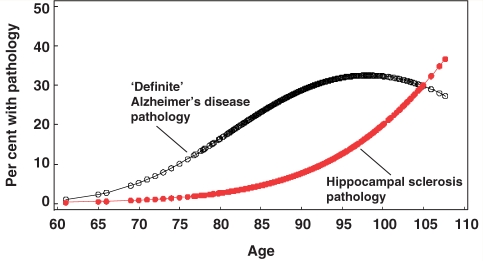
Individuals with hippocampal sclerosis pathology tended to be older than those without hippocampal sclerosis pathology. Whereas the percentage of individuals with hippocampal sclerosis pathology increased progressively with age of death, the percentage of individuals with Braak stage V or VI neurofibrillary pathology and Alzheimer’s disease-type neuritic plaques tapered off after the age of 95 years (Fig. 1). APOE status was independent of risk for hippocampal sclerosis pathology (Supplementary Table 2). In contrast, APOE ε4 carriers among patients with Alzheimer’s disease were over-represented and APOE ε2 carriers were under-represented, as expected (Supplementary Table 2).
Figure 1.
Estimated probability of a pathologically confirmed ‘definite’ Alzheimer’s disease (black curve) and the probability of a hippocampal sclerosis pathology (red curve; n = 106) as a function of age at death. ‘Definite’ Alzheimer’s disease cases (n = 286) had moderate or high densities of neuritic amyloid plaques and Braak stage V or VI. Note that after the age of 95 years, the probability for Alzheimer’s disease-type pathological diagnosis begins to decline but the probability for pathologically confirmed hippocampal sclerosis increases dramatically.
Correlations with TAR DNA binding protein 43 immunohistochemistry
At least two disease processes have been linked with hippocampal sclerosis pathology in later life: aberrant TDP-43 inclusions and vascular insufficiency (Lippa and Dickson, 2004; Attems and Jellinger, 2006; Amador-Ortiz et al., 2007; Probst et al., 2007; Zarow et al., 2008; Thom, 2009). We queried how these factors were associated with hippocampal sclerosis pathology. A convenience sample of 306 cases was evaluated using TDP-43 immunohistochemistry, 79 with hippocampal sclerosis and 227 without hippocampal sclerosis (Table 2). Immunostained slides were read blindly with regard to the histopathological diagnoses (Fig. 2). In all three cohorts sampled, the presence of aberrant TDP-43 was considerably more frequent in hippocampal sclerosis positives than hippocampal sclerosis negatives [estimated odds ratio (OR) 83.5, 95% confidence interval (95% CI) 35.6–195.9]. The distribution of the scored severity of TDP-43 pathology indicated that a range of aberrant TDP-43 changes can be seen in HS-Ageing (Supplementary Fig. 2). In contrast, in surgical pathology cases where sclerotic hippocampi were resected to treat chronic seizures (n = 10; average age 36.8 years), every case without exception was negative for aberrant TDP-43 (Fig. 2E and Supplementary Table 1).
Table 2.
Cases from the UK-Alzheimer’s Disease Centre, Nun Study and Georgia Centenarians: comparison in TDP-43 immunostaining including 79 cases (convenience sample) with hippocampal sclerosis (HS-Ageing) and 227 patients without hippocampal sclerosis (HS-NEG)
| Cohort | Total stained for TDP-43 (n) | Average age at death (years) | HS-Ageing evaluated for TDP-43 (n) | HS-Ageing with TDP+n (%) | HS-NEG evaluated for TDP-43 (N) | HS-NEG with TDP+n (%) |
|---|---|---|---|---|---|---|
| UK-Alzheimer’s Disease Centre | 208 | 84.3 | 23 | 15 (65) | 185 | 15 (5) |
| Nun Study | 48 | 92.8 | 48 | 43 (90) | ||
| Georgia centenarians | 50 | 102.2 | 8 | 8 (100) | 42 | 7 (17) |
| Total | 306 | 88.6 | 79 | 66 (89.9) | 227 | 22 (9.7) |
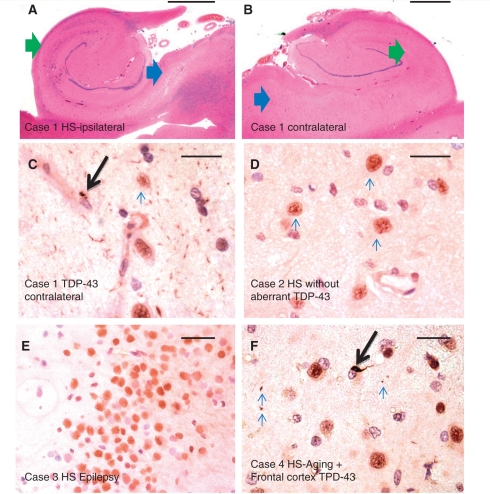
Figure 2.
Features of HS-Ageing and TDP-43 immunohistochemistry. A 97-year-old female (Case 1) had hippocampal features that, with haematoxylin and eosin staining, met criteria for hippocampal sclerosis on the right (A) but not the left (B) side. Green arrows show CA1, blue arrows show subiculum. Immunohistochemistry for TDP-43 showed clear evidence of aberrant TDP-43 on the left side (C), confirming that the process was bilateral. Case 2 (D) is an 88-year-old male with bilateral hippocampal sclerosis pathology but not Alzheimer’s disease or cerebrovascular disease, without aberrant TDP-43 staining. Shown here is the normal pattern of TDP-43 staining in neurons in the CA1 field of the hippocampus. Case 3 (E) is from a 34-year-old female with history of seizures and right mesial temporal sclerosis. Shown is a portion of the dentate granule cells, which like the rest of the hippocampectomy specimen (and all the other hippocampal sclerosis–seizures cases) showed no evidence of aberrant TDP-43 in cytoplasm or neurites. Case 4 (F) is from a 97-year-old APOE 3/3 male with Alzheimer’s disease and bilateral hippocampal sclerosis. Shown is a representative high-power field from frontal lobe (Brodmann area 9), with sparse aberrant TDP-43 immunohistochemistry including an intraneuronal inclusion (arrow) and a few scattered neurites (smaller arrows). Four of 14 stained frontal cortices from cases with HS-Ageing–TDP also showed scattered immunopositivity in frontal cortex. Scale bars = 1 mm (A, B and F), 50 microns (C–E). HS = hippocampal sclerosis.
