Abstract
Study Objectives:
To examine nighttime sleep patterns of persons with dementia showing nocturnal agitation behaviors and to determine whether restless legs syndrome (RLS), periodic limb movements in sleep (PLMS), and obstructive sleep apnea (OSA) are associated with nocturnal agitation behaviors.
Design:
Cross-sectional.
Setting:
General community.
Participants:
59 participants with geriatrician-diagnosed dementia. Participants ages ranged from 66 to 88 years (mean age 79.1; SD 6.0). Mean Mini Mental State Examination (MMSE) score was 20.1 (SD 6.6). MMSE was used to measure baseline cognitive function and not for the diagnosis of dementia.
Interventions:
None.
Measurements and Results:
Sleep was measured by 2 nights of in-home, attended, portable polysomnography (PSG). Nocturnal agitation was measured over 3 additional nights using the Cohen-Mansfield Agitation Inventory modified for direct observations. Two experts independently and via consensus identified probable RLS. Total sleep time in participants was 5.6 h (SD 1.8 h). Mean periodic limb movements in sleep index (PLMI) was 15.29, and a high percentage (49%) had moderate to severe obstructive sleep apnea. Probable RLS was present in 24% of participants. Those with more severe cognitive impairment had longer sleep latency. Severe cognitive impairment, low apnea hypopnea index (AHI), and probable RLS were associated with nocturnal agitation behaviors (R2 = 0.35, F3,55 = 9.40, P < 0.001).
Conclusions:
It appears that probable RLS is associated with nocturnal agitation behaviors in persons with dementia, while OSA and PLMS are not. Further investigation is warranted to determine if treatment of RLS impacts nocturnal agitation behaviors in persons with dementia.
Citation:
Rose KM; Beck C; Tsai PF; Liem PH; Davila DG; Kleban M; Gooneratne NS; Kalra G; Richards KC. Sleep disturbances and nocturnal agitation behaviors in older adults with dementia. SLEEP 2011;34(6):779-786.
Keywords: Sleep, dementia, nocturnal agitation behaviors, probable restless legs syndrome
INTRODUCTION
There are now 5.3 million persons in the United States with Alzheimer disease (AD) and, that figure will increase to 13.2 million by 2050.1 The majority of older adults with AD are cared for in their homes: each year 11 million American family members provide 12.5 billion hours of care to persons with AD.2 Caring for these adults can be particularly taxing in the evening and night because as cognitive abilities decline, sleep duration is reduced and sleep patterns are often fragmented, with frequent awakenings accompanied by agitation behaviors, including general restlessness, screaming, and physical aggressiveness.3,4 Antipsychotic and hypnotic medications have been relatively unsuccessful in treating nocturnal agitation behaviors in older adults with AD and are frequently associated with adverse events, including greater odds for mortality and cardiovascular events,5–7 perhaps because they do not address the precipitating causes. A recent meta-analysis of non-pharmacological interventions designed to reduce agitation in older adults with AD concluded that only sensory interventions such as aromatherapy, thermal bath, and calming music with hand massage were efficacious (standardized mean difference: SMD −1.07; 95% confidence interval: (CI) – 1.76 to −0.38; P = 0.002).8 However, none of these studies addressed agitation that occurred during the evening and night. Thus, there is a need to identify triggers for nocturnal agitation behaviors as a basis for developing effective interventions to prevent or reduce these behaviors.
One category of disorders that may contribute to nocturnal agitation behaviors is that of sleep disorders, several of which are more common in older adults or in older adults with cognitive impairment. Restless legs syndrome (RLS) produces an urge to move the legs that is usually accompanied or caused by uncomfortable and unpleasant sensations in the legs, with symptoms either beginning or worsening during periods of rest or inactivity, most often during the evening or nighttime hours. Persons may complain of inability to fall asleep or to remain asleep because of this disorder. The prevalence of the disorder increases with age9 and is associated with iron deficiency and depression.10 Periodic limb movements in sleep (PLMS) consist of involuntary movements of the legs while asleep and is mostly unnoticed and asymptomatic in this population.11,12 The essential features of obstructive sleep apnea (OSA) are brief repetitive episodes of breathing pauses (apnea) and hypoventilation (hypopnea) which create fragmented nocturnal sleep, due to microarousals, and cause hypoxia. Hypoxia in turn may precipitate or worsen nocturnal confusion.13
The specific aims of this study were to: (1) examine the nighttime sleep patterns of persons with a diagnosis of dementia who have nocturnal agitation behaviors; and (2) determine whether probable RLS, PLMS, and OSA were associated with nocturnal agitation behaviors. We hypothesized that these sleep disorders might trigger nocturnal agitation behaviors. To address these aims, we conducted a study of sleep disorders in 59 older adults with nocturnal agitation behaviors that included objective sleep evaluation with 2 nights of polysomnography (PSG), direct behavioral observations for 19 hours, and detailed assessment of risk factors from both the patient and caregiver.
METHODS
This multivariate, cross-sectional study (Veterans Affairs #NRI01-077; Richards, PI) conducted from 2003-2008, examined the nighttime sleep patterns of persons with a diagnosis of dementia whose caregivers reported that they exhibited nocturnal agitation behaviors. The study protocol was approved by the institutional review board.
