Abstract
The distribution of mucosa-associated bacteria, bifidobacteria and lactobacilli and closely related lactic acid bacteria, in biopsy samples from the ascending, transverse, and descending parts of the colon from four individuals was investigated by denaturing gradient gel electrophoresis (DGGE). Bifidobacterial genus-specific, Lactobacillus group-specific, and universal bacterial primers were used in a nested PCR approach to amplify a fragment of the 16S rRNA gene. DGGE profiles of the bifidobacterial community were relatively simple, with one or two amplicons detected at most sampling sites in the colon. DGGE profiles obtained with Lactobacillus group-specific primers were complex and varied with host and sampling site in the colon. The overall bacterial community varied with host but not sampling site.
The human colon harbors a highly complex microbiota. Interactions between this microbiota and the host influences the health of the host in many ways (5). Bifidobacterium spp. and lactic acid bacteria (LAB), especially Lactobacillus spp., are considered normal residents of the gastrointestinal tract (11, 17). Historically the microbiota of the intestine has been examined by culture-dependent microbiological methods, but microscopic and culture-independent molecular methods have indicated that 60 to 80% of the bacterial population in the human intestine have not been cultured yet, highlighting the need for methods alternative to the traditional cultivation-based methods (6, 8, 16). Denaturing gradient gel electrophoresis (DGGE) is a culture-independent method based on sequence-specific separation of PCR-derived rRNA gene amplicons, which have proven useful in analysis of the intestinal microbiota (4, 15, 19, 21). Our present knowledge of the microbiota associated with the human colon is based on analysis of fecal samples, and only a limited number of studies characterizing the microbiota associated with the human colonic mucosa wall have been carried out. In culture-based studies Bacteroides and Fusobacterium spp. have been found to form the predominant bacterial populations on colonic biopsy samples, with bifidobacteria and lactobacilli being detected in various numbers (3, 7, 14). In a recent study Hold et al. (6) investigated the diversity of mucosa-associated bacteria in the human colon by 16S rRNA gene clone analysis. According to their findings the bacterial community associated with the human colon wall is dominated by bacteria closest related to Bacteroides and Clostridium spp. (6). Zoetendal et al. (21) investigated the distribution of mucosa-associated bacteria along the colon using DGGE and found that the predominant bacterial community associated with the colon wall was host specific and uniformly distributed along the colon. Furthermore the distribution of Lactobacillus-like bacteria along the colon was investigated. The Lactobacillus-like community was found to be simple, with only one amplicon predominating at all sampling sites. In 7 out of 10 individuals no variation in the Lactobacillus-like community was seen along the colon, whereas minor differences with sampling site were observed in 3 out of 10 individuals (21). No culture-independent studies investigating the distribution of Bifidobacterium spp. associated with the human colonic mucosa wall have been published so far.
The aim of the present study was to simultaneously study three groups of bacteria adhering to the colon wall by DGGE: Bifidobacterium spp., Lactobacillus spp. and closely related bacteria, and the overall bacterial population.