We tested a sample of convenience to address whether aberrant TDP-43 was present in the contralateral side in cases that had unilateral hippocampal sclerosis pathology by haematoxylin and eosin stain (eight cases with unilateral hippocampal sclerosis by haematoxylin and eosin of which two were TDP-43 negative). The contralateral sides that were deemed on haematoxylin and eosin to be ‘negative’ for hippocampal sclerosis were immunopositive for aberrant TDP-43 in five of the six TDP-43 positive cases (Fig. 2C and Supplementary Table 3). The two cases that were TDP-43 negative on the ‘affected’ side were also negative for TDP-43 on the contralateral side. In a separate subsample, we tested the frontal cortex (Brodmann area 9, the superior frontal gyrus) to assess whether cases with HS-Ageing-TDP also had aberrant TDP-43 in the frontal cortex. Of 14 cases evaluated with bilateral HS-Ageing and TDP-43, four (29%) had aberrant frontal cortical staining, albeit usually less severe than that seen in the hippocampal portions (Fig. 2F and Supplementary Table 4).
Aberrant TDP-43 immunoreactivity in hippocampus and some frontal cortical sections of cases raises the question of relevance to FTLD-TDP (Armstrong et al., 2010; Baborie et al., 2010). Table 3 compares cases with HS-Ageing (present study) with published studies of FTLD-TDP cohorts (Mackenzie et al., 2006; Sampathu et al., 2006; Davidson et al., 2007; Josephs et al., 2009; Armstrong et al., 2010; Rohrer et al., 2010). Note that a handful of cases with frontotemporal dementia syndromes were excluded from the current study up-front (see above). However, cases with HS-Ageing are distinctly older and lack clinical frontotemporal dementia, primary progressive aphasia, or semantic dementia symptoms, unlike FTLD-TDP cases from our cohorts or elsewhere in the published literature (Table 3).
Table 3.
Comparison of mean age at death and percentage hippocampal sclerosis positive, frontotemporal dementia positive, progressive non-fluent aphasia positive, and semantic dementia positive, between the current case series (bold) and prior case series with frontotemporal lobar dementia with aberrant TDP-43 (FTLD-TDP)
| n | Mean death age in years (SD) | Hippocampal sclerosis pathology (%) | FTD or PNFA clinically (%) | Semantic dementia clinically (%) | |
|---|---|---|---|---|---|
| Current study | |||||
| HS-Ageing (all) | 106 | 92 (7) | 100b | 0 | 0 |
| HS-Ageing–TDPa | 71 | 94 (7) | 100b | 0 | 0 |
| Rohrer et al. (2010) | |||||
| FTLD–TDP type 1c | 9 | 59 (8) | 0 | 0 | 100 |
| FTLD–TDP type 2c | 5 | 59 (11) | 20 | 100 | 0 |
| FTLD–TDP type 3c | 10 | 57 (8) | 10 | 80 | 0 |
| Josephs et al. (2009) | |||||
| FTLD–TDP type 1d | 24 | 76 (10) | 75 | 100 | 0 |
| FTLD–TDP type 2d | 9 | 74 (10) | 56 | 29 | 71 |
| FTLD–TDP type 3d | 6 | 70 (8) | 67 | 100 | 0 |
| Mackenzie et al. (2006)e | |||||
| FTLD–TDP type 1d | 15 | 69 (5) | 93 | 93 | 7 |
| FTLD–TDP type 2d | 9 | 70 (4) | 67 | 22 | 77 |
| FTLD–TDP type 3d | 13 | 59 (11) | 100 | 100 | 0 |
| Armstrong et al. (2009)f | |||||
| FTLD–TDP sporadic | 52 | 71 (11) | 6 | ||
| FTLD–TDP not sporadic | 42 | 70 (9) | 7 | ||
a 71/79 cases with hippocampal sclerosis evaluated for TDP-43 immunohistochemistry were positive.
b 100% is due entirely to inclusion criteria.
c According to the Sampathu scheme for FTLD-TDP typing (Sampathu et al, 2006).
d According to the Mackenzie scheme for FTLD-TDP typing (Mackenzie et al, 2006).
e Displayed also are data from the same patients in Davidson et al. (2007).
f Patients in this study all met clinical criteria for frontotemporal dementia syndrome.
FTD = frontotemporal dementia including behavioural and motor neurone disease variants; PNFA = progressive non-fluent aphasia.
Correlations with brain infarcts and other comorbidities
Another disease mechanism linked to hippocampal sclerosis relates to cerebral perfusion (Ng et al., 1989). We sought to determine if cerebral infarctions occurred independently of hippocampal sclerosis, with or without aberrant TDP-43. Results are shown in Table 4. In the UK-Alzheimer’s Disease Centre cohort, the presence of large infarcts was associated with an increased risk for hippocampal sclerosis pathology (P < 0.04). However, when comparing clinical and pathological parameters between cases with hippocampal sclerosis and controls, we found it critical to control appropriately for patients’ ages because cases with hippocampal sclerosis pathology and cases with pathologically confirmed strokes both tended to be older. Neither vascular risk factors nor pathologically verified brain infarction risk were significantly different in cases with hippocampal sclerosis pathology compared with control individuals who died aged ≥90 years. We also assessed whether hippocampal sclerosis pathology corresponded to increased likelihood of large infarcts or lacunar infarcts in the Nun Study data set. Again, there was no increased risk for hippocampal sclerosis pathology in cases with infarcts after controlling for patients’ ages. There was also no difference in the prevalence of diffuse/neocortical Lewy body pathology in hippocampal sclerosis cases versus controls in either dataset (Supplementary Table 4). There was no added risk for infarcts, Lewy bodies or other clinical or pathological features in those cases with hippocampal sclerosis pathology but not aberrant TDP-43 immunohistochemistry (data not shown). Finally, hippocampal sclerosis pathology was slightly over-represented in cases with Alzheimer’s disease and vice versa; however, there was not good evidence that the interaction effect was powerful or specific despite the overlapping target cell populations for the diseases.
Table 4.