Participants were recruited from 2 geriatric outpatient clinics using the following inclusion criteria: (1) a dementia diagnosis made by a geriatrician; (2) English speaking and living in a private residence with an adult caregiver (spouse, child, friend, or sibling) who spoke English; (3) caregiver report of one or more nocturnal agitation behaviors ≥ 3 times a week; (4) ambulatory; and (5) medically stable, defined as unchanged medications within 30 days and the absence of fever or other symptoms of acute illness. All study participants received the diagnosis of dementia from geriatricians who were practicing in a university setting and whose practice reflected the state of the science in diagnosis of a dementia-type illness during the study time period, although relatively few potential participants had undergone extensive neuropsychological testing or had a consensus diagnosis of probable Alzheimer Disease. Exclusion criteria were current treatment for RLS, PLMS, or OSA.
Study Procedures and Measures
Research assistants (RAs) explained the study to volunteers with dementia, their caregivers, and their legal guardians (if necessary) and then assessed their capacity to provide informed consent by determining whether they could describe the study procedures and potential risks of participation. If either the volunteers or their guardians had the capacity to provide informed consent, the research assistants obtained their written consent. They also obtained assent from the participants each time data were collected. After informed consent, the caregiver completed the Cohen-Mansfield Agitation Inventory (CMAI)14 to describe the frequency of nocturnal agitation behaviors of the person with dementia and completed a RLS diagnostic interview and a participant sleep history. Those who met the inclusion criteria were then scheduled for 2 nights of attended PSG at home (Nights 1-2). If the sleep technician was able to obtain ≥ 4 h of sleep during the 2 PSG nights, the participants were scheduled for 3 additional nights (Nights 3-5) of behavioral observations conducted by RAs in the participant's homes.
The RAs conducted direct observations of participant's nocturnal agitation behaviors using the CMAI.14 This 29-item instrument uses a 7-point rating scale to assess the frequency with which a person shows certain behaviors (1 = never; 7 = several times per hour). Higher scores indicate more frequent occurrence of nocturnal agitation behaviors. Examples of behaviors included in the instrument are pacing, screaming, biting, and general restlessness. Inter-rater reliability of this instrument is 0.92. This instrument has been modified for direct observation and this is the format we used for this study.21 The RAs completed a 1-week training course on identifying and scoring nocturnal agitation behaviors, taught by investigators Drs. Kathy Richards and Cornelia Beck. Following this training, the RAs accurately identified disruptive behaviors at a high level of agreement (r > 0.90) with nurse experts and each other. The RAs recoded randomly selected 2-h segments of video data biannually, with agreement ranging from 0.80-0.95.
The diagnostic standard for RLS is patient recall and report of specific symptoms and associated behaviors. However, this diagnostic method is not feasible for persons with dementia.19 To provide an objective diagnosis of probable RLS, we recruited 2 leading authorities in RLS (Richard Allen, PhD and Wayne Hening, MD, PhD) who independently rated each participant as probable RLS or no RLS based on the following data: (1) diagnoses and medications; (2) caffeine and alcohol intake; (3) chief sleep complaint (from caregiver and/or elder); (4) the caregiver RLS diagnostic interview; (5) PSG data, including the apnea hypopnea index (AHI) and periodic limb movement in sleep index (PLMI); and (6) the research assistant's observations of RLS signs. These criteria follow the guidelines of diagnostic criteria for probable RLS in cognitively impaired elderly.20
The Mini-Mental State Examination23 (MMSE) was used to measure baseline cognitive function in study participants with dementia and not for the diagnosis of dementia. The MMSE is a widely used screening test that measures orientation, memory, and attention but is not used as the sole diagnostic criterion for the diagnosis of dementia. Scores > 25 are generally interpreted as normal, while lower scores on the MMSE indicate more cognitive impairment.22 The MMSE has established reliability and validity.23
The Grass Portable Polysomnography Data Acquisition System (Astro-Med Inc, West Warwick, RI) was used to collect PSG data on sleep patterns, AHI, and other breathing and oxygenation variables, and PLMI on Nights 1-2. Participants slept in their own beds at home, with a bed partner if they usually slept with one, during their habitual sleeping hours. During the sessions, the PSG system and sleep technician remained in the hall outside the participant's bedroom or in an adjoining room. The technician continuously observed the participants to assure their safety and the integrity of the monitoring devices using a small portable video camera that transmitted to a monitor placed outside the room. To assist in the sleep recordings and for descriptive information, the technician noted every 5 minutes whether the participant was behaviorally awake or asleep.
