Abstract
Obesity is typically associated with abnormal eating behaviors. Brain imaging studies in humans implicate the involvement of dopamine (DA)-modulated circuits in pathologic eating behavior(s). Food cues increase striatal extracellular DA, providing evidence for the involvement of DA in the nonhedonic motivational properties of food. Food cues also increase metabolism in the orbitofrontal cortex indicating the association of this region with the motivation for food consumption. Similar to drug-addicted subjects, striatal DA D2 receptor availability is reduced in obese subjects, which may predispose obese subjects to seek food as a means to temporarily compensate for understimulated reward circuits. Decreased DA D2 receptors in the obese subjects are also associated with decreased metabolism in prefrontal regions involved in inhibitory control, which may underlie their inability to control food intake. Gastric stimulation in obese subjects activates cortical and limbic regions involved with self-control, motivation, and memory. These brain regions are also activated during drug craving in drug-addicted subjects. Obese subjects have increased metabolism in the somatosensory cortex, which suggests an enhanced sensitivity to the sensory properties of food. The reduction in DA D2 receptors in obese subjects coupled with the enhanced sensitivity to food palatability could make food their most salient reinforcer putting them at risk for compulsive eating and obesity. The results from these studies suggest that multiple but similar brain circuits are disrupted in obesity and drug addiction and suggest that strategies aimed at improving DA function might be beneficial in the treatment and prevention of obesity.
Keywords: brain dopamine, obesity, positron emission tomography
The prevalence of obesity is increasing worldwide, which varies remarkably across ethnic groups and cultures, and across age groups. In the United States, approximately 90 million Americans are obese. Lately, the prevalence of obesity is leveling off in women but is increasing in men, children, and adolescents.1 Obesity is associated with an increased risk of all-cause morbidity and mortality, which places a sense of urgency to understand the processes that have contributed to this epidemic. Obesity represents the upper end of a bodyweight continuum, rather than a qualitatively different state. Obesity can derive from a variety of causes (ie, genetic, culture, nutrition intake, physical activity).2 Most notably, obesity is more prevalent (10 times more likely) in persons whose parents, brothers, or sisters are obese. Studies in identical twins have clearly demonstrated that genetics play a major role.3 For example, nonidentical twins raised together were less similar in weight than identical twins raised apart. However, despite the importance of genetics, it is likely that the changes in the environment are the main contributors to the rapid escalation and magnitude of the obesity epidemic in recent decades. The nature and nurture interactions associated with obesity are thought to occur after conception but before birth. Maternal nutritional imbalance and metabolic disturbances during pregnancy could affect gene expression and contribute to the development of obesity and diabetes mellitus of offspring in later life.4 Recent experiments have shown that nutritional exposures, stress, or disease state after birth may also result in lifelong remodeling of gene expression.5
Of particular relevance is the environment, which has made food not only widely available but also increasingly more varied and palatable. However, the net effect of overweight and obesity on morbidity and mortality is difficult to quantify. It is likely that a gene-environment interaction(s), in which genetically susceptible individuals respond to an environment with increased availability of palatable energy-dense foods and reduced opportunities for energy expenditure, contribute to the current high prevalence of obesity.6
PERIPHERAL AND CENTRAL SIGNALS IN EATING BEHAVIOR
Food ingestion is modulated by both peripheral and central signals. The hypothalamus and its various circuits including orexin and melanin concentrating hormone producing neurons in the lateral hypothalamus as well as neuropeptide Y/agouti related protein and alpha-melanocyte stimulating hormone producing neurons in the arctuate nucleus are thought to be the principal homeostatic brain regions responsible for the regulation of body weight (Fig. 1A).7 Peripheral hormone signals (ie, ghrelin, peptide YY3–36, leptin) that originate from the gut and fat cells continually inform the brain about the status of acute hunger and satiety.8 The hunger peptide, ghrelin, normally increases during fasting and drops after a meal.9 Ghrelin increases food intake and body weight by stimulating neurons in the hypothalamus. Fasting ghrelin levels are lower in obese individuals and fail to decline after a meal and this may contribute to their overeating.10 Obese individuals often have enlarged adipocytes with a reduced buffering capacity for fat storage. The dysfunction of adipose tissue (particularly abdominal fat) plays an important role in the development of insulin resistance. Adipocytes modulate influx of dietary fat and secrete a variety of hormones (ie, leptin). Leptin signals to the brain the level of body fat stores and induces weight loss by suppressing food intake and by stimulating metabolic rate.11 It is also involved in the neuroendocrine response to starvation, energy expenditure, and reproduction (initiation of human puberty).12 Common forms of obesity in humans are associated with a failure for high leptin levels to suppress feeding and mediate weight loss, which is defined as leptin resistance.11,13 Leptin resistance in the hypothalamus invokes the starvation pathway and promotes food intake. Insulin shares a common central signaling pathway with leptin that regulates energy homeostasis through the hypothalamus. Insulin levels reflect short-term changes in energy intake, whereas leptin levels reflect energy balance over a longer period of time.14 Insulin also acts as an endogenous leptin antagonist. Suppression of insulin ameliorates leptin resistance. Chronically, rises in insulin (ie, insulin resistance) impede leptin signal transduction and propagate obesity.
FIGURE 1.
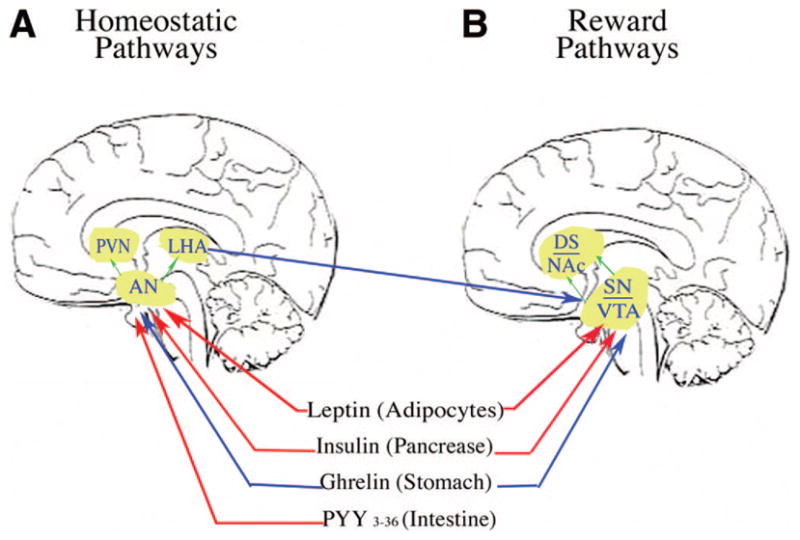
Homeostatic (A) and dopaminergic (reward/motivation) (B) circuits. Red lines depict inhibitory inputs and blue lines depict excitatory inputs. A, Peripheral hormone signals (ie, leptin, ghrelin, insulin, peptide YY) enter the brain directly or indirectly and provide afferent information to the arctuate nucleus (AN), relating to short-term energy metabolism and energy sufficiency. The AN elicits anorexigenic (alpha-melanocyte stimulating hormone, cocaine-amphetamine regulated transcript), and orexigenic (neuropeptide Y, agouti-related protein, gamma-amino butyric acid) signals to the melanocortin 4 receptors in the paraventricular nucleus (PVN) and later hypothalamic area (LHA). Orexin neurons in the LHA send axons to many brain regions including the ventral tegmental areas (VTA). The orexin activates dopamine (DA) neurons in a manner similar to ghrelin. B, The peripheral signals also enter the brain and act on DA neurons in the VTA and substantia nigra (SN) directly. The DA neurons in the VTA send axon projections to the nucleus accumbens (NAc); whereas the DA neurons in the SN send axon projections to the dorsal striatum (DS). Ghrelin activates DA neurons; whereas leptin and insulin inhibit them.
