Abstract
Formation of aberrant protein conformers is a common pathological denominator of different neurodegenerative disorders, such as Alzheimer's disease or prion diseases. Moreover, increasing evidence indicates that soluble oligomers are associated with early pathological alterations and that oligomeric assemblies of different disease-associated proteins may share common structural features. Previous studies revealed that toxic effects of the scrapie prion protein (PrPSc), a β-sheet-rich isoform of the cellular PrP (PrPC), are dependent on neuronal expression of PrPC. In this study, we demonstrate that PrPC has a more general effect in mediating neurotoxic signalling by sensitizing cells to toxic effects of various β-sheet-rich (β) conformers of completely different origins, formed by (i) heterologous PrP, (ii) amyloid β-peptide, (iii) yeast prion proteins or (iv) designed β-peptides. Toxic signalling via PrPC requires the intrinsically disordered N-terminal domain (N-PrP) and the GPI anchor of PrP. We found that the N-terminal domain is important for mediating the interaction of PrPC with β-conformers. Interestingly, a secreted version of N-PrP associated with β-conformers and antagonized their toxic signalling via PrPC. Moreover, PrPC-mediated toxic signalling could be blocked by an NMDA receptor antagonist or an oligomer-specific antibody. Our study indicates that PrPC can mediate toxic signalling of various β-sheet-rich conformers independent of infectious prion propagation, suggesting a pathophysiological role of the prion protein beyond of prion diseases.
Keywords: Alzheimer, intrinsically disordered, NMDA receptor, oligomer, prion
Introduction
Various approaches coming from neuropathology, genetics, animal modelling and biophysics have established a crucial role of protein misfolding in the pathogenesis of different neurodegenerative disorders, such as Alzheimer's disease (AD), Parkinson's disease, polyglutamine expansion diseases and prion diseases. However, there is an ongoing debate about the nature of the harmful proteinaceous species and how toxic conformers selectively damage neuronal populations (reviewed in Haass and Selkoe, 2007; Winklhofer et al, 2008; Ilieva et al, 2009).
The prion protein is best known for its crucial role in the pathogenesis of prion diseases, such as Creutzfeldt–Jakob disease and Gerstmann–Sträussler–Scheinker syndrome in humans, scrapie in sheep and goat, bovine spongiform encephalopathy in cattle and chronic wasting disease in free-ranging deer. In the disease, the cellular isoform of PrP (PrPC) is converted into an aberrantly folded, β-sheet-rich isoform, designated scrapie prion protein (PrPSc). PrPSc is found in extracellular deposits in the diseased brain and has been shown to be the essential constituent of infectious prions (reviewed in Weissmann et al, 1996; Prusiner et al, 1998; Collinge, 2001; Chesebro, 2003). Notably, mice with a targeted disruption of the PrP gene (PRNP) are resistant to prion diseases and do not propagate infectious prions (Büeler et al, 1993). Moreover, based on transmission studies in laboratory animals, it has been proposed that productive propagation of infectious prions involves a direct interaction between PrPC and PrPSc, which is highly dependent on the sequence homology between the two isoforms. As a consequence, PrPSc molecules derived from one species fail to or inefficiently transmit the disease to an organism expressing heterologous PrPC molecules, a feature denoted as a species or transmission barrier (Prusiner et al, 1990; Moore et al, 2005; Collinge and Clarke, 2007). Interestingly, transgenic mouse models revealed that neuronal expression of GPI-anchored PrPC is required to mediate neurotoxic effects of PrPSc (Brandner et al, 1996; Mallucci et al, 2003; Chesebro et al, 2005). We corroborated the important role of PrPC as a mediator of PrPSc-induced toxicity and identified the intrinsically disordered N-terminal domain and the C-terminal GPI anchor of PrPC as essential domains for this activity (Rambold et al, 2008). Recently, it was suggested that PrPC may also have a role in the pathogenesis of AD. It was reported that amyloid β (Aβ)-induced inhibition of long-term potentiation (LTP) and memory impairment in transgenic mouse models of AD requires PrPC (Lauren et al, 2009; Gimbel et al, 2010). AD is the most common neurodegenerative disorder characterized by the deposition of Aβ-peptides in extracellular amyloid plaques and hyperphosphorylated τ in intraneuronal neurofibrillary tangles (reviewed in De Strooper, 2010; Palop and Mucke, 2010). While the exact mechanisms leading to progressive neurodegeneration in AD remain elusive, a variety of experimental studies indicated that soluble oligomeric species of Aβ contribute to synaptic dysfunction (Lambert et al, 1998; Walsh et al, 2002; Lacor et al, 2004; Cleary et al, 2005; Harmeier et al, 2009; Lauren et al, 2009). In addition, there is growing evidence that NMDA receptors are involved in mediating toxic effects of Aβ (reviewed in Ondrejcak et al, 2010). Notably, PrPC can attenuate excitotoxicity by inhibiting NMDA receptors (Khosravani et al, 2008).
Herein, we show that cell surface localized PrPC has the ability to interact with and mediate toxic signalling of β-sheet-rich conformers of different origin. In addition, we show that pro-apoptotic signalling via PrPC induced by various β-sheet-rich conformers is independent of the replication of infectious prions and can be significantly reduced by an NMDA receptor antagonist.
Results
PrPC mediates toxic signalling of homologous and heterologous PrPSc
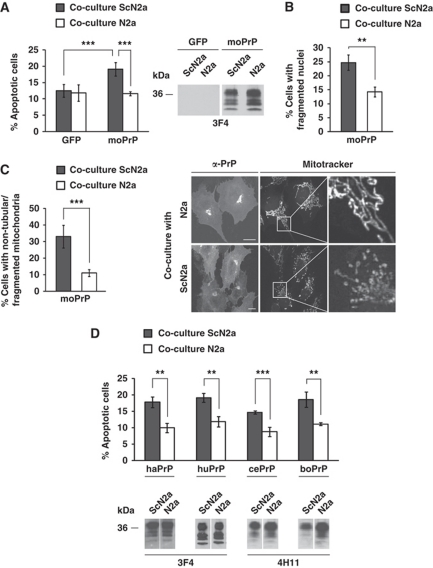
We have recently established a cell culture assay to demonstrate that PrPC localized at the cell surface is a mediator of pro-apoptotic signalling induced by PrPSc (Rambold et al, 2008). This assay is based on the co-cultivation of PrPC-expressing cells with scrapie-infected cells that release PrPSc and infectious prions into the cell culture medium. As illustrated in Figure 1A, co-cultivation of SH-SY5Y cells expressing PrPC with scrapie-infected mouse neuroblastoma (ScN2a) cells increased apoptotic cell death, as determined by activated caspase-3 (Figure 1A; Supplementary Figure S1A) or fragmented nuclei (Figure 1B) in cells expressing mouse PrPC (moPrP). Control SH-SY5Y cells could be co-cultured with ScN2a cells without adverse effects (Figure 1A and B). Interestingly, PrPSc had also an adverse effect on mitochondrial morphology in PrPC-expressing cells. A significant increase in the number of cells harbouring non-tubular/fragmented mitochondria was observed in PrPC-expressing SH-SY5Y cells exposed to PrPSc, but not in cells co-cultivated with N2a cells (Figure 1C). Based on these and previously published studies in mice (Brandner et al, 1996; Mallucci et al, 2003; Chesebro et al, 2005), two plausible scenarios for the toxic effects of PrPSc can be envisaged. Neurotoxicity of PrPSc could be linked to its propagation in neuronal cells, which is dependent on the expression of PrPC. We addressed this possibility, but found no evidence that PK-resistant PrP was formed in mouse PrPC-expressing SH-SY5Y cells during co-cultivation with ScN2a cells (Supplementary Figure S1B). Alternatively, PrPSc elicits a deadly signal through a PrPC-dependent signalling pathway. To experimentally address this scenario, we modified our cell culture model in order to minimize the possibility that propagation of infectious prions occurs. We therefore used SH-SY5Y cells in our co-cultivation assay expressing hamster, human, cervid or bovine PrPC instead of mouse PrPC. Based on previous studies in transgenic mice and cell culture models (Scott et al, 1989, 1997), it is highly unlikely that mouse (mo) PrPSc released by ScN2a cells can induce efficient conversion of heterologous PrPC molecules into PrPSc. Co-cultivation of SH-SY5Y expressing the respective PrPC with uninfected N2a cells had no effect on cellular viability. However, co-cultivation with ScN2a cells revealed that heterologous PrPC, be it of hamster, human, cervid or bovine origin, efficiently mediated toxic signalling of moPrPSc (Figure 1D). Thus, toxic signalling of PrPSc via PrPC appears to be independent of PrPSc propagation.
Figure 1.