The portable PSG system displayed electrographically the electroencephalogram (EEG), electrooculogram (EOG), electromyogram (EMG), electrocardiogram (ECG), airflow, leg movement, respiratory excursion, and arterial oxygen saturation. The sleep technician used standard calibration and recording procedures.15,16 The EEG electrodes were placed at C3, C4, O1, and O2 (International 10-20 system of measurement) and referred to the contralateral mastoid. One registered PSG technologist, blinded to the results of the agitation data and RLS data, scored the recordings for sleep patterns using standardized scoring criteria, with the following exceptions: (1) because persons with dementia have diffuse delta and theta activity in the EEG and landmarks such as sleep spindles have disappeared, the technologist collapsed all NREM stages into one—indeterminate NREM sleep; and (2) because some persons with dementia may not have the usual REM sleep muscle atonia, we disregarded conventional scoring criteria for REM sleep muscle atonia in these individuals and used scoring guidelines for dementia as published by Bliwise, a co-investigator.17 Interrater reliability between the registered polysomnography technologist and Bliwise was assessed at the beginning and annually during data collection and ranged from 0.85 to 0.95. Data for this study were collected prior to the implementation of the revised recording and scoring criteria for PSG.18
The AHI was defined as the number of apneas and hypopneas per sleep hour that resulted in ≥ 4% oxygen desaturation or an EEG alpha wave arousal.16 The desaturation had to occur within 30 sec, and the arousal had to occur within 3 sec of the termination of the apnea or hypopnea. The registered PSG technologist used the following definitions to score these events: (1) obstructive apnea - complete or almost complete cessation (25% of the amplitude at baseline) of airflow ≥ 10 sec with chest or abdominal wall movement present; (2) obstructive hypopnea - reduction in airflow (< 70% of baseline level) for ≥ 10 sec with chest wall or abdominal movement present, terminated by an arousal or followed by a reduction in oxygen saturation ≥ 4%, (3) central apnea – complete or almost complete cessation (approximately 25% of the amplitude of baseline) of airflow ≥ 10 sec with no chest wall or abdominal movement present; and (4) central hypopnea - reduction in airflow (< 70% of baseline level) ≥ 10 sec with no chest wall or abdominal movement present, terminated by an arousal or followed by ≥ 4% reduction in oxygen saturation.16 The sleep technician recorded periodic limb movements from both legs using electrodes placed on the anterior tibialis muscles. The standard definition of PLMS used for scoring was repeated movements of the extremities during sleep that occurred in a sequence of ≥ 4, with the duration of movements from 0.5-5 sec and an inter-movement interval between 5 and 90 sec.16
A RA directly observed the person with dementia when he or she was out of bed and recorded the observations using the CMAI. After the person had gone to bed, the RA observed him or her via a video camera placed in the bedroom and a monitor located in a hallway or room adjacent to the bedroom. The RA noted every 5 min whether the participant was behaviorally awake or asleep. Sleep was defined as a quiet state with eyes closed. The RA scored the presence of behaviors during wake time at 5-min intervals, reflecting the number of episodes of a specific nocturnal agitation behavior per night. We then calculated the total number of episodes of all nocturnal agitation behaviors and divided this number by the exact hours of observation for each participant to calculate the Agitation Behaviors Index, defined as the total number of behaviors divided by the total hours of observation.
RLS induces certain characteristic behavioral signs: rubbing legs and inability to keep legs still. Using an investigator-developed instrument, with coding instructions, RAs continuously observed participants for these behaviors and recorded them from 19:00 to 00:00 (7 pm to 12 midnight) on Night 3, and from 22:00 to 06:00 on Nights 4-5. We collected data on 3 different nights because symptoms may vary from night to night, and thus several nights of observation are more likely to provide a definitive diagnosis. We conducted observations during one early evening because symptoms of RLS peak in the early evening and the first part of the night. The principal investigator (Kathy Richards) trained the RAs in conducting the behavioral observations. The RA raters achieved 0.90-0.95 agreement in rating RLS behaviors using video examples of persons with RLS at the study outset and biannually in rating RLS behaviors. Initial agreement between Drs. Allen and Hening on whether participants had probable RLS or no RLS was 85%. Agreement increased to 100% following discussion of the disputed cases. The experts were blinded to data on nocturnal agitation behaviors.
Statistical Analysis
We conducted a power analysis using Cohen's MRC procedures24 to determine the sample size required to determine whether age, gender, cognitive status, AHI, PLMI, and probable RLS predicted nocturnal agitation behaviors in persons with dementia who were living at home. We estimated that the AHI, the PLMI, and probable RLS would each account for 7% of the variance in nocturnal agitation behaviors. We further estimated that together age, gender, and cognitive status would account for 5% of the variance, based on several studies that showed small to moderate correlations.25,26 For a multiple linear regression model including these 3 covariates with a squared multiple correlation of 0.05, a sample size of 56 provided ≥ 80% power to detect an increase in R2 of ≥ 0.21 by including the predictor variables of AHI, PLMI, and probable-RLS. This estimate was based on an f2 = 0.28 and lambda (λ) = 15.8.
All tests of effects were based on a 5% significance level. For these 59 participants, predictors with significant zero-order correlation (probable-RLS and AHI) partialled by the covariate with significant zero-order correlation (MMSE) produced an effect size, f2 = 0.36 and λ = 18.26, resulting in a power of 95%. The dependent variable, Agitation Behaviors Index, needed a square root transformation and the AHI needed a log (natural log) transformation to improve normality.
Descriptive statistics were generated for demographic and sleep variables for the sample. Polysomnography data for the 2 nights were averaged to increase stability. One-way ANOVAs were used to evaluate differences in demographic and sleep variables based on cognitive status; the Scheffe post hoc test was used to examine group differences. For variables with significant heterogeneity of variance (< 0.001) as confirmed by the Bartlett test, we performed the Kruskal-Wallis test, followed by the nonparametric 2-sample Wilcoxon rank-sum (Mann-Whitney) test.
Associations of each covariate variable (age, gender, and MMSE) and predictor variable (log AHI, PLMI, and probable RLS) with the Agitation Behaviors Index were examined initially in a single predictor regression analysis. Multiple regression procedures, including stepwise selection using Stat v.10, were then used to identify the model with the fewest number of predictors in which each predictor made a significant contribution. The regression model was found adequate by the omitted variable test; all assumptions for the model were met.
