Abstract
During brain development, the neocortex shows periods of enhanced plasticity, which enables the acquisition of knowledge and skills that we use and build on in adult life. Key to persistent modifications of neuronal connectivity and plasticity of the neocortex are molecular changes occurring at the synapse. Here we used isobaric tag for relative and absolute quantification to measure levels of 467 synaptic proteins in a well-established model of plasticity in the mouse visual cortex and the regulation of its critical period. We found that inducing visual cortex plasticity by monocular deprivation during the critical period increased levels of kinases and proteins regulating the actin-cytoskeleton and endocytosis. Upon closure of the critical period with age, proteins associated with transmitter vesicle release and the tubulin- and septin-cytoskeletons increased, whereas actin-regulators decreased in line with augmented synapse stability and efficacy. Maintaining the visual cortex in a plastic state by dark rearing mice into adulthood only partially prevented these changes and increased levels of G-proteins and protein kinase A subunits. This suggests that in contrast to the general belief, dark rearing does not simply delay cortical development but may activate signaling pathways that specifically maintain or increase the plasticity potential of the visual cortex. Altogether, this study identified many novel candidate plasticity proteins and signaling pathways that mediate synaptic plasticity during critical developmental periods or restrict it in adulthood.
Plasticity in the neocortex allows us to learn and adapt to our environment and occurs with active training and passive exposure. In particular during critical periods of development, neuronal connections of the neocortex are highly malleable. Understanding the molecular mechanisms that regulate critical period plasticity is highly relevant because dysregulation of neocortical plasticity during development underlies many disorders of the brain, ranging from a lazy eye to schizophrenia. Knowledge about the molecular events that regulate plasticity may eventually let us control neocortical plasticity during development or reactivate it in adulthood for clinical purposes.
The primary visual cortex (V1)1 is the most frequently used brain area for studying neocortical plasticity. Plasticity of ocular dominance is an especially convenient experimental model. Prolonged occlusion of one eye (monocular deprivation, MD) during the critical period results in a physiological (1) and anatomical (2) overrepresentation of inputs from the open eye at the cost of inputs from the deprived eye. Dark rearing, whereby animals are raised in total darkness from birth, results in a delayed critical period for plasticity of ocular dominance (OD) (3). Because these functional and anatomical changes are well described and can be induced with relative ease, OD plasticity in V1 is highly suitable for identifying cellular and molecular mechanisms involved in neocortical plasticity and its critical period.
Studies in rodents have provided increasing knowledge on the genes and proteins involved in OD plasticity, and the use of both forward- and reverse genetics (4, 5) has been instrumental in this. Also changes in gene expression observed by microarray studies (6, 7) investigating plasticity-manipulating paradigms have generated valuable insights into the molecular processes underlying visual cortex plasticity. In order to study the molecular events intrinsic to the synapse, however, direct approaches to quantitatively address the synaptic proteome are necessary. This can be achieved by assessing fractions biochemically enriched for synaptic membranes. Such an approach has the important advantage that localized events can be revealed that are otherwise hidden in the complexity of molecular changes occurring in other subcellular compartments or in non-neuronal cell types.
Here we performed proteomic analyses using an isobaric tag for relative and absolute quantitation (iTRAQ) and tandem mass spectrometry. We used this approach to identify proteins in the synaptic membrane fraction whose levels are altered by visual experience or age. This method allowed for the labeling of peptides derived from four different experimental paradigms and permitted parallel identification and comparative quantification. We analyzed the synaptic membrane proteome of the binocular area of V1 from mice: (i) during the critical period, (ii) during the critical period while OD plasticity was being induced, (iii) in young adult mice after the critical period, and (iv) in young adult mice in which the critical period was delayed with dark rearing. Direct comparison of these groups enabled us to study the effects of monocular deprivation and age on the synaptic membrane proteome fraction and analyze how dark rearing affected the age-induced changes.
EXPERIMENTAL PROCEDURES
Animals
Throughout the study, male C57BL/6JOlaHsd mice from Harlan Netherlands were used. Mice in group 1 (“P30,” binocular visual cortex isolated at P30), group 2 (“P30-MD,” binocular visual cortex isolated at P30 after 4 days of monocular deprivation) and group 3 (“P46,” binocular visual cortex isolated at P46) were housed on a standard 12 h light-dark cycle. Right eyelids of P30-MD mice were sutured at P26 under isoflurane anesthesia as previously described (8). Mice in group 4 (“P46-DR,” binocular visual cortex isolated at P46 after dark rearing) were housed in the dark from before birth until decapitation. Because decapitation for this group was performed in the dark, tissue collection for P30, P30-MD, and P46 mice was done just before the end of the dark period of the light-dark cycle, to avoid fast effects of light exposure on protein expression. All experiments involving mice were approved by the institutional animal care and use committee of the Royal Netherlands Academy of Arts and Sciences.
Tissue Preparation and Synaptic Membrane Isolation
In order to prepare protein extracts enriched for synaptic membranes, binocular visual cortex was dissected, snap-frozen in liquid nitrogen, and stored at −80 °C until protein isolation. Bilateral binocular V1 was collected, except for P30-MD mice, for which only the binocular visual cortex contralateral to the deprived eye was isolated. Pools of dissected visual cortex (n = 8 hemicortices per treatment, corresponding to four mice in the groups P30, P46, and P46-DR, or to eight P30-MD mice, randomized with regard to litter composition) were homogenized in ice-cold 0.32 m sucrose buffer with 5 mm HEPES at pH 7.4 and protease inhibitor (Roche), and centrifuged at 1000 × g for 10 min at 4 °C to remove debris. Supernatant was loaded on top of a discontinuous sucrose gradient consisting of 1.2 m and 0.85 m sucrose. After ultracentrifugation at 110000 × g for 2 h at 4 °C, the fraction at the interface of 0.85 m and 1.2 m sucrose, containing the synaptosomes, was collected, resuspended, and pelleted by ultracentrifugation at 70000 × g for 30 min at 4 °C. The pellet was subsequently resuspended in a hypotonic HEPES solution and lysed. The resulting synaptic membrane fraction was recovered by ultracentrifugation using the discontinuous sucrose gradient as described earlier. The interface fraction containing the synaptic membranes was collected and pelleted by ultracentrifugation at 70000 × g for 30 min at 4 °C after which the material was redissolved in 5 mm HEPES. For iTRAQ labeling, protein concentrations were determined by means of a Bradford assay (Bio-Rad) after which for each sample, 150 μg of protein was transferred to a fresh tube and dried by SpeedVac.
iTRAQ Labeling, Two-dimensional Liquid-chromatography and Tandem Mass Spectrometry
Synaptic membranes were dissolved in detergent (0.85% RapiGest Waters Corporation, Milford, MA), alkylated with methyl methanethiosulfonate, and digested with trypsin as described (9, 10). Peptides were tagged with the respective iTRAQ reagents (114 = P30; 115 = P30-MD; 116 = P46; 117 = P46-DR). To accommodate four separate pools of tissue of each of the four experimental conditions, a total of four times 4-plex iTRAQ experiments were performed.
Dried iTRAQ samples were separated in the first dimension by a polysulfoethyl A strong cation exchange column (PolyLC), and the second dimension on an analytical capillary C18 column (150 mm × 100 μm i.d. column). The eluate from the C18 column was mixed with matrix (7 mg α-cyano-hydroxycinnaminic acid in 1 ml 50% acetonitril, 0.1% trifluoroacetic acid, 10 mm dicitrate ammonium), delivered at 1.5 μl/min and deposited onto an Applied Biosystems matrix-assisted laser desorption ionization plate by means of a robot (Dionex) once every 15s for a total of 384 spots.
MALDI plate analysis was performed on a 4800 Proteomics Analyzer (Applied Biosystems, Forster City, CA). Peptide collision-induced dissociation was performed at 1 kV with nitrogen collision gas. Tandem MS (MS/MS) spectra were collected from 5000 laser shots. Peptides with a signal to noise ratio over 50 at the MS mode were selected for MS/MS, at a maximum of 30 MS/MS per spot. The precursor mass window was set to a relative resolution of 180. Peaklists were extracted using GPS software (AB Sciex, version 3.6).
