Abstract
Organisms survive changes in the environment by altering their rates of metabolism, growth, and reproduction. At the same time, the system must ensure the stability and functionality of its macromolecules. Fluctuations in the environment are sensed by highly conserved stress responses and homeostatic mechanisms, and of these, the heat shock response (HSR) represents an essential response to acute and chronic proteotoxic damage. However, unlike the strategies employed to maintain the integrity of the genome, protection of the proteome must be tailored to accommodate the normal flux of nonnative proteins and the differences in protein composition between cells, and among individuals. Moreover, adult cells are likely to have significant differences in the rates of synthesis and clearance that are influenced by intrinsic errors in protein expression, genetic polymorphisms, and fluctuations in physiological and environmental conditions. Here, we will address how protein homeostasis (proteostasis) is achieved at the level of the cell and organism, and how the threshold of the stress response is set to detect and combat protein misfolding. For metazoans, the requirement for coordinated function and growth imposes additional constraints on the detection, signaling, and response to misfolding, and requires that the HSR is integrated into various aspects of organismal physiology, such as lifespan. This is achieved by hierarchical regulation of heat shock factor 1 (HSF1) by the metabolic state of the cell and centralized neuronal control that could allow optimal resource allocation between cells and tissues. We will examine how protein folding quality control mechanisms in individual cells may be integrated into a multicellular level of control, and further, even custom-designed to support individual variability and impose additional constraints on evolutionary adaptation.
The heat shock response (HSR), essential for maintaining cellular proteostasis, is integrated into aspects of organismal physiology, such as growth and lifespan. Cell nonautonomous regulation of heat shock factor 1 (HSF1) activity and chaperone expression by the nervous system may set the threshold for the stress responses.
PROTEIN QUALITY CONTROL: AN OVERVIEW
The fidelity of information transfer, from DNA to proteins, requires quality control mechanisms to minimize the propagation of errors. Although it is evident that alterations of even a single base pair of DNA can have far-reaching consequences on selection and survival, necessitating highly accurate quality control mechanisms to identify and repair DNA damage, for proteins, the constraints are less clear. Protein functionality is the consequence of two seemingly incompatible properties—achieving a stable, defined native structure, while maintaining conformational flexibility. A protein has, in principle, all the information from the primary amino acid sequence to achieve the specific fold characteristic of its native state (Anfinsen 1973). For most eukaryotic proteins, however, this is a challenging task because of the long-range contacts, multiple transition states and intermediates that are populated during folding, presence of intrinsically disordered domains, and multidomain structure essential for assembly and function of molecular machines (Jaenicke 1991; Fersht 1995; Wolynes et al. 1995; Plaxco et al. 1998; Stevens and Argon 1999b; Thulasiraman et al. 1999; van den Berg et al. 2000; Brockwell and Radford 2007; Ferreiro et al. 2007). Consequently, many proteins in vivo may only be marginally stable (Somero 1995; DePristo et al. 2005), or acquire stability on assembly with a partner (Sinclair et al. 1994; Demchenko 2001), binding with a ligand (Pratt and Toft 2003; Park and Marqusee 2005), or targeting to a specific subcellular compartment (Deshaies et al. 1988). Thus, the “correct” conformation of a protein becomes a functional definition.
Adding to this complexity, proteins are a renewable resource and therefore exist in a constant state of synthesis and degradation such that the “optimal” folded state of the proteome within a cell is constantly in flux, and highly sensitive to changes in the environment. Moreover, because protein abundance can differ by orders of magnitude (Ghaemmaghami et al. 2003), the challenge to understand the threshold for misfolding is daunting. The constraints on protein quality control mechanisms for optimal folding of proteins expressed as a few copies, in which every copy needs to be functional, would differ vastly from those for a highly expressed protein. Conversely, the production of nonnative proteins should have varying costs for the cell, with the fractional contribution of the highly expressed proteins to the misfolded pool being much higher than for low-copy proteins. Indeed, it has been suggested that the evolutionary pressure on coding sequences of highly expressed proteins is dominated by avoidance of mistranslation-induced misfolding (Drummond and Wilke 2008). It has been estimated that at a frequency of mistranslation of 5 × 10−4, an average 400-residue protein could be expected to contain at least one misincorporated amino acid 18% of the time. In addition to mistranslation, transcriptional errors, mutations and polymorphisms, and incorporation of amino-acid analogs (such as certain antibiotics or plant metabolites), have the potential to affect protein folding (Fig. 1) (Suckow et al. 1996; Stevens and Argon 1999a; Jordanova et al. 2006; Lee et al. 2006; Ng and Henikoff 2006). Coding polymorphisms are estimated to occur at an average of two per coding sequence, providing a constant level of sequence variation between individuals (Sachidanandam et al. 2001). Such sequence alterations can potentially affect not only the stability of different folds, intermediates, and the native state (thermodynamic destabilization), but also change the rates of transitions and thus the folding pathway, including diversity and relative abundance of intermediates, or the final conformation of a protein (kinetic partitioning) (Sinclair et al. 1994; Sanchez et al. 2010). Copy number variation and altered regulation of gene expression can also affect the stoichiometry of subunits and binding partners; premature termination or read-through of transcription or translation may bring about expression of nonnative sized proteins; defects in posttranslational modifications, targeting, and turnover—all of these may ultimately affect the folding of a given protein. Whether these errors are accommodated into a functional protein, and at what cost, or lead to misfolding and/or premature degradation of the protein, they contribute to a critical and likely fluctuating baseline of protein homeostasis in any given cell (Balch et al. 2008; Gidalevitz et al. 2010).
Figure 1.
Cellular protein homeostasis (proteostasis). To maintain proteins in a functionally folded state, cells must find a balance between the intrinsic and extrinsic forces that perturb protein folding and specialized networks of molecular chaperones, folding enzymes, and degradation machinery. Molecular chaperones participate at multiple levels in protein biogenesis: assisting in the de novo folding and protein interactions and preventing deleterious intermolecular interactions.
Protein folding in a cellular environment imposes further challenges, such as vectorial synthesis, macromolecular crowding with its associated risk of inappropriate intermolecular contacts, proximity to membranes, and local variations in ionic strength (i.e., Ca2+ fluxes during signaling) and redox state. It is not surprising then, that efficient and correct folding in vivo is strongly dependent on molecular chaperones (Ellis 1990; Gething and Sambrook 1992; Hartl et al. 1994; Ellis and Hartl 1999; Fink 1999; van den Berg et al. 1999), many of which are essential in eukaryotes. To accomplish optimal protein folding and stability, cells must find a balance between the intrinsic structural properties of proteins and the specialized networks of molecular chaperones, folding enzymes, and degradation machinery (Fig. 1) (Balch et al. 2008), that are regulated by stress-inducible responses (Fig. 3) (Welch 1992; Morimoto et al. 1997; Akerfelt et al. 2010). This integrated proteostasis network serves to achieve the cellular state in which the proteome is both stable and functional (Balch et al. 2008). Thus, one can ask how the proteostasis machinery detects and responds to changes in the protein folding in a milieu of different conformational states that likely coexist at any given time, and how the threshold for the stress-inducible responses is set, such that proteostasis is maintained across diverse cell types over the life history and metabolic states of an organism.
HOW TO MAINTAIN A FUNCTIONAL PROTEOME: CHAPERONE NETWORKS
Molecular chaperones have multiple roles in protein biogenesis: they prevent deleterious intermolecular interactions and facilitate folding and functionality, and regulate a multitude of cellular processes that employ protein conformation dynamics (Figs. 1 and 2) (Nollen and Morimoto 2002; Deuerling and Bukau 2004; Bukau et al. 2006; Ron and Walter 2007; Voisine et al. 2010). Chaperones can show both a considerable specialization and a hierarchical organization into highly interconnected networks (Frydman and Hohfeld 1997; Kelley 1998; Zhao et al. 2005; Sahi and Craig 2007; Kampinga and Craig 2010). For example, distinct groups of chaperones and cochaperones orchestrate a sequence of events that control the folding and maturation of many highly regulated macromolecular complexes such as the steroid hormone receptors (Picard et al. 1990; Pratt and Toft 2003).
Figure 2.
Chaperone levels are actively maintained in cells to accommodate the demands of protein folding. All cells and organisms adjust the expression of chaperones and other cytoprotective genes to adapt to changing environmental conditions and ensure recovery following perturbations to proteostasis. At the molecular level, this is mediated by the transcriptional regulation of HS genes by the heat shock factor 1 (HSF1). (A) HSF1 in unstressed metazoan cells is in an inert, monomeric state, transiently bound to chaperones. (B) The current model for the activation of HSF1 and up-regulation of chaperones is that the increased flux of misfolded and damaged proteins that occurs on heat shock or other proteotoxic stressors is met by a corresponding increase in chaperone levels. (C) The attenuation of the HSR following stress is less well understood. It is unclear what happens to the excess chaperone capacity induced in the cell following the resolution of protein misfolding. In fact, exposure of the cell to a mild environmental stress that causes chaperone induction establishes a hormetic state in which cells are protected from a subsequent lethal stress, perhaps because of the excess of chaperones.