RESULTS
Ninety-four subjects were assessed for eligibility to participate in this study, 74 consented, and 59 of these met all inclusion criteria. The final sample of 59 consisted of 41 men (68.33%) and 18 women (31.67%). Because 7 participants could not tolerate the full PSG data collection procedures (5 could not tolerate the ankle strap used to measure periodic limb movements, and 2 could not tolerate the abdominal belt used to measure respiratory effort), we have incomplete data on these participants. Participants ranged in age from 66 to 88 years with a mean age of 79.1 years (SD 6.0). Twenty-nine had scores on the MMSE indicating mild cognitive impairment (24 males), 23 had moderate cognitive impairment (15 males), and 7 had severe cognitive impairment (2 males). The mean MMSE score was 20.1 (SD 6.6). Participants' MMSE scores were used to categorize their cognitive status as mild cognitive impairment (21-30), moderate cognitive impairment (11-20), or severe cognitive impairment (1-10).27 Although 9 persons scored > 26 on the MMSE, all had a geriatrician diagnosis of dementia and met the criteria for nocturnal agitation behaviors. The MMSE was not used as a diagnostic measure of dementia, but rather it was used as an indicator of severity of cognitive impairment for our sample. Twenty-one percent were prescribed tranquilizer and antipsychotic medications; 30% were taking antidepressant medications (selective serotonin reuptake inhibitors and tricyclics); 38% were receiving vitamin B-12 supplements; and 15% had a documented diagnosis of anemia. The 3 groups we created based upon MMSE values—mild, moderate, and severe impairment—were compared on demographics, sleep patterns, AHI and other breathing and oxygenation variables, and PLMI. Analysis of variance (ANOVA) indicated no significant differences among the groups in age; however, Fisher exact test indicated significant difference between the groups in gender composition (Fisher exact P = 0.035), with the mildly cognitively impaired group having more men than the other groups.
Nighttime Sleep Patterns
Sleep duration of 5.63 h (SD 1.82 h) per night was found, and a sleep efficiency of 66.6%. Only 22% of participants slept ≥ 7 h per night. The sample had a mean PLMI of 15.29 events/h (moderate severity), and a mean AHI of 19.45 events/h (moderate severity). Significant differences between mild, moderate, and severe cognitive impairment groups were found in time to first 10 min of sleep (P ≤ 0.01); persons with severe cognitive impairment took the longest time to fall asleep. Similar patterns were noted for sleep latency and latency to REM sleep; however these differences were not statistically significant. Awakenings due to respiratory events (P = 0.03) and number of sleep stage shifts (P < 0.01) were significantly more frequent in the group with mild cognitive impairment. The AHI showed a decline from the mild to moderate and severe cognitive impairment groups, although the differences were not significant. The groups did not significantly differ on other indices of sleep architecture, total sleep time, hypoxia, or PLMS. Sleep parameters are shown in Table 1.
Table 1.
Demographic and polysomnography variables grouped by severity of cognitive status; mean scores (SD)
| Total N = 59 | Mild MMSE 21-30 (n = 29) | Moderate MMSE 11-20 (n = 23) | Severe MMSE 1-10 (n = 7) | F | P | |
|---|---|---|---|---|---|---|
| Age | 79.1 (6.0) | 78.1 (6.4) | 79.7 (5.0) | 80.4 (8.1) | 0.66 | 0.52 |
| Gender | 3.83 | 0.02* | ||||
| Male | 41 | 24 | 15 | 2 | ||
| Female | 18 | 5 | 8 | 5 | ||
| Sleep Architecture: | ||||||
| Minutes of latency to first epoch of sleep | 43.2 (91.2) | 25.7 (25.0) | 49.5 (104.8) | 98.1 (182.3) | 1.94 | 0.15 |
| Minutes of latency to first 10 min of sleep | 87.4 (144.6) | 43.3 (36.6) | 92.9 (143.9) | 258.1 (281.5) | 7.73 | ≤ 0.01* |
| Minutes of latency to REM sleep | 147.9 (89.83) | 131.8 (71.4) | 161.5 (106.6) | 173.8 (102.4) | 0.95 | 0.39 |
| NREM Sleep | ||||||
| Minutes | 292.7 (96.1) | 293.1 (80.7) | 287.7 (95.21) | 307.3 (160.4) | 0.11 | 0.89 |