Comparison of clinical features and pathological comorbidities between cases with HS-Ageing and hippocampal sclerosis negative cases in two cohorts: UK-Alzheimer’s Disease Centre and Nun Study
| HS-Ageing | n | No HS-Ageing | n | P-valuea | No hippocampal sclerosis aged >89 years | n | P-value a | |
|---|---|---|---|---|---|---|---|---|
| UK-Alzheimer’s disease centre | ||||||||
| Age at death (mean years) | 87.7 | 44 | 82.0 | 521 | 93.4 | 107 | ||
| History of hypertension (%) | 48.4 | 31 | 55.3 | 309 | 0.571 | 59.5 | 111 | 0.308 |
| History of transient ischaemic attacks (%) | 10.7 | 28 | 10.1 | 277 | 1.000 | 14.9 | 94 | 0.760 |
| History of smoking (%) | 44.4 | 27 | 46.5 | 230 | 1.000 | 28.0 | 75 | 0.151 |
| History of brain trauma (%) | 16.7 | 30 | 14.0 | 272 | 0.782 | 10.8 | 93 | 0.521 |
| History of seizures (%) | 9.7 | 31 | 7.1 | 268 | 0.487 | 2.2 | 91 | 0.103 |
| Braak stage V or VI (%) | 54.6 | 44 | 51.1 | 521 | 0.754 | 36.5 | 107 | 0.047 |
| Lewy body disease (%) | 22.7 | 44 | 15.4 | 521 | 0.200 | 12.2 | 107 | 0.134 |
| Amyloid angiopathy (%) | 61.0 | 41 | 64.9 | 502 | 0.614 | 65.1 | 106 | 0.703 |
| Pale infarcts (%) | 6.8 | 44 | 7.1 | 521 | 1.000 | 9.5 | 107 | 0.757 |
| Haemorrhagic infarcts (%) | 4.6 | 44 | 5.8 | 521 | 1.000 | 7.5 | 107 | 0.724 |
| Lacunar infarcts (%) | 6.8 | 44 | 5.8 | 521 | 0.736 | 8.4 | 107 | 1.000 |
| Micro-infarcts (%) | 43.2 | 44 | 36.5 | 521 | 0.417 | 44.9 | 107 | 1.000 |
| Large infarcts (%) | 36.4 | 44 | 21.9 | 521 | 0.039 | 31.8 | 107 | 0.704 |
| Nun Study | ||||||||
| Age at death (mean years) | 92.8 | 56 | 89.5 | 470 | 93.6 | 241 | ||
| Lewy body disease (%) | 16.1 | 56 | 8.7 | 470 | 0.077 | 10.4 | 241 | 0.228 |
| Lacunar infarcts (%) | 46.8 | 47 | 31.1 | 437 | 0.034 | 37.3 | 212 | 0.249 |
| Large infarcts (%) | 17.0 | 47 | 17.8 | 437 | 1.000 | 17.5 | 212 | 1.000 |
a P versus hippocampal sclerosis (Fisher’s exact test).
No significant associations were found between the presence of hippocampal sclerosis pathology and the large majority of other clinical or pathological parameters. For example, there was no increased or decreased risk for HS-Ageing in persons with a history of coronary artery surgery or angioplasty, low- or high-education levels, or in diabetics (data not shown). Unlike other subtypes of neurodegenerative diseases, we also found no gender effect in terms of HS-Ageing risk, with the caveat that most individuals in the over 95-year-old cohort (who have the greatest risk for HS-Ageing) were female.
Correlations with longitudinal cognitive assessments
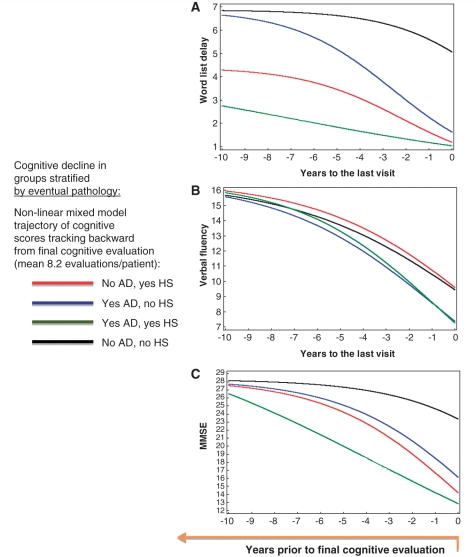
To determine whether particular cognitive tests are able to discriminate patients who will eventually demonstrate HS-Ageing pathologically, retrospective analyses were used from individuals who had serial cognitive assessments in the Nun Study and UK-Alzheimer’s Disease Centre. Cases stratified into groups according to the eventual neuropathological diagnoses: with or without ‘high likelihood’ Alzheimer’s disease pathology [Braak stage V or VI and moderate or severe densities of neuritic amyloid plaques according to the CERAD criteria (1997)], and with or without HS-Ageing pathology. Each hippocampal sclerosis positive case was matched to 1–2 hippocampal sclerosis negative cases on age, gender, education and APOE 4 carrier status. Non-linear mixed effects regression modelling was used to assess the trajectory of different cognitive test scores according to the four groups determined by eventual pathology (Fig. 3). Results show the changes in test scores moving backward in time from the final evaluation (median 9 months prior to death) through the prior 10 years, with an average of 8.2 longitudinal assessments. Verbal fluency test scores were higher, but word list delayed recall scores were lower, in patients with HS-Ageing relative to patients with Alzheimer’s disease pathology only. Word list delayed recall shows effect by hippocampal sclerosis but not Alzheimer’s disease pathology on asymptote, scale and midpoint (P < 0.0001) whereas verbal fluency shows effect by Alzheimer’s disease but not hippocampal sclerosis pathology on asymptote and midpoint (P < 0.0001). MMSE scores provide weaker discriminatory power than the other two tests.
Figure 3.
Linear mixed modelling of world list delay (A), verbal fluency (B) and MMSE (C) scores over time (n = 118 patients) with the goal of developing a biomarker for HS-Ageing. Cognitive assessment scores were modelled starting with the final evaluation and working back for 10 years using the non-linear mixed effects regression model (Martins et al., 2005; Nelson et al., 2009). This approach was used because starting at ‘baseline’ and moving forward required potential insertion of bias to cope with variance in the number of years before the advent of cognitive decline. Eventual HS-Ageing pathology and Alzheimer’s disease (with Braak stage V or VI) pathology, are the two main factors investigated and the fitted curves were plotted accordingly. The data from this three-parameter logistic model suggested an approach that could use cognitive testing as a biomarker for HS-Ageing because verbal fluency scores were higher, but word list delay lower in patients with HS-Ageing pathology (red line) than those with pure Alzheimer’s disease pathology (blue line). Note that MMSE scores provide weaker discriminatory power than the other two tests although, as in the other tests, the impact of HS-Ageing and Alzheimer’s disease are additive in individuals with both pathologies (green line). AD = Alzheimer's disease; HS = hippocampal sclerosis.