The biogenesis of the nascent glucocorticoid receptor (GR) involves initial recognition by the HSP70/HSP40 chaperones (Smith and Toft 1993; Kimura et al. 1995; Dittmar et al. 1998). The cochaperone HOP bridges HSP70 and the HSP90 chaperones (Chen and Smith 1998; Odunuga et al. 2004), switching the immature GR to associate with the HSP90/p23 (or AHA1)/immunophilin chaperones to form the hormone-responsive GR complex (Dittmar et al. 1997; Pratt and Toft 1997; Murphy et al. 2001; Morishima et al. 2003; Harst et al. 2005; Pratt et al. 2006). Interactions of the HSP70 and HSP90 chaperone machines has different functional consequences: the HSP70 complex protects nascent proteins from inappropriate interactions, thus preventing misfolding and aggregation, whereas the HSP90 complex maintains the almost native, metastable hormone receptor in a ligand binding-competent state. As for many other signaling molecules, including kinases, cell cycle regulators, cell death regulators, and nuclear hormone receptors, GR is poised for activation. Genetic and biochemical studies suggest that activation-ready or ligand binding-competent states of such proteins are dependent on HSP90 for their stability and turnover in the absence of activating signal or ligand: the chaperone complex protects the dynamic ligand-binding cleft against misfolding because of the exposure of internal hydrophobic residues, concomitantly facilitating the formation of a stable signaling molecule (Giannoukos et al. 1999; Kaul et al. 2002; Pratt et al. 2008). Activation is coupled with chaperone release, although dynamic interactions with chaperones remain for intracellular movement, nuclear translocation, and binding to chromatin (Davies et al. 2002; Freeman and Yamamoto 2002; Elbi et al. 2004). This hierarchical organization of chaperones and other proteins that regulate protein folding (proteostasis network, PN), therefore, not only allows for various triage decisions to be made during the folding and maturation of proteins in the cell, but also modulates the responses of “primed” signaling pathways.
The functional properties of chaperone networks can adapt to the specific needs of different substrates, such that a chaperone can have different roles depending on identity or conformational state of its substrates, and on cochaperone interactions (McClellan et al. 2005). In this regard, the role of cochaperones and accessory proteins, and the restricted expression of certain chaperones, may be particularly important to define substrate specificity. For instance, the cochaperone dHDJ1, but not dHDJ2, synergized with HSP70 to suppress polyglutamine toxicity in Drosophila (Chan et al. 2000). If the absolute and relative abundance of chaperones and cochaperones influences the availability and activities of different proteins and pathways, changes in composition of the PN could redirect information flow through the intracellular pathways and affect the cellular responses to extracellular signals. Specific pathways may become favored or dysregulated because of alterations in the levels of a particular cochaperone that is specifically required for their regulation (Nollen and Morimoto 2002). For example, increased levels of HSP70, in response to stress, inhibit the Ras/Raf-1 signaling pathway in tissue culture cells by sequestering cochaperone Bag1. This disrupts the stimulatory properties of Bag1 on Raf-1 and results in cell growth arrest (Song et al. 2001). Thus, it is important to understand how cells and organisms respond to altered chaperone and cochaperone levels associated with fluctuating environmental conditions, aging, and disease.
HOW TO MAINTAIN A FUNCTIONAL PROTEOME: ROLE OF STRESS RESPONSES
Numerous conditions lead to an imbalance of proteostasis. Amongst them, the earliest studied were the effects of environmental insults, including elevated temperatures, oxidative stress, and heavy metals that perturb protein biogenesis and cause protein damage (Lindquist 1986; Lindquist and Craig 1988). In all cells and organisms, these conditions result in the induction of ubiquitous cellular responses to environmental stress, including the HSR, that adjust the expression of chaperones and other cytoprotective genes to ensure stress adaptation, recovery, and survival (Figs. 2 and 3) (Wu 1995; Morimoto 1998). At the molecular level, this is mediated by the transcriptional regulation of HS genes by the heat shock factor 1 (HSF1) (Wu et al. 1987; Zimarino and Wu 1987; Akerfelt et al. 2010), proportional to the intensity, duration, and type of stress, and the metabolic state of the cell (Fig. 2) (Zimarino et al. 1990; Abravaya et al. 1991; Gasch et al. 2000; Hahn et al. 2004).
Figure 3.
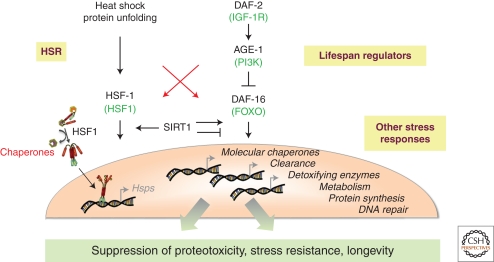
Proteostasis pathways. Multiple interconnected pathways regulate the expression of chaperones and other cytoprotective genes that contribute to maintenance of protein folding homeostasis during growth, development, and aging and under various stress conditions. These complex signaling pathways participate in diverse physiological functions and therefore proteostasis requires precise control over their activities. The cell nonautonomous regulation of the HSR by neurons may allow the integration of stress responses with growth and metabolic state of the animal.
HSF1 in unstressed metazoan cells is in an inert, monomeric state, transiently bound to chaperones (Fig. 2A) (Abravaya et al. 1992; Shi et al. 1998; Zou et al. 1998), and on activation forms a transcriptionally active homotrimer that binds to DNA. HSF1 is regulated by transient interactions with chaperones and posttranslational modifications (PTM), including phosphorylation (Sorger and Pelham 1988; Knauf et al. 1996; Kline and Morimoto 1997; Holmberg et al. 2001; Guettouche et al. 2005), sumoylation (Hietakangas et al. 2003; Anckar and Sistonen 2007), and acetylation (Westerheide et al. 2009). These interactions function as direct regulators and rheostats to determine not only whether HS genes are transcribed, but also the kinetics and duration of their expression (Abravaya et al. 1991, 1992; Wu 1995; Morimoto 1998; Shi et al. 1998; Yao et al. 2006; Anckar and Sistonen 2007). Many organisms express additional HSF genes (Wu 1995; Morimoto 1998; Anckar and Sistonen 2007) that have independent functions, especially in the development of specific organs, but also coordinate their activities with HSF1. Thus, the combination of PTMs, chaperone interactions, and multiple regulators of HSF1 affords multiple levels of control and feedback loops to precisely regulate chaperone levels in the cell, following stress-induced protein misfolding (Fig. 2B).
Although individual steps in the regulation of HSF1 and the HSR have been identified, there are many aspects that remain to be addressed. Among the initial questions was the sensor of heat shock and other stressors that activate HSF1. Numerous groups have suggested that HSF1 itself is the sensor (Mosser et al. 1990; Zhong 1998), however, in vivo, the temperature of HSF1 activation appears to be set by the cell. The current model proposes that the primary signal for activation is the flux of misfolded and damaged proteins that shifts the chaperone equilibrium in the cytoplasm and nucleus, leading to the derepression of HSF1 (Fig. 2B) (Ananthan et al. 1986; Morimoto 1998; Voellmy and Boellmann 2007). More recently, the attenuation of the HSR was shown to be regulated by the activity of the NAD-dependent sirtuin, SIRT1, that also regulates the activity of the FOXO transcription factor DAF-16, thus providing an important link between HSF1, the metabolic state of the cell, and lifespan (Fig. 3) (Westerheide et al. 2009). A recent demonstration that moderate vs. stress-induced complete depletion of chaperone availability differentially regulates mTORC1 assembly provided additional support for coordination between protein misfolding and regulation of metabolism (Qian et al. 2010). This mechanism is proposed to enable mTORC1 to rapidly detect and respond to environmental cues while also sensing intracellular protein misfolding. However, while all cells and organisms readily up-regulate chaperones on exposure to stressful environmental conditions, it is puzzling that the chronic accumulation of misfolded proteins as occurs in conformational diseases does not consistently activate HSR. Therefore, it is possible that multiple levels of regulation in addition to the presence of misfolded proteins trigger HSF1 activation and subsequently the levels of chaperones.
In addition to stress-induced transcription of HS genes, the HSR is also regulated at the posttranscriptional level by mRNA stability (Theodorakis and Morimoto 1987), stress-induced translational control (Banerji et al. 1984), and effects on the activity and subcellular localization of chaperones (Milarski and Morimoto 1986; Welch and Suhan 1986). Moreover, the HSR also down-regulates numerous housekeeping functions of the cell during stress and recovery to reset the cellular clock for cell growth. Within the milieu of numerous cells in an organism, such perturbations could have profound effects on growth, metabolism, development, and perhaps even the evolutionary trajectory of organisms.
