Abstract
In this study, we characterized the metabolome of the human ovary and identified metabolic alternations that coincide with primary epithelial ovarian cancer (EOC) and metastatic tumors resulting from primary ovarian cancer (MOC) using three analytical platforms: gas chromatography mass spectrometry (GC/MS) and liquid chromatography tandem mass spectrometry (LC/MS/MS) using buffer systems and instrument settings to catalog positive or negative ions. The human ovarian metabolome was found to contain 364 biochemicals and upon transformation of the ovary caused changes in energy utilization, altering metabolites associated with glycolysis and β-oxidation of fatty acids—such as carnitine (1.79 fold in EOC, p<0.001; 1.88 fold in MOC, p<0.001), acetylcarnitine (1.75 fold in EOC, p<0.001; 2.39 fold in MOC, p<0.001), and butyrylcarnitine (3.62 fold, p<0.0094 in EOC; 7.88 fold, p<0.001 in MOC). There were also significant changes in phenylalanine catabolism marked by increases in phenylpyruvate (4.21 fold; p = 0.0098) and phenyllactate (195.45 fold; p<0.0023) in EOC. Ovarian cancer also displayed an enhanced oxidative stress response as indicated by increases in 2-aminobutyrate in EOC (1.46 fold, p = 0.0316) and in MOC (2.25 fold, p<0.001) and several isoforms of tocopherols. We have also identified novel metabolites in the ovary, specifically N-acetylasparate and N-acetyl-aspartyl-glutamate, whose role in ovarian physiology has yet to be determined. These data enhance our understanding of the diverse biochemistry of the human ovary and demonstrate metabolic alterations upon transformation. Furthermore, metabolites with significant changes between groups provide insight into biochemical consequences of transformation and are candidate biomarkers of ovarian oncogenesis. Validation studies are warranted to determine whether these compounds have clinical utility in the diagnosis or clinical management of ovarian cancer patients.
Introduction
Ovarian cancer is the most lethal malignancy of the female reproductive system and the 5th cause of cancer death in women. It is estimated that 21,880 women will be diagnosed and 13,850 will die from this disease this year. The five-year survival rate at Stage I is 93.5% but drops to 27.6% at Stage IV, where a majority of cases are diagnosed due to a lack of symptoms at the earlier stages [1]. Current detection strategies include transvaginal ultrasound and blood CA-125 levels. However, both detection methods have shortcomings. With ultrasound, cancer could be mistaken for functional cysts in pre-menopausal women due to the dynamic nature of the ovarian surface [2]. CA-125 has a high false positive rate [2] that can arise from a variety of conditions including endometriosis, fibroids, hemorrhagic ovarian cysts, acute pelvic inflammatory disease, menstruation, first trimester pregnancy, and several other cancer types [3]. In addition, CA-125 is often not detectable in early stage ovarian cancer [4]. Alternative methods are being developed for patients who have normal CA-125 levels but are suspected to having recurrent disease based on clinical symptoms [5]. These methods include other potential biomarkers, the most promising being human epydidimus protein 4 (HE4), [6], [7], [8], [9], [10] despite detection rates of 50–60% in early stage ovarian cancer. A comprehensive study comparing the sensitivity of ovarian cancer biomarkers to discriminate between benign and malignant masses has been described [11] as well as the role of molecular markers in prognosis and therapy reviewed in [12]. It is important that suggested biomarkers have predictive value as indicated by sensitivity of 75% or greater as well as specificity of 99.6% to be able to detect early stage cancer when it is the most treatable [4].
One approach to identify disease biomarkers is to use information-rich analytical tools such as omics-scale biological methods to characterize the composition of the target tissue in health and disease. In this case, it is important to understand the biochemical alterations that are known to occur during neoplastic transformation. The first energy metabolism alteration in cancer cells was described by Otto Warburg, who showed cancer cells preference for glycolysis resulting in the generation of lactate for ATP production over the more efficient process of oxidative phosphorylation by the mitochondria [13]. This requires the cancer cells to increase their glucose uptake through the expression of several isoforms of glucose transporters (GLUT 1 to 9) [14] and to increase their glucose catabolism to compensate for the energy production loss, a fact that can be exploited in the clinical detection of neoplasm by positive emission tomography (PET) imaging [15].
The molecular mechanisms involved in the hyperactive glycolysis have been analyzed and some of key factors identified—including Akt, nuclear factor-κB (NF-κB), hypoxia-inducible factor-1 (HIF1), and p53 [14], [16], [17], [18], [19], [20]. The products of these genes are involved in cellular activation, nutrient import, and protection from apoptosis. These genes are known to interact in complex hierarchical webs. For example, HIF-1 can be modulated by other oncogenes such as Akt [14], K-Ras [21], and Her-2 [22] to increase the expression of several glycolytic enzymes. Other molecular mechanisms include transcriptional regulation by Myc to increase expression of transporters and glycolytic enzymes—in particular GLUT, hexokinase 2, and lactate dehydrogenase [14], [23]—as well as by the phosphoinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway [24], [25], which is commonly overactive in carcinomas [26]. In addition, tumor metabolism differentially expresses glycoltyic isoenzymes, such as pyruvate kinase (PKM2), which can shift between a dimer and a tetramer to adapt to the energy requirements of the cells [27], [28]. However, PKM2 can also be by-passed by the accumulation of phosphoenolpyruvate (PEP) resulting in PEP-dependent phosphorylation and activation of phosphoglycerate mutase which produces pyruvate directly from 3-phosphoglycerate [29]. Vander Heiden et al. [29] hypothesized that this uncouples pyruvate production from ATP generation, maintaining an ATP/AMP ratio that does not inhibit glycolysis, and provides a significant pool of pyruvate as an anabolic precursor. Most of the research on tumor cell metabolism has focused on glucose utilization. When glucose is limited, solid tumors are forced to catabolize alternative substrates such as fatty acids, and amino acids as an alternative energy source.
However, oncogenesis can result in a diverse panel of metabolic alterations that could be tissue specific or generic across human cancers. Therefore, a comprehensive metabolic analysis of solid tumors could reveal valuable metabolites for both early diagnosis of cancer as well as to monitor disease progression and/or recurrence to inform clinical management of cancer patients. These biomarkers could conceivably be used as surrogate endpoints in clinical trials and could suggest new metabolic targets for cancer management as well as provide complementary targets for chemotherapy treatment. Metabolomics is a systematic analytical tool used for identification of biochemical metabolites from cellular processes, a term that includes several types of analyses ranging for nuclear magnetic resonance spectroscopy (NMR), mass spectrometry (MS), tracer-based studies, and metabolic footprinting [30]. While each of these methods has unique advantages, MS has established itself as the high-throughput and industrially stable approach to assess both the composition of diverse sample types as well as changes to that composition following perturbation. Although metabolomics has been around for decades, more recently it has garnered attention as a translational tool for the identification and treatment of cancer in the clinical setting, as well as for drug target development [31].
In a previous study, Denkert et al. [32] used gas chromatography MS/time-of flight (GC-MS/TOF) to compare borderline ovarian tumors to ovarian carcinomas. They identified 114 of 291 (39.1%) compounds and found an increased in proteinogenic amino acids, purines, pyrimides, and lipid membrane precursors in ovarian carcinomas vs. borderline tumors and interpreted these data to mean that carcinomas have higher cell proliferation rates. In addition to tumor metabolic analysis, urine samples from ovarian cancer patients have also been studied. Woo et al. [33] conducted a metabolomic-based study to find urinary biomarkers for ovarian and breast cancer using GC/MS. Two known biomarkers for breast cancer and 3 new biomarkers for ovarian cancer were identified: 1-methyladenosine, 3-methyluridine, and 4-androstene-3,17-dione. The ovarian cancer biomarkers were related to oxidative DNA damage and DNA methylation. Similarly, Slupsky et al. [34] collected urine samples from patients with early- and late-stage breast or ovarian cancer, as well as from healthy women, to obtain a metabolic profile using NMR. The concentration of specific metabolites decreased in patients with cancer, resulting in a unique profile. Alterations in intermediates of the tricarboxylic acid cycle (TCA) as well as molecules relating to energy metabolism and amino acids were observed.