MS/MS spectra search was performed against the mouse SwissProt (release 7 February 2007; ∼15,000 sequences) and and NCBInr (release October 2007; ∼150,000 sequences) databases using Mascot (version 2.2, Matrix Science) and GPS Explorer (version 3.6, Applied Biosystems) software. Searches were performed with cysteine modification by methyl methanethiosulfonate as fixed modifications, oxidation of methionine as variable modification, a precursor mass tolerance of 150 ppm, and a fragment mass tolerance of 0.4 Da while allowing a single site of miscleavage. The false positive rates of peptide identification estimated from decoy database searches were ∼0.05 for all searches (supplemental Table S1). For subsequent analysis only those peptides were included that mapped unique to one protein. Proteins were considered for quantification if at least one unique peptide had a C.I. ≥ 95% and at least two peptides in three out of four experiments were identified.
iTRAQ areas (m/z 114–117) were extracted from raw spectra and corrected for isotopic overlap using GPS explorer. As a low iTRAQ signal is less reliable for quantitation, only peptides with iTRAQ signals above 2000 were included. To compensate for potential variations in the starting amounts of the samples, individual peak areas of each iTRAQ signature peak were log transformed to yield a normal distribution, and normalized to the mean peak area for every sample. The average iTRAQ peak area of all unique peptides annotated to a certain protein was used to determine protein abundance per treatment.
In order to obtain better insight in the concerted changes in protein levels and the underlying biological events, we also inspected proteins that were identified with less stringent criteria (C.I. >85%, 1 or more peptide in each set for quantification). We clearly indicated such proteins in figures and tables. We derived no conclusions about individual proteins identified with these less stringent criteria unless we confirmed these findings by Western blot analysis.
To compare the abundance of proteins across four parallel iTRAQ-based experiments (sets A–D), within each experiment peptide quantity values were standardized to scores around zero by subtracting the mean peak of all four samples. Data from all experimental sets were then combined, and analyzed by Student's t test (independent samples, two-tailed) for each of the four biologically relevant comparisons. As the t test does not take into account the effect of multiple testing, we used the Statistical Analysis of Microarrays package (11), a resampling-based method, to estimate the false-positive rate. By creating randomized data distributions SAM estimates the rate of false positive discoveries. The q-value calculated by SAM for each protein reflects the number of empirically determined false-positives at the significance level of the respective protein. Therefore the false discovery rate (FDR) levels in our results hold information about a single protein and should not be interpreted as a global FDR level. Changes in expression levels were considered significant when the p value was below 0.05 and the respective FDR below 15%. Protein-level quantification data are listed in supplemental Table S4. Peptide-level identification and iTRAQ quantification data of the four iTRAQ experiments in this study are listed in supplemental Tables S7A–D. To establish whether functional categories of proteins were over- or underrepresented among the proteins with increased or decreased levels under different experimental conditions, we categorized all 467 proteins by function (mitochondrial, or regulating the actin cytoskeleton or neurofilament, tubulin, or septin cytoskeletons, synaptic efficacy or signal transduction, supplemental Table S5). Next we performed Chi-square tests followed by Benjamini-Hochberg correction for multiple testing to investigate whether any of the functional categories were over- or -underrepresented under a specific experimental condition.
All mass spectra used in this study are publicly available at the PRIDE PRoteomics IDEntifications database under Accession numbers: 16649–16656 (http://www.ebi.ac.uk/pride/q.do?accession=1664916656) (12).
Western Blotting
Western blots were performed on the four synaptic membrane protein extracts that were also used for the iTRAQ experiments. In addition we analyzed by immunoblotting two to four independent samples from pools of four animals each, kept under the same experimental conditions as the animals for iTRAQ experiments. The required amount of protein to be applied onto the gel was determined individually for each antibody in a set of test runs. Depending on the antibody, between 1 and 5 μg was used. Samples for Western blotting were prepared according to the manufacturer's protocol (NuPage®, Invitrogen) and loaded onto a NuPAGE 4–12% continuous Bis-Tris gel (Invitrogen). Before transfer to PVDF-paper, the gel was soaked in transfer buffer containing 20% MetOH and 0.1% NuPAGE antioxidant for 15 min. PVDF-paper was incubated in 100% methanol for 5 min, in MQ water and subsequently in transfer buffer. Subsequently, proteins were transferred to the PDV-paper overnight at 4 °C. After transfer, the PVDF-paper was rinsed with water, air dried, and kept at 4 °C overnight. It was then reactivated with 100% methanol, washed with MQ water and subsequently with Tris-buffered saline (TBS). After blocking with 1% casein solution in TBS for 1 h, paper was incubated with either of the following primary antibodies in 0.3% casein solution in TBS with 0.1% Tween (TBST) for 2 h at room temperature: mouse-α-Sema4D (BD Transduction Labs, 610670/553005, 1:500), rb-α-SOS-1 (Santa Cruz, Santa Cruz, CA; 1:1000), m-α-Clathrin light chain (SySy, Goettingen, Germany; 113011, 1:250), rb-α-NCAM (Millipore, AB5032, 1:1000), rb-α-Synapsin (Millipore, Billerica, MA; 1:4000), rb-α-Septin-8 (gift of B. Zieger, 1:1000), rb-α-GAT-1 (Millipore, AB1570, 1:1000), rb-α-Ube4b (gift from M. Coleman, Cambridge UK, 1:50), m-α-14–3-3 beta (Santa Cruz, sc-59417, 1:1000), m-α-14–3-3 eta (Millipore, AB9736, 1:2000), rb-α-GABA(A)-R alpha1 (Millipore, AB5609, 1:1000).
Paper was then washed with TBST and incubated for 1 h at room temperature with an infrared IRDye®800CW-labeled secondary antibody (goat-α-mouse-IR (926–32210) or goat-α-rabbit-IR (926–32211), LI-COR Biosciences, Lincoln, NE; 1:5000 in TBST with 0.01% SDS to reduce background). From secondary antibody incubation onwards, papers were protected from light. Papers were washed with TBST and then with TBS, after which they were scanned for secondary antibody fluorescence using the Odyssey® Infrared Imager (LI-COR Biosciences). Relative amounts of fluorescence were quantified using the Odyssey 2.1 software package (LI-COR Biosciences). To test whether the Western blot confirmed the results obtained with iTRAQ, we determined the significance of the Western blotting data by Student's t test (one-tailed, independent samples).
RESULTS
Identification of Proteins Affected by Monocular Deprivation, Age, or Dark Rearing Using iTRAQ
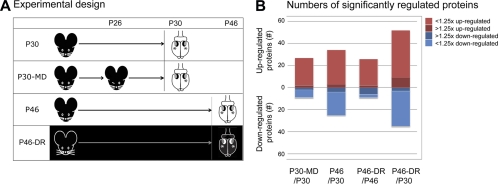
In order to identify synaptic proteins involved in mediating OD plasticity in the visual cortex or regulating its critical period, we performed quantitative proteomics using iTRAQ on fractions containing synaptic membranes derived from the binocular visual cortex from groups of mice kept under four different experimental conditions (Fig. 1A). The first group (P30) contained mice during the peak of the critical period, at P30. The second group (P30-MD) contained mice from the same age that were monocularly deprived from P26 for a period of 4 days. In this group we only used the binocular cortex contralateral to the deprived eye. The third group (P46) contained adolescent mice, in which the peak of the critical period had passed, at P46. The fourth group (P46-DR) contained mice of the same age that were dark reared, and in which the critical period should thus have been delayed. We used four independent sets of mice per experimental condition in order to adequately replicate our findings (sets A–D). We identified a total of 467 proteins with a confidence of more than 95% that were detected under all four conditions, in all four sets and quantified with two or more peptides in at least three sets.
Fig. 1.