Scrapie prions induce apoptosis and interfere with mitochondrial integrity in cells expressing homologous and heterologous PrPC. (A, B) SH-SY5Y cells expressing mouse (mo) PrP or GFP were co-cultured with ScN2a or N2a cells for 16 h. For quantification of apoptotic cell death, SH-SY5Y cells were fixed, permeabilized and stained for active caspase-3 (A) or fragmented nuclei (B). Expression of PrP was analysed by western blotting using the anti-PrP antibody 3F4 (middle panel). (C) Scrapie prions interfere with mitochondrial integrity. SH-SY5Y cells expressing moPrP were co-cultured with ScN2a or N2a cells for 16 h. Cells were stained with MitoTracker Red CMXRos to visualize mitochondria and analysed by fluorescence microscopy (right panel). Cells displaying an intact network of tubular mitochondria were classified as tubular. When this network was disrupted and mitochondria appeared predominantly spherical or rod-like, they were classified as fragmented. For quantification (left panel), the mitochondrial morphology of at least 500 cells per experiment was determined in a blinded manner. Quantifications were based on triplicates of at least three independent experiments. (D) PrPC mediates toxic signalling of heterologous PrPSc. SH-SY5Y cells transiently expressing hamster (ha), human (hu), cervid (ce) or bovine (bo) PrPC were co-cultured with ScN2a or N2a cells for 16 h. Shown is the percentage of apoptotic cells among transfected cells. Expression of transfected constructs was analysed by western blotting using the anti-PrP antibody 4H11 or 3F4. **P<0.005; ***P<0.0005.
PrPC mediates toxic signalling of oligomeric Aβ secreted from transfected cells or prepared by chemical synthesis
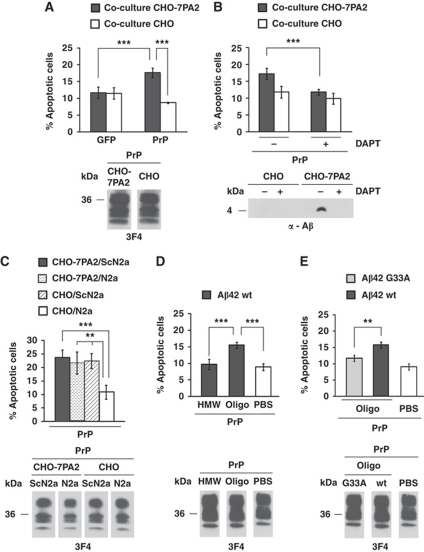
Prompted by the observation that PrPC can mediate toxic signalling of heterologous PrPSc molecules, we investigated whether Aβ could mediate toxic signalling via PrPC. The rationale behind this approach was provided by studies showing that Aβ-induced blockage of LTP and memory impairment in transgenic mouse models of AD requires PrPC (Lauren et al, 2009; Gimbel et al, 2010). We made use of a stably transfected Chinese hamster ovary cell line (CHO-7PA2) that expresses the familial AD mutation V717F in the amyloid precursor protein APP751 and secretes Aβ (Podlisny et al, 1995). Importantly, the presence of secreted oligomeric Aβ in the medium of CHO-7PA2 cells has been shown to potently inhibit LTP in vivo (Walsh et al, 2002; Cleary et al, 2005). SH-SY5Y cells were grown on cover slips and transiently transfected with PrPC. The cover slips were then placed into cell culture dishes with CHO-7PA2 or CHO control cells, and apoptosis of SH-SY5Y cells was analysed after 16 h of co-cultivation. Control SH-SY5Y cells expressing GPI-anchored GFP could be co-cultivated with CHO-7PA2 or CHO cells without adverse effects on cell viability (Figure 2A). Similarly, SH-SY5Y cells expressing PrPC did not show increased apoptosis when co-cultured with control CHO cells. However, a significant increase in apoptotic cell death was observed when SH-SY5Y cells expressing PrPC were co-cultivated with CHO-7PA2 cells (Figure 2A). Notably, the toxic effect of CHO-7PA2 cells was dependent on the generation of Aβ, as co-cultivation with CHO-7PA2 cells pre-treated with the γ-secretase inhibitor DAPT did not induce apoptotic cell death in PrPC-expressing SH-SY5Y cells (Figure 2B). If PrPSc and Aβ mediate toxic signalling via different PrPC-dependent pathways, one would assume that the exposure to both PrPSc and Aβ enhances toxicity. However, the rate of cell death in PrPC-expressing SH-SY5Y co-cultivated with CHO-7PA2 or ScN2a cells was similar to those co-cultivated with both CHO-7PA2 and ScN2a cells (Figure 2C). To provide further evidence for a causal role of Aβ as toxic agent and to characterize the Aβ species mediating toxic signalling via PrPC, we employed high- and low-molecular weight Aβ42 aggregates obtained by size-exclusion chromatography (SEC) (Supplementary Figure S2) (Harmeier et al, 2009). Immediately after elution from the column, equal amounts of Aβ42-peptides (500 nM final concentration) were added to SH-SY5Y cells expressing PrPC. Consistent with our results from the co-cultivation assay, Aβ42 was only toxic to cells expressing PrPC. Moreover, only low-molecular weight oligomeric Aβ42 (oligo) efficiently induced apoptotic cell death in PrPC-expressing SH-SY5Y cells, while high-molecular weight aggregates had no adverse effects on cell viability (Figure 2D). Notably, the same fraction composed of low-molecular weight oligomeric Aβ42 was shown to inhibit LTP in mouse hippocampal slices (Harmeier et al, 2009). In addition, it has been shown that oligomers of Aβ42 G33A, in which G33 of the central GXXXG motif is substituted by A (Munter et al, 2007, 2010), do not inhibit LTP (Harmeier et al, 2009). In line with this observation, oligomers of Aβ42 G33A did not induce a significant increase in cell death in SH-SY5Y cells expressing PrPC (Figure 2E).
Figure 2.
Soluble oligomers of Aβ induce apoptosis in PrPC-expressing cells. (A, B) SH-SY5Y cells expressing the constructs indicated were co-cultivated with CHO-7PA2 or CHO cells for 16 h and apoptotic cell death in SH-SY5Y cells was determined as described in Figure 1A. (A) Soluble oligomers of Aβ secreted by stably transfected cells induce apoptosis in PrPC-expressing cells. GPI-anchored GFP was used as control. Expression of PrPC in CHO-7PA2 or CHO cells was analysed by western blotting using the 3F4 antibody (lower panel). (B) Inhibition of γ-secretase interferes with toxic effects of CHO-7PA2 cells. SH-SY5Y cells expressing PrPC were co-cultured with DAPT-treated (1 μM) CHO-7PA2 or CHO cells. Aβ present in the conditioned medium of CHO-7PA2 or CHO cells was analysed by immunoprecipitation followed by western blotting (lower panel). (C) SH-SY5Y cells expressing moPrPC were co-cultivated with the cell lines indicated. After16 h of co-cultivation, apoptotic cell death in SH-SY5Y cells was determined as described in Figure 1A. Expression of PrPC in co-cultured SH-SY5Y cells was analysed by western blotting using the 3F4 antibody (lower panel). (D) Synthetic Aβ42 oligomers are toxic to cells expressing PrPC. SH-SY5Y cells transiently expressing moPrPC were incubated in the presence of either oligomers (oligo) or high-molecular weight aggregates (HMW) of Aβ42 (500 nM each) for 12 h and apoptotic cell death was determined. Expression of PrPC was analysed by western blotting using the 3F4 antibody (lower panel). (E) Decreased toxicity of oligomers formed by mutant Aβ42 G33A. SH-SY5Y cells expressing moPrPC were incubated in the presence of Aβ42 G33A oligomers (500 nM) as described in (C). Expression of PrPC was monitored by western blotting using the anti-PrP antibody 3F4 (lower panel). **P<0.005; ***P<0.0005.
The intrinsically disordered N-terminal domain and the C-terminal GPI anchor of PrP are required to mediate the toxic effects of Aβ
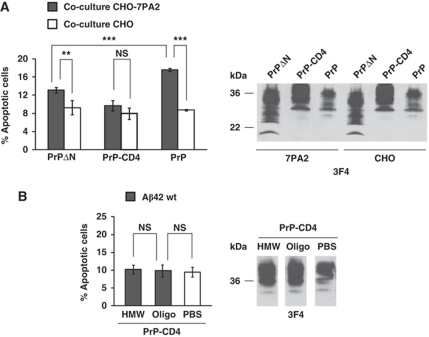
After having established an assay to study PrPC-mediated toxicity of Aβ, we sought to define the domains of PrPC required for the transmission of Aβ toxicity. Our previous study revealed that the intrinsically disordered N-terminal domain and the C-terminal GPI anchor of PrPC are required to mediate toxic signalling of PrPSc (Rambold et al, 2008). Consequently, we tested the ability of Aβ to induce cell death in SH-SY5Y cells either expressing a PrP construct lacking amino acids 27–89 (PrPΔN) or containing a heterologous C-terminal transmembrane domain instead of the GPI anchor (PrP-CD4). Of note, both PrP mutants are complex glycosylated and present at the outer side of the plasma membrane, similarly to wild-type GPI-anchored PrPC (Winklhofer et al, 2003b). Co-cultivation of PrP-expressing SH-SY5Y cells with Aβ-secreting CHO-7PA2 cells revealed that PrP-CD4 was not able to mediate toxic effects of Aβ. Expression of PrPΔN increased apoptotic cell death, but the effect was significantly reduced compared with full-length PrP (Figure 3A). In support of the notion that the GPI anchor of PrP is essential to mediate toxic signalling of Aβ, we could show that oligomers of synthetic Aβ42 did not increase apoptotic cell death in PrP-CD4-expressing cells (Figure 3B).