Prior to this study, however, the metabolome of the normal ovary has not been studied, nor the changes that occur with neoplastic transformation and metastatic disease progression. In the present study, for the first time we report the metabolic profile of the normal human ovary and compare it to the metabolic profile of primary epithelial ovarian cancer (EOC) and metastatic tumors resulting from initial EOC (MOC) using GC/MS and LC/MS/MS.
Results
Identification of metabolites, statistical analysis, and pathway analysis
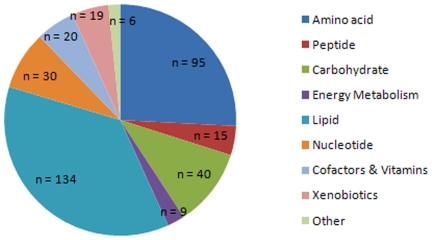
In samples from our three groups (normal, EOC, and MOC), 364 molecules were identified (Table S1) when compared to the Metabolon library containing 1,700 molecules. Identification was based on retention time, charge (m/z), preferred adducts, and fragmentation pattern of the molecule. The comprehensive library allowed for rapid identification with a high fidelity. These compounds included a large variety of classes, ranging from simple amino acids and peptides to carbohydrates, lipids, nucleotides, cofactors and vitamins, and xenobiotics (Fig. 1).
Figure 1. Class distribution of identified metabolites.
n = number of metabolites in each class.
Data is a summation of individuals belonging to a group. Using one-way ANOVA with a Tukey post-test to identify differentially abundant metabolites across the three classes of tissue analyzed, 95 biochemicals were statistically significant and furthermore had a p≤0.05 in at least one of the pairwise comparisons (EOC vs. normal; MOC vs. normal; MOC vs. EOC). The identities of these metabolites are given in Table S2. Using the abundance profiles of these metabolites, supervised principal components analysis (PCA) was performed, yielding good separation of the three groups (Fig. S1).
Using Ingenuity Pathway Analysis (IPA), we identified the top 15 canonical pathways involved in EOC (Table S3) and MOC (Table S4). In almost all cases, these were related to amino acid metabolism and biosynthesis. Also of note, pyrimidine metabolism, purine metabolism, and glycoxylate and decarboxylate metabolism pathways only appeared in the case of MOC.
Metabolic profile of the normal ovary and loss of function upon transformation
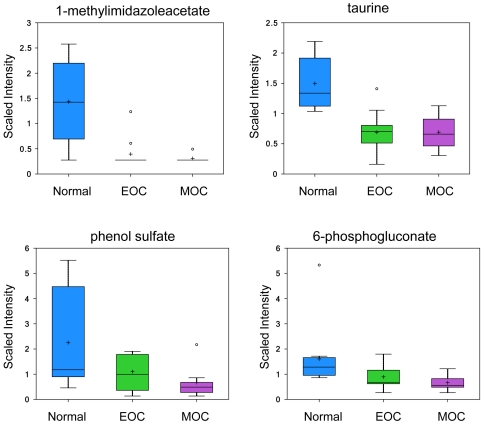
The first principal component separated the non-transformed ovarian samples from the transformed tissues (both EOC and MOC), while the second principal component identified a further set of biochemical alterations that corresponded with metastasis (Figure S1). Evaluation of the loadings plot further classified the compounds into loss/gain-of-function with either transformation or metastasis. Four metabolites were in high abundance in the ovary prior to neoplastic transformation: 1-methylimidazole acetate (−2.06 fold, p<0.001 in EOC; −2.18 fold, p<0.001 in MOC), taurine (−1.75 fold, p<0.001 in EOC, −1.97 fold, p<0.001 in MOC), phenol sulfate (−2.22 fold, p = 0.0535 in EOC; −3.0 fold, p = 0.0217 in MOC), and 6-phosphogluconate (−1.64 fold, p = 0.0538 in EOC, −1.92 fold, p = 0.0264; Fig. 2). These biochemicals have a significant drop in abundance upon transformation. Two of these metabolites (methylimidazoleacetate and 6-phosphogluconate) have previously been associated with normal ovarian function and the drop in their abundance can be considered a loss-of-function associated with transformation.
Figure 2. Significant metabolites present in the normal ovary and are reduced upon neoplastic transformation.
1-methylimidazoleacetate and taurine analyzed by LC/MS positive ion spray; phenol sulfate and 6-phosphogluconate analyzed by LC/MS negative ion spray. Box legend: + inside box represents mean value, bar inside box represents median value, upper bar represents maximum of distribution, lower bar represents minimum of distribution, circle represents extreme data points.
Methylimidazoleacetate is the main metabolite of histamine. This end product of histamine catabolism is formed by N-methylation in the imidazole ring to methylhistamine by histamine methyltransferase and a subsequent oxidative deamination in the side chain by type B monoamine oxidase. From studies it is known that as much as 70–80% of the histamine metabolized in the body is excreted in the urine as methylimidazoleacetate [35]. Thus, urinary methylimidazoleacetate being the major and specific histamine metabolite is a clear marker of any changes in histamine metabolism in the body. Ovarian histamine production occurs by tissue resident mast cells and has been shown to coordinate with ovulation [36].
Taurine is not involved in protein synthesis and/or has limited participation in biochemical pathways outside of peroxisomal formation of N-acyl lipid conjugates, such as bile acids and fatty acids. However, several functions have been demonstrated for taurine—such as osmoregulation, membrane stabilization, detoxification, antioxidation, modulation of ion flux, and as an inhibitory neurotransmitter or neuromodulator [37], [38], [39], [40], [41]. The roles of taurine in the reproductive system are multiple and complex. Taurine is the predominant amino acid in genital secretions—including seminal, uterine, and oviduct fluids [42], [43]. It has been demonstrated that the ovary contains the mRNA of a taurine transporter [44] and that the rat oviduct contains up to 10 µmol taurine/g tissue [45]. Taurine is also present in high concentrations in the rat and human uterus, and its concentration decreases with pregnancy [46], [47]. Despite all these data, the roles of taurine in the female reproductive system are largely unknown and there are no previous studies about its localization in these organs.
Phenol sulfate is a hepatically processed gut microfloral metabolite, and 6-phosphogluconate is an intermediate in the utilization of glucose within the pentose phosphate pathway, potentially signifying that glucose is restricted from entry into the pentose phosphate pathway in the healthy ovary and that upon transformation there is a higher affinity mechanism in place for this mechanism. This interpretation makes sense given that the pentose phosphate pathway produces both ribose for nucleotide biosynthesis, as well as two molar equivalents of NAD(P)H that could mitigate oxidative stress and aid in glutathione recycling.
Six compounds differentiated transformed ovarian tissue independent of whether the cancer was localized or metastatic (PC1>0; PC2 = 0). These compounds included several quaternary amines (betaine, carnitine, and erogthionine), the TCA cycle intermediates malate and fumarate and N-acetylglycine. Increased tissue quaternary amine concentrations are typically due to tissue demand for either choline or carnitine as the transporters for quaternary amines are selective but not specific [48]. In all, thirteen compounds containing quaternary amines were found to have increased tissue abundance in one or both of the ovarian cancer groups compared to the non-transformed ovarian tissue and two choline-containing lysolipids had significantly reduced abundance in the transformed ovarian tissue.