Experimental design and numbers of regulated proteins. A, Mice in the first group (“P30”) were reared under a normal 12 h light/12 dark regime for 30 days. Mice in the second group (“P30-MD”) were reared similarly, but monocularly deprived from P26 for a period of 4 days. Mice in the third group (“P46”) were normally reared for 46 days. Mice in the fourth group (“P46-DR”) were dark reared until P46. The visual cortices from which the binocular zone was collected are indicated with an asterisk (*). B, A total of 467 proteins were identified in all experiments. A modest percentage (7.5–20%) of these proteins were expressed at significantly different levels under the various experimental conditions, and most proteins were regulated less than 1.25-fold. The visual cortex from dark-reared mice at P46 and from normally reared mice at P30 differed most extensively.
We compared the synaptic membrane proteome of P30 with that of P30-MD, P46, and P46-DR, and P46 with P46-DR. Overall, we found that between the different experimental conditions synaptic protein levels differed only to a moderate degree (Fig. 1B). The total numbers of proteins that had significantly (p < 0.05, t test, and false discovery rate (FDR <15%)) different levels between conditions ranged from 35 to 84 (out of the 467 proteins that were considered). Their average changes ranged between 1.16- and 1.28-fold, depending on the experimental condition. Only a small number of proteins had changed levels of more than 1.25-fold (Fig. 1B).
To validate the results, we performed Western blots for 14 proteins and conditions under which we detected significant changes using iTRAQ (supplemental Fig. S1). Of these 14, we confirmed 11, showing expression level changes concordant with the iTRAQ experiment. In all these 11 cases the level changes as observed by Western blot of the samples that were also used for iTRAQ analyses occurred in the same direction as the samples prepared independently (not shown). In most cases, changes in the levels as assessed by Western blot were larger than observed by iTRAQ, which was also described previously (9) This is partially caused by the fact that iTRAQ suffers to some extent from the compression of the quantitation ratios to a ratio of 1 when used with complex samples, such as our synaptic membrane preparation (13).
Synaptic Proteins Regulated by MD
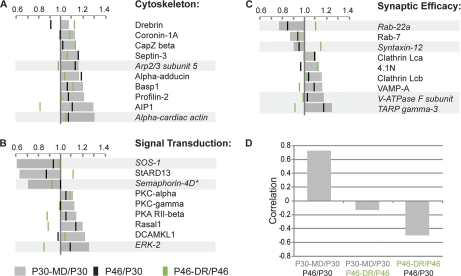
To analyze the effects of MD on synaptic proteins, we compared their relative expression levels in the binocular cortex of P30 and P30-MD mice. During MD, synapses may become stabilized or instead, replaced by new synapses. To obtain insight into which changes in protein levels relate to which of these events, we made two comparisons. First, we compared the significant (p < 0.05 t test, FDR <15%) changes in protein levels induced by MD (Figs. 2A–2C, indicated in gray) with those that occurred with age (P46/P30 ratio, indicated by black bars) and are thus expected to correlate with synapse maturation, which is usually associated with an increase in synapse size, efficacy, and stability. Second, we compared the changes induced by MD (P30 MD/P30 ratio) with those induced by DR (P46-DR/P46 ratio, indicated by green bars), which are expected to correlate with reduced synapse maturity. We found that among the MD regulated proteins, there was an anticorrelation between the changes in levels of proteins caused by dark rearing and by age (corr = −0.50, p < 0.005) (Fig. 2D) indicating that the changes in levels of these proteins indeed represent partially opposing biological events. Interestingly, significant changes in protein levels induced by MD correlated strongly with changes occurring with age (corr = 0.72, p < 0.000001), whereas they showed no significant correlation with changes induced by DR (corr = −0.12, p = 0.473). This suggests that V1 of mice monocularly deprived for 4 days showed a relative increase of mature synapses compared with that of visually undeprived mice.
Fig. 2.
Proteins regulated by monocular deprivation. Proteins are categorized in groups: (A) associated with the cytoskeleton, (B) involved in signal transduction, or (C) regulating synaptic efficacy. Gray bars indicate fold change in relative protein expression levels of individual proteins in monocularly deprived binocular visual cortex compared with the same tissue from normally reared P30 mice. Green lines indicate the relative changes in levels of the same protein with dark rearing (P46-DR/P46) and black lines with age (P46/P30). (D) Changes in protein expression induced by monocular deprivation correlate strongly with those occurring with age. As expected, changes in protein expression caused by age (P46/P30) anticorrelate with those induced by dark rearing (P46-DR/P46). * confidence between 85 and 95%. Proteins quantified with less than two peptides in more than one set are indicated in italics and light gray bars.
We categorized the regulated proteins in groups based on their cellular function, allowing us to obtain a better understanding of the functional implications of the observed changes. Shown in Figs. 2A–2C are changes in levels of proteins in those three categories in which more than five proteins were found to be affected by MD: (a) proteins associated with the cytoskeleton, (b) proteins involved in signal transduction, and (c) proteins known to regulate synaptic efficacy. The latter include neurotransmitter receptors, proteins regulating their trafficking, and proteins involved in vesicle release and recycling. Together, these groups of proteins represent approximately two thirds of all proteins regulated by MD. All proteins regulated by MD, including those that did not fit into one of these three categories are in supplemental Table S2. The latter mostly represent proteins with unknown functions in the central nervous system. For completeness, the relative levels, standard deviations, p values, FDRs and numbers of peptides used for quantitation of all proteins identified with a confidence higher than 85% in all four sets and under all four experimental conditions are included in supplemental Table S4.
Cytoskeleton-associated Proteins
A relatively large number of proteins (p < 0.05 Chi-square test with Benjamini-Hochberg correction compared with all identified proteins, supplemental Table S5) of which synaptic expression was increased after MD are involved in regulating the actin cytoskeleton (Fig. 2A). Several of these also showed higher levels in DR (P46-DR compared with P46), suggesting that these proteins are associated with more immature synapses. These included, among others, the developmentally regulated brain protein Drebrin (14) and Basp1, which has similar and partially overlapping functions in neurite outgrowth (15) as the growth associated protein GAP-43. Other proteins, including Profilin-2, Septin-3, α-Adducin and AIP1, also showed higher levels with age (P30 to P46) suggesting that they are associated with more mature synapses.
Protein Kinases and G-Protein Signaling
MD resulted in increased levels of various kinases at synaptic membranes (Fig. 2B), including several that have been previously implicated in plasticity in V1, such as PKC-α and -γ (16) and the regulatory subunit RII-β of PKA (17). We also found an increase of Rasal1, an inhibitor of Ras-signaling. In contrast, we observed a strongly (1.59×) decreased level of StARD13, an inhibitor of Rho-signaling.
Proteins Regulating Synaptic Efficacy
Only a small set of proteins involved in regulating synaptic strength (Fig. 2C) was altered with MD. Among these were Clathrin light chains A and B, which were both up-regulated after MD. We confirmed this by Western blot analysis (supplemental Fig. S1). This suggests that endocytosis may be activated by MD, which is further supported by our observation that the endocytosis associated proteins Amphiphysin, AP-2 α-1 and AP-2 μ-1 were also significantly (t test, p < 0.05) increased after MD, albeit with an FDR of over 15% (supplemental Table S2). The other proteins in this group showing altered levels after MD did not consistently point toward well-defined biological events.