Figure 3.
Toxic signalling of PrPC is dependent on the intrinsically disordered N-terminal domain and the C-terminal GPI anchor. (A) Apoptotic activity of soluble Aβ oligomers secreted by stably transfected CHO-7PA2 cells is compromised in cells expressing PrP mutants. SH-SY5Y cells expressing the PrP constructs indicated were co-cultivated with CHO-7PA2 or CHO cells and apoptotic cell death in SH-SY5Y cells was determined as described in Figure 1A. Expression of PrP constructs was controlled by western blotting using the 3F4 antibody (right panel). (B) Synthetic Aβ42 oligomers are not toxic to cells expressing PrP-CD4. SH-SY5Y cells expressing PrP-CD4 were incubated with either oligomers (oligo) or high-molecular weight aggregates (HMW) of Aβ42 (500 nM each) for 12 h and apoptotic cell death was determined. **P<0.005; ***P<0.0005; NS, non-significant. Expression of PrP-CD4 was analysed by western blotting using the 3F4 antibody (right panel).
PrPC mediates toxic signalling of β-sheet-rich conformers of a yeast prion protein
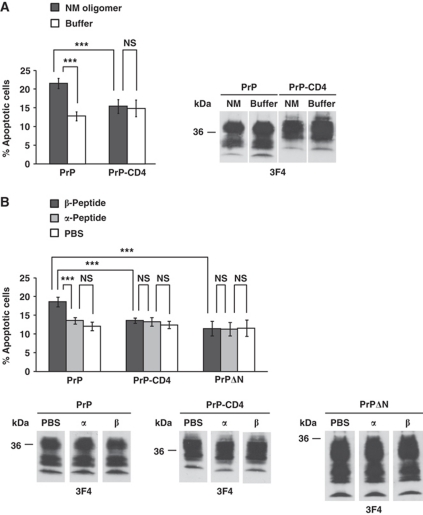
Proteins with prion-like properties have also been identified in fungi (Wickner, 1994). In Saccharomyces cerevisiae, the [PSI+] prion is formed by the essential translation termination factor Sup35 (Glover et al, 1997). The amyloidogenic N-terminal region (N) contains the essential prion-forming determinants, while the C-terminal domain of Sup35 does not contribute to prion behaviour. A charged middle region (M) enhances the solubility of Sup35 in the non-prion form and contributes to the stability of the prion form (i.e. the [PSI+] phenotype). Thus, the N and M regions of Sup35 (designated NM) can serve as a separable prion-forming module (Li and Lindquist, 2000). Similarly to PrP in mammals, prion proteins in fungi typically form highly structured β-sheet-rich fibrils, known as amyloids, upon conversion to the infectious prion form (Shorter and Lindquist, 2005). Consequently, we tested the ability of β-sheet-rich conformers of NM to mediate toxic signalling in SH-SY5Y cells via PrPC. Low-molecular weight NM oligomers, containing dimers, trimers and tetramers, were added to SH-SY5Y cells transiently expressing PrPC and apoptosis was determined after 7 h. PrPC-expressing SH-SY5Y cells exhibited a significantly increased rate of apoptotic cell death, whereas the viability of cells expressing PrP-CD4 was not affected (Figure 4A).
Figure 4.
PrPC mediates toxic signalling of an oligomeric yeast prion protein and of β-sheet-rich oligomers formed by a designed peptide. (A, B) β-sheet-rich conformers of completely different origin induce apoptosis via PrPC. SH-SY5Y cells expressing the PrP constructs indicated were incubated with either an oligomeric yeast prion protein (NM, 500 nM) for 7 h (A) or a synthetic β-peptide or α-peptide (200 nM each) for 12 h (B). Apoptotic cell death was determined as described in Figure 1A. Expression of the PrP constructs was controlled by western blotting using the 3F4 antibody. **P<0.005; ***P<0.0005; NS, non-significant.
PrPC mediates toxic signalling of β-sheet-rich oligomers formed by designed peptides
The ability of PrPC to mediate toxic signalling not only of PrPSc, but also of proteins with completely unrelated primary sequence, such as Aβ and yeast prion oligomers, raised the possibility that the interaction of PrPC with the pathogenic protein conformers is primarily determined by structural features. We followed up this hypothesis by including designed peptides in our analysis that are in either an α-helical or β-sheet conformation. The sequences of these peptides were derived from a previous study, showing that binary patterning of polar and non-polar residues arranged in alternating periodicity can direct proteins to form β-sheet secondary structure (West et al, 1999; Wei et al, 2003). The peptides (200 nM final concentration) were added to SH-SY5Y cells expressing GPI-anchored wild-type PrPC (PrP), PrP with a heterologous C-terminal transmembrane domain (PrP-CD4) or PrPΔN and apoptotic cell death was quantified after 12 h of treatment. Notably, an increase in cell death was only induced in cells expressing GPI-anchored PrPC by a peptide with β-sheet conformation (β-peptide) (Figure 4B). Incubation with a peptide in α-helical conformation (α-peptide) had no adverse effect on cell viability.
Toxic signalling via PrPC can be inhibited by a secreted version of the intrinsically disordered N-terminal domain of PrP
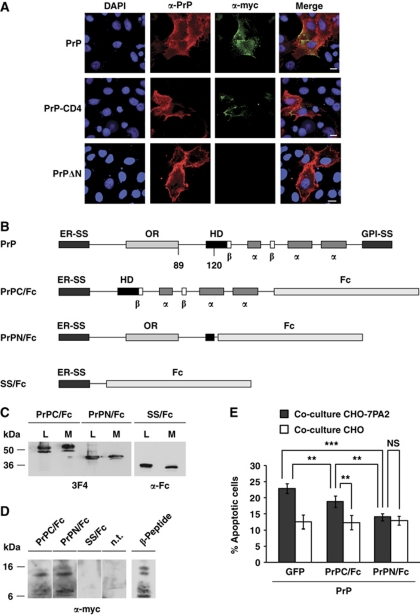
The experiments described above revealed that both PrP mutants, PrP-CD4 and PrPΔN, are impaired in their activity to transduce toxic signalling of β-conformers. To test whether this was due to an impaired binding of the PrP constructs to the β-conformers, we performed indirect immunofluorescence studies and co-immunoprecipitation experiments. Cells expressing wild-type PrPC (PrP), PrP-CD4 or PrPΔN were incubated with the synthetic β-peptide and localization of PrP and the β-peptide was visualized by indirect immunofluorescence. These experiments revealed that full-length PrP efficiently mediates the interaction of the β-peptide with the cells, irrespective of whether PrP is linked via a GPI anchor or a transmembrane domain to the plasma membrane (Figure 5A). However, cells expressing PrPΔN showed a significantly reduced binding to the β-peptide (Figure 5A).
Figure 5.
Toxic signalling can be inhibited by a secreted version of the intrinsically disordered N-terminal domain of PrP. (A) The N-terminal domain of PrP mediates association with β-peptides. SH-SY5Y cells expressing the indicated PrP constructs were grown on cover slips and incubated for 2 h at 37°C with the β-peptide (200 nM). SH-SY5Y cells were fixed and stained with the polyclonal anti-PrP antibody A7 and the monoclonal anti-myc antibody 4A6, recognizing the β-peptide. Cell nuclei were visualized by DAPI. Scale bar, 10 μm. (B) Schematic presentation of PrP and the PrP/Fc fusion constructs analysed. PrPC/Fc, C-terminal domain of PrP fused to the Fc portion of human IgG1; PrPN/Fc, N-terminal domain of PrP fused to the Fc portion; SS/Fc, the ER signal sequence of PrP fused to the Fc portion; ER-SS, ER signal sequence; OR, octarepeat; HD, hydrophobic domain; α, α-helical region; β, β-strand; GPI-SS, GPI signal sequence. (C–E) A secreted version of the N-terminal domain of PrP associates with β-peptides and interferes with their toxic signalling. (C) PrP/Fc fusion proteins are efficiently expressed and secreted into the cell culture medium. N2a cells were transiently transfected with the PrP/Fc fusion constructs. Proteins present in the cell culture medium (M) and cell lysates (L) were analysed by western blotting using the anti-PrP antibody 3F4 or an anti-human IgG antibody. (D) The β-peptide co-precipitates with a secreted version of the intrinsically disordered N-terminal domain of PrP. Conditioned medium from PrP/Fc-expressing N2a cells were mixed with the β-peptide (50 nM) and incubated for 3 h at 4°C. PrP/Fc was purified with protein A-sepharose beads and the pellet was analysed by western blotting. Non-transfected cells (n.t.) were used as control. (E) PrPN/Fc interferes with toxic signalling of Aβ via PrPC. SH-SY5Y cells expressing GPI-anchored PrPC and the indicated constructs were co-cultivated with CHO-7PA2 or CHO cells and apoptotic cell death in SH-SY5Y cells was determined as described in Figure 1A. **P<0:005; ***P<0.0005; NS, non-significant.