Cancer cells have altered carbohydrate metabolism
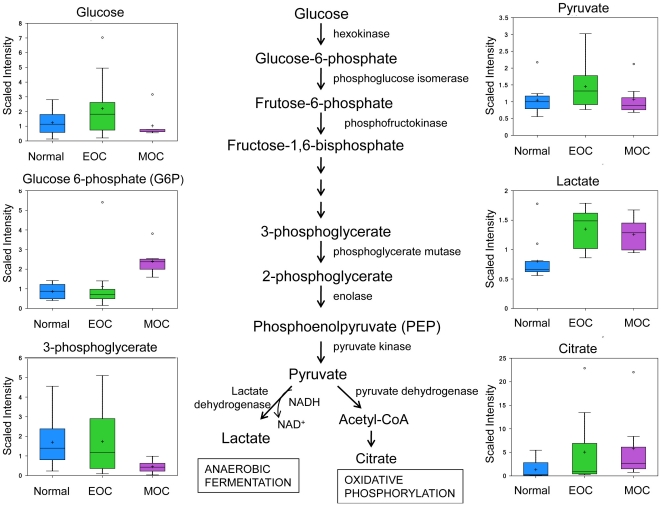
One of the signature hallmarks of cancer is an altered glucose metabolism. In 1929, Otto Warburg first proposed that cancer cells utilized glucose differently than normal cells, preferring glucose for anaeroblic glycolysis instead of oxidative phosphorylation for the generation of ATP [13], resulting in increased lactate production and a lower pH than normal tissue, which in turn impairs DNA repair mechanisms [49]. Our results showed an increase in lactate in both EOC and MOC with a fold change of 1.46 (p<0.001) and 1.37 (p = 0.0076), respectively, when compared to normal ovarian tissue. Only MOC showed an increase in glucose-6-phosphate (2.91 fold, p = 0.0029). There were no significant changes in glucose, pyruvate, acetylphosphate, phosphate, pyrophosphate, or citrate between groups (Fig. 3). The increase in lactate coupled with no significant changes in citrate levels, indicate that glycolysis was not impeded but rather oxidative phosphorylation. Interestingly, another aspect of Warburg metabolism, hexose phosphate abundance, was only elevated in the MOC samples (data not shown).
Figure 3. The glycolytic pathway converting glucose to pyruvate for anaerobic fermation to produce lactate or aerobic respiration of the citric acid for oxidative phosphorylation.
Glucose, glucose-6-phosphate, fructose-6-phosphate, 3-phosphoglycerate, phosphoenolpyruvate (PEP), pyruvate, lactate, and citrate analyzed by GC/MS. Box legend: + inside box represents mean value, bar inside box represents median value, upper bar represents maximum of distribution, lower bar represents minimum of distribution, circle represents extreme data points.
The other carbohydrate with a significant increase in abundance in transformed ovarian tissue was fucose (2.75 fold, p<0.001 in EOC; 1.81 fold, p = 0.0103 in MOC). This finding may be due to a loss of function of specific ovarian glycosylation pathways since it has been demonstrated that normal ovarian tissue expresses a specific protein fucosylation pathway that results in the fucose moiety being directly coupled to the protein through Ser/Thr [50]. This ovary-specific glycosylation pathway is also unique in that the fucose is not the terminal sugar, but an internal sugar in the O-linked oligosaccharide.
Increased fatty acid oxidation in EOC and MOC
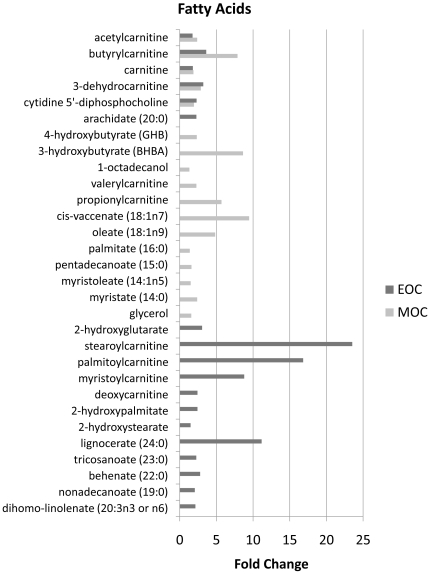
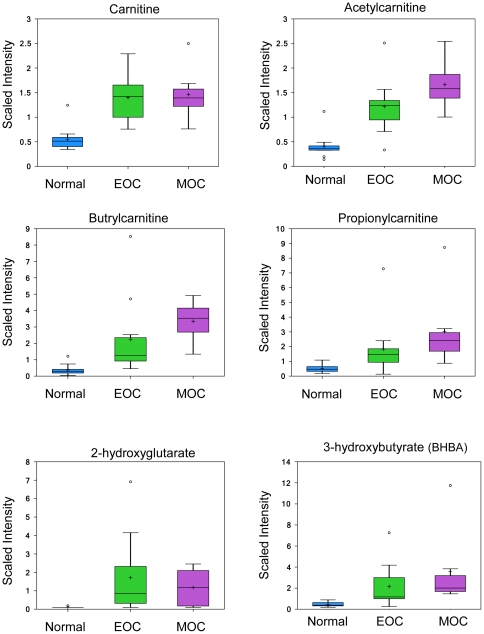
As an alternative to oxidative phosphorylation for ATP production, EOC and MOC prefer to utilize fatty acids as indicated by an increase in several fatty acids (Fig. 4) involved in fatty acid and carnitine metabolism—particularly carnitine (1.79 fold in EOC, p<0.001; 1.88 fold in MOC, p<0.001), acetylcarnitine (1.75 fold in EOC, p<0.001; 2.39 fold in MOC, p<0.001), butyrylcarnitine (3.62 fold, p = 0.0094 in EOC; 7.88 fold, p<0.001 in MOC), and propionylcarnitine increased 5.7 fold (p = 0.0047) in MOC only (Fig. 5). Carnitine has been recognized as a transport protein that delivers fatty acids into the mitochondria for β-oxidation. Endogenous acetylcarnitine has been used as an indicator of mitochondrial health through the balance of acetyl-CoA∶CoA by transferring the acetyl group to carnitine to form acetylcarnitine and thus provide acetyl groups for the synthesis of sterols, fatty acids, and ketone bodies [51].
Figure 4. Upregulated fatty acids in EOC and/or MOC compared to normal ovarian tissue.
Figure 5. Carnitine and fatty acid metabolites.
Carnitine, acetylcarnitine, butrylcarnitine, and propionylcarnitine analyzed by LC/MS positive ion spray. 2-hydroxyglutarate and 3-hydroxybutyrate analyzed by GC/MS. Box legend: + inside box represents mean value, bar inside box represents median value, upper bar represents maximum of distribution, lower bar represents minimum of distribution, circle represents extreme data points.
The ketone body 3-hydroxybutyrate (BHBA) was upregulated 8.63 fold (p = 0.0056) in MOC compared to normal (Fig. 5). In addition, the cytosolic pool of acetyl-CoA is essential of de novo lipogenesis [52]. The excess production of cytoplasmic acetyl-coA compared to the mitochondrial capacity for its incorporation into the TCA cycle is demonstrated by the increased abundance of a panel of N-acetyl amino acids in the cancerous tissues—including N-acetylglutamate, N-acetylglycine, N-acetylthreonine, the neuroactive amino acids N-acetylaspartate (NAA) and N-acetyl-aspartylglutamate (NAAG), the polyamine degradation product N-acetylputrescine, and even N-acetylglucosamine-6-phosphate.
Interestingly, we also found that the recently described oncometabolite, 2-hydroxyglutarate had increased abundance in EOC (3.06 fold, p = 0.0114 in EOC; Fig. 5) [53].