THE PROTEOME: FOLDED, MISFOLDED, OR SOMETHING IN BETWEEN?
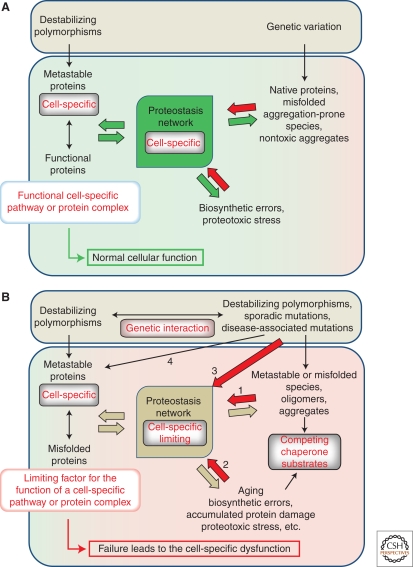
Efficient proteostasis depends on the balance between the “folding capacity” of chaperone networks and the continuous flux of potentially nonnative proteins (Fig. 4A). With sequence variation, biosynthetic errors, and environmental fluctuations contributing to metastability of the proteome, how do cells achieve balance and set the threshold for sensing additional misfolding and induction of the HSR?
Figure 4.
Proteostasis networks must match the misfolded protein load. (A) Protein misfolding because of genetic variation, including destabilizing polymorphisms, biosynthetic errors such as mistranslation, and various proteotoxic stresses is suppressed by the activity of molecular chaperones and other components of Proteostasis network. (B) Dysfunction of cell-specific proteins, protein complexes, or pathways (target pathway, left edge) because of misfolding may result from excessive competition for limiting components of Proteostasis network (1), from other misfolded species, which can be genetically encoded (competing pathway, right edge), for example in familial variants of conformational disease. Alternatively, competition could be generated by biosynthetic errors, proteotoxic stresses, etc. (2), Dysregulation of Proteostasis network itself by mutations and polymorphisms (3), as well as additional “hits” sustained by the target pathway (4), may all lead to the cell-specific dysfunction of the target pathways. Together, the latter three possibilities may contribute to the development of the sporadic variants of conformational disease.
One possibility is that individual sequence variation and biosynthetic errors do not affect folding trajectories or the stability of most cellular proteins, either because of the high initial stability of proteins themselves, or because of the excess buffering capacity in chaperone and proteostasis networks. However, neither of these two options appears to be supported by experimental evidence. Across species, most proteins are only marginally stable, with ΔG values between −3 and −15 kcal mol−1 (Pace et al. 1981; DePristo et al. 2005). This is partly because of entropic penalty imposed by folding, and to the selective pressure to balance structural stability with conformational flexibility necessary for function (Frauenfelder et al. 1991; Zavodszky et al. 1998; Kamerzell and Middaugh 2008; Gardino et al. 2009). Because of this marginal initial stability, most amino acid substitutions are not neutral for either protein stability or function (Pakula and Sauer 1989; Matthews 1993; DePristo et al. 2005), and ∼70% of rare missense alleles in human population are predicted to be mildly deleterious (Kryukov et al. 2007). This interdependence of function and conformational flexibility also argues against the PN having excess capacity. Functionally important conformational changes often involve structural transitions between alternative low energy states (Frauenfelder et al. 1991), potentially populating intermediate states, or exposing binding surfaces. Therefore, suppression of these events may adversely affect cellular function. Indeed, chaperone expression is tightly regulated, and abnormally high cellular levels of HSP70 in Drosophila cells (Feder et al. 1992) and larvae (Krebs and Feder 1997) interfere with growth, development, and survival to adulthood. Another indication of the lack of excess buffering capacity is the failure in Caenorhabditis elegans to maintain metastable proteins in a functional state, when the folding environment is challenged either by expression of destabilized, aggregation-prone mutant protein (Gidalevitz et al. 2006, 2009), or by aging (Fig. 4B) (Ben-Zvi et al. 2009).
Another strategy for maintaining proteostasis is through disposal of defective proteins. It has been suggested that up to 30% of newly synthesized proteins are directly targeted to proteasomal degradation, presumably because of being defective (Princiotta et al. 2003). Proteins in nonproductive chaperone cycles that are kinetically trapped would also be preferentially targeted for degradation (Skowronek et al. 1998; Wickner et al. 1999; Connell et al. 2001; McClellan et al. 2005). For example, whether the slow folding substrate, CFTR, reaches its native state before being targeted to degradation appears to be determined by the relative abundance of cochaperones CHIP and HDJ-2 (Meacham et al. 2001). It is not clear, however, whether such triage only applies to severely folding-deficient and stalled proteins. For example, the relative abundance of HDJ-2 over CHIP in the cell is suggested to favor folding of CFTR over degradation (Meacham et al. 2001).
An alternate explanation for maintenance of proteostasis within cells despite the high probability of misfolding is that the folding environment is finely tuned to the specific needs of a given cell and tissue. Proteostasis then becomes a matter of both changes in chaperone capacity and the flux of folding substrates. A certain perspective can be gained by considering the buffering of metastable proteins by molecular chaperones (Fig. 4A). The chaperone machine, GroEL/ES, can suppress detrimental phenotypes caused by the temperature-induced destabilization of metastable proteins (Van Dyk et al. 1989). More recently, it has been shown that certain alleles, or sequence variants, that code for destabilized mutant proteins could be maintained within organisms as long as they were within the proteostatic capacity of the organisms. Once proteostasis was perturbed, either by elevated temperatures, limitation of a chaperone activity (Rutherford and Lindquist 1998; Queitsch et al. 2002; Yeyati et al. 2007), expression of another misfolded protein (Gidalevitz et al. 2006, 2009), or aging (Ben-Zvi et al. 2009), the phenotypic effects of the metastable allele became apparent (Fig. 4B). This suggests a view of the cellular proteome not as a collection of invariant, crystallographic-like native states, occupying narrow minima at the bottom of their folding funnels, and a collection of discrete alternative nonnative states (Dill and Chan 1997; Clark 2004; Bartlett and Radford 2009), but rather as a continuum of perhaps imperfect, near-native states occupying a broad basin at the bottom of the folding funnel, but which are able to assume the functional conformation on the completion of folding, i.e., on binding to a partner protein, or reaching their cellular destinations. The latter view reconciles the constant flux of variant proteins, and is achieved by setting the capacity of the chaperone and proteostasis networks to precisely accommodate this flux (Fig. 2A,B). Because there is no excess capacity, folding in the cell is exquisitely sensitive to any environmental perturbation, or to competition for the specific, limiting folding resources by a misfolded or aggregation-prone protein (Gidalevitz et al. 2010). Exposure of the cell to a mild environmental stress causes chaperone induction and establishes a hormetic state in which chaperones transiently accumulate in excess of folding requirements, thus conferring remarkable protection against a subsequent lethal stress (Fig. 2C).
ORGANISMAL CONTROL: CELL NONAUTONOMOUS REGULATION OF PROTEOSTASIS
Evidence for additional levels of control of proteostasis has been shown in the metazoan C. elegans. Polyglutamine aggregation in muscle cells was shown to be affected by cholinergic signaling in animals defective for the neuron-specific transcription factor unc-30 that regulates the synthesis of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) (Garcia et al. 2007). Either defective GABA signaling or increased acetylcholine (ACh) signaling in mutant animals caused a general imbalance in protein homeostasis in postsynaptic muscle cells, revealing that an imbalance in neuronal signaling had cell nonautonomous consequences on protein homeostasis. Moreover, exposure to GABA antagonists or ACh agonists had similar effects, suggesting that toxins that act at the neuromuscular junction can be potent modifiers of protein conformational disorders (Garcia et al. 2007). These results show the importance of intercellular communication in intracellular protein homeostasis.