| % of TIB | 57.5 (15.3) | 58.6 (12.4) | 57.0 (15.5) | 54.69 (25.7) | 0.20 | 0.82 |
| % of TST | 85.9 (10.5) | 86.2 (7.6) | 85.9 (10.7) | 85.2 (19.4) | 0.04 | 0.96 |
| REM Sleep | ||||||
| Minutes | 45.6 (28.9) | 48.6 (27.9) | 44.7 (30.4) | 35.6 (29.8) | 0.58 | 0.56 |
| % of TIB | 9.0 (5.9) | 10.0 (6.1) | 8.7 (5.6) | 6.2 (5.0) | 1.29 | 0.28 |
| % of TST | 12.5 (7.43) | 13.8 (7.55) | 12.2 (7.2) | 7.6 (6.6) | 2.03 | 0.14 |
| TST | ||||||
| Minutes | 338.3 (109.2) | 341.7 (89.0) | 332.4 (113.3) | 342.9 (177.9) | 0.05 | 0.94 |
| Total Wake Time | ||||||
| Minutes | 151.6 (71.8) | 152.8 (68.6) | 154.7 (77.3) | 136.5 (76.1) | 0.17 | 0.84 |
| Awakenings, No. | 34.7 (16.3) | 39.6 (19.8) | 31.8 (10.4) | 23.3 (5.4) | 3.75 | 0.02* |
| Spontaneous | 14.6 (8.5) | 14.6 (9.6) | 14.4 (7.8) | 15.2 (7.4) | 0.02 | 0.97 |
| Bathroom | 1.9 (1.8) | 2.2 (1.4) | 1.8 (2.3) | 0.9 (0.7) | 1.54 | 0.22 |
| Respiratory | 18.2 (16.1) | 22.8 (19.0) | 15.5 (11.6) | 7.1 (6.0) | 3.44 | 0.03* |
| Sleep efficiency | 66.6 (18.0) | 68.6 (14.7) | 65.8 (18.6) | 60.8 (28.5) | 0.57 | 0.57 |
| TIB | 490.0 (78.5) | 494.6 (59.3) | 487.3 (82.8) | 479.4 (134.8) | 0.12 | 0.88 |
| Stage shifts, No. | 83.2 (36.5) | 99.2 (40.3) | 71.6 (23.0) | 53.1 (22.0) | 7.90 | ≤ 0.01* |
| PLMI | 15.3 (19.2) | 13.6 (18.1) | 15.4 (19.2) | 25.3 (26.9) | 0.89 | 0.41 |
| PLMI with arousals | 4.8 (6.9) | 3.9 (6.1) | 4.7 (6.9) | 9.5 (9.2) | 1.73 | 0.18 |
| AHI | 19.5(14.9) | 21.6 (16.9) | 18.5 (12.8) | 13.0 (12.1) | 0.90 | 0.41 |
| SpO2 nadir, % | 86.7 (4.8) | 86.3 (4.3) | 86.7 (5.2) | 89.7 (6.2) | 1.05 | 0.35 |
NREM, non-rapid eye movement; REM, rapid eye movement; TIB, time in bed; TST, total sleep time; PLMI, periodic limb movement in sleep index; AHI, apnea hypopnea index.
significant χ2
Predictors of nocturnal agitation behaviors
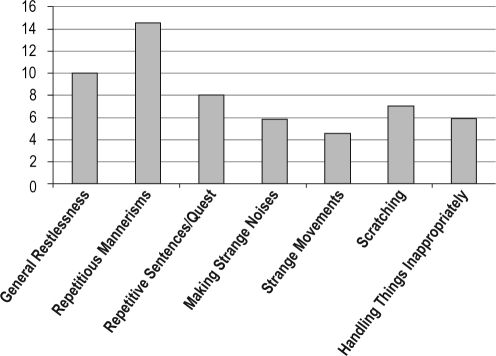
The mean Agitation Behaviors Index was 2.97 (SD 3.3); the range was 0–14.9. The types and frequencies of nighttime behaviors observed are shown in Figure 1. The most frequent agitation behaviors were repetitive mannerisms, general restlessness, repetitive sentences/questions, and scratching. Bivariate correlations of the covariates and predictor variables with the Agitation Behaviors Index showed significant relationships for MMSE (P = 0.01), log AHI (P = 0.01), and probable RLS (P = 0.01) (see Table 2). Age, gender, and PLMI were not correlated with nocturnal agitation behaviors (P > 0.05).
Figure 1.
Most common agitation behaviors and number of participants with the behaviors. N = 59.
Table 2.
Bivariate correlation of predictors and Agitation Behaviors Index
| Predictor Variable | Mean | SD | Min | Max | R | P |
|---|---|---|---|---|---|---|
| Age | 79.1 | 6.0 | 66 | 89 | 0.003 | 0.98 |
| Gender | 0.012 | 0.19 | ||||
| MMSE | 20.0 | 6.0 | 0 | 30 | -0.33 | 0.01 |
| Apnea hypopnea index | 19.5 | 14.9 | 1.1 | 67.6 | -0.37 | 0.01 |
| Periodic limb movements index | 15.3 | 19.2 | 0 | 72.6 | 0.18 | 0.18 |
| Probable restless legs syndrome | 0.31 | 0.01 | ||||
| Outcome Variables | ||||||
| Agitation Behaviors Index | 3.0 | 3.3 | 0 | 14.9 | ||
| Square Root Agitation Behaviors Index | 1.5 | 0.9 | 0 | 3.86 |
Multiple regression models
To determine whether probable RLS, PLMS, and OSA predicted nocturnal agitation behaviors, we regressed the Agitation Behaviors Index on the predictors of probable RLS, the PLMI, and the AHI, partialled by a covariate set of age, gender, and MMSE. The Agitation Behaviors Index regressed on the covariate set produced an R2 = 0.117 (P = 0.080).
When the nonsignificant variables were eliminated from the covariate and predictor sets, the covariate set contained only MMSE, which produced an R2 = 0.107 (P = 0.012) with the Agitation Behaviors Index. The multiple correlations of the Agitation Behaviors Index regressed on AHI and probable RLS, controlling for MMSE, had an R2 = 0.35 (P = 0.001).
As shown in Table 3, cognitive status (MMSE), the AHI, and probable RLS were significantly associated with nocturnal agitation behaviors (R2 = 0.35, F3,55 = 9.40, P < 0.001). This model had a high effect size (0.36) and a power of 95%.
Table 3.