Because the results from the statistical modelling showed the verbal fluency and word list delayed recall could help discriminate cases with eventual HS-Ageing pathology from those with Alzheimer’s disease, we evaluated these tests at the baseline examination and in a time window 5.5–6.5 years prior to death (Supplementary Fig. 3). The latter time-point was selected because—for most patients—it was after the advent of symptoms but before ‘end-stage’ cognitive manifestations. The results of these additional analyses are shown in Table 5. Even at the baseline examination, the individuals with eventual HS-Ageing pathology tended to have higher verbal fluency test scores and lower word list delayed recall test scores relative to patients who eventually developed Alzheimer’s disease pathology (Supplementary Fig. 3). The baseline ANCOVA (adjusted for study group) results show a significant effect related to neuropathological diagnosis for HS-Ageing for word list delayed recall (P = 0.009) and the ratio of word list delayed recall:verbal fluency (P = 0.012). At a later time-point, 5.5–6.5 years prior to death, the ANCOVA (adjusted for study group) results show a significant effect due to neuropathological diagnosis for HS-Ageing for the MMSE (P ≤ 0.015), word list delayed recall (P < 0.0004) and word list delayed recall/verbal fluency (P < 0.005). The results of comparing the subsample of hippocampal sclerosis positive/Alzheimer’s disease negative and hippocampal sclerosis negative/Alzheimer’s disease positive cases only was also P < 0.05 at both time-points (data not shown). The ratio of word list delayed recall/verbal fluency was significantly different both at baseline and at 5.5–6.5 years before death when comparing groups with and without HS-Ageing (Supplementary Fig. 3). However, there was overlap in the values such that this ratio cannot be used to definitively identify individuals who would eventually manifest the pathology.
Table 5.
Cases from the UK-Alzheimer’s Disease Centre and Nun Study matched for age, gender, APOE allele frequencies and education level: comparison on neuropsychological test scores by pathological diagnosis
| HS-Ageing negative | HS-Ageing positive | |||||||
|---|---|---|---|---|---|---|---|---|
| Alzheimer’s disease negative | n | Alzheimer’s disease positive | n | Alzheimer’s disease negative | n | Alzheimer’s disease positive | n | |
| At intake | ||||||||
| Test scores (average ± SEM) | ||||||||
| MMSE | 27.8 ± 0.3 | 52 | 27.5 ± 0.4 | 23 | 27.6 ± 0.4 | 30 | 26.2 ± 0.6 | 13 |
| Verbal fluency | 16.7 ± 0.6 | 52 | 15.1 ± 0.9 | 23 | 16.3 ± 0.8 | 30 | 14.5 ± 1.2 | 13 |
| Word list delay | 5.9 ± 0.4 | 52 | 5.7 ± 0.5 | 23 | 5.1 ± 0.5 | 30 | 3.7 ± 0.7 | 13 |
| Word list delay/verbal fluency | 0.36 ± 0.02 | 52 | 0.39 ± 0.04 | 23 | 0.32 ± 0.03 | 30 | 0.26 ± 0.05 | 13 |
| 5.5–6.5 years prior to death | ||||||||
| Test scores (average ± SEM) | ||||||||
| MMSE | 27.6 ± 0.9 | 37 | 25.0 ± 1.6 | 12 | 25.4 ± 1.1 | 25 | 18.7 ± 2.7 | 4 |
| Verbal fluency | 16.5 ± 0.9 | 37 | 12.9 ± 1.6 | 12 | 13.9 ± 1.1 | 25 | 12.0 ± 2.7 | 4 |
| Word list delay | 6.8 ± 0.4 | 37 | 5.7 ± 0.8 | 11 | 4.2 ± 0.5 | 25 | 2.1 ± 1.3 | 4 |
| Word list delay/verbal fluency | 0.42 ± 0.03 | 37 | 0.43 ± 0.05 | 10 | 0.30 ± 0.04 | 24 | 0.20 ± 0.10 | 3 |
Table 6 shows the results of the MMSE, verbal fluency, word list delayed recall, and word list delayed recall/verbal fluency tests for cases with HS-Ageing stratified by whether or not aberrant TDP-43 staining was detected. Note that the cases without aberrant TDP-43 are relatively few (n = 4 and 6 at the two time-points) so this comparison is poorly powered for statistical purposes. These results do not provide a definitive answer to whether the cases with HS-Ageing TDP can be confidently differentiated from cases without aberrant TDP-43 immunostaining.
Table 6.
Cases from the UK-Alzheimer’s Disease Centre and Nun Study: comparison of cases with HS-Ageing only on neuropsychological test scores stratified by whether or not aberrant TDP-43 was found by pathology
| HS-Ageing TDP positive | HS-Ageing TDP negative | P-value | |
|---|---|---|---|
| At intake (among cases stained for TDP-43) | (n = 28) | (n = 6) | |
| Test scores (average ± SEM) | |||
| MMSE | 26.9 ± 0.5 | 27.5 ± 0.8 | 0.574 |
| Verbal fluency | 14.2 ± 0.7 | 15.8 ± 1.4 | 0.324 |
| Word list delay | 3.8 ± 0.5 | 5.8 ± 0.7 | 0.060 |
| Word list delay/verbal fluency | 0.3 ± 0.03 | 0.4 ± 0.1 | 0.167 |
| 5.5–6.5 years prior to death (among cases stained for TDP-43) | (n = 19) | (n = 4) | |
| Test scores (average ± SEM) | |||
| MMSE | 24.7 ± 1.6 | 25.0 ± 1.9 | 0.932 |
| Verbal fluency | 12.2 ± 1.2 | 16.8 ± 1.9 | 0.107 |
| Word list delay | 3.1 ± 0.5 | 6.5 ± 1.3 | 0.010 |
| Word list delay/verbal fluency | 0.3 ± 0.04 | 0.4 ± 0.1 | 0.179 |
Discussion
Autopsy-verified hippocampal sclerosis cases (n = 106, with 10 extra surgical pathology cases) and controls (n = 1004) from three large autopsy series were evaluated with the intention of better understanding the clinical and pathological parameters associated with hippocampal sclerosis in ageing. Our data support prior work linking HS-Ageing with aberrant TDP-43 immunohistochemical staining. Each additional year beyond the age of 95 years was associated with the increased risk for HS-Ageing pathology but not with increased Alzheimer’s disease pathology. Our data did not provide support for the pathogenetic connection between vascular factors and HS-Ageing. We found that we could determine a cognitive test profile with group-level differences between persons that manifest HS-Ageing pathology versus those that would develop Alzheimer’s disease. We conclude that the clinical and pathological features of HS-Ageing indicate a discrete brain disease with high prevalence in the studied autopsy cohorts.