Additional evidence to suggest a more complex level of regulation comes from evidence that the HSR is not cell autonomous (Prahlad et al. 2008). C. elegans deficient for the two AFD thermosensory neurons, among the 959 cells of the organism, did not induce a HSR in nonneuronal cells on exposure to HS. In these AFD-deficient animals, HSF1 and the HS genes were functional, as HSF1 could be induced by exposure to an alternate stress, the heavy metal cadmium. This specificity, whereby neurons that regulate the behavioral response of an organism to its environment also regulate chaperone expression was unexpected, and may even suggest that different sensory neurons control the organismal response to different environmental stressors (Prahlad et al. 2008). Moreover, the cell nonautonomous regulation of the HSR by the thermosensory AFD neurons was also dependent on the metabolic status. These results suggest that the cellular machinery for HSP induction on heat shock is under the negative regulation of (at least) two mutually inhibitory neurohormonal pathways: a temperature-sensing pathway and a growth-regulated pathway. Disruption of either pathway results in a net inhibition of HS-dependent HSP transcription; the presence or absence of both pathways allows the cellular homeostatic mechanisms to express HSPs on heat stress. The downstream target of the AFDs appears to be HSF1, although how HSF1 is regulated by the AFD neurons remains unresolved (Prahlad et al. 2008; Prahlad and Morimoto 2009). Data from other studies on nutrient dependent signaling in C. elegans suggests that the growth related signal may act through insulin-like signaling pathway (ILS) FOXO transcription factor, DAF-16 (Alcedo and Kenyon 2004), suggesting that, as in mammalian tissue culture cells or yeast (Morano et al. 1999; Anckar and Sistonen 2007), organismal growth and HSF1-dependent expression of chaperones may be mutually antagonistic (Fig. 3). Thus, under certain growth or metabolic conditions, neuronal signaling appears capable of overriding the cell autonomous up-regulation of HSPs expected to occur in response to stress-induced cellular protein damage. Nearly all aspects of C. elegans growth and development, including the duration of adult life-span and the development of stress resistant states of the organism, are affected by the environment (Devaney 2006; Gutteling et al. 2007) and coordinated via neuroendocrine signaling pathways. The interaction of ILS with HSF1 in regulation of longevity could therefore represent an important molecular strategy to couple the regulation of organismal functions with an ancient genetic switch that governs the ability of cells to sense and respond to stress (Fig. 3).
The cell nonautonomous regulation of stress responses by neurons has recently gained support from another study showing that the mitochondrial stress response, central to regulating longevity, is also under cell nonautonomous control (Durieux et al. 2011). Cell nonautonomous regulation of chaperones may in fact be a more general feature of metazoan control, although the type of regulation itself may differ based on the ecology and life history of the organism (Feder and Hofmann 1999). In this regard, restraint stress in rodents (Blake et al. 1991; Fawcett et al. 1994) results in activation of the hypothalamic-pitutary-adrenal axis and ACTH-dependent up-regulation of specific HSPs in the thoracic aorta, endothelial cells, and adrenal cortex of rats. This induction of HSPs is HSF1-dependent and markedly declines with age (Fawcett et al. 1994). More recently, it was shown that temperature entrainment of the circadian rhythm in peripheral tissues of rodents was HSF1 dependent (Buhr et al. 2010). This temperature entrainment was masked by the activity of the SCN, which is not temperature responsive, suggesting again that there may be centralized control of HSF1 activity in mammals.
A major advantage of centralized control of proteostasis is the ability of the organism to control resource allocation in a manner that best suits its physiological needs and environmental niche. Thus, the nervous system, because of its role in regulating behavior, metabolism, longevity and reproduction, and the HSR, may also determine the extent of protein damage that can be tolerated by cells. Neuronal control of proteostasis and HSF1 activity may also provide a partial explanation for why, in diseases of protein conformation such as Huntington’s disease, Parkinson’s disease, Alzheimer’s disease, certain cancers, and type II diabetes, cells accumulate heterogeneous populations of misfolded proteins leading to cell death, yet do not consistently and sufficiently activate their heat shock response.
ORGANISMAL CONTROL: CHAPERONE SPECIALIZATION TO ACCOMMODATE DIFFERENCES BETWEEN CELL TYPES AND ORGANISMS
An important aspect in considering organism-level regulation of proteostasis is the role of variation. No two cell-types within an organism, and no two individuals within a population are likely to have the same proteome and PN (Pollak et al. 2006). In C. elegans, this was suggested by the broad variation in the HSR observed in isogenic populations of animals (Yashin et al. 2002; Rea et al. 2005; Wu et al. 2006). This variation in the HSR was suggested to be predictive of individual lifespan post stress, with better survival in animals with a stronger HSR. The source of this variability could be caused by the inter-individual variation in the composition and state of the proteome and differences in the ability to sense and integrate the environmental signal. Furthermore, it is unclear whether the difference in stress induction is constant across different cells and tissues of an individual, consistent with organism-level regulation of HSR, or whether stochasticity is also present between cells.
An interesting question is whether variability in the composition of the proteome is adaptive or detrimental. An indication of adaptive value is provided by the ability of certain Candida albicans species to decode the standard leucine CUG codon as serine: not only are these C. albicans species naturally stress resistant, but transferring this ability to a Saccharomyces cerevisiae resulted in triggering the general stress response and expression of stress proteins, which, in turn, created a competitive edge under stress conditions (Santos et al. 1999). On the other hand, if the proteostasis networks are indeed operating at near capacity, excess variation may lead to chronic misfolding and thus be strongly detrimental. For example, a mouse sti mutation in the tyrosyl-tRNA synthetase, a model for a subtype of Charcot-Marie-Tooth neuropathy (Jordanova et al. 2006), leads to the production of heterogeneous misfolded proteins, accompanied by increased expression of chaperones in the cytoplasm and the endoplasmic reticulum (ER) (Lee et al. 2006). Although an observed increase in chaperone expression suggests that adaptive transcriptional responses are indeed activated, the cellular dysfunction and neurodegeneration in this mouse model indicate that chronic protein misfolding may overwhelm the proteostasis networks. Thus, we expect that evolutionary pressure must have acted to maintain the balance between a potential adaptive value and the detrimental effects of sequence variation.
Functional specialization of different cell-types in a metazoan implies both a different set of expressed proteins, and different intracellular conditions in which these proteins have to operate. Thus, the composition and regulation of proteostasis networks, and the activities of various stress response pathways, may match the functionality of a given cell (Fig. 4A). There are clear indications of cell-type specific expression of some molecular chaperones and components of degradation machinery (Powers et al. 2009), although we lack a comprehensive definition of tissue- and cell-specific expression patterns, particularly during organismal development and aging. Studies on cell differentiation suggest that expression of specialized chaperone networks may be coordinated by the same developmental programs that control the expression of cell-specific proteomes. For example, induction of immunoglobulin production during plasma cell differentiation is pre-empted by up-regulation of mitochondrial and cytosolic chaperones, and ER resident folding factors and redox balance proteins (van Anken et al. 2003), thus preparing the cell for the massive expression of Ig molecules (Hu et al. 2009). Similarly, an inability to activate the appropriate stress response and induce chaperone expression severely compromises insulin-producing β-cell survival (Harding et al. 2001), and blocks β-cell development in Wolcott-Rallison syndrome of infantile diabetes (Delepine et al. 2000). This suggests that the correspondence between the composition of the proteome and cell-type specific chaperone requirements may be dictated by the client proteins expressed in these cells.
This view can be further illustrated by the cell-type and substrate-dependent consequences of inactivation of specific chaperones in multicellular organisms. Reduced expression of HSP90α1, but not of HSP90α2 in zebrafish leads to defects in myosin folding and assembly, and thus paralyzed embryos (Du et al. 2008), whereas HSP90β null mutant mouse embryos fail to form a fetal placental labyrinth (Voss et al. 2000). The knockout of the ER chaperone GRP94 results in failure of mesoderm formation, and an inability of the mutant ES cells to give rise to muscle cells, because of the failure of folding and secretion of IGF-II (Wanderling et al. 2007). Suppression of CCT activity in mouse photoreceptors by expression of dominant-negative cochaperone PhLP results in malformation of the cellular compartment responsible for light detection, and triggers rapid retinal degeneration (Posokhova et al. 2010). These specific phenotypes support the view that even though most molecular chaperones interact with, and assist in the folding of, multiple diverse protein substrates, some proteins may be strictly dependent on the activity of a specific chaperone and themselves be essential for a cell- or tissue-specific function or a developmental process (Fig. 4A). Dysfunction of such proteins and of the pathways in which these proteins act may then be a consequence of proteotoxic stress or compromise proteostasis networks and stress responses, potentially contributing to disease and aging. Given these considerations, definition of tissue- and cell-specific expression of molecular chaperones, during development and aging, and of their substrate repertoire, should contribute substantially to our understanding of biology and disease.
NATURAL GENETIC VARIATION: POLYMORPHISMS AND MUTATIONS
The role of the natural genetic variation in generating proteome variation is supported not only by the predictive computational and modeling approaches, but also by direct measurements (Klose et al. 2002; Foss et al. 2007). A recent study examined 46 coding polymorphisms for 16 human enzymes with known three-dimensional structures and found that a high proportion (48%) of these natural variants results in altered thermal stability and, in some cases, catalytic efficiency or allosteric regulation (Allali-Hassani et al. 2009). From the considerations discussed above, we speculate that altered thermostability leading to a phenotypic outcome may depend on factors such as the “strength” of the chaperone network in a given individual, influences by polymorphisms in chaperone or stress regulatory genes and imbalanced coexpression of chaperones and cochaperones, environmental influences and exposures to proteotoxic stresses, and the presence of other destabilized or misfolded proteins, particularly those acting in the same pathway or competing for the same chaperones (Fig. 4B).