Best fitting multiple linear regression model for nocturnal agitation behaviors for 59 subjects
| Variable | R (P value) | Coefficient | Standard Error | 95% CI |
P Value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Probable RLS | 0.31 (0.02) | 0.70 | 0.23 | 0.233 | 1.173 | 0.004 |
| MMSE | -0.33 (0.012) | -0.04 | 0.007 | -0.066 | -0.010 | 0.012 |
| Log AHI | -0.37 (0.004) | 0.11 | 0.11 | -0.542 | -0.107 | 0.004 |
| Intercept | 2.95 | 0.39 | 0.39 | 2.15 | 3.73 | ≤ 0.001 |
R2 = 0.347; P < 0.001. R2 is the proportion of the variation in nocturnal agitation behaviors explained by the model.
P-RLS, probable restless legs syndrome; MMSE, Mini Mental Status Examination scores; Log AHI, log transformation of apnea-hypopnea index.
DISCUSSION
In this study, older adults who had a diagnosis of dementia and whose caregiver reported nocturnal agitation behaviors experienced substantial nocturnal sleep disturbance, including short total sleep time, poor sleep efficiency, and multiple nighttime awakenings. Further, severe cognitive impairment, a low AHI, and probable RLS were significantly associated with the frequency of nocturnal agitation behaviors. These findings are important as they are derived from the only research, to our knowledge, that has used an objective measure of RLS to examine the association of sleep disorders with nocturnal agitation in this population. Our findings indicate that RLS may be related to nocturnal agitation behaviors in persons with cognitive impairment.
The finding of substantial nocturnal sleep disturbances is consistent with those from a multi-site clinical trial that investigated sleep disturbances using wrist actigraphy in 157 older adults with dementia who slept < 7 hours per night.28 Baseline sleep parameters for those participants showed a total sleep time of 5.8 hours (SD 1.3 h); sleep efficiency of 69% (SD 11%); and wake after sleep onset of 2.0 hours (SD 1.0 h). In comparison, a majority of older adults without mental illness, sleep complaints, or cognitive impairment have been found by PSG to sleep > 6 hours, and to have sleep efficiencies > 80%.29 While total sleep time, total minutes of awake, and sleep efficiency were far worse in our sample than in the reported norms for persons without cognitive impairment, we did not find statistically significant differences based on severity of cognitive status in these variables. We did find significant differences among mild, moderate, and severe cognitive impairment groups in time to first 10 minutes of sleep (P < 0.01), with persons with severe cognitive impairment taking the longest to fall asleep.
We found that 24% of cognitively impaired older adults with nocturnal agitation behaviors had probable RLS, which is higher than the reported 4% to 11% prevalence in the older adult population.30 Thirty-seven percent of our sample had a PLMI ≥ 15. This figure is slightly lower than that of 52% reported in community-dwelling older women.31 In another study of older adults with dementia, in which proxy reports from caregivers were used to complete the Mayo Sleep Questionnaire, 20.7% had probable RLS and 11.3% experienced PLMS.32 Both depression and iron deficiency have been associated with RLS and periodic limb movement disorder.10 In our study, 30% of participants were prescribed selective serotonin uptake inhibitors for treatment of depression and 15% had documented anemia.
It is common for older adults to have OSA, with prevalence rates of 20% to 25% reported.33 Among those with dementia, the prevalence is higher.34 OSA creates intermittent hypoxia during sleep, leading to sleep fragmentation due to recurrent nocturnal arousals. Therefore, one would expect to find a linear relationship between the number of apnea hypopnea episodes and the number of awake nocturnal agitation behaviors; however, in this study as the AHI increased, the Agitation Behavior Index actually decreased. Our findings lend support to those of another study that used 1 night of portable recording plus actigraphy and nurse-rated agitation behaviors, which found that sleep apnea was associated with daytime agitation, but not nighttime agitation behaviors in participants with dementia (MMSE mean score of 6.5) who resided in nursing homes.34 Further, neither evening nor nighttime aggression was associated with any measures of sleep disordered breathing (AHI). In this context, it is worthwhile to consider that an arousal is not necessarily identical to an awakening. A nocturnal arousal associated with apnea or hypopnea is defined as at least three seconds of altered electroencephalographic activity. While it can lead to an actual nocturnal awakening, this is not necessarily the case, and in the majority of cases, the patient returns to sleep immediately after an arousal. It may be that at higher AHI levels, there are higher levels of sleepiness that lead to a reduction in the number of nocturnal awakenings and fewer agitation behaviors. This should be investigated in future studies.
We found that a low AHI, probable RLS, and more severe cognitive impairment, based upon MMSE score, were significantly associated with nocturnal agitation behaviors. While we did not measure daytime agitation behaviors in our study, our findings are in alignment to those of another study,32 which found that aggressive agitation using the Cohen-Mansfield Agitation Inventory and manual manipulation behaviors during the day but not during the night were greater in persons with AD with more severe sleep disordered breathing, while search and wandering agitation behaviors decreased with more severe sleep disordered breathing. Similar to our findings that more severe cognitive impairment (low MMSE scores) were significantly associated with nighttime agitation behaviors, Tsoi et al. found that scores on the MMSE were not predictors of behavioral disturbances in patients diagnosed with mild to moderate dementia, but baseline scores on executive function did predict disturbed behavior and this association persisted at follow-up evaluations over 3-6 years.35
Interestingly, Alzheimer disease and RLS have certain risk factors in common: advanced age, depression, anxiety, smoking, and hypertension.36,37 RLS is known to interfere with sleep onset and maintenance. Studies have found that persons with RLS score lower on measures of cognition than controls matched for age and education, although these findings have not always been replicated in other studies.38,39 Our findings indicate that probable RLS in older adults with dementia is associated with nocturnal agitation behaviors. Pending additional longitudinal studies supporting the hypothesis that RLS plays a role in the development of nocturnal agitation in dementia, RLS should be screened for and treated in this population. Our findings also point to the need for validation of objective diagnostic measures for RLS, since the current diagnostic standard is clinical interview, which is not feasible in this population. Future research is needed to determine whether treatment of RLS can reduce nocturnal agitation.