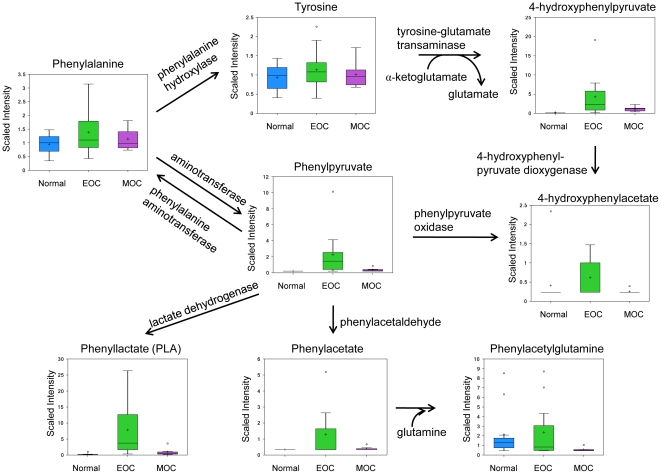
Enhanced phenylalanine catabolism
Phenylalanine catabolism also results in the production of ketones, namely phenylpyruvate and 4-hydroxypyruvate. Major metabolites of phenylalanine catabolism were significantly increased in EOC compared to normal (Fig. 6). Phenylpyruvate increased 4.21 fold (p = 0.0098) in EOC only compared to normal but decreased −3.68 fold (p = 0.036) in MOC compared to EOC. Phenyllactate (PLA) increased 195.45 fold (p<0.0023) in EOC, a finding which is typically attributed to insufficient activity of phenylalanine hydroxylase [54]. Phenylacetate increased 1.93 fold (p = 0.0203) in EOC compared to normal only, whereas 4-hydroxyphenylpyruvate was increased 17.82 fold (p = 0.0069) in EOC compared to normal. Phenylalanine, tyrosine, phenylacetylglutamine, and 4-hydroxyphenylacetate were not significantly changed. Phenylalanine and its major metabolites—phenylpyruvate, PLA, and phenylacetate—induce oxidative stress in the hippocampus and cerebral cortrex via generation of reactive oxygen species, which was mitigated by α-tocopherol [55]. Phenylacetate has also been shown to have an inhibitory growth effect in ovarian cancer cell lines [56], whereas PLA can promote growth [57]. Therefore, it seems reasonable that ovarian cancer would favor the production of PLA over other alternative metabolites, consistent with our results. These metabolites are generated by transamination of phenylalanine and subsequent oxidation of the phenylpyruvate.
Figure 6. Phenylalanine metabolic pathway.
Phenylalanine and tyrosine analyzed by LC/MS pos.; phenylpyruvate, phenyllacetate, phenylacetylglutamine, phenylactate, and 4-hydroxyphenylpyruvate via LC/MS negative ion spray; 4-hydroxyphenylacetate via GC/MS. Box legend: + inside box represents mean value, bar inside box represents median value, upper bar represents maximum of distribution, lower bar represents minimum of distribution, circle represents extreme data points.
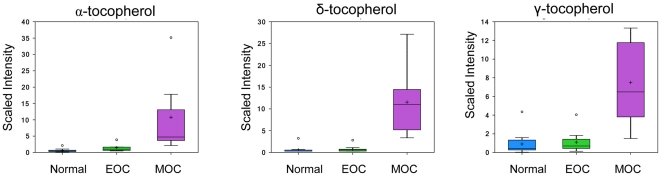
Increased levels of tocopherols in MOC
There are four main isoforms of tocopherols: α, β, δ, and γ, with α-tocopherol being the most biologically active form, accounting for approximately 90% of the Vitamin E found in animal tissues, where it serves as an antioxidant to quench free radicals and terminate lipid peroxidation [58], [59]. Hence it serves as an effective defense against radiation, which generates free radicals from water or biomolecules [60]. Metabolomic analysis showed a significant increase in α-, δ-, and γ-tocopherol levels in MOC. α-tocopherol increased 1,160.41 fold (p<0.001) compared to normal and 627.67 fold (p = 0.0023) compared to EOC. δ-tocopherol increased 1,775.51 fold (p<0.001) compared to normal and 1,950.08 fold (p<0.001) compared to EOC. γ-tocopherol increased 95.74 fold (p<0.001) compared to normal and 83.79 fold (p<0.001) compared to EOC (Fig. 7). As tocopherols are fat soluble, they are carried in the blood packaged in lipoproteins, mainly LDL and HDL, whereupon they are be transported to tissue and undergo uptake by the same mechanism by which lipids are delivered [58]. Uptake in the normal ovary is regulated by lipoprotein receptors [59]. Tocopherols have also been implicated in suppression of the immune system responsiveness by decreasing the reactive oxygen species and/or altering arachidonic acid metabolites [61]. Therefore, it seems reasonable that metastatic cancer would accumulate them to suppress the immune response and provide a defense against radiation treatment for cancer.
Figure 7. Three of the main tocopherol levels in normal, EOC, and MOC.
Tocopherols analyzed via GC/MS. Box legend: + inside box represents mean value, bar inside box represents median value, upper bar represents maximum of distribution, lower bar represents minimum of distribution, circle represents extreme data points.
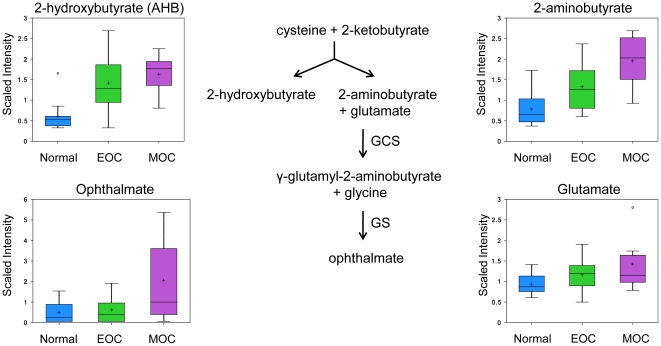
Enhanced oxidative stress response
Ophthalmate is an analog of the reduced form of glutathione (GSH) with the thiol group of GSH replaced with a methyl group. Ophthalmate can be synthesized from 2-aminobutyrate and glutamate by the enzyme γ-glutamyl cysteine synthetase (GCS) to form γ-glutamyl-2-aminobutyrate [62], which can be catalyzed by glutathione synthetase (GS) to form ophthalmate [63] (Fig. 7). Ophthalmate has been indicated as a biomarker of oxidative stress as insufficient levels of GSH results in ophthalmate synthesis through activation of GCS. GSH is one of the most abundant intracellular antioxidants that protects the mitochondria from endogenous oxygen radicals [64] and also keeps enzymes and other cellular compounds in a reduced state [65], making it one of the most important cellular antioxidants, as its depletion leads to cell death. GSH has also been implicated in chemotherapy resistance through the activation of multi-drug resistant transporter 1 (MDR-1) [66], [67].
The oxidized form of glutathione (GSSG), GSH, γ-glutamyl-2-aminobutyrate, and ophthalmate can be detected in the serum in mice [67] so all have the potential for biomarkers. However, to date 2-aminobutyrate and ophthalmate have not been investigated in ovarian cancer. Here we report of the first time, significant increases of 2-aminobutyrate in both EOC (1.46 fold, p = 0.0316) and MOC (2.25 fold, p<0.001), suggesting an enhanced oxidative response. Ophthalmate increased 2.94 fold in MOC (p = 0.0128; Fig. 8). However, there were no significant changes in glutathione (reduced and oxidized states) or glutamate.
Figure 8. Ophthalmate biosynthesis pathway.
2-hydroxybutyrate and 2-aminobutyrate analyzed via GC/MS. Box plot of glutamate and ophthalmate analyzed by LC/MS positive ion spray. Box legend: + inside box represents mean value, bar inside box represents median value, upper bar represents maximum of distribution, lower bar represents minimum of distribution, circle represents extreme data points.