To test for a direct interaction between PrP and the β-peptide, we expressed various secreted PrP constructs devoid of a GPI anchor and analysed a possible interaction of secreted PrP with the β-peptide by co-immunoprecipitation experiments. Our previous studies revealed that the isolated N-terminal domain of PrP cannot be expressed as a secreted protein in mammalian cells, since ER import of such a C-terminally truncated PrP construct is abrogated (Heske et al, 2004; Miesbauer et al, 2009). We therefore generated fusion proteins composed of the isolated N- or C-terminal domains of PrP and the Fc portion of human IgG1 (Figure 5B). Western blots illustrated that all PrP/Fc fusion proteins are efficiently expressed and secreted into the cell culture media (Figure 5C). The conditioned media from PrP/Fc-expressing cells were mixed with the β-peptide (50 nM) and incubated for 3 h at 4°C. PrP/Fc was purified with protein A-sepharose beads and the immunopellet was analysed by western blotting (Figure 5D). The β-peptide efficiently co-precipitated with PrPN/Fc and to a lesser extent with PrPC/Fc, but not with the Fc part alone (Figure 5D), supporting the conclusion that an interaction with the β-conformers is mainly mediated by the N-terminal domain of PrP.
Finally, we tested the ability of PrPN/Fc to interfere with toxic signalling of Aβ via PrPC. To this end, we employed the co-cultivation assay of PrPC-expressing SH-SY5Y cells with Aβ-secreting CHO-7PA2 cells. Indeed, co-expression of the secreted N-terminal domain of PrP (PrPN/Fc) significantly reduced toxic signalling of Aβ via PrPC (Figure 5E).
Toxic signalling via PrPC can be inhibited by an oligomer-specific antibody and an NMDA receptor antagonist
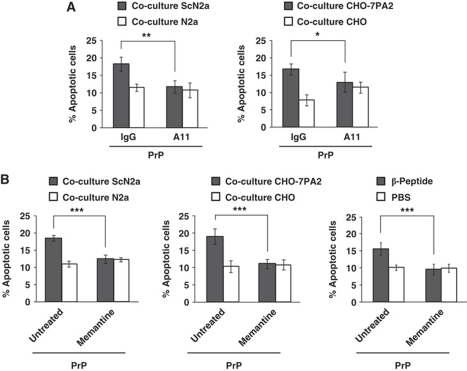
The experiments described above indicated that PrPC is a potent mediator of toxic signalling of β-sheet-rich conformers formed by polypeptides of different origins. Most likely, these polypeptides share common structural features that are recognized or bound by PrPC. This idea is supported by the finding that the conformation-dependent antibody A11 recognizes toxic oligomers formed by both Aβ- and PrP-peptides (Kayed et al, 2003). To test whether A11 could interfere with toxic signalling of Aβ and PrPSc, SH-SY5Y cells expressing GPI-anchored PrPC were co-cultured with either ScN2a or CHO-7PA2 cells in the presence or absence of A11. This assay revealed that the addition of A11 to the cell culture medium significantly interfered with toxic effects of both Aβ and PrPSc on PrPC-expressing cells (Figure 6A).
Figure 6.
An oligomer-specific antibody and an NMDA receptor antagonist prevent toxic signalling of Aβ oligomers, PrPSc and β-peptide. (A, B) SH-SY5Y cells expressing GPI-anchored PrPC were co-cultivated with the cell lines indicated or exposed to β-peptide (200 nM). Apoptotic cell death in SH-SY5Y cells was analysed after 16 h. Cells were co-cultured in the presence of the oligomer-specific polyclonal antibody A11 (1 μg/ml) (A) or the NMDA receptor antagonist memantine (10 μM) (B). An unspecific rabbit antiserum (IgG) or water (untreated) served as controls. *P<0.05; **P<0.005; ***P<0.0005.
To define components downstream of PrPC involved in toxic signalling of PrPSc and Aβ, we performed a co-culture assay in the presence of memantine, an NMDA receptor antagonist. The rationale behind this approach is based on the findings that NMDA receptors are involved in mediating toxic effects of Aβ (reviewed in Ondrejcak et al, 2010), that NMDA receptor antagonists can block PrPSc-induced toxicity (Muller et al, 1993) and that PrPC can attenuate excitotoxicity by inhibiting NMDA receptors (Khosravani et al, 2008). PrPC-expressing SH-SY5Y cells were pre-incubated with memantine for 1 h and either co-cultivated with ScN2a or CHO-7PA2 cells or treated with the β-peptide. Memantine treatment had no effect on the levels of GPI-anchored PrPC at the plasma membrane or Aβ in the cell culture media (data not shown); however, the toxic effects of PrPSc, Aβ- and β-peptide on PrPC-expressing cells were significantly reduced by a pharmacological blockage of NMDA receptor activity (Figure 6B).
Primary neurons devoid of PrPC are less vulnerable to toxic effects of PrPSc and β-peptides
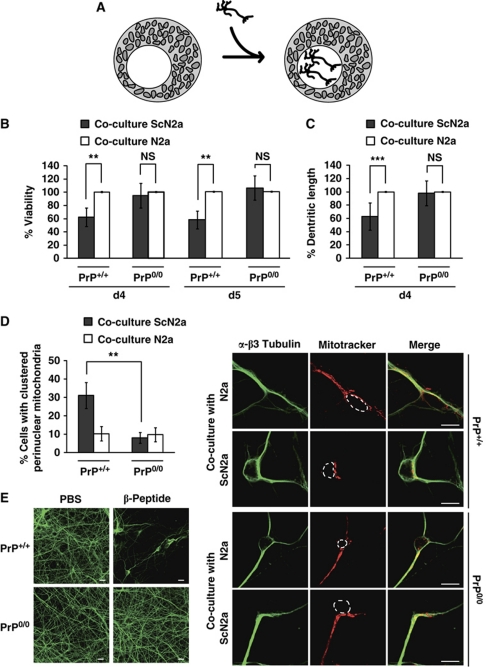
To validate our findings in mammalian neurons, we prepared primary neuronal cultures from the cortical region of mouse embryos at E14.5–E15.5 days of gestation. Primary neurons, derived either from Prnp0/0 mice or from the corresponding wild-type line expressing PrPC, were co-cultivated with ScN2a cells or their uninfected counterpart (N2a) (Figure 7A). First, cell viability was determined after 4 and 5 days in co-culture. As shown in Figure 7B, the viability of PrPC-expressing neurons was significantly impaired when co-cultured with ScN2a cells. On the other hand, no differences in viability were observed between PrP0/0 neurons cultivated with either ScN2a or N2a cells. In addition, a significant reduction in dendritic length was observed in PrPC-expressing neurons co-cultivated with ScN2a cells when compared with neurons co-cultivated with N2a cells, while the exposure of PrP0/0 neurons to PrPSc had no impact on dendritic length (Figure 7C). Furthermore, we assessed the distribution of mitochondria in primary neurons. Exposure of PrPC-expressing neurons to PrPSc significantly increased the number of cells harbouring perinuclearly clustered mitochondria. Importantly, in PrP0/0 neurons, the number of cells with clustered perinuclear mitochondria was not increased upon co-culturing with ScN2a cells (Figure 7D). In another approach we analysed the toxic effects of the designed β-peptide added to the cell culture medium of primary PrP0/0 or PrP+/+ cortical neurons. Cortical neurons were cultivated on poly-L-lysine-coated cover slips and β-peptide (2 or 5 μM) was added on days 4 and 5. At day 6 cells were fixed, permeabilized, stained for β3 tubulin and examined by fluorescence microscopy. Remarkably, the β-peptide caused neuronal cell loss only in PrPC-expressing neurons (Figure 7E). In conclusion, these experiments revealed that PrPC sensitizes primary cortical neurons to toxic effects of β-sheet-rich conformers.
Figure 7.