This study had some limitations. First, although we attempted to collect data on measures of anxiety, depression, and pain, we have incomplete data because cognitive impairment limited patient reporting and therefore we could not include the data in our analyses. Seven of our potential study participants became too agitated during the PSG procedures to continue with the study, so it may be that we have no data on the most severely agitated subjects and this may have resulted in underreporting of some of the risk relationships we observed. It is also possible that findings were affected by the presence of observers and videotaping equipment during data collection. Last, because we only included persons with dementia whose caregivers reported nocturnal agitation in our study sample, we cannot generalize our findings to those dementia patients without reported nocturnal agitation.
Despite these limitations, this study provides unique information on nocturnal agitation behaviors and sleep patterns among adults diagnosed with dementia. Self-report data indicate that nocturnal agitation behaviors are a major reason caregivers place demented family members in nursing homes.40
Our data on sleep patterns and agitation behaviors provide a rare image of the nighttime events dealt with by caregivers on a routine basis. Future research should determine whether RLS predicts early neurocognitive degeneration in older adults and if so, identify potential mechanisms for this relationship. The high prevalence of RLS in older adults with early neurocognitive degeneration may be related to interacting or shared pathophysiology, such as iron deficiency and depression, or perhaps shared genetic characteristics, such as the APOE 4 allele. Chronic sleep loss in persons with RLS, including sleep fragmentation and reduced sleep duration, also may accelerate neurocognitive degeneration. If the specific sleep disorders of older adults who have dementia and nocturnal agitation are identified, interventions may be developed to delay or prevent institutionalization, resulting in significant cost savings and improving the quality of life of these older adults and their caregivers.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Davila serves on the board of directors for the National Sleep Foundation. Dr. Gooneratne has received grant support from Takeda. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors wish to acknowledge Corinne Lambert and Susan Jegley for their work on this project. Funding for this study was received through the Department of Veterans Affairs #NRI01-077-1; Kathy Richards, Principal Investigator. The work was performed at the University of Arkansas for Medical Sciences in Little Rock, AR. Dr. Rose received support for the work through the John A. Hartford Foundation's Building Academic Geriatric Nursing Capacity Award Program.
REFERENCES
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer's Association. 2010 Alzheimer's disease facts and figures. Alzheimers Dement. 2010;6:158–94. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 3.McCurry SM, Vitiello MV, Gibbons LE, Logsdon RG, Teri L. Factors associated with caregiver reports of sleep disturbances in persons with dementia. Am J Geriatr Psychiatry. 2006;14:112–20. doi: 10.1097/01.JGP.0000192499.25940.da. [DOI] [PubMed] [Google Scholar]
- 4.Ancoli-Israel S, Vitiello MV. Sleep in dementia. Am J Geriatr Psychiatry. 2006;14:91–4. doi: 10.1097/01.JGP.0000200973.93494.aa. [DOI] [PubMed] [Google Scholar]
- 5.Rosenheck RA, Leslie DL, Sindelar JL, et al. Clinical Antipsychotic Trial of Intervention Effectiveness-Alzheimer's Disease (CATIE-AD) investigators. Cost-benefit analysis of second-generation antipsychotics and placebo in a randomized trial of the treatment of psychosis and aggression in Alzheimer disease. Arch Gen Psychiatry. 2007;64:1259–68. doi: 10.1001/archpsyc.64.11.1259. [DOI] [PubMed] [Google Scholar]
- 6.Tariot PN. Review: atypical antipsychotics may be useful in treating behavioural and psychological symptoms of dementia but cause adverse effects. Evid Based Ment Health. 2005;8:16. doi: 10.1136/ebmh.8.1.16. [DOI] [PubMed] [Google Scholar]
- 7.Ballard C, Hanney ML, Theodoulou M, et al. The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol. 2009;8:151–7. doi: 10.1016/S1474-4422(08)70295-3. [DOI] [PubMed] [Google Scholar]
- 8.Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13:512–20. doi: 10.1080/13607860902774394. [DOI] [PubMed] [Google Scholar]
- 9.Tison F, Crochard A, Leger D, Bouee S, Lainey E, El Hasnaoui A. Epidemiology of restless legs syndrome in French adults: a nationwide survey: the INSTANT Study. Neurology. 2005;65:239–46. doi: 10.1212/01.wnl.0000168910.48309.4a. [DOI] [PubMed] [Google Scholar]
- 10.Lee HB, Hening WA, Allen RP, et al. Restless legs syndrome is associated with DSM-IV major depressive disorder and panic disorder in the community. J Neuropsychiatry Clin Neurosci. 2008;20:101–5. doi: 10.1176/jnp.2008.20.1.101. [DOI] [PubMed] [Google Scholar]
- 11.Dickel M, Mosko S. Morbidity cut-offs for sleep apnea and periodic limb movements in predicting sleep complaints in seniors. Sleep. 1990;2:155–66. doi: 10.1093/sleep/13.2.155. [DOI] [PubMed] [Google Scholar]