The mesencephalic dopamine (DA) system regulates pleasurable and motivating responses to food intake and stimuli,15,16 which affects and alters behavioral components of energy homeostasis. The mesencephalic DA system can respond to food stimuli even in the presence of postprandial satiety factors.17 When that occurs the regulation of eating behavior can be switched from a homeostatic state to an hedonic corticolimbic state. In addition, other mechanisms modulate eating behavior such as stress, which increases consumption of high energy density food,18 also contributing to obesity.19 The present article discusses the role that DA pathways may play in obesity.
NEUROBIOLOGY OF EATING BEHAVIOR
Behavioral studies show similarities among certain patterns of overeating and other excessive behaviors such as drinking too much alcohol and compulsive gambling. These behaviors activate brain circuitry that involves reward, motivation, decision-making, learning, and memory. Some ingredients in palatable food (ie, sugar, corn oil) can be a subject to compulsive consumption, which we term abuse and can lead to a natural form of loss of controll over their intake, which is akin to what is observed with addiction.20,21 Indeed, ingestion of sugar induces brain release of opioids and DA, which are neurotransmitters traditionally associated with the rewarding effects of drugs of abuse. In certain conditions (ie, intermittent, excessive sugar intake), rats can display behavioral and neurochemical changes that resemble those observed in animal models of drug dependence.22 From an evolutionary perspective, animals would benefit from a neural mechanism (circuitry) that supports an animal’s ability to pursue natural rewards (food, water, sex). These circuits, however, are sometimes dysfunctional leading to various types of disorders.
Endogenous opioids are expressed throughout the limbic system and contribute to processing of reinforcing signals, and palatable foods increases endogenous opioid gene expression.23 Furthermore, injection of mu-opioid agonists in the nucleus accumbens potentiates intake of palatable foods.24 Opioid antagonists, on the other hand, reduce food ratings of pleasantness without affecting hunger.25 It is likely that the opioid system is involved with the liking and the pleasurable responses to food that might augment the intake of highly palatable foods such as those consumed in a high fat and sugar diet.26
DA is a neurotransmitter known to play a major role in motivation that is involved with reward and prediction of reward. The mesocorticolimbic DA system projects from the ventral tegmental area to the nucleus accumbens (NAc), with inputs from various components of the limbic system including the amygdala, hippocampus, hypothalamus, striatum, orbitofrontal cortex (OFC), and the prefrontal cortex. NAc DA has been shown to mediate the reinforcing effects of natural rewards (ie, sucrose).27 DA pathways make food more reinforcing and is also associated with the reinforcing responses to drugs of abuse (ie, alcohol, methamphetamine, cocaine, heroine).28 Other neurotransmitters (eg, acetylcholine, GABA, and glutamine) that modulate DA pathways are also involved in eating behaviors.29
BRAIN DA SYSTEM AND EATING BEHAVIOR
DA regulates food intake via the mesolimbic circuitry apparently by modulating appetitive motivational processes.30 There are projections from the NAc to the hypothalamus that directly regulate feeding.31 Other forebrain DA projects are also involved. DAnergic pathways are critical for survival since they help influence the fundamental drive for eating. Brain DA systems are necessary for wanting incentives, which is a distinct component of motivation and reinforcement.32 It is one of the natural reinforcing mechanisms that motivate an animal to perform and seek a given behavior. The mesolimbic DA system mediates incentive learning and reinforcement mechanisms associated with positive reward such as palatable food in a hungry animal.32
DAergic neurotransmission is mediated by 5 distinct receptor subtypes, which are classified into 2 main classes of receptors termed D1-like (D1 and D5) and D2-like (D2, D3, and D4). The location and function of these receptor subtypes are listed in Table 1. In the case of drug self-administration, activation of D2-like receptors has been shown to mediate the incentive to seek further cocaine reinforcement in animals. In contrast, D1-like receptors mediate a reduction in the drive to seek further cocaine reinforcement.33 Both the D1- and D2-like receptors act synergistically when regulating feeding behaviors. Nevertheless, the precise involvement of DA receptor subtypes in mediating eating behavior is still not clear. DA D1-like receptors play a role in motivation to work for reward-related learning and translation of new reward to action.34,35 No human imaging studies have assessed the involvement of D1 receptors on eating behaviors yet. Animal studies showed that infusion of DA D1 receptor antagonists in the NAc shell impaired associative gustatory (ie, taste) learning and blunted the rewarding effects of palatable food.36 Selective D1 receptor agonist can enhance preference of high-palpability food over regular maintenance diet.37 The role of DA D5 receptors on eating behaviors is not established because of the lack of selective ligand that can discriminate between D1 and D5 receptors.
TABLE 1.
Location and Function of Dopamine (DA) Receptor Subtypes
| DA Receptors | Location (Reviewed in Ref. 38) | Function |
|---|---|---|
| D1-like | ||
| D1/D5 | Caudate nucleus, prefrontal, premotor, cingulate and entorhinal cortices, hippocampus, dentate gyrus, substantia nigra | Gustatory learning, food preference36,37 |
| D2-like | ||
| D2 | Predominantly in the striatum, in the core of nucleus accumbens (NAc), and in the olfactory tubercle. It also presents in the prefrontal, cingulate, temporal and enthorinal cortices, amygdala, hippocampus, hypothalamus, substantia nigra pars compacta, ventral tegmental area (VTA) | Food seeking, prediction, expectation, motivation (reviewed in Ref. 30) |
| D3 | Predominantly in limbic brain regions, ie, NAc, islands of Calleja, VTA, substantia nigra, internal globus pallidus, ventral pallidum and lower levels in dorsal striatum, septum, amygdala, hippocampus | Relapse, food-related cue reintroduction or reinstatement (reviewed in Ref. 50) |
| D4 | Predominantly in frontal cortex, amygdala, hippocampus, hypothalamus, mesencephalon, and low levels in basal ganglia | Inhibitory control, satiety 59,60 |
The D2 receptors have been associated with feeding and addictive behaviors in animal and human studies. D2 receptors play a role in reward seeking, prediction, expectation, and motivation.30 Food seeking is initiated by hunger; however, it is food-predictive cues that activate and motivate animals. Many of the animal studies were evaluated using mixed D2/D3 receptor antagonists or agonists.38 D2 receptor antagonists block food seeking behaviors that depend on history association (reinforcement) between the cues and the reward they predict as well as on palatable foods they like.39 When food is no longer priming and rewarding for an animal, D2 agonists can be used to reinstate extinguished reward seeing behavior.40 Human imaging studies of eating behaviors have mainly used positron emission tomography (PET) studies with [11C]raclopride, a reversible DA D2/D3 receptor radioligand, which binds at D2 and D3 receptors with similar affinity. A human PET study with [11C]raclopride that measured DA releases in the striatum after consumption of a favorite food showed that the amount of DA release was correlated with the ratings of meal pleasantness.41 Food deprivation potentiates the rewarding effects of food.42 During fasting, the role of DA is not selective for food, but rather signals the salience for a variety of potential biologic rewards and cues that predict rewards.43 Chronic food deprivation also potentiates the rewarding effects of most addictive drugs.44 The striatum, OFC, and amygdala, which are brain regions that receive DA projections are activated during the expectation of food.45 In fact, using PET and [11C]raclopride to evaluate changes in extracellular DA in striatum in response to food-cues (presentation of palatable food) in food-deprived subjects, we showed significant increases in extracellular DA in the dorsal striatum but not in the ventral striatum (where the NAc is located).46 The DA increases were significantly correlated with the increases in self-reports of hunger and desire for food. These results provided evidence of conditioned-cue reaction in the dorsal striatum. The involvement of DA in the dorsal striatum seems to be crucial for enabling the motivation required to consume the food that is necessary for survival.47,48 It is different from the activation in the NAc, which may be related more to motivation associated with food palatability.30,49
It has been postulated that D3 receptors might be involved in drug dependence and addiction.50 Recently, several selective D3 receptor antagonists were developed. These antagonists have higher selectivity for the D3 receptor compared with other DA receptors.50 Administration of a selective D3 receptor antagonist prevented nicotine-triggered relapse to nicotine-seeking behavior.51 It also attenuated sucrose-seeking behavior induced by sucrose-associated cue reintroduction in the rodent.52 We have also shown that D3 receptor antagonists decrease food intake in rats.53 Several selective D3 receptor PET radioligands have been developed54–56 but none to our knowledge has been used to investigate eating behavior and obesity in humans. The D4 receptors are predominantly located in cortical regions in both pyramidal and GABAergic cells,57 in striatal neurons and in hypothalamus.58 It is believed to act as an inhibitory postsynaptic receptor controlling the neurons of the frontal cortex and striatum.59 These receptors may play a role influencing satiety.60
DOPAMINE AND THE SENSORY EXPERIENCE OF FOOD
Sensory processing of food and food-related cues plays an important role in the motivation for food and it is especially important in the selection of a varied diet. Sensory inputs of taste, vision, olfaction, temperature, and texture are first sent to the primary sensory cortices (ie, insula, primary visual cortex, pyriform, primary somatosensory cortex) and then to the OFC and amygdala.61 The hedonic reward value of food is closely linked to the sensory perception of the food. The relation of DA in these brain regions during sensory perception of food will be discussed.