Primary neurons lacking PrPC are less vulnerable to toxic effects of PrPSc or β-peptide. (A) Schematic model of the co-cultivation assay. Primary neurons were plated on a coated cover slips located in a cell culture dish with either N2a or ScN2a cells. (B–D) Primary neuronal cultures prepared from cortices of PrP0/0 or PrP+/+ mouse embryos (E14.5–E15.5) were co-cultured with N2a or ScN2a cells for 4 or 5 days. (B) Viability of primary cortical neurons is impaired by co-cultivation with ScN2a cells dependent on PrPC expression. To analyse neuronal viability, MAP2-positive cells were determined in an area of 1 mm2 by fluorescence microscopy. Shown is the percentage of viable neurons co-cultured with ScN2a cells in comparison to primary neurons co-cultured with N2a cells. Viability of neurons co-cultured with N2a cells was set as 100%. (C) Dendritic lengths of PrPC-expressing primary neurons are reduced after co-cultivation with ScN2a cells. After 4 days in co-culture, dendritic lengths of at least six MAP2-positive primary neurons were quantified using a Zeiss LSM Image program. Shown are relative alterations in dendritic length of primary neurons co-cultured with ScN2a cells in comparison to primary neurons co-cultured with N2a cells (set as 100%). (D) Co-cultivation with ScN2a cells induces perinuclear mitochondrial clustering in PrPC-expressing primary cortical neurons. Co-cultured primary cortical neurons were stained at day 4 with MitoTracker Red CMXRos to visualize mitochondria and analysed by fluorescence microscopy (right panel). β3 Tubulin was used as a neuronal marker. The cell nuclei are indicated by dotted lines. (Left panel) Shown is the percentage of neurons displaying clustered perinuclear mitochondria. For quantification, mitochondria of at least 500 cells per experiment were determined in a blinded manner. Quantifications were based on triplicates of at least three independent experiments. (E) Treatment of primary cortical neurons with the designed β-peptide causes neuronal cell loss only in PrPC-expressing neurons. Primary cortical neurons from PrP0/0 or PrP+/+ mice were incubated with β-peptide (2 or 5 μM) on days 4 and 5. At day 6 neurons were fixed, permeabilized and analysed by indirect immunofluorescence using an anti-β3 tubulin antibody. The experiment was performed in triplictate and fluorescence images of one representative experiment are shown. **P<0.005; ***P<0.0005, NS, non-significant.
Discussion
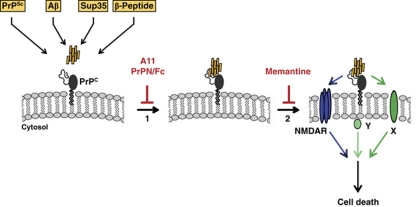
Our work revealed that PrPC localized at the cell surface interacts with and mediates toxic signalling of β-sheet-rich conformers of completely different origin (summarized in the model shown in Figure 8). Both the interaction of PrPC with β-sheet-rich conformers and induction of pro-apoptotic signalling are dependent on the intrinsically disordered N-terminal domain of PrPC and the C-terminal GPI anchor.
Figure 8.
Putative model of oligomer-induced toxic signalling via PrPC. PrPC at the plasma membrane can physically interact with β-sheet-rich conformers of different origin, such as PrPSc, amyloid β (Aβ), yeast prion protein (Sup35) or designed peptides (β-peptide) (step 1). Interaction of PrPC with the β-sheet-rich conformers can be inhibited by an oligomer-specific antibody (A11), or a secreted version of the intrinsically disordered N-terminal domain of PrPC (PrPN/Fc). The PrP/β-sheet complex can then induce apoptotic signalling (step 2). Toxic signalling via PrPC is dependent on the GPI anchor of PrP and can be inhibited by the NMDA receptor antagonist memantine. Since PrPC has no direct contact to the cytosolic compartment, it is plausible to assume that intracellular signal transmission involves additional cellular factors, such as the NMDA receptor (NMDAR), or a different transmembrane protein (X), or a cytosolic protein associated with lipid rafts (Y).
A critical step in the pathogenesis of prion diseases is a direct interaction of PrPC with PrPSc, through which PrPSc forces PrPC to adopt the pathogenic conformation. An efficient conformational transition of PrPC into PrPSc is highly dependent on the sequence homology between the two isoforms. As a consequence, an inter-species transmission of prion diseases is extremely difficult, a phenomenon denoted as species or transmission barrier (Prusiner et al, 1990). Remarkably, our data revealed that PrPC can mediate toxic signalling of PrPSc without being converted into PrPSc: hamster, human, cervid or bovine PrPC-mediated toxic signalling of mouse PrPSc as efficient as mouse PrPC, whereas mouse PrPSc could convert these heterologous PrPC species into PrPSc. We also showed that β-sheet-rich conformers of completely different origin, formed by the Aβ-peptide, a yeast prion protein or a designed peptide, can induce apoptotic signalling through PrPC. Taken together, these results strongly suggest that PrPC has an intrinsic ability to interact with β-sheet-rich conformers independent of their primary sequence. Notably, the oligomer-specific antibody A11 could block PrPC-mediated toxic signalling of both PrPSc and Aβ, supporting the idea that different toxic β-conformers share common structural features, which are recognized and bound by PrPC.
Employing different PrP mutants, such as PrP-CD4 and PrPΔN, we were able to define domains of PrPC required for signalling activity. What could be the role of the C-terminal GPI anchor and the intrinsically disordered N-terminal domain in toxic signal transduction via PrPC? PrP-CD4, which is anchored to the plasma membrane by a heterologous C-terminal transmembrane domain, binds to β-peptides, but cannot mediate toxic effects. In contrast to GPI-anchored PrPC, PrP-CD4 does not localize to detergent-insoluble microdomains at the plasma membrane (Rambold et al, 2008). These data suggest that targeting of PrPC to detergent-insoluble microdomains is a pre-requisite to induce intracellular signalling pathways. PrPΔN lacks amino acids 27–89 and thereby most of the intrinsically disordered domain. Notably, intrinsically disordered domains have been shown to be involved in protein–protein interactions (Tompa et al, 2009). Indeed, our experiments revealed that the interaction of β-conformers with PrPC-expressing cells is reduced by deleting the N-terminal domain of PrP. Moreover, a secreted version of the N-terminal domain of PrP (PrPN/Fc) efficiently interacted with β-conformers and interfered with toxic signalling via PrPC.
Interestingly, PrPC can also activate neuroprotective signalling pathways (McLennan et al, 2004; Shyu et al, 2005; Spudich et al, 2005; Weise et al, 2006; Mitteregger et al, 2007), and both the C-terminal GPI anchor and the unstructured N-terminal domain are required for this physiological activity (Mitteregger et al, 2007; Rambold et al, 2008). Thus, PrPC might act as a signalling molecule at the cell surface to promote stress-protective signalling under physiological conditions, which can be switched to toxic signalling through an interaction with β-sheet-rich conformers. Similarly to other GPI-anchored proteins involved in signal transduction, PrPC most probably acts as a co-receptor in concert with a transmembrane protein to transduce the signal into the cell. Unfortunately, little is known about signalling pathways downstream of PrPC. While different PrPC-dependent pathways and -interacting proteins have been described (reviewed in Caughey and Baron, 2006), experimental evidence for a direct role of these components in PrPSc- or Aβ-induced toxicity is missing. Our study presents evidence that Aβ-, PrPSc- and β-peptide-induced toxicity is significantly suppressed by pharmacologically inhibiting NMDA-type glutamate receptor activity. PrPC has previously been reported to attenuate excitotoxicity by inhibiting NMDA receptors (Khosravani et al, 2008). In addition, there is increasing evidence that excitotoxicity mediated by NMDA receptors has a crucial role in the pathogenesis of AD. For example, it has been shown that Aβ-mediated spine loss requires activity of NMDA-type glutamate receptors (Shankar et al, 2007). All in all, our data provide a scientific rationale for a beneficial effect of NMDA receptor antagonists in a more wider range of neurodegenerative disorders associated with the formation of β-sheet-rich conformers, given that they are applied at an early stage of the disease before irreversible neuronal loss has occurred.