- 12.Gehrman P, Stepnowsky C, Cohen-Zion M, Marler M, Kripke D, Ancoli-Israel S. Long-term follow-up of periodic limb movements in sleep in older adults. Sleep. 2002;25:340–3. doi: 10.1093/sleep/25.3.340. [DOI] [PubMed] [Google Scholar]
- 13.Cao M, Guilleminault HC, Kushida C. Clinical features and evaluation of obstructive sleep apnea and upper airway resistance syndrome. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. 5th ed. St. Louis: Saunders; 2011. p. 1206. [Google Scholar]
- 14.Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol. 1989;44:M77–84. doi: 10.1093/geronj/44.3.m77. [DOI] [PubMed] [Google Scholar]
- 15.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 16.Montplaisir J, Nicholas A, Godbout R, Walters A. Restless legs syndrome and periodic leg movement disorder. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. 3rd ed. Philadelphia: WB Saunders; 2000. p. 742. [Google Scholar]
- 17.Bliwise DL. Dementia. In: Kryger MH, Roth T, Dement WC, editors. Principles and practices of sleep medicine. 3rd ed. Philadelphia: WB Saunders Company; 2000. p. 1058. [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson A, et al., editors. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. 1st ed. Westchester, IL: American Association of Sleep Medicine; 2007. [Google Scholar]
- 19.Richards K, Shue V, Beck C, Lambert C, Bliwise D. Restless legs syndrome risk factors, behaviors, and diagnoses in persons with early to moderate dementia and sleep disturbance. Behav Sleep Med. 2010;8:48–61. doi: 10.1080/15402000903425769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen R, Picchietti D, Hening W, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 21.Chrisman M, Tabar D, Whall AL, Booth DE. Agitated behavior in the cognitively impaired elderly. J Gerontol Nurs. 1991;17:9–13. doi: 10.3928/0098-9134-19911201-04. [DOI] [PubMed] [Google Scholar]
- 22.Tombaugh T. N., McDowell I., Kristjansson B., Hubley A. M. Mini-Mental State Examination(MMSE) and the Modified MMSE (3MS): a psychometric comparison and normative data. Psychological Assessment. 8:48–59. [Google Scholar]
- 23.Folstein M, Folstein S, McHugh P. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical power for the behavioral sciences. 2nd ed. New Jersey: Lawrence Erlbaum; 1988. [Google Scholar]
- 25.Cohen-Mansfield J, Libin A. Verbal and physical non-aggressive agitated behaviors in elderly persons with dementia: robustness of syndromes. J Psychiatr Res. 2005;39:325–32. doi: 10.1016/j.jpsychires.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Savva G, Zaccai J. Prevalence, correlates, and course of behavioural and psychological symptoms of dementia in the population. Br J Psychiatry. 2009;194:212–9. doi: 10.1192/bjp.bp.108.049619. [DOI] [PubMed] [Google Scholar]
- 27.Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurz A. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry. 2006;14:139–44. doi: 10.1097/01.JGP.0000192478.82189.a8. [DOI] [PubMed] [Google Scholar]
- 28.Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer's disease. Sleep. 2003;26:893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 30.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53:547–54. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 31.Claman D, Redline S, Blackwell T, et al. Prevalence and correlates of periodic limb movements in older women. J Clin Sleep Med. 2006;2:438–45. [PubMed] [Google Scholar]
- 32.Rongve A, Boeve B, Aarsland D. Frequency and correlates of caregiver-reported sleep disturbances in a sample of persons with early dementia. J Am Geriatr Soc. 2010;58:480–6. doi: 10.1111/j.1532-5415.2010.02733.x. [DOI] [PubMed] [Google Scholar]
- 33.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 34.Gehrman PR, Martin JL, Shochat T, Nolan S, Corey-Bloom J, Ancoli-Israel S. Sleep-disordered breathing and agitation in institutionalized adults with Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:426–33. [PubMed] [Google Scholar]
- 35.Tsoi T, Baillon S, Lindesay J. Early frontal executive impairment as a predictor of subsequent behavior disturbance in dementia. Am J Geriatr Psychiatry. 2008;16:102–8. doi: 10.1097/JGP.0b013e318151fb42. [DOI] [PubMed] [Google Scholar]
- 36.Rothdach AJ, Trenkwalder C, Haberstock J, Keil U, Berger K. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Memory and Morbidity in Augsburg Elderly. Neurology. 2000;54:1064–8. doi: 10.1212/wnl.54.5.1064. [DOI] [PubMed] [Google Scholar]
- 37.Phillips B, Hening W, Britz P, Mannino D. Prevalence and correlates of restless legs syndrome: results from the 2005 National Sleep Foundation Poll. Chest. 2006;129:76–80. doi: 10.1378/chest.129.1.76. [DOI] [PubMed] [Google Scholar]
- 38.Gamaldo CE, Benbrook AR, Allen RP, Oguntimein O, Earley CJ. A further evaluation of the cognitive deficits associated with restless legs syndrome (RLS) Sleep Med. 2008;9:500–5. doi: 10.1016/j.sleep.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearson VE, Allen RP, Dean T, Gamaldo CE, Lesage SR, Earley CJ. Cognitive deficits associated with restless legs syndrome (RLS) Sleep Med. 2006;7:25–30. doi: 10.1016/j.sleep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Beeri M, Werner P, Davidson M, Noy S. The cost of behavioral and psychological symptoms of dementia in community-dwelling Alzheimer's Disease patients. Int J Geriatr Psychiatry. 2002;17:403–9. doi: 10.1002/gps.490. [DOI] [PubMed] [Google Scholar]