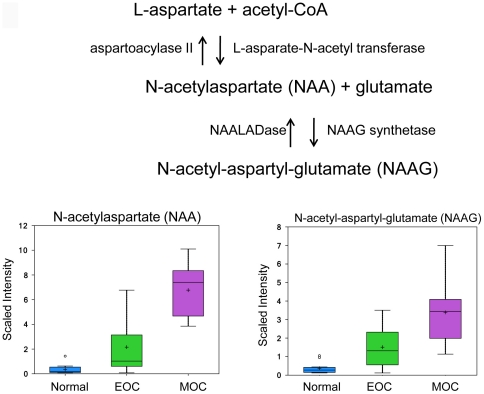
Increased production of NAA and NAAG
N-acetylasparate (NAA) is a free amino acid found in the brain at very high concentrations that functions as an osmolyte in fluid balance to protect neurons against osmotic stress [68], [69]. It is also thought to serve as a source of acetate for lipid and myelin synthesis [70] and contribute glutamate for energy production in the neuronal mitochondria through a series of reactions [71], [72]. NAA is synthesized from L-asparate and acetyl-CoA by the enzyme L-asparate-N-acetyl transferase and hydrolyzed by the enzyme aspartoacylase II. NAA serves as a precursor for N-ascetyl-aspartyl-glutamate (NAAG) using the enzyme NAA synthetase (Fig. 8). Due to its packaging with glutamate, the physiological role for NAAG has been difficult to identify, however, it fulfills the criteria of a neurotransmitter as it is packaged into synaptic vesicles and released in a Ca2+-dependent manner from nerve terminals [73]. It has been proposed to serve as a shuttle for glutamate to activate the glutamate receptor mGluR3 due to the cytotoxic nature of glutamate [74], [75]. More recently, it has been used to diagnose brain disorders. Moreover, NAA concentrations have been found in a patient with ovarian mucinous cystadenoma [76] and in ovarian cyst fluid of serous ovarian tumors [77], although a physiological role in the ovary has not been determined. Here we report that NAA and NAAG, two free amino acids, were detected in the normal ovary with significantly increased levels in EOC showing a fold change of 3.50 (p = 0.0301) and 2.19 (p = 0.0352), respectively, with further increases in MOC with a fold change of 85.60 fold (p<0.001) and 8.09 (p<0.001), respectively (Fig. 9).
Figure 9. NAA and NAAG biosynthesis pathway.
NAA and NAAG analyzed via LC/MS negative and LC/MS positive ion spray, respectively. Box legend: + inside box represents mean value, bar inside box represents median value, upper bar represents maximum of distribution, lower bar represents minimum of distribution, circle represents extreme data points.
NAAG is broken down by N-acetylated alpha-linked acidic dipeptidase (NAALADase), a NAAG-specific catabolic enzyme [78]. NAALADase is composed of three family members: NAALADase I, NAALADase L, and NAALADase II. NAALADase II has been found to be highly expressed in the ovary when identified by Northern blot and reverse-transcription PCR [79].
Discussion
Metabolomics has been widely used to identify biomarkers for various disease states—including diabetes [80] and atherosclerosis [81]—using blood, urine, cells, and tissue. Of import, metabolomics has provided a comprehensive technique to identify biomarkers for cancer—including breast [82], [83], [84], [85], ovarian [32], [33], [86], prostate [87], [88], colorectal [89], [90], [91], and gastric cancer [92]. Identification of biomarkers is of the utmost importance as it can help diagnose diseases at an earlier stage, leading to a better prognostic outcome, when used in conjunction with existing methods, such as transvaginal ultrasound in the case of ovarian cancer.
In this study, the comprehensive metabolic profile of normal ovaries, EOC, and MOC were compared using GC/MS or LC/MS/MS. Significant changes in energy utilization were detected as well as an enhanced oxidative stress response. Similar to the study by Denkert et al. [32], we have found increased levels of amino acids in EOC and MOC vs. normal. However, contrary to their study, the lysolipid levels were either not significantly changed or downregulated in EOC and/or MOC. Woo et al. [33] found urinary biomarkers for ovarian cancer using GC/MS related to DNA oxidative damage and DNA methylation: 1-methyladenosine, 3-methyluridine, and 4-androstene-3,17-dione. We found no significant changes in N1-methyladenosine or 4-androstene-beta,17beta diol disulfate based on tissue samples, perhaps due to the high amount of GSSG found in our samples coupled with a reduced amount of GSH, protecting the tissue from oxidative damage or from a more efficient excretion of the metabolites in these patients. We also found increased tocopherols in MOC, which are best known for their antioxidant properties. More recently, urine metabolite profiling in breast and ovarian cancer showed that metabolite concentrations correlated with both cancers compared to healthy individuals [34]. Consistent with the aforementioned study, Odunsi et al. [86] were able to separate sera from healthy individuals from that of EOC patients using (1)H-NMR spectroscopy. Applying unsupervised PCA analysis as well as supervised Soft Independent Modeling of Class Analogy (SIMCA) allowed for pattern recognition. They were able to correctly identify increases in alanine, valine, glucose, and 3-hydroxybutyrate (a ketone body) in EOC sera. In our samples, we showed an increase of 3-hydroxybutyrate in MOC with a borderline significant increase in EOC. However, the other metabolites were not significantly altered.
Additional metabolic analysis in colorectal tissue [89], [91] and in gastric cancer metastases [92] samples showed increases in glycolysis shown by the increase in lactate and fatty acid metabolism, and decreases in TCA intermediates. Lactate and phenylalanine have yielded satisfactory sensitivity and accuracy in differentiating oral squamous cell carcinoma from oral leukoplakia [93]; however, these metabolites could be generic across multiple cancers.
One of the proposed biomarkers for tumor progression and invasiveness in prostate cancer is sarcosine, also known as N-methylglycine [94], although there is some debate whether sarcosine can be detected in the serum [95] or urine [96]. Sarcosine can be synthesized from glycine by the enzyme glycine N-methylransferase or from dimethylglycine by the enzyme dimethylglycine dehydrogenase, which in turn is demethylated from betaine. In our samples, we found a significant increase in betaine in both EOC and MOC compared to normal. However, dimethylglycine and sarcosine were not significantly different, possibly due to a high biological variability between samples, which could be clarified with an increased sample size.
Based on the data we have collected, we have identified possible candidates for biomarker analysis for both preliminary cancer diagnosis and metastatic disease progression. The dramatic increase of tocopherols in MOC makes these molecules attractive candidates for aggressiveness and/or progression. Further investigation is needed to determine the potential utility of these initial findings.
Materials and Methods
Histopathology
For metabolic profiling, 30 patient tissues were obtained from University of Alabama, Birmingham, Comprehensive Cancer Center and stored at −80°C. Type and stage was determined by evaluation by a pathologist. Tissues included 12 normal ovarian samples (mean age 48.6±7.6 years), 11 primary ovarian adenocarcinomas ranging from Stage I through Stage IIIC (mean age 56.5±9.8 years), and 7 metastatic tumors in the omentum resulting from initial ovarian adenocarcinomas ranging from Stage IIIC through Stage IV (mean age 62±8.2 years). Patients with previous diseases and treatments were excluded from the study.
Metabolic Profiling
100 µg of frozen biopsy tissue was submitted to Metabolon, Inc. (Durham, NC) for sample extraction and analysis. In brief, Metabolon performed cold methanol extraction of mechanically disaggregated tissue samples and these extracts were split into three aliquots. The reproducibility of the extraction protocol was assessed by the recovery of xenobiotic compounds spiked into every tissue sample prior to extraction. These aliquots were processed and characterized by one of the three analytical methods previously described: UHPLC-ESI-MS/MS in the positive ion mode, UHPLC-ESI-MS/MS in the negative ion mode and sialylation followed by GC-EI-MS. Chromatographic timelines were standardized using a series of xenobiotics that elute at specified intervals throughout each chromatographic run. The technical variability of each analytical platform was assessed by repeated characterization of a pooled standard that contained an aliquot of each sample within the study. The platform for sample analysis has been described in detail [97], [98]. However, the combination of the Metabolon platform with ovarian tissue is novel.
Supporting Information
Supervised PCA separated normal ovarian tissue from ovarian cancer (PC1; blue→green and purple) and localized tumor from metastasis (PC2; green→purple).
(TIF)
List of metabolites identified through mass spectrometry.
(XLS)
ANOVA analysis of metabolites grouped by class of compound.
(XLS)
Ingenuity pathway analysis for the top 15 canonical pathways for EOC.
(XLS)
Ingenuity pathway analysis for the top 15 canonical pathways for MOC.