Materials and methods
Plasmids, antibodies and reagents
All expression constructs have been described previously (Rambold et al, 2008). Amino acid numbers refer to mouse Prion Protein sequence (GenBank™ accession number NP 035300), hamster Prion Protein sequence (GenBank™ accession number P04273), human Prion Protein sequence (GenBank™ accession number AAA60182), cervid Prion Protein sequence (GenBank™ accession number ABW79904) or bovine Prion Protein sequence (GenBank™ accession number AAQ64648). As transfection marker the EYFP-C1 vector (Clontech) was used. The following antibodies were used: mouse monoclonal anti-PrP 3F4 antibody (Kascsak et al, 1987), mouse monoclonal anti-PrP 4H11 antibody (Ertmer et al, 2004), rabbit polyclonal anti-PrP antibody A7 (Winklhofer et al, 2003a), mouse monoclonal anti-myc 4A6 antibody (Millipore), rabbit polyclonal anti-active caspase-3 antibody (Promega), mouse monoclonal anti-MAP2 antibody (Sigma-Aldrich), rabbit polyclonal anti-β3 tubulin antibody (Abcam), fluorescent dye-labelled anti-rabbit IgG antibody Alexa Flour 555 (Invitrogen), fluorescent dye-labelled anti-mouse IgG antibody Alexa Flour 555 (Invitrogen), fluorescent dye-labelled anti-mouse IgG antibody Alexa Flour 488 (Invitrogen), fluorescent dye-labelled anti-rabbit IgG antibody Alexa Flour 488 (Invitrogen), horseradish peroxidase-conjugated anti-mouse IgG antibody (Amersham, Promega), horseradish peroxidase-conjugated anti-human IgG antibody (Promega), A11 polyclonal antibody (Kayed et al, 2003), rat monoclonal anti-Aβ antibody 2D8 (Shirotani et al, 2007), rabbit polyclonal anti-Aβ antibody 3552 (Yamasaki et al, 2006). The following reagents were used: DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester), memantine hydrochlorid (Sigma), MitoTracker Red CMXRos (Invitrogen), Proteinase K (Sigma-Aldrich), TO-PRO-3 iodide (642/661) (Invitrogen), poly-L-lysine hydrobromide (Sigma-Aldrich). The mounting medium Mowiol (Calbiochem) was supplemented with DAPI (4′,6-diamidino-2-phenylindole; Sigma-Aldrich).
Cell lysis, immunoprecipitation and western blot analysis
As described earlier (Tatzelt et al, 1996), cells were washed twice with cold phosphate-buffered saline (PBS), scraped off the plate and lysed in cold detergent buffer (0.5% Triton X-100, 0.5% sodium deoxycholat in PBS). Post-nuclear supernatants or secreted and TCA-precipitated proteins were boiled with Laemmli sample buffer and analysed by western blotting as described previously (Winklhofer and Tatzelt, 2000). Aβ oligomers in conditioned medium of CHO-7PA2 were analysed by immunoprecipitation with the polyclonal antibody 3552 followed by western blotting using the monoclonal antibody 2D8.
Cell culture, transfection, co-cultivation, γ-secretase inhibitor experiments and NMDA receptor antagonist treatment
Cells were cultivated and transfected as described earlier (Winklhofer et al, 2003b). Stably transfected Chinese hamster ovary cells (CHO-7PA2) that express the familial AD mutation V717F in the amyloid precursor protein APP751 and secrete Aβ were described earlier (Podlisny et al, 1995). The human SH-SY5Y cell line (DSMZ number ACC 209) is a subline of bone marrow biopsy-derived SK-N-SH cells. For co-cultivation experiments, SH-SY5Y cells were grown on glass cover slips and transfected with Lipofectamine (Invitrogen). Two hours after transfection, cover slips were transferred into dishes containing a 90% confluent cell layer of ScN2a, N2a, CHO-7PA2 or CHO cells. For co-cultivation, SH-SY5Y cells were cultivated in a combination of CHO-7PA2/ScN2a, CHO-7PA2/N2a, CHO/ScN2a or CHO/N2a cells. After 16 h of co-cultivation, either apoptotic cell death or mitochondrial morphology was analysed (see below). To block Aβ generation, CHO-7PA2 cells were pre-treated for 24 h with DAPT (1 μM) before co-cultivation. To interfere with NMDA receptor activation, transfected SH-SY5Y cells were pre-treated for 1 h with memantine (10 μM) before co-cultivation. Memantine (10 μM) was also present during the co-cultivation.
Aβ42-peptides, SEC
As described earlier (Harmeier et al, 2009), synthetic Aβ42 and Aβ42 G33A peptides (PSL, Peptide Speciality Laboratories GmbH) were monomerized in formic acid and subsequently re-dissolved in water containing 0.1% ammonia. The dissolved peptides were loaded onto a Superose 12 (10/300 HR) column (Amersham Bioscience), and 1 ml fractions were collected in PBS as running buffer at a flow rate of 0.5 ml/min. Peptide concentrations were determined using spectrophotometric methods, and the aggregation state was further determined by SDS–PAGE and western blotting. Equal amounts (final concentration 500 nM) of oligomers or high-molecular weight aggregates of Aβ42 were applied on transiently transfected SH-SY5Y cells, and apoptotic cell death was analysed after 12 h.
Yeast prion protein NM: generation and treatment conditions
The NM stock solution was prepared as described (Scheibel et al, 2001). For the preparation of dityrosine-crosslinked NM oligomers, the NM stock solution was diluted to a final concentration of 15 μM in 20 mM Tris, 150 mM sodium chloride, 0.5 mM coppers and 0.5 mM zinc sulphate. To this solution, 0.1 mg/ml of superoxide dismutase (Sigma-Aldrich) was added. Using a Fluorolog fluorimeter (HORIBA Jobin Yvon), the prepared NM solution was crosslinked via excitation at 280 nm for 2 h at room temperature while stirring. Subsequently, an equivalent volume of 6 M guanidinium hydrochloride (Fluka) was added to the solution. For isolation of low-molecular weight crosslinked NM oligomers, the NM solution was first filtered through a 100-kDa Amicon Ultra Centrifugal Filter (Millipore) and the flow through was concentrated using a 3-kDa Amicon Ultra Centrifugal Filter (Millipore). Transiently transfected SH-SY5Y cells were treated with either the low-molecular weight crosslinked NM (final concentration 500 nM) or buffer (10 mM Tris, 75 mM sodium chloride, 3 M guanidinium–HCl, 250 μM copper sulphate, 250 μM zinc sulphate) as control, and apoptotic cell death was analysed after 7 h of treatment.
β- and α-peptides: generation and treatment conditions
The N-terminally myc-tagged β-sheet peptide (ATGTGTGAACAAAAGCTTATTTCTGAAGAAGACTTGGGTATGCAGATCTCCATGGATTATGAGATCAAGTTCCACGGTGATGGTGATAACTTCGACCTCAACCTCGACGATTCTGGTGGTGATCTTCAACTTCAGATCCGCGGCCCGGGTGGCCGTGTCCACGTCCACATCCACAGTAGTAGTGGTAAGGTCGACTTCCACGTCAACAACGACGGCGGCGATGTTGAAGTTAAAATGCACTAG) coding for MCEQKLISEEDLGMQISMDYEIKFHGDGDNFDLNLDDSGGDLQLQIRGPGGRVHVHIHSSSGKVDFHVNNDGGDVEVKMH and the α-helical peptide (ATGTGTGAACAAAAGCTTATTTCTGAAGAAGACTTGGGTATGTATGGCAAGTTGAACGACCTGCTGGAAGACTTGCAAGAGGTGCTGAAGAACCTCCACAAAAACTGGCACGGTGGCAAAGACAACCTGCACGACGTCGACAACCACTTGCAGAACGTCATCGAAGACATCCACGACTTCATGCAAGGCGGTGGCAGCGGCGGCAAGCTGCAAGAGATGATGAAAGAGTTCCAACAGGTGTTGGACGAACTCAACAACCACTTGCAAGGCGGTAAACACACCGTGCACCACATCGAACAAAACATCAAAGAGATCTTCCACCACTTGGAAGAGCTCGTCCACCGTTAG) coding for MCEQKLISEEDLGMYGKLNDLLEDLQEVLKNLHKNWHGGKDNLHDVDNHLQNVIEDIHDFMQGGGSGGKLQEMMKEFQQVLDELNNHLQGGKHTVHHIEQNIKEIFHHLEELVHR were expressed in Escherichia coli BL21 (DE3) using the pTrcHis vector or the pProEx HTb, respectively. The β-sheet peptide was mainly present in inclusion bodies and purified using a MonoQ 10/100 HR 16/10 column (0–1 M NaCl gradient in 8 M urea) followed by SEC (Sephacryl S-300, HiPreP 26/60) in 8 M urea, 100 mM NaCl (pH 7.5). After dialysis against 100 mM NaCl, 25 mM sodium phosphate, refolded soluble oligomers were purified by SEC. The α-helical peptide was purified under non-denaturing conditions using Ni-NTA agarose (Qiagen). β- or α-Peptides (200 nM each) were applied to transiently transfected SH-SY5Y cells and apoptotic cell death was analysed after 12 h. To interfere with NMDA receptor activation, transfected SH-SY5Y cells were pre-treated for 1 h with memantine (10 μM) before application of β-peptide. To investigate a possible direct interaction with PrP, the β- or α-peptides (final concentration 200 nM) were added to transiently transfected SH-SY5Y cells. After 2 h incubation, SH-SY5Y cells were fixed and stained with the polyclonal anti-PrP antibody A7 and the monoclonal anti-myc antibody 4A6 overnight at 4°C, followed by an incubation with the fluorescently labelled secondary antibodies Alexa Flour 555 and Alexa Flour 488 for 1 h at room temperature. Cells were then mounted onto glass slides and examined by fluorescence microscopy using a Zeiss Axiovert 200 M microscope (Carl Zeiss). Cell nuclei were visualized by DAPI. In addition, conditioned media from PrP/Fc-expressing N2a cells were mixed with the β-peptide (50 nM final concentration) and incubated for 3 h at 4°C. PrP/Fc was purified with protein A-sepharose beads and the pellet was analysed by western blotting using anti-myc 4A6 antibody.