To gain better insight into the broader biological context of the observed changes we also investigated the proteins that were quantified with less stringent criteria (C.I. >85% with 1 or more peptides in all four sets). These proteins are marked in Fig. 2 by a light gray bar (if quantified by fewer peptides) and/or an asterisk (if the C.I. was between 85 and 95%). Overall, these added proteins fit well in the overall molecular portrait. Among the cytoskeletal proteins, two more actin-related proteins were identified whose levels were higher after MD (Fig. 2A). Among the signaling proteins with increased levels after MD, ERK-2 was now detected, another well-known kinase involved in OD plasticity (18). Two additional signaling proteins were identified with strongly reduced expression levels after MD (Fig. 2B). One was SOS-1 (1.64x, confirmed by Western blot, supplemental Fig. S1), an important activator of Ras-signaling which seems consistent with the increased levels of Rasal1, an inhibitor of Ras-signaling. Interestingly, the second protein with strongly reduced expression was Semaphorin-4D (1.41x, confirmed by Western blot, supplemental Fig. S1). This protein induces axonal growth cone collapse and increases spine density upon interacting with its receptor PlexinB1, which inhibits Ras-signaling and activates Rho-signaling (19, 20). We currently do not know whether there is a link between Semaphorin 4D-signaling and the observed changes in the regulators of Rho -signaling (SOS-1), and Ras-signaling (Rasal1 and StARD13). The additional proteins in the last group involved in regulating synaptic efficacy, did not provide a consistent picture.
Taken together, MD caused elevated levels of kinases and cytoskeleton-associated proteins, (see Fig. 5A). A consistent increase or decrease in proteins determining synaptic strength could not be detected. Strikingly, we noticed that significant changes in protein levels induced by MD correlated strongly with changes in the levels of these proteins occurring with age. As 4 days of MD is associated predominantly by synapse loss (21) it is possible that synapses that are not eliminated are of a more mature signature. This would result in a relative increase of spine maturity after deprivation, which may explain the observed correlation between MD- and age-induced changes in protein levels.
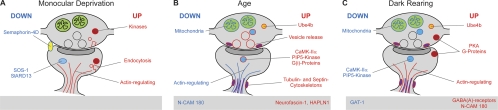
Fig. 5.
Overview of changes in synaptic proteins. Pre- and postsynaptic changes that probably occur in excitatory synapses are indicated in the bouton and spine head respectively. Changes expected to occur predominantly in inhibitory synapses are indicated in the bar below. A, With monocular deprivation, the G-proteins SOS-1 and StARD13 are strongly decreased, as is Semaphorin-4D. Actin-associated proteins, Clathrin light chains and various kinases show higher expression. Proteins indicated in the lower bar are believed to act mainly at inhibitory synapses. B, With age, Actin-associated proteins diminish, as do mitochondrial proteins and N-CAM 180. Proteins that increase levels with age include proteins associated with the Tubulin and Septin cytoskeletons, a select group of G-proteins and kinases, proteins involved in vesicle release and proteins associated with the extracellular matrix, including Neurofascin and Hapln1. C, Dark rearing causes decrease of some age regulated kinases, but also the further decrease of mitochondrial proteins and GAT-1. It increases levels of proteins associated with the Actin cytoskeleton, subunits of Protein Kinase A and G-proteins, Ube4b, GABA(A) receptors and N-CAM 180.
Synaptic Proteins Regulated with Age or Dark Rearing
In order to obtain insight into the molecular events involved in the regulation of the critical period, we compared synaptic proteins of binocular visual cortices of P30 mice with P46 and P46-DR mice. To identify proteins that were possibly involved in maintaining or activating plasticity in the dark-reared visual cortex, we categorized the synaptic proteins in three groups. The first were proteins whose expression at synaptic membranes altered with age (P30 versus P46), but not upon dark rearing (P30 versus P46-DR). These proteins represent the view that dark rearing prevents specific changes in the visual cortex that normally occur with age and cause the closure of the critical period. The second group contained proteins of which expression was also regulated with age, whereas dark rearing did not prevent this. These proteins may well affect OD plasticity, but are unlikely to be involved in preventing closure of the critical period by dark rearing. The third group contained proteins of which levels did not change with age, but differed in dark-reared mice from the situation in nondeprived P30 or P46 mice. These proteins are unlikely to be involved in critical period closure with age, but may represent signaling pathways that prevent closure in the dark-reared mice. We found that at P46, 69 proteins were expressed at levels significantly different from the situation at P30 (Fig. 1B). After dark rearing, levels of 29 of these proteins were not different from the situation at P30, whereas levels of the other 40 remained different from those at P30. However, dark rearing also induced many changes in levels of proteins that did not change with age, together causing the P46-DR and P30 groups to be the groups most different from each other (Fig. 1B). Changes in the expression of proteins involved in regulating the cytoskeleton, synaptic efficacy, intracellular signaling, or mitochondrial proteins are discussed below. These represented more than 60% of all differentially expressed proteins. Supplemental Table S3 shows all proteins with altered levels.
Cytoskeleton-associated Proteins
Similar to the situation in V1 after MD, consistent changes occurred in the level of expression of cytoskeletal proteins with age. We found reduced levels of actin cytoskeleton-regulating proteins with age (p < 0.05 Chi/square test with Benjamini/Hochberg correction compared with all identified proteins), which was partially prevented by dark rearing (Fig. 3A). This included proteins typically expressed in neurons undergoing synaptogenesis, such as Drebrin (14), GAP-43 (15), and delta-2 Catenin (22). α-Adducin was the only actin-associated protein whose level increased with age. The levels of actin cytoskeleton-regulating proteins whose age-dependent decrease was not reversed by dark rearing (Fig. 3B) were those of Cofilin and β-Catenin. Interestingly, dark rearing also increased the levels of actin cytoskeleton-regulating proteins that did not decrease with age (Fig. 3C). Only one such protein, MARCKS, showed the opposite behavior and was actually decreased upon dark rearing. In contrast, we detected increased levels of proteins associated with the tubulin and neurofilament cytoskeletons with age (Fig. 3B). These changes were not prevented by dark rearing. We noticed a similar trend for Septins, which have recently been found to form filaments localized at the neck of dendritic spines (23) and at the presynapse (24). The overrepresentation of tubulin-, neurofilament-, and septin-cytoskeleton associated proteins with age was significant (p < 0.0001, Chi-square test with Benjamini-Hochberg correction). Proteins identified or quantified with less stringent criteria (C.I. between 85–95%, indicated with an asterisk, and/or quantified with one peptide or more in each set, indicated with gray bars) were two additional tubulin-skeleton related proteins and various actin-associated proteins that changed their levels in the same direction as other members of these groups.
Fig. 3.
Proteins regulated by age or altered visual experience. Proteins are again categorized (A–C) associated with the cytoskeleton, (D–F) involved in signal transduction, or (G–I) regulating synaptic efficacy. (J, K) Proteins that do not belong to these categories but are differentially expressed more than 1.25-fold are also shown. Color intensity indicates the level of regulation. Lower expression is indicated in blue, whereas higher expression is indicated in red. The upper panels (A, D, G) show proteins whose expression in visual cortex at P30 is significantly different from that at P46 (left column), whereas expression in dark-reared visual cortex at P46 is not different from that of P46 or P30 visual cortex from normally reared mice. Middle panels (B, E, H) represent proteins different with age, also if dark reared. Lower panels (C, F, I) show those proteins that are not regulated with age, but whose expression in visual cortex from P46 dark-reared mice differs from that in normally reared mice at P30 or P46. The strongly differentially expressed proteins are categorized in a similar fashion, with the left panel (J) showing the age and dark rearing regulated proteins and one age-only regulated protein, and the right panel (K) showing the proteins only affected by dark rearing. * confidence between 85% and 95%. Proteins quantified with less than two peptides in more than one set are indicated in italics and light gray bars. The order of the proteins as shown is determined by hierarchical clustering using average linkage (Multiexperiment viewer, TM4 software).
Protein Kinases and G-Protein Signaling
We found that levels of several kinases and proteins involved in G-protein signaling were increased with age, which in most cases was prevented by dark rearing (Fig. 3D–3E). These included CaMK-II α and PIP5K1-γ. We were surprised, however, by the relatively large number of proteins (p < 0.01 Chi-square test with Benjamini-Hochberg correction compared with all identified proteins) involved in PKA- and G-protein-signaling that were not regulated with age but whose levels were significantly higher upon dark rearing than in P30- or P46 nondeprived visual cortex (Fig. 3F). These proteins included PKA C-α, the regulatory subunit RII-α, the PKA anchor protein AKAP150, and the small GTPase H-Ras. Signaling proteins that were decreased upon dark rearing included Ephexin-1, a Rho-type guanine nucleotide exchange factor involved in Eph signaling, as well as Calmodulin. Dark rearing also caused a strong decrease (1.37×) in the integrin-associated protein SHPS-1. This protein is involved in IGF-I signaling (25), which has recently been found to affect OD plasticity (7) and visual cortical development (26). When using less stringent criteria for selecting the proteins with altered levels, PKA C-β was also found to be increased as were various additional G-proteins.