Prior studies have found that hippocampal sclerosis pathology is detected in the brains of between 0.4% and 26% of elderly individuals (Dickson et al., 1994; Ala et al., 2000; Crystal et al., 2000; Jellinger, 2000; Barker et al., 2002; Leverenz et al., 2002; Petrovitch et al., 2005; Chui et al., 2006; Josephs et al., 2007; Nelson et al., 2007; Saito and Murayama, 2007; Sonnen et al., 2007; Zarow et al., 2008; Schneider et al., 2009). The variability in apparent hippocampal sclerosis prevalence may relate to differing cohort and study characteristics: patients’ ages and ante-mortem dementia severity, the thoroughness of the tissue sampling (not all studies noted that bilateral hippocampi were evaluated) and different diagnostic practices of individual neuropathologists involved in the studies. Although the true epidemiological prevalence of HS-Ageing is not known, the range of ∼8–18% prevalence in some large cohorts of aged individuals provides an approximation (Barker et al., 2002; Leverenz et al., 2002; Petrovitch et al., 2005; Chui et al., 2006). In the current study, 9.5% of cases had hippocampal sclerosis pathology (106/1110). This proportion may be lower than other autopsy series because more than half of the individuals in our sample had neither Alzheimer’s disease nor HS-Ageing pathology (625/1110, 57%); many were non-demented before death (overall mean final MMSE = 22.3, n = 296 with final MMSE = 26 or higher).
There was a lack of definite association between the presence of HS-Ageing on pathology and cerebrovascular disease or risk factors. This agrees with some prior autopsy series (Jellinger, 1994; Leverenz et al., 2002; Hatanpaa et al., 2004) but not all (Dickson et al., 1994; Corey-Bloom et al., 1997; Kril et al., 2002). As a practical point, both brain infarcts and HS-Ageing pathologies increase in advanced age and failing to incorporate this expectation by using sufficiently aged comparison groups will lead to a spurious correlation (as occurs in both the UK-Alzheimer’s Disease Centre and Nun Study data sets). In summary, we find that few if any individuals in our data sets harboured hippocampal sclerosis pathology due to vascular factors.
Two of the findings in the present study also agree with prior published literature: hippocampal sclerosis pathology increases in extreme advanced age and is associated with aberrant TDP-43 immunohistochemistry. A prior study evaluated 13 patients with hippocampal sclerosis and observed that these patients died at advanced ages (Dickson et al., 1994). However, the impact of ageing in hippocampal sclerosis prevalence was less obvious in other reports (Leverenz et al., 2002; Hatanpaa et al., 2004).
The positive correlation between dementia prevalence and ageing is widely accepted. However, specific neurodegenerative diseases tend to be most prevalent within particular ranges of the human ageing spectrum. Most brains in advanced age harbour at least incipient vascular disease, Alzheimer’s disease pathology, or synucleinopathies, paralleling the trend for impaired normative outcomes of cognitive tests (Petrovitch et al., 2005; Nelson et al., 2007, 2009; Schneider et al., 2007, 2009). Some cerebrovascular pathology is the norm (>75% prevalence) in individuals over the age of 90 years (van Dijk et al., 2002; Petrovitch et al., 2005; Nelson et al., 2007). This explanation may account for the finding that ‘pure’ hippocampal sclerosis pathology—with mean age of death 91.7 years in the current study—is not common (Ala et al., 2000; Jellinger, 2000; Barker et al., 2002; Attems and Jellinger, 2006). Individuals with Alzheimer’s disease pathology, but lacking HS-Ageing, tend to die younger (mean age at death 84.4 years in the current study) and thus have less concomitant pathology (Barker et al., 2002; Nelson et al., 2007). The prevalence of HS-Ageing pathology increases dramatically, while the prevalence of Alzheimer’s disease-type pathology declines, among individuals in our research cohort who died beyond the age of 95 years (Fig. 1). This pattern suggests a new insight into this expanding demographic cohort. Advanced age may indeed be the strongest risk factor for dementia, and Alzheimer’s disease the most common disease underlying dementia. However, the increased risk for dementia in extreme old age may be conferred largely by vascular disease and HS-Ageing, not by Alzheimer’s disease.
In addition to being linked to advanced age, hippocampal sclerosis pathology is also associated strongly with aberrant TDP-43 immunohistochemistry (Neumann et al., 2006; Amador-Ortiz et al., 2007; Josephs et al., 2008). In the current study, we confirm that ∼90% of patients with hippocampal sclerosis had aberrant TDP-43 immunohistochemical staining, in comparison to ∼10% in older controls irrespective of the presence of other pathologies. The interface between HS-Ageing and TDP-43-positive FTLD has not been well defined (Hatanpaa et al., 2004; Amador-Ortiz et al., 2007; Probst et al., 2007); clearly, cases with HS-Ageing do not fit neatly into existing FTLD classification (Cairns et al., 2007). A prior study reported that individuals with hippocampal sclerosis pathology frequently met clinical diagnostic criteria for frontotemporal dementia (Blass et al., 2004) but this was not the case in our research groups. The enormous age difference in subjects studied [Blass et al. (2004) had a mean age of 68.1 years versus 91.7 years in the present study] probably explains the difference in the underlying disease (Table 3). It remains to be seen whether the TDP-43 abnormalities are causally linked with HS-Ageing pathology. A speculative hypothesis, dovetailing on the recently described association between TDP-43 pathology and chronic trauma-induced encephalopathy (King et al., 2010; McKee et al., 2010), and the fact that hippocampal sclerosis-like pathology is observed in some blunt trauma cases (Kotapka et al., 1992), is that aberrant TDP-43 with hippocampal sclerosis pathology in advanced age may reflect physical wear and tear.