The insular cortex is involved in the interceptive sense of the body and in emotional awareness.62 Our imaging study in which we used balloon extension to mimic the gastric distension that occurs during normal food intake showed activation of the posterior insula, which implicates its role in the awareness of body state.63 Indeed, in smokers, damage to the insula disrupts their physiologic urge to smoke.64 The insula is the primary gustatory area, which participates in many aspects of eating behavior such as taste. DA plays an important role in the tasting of palatable foods, which is mediated through the insula.65 Animal studies have shown that tasting sucrose increases DA release in the NAc.66 Lesions in the ventral tegmental area reduced consumption of a preferred sucrose solution.67 Human imaging studies have showed that tasting palatable foods activated the insula and midbrain areas.68,69 However, the human brain can distinguish the calorie content of the sweet solution unconsciously. For example, when normal weight women tasted sweetener with calories (sucrose), both the insula and DAnergic midbrain areas were activated, whereas when they tasted sweetener without calories (sucralose), they only activated the insula.69 Obese subjects have greater activation in the insula than normal controls when tasting a liquid meal that consists of sugar and fat.68 In contrast, subjects who have recovered from anorexia nervosa show less activation in the insula when tasting sucrose and no association of feelings of pleasantness with insular activation as observed in the normal controls.70 It is likely that dysregulation of the insula in response to the taste might be involved in disturbances in appetite regulation.
There is limited literature that addresses the role of the primary somatosensory cortex in food intake and obesity. Activation of the somatosensory cortex was reported in an imaging study of normal weight women during the viewing of images of low caloric foods.71 Using PET and [18F]fluoro-deoxyglucose (FDG) to measure regional brain glucose metabolism (marker of brain function), we showed that morbidly obese subjects had higher than normal baseline metabolism in the somatosensory cortex (Fig. 2).72 There is evidence that the somatosensory cortex influences brain DA activity73,74 including regulating amphetamine-induced striatal DA release.75 DA also modulates the somatosensory cortex in the human brain.76 Moreover, we recently showed an association between striatal D2 receptors availability and glucose metabolism in the somatosensory cortex of obese subjects.77 Since DA stimulation signals saliency and facilitates conditioning,78 DA’s modulation of the somatosensory cortex to food stimuli might enhance their saliency, which is likely to play a role in the formation of conditioned associations between food and food-related environmental cues.
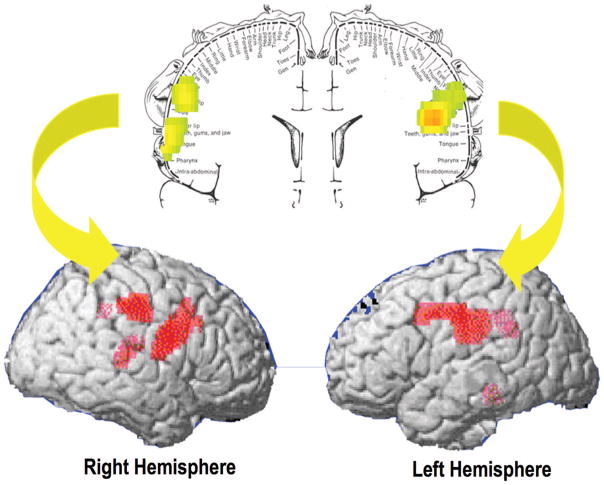
FIGURE 2.
Color-coded statistical parameter map (SPM) result displayed in a coronal plane with a superimposed diagram of the somatosensory homunculus with its corresponding three-dimensional (3D) rendered SPM images show the areas with higher metabolism in obese than in lean subjects. The areas that are significantly higher are displayed in red and are superimposed into the surface of 3D reconstructed brain magnetic resonance images (grayscale). Obese subjects have higher metabolism than lean subjects in the somatosensory areas where the mouth, lips and tongue are represented. (Adapted from Neuroreport. 2002;13:1151–1155.)
The OFC, which is in part regulated by DA activity, is a key brain region for controlling behaviors and for salience attribution including the value of food.79,80 As such, it determines the pleasantness and palatability of food as a function of its context. Using PET and FDG in normal weight individuals, we showed that exposure to food-cues (same paradigm as the one with which we shoed that cues increase DA in dorsal striatum) increased metabolism in OFC and that these increases were associated with the perception of hunger and the desire for food.81 The enhanced OFC activation by the food stimulation are likely to reflect downstream DAergic effects and are likely to participate in DA’s involvement in the drive for food consumption. The OFC participates in learning stimulus-reinforcement associations and conditioning.82,83 It also participates in conditioned cues elicited feeding.84 Thus its activation secondary to food-induced DA stimulation could result in an intense motivation to consume food. Dysfunction of the OFC is associated with compulsive behaviors including overeating.85 This is relevant because food-induced conditioned responses likely contribute to overeating irrespective of hunger signals.86
The amygdala is another brain region involved in eating behavior. More specifically, there is evidence that it is involved with learning and recognition of the biologic significance of objects during food procurement.87 Extracellular DA levels in the amygdala were increased in a preclinical study of food intake after a brief period of fasting.88 Functional neuroimaging studies using PET and functional magnetic resonance imaging (fMRI) have shown activation of the amygdala with food-related stimuli, tastes, and odors.89–91 The amygdala is also involved with the emotional component of food intake. Stress-induced amygdala activation can be dampened by the ingestion of energy-dense food.18 The amygdala receives interoceptive signals from the visceral organs. In a study in which we assessed with fMRI the brain activation response to gastric distention, we showed an association between activation in the amygdala and subjective feelings of fullness.63 We also found that the subjects with higher body mass index (BMI) had less activation in the amygdala during gastric distention. It is likely that perception mediated by the amygdala could influence the content and volumes of food consumed in a given meal.