Proteins Regulating Synaptic Efficacy
The group of synaptic membrane associated proteins that were up-regulated with age were dominated by a large set of presynaptic proteins well known to be involved in vesicle release and recycling, including Synapsins-1 and −2 (confirmed by Western blot, supplemental Fig. S1), Syntaxin-1B, NSF, Munc-18, Synaptogyrin-1, Synaptotagmin-2, Clathrin Lca and AP-2 (Figs. 3G–3H). Dark rearing only partially prevented this (Fig. 3G), for example in the case of Synapsin-1, Syntaxin-1B, and Synaptogyrin-1. Interestingly, no postsynaptic density-associated proteins involved in AMPAR trafficking showed increased levels with age, except for Adam22 (and interestingly one of its ligands, LGI1). With dark rearing, several postsynaptic proteins regulating synaptic strength showed altered levels, whereas they were not changed with age (Fig. 3I). Levels of two proteins involved in reducing synaptic strength, mGluR2 (27) and Synaptojanin-1 (28), and one that increases synaptic strength (4.1N) (29) were increased upon dark rearing. Interestingly, Syntaxin-1A was not altered with age, but increased with dark rearing, whereas Syntaxin-1B increased with age, which was reversed by dark-rearing. Overall, the changes in proteins regulating synaptic strength were consistent with an increase in proteins involved in vesicle release with age, whereas dark rearing partially reversed this trend.
Dark rearing has been shown to delay the development of perisomatic GABAergic innervation (30), which plays an essential role in initiating the critical period (31). We were therefore surprised to find an up-regulation of the GABA(A) receptor α-1 with DR. The change in GABA(A) receptor α-1 could not be confirmed by Western blot, however, potentially because it was too small to detect (supplemental Fig. S1). Moreover, we found a decrease in the level of the GABA reuptake protein GAT-1 (confirmed by Western blot, supplemental Fig. S1), which is expected to increase synaptic and extrasynaptic GABA levels.
When lowering the selection criteria for the regulated proteins, the overall picture did not alter. GABA(A) receptor β-1 showed similar changes with dark rearing as the α-1 subunit. Interestingly, several proteins found to postsynaptically regulate glutamatergic synaptic transmission were now detected among the proteins altered with age. These included the strongly (1.25×) increased TARP γ3 - a protein regulating AMPAR trafficking, the kainate receptor Grik5, and a regulator of NMDA receptors, Cask and its interactor Caskin-1. The latter three proteins are also present in the presynapse.
Mitochondrial Proteins
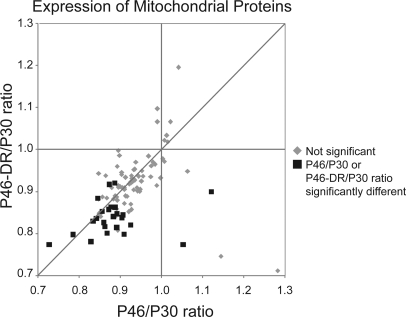
Among the proteins whose levels were changed most consistently were mitochondrial proteins (Fig. 4 and supplemental Table S6), which may be derived from membranes of synapse-resident mitochondria. As synaptosomal membrane fractions result in an enrichment of synaptic proteins but are not pure, it is possible that mitochondria derived from other cellular compartments also contribute to the observed changes. With age, nine proteins were significantly down-regulated. Although in P46-DR mice, five proteins were not significantly different from P30, expression of many other mitochondrial proteins decreased further resulting in a total of 19 proteins that were significantly decreased with dark rearing. This trend toward down-regulation of mitochondrial proteins with age and/or dark rearing did not only reflect a specific subset but was visible in over 85% of all mitochondrial proteins identified, although it was not always significant (Fig. 4). Moreover, the mitochondrial proteins were overrepresented among those reduced with age (p < 0.05 Chi-square test with Benjamini-Hochberg correction) or reduced with DR but not with age (p < 0.0001) compared with all identified proteins. This suggests that there was an actual reduction of mitochondrial content in synapses under these conditions.
Fig. 4.
Levels of mitochondrial proteins decrease with age, and more so by dark rearing. Scatter plot of relative changes in expression levels of mitochondrial proteins with age (x axis, P46/P30) or with age and dark rearing (P46-DR/P30, y axis). Black squares indicate proteins that are significantly affected with age or with dark rearing. All other identified mitochondrial proteins are represented by gray diamonds.
Strongly Regulated Proteins of Special Interest
There were several strongly regulated (>1.25×) proteins that did not fit the categories described above (Figs. 3J, 3K). Three of these were proteins associated with the extracellular matrix (ECM), which has been implicated in limiting plasticity after the critical period (32). With age we found up-regulation of Neurofascin-1, a critical component of the ECM around the axon initial segment (33), and Hapln1 (Hyaluronan associated link protein1, also known as Cartilage linking protein 1) a crucial factor for the initial assembly of perineuronal nets (34). The adhesion molecule N-CAM 180 showed strongly (1.28×) decreased levels with age, which was partially prevented by DR (confirmed by Western blot, supplemental Fig. S1). N-CAM 180 is a protein that is strongly polysialylated (PSA) and interacts with the ECM. It was recently found to inhibit the development of inhibitory synapses and delay the onset of the critical period (35).
When using the less stringent selection criteria, various other proteins with poorly described functions in the brain were detected, such as XTRP2, FAM5A, Hsp70 12B, ANKFY1, and PKM2. Interestingly, we also identified two ubiquitin ligases implicated in axon outgrowth and degeneration: Ube4b and Rpm-1 also known as highwire and MYCBP2. In P46-DR mice we observed a strong decrease in levels (1.26× versus P46 and 1.45× versus P30) of the E3 ubiquitin ligase Rpm-1. This protein has been implicated in presynaptic growth and inhibition of axon outgrowth (36). More recently, it was discovered that Rpm-1 down-regulates the dual leucine zipper kinase DLK-1, a kinase, which mediates axon degeneration in Caenorhabditis elegans and Drosophila (37). In mice DLK-1 has been shown to induce Wallerian axon degeneration (38), the disintegration of an axon after its separation from the cell body (39). In our analysis, DLK-1 itself was detected only in three out of four experiments. Interestingly, it showed the opposite trend from Rpm-1, with a slightly higher expression with MD (1.06× n.s.), age (1.2× p = 0.0036) and DR (1.26× p = 0.0024).
Another protein in the ubiquitin pathway, Ube4b was also increased in level after MD (1.17×, n.s.) with age (1.3×) and even more so upon DR (1.7×). This was confirmed by Western blot (supplemental Fig. S1). Ube4b has been implicated in protection of neurons from axonal degeneration (40). Moreover, a mutation in the Ube4b gene locus has been identified in mice results resulting in strongly delayed Wallerian degeneration of axons and their synapses (39). This so-called WldS-mutation encompasses a gene triplication resulting in the production of a fusion protein consisting of the first 70AA of Ube4b and the nicotinamide mononucleotide adenylyl transferase NMNAT-1, an NAD+ synthase. Plasticity in V1 may therefore depend on those signaling molecules that also regulate Wallerian axon degeneration.