(XLS)
Acknowledgments
The authors would like to thank Kathy McFarland for her excellent secretarial assistance.
Footnotes
Competing Interests: JM is an employee of Metabolon, Inc. and was responsible for preliminary analysis of the data and final editing of the manuscript. However, as part of the University of Louisville's contract with Metabolon, Inc., he does not have any financial benefit or retention of invention or ownership rights, patentable or not. This does not affect adherence to PLoS ONE policies on sharing data and material.
Funding: The funds used to perform this work were supported by a NIH/NCI CA124630 research Grant to SSK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Metabolon was paid for the service peformed in metabolites profiling according to signed contract.
References
- 1.National Cancer Institute. Stat Fact Sheet. [ http://seer.cancer.gov/statfacts/html/ovary.html] Accessed 2011 Jan 18.
- 2.Nossov V, Amneus M, Su F, Lang J, Janco J, et al. The early detection of ovarian cancer: from traditional methods to proteomics. Can we really do better than serum CA-125? Am J Obstret Gynecol. 2008;199:215–223. doi: 10.1016/j.ajog.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Neesham D. Ovarian cancer screening. Austr Fam Physician. 2007;36:126–128. [PubMed] [Google Scholar]
- 4.Cesario S. Advances in the early detection of ovarian cancer: How to hear the whispers early. Nurs Womens Health. 14:222–234. doi: 10.1111/j.1751-486X.2010.01543.x. [DOI] [PubMed] [Google Scholar]
- 5.Bhosale P, Peungjesada S, Wei W, Levenback CF, Schmeler K, et al. Clinical utility of positron emission tomography/computed tomography in the evaluation of suspected recurrent ovarian cancer in the setting of normal CA-125 levels. Int J Gynecol Cancer. 20:936–944. doi: 10.1111/IGC.0b013e3181e82a7f. [DOI] [PubMed] [Google Scholar]
- 6.Lin B, White JT, Wu J, Lele S, Old LJ, et al. Deep depletion of abundant serum proteins reveals low-abundant proteins as potential biomarkers for human ovarian cancer. Proteomics Clin Appl. 2009;3:853–861. doi: 10.1002/prca.200800141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhen H, Yang S, Wu H, Wang S, Lv J, et al. LyGDI is a promising biomarker for ovarian cancer. Int J Gynecol Cancer. 20:316–322. doi: 10.1111/IGC.0b013e3181d0b02d. [DOI] [PubMed] [Google Scholar]
- 8.Kothandaraman N, Bajic VB, Brendan PN, Huak CY, Keow PB, et al. E2F5 status significantly improves malignancy diagnosis of epithelial ovarian cancer. BMC Cancer. 10:64. doi: 10.1186/1471-2407-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellstrom I, Hellstrom K. SMRP and HE4 as biomarkers for ovarian carcinoma when used alone and in combination with CA125 and/or each other. Adv Exp Med Biol. 2008;622:15–21. doi: 10.1007/978-0-387-68969-2_2. [DOI] [PubMed] [Google Scholar]
- 10.Lowe K, Shah C, Wallace E, Anderson G, Paley P, et al. Effects of personal characteristics of serum CA125, mesothelin, and HE4 levels in healthy postmenopausal women at high-risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:5480–5487. doi: 10.1158/1055-9965.EPI-08-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nolen B, Velikokhatnaya L, Marrangoni A, De Geest K, Lomakin A, et al. Serum biomarker panels for the discrimination of benign from malignant cases in patients with an adnexal mass. Gynecol Oncol. 117:440–445. doi: 10.1016/j.ygyno.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zagouri F, Dimopoulos MA, Bournakis E, Papadimitriou CA. Molecular markers in epithelial ovarian cancer: their role in prognosis and therapy. Eur J Gynaecol Oncol. 31:268–277. [PubMed] [Google Scholar]
- 13.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 14.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 330:1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 15.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 16.Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 17.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Kondoh H, Lleonart ME, Gil J, Wang J, Degan P, et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65:177–185. [PubMed] [Google Scholar]
- 19.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 20.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, et al. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi H, Pino MS, Zeng M, Shirasawa S, Chung DC. Oncogenic KRAS and BRAF differentially regulate hypoxia-inducible factor-1alpha and -2alpha in colon cancer. Cancer Res. 2009;69:8499–8506. doi: 10.1158/0008-5472.CAN-09-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimpi S, Nilsson JA. Metabolic enzymes regulated by the Myc oncogene are possible targets for chemotherapy or chemoprevention. Biochem Soc Trans. 2007;35:305–310. doi: 10.1042/BST0350305. [DOI] [PubMed] [Google Scholar]
- 24.Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005;24:7435–7442. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- 25.Rossignol R, Gilkerson R, Aggeler R, Yamagata K, Remington SJ, et al. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res. 2004;64:985–993. doi: 10.1158/0008-5472.can-03-1101. [DOI] [PubMed] [Google Scholar]
- 26.Tokunaga E, Oki E, Egashira A, Sadanaga N, Morita M, et al. Deregulation of the Akt pathway in human cancer. Curr Cancer Drug Targets. 2008;8:27–36. doi: 10.2174/156800908783497140. [DOI] [PubMed] [Google Scholar]
- 27.Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Mazurek S, Eigenbrodt E. The tumor metabolome. Anticancer Res. 2003;23:1149–1154. [PubMed] [Google Scholar]
- 29.Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spratlin JL, Serkova NJ, Eckhardt SG. Clinical applications of metabolomics in oncology: a review. Clin Cancer Res. 2009;15:431–440. doi: 10.1158/1078-0432.CCR-08-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffin JL, Shockcor JP. Metabolic profiles of cancer cells. Nat Rev Cancer. 2004;4:551–561. doi: 10.1038/nrc1390. [DOI] [PubMed] [Google Scholar]
- 32.Denkert C, Budczies J, Kind T, Weichert W, Tablack P, et al. Mass spectrometry-based metabolic profiling reveals different metabolite patterns in invasive ovarian carcinomas and ovarian borderline tumors. Cancer Res. 2006;66:10795–10804. doi: 10.1158/0008-5472.CAN-06-0755. [DOI] [PubMed] [Google Scholar]
- 33.Woo HM, Kim KM, Choi MH, Jung BH, Lee J, et al. Mass spectrometry based metabolomic approaches in urinary biomarker study of women's cancers. Clin Chim Acta. 2009;400:63–69. doi: 10.1016/j.cca.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Slupsky CM, Steed H, Wells TH, Dabbs K, Schepansky A, et al. Urine metabolite analysis offers potential early diagnosis of ovarian and breast cancers. Clin Cancer Res. 16:5835–5841. doi: 10.1158/1078-0432.CCR-10-1434. [DOI] [PubMed] [Google Scholar]
- 35.Ham EA, Schayer RW. Isolation of 1-methylimidazole-4-acetic acid, a metabolic product of histamine, from human urine. Biochim Biophys Acta. 1963;71:208–209. doi: 10.1016/0006-3002(63)91008-4. [DOI] [PubMed] [Google Scholar]
- 36.Szelag A, Merwid-Lad A, Trocha M. [Histamine receptors in the female reproductive system. Part I. Role of the mast cells and histamine in female reproductive system]. Ginekol Pol. 2002;73:627–635. [PubMed] [Google Scholar]