INTERACTION BETWEEN PERIPHERAL METABOLIC SIGNALS AND BRAIN DA SYSTEM
Many peripheral metabolic signals directly or indirectly interact with DA pathways. Highly palatable foods can override internal homeostatic mechanisms through action on brain DA pathways and lead to overeating and obesity.17 Simple carbohydrates such as sugar are a major nutritional source and contribute to about one-fourth of total energy intake. Animal studies have demonstrated that glucose modulates DA neuronal activity in the ventral tegmental area and sub-stantia nigra directly. The midbrain DA neurons also interact with insulin, leptin, and ghrelin.11,92,93 Ghrelin activates DA neurons; whereas leptin and insulin inhibit them (Fig. 1B). Food restriction increases circulating ghrelin released from the stomach and activates the mesolimbic system increasing DA release in the NAc.93 An fMRI study showed that infusion of ghrelin to healthy subjects enhanced activation to food cues in brain regions involved in hedonic and incentive responses.94 Insulin stimulates glucose metabolism directly, functioning as a neurotransmitter or stimulating neuronal glucose uptake indirectly. There is evidence that brain insulin plays a role in feeding behavior, sensory processing, and cognitive function.95–97 Laboratory animals with disruption of brain insulin receptors show enhanced feeding.98 A recent human study using PET-FDG showed that brain insulin resistance coexists in subjects with peripheral insulin resistance, especially in the striatum and insula (regions that relate to appetite and reward).99 Insulin resistance in these brain regions in subjects with insulin resistance may require much higher levels of insulin to experience the reward and the interoceptive sensations of eating. Leptin also plays a role in regulating eating behavior in part through regulation of the DA pathway (but also the cannabinoid system). An fMRI study showed that leptin could diminish food reward and enhance the response to satiety signals generated during food consumption through the modulation of neuronal activity in the striatum in leptin-deficient human subjects.100 Thus, insulin and leptin can act complementarily to modify the DA pathway and alter eating behaviors. Leptin and insulin resistance in the brain DA pathways makes food intake a more potent reward and promotes palatable food intake.101
BRAIN DA AND OBESITY
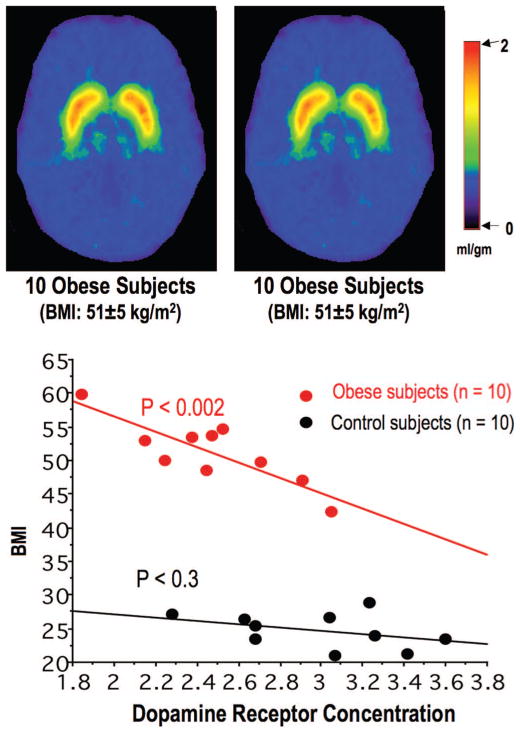
The involvement of DA in overeating and obesity has also been reported in rodent models of obesity.102–105 Treatment with DA agonists in obese rodents induced weight loss, presumably through DA D2- and DA D1-like receptor activations.106 Humans, chronically treated with antipsychotic drugs (D2R antagonists) are at a greater risk of weight gain and obesity, which is mediated in part by blockade of D2R.30 Administration of DA agonists in obese mice normalizes their hyperphagia.105 Our PET studies with [11C]raclopride have documented a reduction in striatal D2/D3 receptor availability in obese subjects.107 The BMI of the obese subjects was between 42 and 60 (body weight: 274–416 lb) and their body weight remained stable before the study. The scans were done after subjects fasted for 17–19 hours and under resting conditions (no stimulation, eyes open, minimal noise exposure). In obese subjects but not in controls, D2/D3 receptor availability was inversely related to BMI (Fig. 3). To assess if low D2/D3 receptors in obesity reflected the consequences of food over-consumption as opposed to a vulnerability that preceded obesity, we assessed the effect of food intake on D2/D3 receptor in Zucker rats (a genetically leptin deficient rodent model of obesity) using autoradiography.108 The animals had free assess to food for 3 months and the D2/D3 receptor levels were evaluated at 4 months old. Results showed that Zucker obese (fa/fa) rats had lower D2/D3 receptor levels than the lean (Fa/Fa or Fa/fa) rats and that food restriction increased D2/D3 receptors both in the lean and the obese rats indicating that low D2/D3 reflects in part the consequences of food over-consumption. Similar to the human study, we also found an inverse correlation of D2/D3 receptor levels and body weight in these obese rats. The relationship between BMI and brain DA transporter (DAT) levels has also been investigated. Rodent studies demonstrated significant decreases in DAT densities in the striatum of obese mice.104,109 In humans, a recent study using single photon emission tomography and [99mTc] TRODAT-1 to study 50 Asians (BMI: 18.7–30.6) in resting state showed that BMI was inversely associated with striatal DAT availability.110 These studies suggest the involvement of an understimulated DA system in excessive weight gain. Since the DA pathways have been implicated in reward (predict reward) and motivation, these studies suggest that deficiency in DA pathways may lead to pathologic eating as a means to compensate for an understimulated reward system.
FIGURE 3.
Group averaged images of [11C]raclopride PET scans for obese and control subjects at the level of the basal ganglia. The images are scaled with respect to the maximum value (distribution volume) obtained on the control subjects and presented using the rainbow scale. Red represents the highest value (2.0) and dark violet represents the lowest value (0 mL/gm). The obese subjects have lower D2R availability when compared with the control subjects. Linear regression between D2R availability (Bmax/Kd) and BMI shows that the D2R availability levels were inversely related to body mass index (BMI) in the obese subjects but not in the control subjects. (Adapted from Lancet. 2001;357:354–357.)
INHIBITORY CONTROL AND OBESITY
In addition to the hedonic reward responses, DA also plays an important role in inhibitory control. Disruption of inhibitory control may contribute to behavioral disorders such as addiction. There are several genes related to DA transmission that play important roles in drug reward and inhibitory control.111 For example, polymorphisms in the D2 receptor gene in healthy subjects are associated with behavioral measures of inhibitory control. Individuals with the gene variant that is linked with lower D2 receptor expression had lower inhibitory control than individuals with the gene variant associated with higher D2 receptor expression.112 These behavioral responses are associated with differences in activation of the cingulate gyrus and dorsolateral prefrontal cortex, which are brain regions that have been implicated in various components of inhibitory control.113 Prefrontal regions also participate in the inhibition of tendencies for inappropriate behavioral responses.114 The significant association between D2R availability and metabolism in prefrontal regions is observed in our studies in drug-addicted subjects (cocaine, methamphetamine, and alcohol).115–117 We found that the reduction in D2R availability in these subjects was associated with decreased metabolism in prefrontal cortical regions,118 which are involved in regulating impulse control, self-monitoring, and goal-directed behaviors.119,120 A similar observation was documented in individuals at high familial risk for alcoholism.121 These behaviors could influence the ability of an individual to self-regulate his/her eating behavior. Previous work with PET using [11C]raclopride, [11C]d-threo-methylphenidate (to measure DAT availability) and FDG to evaluate the association between DA activity and brain metabolism in morbidly obese subjects (BMI >40 kg/m2)77 found that D2/D3 receptor but not DAT were associated with glucose metabolism in dorsolateral prefrontal, orbitofrontal, and cingulate cortices. The findings suggested that D2/D3 receptor-mediated dysregulation of regions implicated in inhibitory control in the obese subjects may underlie their inability to control food intake despite their conscious attempts to do so. This led us to consider the possibility that the low D2/D3 receptor modulation of the risk for overeating in the obese subjects could also be driven by its regulation of the prefrontal cortex.