Comparison of Proteomics Data to Published Microarray Studies
In order to see whether our proteomics approach identified similar proteins with MD or DR as identified with a previously published microarray approach we compared our results with those from Tropea et al. (7) (supplemental Table S8 and supplemental Fig. S2). In this study, MD was performed from p23 until p27, and dark rearing from birth to p27. We found that there was 2.5-fold enrichment of proteins whose message was also found to be significantly regulated with MD (13 of 51 proteins, compared with 3748 out of 36,902 genes, p = 0.0003, Chi-square test), and a 2.7-fold enrichment for DR (10 of 51 proteins, compared with 2680 out of 36,902, genes p = 0.0007, Chi-square test). At the same time, most proteins (75% for MD and 80% for DR) whose levels were changed did not show a concomitant change at the mRNA level underlining the importance of analyzing synaptic proteins directly. The proteins whose levels were changed at both the mRNA and the protein level showed a correlation between these levels with the DR paradigm (0.59, one tailed p < 0.05) but not with the MD paradigm (0.05, one tailed p = 0.44). When proteins quantified with less stringent criteria were included in the analysis, the results did not change much. The enrichment was then 3.5-fold for MD and 2.8-fold for DR, Moreover, 65% of proteins with changed levels in the MD group did not show a concomitant change at the mRNA level. This was 80% with DR. The correlation for altered mRNA and protein levels remained 0.59 (one tailed p < 0.01) for DR and became −0.06 (one tailed p = 0.38) for MD.
DISCUSSION
This study provides a comprehensive insight into the changes in levels of synaptic membrane proteins that accompany OD plasticity in the visual cortex (for an overview see Fig. 5). We found that differences in protein levels after MD, with aging and upon dark rearing revealed distinct cellular processes that may underlie plasticity. Some of these have been previously observed to be associated with plasticity in V1 through forward or reverse genetics approaches or gene expression studies, whereas many others represent novel mechanisms regulating critical period plasticity.
Monocular Deprivation Alters Levels of Intracellular Signaling Proteins, Regulators of the Actin Cytoskeleton and Endocytosis Related Proteins
OD plasticity is associated with the growth and retraction of axons and the loss and gain of dendritic spines. Upon MD during the critical period, we found that levels of proteins involved in regulating the actin cytoskeleton were increased, which may well reflect these structural changes of synapses in OD plasticity (Fig. 5A). MD also resulted in increased levels of PKC and the RII-β subunit of PKA, two kinases that have been implicated in plasticity in the visual cortex (16, 17). Potentially new candidate proteins that regulate OD plasticity are the Rho- and Ras regulating G-Proteins SOS-1 and StARD13 that were strongly decreased by MD. This was accompanied by a similarly strong decrease in Semaphorin-4D. This protein has been found to affect GABAergic synapse formation (41) but also shown to affect Rho- and Ras signaling through its receptor Plexin1, which mediates spine stability (19) and axonal growth cone collapse (20). The lower levels of Semaphorin-4D may thus stimulate axon growth and spine turnover during OD plasticity.
We also observed an increase in proteins involved in Clathrin-dependent endocytosis upon MD. This is possibly related to its role in AMPA-receptor recycling, which has recently been found to be essential for OD plasticity (42), but may also reflect changes in vesicle release or an increase in membrane trafficking accompanying structural changes. In a previous study it was found that cell surface AMPA receptors were reduced after 1 day of MD in rats (43). Although we did not directly assess surface AMPA receptors, we have observed previously that iTRAQ quantitation of synaptic membrane derived proteins does allow the detection of changes in AMPA receptor levels (9). We therefore expected to observe changes in the synaptic proteome consistent with an overall decrease of synaptic efficacy upon MD. However, we observed no changes in AMPA-receptor subunits after 4 days of MD. In fact, the observed changes in protein levels were positively correlated with the changes in these proteins that occurred with age (between P30 and P46). This suggests that after MD, dendritic spines in the visual cortex become more mature, which is usually associated with an increase in their size, efficacy, and stability. These observations may be explained by increased spine turnover during OD plasticity. The early decrease of AMPA receptors are thought to precede the actual loss of spines (43), which occurs after 3–4 days of MD (21). Small spines with lower levels of AMPA receptors are likely to be lost preferentially, effectively causing a relative increase of more mature spines.
Age-related Changes in Proteins Regulating Vesicle Release and the Cytoskeleton
With age, we observed an increase in proteins involved in vesicle release such as Synapsins-1 and −2, NSF, MUNC-18, Synaptogyrin-1, and Syntaxin-1B (Fig. 5B). This suggests that synaptic efficacy increases in V1 during development by boosting the potential for neurotransmitter release, which is in line with a previous study showing a Synapsin-dependent increase of synaptic vesicles and their recruitment in the developing hippocampus (44). The increase of these synaptic proteins was partially prevented by dark rearing (Fig. 5C), but the general trend was not altered.
Very consistent changes occurred in the levels of cytoskeleton-associated proteins. Although actin cytoskeleton-regulating proteins decreased with age, proteins associated with the tubulin, neurofilament, and septin cytoskeletons increased. Dark rearing did not prevent the increase of these latter proteins. However, the lower level of many of the actin-associated proteins with age was prevented by dark rearing. Moreover, several actin-associated proteins whose expression levels did not change with age were higher upon dark rearing. Dark rearing thus prevents specific aspects of synapse maturation in V1, whereas other aspects continue also in the absence of visual input. The increase in actin-cytoskeleton-regulating proteins is in line with the idea that dark rearing increases spine motility (45), which may underlie the increased levels of plasticity that can be induced during the critical period. Recent evidence suggests that microtubules play an important role in the development and plasticity of dendritic spines (46). Their function in critical period plasticity remains to be determined. We also found an increased expression of various septins, which have been associated with mature synapses and found to be expressed at spine necks (23) and at presynaptic sites (24).
Dark Rearing Increases PKA Subunits and G-Proteins
We found that age and DR affected the expression of signaling proteins such as kinases and G-proteins. Some of these proteins changed with age such as CaMK-II alpha, PIP5K1-gamma and a few G-proteins whose function in synaptic plasticity are unknown, and most of these changes were prevented by dark rearing. These changes may well be caused by synaptic activity dependent recruitment of these proteins to the synaptic membrane, as has been shown previously for CaMK-II α (47). Most interestingly, however, a large set of G-proteins, including the small GTPase H-Ras, and several PKA-subunits were not altered with age, but showed increased levels in the dark-reared visual cortex. It has been shown previously that in the absence of RII-alpha or upon inhibition of PKA, OD plasticity is reduced, whereas cholera toxin (a Gs-protein stimulant), forskolin (a Gs-protein-independent activator of adenylate cyclase), and dibutyryl cAMP (a cAMP analog) all stimulate OD plasticity in the adult visual cortex (48). Also the expression of an active form of CREB, a target of PKA, increases adult plasticity (49). Thus, the up-regulation of PKA and G-protein signaling proteins may represent a mechanism by which dark rearing increases the potential for plasticity in the adult visual cortex.
Influence of Age and Dark Rearing on Proteins Associated with Inhibition and the Extracellular Matrix
In recent years, the development of the extracellular matrix (32) and GABAergic innervation (31) have been implicated in the onset and closure of the critical period. Among the proteins that were expressed at different levels with age or dark rearing we found several proteins that are involved in these events. Among the proteins whose expression levels changed most strongly with age was Hapln1, a protein expressed by parvalbumin-expressing interneurons and believed to be essential for the formation of perineuronal nets (34). Its involvement in visual cortex plasticity has recently been identified (50). A second ECM protein strongly increased with age was neurofascin-1, a protein that accumulates around the axon initial segment and is indispensible for setting up inhibitory input onto this axon segment (33). Dark rearing did not decrease expression of these proteins suggesting that they may not be the primary regulators of visual input dependent ECM formation. We also noticed a strong decrease of N-CAM 180 with age, which was partially reversed with DR. During early postnatal life, N-CAM 180 is heavily polysialylated and interacts extensively with the ECM. It has recently been shown that upon eye opening expression of PSA-N-CAM quickly decreases, allowing the formation of perisomatic inhibitory inputs onto pyramidal neurons, which in turn initiates the critical period (35). The age-dependent decrease of N-CAM 180 and its reversal by dark rearing may thus be part of this process. Counter-intuitively, but in line with previous work (7, 51) we detected an increase in GABA(A) receptor α-1 with dark rearing and a decrease in GAT-1, a protein responsible for GABA reuptake. Together, these changes are expected to increase inhibitory input, which seems to contradict studies showing that dark rearing delays the development of inhibitory innervation (30). A possible explanation may be that dark rearing delays inhibitory synapse formation (52), but that this is partially compensated for by an increased sensitivity of postsynaptic neurons to inhibitory input.