Preparation, co-cultivation and treatment of primary cortical neurons
Prnp0/0 mice (Büeler et al, 1992) were on a C57-129Sv background or had been backcrossed on a C57Bl/6 background for over 10 generations. Primary cortical neurons were prepared from C57-129Sv/PrP+/+ or C57-129Sv/PrP0/0 mouse embryos at E14.5–E15.5 days of gestation. Briefly, cortical neurons were cultured in 3.5 cm dishes (1.4 million cells/dish) containing poly-L-lysine (100 μg/ml)-coated cover slips in Neurobasal medium (Invitrogen) supplemented with basic fibroblast growth factor (10 ng/ml), nerve growth factor (10 ng/ml), B27 supplement minus AO 50 × (1 ×) and L-glutamin (0.5 mM). For co-cultivation experiments, cortical neurons were seeded on poly-L-lysine-coated cover slips placed into a dish with a 20% confluent cell layer of either ScN2a or N2a cells. After 4 or 5 days in co-culture, primary neurons on glass cover slips were fixed with 3.7% paraformaldehyde for 20 min, washed and permeabilized with 0.2% Triton X-100 in PBS for 10 min at room temperature. Fixed cells were incubated with an anti-MAP2 antibody overnight at 4°C, followed by an incubation with the fluorescently labelled secondary antibody Alexa Flour 488 for 1 h at room temperature. Cell nuclei were visualized by TO-PRO-3 iodide (642/661). To analyse cell viability, MAP2-positive cells were examined by fluorescence microscopy using a Zeiss Axiovert 200 M microscope (Carl Zeiss). Dendritic lengths of at least six primary neurons co-cultured for 4 days were quantified in an area of 1 mm2 using a Zeiss LSM Image program. To examine mitochondrial morphology, cortical neurons were first incubated with MitoTracker Red CMXRos (Invitrogen) (final concentration 250 nM) before fixation. The number of cells with clustered perinuclear mitochondria out of at least 500 cells was determined. β3 Tubulin was used as a neuronal marker. All experiments were performed in a blinded manner, and quantifications were based on at least three independent experiments. To analyse toxicity of β-peptides, cortical neurons were cultivated on poly-L-lysine-coated cover slips. β-Peptides (2 or 5 μM) were added at days 4 and 5. At day 6, cells were fixed, permeabilized and incubated with an anti-β3 tubulin antibody overnight at 4°C, followed by an incubation with the fluorescently labelled secondary antibody Alexa Flour 488 for 1 h at room temperature. Cells were examined by fluorescence microscopy using a Zeiss Axiovert 200 M microscope (Carl Zeiss). Expression of PrP in neuronal cultures was analysed by immunofluorescence using the monoclonal anti-PrP antibody 4H11 and a polyclonal anti-β3 tubulin antibody as neuronal marker. In addition, western blotting was performed using the monoclonal anti-PrP antibody 4H11 (Supplementary Figure S3A).
Apoptosis assay and mitochondria morphology assay
In all, 16 h after co-cultivation, SH-SY5Y cells were fixed on glass cover slips with 3.7% paraformaldehyde for 20 min, washed and permeabilized with 0.2% Triton X-100 in PBS for 10 min at room temperature. Fixed cells were incubated with an anti-active caspase-3 antibody overnight at 4°C, followed by an incubation with the fluorescently labelled secondary antibody Alexa Fluor 555 for 1 h at room temperature. Cells were then mounted onto glass slides and examined by fluorescence microscopy using a Zeiss Axiovert 200 M microscope (Carl Zeiss). The numbers of cells positive for activated caspase-3 or cells with fragmented nuclei out of at least 1000 transfected cells were determined in a blinded manner. To analyse mitochondrial morphology after co-cultivation, SH-SY5Y cells were incubated with MitoTracker Red CMXRos (Invitrogen) (final concentration 100 nM) before fixation. The number of cells with non-tubular/fragmented mitochondria out of at least 500 transfected cells was determined. All quantifications were based on at least three independent experiments.
PrPSc propagation assay
After co-cultivation, cells were scraped off the plate, the cell pellets were washed twice with cold PBS and lysed in cold detergent buffer (0.5% Triton X-100, 0.5% sodium deoxycholat in PBS). The lysates were centrifuged to generate detergent-soluble and -insoluble fractions. The insoluble fractions were digested with proteinase K (10 μg/ml final concentration) for 0.5 h at 37°C. The reaction was terminated by the addition of PMSF and then both fractions were adjusted to 0.5% sarkosyl. PrP was immunoprecipitated using the monoclonal anti-PrP antibodies 3F4 (specific for the transfected PrP in the SH-SY5Y cells) or 4H11. Immunoprecipitated proteins were boiled in Laemmli sample buffer and analysed by western blotting using the monoclonal anti-PrP antibody 4H11.
Statistical analysis
Quantifications were based on at least three independent experiments. Data were shown as means±s.e. Statistical analysis was performed using Student's t-test. P-values are as follows: *P<0.05; **P<0.005; ***P<0.0005.
Supplementary Material
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 596, GRK 1123), the Max Planck Society and the BMBF (BioDisc, DIP5.1), the HHMI (to SL) and the NIH (GM25874 to SL and NS067782 to JLG). JT gratefully acknowledges support by the German Center for Neurodegenerative Diseases (DZNE). We are grateful to Dominic Walsh and Dennis Selkoe for providing the CHO-7PA2 cells, Charlie Glabe for the A11 antibody and Christian Haass for the Aβ antibodies and DAPT.
Footnotes
The authors declare that they have no conflict of interest.
References
- Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A (1996) Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature 379: 339–343 [DOI] [PubMed] [Google Scholar]
- Büeler H, Aguzzi A, Sailer A, Greiner R-A, Autenried P, Aguet M, Weissmann C (1993) Mice devoid of PrP are resistant to scrapie. Cell 73: 1339–1347 [DOI] [PubMed] [Google Scholar]
- Büeler H, Fischer M, Lang Y, Bluethmann H, Lipp H-P, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C (1992) Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356: 577–582 [DOI] [PubMed] [Google Scholar]
- Caughey B, Baron GS (2006) Prions and their partners in crime. Nature 443: 803–810 [DOI] [PubMed] [Google Scholar]
- Chesebro B (2003) Introduction to the transmissible spongiform encephalopathies or prion diseases. Br Med Bull 66: 1–20 [DOI] [PubMed] [Google Scholar]
- Chesebro B, Trifilo M, Race R, Meade-White K, Teng C, LaCasse R, Raymond L, Favara C, Baron G, Priola S, Caughey B, Masliah E, Oldstone M (2005) Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 308: 1435–1439 [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH (2005) Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci 8: 79–84 [DOI] [PubMed] [Google Scholar]
- Collinge J (2001) Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci 24: 519–550 [DOI] [PubMed] [Google Scholar]
- Collinge J, Clarke AR (2007) A general model of prion strains and their pathogenicity. Science 318: 930–936 [DOI] [PubMed] [Google Scholar]
- De Strooper B (2010) Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol Rev 90: 465–494 [DOI] [PubMed] [Google Scholar]
- Ertmer A, Gilch S, Yun SW, Flechsig E, Klebl B, Stein-Gerlach M, Klein MA, Schatzl HM (2004) The tyrosine kinase inhibitor STI571 induces cellular clearance of PrPSc in prion-infected cells. J Biol Chem 279: 41918–41927 [DOI] [PubMed] [Google Scholar]
- Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Lauren J, Gimbel ZA, Strittmatter SM (2010) Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci 30: 6367–6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover JR, Kowal AS, Schirmer EC, Patino MM, Liu JJ, Lindquist S (1997) Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell 89: 811–819 [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol 8: 101–112 [DOI] [PubMed] [Google Scholar]
- Harmeier A, Wozny C, Rost BR, Munter LM, Hua H, Georgiev O, Beyermann M, Hildebrand PW, Weise C, Schaffner W, Schmitz D, Multhaup G (2009) Role of amyloid-beta glycine 33 in oligomerization, toxicity, and neuronal plasticity. J Neurosci 29: 7582–7590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heske J, Heller U, Winklhofer KF, Tatzelt J (2004) The C-terminal domain of the prion protein is necessary and sufficient for import into the endoplasmic reticulum. J Biol Chem 279: 5435–5443 [DOI] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW (2009) Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol 187: 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kascsak RJ, Rubenstein R, Merz PA, Tonna-DeMasi M, Fersko R, Carp RI, Wisniewski HM, Diringer H (1987) Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol 61: 3688–3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300: 486–489 [DOI] [PubMed] [Google Scholar]