MEMORY AND OBESITY
The susceptibility to gain weight is in part due to the variability in individual responses to environmental triggers such as caloric content of food. The intense desire to eat a specific food or food craving is an important factor influencing appetite control. Food craving is a learned appetite for energy through the reinforcing effects of eating a specific food when hungry.79 It is a common event that is frequently reported across all ages. Nevertheless, food craving can also be induced by food cues and sensory stimulation regardless of the state of satiety indicating that conditioning is independent of the metabolic need for food.122 Functional brain imaging studies have shown that the desire to eat a specific food was associated with activation of the hippocampus, which is likely to reflect its involvement storing and retrieving the memories for the desired food.123,124 The hippocampus connects with brain regions involved in satiety and hunger signals including the hypothalamus and insula. In our studies using gastric stimulation and gastric distention, we showed activation of the hippocampus presumably from downstream stimulation of the vagus nerve and the solitary nucleus.63,125 In these studies, we showed that the activation of the hippocampus was associated with a sensation of fullness. These findings suggest a functional connection between the hippocampus and peripheral organs such as the stomach in the regulation of food intake. The hippocampus also modulates the saliency of stimuli through regulation of DA release in the NAc126 and is involved in incentive motivation.127 It also regulates activity in prefrontal regions involved with inhibitory control.128 An imaging study showed that tasting a liquid meal resulted in decreased activity in the posterior hippocampus in obese and previously obese but not in lean subjects. Persistence of abnormal neuronal response in the hippocampus in the previously obese was associated with their susceptibility to relapse. These findings implicate the hippocampus in the neurobiology of obesity.129 Obese subjects are reported to crave energy-dense foods that make them susceptible to gain weight.130
IMPLICATIONS FOR TREATMENT
Since the development of obesity involves multiple brain circuits (ie, reward, motivation, learning, memory, inhibitory control),15 the prevention and treatment of obesity should be comprehensive and use a multimodal approach. Lifestyle modification (ie, education concerning nutrition, aerobic exercise, effective stress reduction) should be initiated in early childhood and ideally prevention interventions should start during pregnancy. Chronic reduced food intake has been reported to have health benefits, which include modulating the brain DA system. Our recent study in Zucker rats that were chronically food restricted for 3 months had higher D2/D3 receptor levels than the rats with unrestricted food access. Chronic food restriction may also attenuate the age-induced loss of D2/D3 receptor.108 These findings are consistent with preclinical studies reporting that chronic food restriction affects behavior, motor, reward, and slows the aging process.43,131,132 Dietary modifications that reduce energy intake remain central to any weight loss strategy. A study that compared the effectiveness of popular diet programs on the market found a trend of using low carbohydrate, low saturated fat, moderate unsaturated fat, and high protein as an effective diet strategy.133,134 However, many people lose weight initially but start gaining weight after a period of weight loss.135 The food industry should be given incentives to develop low calorie foods that are more attractive, palatable, and affordable so that people can adhere to diet programs for a long time.136 Diet strategies that emphasize social support and family-base counseling are also important to have a successful weight maintenance program.137
Increased physical activity even with minimal impact exercise has been shown to produce measurable improvement in fitness. Exercise generates a number of metabolic, hormonal, and neuronal signals that reach the brain. A high level of fitness is associated with decreases in all causes of mortality in both normal weight and obese individuals. Exercise on a treadmill significantly increases DA release in rat striatum.138 Laboratory animals underwent endurance exercise training (treadmill running, 1 hour per day, 5 days per week for 12 weeks) increase DA metabolism and DA D2 receptor levels in the striatum.139 Animals exercised voluntarily in their cages using a running wheel for 10 days showed enhanced neurogenesis in the hippocampus.140 The effects of physical exercise to human brain function were reported in a brain MRI study that compared the brain volume in a group of healthy but sedentary older individuals (60–79 years old) after 6 months of aerobic exercise training.141 The intervention improved their cardiorespiratory fitness. It also increased their brain volume in both gray and white matter regions. The participants with the greater daily aerobic fitness activity had larger volumes in prefrontal cortices that typically show substantial age-related deterioration. These changes were not observed in the control subjects who participated in nonaerobic exercise (ie, stretching, toning). It is likely that aerobic fitness activity benefits DA function and cognition. Indeed, studies in older individuals have documented that physical activity improved cognitive function.142–145 Fitness training has selective effects on cognitive function that are greatest on executive control processes (ie, planning, working memory, inhibitory control), which usually declines with age.146 Many obese individuals who successfully maintain long-term weight loss report actively engaging in physical activity.147 Their success rate may be in part due to the fact that exercise prevents the reduction in metabolic rate, which usually accompanies chronic weight loss.148 A well-designed aerobic exercise program can modulate motivation, reduce psychologic stress, and enhance cognitive function all of which can help an individual to maintain weight control.149
Drug therapies, in addition to lifestyle changes, are being developed to assist in weight loss in combination with lifestyle management to improve weight loss maintenance and to reduce obesity-related medical consequences. There are a number of targets for drug therapies. Many small molecules and peptides that target the hypothalamus have been reported to increase satiety, reduce food intake, and balance energy homeostasis in rodent models.150,151 However, some of these molecules when tested on clinical trials failed to show meaningful weight loss.152 Peptide YY3–36 (PYY), a physiological gut-derived satiety signal has shown promising results in increasing satiety and reducing food intake in humans.153 An imaging study showed that infusion of PYY modulates neural activity in corticolimbic, cognitive, and homeostatic brain regions.17 In this study, the fasting participants were infused with PYY or saline during 90 minutes of fMRI scanning. The fMRI signal changes in the hypothalamus and OFC extracted from time series data were compared with subsequent caloric intake for each subject on the PYY and saline days. On the saline day, the subjects were fasted and had lower plasma levels of PYY, the change in the hypothalamus correlated with subsequent caloric intake. In contrast, on the PYY day that the high plasma levels of PYY mimicked fed state, the changes in the OFC predicted caloric intake independently of meal-related sensory experience; whereas hypothalamic signal changes did not. Thus, the regulation of eating behaviors could be easily switched from a homeostatic state to a hedonic corticolimbic state. Therefore, the strategy to treat obesity should include agents that modulate the hedonic state of food intake. In fact, several medications with properties of DA reuptake inhibitor (ie, Bupropion), opioid antagonist (ie, Naltrexone), or combination of other drugs that modulate DA activity (ie, Zonisamide, Topiramate) have been reported to promote weight loss in obese subjects.154–156 The efficacy of these medications on long-term weight maintenance needs further evaluation.
CONCLUSION
Obesity reflects an imbalance between energy intake and expenditure that is mediated by the interaction of energy homeostasis and hedonic food intake behavior. DA plays an important role in circuits (ie, motivation, reward, learning, inhibition control) that regulate abnormal eating behavior. Brain imaging studies show that obese individuals have significantly lower D2/D3 receptor levels, which make them less sensitive to reward stimuli, which in turn would make them more vulnerable to food intake as a means to temporarily compensate for this deficit. The decreased D2/D3 receptor levels are also associated with decreased metabolism in brain regions involved with inhibitory control and processing food palatability. This may underlie the inability to control food intake in the obese individuals while facing incentive salience such as exposure to highly palatable food. The results from these studies have implications for the treatment of obesity since they suggest that strategies aimed at improving brain DA function might be beneficial in the treatment and prevention of obesity.
Acknowledgments
The authors also thank the scientific and technical staffs at the Brookhaven Center for Translational Neuroimaging for their support of these research studies as well as the individuals who volunteered for these studies.
Supported in part by grants from the U.S. Department of Energy OBER (DE-ACO2-76CH00016), the National Institute on Drug Abuse (5RO1DA006891-14, 5RO1DA6278-16, 5R21, DA018457-2), the National Institute on Alcohol Abuse and Alcoholism (RO1AA9481-11 & Y1AA3009), and by the General Clinical Research Center at Stony Brook University Hospital (NIH MO1RR 10710).