Signals Regulating Wallerian Degeneration are Altered With Age and Dark Rearing
Finally, we found that proteins implicated in Wallerian axon degeneration were altered with age and dark rearing. The exact signaling pathways that regulate this active form of axon degeneration are still under intense investigation. Several mechanisms have been implicated and include reduced mitochondrial activity (53), alterations in the ubiquitin/proteasome system (54), increased microtubule acetylation through SIRT proteins (55), and the increased expression of DLK-1 by reduced activity of the ubiquitin ligase Rpm-1 (38). We found that with DR, expression of many mitochondrial proteins was reduced. In contrast, we detected a strong increase of the ubiquitin conjugation factor Ube4b under these conditions. A fusion protein consisting of the N-terminal part of Ube4b and the NAD synthase NMNAT-1 is expressed in WldS mutant mice in which Wallerian axon degeneration is strongly diminished (39). These results show that signaling cascades implicated in Wallerian degeneration are also active during the regulation of the critical period, and may thus be involved in mediating experience dependent plasticity in V1.
Altogether, this study provides unprecedented insight into the changes that occur in the synaptic membrane proteome during OD plasticity and with the regulation of the critical period. The results show that dark rearing does not simply halt cortical development, but alters various signaling cascades different from those that change with age. Proteomic changes induced by MD are different from those induced by DR, and correlate better with those occurring with age possibly because of pruning of immature synapses during the first days of OD plasticity. Our study reveals many novel signaling pathways potentially involved in cortical plasticity. We anticipate that future research on these signaling pathways will significantly advance our understanding of the molecular mechanisms underlying synaptic cortical plasticity.
Acknowledgments
We thank N. Saiepour for technical assistance, D. van Versendaal and Dr. C. Lohmann for critical reading of the manuscript, Dr. B. Ziegler for providing the anti- Septin-8 antibody, Dr. M. Coleman for discussions and providing the Ube4b antibody.
Footnotes
* This work was supported by a research support grant and a Vidi grant from the Netherlands Organization for Health Research and Development (ZonMW). C.N.L. is supported by the Netherlands Academy of Arts and Sciences (KNAW) and ZonMW. M.D., J.M.H., J.A.H., K.W.L., A.B.S., and C.N.L. were supported by Bsik grant 03053 from SenterNovem.
 This article contains supplemental Tables S1–S8 and Figs. S1 and S2.
This article contains supplemental Tables S1–S8 and Figs. S1 and S2.
1 The abbreviations used are:
- V1
- primary visual cortex
- iTRAQ
- isobaric tag for relative and absolute quantitation
- MD
- monocular deprivation
- OD
- ocular dominance
- FDR
- false discovery rate
- ECM
- extracellular matrix.
REFERENCES
- 1. Gordon J. A., Stryker M. P. (1996) Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J. Neurosci. 16, 3274–3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Antonini A., Fagiolini M., Stryker M. P. (1999) Anatomical correlates of functional plasticity in mouse visual cortex. J. Neurosci. 19, 4388–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cynader M. (1983) Prolonged sensitivity to monocular deprivation in dark-reared cats: effects of age and visual exposure. Brain Res. 284, 155–164 [DOI] [PubMed] [Google Scholar]
- 4. Heimel J. A., Hermans J. M., Sommeijer J. P., Levelt C. N. (2008) Genetic control of experience-dependent plasticity in the visual cortex. Genes Brain Behav. 7, 915–923 [DOI] [PubMed] [Google Scholar]
- 5. Nedivi E. (1999) Molecular analysis of developmental plasticity in neocortex. J. Neurobiol. 41, 135–147 [PMC free article] [PubMed] [Google Scholar]
- 6. Majdan M., Shatz C. J. (2006) Effects of visual experience on activity-dependent gene regulation in cortex. Nat. Neurosci. 9, 650–659 [DOI] [PubMed] [Google Scholar]
- 7. Tropea D., Kreiman G., Lyckman A., Mukherjee S., Yu H., Horng S., Sur M. (2006) Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nat. Neurosci. 9, 660–668 [DOI] [PubMed] [Google Scholar]
- 8. Heimel J. A., Hartman R. J., Hermans J. M., Levelt C. N. (2007) Screening mouse vision with intrinsic signal optical imaging. Eur. J. Neurosci. 25, 795–804 [DOI] [PubMed] [Google Scholar]
- 9. Van den Oever M. C., Goriounova N. A., Wan L. K., Van der Schors R. C., Binnekade R., Schoffelmeer A. N., Mansvelder H. D., Smit A. B., Spijker S., De Vries T. J. (2008) Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat. Neurosci. [DOI] [PubMed] [Google Scholar]
- 10. Li K. W., Miller S., Klychnikov O., Loos M., Stahl-Zeng J., Spijker S., Mayford M., Smit A. B. (2007) Quantitative proteomics and protein network analysis of hippocampal synapses of CaMKIIalpha mutant mice. J. Proteome. Res. 6, 3127–3133 [DOI] [PubMed] [Google Scholar]
- 11. Tusher V. G., Tibshirani R., Chu G. (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U.S.A. 98, 5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vizcaíno J. A., Côté R., Reisinger F., Barsnes H., Foster J. M., Rameseder J., Hermjakob H., Martens L. (2010) The Proteomics Identifications database: 2010 update. Nucleic Acids Res. 38, D736-D742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ow S. Y., Noirel J., Cardona T., Taton A., Lindblad P., Stensjö K., Wright P. C. (2009) Quantitative overview of N2 fixation in Nostoc punctiforme ATCC 29133 through cellular enrichments and iTRAQ shotgun proteomics. J. Proteome. Res. 8, 187–198 [DOI] [PubMed] [Google Scholar]
- 14. Imamura K., Shirao T., Mori K., Obata K. (1992) Changes of drebrin expression in the visual cortex of the cat during development. Neurosci. Res. 13, 33–41 [DOI] [PubMed] [Google Scholar]
- 15. Frey D., Laux T., Xu L., Schneider C., Caroni P. (2000) Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity. J. Cell Biol. 149, 1443–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schrader L. A., Perrett S. P., Ye L., Friedlander M. J. (2004) Substrates for coincidence detection and calcium signaling for induction of synaptic potentiation in the neonatal visual cortex. J. Neurophysiol. 91, 2747–2764 [DOI] [PubMed] [Google Scholar]
- 17. Fischer Q. S., Beaver C. J., Yang Y., Rao Y., Jakobsdottir K. B., Storm D. R., McKnight G. S., Daw N. W. (2004) Requirement for the RIIbeta isoform of PKA, but not calcium-stimulated adenylyl cyclase, in visual cortical plasticity. J. Neurosci. 24, 9049–9058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di, Cristo G., Berardi N., Cancedda L., Pizzorusso T., Putignano E., Ratto G. M., Maffei L. (2001) Requirement of ERK activation for visual cortical plasticity. Science 292, 2337–2340 [DOI] [PubMed] [Google Scholar]
- 19. Lin X., Ogiya M., Takahara M., Yamaguchi W., Furuyama T., Tanaka H., Tohyama M., Inagaki S. (2007) Sema4D-plexin-B1 implicated in regulation of dendritic spine density through RhoA/ROCK pathway. Neurosci. Lett. 428, 1–6 [DOI] [PubMed] [Google Scholar]
- 20. Ito Y., Oinuma I., Katoh H., Kaibuchi K., Negishi M. (2006) Sema4D/plexin-B1 activates GSK-3beta through R-Ras GAP activity, inducing growth cone collapse. EMBO Rep. 7, 704–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mataga N., Mizuguchi Y., Hensch T. K. (2004) Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron 44, 1031–1041 [DOI] [PubMed] [Google Scholar]