Recent studies in C. elegans have shown that this phenomenon has broad physiological relevance. Temperature-sensitive (ts) metastable proteins begin to lose their function and cause detrimental phenotypes as the organism ages and its proteostasis-regulating pathways begin to fail, even though the animals are grown at the permissive conditions (Ben-Zvi et al. 2009). Increasing the activity of either HSF1, or DAF-16, suppressed the misfolding of these metastable proteins, and restored cellular proteostasis. The suppression of misfolding of metastable proteins in the young animals, or by activation of proteostasis regulators, is reminiscent of the ability of molecular chaperone HSP90 to buffer phenotypic variation because of cryptic mutations (Rutherford and Lindquist 1998). These cryptic mutations, similar to the ts mutations in C. elegans, were proposed to be exposed only under (proteotoxic) stress conditions, when the functional availability of HSP90 is limited. Indeed, the phenotypes exposed by the limitation of HSP90 in Drosophila correlated to specific genetic backgrounds, and were also affected by the temperature. A study in zebra fish showed that developmental phenotypes commonly observed on HSP90 limitation reflected underlying polymorphisms, whose frequency was strain-specific, whereas phenotypes that were rarely seen were unique to specific mutant carrier strains (Yeyati et al. 2007). Thus, it was suggested that a similar buffering of underlying polymorphisms may explain an incomplete penetrance observed in human disease.
The direct evidence that underlying coding polymorphisms have a potential to significantly contribute to conformational disease was recently obtained in C. elegans: expression of either extended polyQ or mutant SOD1 proteins in muscle or neuronal cells of C. elegans lead to the exposure of the ts phenotype at permissive conditions, mediated by the misfolding and loss-of function of ts mutant protein present in the same cell (Gidalevitz et al. 2006, 2009). Furthermore, the misfolding of ts proteins further increased aggregation of the polyQ proteins, thus amplifying the disruption of proteostasis. This effect was most likely caused by the depletion, by the polyQ or mutant SOD1 proteins, of components of chaperone networks that are necessary for maintaining metastable proteins in their folded and functional conformations (Fig. 4B) (Van Dyk et al. 1989; Brown et al. 1997). A recent finding that many of the modifiers of toxicity of polyQ-expanded ataxin-3 in Drosophila also rescue the generic toxicity of protein misfolding caused by the reduced function of HSP70 (Bilen and Bonini 2007) strongly supports the disruption of proteostasis as a mechanism of toxicity. An insight into a potential mechanism by which aggregation-prone proteins may affect the chaperone availability was provided by demonstration that α-synuclein oligomers in vitro inhibited the refolding activity of the HSP70/40 chaperone machinery toward heat- or cold-denatured substrate proteins (Hinault et al. 2010). This depletion of chaperone activity was caused by transient weak interactions of α-Synuclein oligomers specifically with HSP40 cochaperones, without their recruitment into the oligomers.
Additional studies in C. elegans have shown that although polyQ and mutSOD1 proteins were triggering, or accelerating, the onset of toxicity by disrupting proteostasis, it was the nature of the destabilizing sequence variants present in the genetic background that determined the specific phenotypes (Gidalevitz et al. 2006, 2009). Thus, at least in this model, mild folding variants in the genetic background function to both modulate the expression of toxicity of the aggregation-prone protein and to channel specific phenotypes (Gidalevitz et al. 2010). A parallel could be drawn between environmental stress (in this case—a mild temperature increase), organismal aging, and the expression of the aggregation-prone proteins, all leading to the same phenotypic outcome mediated by their effects on the folding, stability, and the functionality of ts metastable proteins (Fig. 4B). Similar destabilization of susceptible proteins, that are either naturally highly dependent on molecular chaperones for their folding, stability, or activity, or are encoded by destabilizing polymorphisms, could then be invoked to suggest an integrative model for conformational disease. In this model, the dysregulation of protein folding homeostasis may represent an outcome of either expression of an aggregation-prone mutant protein (in familial disease), or early molecular events in aging (in sporadic disease), with an ability to amplify the protein damage cascade in age-related conformational diseases, while the complement of mutations and polymorphisms, together with the life history of an organism (environmental stress exposure, metabolic state, etc.), set the threshold for the onset of dysfunction and direct specific phenotypes (Fig. 4B).
PERSPECTIVES
The examination of how cells maintain the correct folding and function of their proteins in a fluctuating environment has made much progress since its inception, but many questions still remain. Although protein misfolding is perhaps the primary trigger for inducing stress responses at the cellular level, it is unclear whether special proteins function as sentinels to signal or detect stress, or whether there is a certain level of bulk misfolding that occurs before stress responses are activated. Given the dynamic nature of the proteome, it is also unclear how alternate conformations, such as misfolded species, can be detected in a sea of potentially nonnative intermediates during ensemble folding. Moreover, the heat shock response itself depends on numerous factors including the developmental state of the organism, its growth, metabolism and age, and individual variation in its proteome. Thus how stress is sensed and transduced to regulation of HSF1 may vary among individuals, from cell to cell within tissues, and even within a single cell throughout lifetime. Cell nonautonomous regulation of HSF1 activity and chaperone expression by the nervous system may set the threshold for the stress responses in a manner that optimizes survival of the organism, perhaps even at the cost of individual cells. The inadequate response to chronic disruption of proteostasis may represent a common trigger in disparate conformational diseases, whereas chaperone-dependent proteins and pathways may channel specific phenotypes. These questions will certainly be better examined as stress responses are studied at the organismal level, and as the proteome variation within populations and its consequences in susceptibility to misfolding and to diseases of protein conformation become apparent.
ACKNOWLEDGMENTS
This was supported by NIH grants GM038109, GM081192, AG026647, and NS047331 (to R.I.M.).
Footnotes
Editors: Richard Morimoto, Dennis Selkoe, and Jeffrey Kelly
Additional Perspectives on Protein Homeostasis available at www.cshperspectives.org
REFERENCES
- Abravaya K, Phillips B, Morimoto RI 1991. Attenuation of the heat shock response in HeLa cells is mediated by the release of bound heat shock transcription factor and is modulated by changes in growth and in heat shock temperatures. Genes Dev 5: 2117–2127 [DOI] [PubMed] [Google Scholar]
- Abravaya K, Myers MP, Murphy SP, Morimoto RI 1992. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev 6: 1153–1164 [DOI] [PubMed] [Google Scholar]
- Akerfelt M, Morimoto RI, Sistonen L 2010. Heat shock factors: Integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 11: 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcedo J, Kenyon C 2004. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron 41: 45–55 [DOI] [PubMed] [Google Scholar]
- Allali-Hassani A, Wasney GA, Chau I, Hong BS, Senisterra G, Loppnau P, Shi Z, Moult J, Edwards AM, Arrowsmith CH, et al. 2009. A survey of proteins encoded by non-synonymous single nucleotide polymorphisms reveals a significant fraction with altered stability and activity. Biochem J 424: 15–26 [DOI] [PubMed] [Google Scholar]
- Ananthan J, Goldberg AL, Voellmy R 1986. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science 232: 522–524 [DOI] [PubMed] [Google Scholar]
- Anckar J, Sistonen L 2007. Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv Exp Med Biol 594: 78–88 [DOI] [PubMed] [Google Scholar]
- Anfinsen CB 1973. Principles that govern the folding of protein chains. Science 181: 223–230 [DOI] [PubMed] [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, Kelly JW 2008. Adapting proteostasis for disease intervention. Science 319: 916–919 [DOI] [PubMed] [Google Scholar]
- Banerji SS, Theodorakis NG, Morimoto RI 1984. Heat shock-induced translational control of HSP70 and globin synthesis in chicken reticulocytes. Mol Cell Biol 4: 2437–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett AI, Radford SE 2009. An expanding arsenal of experimental methods yields an explosion of insights into protein folding mechanisms. Nat Struct Mol Biol 16: 582–588 [DOI] [PubMed] [Google Scholar]