References
- 1.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Bessesen DH. Update on obesity. J Clin Endocrinol Metab. 2008;93:2027–2034. doi: 10.1210/jc.2008-0520. [DOI] [PubMed] [Google Scholar]
- 3.Segal NL, Allison DB. Twins and virtual twins: bases of relative body weight revisited. Int J Obes Relat Metab Disord. 2002;26:437–441. doi: 10.1038/sj.ijo.0801941. [DOI] [PubMed] [Google Scholar]
- 4.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113:1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 5.Gallou-Kabani C, Junien C. Nutritional epigenomics of metabolic syndrome: new perspective against the epidemic. Diabetes. 2005;54:1899–1906. doi: 10.2337/diabetes.54.7.1899. [DOI] [PubMed] [Google Scholar]
- 6.Mietus-Snyder ML, Lustig RH. Childhood obesity: adrift in the “limbic triangle”. Annu Rev Med. 2008;59:147–162. doi: 10.1146/annurev.med.59.103106.105628. [DOI] [PubMed] [Google Scholar]
- 7.Morrison CD, Berthoud HR. Neurobiology of nutrition and obesity. Nutr Rev. 2007;65(12 Pt 1):517–534. doi: 10.1301/nr.2007.dec.517-534. [DOI] [PubMed] [Google Scholar]
- 8.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil. 2008;20 (Suppl 1):64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wren AM. Gut and hormones and obesity. Front Horm Res. 2008;36:165–181. doi: 10.1159/000115364. [DOI] [PubMed] [Google Scholar]
- 11.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 12.Ross MG, Desai M. Gestational programming: population survival effects of drought and famine during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288:R25–R33. doi: 10.1152/ajpregu.00418.2004. [DOI] [PubMed] [Google Scholar]
- 13.Lustig RH. Childhood obesity: behavioral aberration or biochemical drive? Reinterpreting the First Law of Thermodynamics. Nat Clin Pract Endocrinol Metab. 2006;2:447–458. doi: 10.1038/ncpendmet0220. [DOI] [PubMed] [Google Scholar]
- 14.Ahima RS, Lazar MA. Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol. 2008;22:1023–1031. doi: 10.1210/me.2007-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkow ND, Wang GJ, Fowler JS, et al. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3109–3111. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 17.Batterham RL, Ffytche DH, Rosenthal JM, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 18.Dallman MF, Pecoraro N, Akana SF, et al. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci USA. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 21.Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1236–R1239. doi: 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- 22.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolley JD, Lee BS, Fields HL. Nucleus accumbens opioids regulate flavor-based preferences in food consumption. Neuroscience. 2006;143:309–317. doi: 10.1016/j.neuroscience.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 25.Yeomans MR, Gray RW. Effects of naltrexone on food intake and changes in subjective appetite during eating: evidence for opioid involvement in the appetizer effect. Physiol Behav. 1997;62:15–21. doi: 10.1016/s0031-9384(97)00101-7. [DOI] [PubMed] [Google Scholar]
- 26.Will MJ, Pratt WE, Kelley AE. Pharmacological characterization of high-fat feeding induced by opioid stimulation of the ventral striatum. Physiol Behav. 2006;89:226–234. doi: 10.1016/j.physbeh.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Smith GP. Accumbens dopamine mediates the rewarding effect of orosensory stimulation by sucrose. Appetite. 2004;43:11–13. doi: 10.1016/j.appet.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Kelley AE, Baldo BA, Pratt WE, et al. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 30.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- 32.Robinson S, Rainwater AJ, Hnasko TS, et al. Viral restoration of dopamine signaling to the dorsal striatum restores instrumental conditioning to dopamine-deficient mice. Psychopharmacology (Berl) 2007;191:567–578. doi: 10.1007/s00213-006-0579-9. [DOI] [PubMed] [Google Scholar]
- 33.Self DW, Barnhart WJ, Lehman DA, et al. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- 34.Trevitt JT, Carlson BB, Nowend K, et al. Substantia nigra pars reticulata is a highly potent site of action for the behavioral effects of the D1 antagonist SCH 23390 in the rat. Psychopharmacology (Berl) 2001;156:32–41. doi: 10.1007/s002130100708. [DOI] [PubMed] [Google Scholar]
- 35.Fiorino DF, Coury A, Fibiger HC, et al. Electrical stimulation of reward sites in the ventral tegmental area increases dopamine transmission in the nucleus accumbens of the rat. Behav Brain Res. 1993;55:131–141. doi: 10.1016/0166-4328(93)90109-4. [DOI] [PubMed] [Google Scholar]
- 36.Fenu S, Bassareo V, Di Chiara G. A role for dopamine D1 receptors of the nucleus accumbens shell in conditioned taste aversion learning. J Neurosci. 2001;21:6897–6904. doi: 10.1523/JNEUROSCI.21-17-06897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper SJ, Al-Naser HA. Dopaminergic control of food choice: contrasting effects of SKF 38393 and quinpirole on high-palatability food preference in the rat. Neuropharmacology. 2006;50:953–963. doi: 10.1016/j.neuropharm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Missale C, Nash SR, Robinson SW, et al. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 39.McFarland K, Ettenberg A. Haloperidol does not affect motivational processes in an operant runway model of food-seeking behavior. Behav Neurosci. 1998;112:630–635. doi: 10.1037//0735-7044.112.3.630. [DOI] [PubMed] [Google Scholar]
- 40.Wise RA, Murray A, Bozarth MA. Bromocriptine self-administration and bromocriptine-reinstatement of cocaine-trained and heroin-trained lever pressing in rats. Psychopharmacology (Berl) 1990;100:355–360. doi: 10.1007/BF02244606. [DOI] [PubMed] [Google Scholar]
- 41.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 42.Cameron JD, Goldfield GS, Cyr MJ, et al. The effects of prolonged caloric restriction leading to weight-loss on food hedonics and reinforcement. Physiol Behav. 2008;94:474–480. doi: 10.1016/j.physbeh.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 43.Carr KD. Chronic food restriction: enhancing effects on drug reward and striatal cell signaling. Physiol Behav. 2007;91:459–472. doi: 10.1016/j.physbeh.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 44.Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- 45.Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Curr Opin Neurobiol. 2004;14:139–147. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Wang GJ, Fowler JS, et al. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- 47.Sotak BN, Hnasko TS, Robinson S, et al. Dysregulation of dopamine signaling in the dorsal striatum inhibits feeding. Brain Res. 2005;1061:88–96. doi: 10.1016/j.brainres.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 48.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szczypka MS, Kwok K, Brot MD, et al. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 50.Heidbreder CA, Gardner EL, Xi ZX, et al. The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andreoli M, Tessari M, Pilla M, et al. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology. 2003;28:1272–1280. doi: 10.1038/sj.npp.1300183. [DOI] [PubMed] [Google Scholar]
- 52.Cervo L, Cocco A, Petrella C, et al. Selective antagonism at dopamine D3 receptors attenuates cocaine-seeking behaviour in the rat. Int J Neuropsychopharmacol. 2007;10:167–181. doi: 10.1017/S1461145705006449. [DOI] [PubMed] [Google Scholar]
- 53.Thanos PK, Michaelides M, Ho CW, et al. The effects of two highly selective dopamine D3 receptor antagonists (SB-277011A and NGB-2904) on food self-administration in a rodent model of obesity. Pharmacol Biochem Behav. 2008;89:499–507. doi: 10.1016/j.pbb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hocke C, Prante O, Salama I, et al. 18F-Labeled FAUC 346 and BP 897 derivatives as subtype-selective potential PET radioligands for the dopamine D3 receptor. Chem Med Chem. 2008;3:788–793. doi: 10.1002/cmdc.200700327. [DOI] [PubMed] [Google Scholar]
- 55.Narendran R, Slifstein M, Guillin O, et al. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse. 2006;60:485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- 56.Prante O, Tietze R, Hocke C, et al. Synthesis, radiofluorination, and in vitro evaluation of pyrazolo[1,5-a]pyridine-based dopamine D4 receptor ligands: discovery of an inverse agonist radioligand for PET. J Med Chem. 2008;51:1800–1810. doi: 10.1021/jm701375u. [DOI] [PubMed] [Google Scholar]