Biopsy samples were taken from the ascending, transverse, and descending parts of the colons of four individuals (three men aged 36, 41, and 50 years, respectively, and one woman, aged 30 years) who underwent colonoscopy due to previous removal of benign colonic polyps or a family history of polyps. The volunteers considered themselves healthy and did not follow any special dietary routines, and none had recently received any antibiotic treatment. The study was approved by the Ethical Committee of the Municipality of Copenhagen and Frederiksberg (KF 01-388-97) and the Danish Medicines Agency. Evacuation of the colon before the biopsy sampling was induced by two doses of oral laxatives (10 mg of bisacodyl [Nordic Drugs, Limhamm, Sweden] and 45 ml of a liquid combination of K2HPO4 and KH2PO4 orally). After sampling the biopsy samples were transferred to tubes containing 3 ml of isotonic saline and washed in order to remove any fecal debris. This washing step was subsequently repeated twice, making it highly likely that the investigated bacteria were truly associated with the colon wall. Bacterial cells still adhering to the biopsy samples were separated from the samples by transferring the samples to a FastPreb vial (Bio101, Vista, Calif.), adding 1 ml of saline peptone solution (SPS), and then rigorously shaking the samples (45 s, speed 4) in a FastPreb instrument (Bio101). To extract bacterial DNA, 0.8 ml of SPS containing a biopsy sample was centrifuged (14,000 × g, 5 min, 4°C). DNA was extracted from the resulting pellet with a QIAamp DNA stool minikit (Qiagen, Hilden, Germany) by following the manufacturer's instructions (lysis temperature, 95°C). The isolated DNA was subsequently amplified on a Biometra Trio-Thermoblock (Biotron, Göttingen, Germany). PCR was performed by a nested approach modified from that of Boon et al. (2). Primers Bif164f and Bif662r (15), specific for Bifidobacterium spp., 7f and Lab677r (4), specific for Lactobacillus spp. and closely related organisms (Lactobacillus, Leuconostoc, Weissella, Pediococcus, and Aerococcus spp., here as a whole termed Lactobacillus-like), and the universal bacterial primers 7f and 1510r (15) were used as first-round primers. In the second PCR round, the obtained fragments were reamplified with universal bacterial primers P338fGC and P518r, amplifying the V3 region of the 16S rRNA gene (2). Primer sequences and PCR protocols were as stated in the original publications except that an annealing temperature of 60°C was used with primers Bif164f and Bif662r. All primers were purchased from DNA Technologies, Aarhus, Denmark. The final levels of reaction mixture constituents were as follows: 1.25 U of Taq DNA polymerase (Promega, Madison, Wis.), 1× PCR buffer (MgCl2 free; Amersham Biosciences, Piscataway, N.J.), 200 μM (each) deoxynucleotide triphosphate (Amersham Biosciences), 1.5 mM MgCl2, 0.1 μM (each) primer, 1 μl of DNA template, and sterile MilliQ water for adjustment of the volume to 50 μl. The DGGE analysis was basically performed as described by Muyzer et al. (12) with a DCode System apparatus (Bio-Rad, Hercules, Calif.). Polyacrylamide gels (8% [wt/vol] acrylamide-bisacrylamide [37.5:1]) in 1× Tris-acetate-EDTA (TAE) buffer were prepared with a Bio-Rad gradient delivery system (model 475) by using solutions containing 40 and 60% denaturant (100% denaturant corresponds to 7 M urea and 40% [vol/vol] formamide). Gels were run at 60°C for 16 h at a constant voltage of 70 V. After electrophoresis gels were stained with SYBR-GOLD (Molecular Probes, Eugene, Oreg.) for 20 min and photographed. DNA fragments from selected bands were excised from the DGGE gels and identified by sequencing basically as described previously (13) with primers P338fGC and P518r (2) and a CEQ 2000 dye terminator cycle sequencing kit (Beckman Coulter, Fullerton, Calif.). Sequences were manually corrected and aligned to 16S rRNA gene sequences obtained from the GenBank database with the BLAST algorithm (1). Cluster analysis of the DGGE profiles was performed basically as outlined previously (21) with GelCompar, version 4.0 (Applied Maths, Kortrijk, Belgium). Culture-independent results were compared with culture-based isolations. Following separation of the bacterial cells from the biopsy samples as described above 10-fold dilutions were prepared in SPS. From appropriate dilutions 100 μl was spread onto MRS agar (Merck, Darmstadt, Germany) and incubated anaerobically at 37°C for 4 days (GasPak system; Microbiology Systems, Cockeysville, Md.). Based on colony morphology and microscopy, assumed Bifidobacterium and Lactobacillus colonies were restreaked onto MRS for purification. Isolates were stored at −80°C in MRS broth (Merck) containing 20% (vol/vol) glycerol. Isolates of assumed lactobacilli and bifidobacteria were identified by sequencing the V6 to V8 region of the 16S rRNA gene. DNA was extracted from overnight cultures by bead beating, and the DNA was amplified and sequenced with primers 968fGC and 1401r (DNA Technologies) (20) as described above. Pure culture DNA was furthermore amplified with primers P338fGC and 518r (2) and analyzed by DGGE to compare their electrophoretic mobilities to the DGGE profiles of the biopsy samples.