Whereas the pathological data may be biologically informative, there is a practical need for improved clinical detection of HS-Ageing to enable better management of both patients with HS-Ageing and Alzheimer’s disease. We found that the neuropsychological profiles of individuals with incipient HS-Ageing differed systematically, even in the earliest stages of cognitive decline, relative to individuals who would eventually die with advanced Alzheimer’s disease pathology. Individuals with presumed incipient HS-Ageing had higher verbal fluency scores and lower word list delayed recall scores, which is informative because the former relies more on neocortical function and the latter on brain function referent more directly to the hippocampal formation. Although Alzheimer’s disease often presents with memory complaints, the disease does not reach its more severe clinical stages until there is appreciable involvement of the neocortex (Arriagada et al., 1992; Nelson et al., 2009; Dolan et al., 2010). Although the verbal fluency and word list delayed recall tests could be used to differentiate between groups with and without HS-Ageing, this ratio could not discriminate between individuals because there was too much overlap between groups. Further, Alzheimer’s disease and HS-Ageing clinical trajectories over time are probably different from each other, which is an important caveat and potential confounder in our analyses. Our data serve only as an initial indicator that may be honed for more specificity in the future.
The present study is anchored in the analyses of autopsy-verified cases. The requirement for autopsy diagnosis introduces potential biases because autopsy series never achieve ideal parameters for an epidemiological cohort. In the present study, all the neuropathology was performed at the same research centre, which is a key study design element. There has not been a consensus report by a panel of experts about how to diagnose hippocampal sclerosis. Consensus guidelines are important; in cases with Alzheimer’s disease-like pathology that fall outside well-demarcated consensus guidelines, diagnoses are far more varied among neuropathologists than where clear consensus guidelines exist (Nelson et al., 2010). Hippocampal sclerosis may be more explicitly defined to incorporate different subtypes (Table 7). Hippocampal sclerosis–tau (Beach et al., 2003; Miki et al., 2009), hippocampal sclerosis–cerebrovascular disease (Ng et al., 1989; Horn and Schlote, 1992; Kotapka et al., 1992; Kril et al., 2002), and hippocampal sclerosis–FTLD (Barker et al., 2002; Blass et al., 2004; Hatanpaa et al., 2004) are conditions where hippocampal cell death and astrocytosis occur apparently through different mechanisms. Our findings are inconclusive as to whether there is synergy between Alzheimer’s disease and hippocampal sclerosis pathologies, but indicate that if there is synergy it is a relatively weak effect and independent of APOE status, so it is too early to designate a ‘hippocampal sclerosis–Alzheimer’s disease’ subtype. It also may be helpful to underscore the contrast between HS-Ageing and the pathology associated with seizures (Thom, 2009), which may be designated hippocampal sclerosis–seizures, and is not accompanied by aberrant TDP-43 (Lee et al., 2008). Better methods, e.g. biomarkers, are required to diagnose HS-Ageing clinically. In the meantime, we are increasingly impressed by the prevalence and impact of this TDP-43-linked neurodegenerative disease in the aged population.
Table 7.
Hippocampal sclerosis is associated with diverse underlying and accompanying brain diseases with at least five distinct hippocampal sclerosis subtypes
| Subtype | Characteristics |
|---|---|
| Associated with advanced age (HS-Ageing and HS-Ageing–TPD) | Highest prevalence in ‘oldest-old’a |
| Aberrant TDP-43 in ∼90% of casesa | |
| TDP-43 bilateral even if routine haematoxylin and eosin histology not | |
| Still not known whether TPD-43 is causative | |
| No proven relation to strokea | |
| Dementia, not usually seizuresa | |
| Association with Alzheimer’s disease is weak or non-existenta | |
| APOE alleles do not alter riska | |
| Cognitive tests: word list delay/verbal fluency ratio | |
| With seizures (HS–Sz) | Younger persons |
| Seizures, not usually dementia | |
| Often unilateral | |
| No aberrant TDP-43a | |
| With tauopathy (HS–tau) | Non-Alzheimer’s disease tauopathy |
| May include argyrophilic grain disease or progressive supranuclear palsy | |
| With non-tauopathy frontotemporal dementia (HS–FTD) | Multiple possible aetiologies |
| Accompanied by frontotemporal dementia clinical syndrome | |
| With cerebrovascular disease (HS–CVD) | May coexist with other cerebrovascular sequelae |
| Probably not progressive | |
| Similar pathology can be seen in hypoglycaemia, trauma |
a Focus of current study.
Funding
National Institutes of Health (grants R01 NS061933, R01 AG19241, K08 NS050110, P01 AG17553, P30 AG028383, K24 AG027841 and U01 AG016976).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
Dr William R. Markesbery performed the neuropathological evaluations for the great majority of cases and controls before his death in January 2010. The authors are deeply grateful to all of the study volunteers. The authors thank Sonya Anderson, Ann Tudor and Dr Huaichen Liu for technical support; Greg Cooper, MD, Nancy Stiles, MD and Allison Caban-Holt, PhD for the clinical evaluations; and Daron Davis, MD and Stephen W. Scheff, PhD, for pathological evaluations.