- 57.Mrzljak L, Bergson C, Pappy M, et al. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- 58.Rivera A, Cuellar B, Giron FJ, et al. Dopamine D4 receptors are heterogeneously distributed in the striosomes/matrix compartments of the striatum. J Neurochem. 2002;80:219–229. doi: 10.1046/j.0022-3042.2001.00702.x. [DOI] [PubMed] [Google Scholar]
- 59.Oak JN, Oldenhof J, Van Tol HH. The dopamine D(4) receptor: one decade of research. Eur J Pharmacol. 2000;405:303–327. doi: 10.1016/s0014-2999(00)00562-8. [DOI] [PubMed] [Google Scholar]
- 60.Huang XF, Yu Y, Zavitsanou K, et al. Differential expression of dopamine D2 and D4 receptor and tyrosine hydroxylase mRNA in mice prone, or resistant, to chronic high-fat diet-induced obesity. Brain Res Mol Brain Res. 2005;135:150–161. doi: 10.1016/j.molbrainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 61.Rolls ET. Sensory processing in the brain related to the control of food intake. Proc Nutr Soc. 2007;66:96–112. doi: 10.1017/S0029665107005332. [DOI] [PubMed] [Google Scholar]
- 62.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 63.Wang GJ, Tomasi D, Backus W, et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage. 2008;39:1824–1831. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 64.Naqvi NH, Rudrauf D, Damasio H, et al. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hajnal A, Norgren R. Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav. 2005;84:363–369. doi: 10.1016/j.physbeh.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 66.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 67.Shimura T, Kamada Y, Yamamoto T. Ventral tegmental lesions reduce overconsumption of normally preferred taste fluid in rats. Behav Brain Res. 2002;134:123–130. doi: 10.1016/s0166-4328(01)00461-2. [DOI] [PubMed] [Google Scholar]
- 68.DelParigi A, Chen K, Salbe AD, et al. Sensory experience of food and obesity: a positron emission tomography study of the brain regions affected by tasting a liquid meal after a prolonged fast. Neuroimage. 2005;24:436–443. doi: 10.1016/j.neuroimage.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 69.Frank GK, Oberndorfer TA, Simmons AN, et al. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage. 2008;39:1559–1569. doi: 10.1016/j.neuroimage.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 70.Wagner A, Aizenstein H, Mazurkewicz L, et al. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology. 2008;33:513–523. doi: 10.1038/sj.npp.1301443. [DOI] [PubMed] [Google Scholar]
- 71.Killgore WD, Young AD, Femia LA, et al. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 72.Wang GJ, Volkow ND, Felder C, et al. Enhanced resting activity of the oral somatosensory cortex in obese subjects. Neuroreport. 2002;13:1151–1155. doi: 10.1097/00001756-200207020-00016. [DOI] [PubMed] [Google Scholar]
- 73.Huttunen J, Kahkonen S, Kaakkola S, et al. Effects of an acute D2-dopaminergic blockade on the somatosensory cortical responses in healthy humans: evidence from evoked magnetic fields. Neuroreport. 2003;14:1609–1612. doi: 10.1097/00001756-200308260-00013. [DOI] [PubMed] [Google Scholar]
- 74.Rossini PM, Bassetti MA, Pasqualetti P. Median nerve somatosensory evoked potentials. Apomorphine-induced transient potentiation of frontal components in Parkinson’s disease and in parkinsonism. Electroencephalogr Clin Neurophysiol. 1995;96:236–247. doi: 10.1016/0168-5597(94)00292-m. [DOI] [PubMed] [Google Scholar]
- 75.Chen YI, Ren J, Wang FN, et al. Inhibition of stimulated dopamine release and hemodynamic response in the brain through electrical stimulation of rat forepaw. Neurosci Lett. 2008;431:231–235. doi: 10.1016/j.neulet.2007.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuo MF, Paulus W, Nitsche MA. Boosting focally-induced brain plasticity by dopamine. Cereb Cortex. 2008;18:648–651. doi: 10.1093/cercor/bhm098. [DOI] [PubMed] [Google Scholar]
- 77.Volkow ND, Wang GJ, Telang F, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zink CF, Pagnoni G, Martin ME, et al. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23:8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rolls ET, McCabe C. Enhanced affective brain representations of chocolate in cravers vs. non-cravers. Eur J Neurosci. 2007;26:1067–1076. doi: 10.1111/j.1460-9568.2007.05724.x. [DOI] [PubMed] [Google Scholar]
- 80.Grabenhorst F, Rolls ET, Bilderbeck A. How cognition modulates affective responses to taste and flavor: top-down influences on the orbitofrontal and pregenual cingulate cortices. Cereb Cortex. 2008;18:1549–1559. doi: 10.1093/cercor/bhm185. [DOI] [PubMed] [Google Scholar]
- 81.Wang GJ, Volkow ND, Telang F, et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21:1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 82.Cox SM, Andrade A, Johnsrude IS. Learning to like: a role for human orbitofrontal cortex in conditioned reward. J Neurosci. 2005;25:2733–2740. doi: 10.1523/JNEUROSCI.3360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 85.Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. Eur J Neurosci. 2007;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- 86.Ogden J, Wardle J. Cognitive restraint and sensitivity to cues for hunger and satiety. Physiol Behav. 1990;47:477–481. doi: 10.1016/0031-9384(90)90112-h. [DOI] [PubMed] [Google Scholar]
- 87.Petrovich GD, Gallagher M. Amygdala subsystems and control of feeding behavior by learned cues. Ann N Y Acad Sci. 2003;985:251–262. doi: 10.1111/j.1749-6632.2003.tb07086.x. [DOI] [PubMed] [Google Scholar]
- 88.Fallon S, Shearman E, Sershen H, et al. Food reward-induced neurotransmitter changes in cognitive brain regions. Neurochem Res. 2007;32:1772–1782. doi: 10.1007/s11064-007-9343-8. [DOI] [PubMed] [Google Scholar]
- 89.Del Parigi A, Chen K, Salbe AD, et al. Tasting a liquid meal after a prolonged fast is associated with preferential activation of the left hemisphere. Neuroreport. 2002;13:1141–1145. doi: 10.1097/00001756-200207020-00014. [DOI] [PubMed] [Google Scholar]
- 90.Small DM, Prescott J. Odor/taste integration and the perception of flavor. Exp Brain Res. 2005;166:345–357. doi: 10.1007/s00221-005-2376-9. [DOI] [PubMed] [Google Scholar]
- 91.Smeets PA, de Graaf C, Stafleu A, et al. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr. 2006;83:1297–1305. doi: 10.1093/ajcn/83.6.1297. [DOI] [PubMed] [Google Scholar]
- 92.Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 93.Abizaid A, Liu ZW, Andrews ZB, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malik S, McGlone F, Bedrossian D, et al. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 95.Brody S, Keller U, Degen L, et al. Selective processing of food words during insulin-induced hypoglycemia in healthy humans. Psychopharmacology (Berl) 2004;173:217–220. doi: 10.1007/s00213-003-1722-5. [DOI] [PubMed] [Google Scholar]
- 96.Rotte M, Baerecke C, Pottag G, et al. Insulin affects the neuronal response in the medial temporal lobe in humans. Neuroendocrinology. 2005;81:49–55. doi: 10.1159/000084874. [DOI] [PubMed] [Google Scholar]
- 97.Schultes B, Peters A, Kern W, et al. Processing of food stimuli is selectively enhanced during insulin-induced hypoglycemia in healthy men. Psychoneuroendocrinology. 2005;30:496–504. doi: 10.1016/j.psyneuen.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 98.Bruning JC, Gautam D, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 99.Anthony K, Reed LJ, Dunn JT, et al. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: the cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes. 2006;55:2986–2992. doi: 10.2337/db06-0376. [DOI] [PubMed] [Google Scholar]
- 100.Farooqi IS, Bullmore E, Keogh J, et al. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Figlewicz DP, Bennett JL, Naleid AM, et al. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav. 2006;89:611–616. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 102.Meguid MM, Fetissov SO, Blaha V, et al. Dopamine and serotonin VMN release is related to feeding status in obese and lean Zucker rats. Neuroreport. 2000;11:2069–2072. doi: 10.1097/00001756-200007140-00002. [DOI] [PubMed] [Google Scholar]
- 103.Hamdi A, Porter J, Prasad C. Decreased striatal D2 dopamine receptors in obese Zucker rats: changes during aging. Brain Res. 1992;589:338–340. doi: 10.1016/0006-8993(92)91296-q. [DOI] [PubMed] [Google Scholar]
- 104.Geiger BM, Behr GG, Frank LE, et al. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 2008;22:2740–2746. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bina KG, Cincotta AH. Dopaminergic agonists normalize elevated hypothalamic neuropeptide Y and corticotropin-releasing hormone, body weight gain, and hyperglycemia in ob/ob mice. Neuroendocrinology. 2000;71:68–78. doi: 10.1159/000054522. [DOI] [PubMed] [Google Scholar]