- 22. Matter C., Pribadi M., Liu X., Trachtenberg J. T. (2009) Delta-catenin is required for the maintenance of neural structure and function in mature cortex in vivo. Neuron 64, 320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tada T., Simonetta A., Batterton M., Kinoshita M., Edbauer D., Sheng M. (2007) Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr. Biol. 17, 1752–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xue J., Tsang C. W., Gai W. P., Malladi C. S., Trimble W. S., Rostas J. A., Robinson P. J. (2004) Septin 3 (G-septin) is a developmentally regulated phosphoprotein enriched in presynaptic nerve terminals. J. Neurochem. 91, 579–590 [DOI] [PubMed] [Google Scholar]
- 25. Maile L. A., Capps B. E., Miller E. C., Aday A. W., Clemmons D. R. (2008) Integrin-associated protein association with SRC homology 2 domain containing tyrosine phosphatase substrate 1 regulates igf-I signaling in vivo. Diabetes 57, 2637–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ciucci F., Putignano E., Baroncelli L., Landi S., Berardi N., Maffei L. (2007) Insulin-like growth factor 1 (IGF-1) mediates the effects of enriched environment (EE) on visual cortical development. PLoS. One. 2, e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Renger J. J., Hartman K. N., Tsuchimoto Y., Yokoi M., Nakanishi S., Hensch T. K. (2002) Experience-dependent plasticity without long-term depression by type 2 metabotropic glutamate receptors in developing visual cortex. Proc. Natl. Acad. Sci. U.S.A. 99, 1041–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gong L. W., De Camilli P. (2008) Regulation of postsynaptic AMPA responses by synaptojanin 1. Proc. Natl. Acad. Sci. U.S.A. 105, 17561–17566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin D. T., Makino Y., Sharma K., Hayashi T., Neve R., Takamiya K., Huganir R. L. (2009) Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat. Neurosci. 12, 879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morales B., Choi S. Y., Kirkwood A. (2002) Dark rearing alters the development of GABAergic transmission in visual cortex. J. Neurosci. 22, 8084–8090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hensch T. K., Fagiolini M., Mataga N., Stryker M. P., Baekkeskov S., Kash S. F. (1998) Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282, 1504–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pizzorusso T., Medini P., Berardi N., Chierzi S., Fawcett J. W., Maffei L. (2002) Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248–1251 [DOI] [PubMed] [Google Scholar]
- 33. Ango F., Di Cristo G., Higashiyama H., Bennett V., Wu P., Huang Z. J. (2004) Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell 119, 257–272 [DOI] [PubMed] [Google Scholar]
- 34. Carulli D., Rhodes K. E., Fawcett J. W. (2007) Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J. Comp Neurol. 501, 83–94 [DOI] [PubMed] [Google Scholar]
- 35. Di Cristo G., Chattopadhyaya B., Kuhlman S. J., Fu Y., Bélanger M. C., Wu C. Z., Rutishauser U., Maffei L., Huang Z. J. (2007) Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat. Neurosci. 10, 1569–1577 [DOI] [PubMed] [Google Scholar]
- 36. Li H., Kulkarni G., Wadsworth W. G. (2008) RPM-1, a Caenorhabditis elegans protein that functions in presynaptic differentiation, negatively regulates axon outgrowth by controlling SAX-3/robo and UNC-5/UNC5 activity. J. Neurosci. 28, 3595–3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakata K., Abrams B., Grill B., Goncharov A., Huang X., Chisholm A. D., Jin Y. (2005) Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell 120, 407–420 [DOI] [PubMed] [Google Scholar]
- 38. Miller B. R., Press C., Daniels R. W., Sasaki Y., Milbrandt J., DiAntonio A. (2009) A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat. Neurosci. 12, 387–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coleman M. P., Conforti L., Buckmaster E. A., Tarlton A., Ewing R. M., Brown M. C., Lyon M. F., Perry V. H. (1998) An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proc. Natl. Acad. Sci. U.S.A. 95, 9985–9990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaneko-Oshikawa C., Nakagawa T., Yamada M., Yoshikawa H., Matsumoto M., Yada M., Hatakeyama S., Nakayama K., Nakayama K. I. (2005) Mammalian E4 is required for cardiac development and maintenance of the nervous system. Mol. Cell. Biol. 25, 10953–10964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paradis S., Harrar D. B., Lin Y., Koon A. C., Hauser J. L., Griffith E. C., Zhu L., Brass L. F., Chen C., Greenberg M. E. (2007) An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron 53, 217–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yoon B. J., Smith G. B., Heynen A. J., Neve R. L., Bear M. F. (2009) Essential role for a long-term depression mechanism in ocular dominance plasticity. Proc. Natl. Acad. Sci. U.S.A. 106, 9860–9865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heynen A. J., Yoon B. J., Liu C. H., Chung H. J., Huganir R. L., Bear M. F. (2003) Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat. Neurosci. 6, 854–862 [DOI] [PubMed] [Google Scholar]
- 44. Bogen I. L., Jensen V., Hvalby O., Walaas S. I. (2009) Synapsin-dependent development of glutamatergic synaptic vesicles and presynaptic plasticity in postnatal mouse brain. Neuroscience 158, 231–241 [DOI] [PubMed] [Google Scholar]
- 45. Majewska A., Sur M. (2003) Motility of dendritic spines in visual cortex in vivo: changes during the critical period and effects of visual deprivation. Proc. Natl. Acad. Sci. U.S.A. 100, 16024–16029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jaworski J., Kapitein L. C., Gouveia S. M., Dortland B. R., Wulf P. S., Grigoriev I., Camera P., Spangler S. A., Di Stefano P., Demmers J., Krugers H., Defilippi P., Akhmanova A., Hoogenraad C. C. (2009) Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron 61, 85–100 [DOI] [PubMed] [Google Scholar]
- 47. Elgersma Y., Fedorov N. B., Ikonen S., Choi E. S., Elgersma M., Carvalho O. M., Giese K. P., Silva A. J. (2002) Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning. Neuron 36, 493–505 [DOI] [PubMed] [Google Scholar]
- 48. Imamura K., Kasamatsu T., Shirokawa T., Ohashi T. (1999) Restoration of ocular dominance plasticity mediated by adenosine 3′,5′-monophosphate in adult visual cortex. Proc. Biol. Sci. 266, 1507–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pham T. A., Graham S. J., Suzuki S., Barco A., Kandel E. R., Gordon B., Lickey M. E. (2004) A semi-persistent adult ocular dominance plasticity in visual cortex is stabilized by activated CREB. Learn. Mem. 11, 738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carulli D., Pizzorusso T., Kwok J. C., Putignano E., Poli A., Forostyak S., Andrews M. R., Deepa S. S., Glant T. T., Fawcett J. W. (2010) Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 133, 2331–2347 [DOI] [PubMed] [Google Scholar]
- 51. Chen L., Yang C., Mower G. D. (2001) Developmental changes in the expression of GABA(A) receptor subunits (alpha(1), alpha(2), alpha(3)) in the cat visual cortex and the effects of dark rearing. Brain Res. Mol. Brain Res. 88, 135–143 [DOI] [PubMed] [Google Scholar]
- 52. Chattopadhyaya B., Di Cristo G., Higashiyama H., Knott G. W., Kuhlman S. J., Welker E., Huang Z. J. (2004) Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J. Neurosci. 24, 9598–9611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yahata N., Yuasa S., Araki T. (2009) Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J. Neurosci. 29, 6276–6284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhai Q., Wang J., Kim A., Liu Q., Watts R., Hoopfer E., Mitchison T., Luo L., He Z. (2003) Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron 39, 217–225 [DOI] [PubMed] [Google Scholar]
- 55. Suzuki K., Koike T. (2007) Mammalian Sir2-related protein (SIRT) 2-mediated modulation of resistance to axonal degeneration in slow Wallerian degeneration mice: a crucial role of tubulin deacetylation. Neuroscience 147, 599–612 [DOI] [PubMed] [Google Scholar]