- Khosravani H, Zhang Y, Tsutsui S, Hameed S, Altier C, Hamid J, Chen L, Villemaire M, Ali Z, Jirik FR, Zamponi GW (2008) Prion protein attenuates excitotoxicity by inhibiting NMDA receptors. J Cell Biol 181: 551–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL (2004) Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J Neurosci 24: 10191–10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA 95: 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM (2009) Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 457: 1128–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lindquist S (2000) Creating a protein-based element of inheritance. Science 287: 661–664 [DOI] [PubMed] [Google Scholar]
- Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, Collinge J (2003) Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science 302: 871–874 [DOI] [PubMed] [Google Scholar]
- McLennan NF, Brennan PM, McNeill A, Davies I, Fotheringham A, Rennison KA, Ritchie D, Brannan F, Head MW, Ironside JW, Williams A, Bell JE (2004) Prion protein accumulation and neuroprotection in hypoxic brain damage. Am J Pathol 165: 227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesbauer M, Pfeiffer NV, Rambold AS, Muller V, Kiachopoulos S, Winklhofer KF, Tatzelt J (2009) Alpha-helical domains promote translocation of intrinsically disordered polypeptides into the endoplasmic reticulum. J Biol Chem 284: 24384–24393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitteregger G, Vosko M, Krebs B, Xiang W, Kohlmannsperger V, Nolting S, Hamann GF, Kretzschmar HA (2007) The role of the octarepeat region in neuroprotective function of the cellular prion protein. Brain Pathol 17: 174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RA, Vorberg I, Priola SA (2005) Species barriers in prion diseases—brief review. Arch Virol Suppl 187–202 [DOI] [PubMed] [Google Scholar]
- Muller WE, Ushijima H, Schroder HC, Forrest JM, Schatton WF, Rytik PG, Heffner-Lauc M (1993) Cytoprotective effect of NMDA receptor antagonists on prion protein (PrionSc)-induced toxicity in rat cortical cell cultures. Eur J Pharmacol 246: 261–267 [DOI] [PubMed] [Google Scholar]
- Munter LM, Botev A, Richter L, Hildebrand PW, Althoff V, Weise C, Kaden D, Multhaup G (2010) Aberrant amyloid precursor protein (APP) processing in hereditary forms of Alzheimer disease caused by APP familial Alzheimer disease mutations can be rescued by mutations in the APP GxxxG motif. J Biol Chem 285: 21636–21643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munter LM, Voigt P, Harmeier A, Kaden D, Gottschalk KE, Weise C, Pipkorn R, Schaefer M, Langosch D, Multhaup G (2007) GxxxG motifs within the amyloid precursor protein transmembrane sequence are critical for the etiology of Abeta42. EMBO J 26: 1702–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondrejcak T, Klyubin I, Hu NW, Barry AE, Cullen WK, Rowan MJ (2010) Alzheimer's disease amyloid beta-protein and synaptic function. Neuromolecular Med 12: 13–26 [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L (2010) Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat Neurosci 13: 812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydell RE, Teplow DB, Selkoe DJ (1995) Aggregation of secreted amyloid beta-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem 270: 9564–9570 [DOI] [PubMed] [Google Scholar]
- Prusiner SB, Scott M, Foster D, Pan K-M, Groth D, Mirenda C, Torchia M, Yang S-L, Serban D, Carlson GA, Hoppe PC, Westaway D, DeArmond SJ (1990) Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 63: 673–686 [DOI] [PubMed] [Google Scholar]
- Prusiner SB, Scott MR, DeArmond SJ, Cohen FE (1998) Prion protein biology. Cell 93: 337–348 [DOI] [PubMed] [Google Scholar]
- Rambold AS, Müller V, Ron U, Ben-Tal N, Winklhofer KF, Tatzelt J (2008) Stress-protective activity of prion protein is corrupted by scrapie-prions. EMBO J 27: 1974–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel T, Kowal AS, Bloom JD, Lindquist SL (2001) Bidirectional amyloid fiber growth for a yeast prion determinant. Curr Biol 11: 366–369 [DOI] [PubMed] [Google Scholar]
- Scott M, Foster D, Mirenda C, Serban D, Coufal F, Walchli M, Torchia M, Groth D, Carlson G, DeArmond SJ, Westaway D, Prusiner SB (1989) Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell 59: 847–857 [DOI] [PubMed] [Google Scholar]
- Scott MR, Safar J, Telling G, Nguyen O, Groth D, Torchia M, Koehler R, Tremblay P, Walther D, Cohen FE, DeArmond SJ, Prusiner SB (1997) Identification of a prion protein epitope modulating transmission of bovine spongiform encephalopathy prions to transgenic mice. Proc Natl Acad Sci USA 94: 14279–14284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL (2007) Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci 27: 2866–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirotani K, Tomioka M, Kremmer E, Haass C, Steiner H (2007) Pathological activity of familial Alzheimer's disease-associated mutant presenilin can be executed by six different gamma-secretase complexes. Neurobiol Dis 27: 102–107 [DOI] [PubMed] [Google Scholar]
- Shorter J, Lindquist S (2005) Prions as adaptive conduits of memory and inheritance. Nat Rev Genet 6: 435–450 [DOI] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Chiang MF, Ding DC, Li KW, Chen SF, Yang HI, Li H (2005) Overexpression of PrPC by adenovirus-mediated gene targeting reduces ischemic injury in a stroke rat model. J Neurosci 25: 8967–8977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich A, Frigg R, Kilic E, Kilic U, Oesch B, Raeber A, Bassetti CL, Hermann DM (2005) Aggravation of ischemic brain injury by prion protein deficiency: role of ERK-1/-2 and STAT-1. Neurobiol Dis 20: 442–449 [DOI] [PubMed] [Google Scholar]
- Tatzelt J, Prusiner SB, Welch WJ (1996) Chemical chaperones interfere with the formation of scrapie prion protein. EMBO J 15: 6363–6373 [PMC free article] [PubMed] [Google Scholar]
- Tompa P, Fuxreiter M, Oldfield CJ, Simon I, Dunker AK, Uversky VN (2009) Close encounters of the third kind: disordered domains and the interactions of proteins. Bioessays 31: 328–335 [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ (2002) Naturally secreted oligomers of amyloid b protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416: 535–539 [DOI] [PubMed] [Google Scholar]
- Wei Y, Liu T, Sazinsky SL, Moffet DA, Pelczer I, Hecht MH (2003) Stably folded de novo proteins from a designed combinatorial library. Protein Sci 12: 92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise J, Sandau R, Schwarting S, Crome O, Wrede A, Schulz-Schaeffer W, Zerr I, Bahr M (2006) Deletion of cellular prion protein results in reduced Akt activation, enhanced postischemic caspase-3 activation, and exacerbation of ischemic brain injury. Stroke 37: 1296–1300 [DOI] [PubMed] [Google Scholar]
- Weissmann C, Fischer M, Raeber A, Büeler H, Sailer A, Shmerling D, Rülicke T, Brandner S, Aguzzi A (1996) The role of PrP in pathogenesis of experimental scrapie. Cold Spring Harb Symp Quant Biol 61: 511–522 [PubMed] [Google Scholar]
- West MW, Wang W, Patterson J, Mancias JD, Beasley JR, Hecht MH (1999) De novo amyloid proteins from designed combinatorial libraries. Proc Natl Acad Sci USA 96: 11211–11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB (1994) [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae [see comments]. Science 264: 566–569 [DOI] [PubMed] [Google Scholar]
- Winklhofer KF, Heller U, Reintjes A, Tatzelt J (2003a) Inhibition of complex glycosylation increases formation of PrPSc. Traffic 4: 313–322 [DOI] [PubMed] [Google Scholar]
- Winklhofer KF, Heske J, Heller U, Reintjes A, Muranji W, Moarefi I, Tatzelt J (2003b) Determinants of the in vivo-folding of the prion protein: a bipartite function of helix 1 in folding and aggregation. J Biol Chem 278: 14961–14970 [DOI] [PubMed] [Google Scholar]
- Winklhofer KF, Tatzelt J (2000) Cationic lipopolyamines induce degradation of PrPSc in scrapie-infected mouse neuroblastoma cells. Biol Chem 381: 463–469 [DOI] [PubMed] [Google Scholar]
- Winklhofer KF, Tatzelt J, Haass C (2008) The two faces of protein misfolding: gain and loss of function in neurodegenerative diseases. EMBO J 27: 336–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki A, Eimer S, Okochi M, Smialowska A, Kaether C, Baumeister R, Haass C, Steiner H (2006) The GxGD motif of presenilin contributes to catalytic function and substrate identification of gamma-secretase. J Neurosci 26: 3821–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.