Glossary
Abbreviations
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
- FTLD
frontotemporal lobar degeneration
- HS-Ageing
hippocampal sclerosis associated with ageing
- MMSE
mini-mental state examination
- TDP-43
TAR DNA binding protein 43
References
- Ala TA, Beh GO, Frey WH., 2nd Pure hippocampal sclerosis: a rare cause of dementia mimicking Alzheimer’s disease. Neurology. 2000;54:843–8. doi: 10.1212/wnl.54.4.843. [DOI] [PubMed] [Google Scholar]
- Amador-Ortiz C, Ahmed Z, Zehr C, Dickson DW. Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol. 2007a;113:245–52. doi: 10.1007/s00401-006-0183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann. Neurol. 2007b;61:435–45. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RA, Ellis W, Hamilton RL, Mackenzie IR, Hedreen J, Gearing M, et al. Neuropathological heterogeneity in frontotemporal lobar degeneration with TDP-43 proteinopathy: a quantitative study of 94 cases using principal components analysis. J Neural Transm. 2010;117:227–39. doi: 10.1007/s00702-009-0350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42(3 Pt 1):631–9. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Attems J, Jellinger KA. Hippocampal sclerosis in Alzheimer disease and other dementias. Neurology. 2006;66:775. doi: 10.1212/01.wnl.0000200959.50898.26. [DOI] [PubMed] [Google Scholar]
- Baborie A, Griffiths TD, Jaros E, McKeith IG, Burn DJ, Richardson A, et al. Pathological correlates of frontotemporal lobar degeneration in the elderly. Acta Neuropathol. 2010 doi: 10.1007/s00401-010-0765-z. [DOI] [PubMed] [Google Scholar]
- Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Disease and Associated Disorders. 2002;16:203–12. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- Beach TG, Sue L, Scott S, Layne K, Newell A, Walker D, et al. Hippocampal sclerosis dementia with tauopathy. Brain Pathol. 2003;13:263–78. doi: 10.1111/j.1750-3639.2003.tb00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass DM, Hatanpaa KJ, Brandt J, Rao V, Steinberg M, Troncoso JC, et al. Dementia in hippocampal sclerosis resembles frontotemporal dementia more than Alzheimer disease. Neurology. 2004;63:492–7. doi: 10.1212/01.wnl.0000133008.89613.82. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui HC, Zarow C, Mack WJ, Ellis WG, Zheng L, Jagust WJ, et al. Cognitive impact of subcortical vascular and Alzheimer’s disease pathology. Ann Neurol. 2006;60:677–87. doi: 10.1002/ana.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AW, White CL, 3rd, Manz HJ, Parhad IM, Curry B, Whitehouse PJ, et al. Primary degenerative dementia without Alzheimer pathology. Can J Neurol Sci. 1986;13(Suppl 4):462–70. doi: 10.1017/s0317167100037136. [DOI] [PubMed] [Google Scholar]
- Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. . The National Institute on Ageing, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Age. 1997;18(Suppl 4):S1–2. [PubMed] [Google Scholar]
- Corey-Bloom J, Sabbagh MN, Bondi MW, Hansen L, Alford MF, Masliah E, et al. Hippocampal sclerosis contributes to dementia in the elderly. Neurology. 1997;48:154–60. doi: 10.1212/wnl.48.1.154. [DOI] [PubMed] [Google Scholar]
- Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB. The relative frequency of "dementia of unknown etiology" increases with age and is nearly 50% in nonagenarians. Arch Neurol. 2000;57:713–9. doi: 10.1001/archneur.57.5.713. [DOI] [PubMed] [Google Scholar]
- Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58:376–88. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- Davidson Y, Kelley T, Mackenzie IR, Pickering-Brown S, Du Plessis D, Neary D, et al. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol. 2007;113:521–33. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, et al. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol. 1994;88:212–21. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- Dolan D, Troncoso J, Resnick SM, Crain BJ, Zonderman AB, O’Brien RJ. Age, Alzheimer’s disease and dementia in the Baltimore longitudinal study of ageing. Brain. 2010;133(Pt 8):2225–31. doi: 10.1093/brain/awq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gosche KM, Mortimer JA, Smith CD, Markesbery WR, Snowdon DA. Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology. 2002;58(10):1476–82. doi: 10.1212/wnl.58.10.1476. [DOI] [PubMed] [Google Scholar]
- Hatanpaa KJ, Blass DM, Pletnikova O, Crain BJ, Bigio EH, Hedreen JC, et al. Most cases of dementia with hippocampal sclerosis may represent frontotemporal dementia. Neurology. 2004;63:538–42. doi: 10.1212/01.wnl.0000129543.46734.c0. [DOI] [PubMed] [Google Scholar]
- Hensley B, Martin P, MacDonald M, Poon L, Jazwinski SM, Green RC, et al. Family history and adaptation among centenarians and octogenarians. Gerontology. 2010;56:83–7. doi: 10.1159/000271955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M, Schlote W. Delayed neuronal death and delayed neuronal recovery in the human brain following global ischemia. Acta Neuropathol. 1992;85:79–87. doi: 10.1007/BF00304636. [DOI] [PubMed] [Google Scholar]
- Jellinger K. Pure hippocampal sclerosis: a rare cause of dementia mimicking Alzheimer’s disease. Neurology. 2000;55:739–40. doi: 10.1212/wnl.55.5.735-d. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Hippocampal sclerosis: a common pathological feature of dementia in very old humans. Acta Neuropathol. 1994;88:599. doi: 10.1007/BF00296500. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci. 2007;257:80–7. doi: 10.1016/j.jns.2007.01.045. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Ahmed Z, Katsuse O, Parisi JF, Boeve BF, Knopman DS, et al. Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol. 2007;66:142–51. doi: 10.1097/nen.0b013e31803020cf. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Stroh A, Dugger B, Dickson DW. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol. 2009;118:349–58. doi: 10.1007/s00401-009-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70:1850–57. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Sweeney F, Bodi I, Troakes C, Maekawa S, Al-Sarraj S. Abnormal TDP-43 expression is identified in the neocortex in cases of dementia pugilistica, but is mainly confined to the limbic system when identified in high and moderate stages of Alzheimer’s disease. Neuropathology. 2010;30:408–19. doi: 10.1111/j.1440-1789.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- Kotapka MJ, Graham DI, Adams JH, Gennarelli TA. Hippocampal pathology in fatal non-missile human head injury. Acta Neuropathol. 1992;83:530–4. doi: 10.1007/BF00310031. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Patel S, Harding AJ, Halliday GM. Patients with vascular dementia due to microvascular pathology have significant hippocampal neuronal loss. J Neurol Neurosurg Psychiatry. 2002;72:747–51. doi: 10.1136/jnnp.72.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, Lee VM, Trojanowski JQ, Neumann M. TDP-43 immunoreactivity in anoxic, ischemic and neoplastic lesions of the central nervous system. Acta Neuropathol. 2008;115:305–11. doi: 10.1007/s00401-007-0331-5. [DOI] [PubMed] [Google Scholar]