The nested-PCR approach was chosen in order to obtain stronger bands in the denaturing gradient gels. Furthermore the chosen approach allows comparison between DGGE patterns obtained with different specific primers because the same 16S rRNA gene fragment was amplified in the second PCR round. DGGE of rRNA gene amplicons obtained with Bifidobacterium-specific primers revealed that the bifidobacterial community associated with the colonic wall in the four individuals examined here was simple, with zero to two amplicons being detected at each sampling site (Fig. 1). Some variations in DGGE patterns were observed with host and sampling site (Fig. 1), but the numbers of samples and observed bands are too small to allow definite conclusions. Due to the simple Bifidobacterium-specific DGGE profiles, no cluster analysis was performed. Bifidobacterium longum and Bifidobacterium bifidum were detected at the same sampling sites by conventional plating methods as well as DGGE (data not shown). Amplicons most closely related to Bifidobacterium adolescentis and Bifidobacterium ruminantium were detected by DGGE but not by conventional plating. Likewise, Matsuki et al. (9) frequently detected B. adolescentis in fecal samples by direct species- and group-specific PCR, but not by conventional plating followed by specific PCR. No bands were detected in the ascending and descending parts of the colon from individual 3 and the ascending part of the colon from individual 4 (Fig. 1). Nevertheless B. longum was detected in the ascending part of the colon by plating in both individuals (data not shown). This was probably because B. longum was present in numbers below the detection limit of the PCR-DGGE method. All bifidobacterial isolates analyzed by DGGE migrated as expected compared to identified fragments in the DGGE patterns of the biopsy specimens (data not shown).
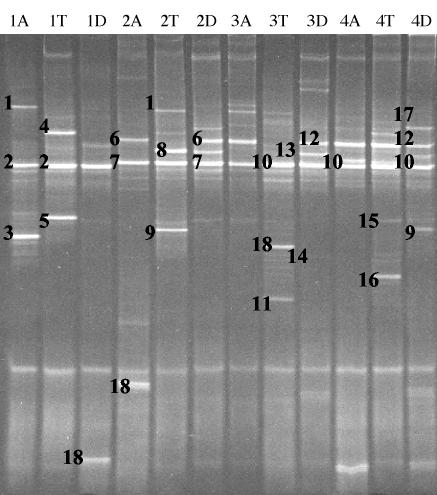
FIG. 1.
DGGE profiles representing 16S rRNA gene fragments of Bifidobacterium spp. from the ascending (A), transcending (T), and descending (D) parts of the colon wall from individuals 1 to 4. The closest relatives of the fragments sequenced (percentages of identical nucleotides compared to the sequences retrieved from the GenBank database) are as follows: B. adolescentis and B. ruminantium (1; 96.5%), B. longum and B. infantis (2; 99.4%), B. adolescentis and B. ruminantium (3; 99.4%), and B. bifidum (4; 100%).
The DGGE patterns representing the Lactobacillus-like community associated with the human colonic mucosa wall were relatively complex, with several amplicons detected at all sampling sites (Fig. 2). Cluster analysis (Fig. 3) did not reveal any grouping with either host or sampling site, indicating that the Lactobacillus-like community varies with host as well as sampling site in the colon in the four individuals examined here. In agreement with the specificity of the chosen primer all identified bands represented Lactobacillus, Leuconostoc, Weissella, or Aerococcus spp. with the exception of a band detected at all sampling sites in volunteer 1, which was most closely related to Eubacterium biforme (Fig. 2, band 2). No Pediococcus spp. were detected. The chosen primer set specific for Lactobacillus-like bacteria was developed and evaluated by Heilig et al. (4), who also observed the unintended amplification of E. biforme-like amplicons, but otherwise the primer set was found to be specific for Lactobacillus-like bacteria (4). Amplicons most closely related to the genus Weissella (Fig. 2, band 7) were detected in all parts of the colon in individual 2. A band most closely related to Leuconostoc citreum (Fig. 2, band 10) was likewise detected in all parts of the colon in volunteers 3 and 4. Various Lactobacillus spp. are generally considered to form the major part of the fecal LAB community (17). Our results indicate that, as seen from Fig. 2, LAB other than Lactobacillus might form a significant part of the mucosa-associated LAB, as leuconostocs and not lactobacilli were detected in most samples. Using conventional plating methods a few Lactobacillus spp. (Lactobacillus casei group and Lactobacillus ruminis, data not shown) but no Leuconostoc, Weissella, Pediococcus, or Aerococcus spp. were isolated. This was probably because we aimed specifically at isolating Lactobacillus spp. All isolates migrated as expected when analyzed by DGGE. The L. casei group isolates had a migration pattern different from those of all fragments in the DGGE patterns of the biopsy specimens (data not shown). This was expected as the L. casei group was detected only by the cultivation-based method. The fact that only a few lactobacilli were detected in the cultivation-based part of the study could support our observation from the DGGE profiles that lactobacilli, at least in the volunteers examined here, do not form the major part of the LAB population associated with the human colon wall. As mentioned above, in a previous investigation of the Lactobacillus-like community on human colonic biopsy samples by DGGE it was reported that the DGGE profiles were simple and low in diversity (21). The DGGE profiles obtained in this study differed from these results, as variations with sampling site as well as host were seen (Fig. 2 and 3). Furthermore several amplicons predominated in all samples. The same Lactobacillus-specific primer was used in both studies, but differences in, e.g., DNA extraction methods might explain the different observations. Natural variations among the subjects examined, as previously reported on the basis of fecal samples, might be another explanation (18).