- Ben-Zvi A, Miller EA, Morimoto RI 2009. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci 106: 14914–14919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilen J, Bonini NM 2007. Genome-wide screen for modifiers of ataxin-3 neurodegeneration in Drosophila. PLoS Genet 3: 1950–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake MJ, Udelsman R, Feulner GJ, Norton DD, Holbrook NJ 1991. Stress-induced heat shock protein 70 expression in adrenal cortex: An adrenocorticotropic hormone-sensitive, age-dependent response. Proc Natl Acad Sci 88: 9873–9877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockwell DJ, Radford SE 2007. Intermediates: Ubiquitous species on folding energy landscapes? Curr Opin Struct Biol 17: 30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CR, Hong-Brown LQ, Welch WJ 1997. Correcting temperature-sensitive protein folding defects. J Clin Invest 99: 1432–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, Yoo SH, Takahashi JS 2010. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330: 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B, Weissman J, Horwich A 2006. Molecular chaperones and protein quality control. Cell 125: 443–451 [DOI] [PubMed] [Google Scholar]
- Chan HY, Warrick JM, Gray-Board GL, Paulson HL, Bonini NM 2000. Mechanisms of chaperone suppression of polyglutamine disease: Selectivity, synergy and modulation of protein solubility in Drosophila. Hum Mol Genet 9: 2811–2820 [DOI] [PubMed] [Google Scholar]
- Chen S, Smith DF 1998. Hop as an adaptor in the heat shock protein 70 (Hsp70) and hsp90 chaperone machinery. J Biol Chem 273: 35194–35200 [DOI] [PubMed] [Google Scholar]
- Clark PL 2004. Protein folding in the cell: Reshaping the folding funnel. Trends Biochem Sci 29: 527–534 [DOI] [PubMed] [Google Scholar]
- Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, Patterson C 2001. The co-chaperone CHip regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol 3: 93–96 [DOI] [PubMed] [Google Scholar]
- Davies TH, Ning YM, Sanchez ER 2002. A new first step in activation of steroid receptors: Hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem 277: 4597–4600 [DOI] [PubMed] [Google Scholar]
- Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C 2000. EIF2AK3, encoding translation initiation factor 2-α kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet 25: 406–409 [DOI] [PubMed] [Google Scholar]
- Demchenko AP 2001. Recognition between flexible protein molecules: Induced and assisted folding. J Mol Recognit 14: 42–61 [DOI] [PubMed] [Google Scholar]
- DePristo MA, Weinreich DM, Hartl DL 2005. Missense meanderings in sequence space: A biophysical view of protein evolution. Nat Rev Genet 6: 678–687 [DOI] [PubMed] [Google Scholar]
- Deshaies RJ, Koch BD, Werner-Washburne M, Craig EA, Schekman R 1988. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature 332: 800–805 [DOI] [PubMed] [Google Scholar]
- Deuerling E, Bukau B 2004. Chaperone-assisted folding of newly synthesized proteins in the cytosol. Crit Rev Biochem Mol Biol 39: 261–277 [DOI] [PubMed] [Google Scholar]
- Devaney E 2006. Thermoregulation in the life cycle of nematodes. Int J Parasitol 36: 641–649 [DOI] [PubMed] [Google Scholar]
- Dill KA, Chan HS 1997. From Levinthal to pathways to funnels. Nat Struct Biol 4: 10–19 [DOI] [PubMed] [Google Scholar]
- Dittmar KD, Banach M, Galigniana MD, Pratt WB 1998. The role of DnaJ-like proteins in glucocorticoid receptor.hsp90 heterocomplex assembly by the reconstituted hsp90.p60.hsp70 foldosome complex. J Biol Chem 273: 7358–7366 [DOI] [PubMed] [Google Scholar]
- Dittmar KD, Demady DR, Stancato LF, Krishna P, Pratt WB 1997. Folding of the glucocorticoid receptor by the heat shock protein (hsp) 90-based chaperone machinery. The role of p23 is to stabilize receptor.hsp90 heterocomplexes formed by hsp90.p60.hsp70. J Biol Chem 272: 21213–21220 [DOI] [PubMed] [Google Scholar]
- Drummond DA, Wilke CO 2008. Mistranslation-induced protein misfolding as a dominant constraint on coding-sequence evolution. Cell 134: 341–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du SJ, Li H, Bian Y, Zhong Y 2008. Heat-shock protein 90α1 is required for organized myofibril assembly in skeletal muscles of zebrafish embryos. Proc Natl Acad Sci 105: 554–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux J, Wolff S, Dillin A 2011. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144: 79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbi C, Walker DA, Romero G, Sullivan WP, Toft DO, Hager GL, DeFranco DB 2004. Molecular chaperones function as steroid receptor nuclear mobility factors. Proc Natl Acad Sci 101: 2876–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ 1990. The molecular chaperone concept. Semin Cell Biol 1: 1–9 [PubMed] [Google Scholar]
- Ellis RJ, Hartl FU 1999. Principles of protein folding in the cellular environment. Curr Opin Struct Biol 9: 102–110 [DOI] [PubMed] [Google Scholar]
- Fawcett TW, Sylvester SL, Sarge KD, Morimoto RI, Holbrook NJ 1994. Effects of neurohormonal stress and aging on the activation of mammalian heat shock factor 1. J Biol Chem 269: 32272–32278 [PubMed] [Google Scholar]
- Feder ME, Hofmann GE 1999. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu Rev Physiol 61: 243–282 [DOI] [PubMed] [Google Scholar]
- Feder JH, Rossi JM, Solomon J, Solomon N, Lindquist S 1992. The consequences of expressing hsp70 in Drosophila cells at normal temperatures. Genes Dev 6: 1402–1413 [DOI] [PubMed] [Google Scholar]
- Ferreiro DU, Hegler JA, Komives EA, Wolynes PG 2007. Localizing frustration in native proteins and protein assemblies. Proc Natl Acad Sci 104: 19819–19824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht AR 1995. Characterizing transition states in protein folding: An essential step in the puzzle. Curr Opin Struct Biol 5: 79–84 [DOI] [PubMed] [Google Scholar]
- Fink AL 1999. Chaperone-mediated protein folding. Physiol Rev 79: 425–449 [DOI] [PubMed] [Google Scholar]
- Foss EJ, Radulovic D, Shaffer SA, Ruderfer DM, Bedalov A, Goodlett DR, Kruglyak L 2007. Genetic basis of proteome variation in yeast. Nat Genet 39: 1369–1375 [DOI] [PubMed] [Google Scholar]
- Frauenfelder H, Sligar SG, Wolynes PG 1991. The energy landscapes and motions of proteins. Science 254: 1598–1603 [DOI] [PubMed] [Google Scholar]
- Freeman BC, Yamamoto KR 2002. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science 296: 2232–2235 [DOI] [PubMed] [Google Scholar]
- Frydman J, Hohfeld J 1997. Chaperones get in touch: The Hip-Hop connection. Trends Biochem Sci 22: 87–92 [DOI] [PubMed] [Google Scholar]
- Garcia SM, Casanueva MO, Silva MC, Amaral MD, Morimoto RI 2007. Neuronal signaling modulates protein homeostasis in Caenorhabditis elegans post-synaptic muscle cells. Genes Dev 21: 3006–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardino AK, Villali J, Kivenson A, Lei M, Liu CF, Steindel P, Eisenmesser EZ, Labeikovsky W, Wolf-Watz M, Clarkson MW, et al. 2009. Transient non-native hydrogen bonds promote activation of a signaling protein. Cell 139: 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell 11: 4241–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J 1992. Protein folding in the cell. Nature 355: 33–45 [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS 2003. Global analysis of protein expression in yeast. Nature 425: 737–741 [DOI] [PubMed] [Google Scholar]
- Giannoukos G, Silverstein AM, Pratt WB, Simons SS Jr 1999. The seven amino acids (547–553) of rat glucocorticoid receptor required for steroid and hsp90 binding contain a functionally independent LXXLL motif that is critical for steroid binding. J Biol Chem 274: 36527–36536 [DOI] [PubMed] [Google Scholar]
- Gidalevitz T, Kikis EA, Morimoto RI 2010. A cellular perspective on conformational disease: The role of genetic background and proteostasis networks. Curr Opin Struct Biol 20: 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI 2006. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science 311: 1471–1474 [DOI] [PubMed] [Google Scholar]
- Gidalevitz T, Krupinski T, Garcia S, Morimoto RI 2009. Destabilizing protein polymorphisms in the genetic background direct phenotypic expression of mutant SOD1 toxicity. PLoS Genet 5: e1000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guettouche T, Boellmann F, Lane WS, Voellmy R 2005. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteling EW, Doroszuk A, Riksen JA, Prokop Z, Reszka J, Kammenga JE 2007. Environmental influence on the genetic correlations between life-history traits in Caenorhabditis elegans. Heredity 98: 206–213 [DOI] [PubMed] [Google Scholar]
- Hahn JS, Hu Z, Thiele DJ, Iyer VR 2004. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol Cell Biol 24: 5249–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D 2001. Diabetes mellitus and exocrine pancreatic dysfunction in Perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell 7: 1153–1163 [DOI] [PubMed] [Google Scholar]