- 106.Pijl H. Reduced dopaminergic tone in hypothalamic neural circuits: expression of a “thrifty” genotype underlying the metabolic syndrome? Eur J Pharmacol. 2003;480:125–131. doi: 10.1016/j.ejphar.2003.08.100. [DOI] [PubMed] [Google Scholar]
- 107.Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 108.Thanos PK, Michaelides M, Piyis YK, et al. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62:50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- 109.Huang XF, Zavitsanou K, Huang X, et al. Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behav Brain Res. 2006;175:415–419. doi: 10.1016/j.bbr.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 110.Chen PS, Yang YK, Yeh TL, et al. Correlation between body mass index and striatal dopamine transporter availability in healthy volunteers—a SPECT study. Neuroimage. 2008;40:275–279. doi: 10.1016/j.neuroimage.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 111.Hurd YL. Perspectives on current directions in the neurobiology of addiction disorders relevant to genetic risk factors. CNS Spectr. 2006;11:855–862. doi: 10.1017/s1092852900015005. [DOI] [PubMed] [Google Scholar]
- 112.Klein TA, Neumann J, Reuter M, et al. Genetically determined differences in learning from errors. Science. 2007;318:1642–1645. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- 113.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 114.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Volkow ND, Chang L, Wang GJ, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 116.Volkow ND, Fowler JS, Wang GJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 117.Volkow ND, Wang GJ, Telang F, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Volkow ND, Wang GJ, Telang F, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grace AA, Floresco SB, Goto Y, et al. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 120.Brewer JA, Potenza MN. The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol. 2008;75:63–75. doi: 10.1016/j.bcp.2007.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Volkow ND, Wang GJ, Begleiter H, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- 122.Fedoroff I, Polivy J, Herman CP. The specificity of restrained versus unrestrained eaters’ responses to food cues: general desire to eat, or craving for the cued food? Appetite. 2003;41:7–13. doi: 10.1016/s0195-6663(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 123.Pelchat ML, Johnson A, Chan R, et al. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 124.Thanos PK, Michaelides M, Gispert JD, et al. Differences in response to food stimuli in a rat model of obesity: in-vivo assessment of brain glucose metabolism. Int J Obes (Lond) 2008;32:1171–1179. doi: 10.1038/ijo.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang GJ, Yang J, Volkow ND, et al. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc Natl Acad Sci USA. 2006;103:15641–15645. doi: 10.1073/pnas.0601977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 127.Tracy AL, Jarrard LE, Davidson TL. The hippocampus and motivation revisited: appetite and activity. Behav Brain Res. 2001;127:13–23. doi: 10.1016/s0166-4328(01)00364-3. [DOI] [PubMed] [Google Scholar]
- 128.Peleg-Raibstein D, Pezze MA, Ferger B, et al. Activation of dopaminergic neurotransmission in the medial prefrontal cortex by N-methyl-D-aspartate stimulation of the ventral hippocampus in rats. Neuroscience. 2005;132:219–232. doi: 10.1016/j.neuroscience.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 129.DelParigi A, Chen K, Salbe AD, et al. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord. 2004;28:370–377. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- 130.Gilhooly CH, Das SK, Golden JK, et al. Food cravings and energy regulation: the characteristics of craved foods and their relationship with eating behaviors and weight change during 6 months of dietary energy restriction. Int J Obes (Lond) 2007;31:1849–1858. doi: 10.1038/sj.ijo.0803672. [DOI] [PubMed] [Google Scholar]
- 131.Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5:332–353. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ingram DK, Chefer S, Matochik J, et al. Aging and caloric restriction in nonhuman primates: behavioral and in vivo brain imaging studies. Ann N Y Acad Sci. 2001;928:316–326. doi: 10.1111/j.1749-6632.2001.tb05661.x. [DOI] [PubMed] [Google Scholar]
- 133.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 134.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 135.Mark AL. Dietary therapy for obesity is a failure and pharmacotherapy is the future: a point of view. Clin Exp Pharmacol Physiol. 2006;33:857–862. doi: 10.1111/j.1440-1681.2006.04454.x. [DOI] [PubMed] [Google Scholar]
- 136.Dansinger ML, Gleason JA, Griffith JL, et al. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 137.Wilfley DE, Stein RI, Saelens BE, et al. Efficacy of maintenance treatment approaches for childhood overweight: a randomized controlled trial. JAMA. 2007;298:1661–1673. doi: 10.1001/jama.298.14.1661. [DOI] [PubMed] [Google Scholar]
- 138.Hattori S, Naoi M, Nishino H. Striatal dopamine turnover during treadmill running in the rat: relation to the speed of running. Brain Res Bull. 1994;35:41–49. doi: 10.1016/0361-9230(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 139.MacRae PG, Spirduso WW, Cartee GD, et al. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolite levels. Neurosci Lett. 1987;79:138–144. doi: 10.1016/0304-3940(87)90686-0. [DOI] [PubMed] [Google Scholar]
- 140.Farmer J, Zhao X, van Praag H, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 141.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 142.Angevaren M, Aufdemkampe G, Verhaar HJ, et al. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008:CD005381. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- 143.Taaffe DR, Irie F, Masaki KH, et al. Physical activity, physical function, and incident dementia in elderly men: the Honolulu-Asia Aging Study. J Gerontol A Biol Sci Med Sci. 2008;63:529–535. doi: 10.1093/gerona/63.5.529. [DOI] [PubMed] [Google Scholar]
- 144.Jedrziewski MK, Lee VM, Trojanowski JQ. Physical activity and cognitive health. Alzheimers Dement. 2007;3:98–108. doi: 10.1016/j.jalz.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101:1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 146.Kramer AF, Colcombe SJ, McAuley E, et al. Enhancing brain and cognitive function of older adults through fitness training. J Mol Neurosci. 2003;20:213–221. doi: 10.1385/JMN:20:3:213. [DOI] [PubMed] [Google Scholar]
- 147.Klem ML, Wing RR, McGuire MT, et al. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr. 1997;66:239–246. doi: 10.1093/ajcn/66.2.239. [DOI] [PubMed] [Google Scholar]
- 148.Wyatt HR, Grunwald GK, Seagle HM, et al. Resting energy expenditure in reduced-obese subjects in the National Weight Control Registry. Am J Clin Nutr. 1999;69:1189–1193. doi: 10.1093/ajcn/69.6.1189. [DOI] [PubMed] [Google Scholar]
- 149.Segar ML, Eccles JS, Richardson CR. Type of physical activity goal influences participation in healthy midlife women. Womens Health Issues. 2008;18:281–291. doi: 10.1016/j.whi.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 150.Harrold JA, Halford JC. The hypothalamus and obesity. Recent Patents CNS Drug Discov. 2006;1:305–314. doi: 10.2174/157488906778773616. [DOI] [PubMed] [Google Scholar]
- 151.Aronne LJ, Thornton-Jones ZD. New targets for obesity pharmacotherapy. Clin Pharmacol Ther. 2007;81:748–752. doi: 10.1038/sj.clpt.6100163. [DOI] [PubMed] [Google Scholar]
- 152.Erondu N, Addy C, Lu K, et al. NPY5R antagonism does not augment the weight loss efficacy of orlistat or sibutramine. Obesity (Silver Spring) 2007;15:2027–2042. doi: 10.1038/oby.2007.242. [DOI] [PubMed] [Google Scholar]
- 153.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 154.Gadde KM, Yonish GM, Foust MS, et al. Combination therapy of zonisamide and bupropion for weight reduction in obese women: a preliminary, randomized, open-label study. J Clin Psychiatry. 2007;68:1226–1229. doi: 10.4088/jcp.v68n0809. [DOI] [PubMed] [Google Scholar]
- 155.Gadde KM, Franciscy DM, Wagner HR, II, et al. Zonisamide for weight loss in obese adults: a randomized controlled trial. JAMA. 2003;289:1820–1825. doi: 10.1001/jama.289.14.1820. [DOI] [PubMed] [Google Scholar]
- 156.Stenlof K, Rossner S, Vercruysse F, et al. Topiramate in the treatment of obese subjects with drug-naive type 2 diabetes. Diabetes Obes Metab. 2007;9:360–368. doi: 10.1111/j.1463-1326.2006.00618.x. [DOI] [PubMed] [Google Scholar]