- 37.Solis JM, Herranz AS, Herreras O, Lerma J, Martin del Rio R. Does taurine act as an osmoregulatory substance in the rat brain? Neurosci Lett. 1988;91:53–58. doi: 10.1016/0304-3940(88)90248-0. [DOI] [PubMed] [Google Scholar]
- 38.Huxtable RJ. Taurine in the central nervous system and the mammalian actions of taurine. Prog Neurobiol. 1989;32:471–533. doi: 10.1016/0301-0082(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 39.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 40.Martin del Rio R, Herranz AS, Herreras O, Menendez N, Solis JM. Possible osmoregulatory role of taurine in the cellular swelling evoked by weak organic acids in the rat hippocampus. Prog Clin Biol Res. 1990;351:357–368. [PubMed] [Google Scholar]
- 41.Galarreta M, Bustamante J, del Rio RM, Solis JM. A new neuromodulatory action of taurine: long-lasting increase of synaptic potentials. Adv Exp Med Biol. 1996;403:463–471. doi: 10.1007/978-1-4899-0182-8_50. [DOI] [PubMed] [Google Scholar]
- 42.Casslen BG. Free amino acids in human uterine fluid. Possible role of high taurine concentration. J Reprod Med. 1987;32:181–184. [PubMed] [Google Scholar]
- 43.Guerin P, Guillaud J, Menezo Y. Hypotaurine in spermatozoa and genital secretions and its production by oviduct epithelial cells in vitro. Hum Reprod. 1995;10:866–872. doi: 10.1093/oxfordjournals.humrep.a136052. [DOI] [PubMed] [Google Scholar]
- 44.Jhiang SM, Fithian L, Smanik P, McGill J, Tong Q, et al. Cloning of the human taurine transporter and characterization of taurine uptake in thyroid cells. FEBS Lett. 1993;318:139–144. doi: 10.1016/0014-5793(93)80008-i. [DOI] [PubMed] [Google Scholar]
- 45.Orensanz LM, Fernandez I, Martin del Rio R, Storm-Mathisen J. Gamma-aminobutyric acid in the rat oviduct. Adv Biochem Psychopharmacol. 1986;42:265–274. [PubMed] [Google Scholar]
- 46.Phoenix J, Wray S. Changes in human and rat uterine phosphoethanolamine and taurine with pregnancy and parturition. Exp Physiol. 1994;79:601–604. doi: 10.1113/expphysiol.1994.sp003794. [DOI] [PubMed] [Google Scholar]
- 47.Turner O, Phoenix J, Wray S. Developmental and gestational changes of phosphoethanolamine and taurine in rat brain, striated and smooth muscle. Exp Physiol. 1994;79:681–689. doi: 10.1113/expphysiol.1994.sp003800. [DOI] [PubMed] [Google Scholar]
- 48.Grundemann D, Harlfinger S, Golz S, Geerts A, Lazar A, et al. Discovery of the ergothioneine transporter. Proc Natl Acad Sci U S A. 2005;102:5256–5261. doi: 10.1073/pnas.0408624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan J, Narayanan L, Rockwell S, Glazer PM. Diminished DNA repair and elevated mutagenesis in mammalian cells exposed to hypoxia and low pH. Cancer Res. 2000;60:4372–4376. [PubMed] [Google Scholar]
- 50.Moloney DJ, Haltiwanger RS. The O-linked fucose glycosylation pathway: identification and characterization of a uridine diphosphoglucose: fucose-beta1,3-glucosyltransferase activity from Chinese hamster ovary cells. Glycobiology. 1999;9:679–687. doi: 10.1093/glycob/9.7.679. [DOI] [PubMed] [Google Scholar]
- 51.Rosca MG, Lemieux H, Hoppel CL. Mitochondria in the elderly: Is acetylcarnitine a rejuvenator? Adv Drug Deliv Rev. 2009;61:1332–1342. doi: 10.1016/j.addr.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong DA, Bassilian S, Lim S, Paul Lee WN. Coordination of peroxisomal beta-oxidation and fatty acid elongation in HepG2 cells. J Biol Chem. 2004;279:41302–41309. doi: 10.1074/jbc.M406766200. [DOI] [PubMed] [Google Scholar]
- 53.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iijima S, Ishii A, Miyakoshi T, Odaira T, Musha M. Studies on the experimental phenylketonuria in rats. Tohoku J Exp Med. 1975;117:167–178. doi: 10.1620/tjem.117.167. [DOI] [PubMed] [Google Scholar]
- 55.Fernandes CG, Leipnitz G, Seminotti B, Amaral AU, Zanatta A, et al. Experimental evidence that phenylalanine provokes oxidative stress in hippocampus and cerebral cortex of developing rats. Cell Mol Neurobiol. 30:317–326. doi: 10.1007/s10571-009-9455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Melichar B, Ferrandina G, Verschraegen CF, Loercher A, Abbruzzese JL, et al. Growth inhibitory effects of aromatic fatty acids on ovarian tumor cell lines. Clin Cancer Res. 1998;4:3069–3076. [PubMed] [Google Scholar]
- 57.Collier VU, Butler DO, Mitch WE. Metabolic effects of L-phenyllactate in perfused kidney, liver, and muscle. Am J Physiol. 1980;238:E450–457. doi: 10.1152/ajpendo.1980.238.5.E450. [DOI] [PubMed] [Google Scholar]
- 58.Debier C, Larondelle Y. Vitamins A and E: metabolism, roles and transfer to offspring. Br J Nutr. 2005;93:153–174. doi: 10.1079/bjn20041308. [DOI] [PubMed] [Google Scholar]
- 59.Aten RF, Kolodecik TR, Behrman HR. Ovarian vitamin E accumulation: evidence for a role of lipoproteins. Endocrinology. 1994;135:533–539. doi: 10.1210/endo.135.2.8033800. [DOI] [PubMed] [Google Scholar]
- 60.Odin AP. Vitamins as antimutagens: advantages and some possible mechanisms of antimutagenic action. Mutat Res. 1997;386:39–67. doi: 10.1016/s1383-5742(96)00044-0. [DOI] [PubMed] [Google Scholar]
- 61.Meydani SN, Tengerdy RP. Vitamin E in Health and Disease. In: Packer L, Fuchs J, editors. Vitamin E and immune response. New York: Marcel Dekker; 1992. pp. 549–563. [Google Scholar]
- 62.Huang CS, Moore WR, Meister A. On the active site thiol of gamma-glutamylcysteine synthetase: relationships to catalysis, inhibition, and regulation. Proc Natl Acad Sci U S A. 1988;85:2464–2468. doi: 10.1073/pnas.85.8.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oppenheimer L, Wellner VP, Griffith OW, Meister A. Glutathione synthetase. Purification from rat kidney and mapping of the substrate binding sites. J Biol Chem. 1979;254:5184–5190. [PubMed] [Google Scholar]
- 64.Yang CS, Chen WY, Tsai PJ, Cheng FC, Kuo JS. Effect of diethylmaleate on liver extracellular glutathione levels before and after global liver ischemia in anesthetized rats. Biochem Pharmacol. 1997;53:357–361. doi: 10.1016/s0006-2952(96)00729-0. [DOI] [PubMed] [Google Scholar]
- 65.Meister A. Glutathione metabolism. Methods Enzymol. 1995;251:3–7. doi: 10.1016/0076-6879(95)51106-7. [DOI] [PubMed] [Google Scholar]
- 66.Shim GS, Manandhar S, Shin DH, Kim TH, Kwak MK. Acquisition of doxorubicin resistance in ovarian carcinoma cells accompanies activation of the NRF2 pathway. Free Radic Biol Med. 2009;47:1619–1631. doi: 10.1016/j.freeradbiomed.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 67.Soga T, Baran R, Suematsu M, Ueno Y, Ikeda S, et al. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J Biol Chem. 2006;281:16768–16776. doi: 10.1074/jbc.M601876200. [DOI] [PubMed] [Google Scholar]
- 68.Taylor DL, Davies SE, Obrenovitch TP, Doheny MH, Patsalos PN, et al. Investigation into the role of N-acetylaspartate in cerebral osmoregulation. J Neurochem. 1995;65:275–281. doi: 10.1046/j.1471-4159.1995.65010275.x. [DOI] [PubMed] [Google Scholar]
- 69.Sager TN, Fink-Jensen A, Hansen AJ. Transient elevation of interstitial N-acetylaspartate in reversible global brain ischemia. J Neurochem. 1997;68:675–682. doi: 10.1046/j.1471-4159.1997.68020675.x. [DOI] [PubMed] [Google Scholar]