- Harst A, Lin H, Obermann WM 2005. Aha1 competes with Hop, p50 and p23 for binding to the molecular chaperone Hsp90 and contributes to kinase and hormone receptor activation. Biochem J 387: 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU, Hlodan R, Langer T 1994. Molecular chaperones in protein folding: The art of avoiding sticky situations. Trends Biochem Sci 19: 20–25 [DOI] [PubMed] [Google Scholar]
- Hietakangas V, Ahlskog JK, Jakobsson AM, Hellesuo M, Sahlberg NM, Holmberg CI, Mikhailov A, Palvimo JJ, Pirkkala L, Sistonen L 2003. Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol Cell Biol 23: 2953–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinault MP, Cuendet AF, Mattoo RU, Mensi M, Dietler G, Lashuel HA, Goloubinoff P 2010. Stable α-synuclein oligomers strongly inhibit chaperone activity of the Hsp70 system by weak interactions with J-domain co-chaperones. J Biol Chem 285: 38173–38182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg CI, Hietakangas V, Mikhailov A, Rantanen JO, Kallio M, Meinander A, Hellman J, Morrice N, MacKintosh C, Morimoto RI, et al. 2001. Phosphorylation of serine 230 promotes inducible transcriptional activity of heat shock factor 1. EMBO J 20: 3800–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CC, Dougan SK, McGehee AM, Love JC, Ploegh HL 2009. XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells. EMBO J 28: 1624–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke R 1991. Protein folding: Local structures, domains, subunits, and assemblies. Biochemistry 30: 3147–3161 [DOI] [PubMed] [Google Scholar]
- Jordanova A, Irobi J, Thomas FP, Van Dijck P, Meerschaert K, Dewil M, Dierick I, Jacobs A, De Vriendt E, Guergueltcheva V, et al. 2006. Disrupted function and axonal distribution of mutant tyrosyl-tRNA synthetase in dominant intermediate Charcot-Marie-Tooth neuropathy. Nat Genet 38: 197–202 [DOI] [PubMed] [Google Scholar]
- Kamerzell TJ, Middaugh CR 2008. The complex inter-relationships between protein flexibility and stability. J Pharm Sci 97: 3494–3517 [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Craig EA 2010. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11: 579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S, Murphy PJ, Chen J, Brown L, Pratt WB, Simons SS Jr 2002. Mutations at positions 547–553 of rat glucocorticoid receptors reveal that hsp90 binding requires the presence, but not defined composition, of a seven-amino acid sequence at the amino terminus of the ligand binding domain. J Biol Chem 277: 36223–36232 [DOI] [PubMed] [Google Scholar]
- Kelley WL 1998. The J-domain family and the recruitment of chaperone power. Trends Biochem Sci 23: 222–227 [DOI] [PubMed] [Google Scholar]
- Kimura Y, Yahara I, Lindquist S 1995. Role of the protein chaperone YDJ1 in establishing Hsp90-mediated signal transduction pathways. Science 268: 1362–1365 [DOI] [PubMed] [Google Scholar]
- Kline MP, Morimoto RI 1997. Repression of the heat shock factor 1 transcriptional activation domain is modulated by constitutive phosphorylation. Mol Cell Biol 17: 2107–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose J, Nock C, Herrmann M, Stuhler K, Marcus K, Bluggel M, Krause E, Schalkwyk LC, Rastan S, Brown SD, et al. 2002. Genetic analysis of the mouse brain proteome. Nat Genet 30: 385–393 [DOI] [PubMed] [Google Scholar]
- Knauf U, Newton EM, Kyriakis J, Kingston RE 1996. Repression of human heat shock factor 1 activity at control temperature by phosphorylation. Genes Dev 10: 2782–2793 [DOI] [PubMed] [Google Scholar]
- Krebs RA, Feder ME 1997. Deleterious consequences of Hsp70 overexpression in Drosophila melanogaster larvae. Cell Stress Chaperones 2: 60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryukov GV, Pennacchio LA, Sunyaev SR 2007. Most rare missense alleles are deleterious in humans: Implications for complex disease and association studies. Am J Hum Genet 80: 727–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Beebe K, Nangle LA, Jang J, Longo-Guess CM, Cook SA, Davisson MT, Sundberg JP, Schimmel P, Ackerman SL 2006. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature 443: 50–55 [DOI] [PubMed] [Google Scholar]
- Lindquist S 1986. The heat-shock response. Annu Rev Biochem 55: 1151–1191 [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA 1988. The heat-shock proteins. Annu Rev Genet 22: 631–677 [DOI] [PubMed] [Google Scholar]
- Matthews BW 1993. Structural and genetic analysis of protein stability. Annu Rev Biochem 62: 139–160 [DOI] [PubMed] [Google Scholar]
- McClellan AJ, Scott MD, Frydman J 2005. Folding and quality control of the VHL tumor suppressor proceed through distinct chaperone pathways. Cell 121: 739–748 [DOI] [PubMed] [Google Scholar]
- Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM 2001. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol 3: 100–105 [DOI] [PubMed] [Google Scholar]
- Milarski KL, Morimoto RI 1986. Expression of human HSP70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci 83: 9517–9521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano KA, Santoro N, Koch KA, Thiele DJ 1999. A trans-activation domain in yeast heat shock transcription factor is essential for cell cycle progression during stress. Mol Cell Biol 19: 402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI 1998. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12: 3788–3796 [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Kline MP, Bimston DN, Cotto JJ 1997. The heat-shock response: Regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem 32: 17–29 [PubMed] [Google Scholar]
- Morishima Y, Kanelakis KC, Murphy PJ, Lowe ER, Jenkins GJ, Osawa Y, Sunahara RK, Pratt WB 2003. The hsp90 cochaperone p23 is the limiting component of the multiprotein hsp90/hsp70-based chaperone system in vivo where it acts to stabilize the client protein: hsp90 complex. J Biol Chem 278: 48754–48763 [DOI] [PubMed] [Google Scholar]
- Mosser DD, Kotzbauer PT, Sarge KD, Morimoto RI 1990. In vitro activation of heat shock transcription factor DNA-binding by calcium and biochemical conditions that affect protein conformation. Proc Natl Acad Sci 87: 3748–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PJ, Kanelakis KC, Galigniana MD, Morishima Y, Pratt WB 2001. Stoichiometry, abundance, and functional significance of the hsp90/hsp70-based multiprotein chaperone machinery in reticulocyte lysate. J Biol Chem 276: 30092–30098 [DOI] [PubMed] [Google Scholar]
- Ng PC, Henikoff S 2006. Predicting the effects of amino acid substitutions on protein function. Annu Rev Genomics Hum Genet 7: 61–80 [DOI] [PubMed] [Google Scholar]
- Nollen EA, Morimoto RI 2002. Chaperoning signaling pathways: Molecular chaperones as stress-sensing ‘heat shock’ proteins. J Cell Sci 115: 2809–2816 [DOI] [PubMed] [Google Scholar]
- Odunuga OO, Longshaw VM, Blatch GL 2004. Hop: More than an Hsp70/Hsp90 adaptor protein. Bioessays 26: 1058–1068 [DOI] [PubMed] [Google Scholar]
- Pace CN, Fisher LM, Cupo JF 1981. Globular protein stability: Aspects of interest in protein turnover. Acta Biol Med Ger 40: 1385–1392 [PubMed] [Google Scholar]
- Pakula AA, Sauer RT 1989. Genetic analysis of protein stability and function. Annu Rev Genet 23: 289–310 [DOI] [PubMed] [Google Scholar]
- Park C, Marqusee S 2005. Pulse proteolysis: A simple method for quantitative determination of protein stability and ligand binding. Nat Methods 2: 207–212 [DOI] [PubMed] [Google Scholar]
- Picard D, Khursheed B, Garabedian MJ, Fortin MG, Lindquist S, Yamamoto KR 1990. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature 348: 166–168 [DOI] [PubMed] [Google Scholar]
- Plaxco KW, Simons KT, Baker D 1998. Contact order, transition state placement and the refolding rates of single domain proteins. J Mol Biol 277: 985–994 [DOI] [PubMed] [Google Scholar]
- Pollak DD, John J, Bubna-Littitz H, Schneider A, Hoeger H, Lubec G 2006. Components of the protein quality control system are expressed in a strain-dependent manner in the mouse hippocampus. Neurochem Int 49: 500–507 [DOI] [PubMed] [Google Scholar]
- Posokhova E, Song H, Belcastro M, Higgins L, Bigley LR, Michaud NA, Martemyanov KA, Sokolov M 2010. Disruption of the Chaperonin containing TCP-1 function affects protein networks essential for rod outer segment morphogenesis and survival. Mol Cell Proteomics 10: M110 000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE 2009. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem 78: 959–991 [DOI] [PubMed] [Google Scholar]
- Prahlad V, Cornelius T, Morimoto RI 2008. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science 320: 811–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahlad V, Morimoto RI 2009. Integrating the stress response: Lessons for neurodegenerative diseases from C. elegans. Trends Cell Biol 19: 52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB, Toft DO 1997. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev 18: 306–360 [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO 2003. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 228: 111–133 [DOI] [PubMed] [Google Scholar]