FIG. 2.
DGGE profiles representing 16S rRNA gene fragments of Lactobacillus spp. and related LAB from the ascending (A), transcending (T), and descending (D) parts of the colon wall from individuals 1 to 4. The closest relatives of fragments sequenced (percentages of identical nucleotides compared to sequences retrieved from the GenBank database) are as follows: Lactobacillus fermentum (1; 94.5%), E. biforme (2; 99.5%); Leuconostoc carnosum (3; 97.8%); Aerococcus viridans (4; 97.2%); Aerococcus sanguinicola (5; 97.3%); Weissella kimchii, Weissella cibaria, and Weissella confusa (6 and 7; 99.5 and 98.0%, respectively); L. fermentum (8; 96.8%); Leuconostoc citreum (9; 98.9%); L. citreum (10; 98.9%); L. citreum (11; 94.6%); Leuconostoc kimchii (12; 98.8%); Leuconostoc fallax (13; 99.5%); Lactobacillus iners (14; 99.4%); Weissella soli (15; 95.1%); L. ruminis (16; 96.2%); W. soli (17; 95.1%). 18, no sequence obtained or the fragment did not migrate as an original band after reamplification.
FIG. 3.
Dendrogram derived from DGGE analysis of the Lactobacillus-like population from the ascending (A), transcending (T), and descending (D) parts of the colon wall from individuals 1 to 4 based on Dice's coefficient of similarity with the unweighted pair group method with arithmetic averages clustering algorithm.
DGGE profiles of PCR amplicons derived with universal bacterial primers were complex (Fig. 4). Cluster analysis revealed that the DGGE patterns were host specific and did not vary with sampling site (data not shown). This is in agreement with previous results (21). All obtained sequences, except the amplicon closely related to B. bifidum, were less than 97% identical to known sequences (Fig. 4). This implies that the amplicons represent unknown species.
FIG. 4.
DGGE profiles representing 16S rRNA gene fragments of the predominating bacteria from the ascending (A), transcending (T), and descending (D) parts of the colon wall from individuals 1 to 4. Closest relatives of fragments sequenced (percentages of identical nucleotides compared to sequence retrieved from the GenBank database) are as follows: Bacteroides vulgatus (1; 95.0%), Bacteroides vulgatus (2; 95.6%); Prevotella enoeca (3; 91.7%); B. bifidum (4; 100%); Bacteroides distasonis (5; 96.1%). 6, no sequence obtained.
Some bifidobacteria and LAB are believed to possess probiotic, health-promoting effects. Some authors consider adherence to the colon wall a desirable trait of these probiotic organisms, as this might enhance their potential to colonize the colon (10). Our finding that the bifidobacterial and Lactobacillus-like microbiota associated with the human colonic mucosa wall vary with host and/or position in the colon raises the question of whether it is at all possible to find a common probiotic organism capable of colonizing all individuals of a given population? Our results do not give a conclusive answer but highlight the need for further characterization of the mucosa-associated bacteria in the human colon.
Nucleotide sequence accession numbers.
The sequences determined in this study have been assigned GenBank accession no. AY267911 to AY267947, AY273784, and AY273785.