- 70.Namboodiri AM, Peethambaran A, Mathew R, Sambhu PA, Hershfield J, et al. Canavan disease and the role of N-acetylaspartate in myelin synthesis. Mol Cell Endocrinol. 2006;252:216–223. doi: 10.1016/j.mce.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 71.Clark JF, Doepke A, Filosa JA, Wardle RL, Lu A, et al. N-acetylaspartate as a reservoir for glutamate. Med Hypotheses. 2006;67:506–512. doi: 10.1016/j.mehy.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 72.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coyle JT. The nagging question of the function of N-acetylaspartylglutamate. Neurobiol Dis. 1997;4:231–238. doi: 10.1006/nbdi.1997.0153. [DOI] [PubMed] [Google Scholar]
- 74.Baslow MH. A novel key-lock mechanism for inactivating amino acid neurotransmitters during transit across extracellular space. Amino Acids. 38:51–55. doi: 10.1007/s00726-009-0232-0. [DOI] [PubMed] [Google Scholar]
- 75.Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, et al. N-acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69:174–181. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]
- 76.Hascalik S, Celik O, Sarac K, Alkan A, Mizrak B. Clinical significance of N-acetyl-L-aspartate resonance in ovarian mucinous cystadenoma. Int J Gynecol Cancer. 2006;16:423–426. doi: 10.1111/j.1525-1438.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 77.Kolwijck E, Wevers RA, Engelke UF, Woudenberg J, Bulten J, et al. Ovarian cyst fluid of serous ovarian tumors contains large quantities of the brain amino acid N-acetylaspartate. PLoS ONE. 5:e10293. doi: 10.1371/journal.pone.0010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robinson MB, Blakely RD, Coyle JT. Quisqualate selectively inhibits a brain peptidase which cleaves N-acetyl-L-aspartyl-L-glutamate in vitro. Eur J Pharmacol. 1986;130:345–347. doi: 10.1016/0014-2999(86)90291-8. [DOI] [PubMed] [Google Scholar]
- 79.Pangalos MN, Neefs JM, Somers M, Verhasselt P, Bekkers M, et al. Isolation and expression of novel human glutamate carboxypeptidases with N-acetylated alpha-linked acidic dipeptidase and dipeptidyl peptidase IV activity. J Biol Chem. 1999;274:8470–8483. doi: 10.1074/jbc.274.13.8470. [DOI] [PubMed] [Google Scholar]
- 80.Lanza IR, Zhang S, Ward LE, Karakelides H, Raftery D, et al. Quantitative metabolomics by H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PLoS ONE. 5:e10538. doi: 10.1371/journal.pone.0010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen X, Liu L, Palacios G, Gao J, Zhang N, et al. Plasma metabolomics reveals biomarkers of the atherosclerosis. J Sep Sci. [DOI] [PubMed]
- 82.Frickenschmidt A, Frohlich H, Bullinger D, Zell A, Laufer S, et al. Metabonomics in cancer diagnosis: mass spectrometry-based profiling of urinary nucleosides from breast cancer patients. Biomarkers. 2008;13:435–449. doi: 10.1080/13547500802012858. [DOI] [PubMed] [Google Scholar]
- 83.Bathen TF, Jensen LR, Sitter B, Fjosne HE, Halgunset J, et al. MR-determined metabolic phenotype of breast cancer in prediction of lymphatic spread, grade, and hormone status. Breast Cancer Res Treat. 2007;104:181–189. doi: 10.1007/s10549-006-9400-z. [DOI] [PubMed] [Google Scholar]
- 84.Sitter B, Lundgren S, Bathen TF, Halgunset J, Fjosne HE, et al. Comparison of HR MAS MR spectroscopic profiles of breast cancer tissue with clinical parameters. NMR Biomed. 2006;19:30–40. doi: 10.1002/nbm.992. [DOI] [PubMed] [Google Scholar]
- 85.Mountford CE, Somorjai RL, Malycha P, Gluch L, Lean C, et al. Diagnosis and prognosis of breast cancer by magnetic resonance spectroscopy of fine-needle aspirates analysed using a statistical classification strategy. Br J Surg. 2001;88:1234–1240. doi: 10.1046/j.0007-1323.2001.01864.x. [DOI] [PubMed] [Google Scholar]
- 86.Odunsi K, Wollman RM, Ambrosone CB, Hutson A, McCann SE, et al. Detection of epithelial ovarian cancer using 1H-NMR-based metabonomics. Int J Cancer. 2005;113:782–788. doi: 10.1002/ijc.20651. [DOI] [PubMed] [Google Scholar]
- 87.Osl M, Dreiseitl S, Pfeifer B, Weinberger K, Klocker H, et al. A new rule-based algorithm for identifying metabolic markers in prostate cancer using tandem mass spectrometry. Bioinformatics. 2008;24:2908–2914. doi: 10.1093/bioinformatics/btn506. [DOI] [PubMed] [Google Scholar]
- 88.Cheng LL, Burns MA, Taylor JL, He W, Halpern EF, et al. Metabolic characterization of human prostate cancer with tissue magnetic resonance spectroscopy. Cancer Res. 2005;65:3030–3034. doi: 10.1158/0008-5472.CAN-04-4106. [DOI] [PubMed] [Google Scholar]
- 89.Denkert C, Budczies J, Weichert W, Wohlgemuth G, Scholz M, et al. Metabolite profiling of human colon carcinoma–deregulation of TCA cycle and amino acid turnover. Mol Cancer. 2008;7:72. doi: 10.1186/1476-4598-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Monleon D, Morales JM, Barrasa A, Lopez JA, Vazquez C, et al. Metabolite profiling of fecal water extracts from human colorectal cancer. NMR Biomed. 2009;22:342–348. doi: 10.1002/nbm.1345. [DOI] [PubMed] [Google Scholar]
- 91.Chan EC, Koh PK, Mal M, Cheah PY, Eu KW, et al. Metabolic profiling of human colorectal cancer using high-resolution magic angle spinning nuclear magnetic resonance (HR-MAS NMR) spectroscopy and gas chromatography mass spectrometry (GC/MS). J Proteome Res. 2009;8:352–361. doi: 10.1021/pr8006232. [DOI] [PubMed] [Google Scholar]
- 92.Chen JL, Tang HQ, Hu JD, Fan J, Hong J, et al. Metabolomics of gastric cancer metastasis detected by gas chromatography and mass spectrometry. World J Gastroenterol. 16:5874–5880. doi: 10.3748/wjg.v16.i46.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei J, Xie G, Zhou Z, Shi P, Qiu Y, et al. Salivary metabolite signatures of oral cancer and leukoplakia. Int J Cancer. [DOI] [PubMed]
- 94.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 95.Struys EA, Heijboer AC, van Moorselaar J, Jakobs C, Blankenstein MA. Serum sarcosine is not a marker for prostate cancer. Ann Clin Biochem. 47:282. doi: 10.1258/acb.2010.009270. [DOI] [PubMed] [Google Scholar]
- 96.Jentzmik F, Stephan C, Miller K, Schrader M, Erbersdobler A, et al. Sarcosine in urine after digital rectal examination fails as a marker in prostate cancer detection and identification of aggressive tumours. Eur Urol. 58:12–18; discussion 20-11. doi: 10.1016/j.eururo.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 97.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 98.Ohta T, Masutomi N, Tsutsui N, Sakairi T, Mitchell M, et al. Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicol Pathol. 2009;37:521–535. doi: 10.1177/0192623309336152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supervised PCA separated normal ovarian tissue from ovarian cancer (PC1; blue→green and purple) and localized tumor from metastasis (PC2; green→purple).
(TIF)
List of metabolites identified through mass spectrometry.
(XLS)
ANOVA analysis of metabolites grouped by class of compound.
(XLS)
Ingenuity pathway analysis for the top 15 canonical pathways for EOC.
(XLS)
Ingenuity pathway analysis for the top 15 canonical pathways for MOC.
(XLS)