- Leverenz JB, Agustin CM, Tsuang D, Peskind ER, Edland SD, Nochlin D, et al. Clinical and neuropathological characteristics of hippocampal sclerosis: a community-based study. Arch Neurol. 2002;59(7):1099–106. doi: 10.1001/archneur.59.7.1099. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Dickson DW. Hippocampal sclerosis dementia: expanding the phenotypes of frontotemporal dementias? Neurology. 2004;63:414–5. doi: 10.1212/01.wnl.0000136241.71716.72. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol. 2006;112:539–49. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010;69:918–29. doi: 10.1097/NEN.0b013e3181ee7d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins CA, Oulhaj A, de Jager CA, Williams JH. APOE alleles predict the rate of cognitive decline in Alzheimer disease: a nonlinear model. Neurology. 2005;65:1888–93. doi: 10.1212/01.wnl.0000188871.74093.12. [DOI] [PubMed] [Google Scholar]
- Miki Y, Mori F, Hori E, Kaimori M, Wakabayashi K. Hippocampal sclerosis with four-repeat tau-positive round inclusions in the dentate gyrus: a new type of four-repeat tauopathy. Acta Neuropathol. 2009;117:713–8. doi: 10.1007/s00401-009-0531-2. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Scheff SW, Schmitt FA, Kryscio RJ, Jicha GA, et al. Alzheimer’s-type neuropathology in the precuneus is not increased relative to other areas of neocortex across a range of cognitive impairment. Neuroscience Lett. 2009a;450:336–9. doi: 10.1016/j.neulet.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Santacruz K, et al. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J Neuropathol Exp Neurol. 2009b;68:774–84. doi: 10.1097/NEN.0b013e3181aacbe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2008;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009c;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles "do count" when stageing disease severity. J Neuropathol Exp Neurol. 2007;66:1136–46. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Kryscio RJ, Abner EL, Schmitt FA, Jicha GA, Mendiondo MS, et al. Acetylcholinesterase inhibitor treatment is associated with relatively slow cognitive decline in patients with Alzheimer’s disease and AD + DLB. J Alzheimers Dis. 2009d;16:29–34. doi: 10.3233/JAD-2009-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Kukull WA, Frosch MP. Thinking outside the box: Alzheimer-type neuropathology that does not map directly onto current consensus recommendations. J Neuropathol Exp Neurol. 2010;69:449–54. doi: 10.1097/NEN.0b013e3181d8db07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Ng T, Graham DI, Adams JH, Ford I. Changes in the hippocampus and the cerebellum resulting from hypoxic insults: frequency and distribution. Acta Neuropathol. 1989;78:438–43. doi: 10.1007/BF00688181. [DOI] [PubMed] [Google Scholar]
- Petrovitch H, Ross GW, Steinhorn SC, Abbott RD, Markesbery W, Davis D, et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- Poon LW, Clayton GM, Martin P, Johnson MA, Courtenay BC, Sweaney AL, et al. The Georgia Centenarian Study. Int J Ageing Hum Dev. 1992;34:1–17. doi: 10.2190/8M7H-CJL7-6K5T-UMFV. [DOI] [PubMed] [Google Scholar]
- Poon LW, Jazwinski SM, Green RC, Woodard JL, Martin P, Rodgers WL, et al. Methodological considerations in studying centenarians: lessons learned from the Georgia centenarian studies. In: Poon LW, Perls TT, editors. Annual review of gerontology and geriatrics: biopsychosocial approaches to longevity 2007 ed. Vol. 27. New York: Springer; 2007. pp. 231–64. [PMC free article] [PubMed] [Google Scholar]
- Probst A, Taylor KI, Tolnay M. Hippocampal sclerosis dementia: a reappraisal. Acta Neuropathol. 2007;114:335–45. doi: 10.1007/s00401-007-0262-1. [DOI] [PubMed] [Google Scholar]
- Riley KP, Snowdon DA, Markesbery WR. Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–77. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Geser F, Zhou J, Gennatas ED, Sidhu M, Trojanowski JQ, et al. TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology. 2010;75:2204–11. doi: 10.1212/WNL.0b013e318202038c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Murayama S. Neuropathology of mild cognitive impairment. Neuropathology. 2007;27:578–84. doi: 10.1111/j.1440-1789.2007.00806.x. [DOI] [PubMed] [Google Scholar]
- Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, et al. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169:1343–52. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009a;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009b;66:200–8. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical“ AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–6. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- Schmitt FA, Wetherby MM, Wekstein DR, Dearth CM, Markesbery WR. Brain donation in normal ageing: procedures, motivations, and donor characteristics from the Biologically Resilient Adults in Neurological Studies (BRAiNS) Project. Gerontologist. 2001;41:716–22. doi: 10.1093/geront/41.6.716. [DOI] [PubMed] [Google Scholar]
- Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, et al. Pathological correlates of dementia in a longitudinal, population-based sample of ageing. Ann Neurol. 2007;62:406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- Thom M. Hippocampal sclerosis: progress since Sommer. Brain Pathol. 2009;19:565–72. doi: 10.1111/j.1750-3639.2008.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk EJ, Prins ND, Vermeer SE, Koudstaal PJ, Breteler MM. Frequency of white matter lesions and silent lacunar infarcts. J Neural Transm Suppl. 2002;62:25–39. doi: 10.1007/978-3-7091-6139-5_2. [DOI] [PubMed] [Google Scholar]
- Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and stageing of dementia in Alzheimer’s disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer’s Disease. Arch Neurol. 1992;49:448–52. doi: 10.1001/archneur.1992.00530290030008. [DOI] [PubMed] [Google Scholar]
- Wolf DS, Gearing M, Snowdon DA, Mori H, Markesbery WR, Mirra SS. Progression of regional neuropathology in Alzheimer disease and normal elderly: findings from the Nun study. Alzheimer Dis Assoc Disorders. 1999;13:226–31. doi: 10.1097/00002093-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Zarow C, Sitzer TE, Chui HC. Understanding hippocampal sclerosis in the elderly: epidemiology, characterization, and diagnostic issues. Curr Neurol Neurosci Rep. 2008;8:363–70. doi: 10.1007/s11910-008-0057-3. [DOI] [PubMed] [Google Scholar]