Acknowledgments
The financial support provided by the Danish Dairy Research Foundation (Danish Dairy Board) and the Danish Research and Development Program for Food Technology is highly appreciated.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boon, N., W. de Windt, W. Verstraete, and E. M. Top. 2002. Evaluation of nested PCR-DGGE (denaturing gradient gel electrophoresis) with group-specific 16S rRNA primers for the analysis of bacterial communities from different wastewater treatment plants. FEMS Microbiol. Ecol. 39:101-112. [DOI] [PubMed] [Google Scholar]
- 3.Croucher, S. C., A. P. Houston, C. E. Bayliss, and R. J. Turner. 1983. Bacterial populations associated with different regions of the human colon wall. Appl. Environ. Microbiol. 45:1025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heilig, H. G. H. J., E. G. Zoetendal, E. E. Vaughan, P. Marteau, A. D. L. Akkermans, and W. M. de Vos. 2002. Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl. Environ. Microbiol. 68:114-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hentschel, U., U. Dobrindt, and M. Steinert. 2003. Commensal bacteria make a difference. Trends Microbiol. 11:148-150. [DOI] [PubMed] [Google Scholar]
- 6.Hold, G. L., S. E. Pryde, V. J. Russell, E. Furrie, and H. J. Flint. 2002. Assessment of microbial diversity in human colonic samples by 16S rDNA sequence analysis. FEMS Microbiol. Ecol. 39:33-39. [DOI] [PubMed] [Google Scholar]
- 7.Johansson, M.-L., G. Molin, B. Jeppsson, S. Nobaek, S. Ahrné, and S. Bengmark. 1993. Administration of different Lactobacillus strains in fermented oatmeal soup: in vivo colonization of human intestinal mucosa and effect on the indigenous flora. Appl. Environ. Microbiol. 59:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langendijk, P. S., F. Schut, G. J. Jansen, G. C. Raangs, G. R. Kamphuis, M. H. F. Wilkinson, and G. W. Welling. 1995. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 61:3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuki, T., K. Watanabe, R. Tanaka, M. Fukuda, and H. Oyaizu. 1999. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl. Environ. Microbiol. 65:4506-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattila-Sandholm, T., J. Mättö, and M. Saarela. 1999. Lactic acid bacteria with health claims—interactions and interference with gastrointestinal flora. Int. Dairy J. 9:25-35. [Google Scholar]
- 11.McCartney, A. L., W. Wenzhi, and G. W. Tannock. 1996. Molecular analysis of the composition of the bifidobacterial and Lactobacillus microflora in humans. Appl. Environ. Microbiol. 62:4608-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muyzer, G., E. C. de Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omar, N. B., and F. Ampe. 2000. Microbial community dynamics during production of the Mexican fermented maize dough pozol. Appl. Environ. Microbiol. 66:3664-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poxton, I. R., R. Brown, A. Sawyerr, and A. Ferguson. 1997. Mucosa-associated bacterial flora of the human colon. J. Med. Microbiol. 46:83-91. [DOI] [PubMed] [Google Scholar]
- 15.Satokari, R. M., E. E. Vaughan, W. M. Akkermans-van Vliet, M. Saarela, and W. M. de Vos. 2001. Bifidobacterial diversity in human feces detected by genus-specific PCR and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67:504-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suau, A., R. Bonnet, M. Sutren, J.-J. Godon, G. R. Gibson, M. D. Collins, and J. Doré. 1999. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 65:4799-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tannock, G. W. 1995. Microecology of the gastrointestinal tract in relation to lactic acid bacteria. Int. Dairy J. 5:1059-1070. [Google Scholar]
- 18.Tannock, G. W., K. Munro, H. J. M. Harmsen, G. W. Welling, J. Smart, and P. K. Gopal. 2000. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl. Environ. Microbiol. 66:2578-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter, J., C. Hertel, G. W. Tannock, C. M. Lis, K. Munro, and W. P. Hammes. 2001. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67:2578-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoetendal, E. G., A. D. L. Akkermans, and W. M. de Vos. 1998. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable host-specific communities of active bacteria. Appl. Environ. Microbiol. 64:3854-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zoetendal, E. G., A. von Wright, T. Vilpponen-Salmela, K. Ben-Amor, A. D. L. Akkermans, and W. M. de Vos. 2002. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl. Environ. Microbiol. 68:3401-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]