- Pratt WB, Morishima Y, Osawa Y 2008. The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts. J Biol Chem 283: 22885–22889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB, Morishima Y, Murphy M, Harrell M 2006. Chaperoning of glucocorticoid receptors. Handb Exp Pharmacol 172: 111–138 [DOI] [PubMed] [Google Scholar]
- Princiotta MF, Finzi D, Qian SB, Gibbs J, Schuchmann S, Buttgereit F, Bennink JR, Yewdell JW 2003. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity 18: 343–354 [DOI] [PubMed] [Google Scholar]
- Qian SB, Zhang X, Sun J, Bennink JR, Yewdell JW, Patterson C 2010. mTORC1 links protein quality and quantity control by sensing chaperone availability. J Biol Chem 285: 27385–27395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queitsch C, Sangster TA, Lindquist S 2002. Hsp90 as a capacitor of phenotypic variation. Nature 417: 618–624 [DOI] [PubMed] [Google Scholar]
- Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE 2005. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Genet 37: 894–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529 [DOI] [PubMed] [Google Scholar]
- Rutherford SL, Lindquist S 1998. Hsp90 as a capacitor for morphological evolution. Nature 396: 336–342 [DOI] [PubMed] [Google Scholar]
- Sachidanandam R, Weissman D, Schmidt SC, Kakol JM, Stein LD, Marth G, Sherry S, Mullikin JC, Mortimore BJ, Willey DL, et al. 2001. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature 409: 928–933 [DOI] [PubMed] [Google Scholar]
- Sahi C, Craig EA 2007. Network of general and specialty J protein chaperones of the yeast cytosol. Proc Natl Acad Sci 104: 7163–7168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez IE, Ferreiro DU, Gay Gde P 2010. Mutational analysis of kinetic partitioning in protein folding and protein-DNA binding. Protein Eng Des Sel 24: 179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos MA, Cheesman C, Costa V, Moradas-Ferreira P, Tuite MF 1999. Selective advantages created by codon ambiguity allowed for the evolution of an alternative genetic code in Candida spp. Mol Microbiol 31: 937–947 [DOI] [PubMed] [Google Scholar]
- Shi Y, Mosser DD, Morimoto RI 1998. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev 12: 654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JF, Ziegler MM, Baldwin TO 1994. Kinetic partitioning during protein folding yields multiple native states. Nat Struct Biol 1: 320–326 [DOI] [PubMed] [Google Scholar]
- Skowronek MH, Hendershot LM, Haas IG 1998. The variable domain of nonassembled Ig light chains determines both their half-life and binding to the chaperone BiP. Proc Natl Acad Sci 95: 1574–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF, Toft DO 1993. Steroid receptors and their associated proteins. Mol Endocrinol 7: 4–11 [DOI] [PubMed] [Google Scholar]
- Somero GN 1995. Proteins and temperature. Annu Rev Physiol 57: 43–68 [DOI] [PubMed] [Google Scholar]
- Song J, Takeda M, Morimoto RI 2001. Bag1-Hsp70 mediates a physiological stress signalling pathway that regulates Raf-1/ERK and cell growth. Nat Cell Biol 3: 276–282 [DOI] [PubMed] [Google Scholar]
- Sorger PK, Pelham HR 1988. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell 54: 855–864 [DOI] [PubMed] [Google Scholar]
- Stevens FJ, Argon Y 1999a. Pathogenic light chains and the B-cell repertoire. Immunol Today 20: 451–457 [DOI] [PubMed] [Google Scholar]
- Stevens FJ, Argon Y 1999b. Protein folding in the ER. Semin Cell Dev Biol 10: 443–454 [DOI] [PubMed] [Google Scholar]
- Suckow J, Markiewicz P, Kleina LG, Miller J, Kisters-Woike B, Muller-Hill B 1996. Genetic studies of the Lac repressor. XV: 4000 single amino acid substitutions and analysis of the resulting phenotypes on the basis of the protein structure. J Mol Biol 261: 509–523 [DOI] [PubMed] [Google Scholar]
- Theodorakis NG, Morimoto RI 1987. Posttranscriptional regulation of hsp70 expression in human cells: Effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol 7: 4357–4368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulasiraman V, Yang CF, Frydman J 1999. In vivo newly translated polypeptides are sequestered in a protected folding environment. EMBO J 18: 85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Anken E, Romijn EP, Maggioni C, Mezghrani A, Sitia R, Braakman I, Heck AJ 2003. Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity 18: 243–253 [DOI] [PubMed] [Google Scholar]
- van den Berg B, Ellis RJ, Dobson CM 1999. Effects of macromolecular crowding on protein folding and aggregation. EMBO J 18: 6927–6933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg B, Wain R, Dobson CM, Ellis RJ 2000. Macromolecular crowding perturbs protein refolding kinetics: Implications for folding inside the cell. EMBO J 19: 3870–3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyk TK, Gatenby AA, LaRossa RA 1989. Demonstration by genetic suppression of interaction of GroE products with many proteins. Nature 342: 451–453 [DOI] [PubMed] [Google Scholar]
- Voellmy R, Boellmann F 2007. Chaperone regulation of the heat shock protein response. Adv Exp Med Biol 594: 89–99 [DOI] [PubMed] [Google Scholar]
- Voisine C, Pedersen JS, Morimoto RI 2010. Chaperone networks: Tipping the balance in protein folding diseases. Neurobiol Dis 40: 12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss AK, Thomas T, Gruss P 2000. Mice lacking HSP90β fail to develop a placental labyrinth. Development 127: 1–11 [DOI] [PubMed] [Google Scholar]
- Wanderling S, Simen BB, Ostrovsky O, Ahmed NT, Vogen SM, Gidalevitz T, Argon Y 2007. GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion. Mol Biol Cell 18: 3764–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch WJ 1992. Mammalian stress response: Cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev 72: 1063–1081 [DOI] [PubMed] [Google Scholar]
- Welch WJ, Suhan JP 1986. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol 103: 2035–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerheide SD, Anckar J, Stevens SM Jr, Sistonen L, Morimoto RI 2009. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 323: 1063–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S, Maurizi MR, Gottesman S 1999. Posttranslational quality control: Folding, refolding, and degrading proteins. Science 286: 1888–1893 [DOI] [PubMed] [Google Scholar]
- Wolynes PG, Onuchic JN, Thirumalai D 1995. Navigating the folding routes. Science 267: 1619–1620 [DOI] [PubMed] [Google Scholar]
- Wu C 1995. Heat shock transcription factors: Structure and regulation. Annu Rev Cell Dev Biol 11: 441–469 [DOI] [PubMed] [Google Scholar]
- Wu D, Rea SL, Yashin AI, Johnson TE 2006. Visualizing hidden heterogeneity in isogenic populations of C. elegans. Exp Gerontol 41: 261–270 [DOI] [PubMed] [Google Scholar]
- Wu C, Wilson S, Walker B, Dawid I, Paisley T, Zimarino V, Ueda H 1987. Purification and properties of Drosophila heat shock activator protein. Science 238: 1247–1253 [DOI] [PubMed] [Google Scholar]
- Yao J, Munson KM, Webb WW, Lis JT 2006. Dynamics of heat shock factor association with native gene loci in living cells. Nature 442: 1050–1053 [DOI] [PubMed] [Google Scholar]
- Yashin AI, Cypser JW, Johnson TE, Michalski AI, Boyko SI, Novoseltsev VN 2002. Heat shock changes the heterogeneity distribution in populations of Caenorhabditis elegans: Does it tell us anything about the biological mechanism of stress response? J Gerontol A Biol Sci Med Sci 57: B83–92 [DOI] [PubMed] [Google Scholar]
- Yeyati PL, Bancewicz RM, Maule J, van Heyningen V 2007. Hsp90 selectively modulates phenotype in vertebrate development. PLoS Genet 3: e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavodszky P, Kardos J, Svingor, Petsko GA 1998. Adjustment of conformational flexibility is a key event in the thermal adaptation of proteins. Proc Natl Acad Sci 95: 7406–7411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, Krogan N, Cagney G, Mai D, Greenblatt J, et al. 2005. Navigating the chaperone network: An integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120: 715–727 [DOI] [PubMed] [Google Scholar]
- Zhong M, Orosz A, Wu C 1998. Direct sensing of heat and oxidation by Drosophila heat shock transcription factor. Mol Cell 2: 101–108 [DOI] [PubMed] [Google Scholar]
- Zimarino V, Wu C 1987. Induction of sequence-specific binding of Drosophila heat shock activator protein without protein synthesis. Nature 327: 727–730 [DOI] [PubMed] [Google Scholar]
- Zimarino V, Tsai C, Wu C 1990. Complex modes of heat shock factor activation. Mol Cell Biol 10: 752–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R 1998. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94: 471–480 [DOI] [PubMed] [Google Scholar]