Abstract
We have previously demonstrated that receptors for hematopoietic growth factors, stem cell factor (SCF) and granulocyte-colony stimulating factor (G-CSF) are expressed in the neurons and the neural progenitor cells (NPCs) in adult rat brain, and systemic administration of SCF and G-CSF in the first week after induction of cortical brain ischemia (3 hrs-7d post-ischemia) significantly improve functional outcome, augment NPC proliferation, and reduce infarct volume in rats. The purpose of the present study was to determine whether SCF and G-CSF pass through the blood brain barrier (BBB) in intact rats. The growth factors were labeled with iodine (I125), a radioactive compound. I125-SCF and I125-G-CSF were intravenously administered, and the concentrations of I125-SCF and I125-G-CSF in the blood plasma and the brain were determined at 10, 30, 60, and 120 minutes after injection. We observed that both SCF and G-CSF were slowly and continuously, in the same rate, transported from the blood stream to the brain. In addition, both immunofluorescent staining and western blots showed that receptors for SCF and G-CSF were expressed in the capillaries of adult rat brain, suggesting that SCF and G-CSF entry to the brain may be mediated via receptor-mediated transport, one of the endogenous transports in the BBB. These data indicate that both SCF and G-CSF were able to pass through the BBB in intact animals. This observation will help in further exploring the physiological role of peripheral SCF and G-CSF in the brain and therapeutic possibility to chronic stroke.
Keywords: SCF, G-CSF, brain, hematopoietic growth factor, blood brain barrier
Introduction
SCF and G-CSF are essential hematopoietic growth factors. SCF (also termed master cell growth factor, kit ligand, and steel factor) binds to its receptor, cKit, regulating diverse biological functions such as hematopoiesis, gametogenesis and melanogenesis (Williams and Lyman, 1991; Galli et al., 1994). G-CSF binds its specific receptor, GCSFR, controlling proliferation, differentiation and maturation of the precursor cells of neutrophilic granulocytes (Welter et al., 1996; Hartung, 1998). However, accumulating evidence has demonstrated that SCF and G-CSF also participate in neuronal function, neurogenesis, and neuroprotection. Transgenic mice with mutation of the genes encoding SCF and cKit show a deficit in spatial learning and memory and long-term potentiation (Motro et al., 1996; Katafuchi et al., 2000). cKit has been reported to be expressed in the neurogenic zones, and intraventricular administration of SCF augments NPC proliferation and neuronal regeneration (Jin et al, 2002). Kim et al., reported that G-CSF plays a role in proliferation and differentiation of neural stem cells in vitro (Kim et al., 2004). Recently, neuroprotective effects of SCF/cKit binding in cortical neurons were evidenced in an in vitro study (Dhandapani et al., 2005). G-CSF has been shown to protect neurons from acute focal ischemic injury in rats (Schabitz et al., 2003; Six et al., 2003, Shyu et al., 2004). Our early studies demonstrated that receptors for both SCF and G-CSF existed in the neurons and neurogenic regions in adult brain, and subcutaneous administration of SCF and G-CSF in the acute phase of focal brain ischemia led to increase in NPC proliferation, reduction in infarction size, and functional improvement (Zhao et al., unpublished observations). The BBB is the barrier isolating the brain from the circulation system and limiting blood-borne chemicals entry to the brain. During the acute phase of brain ischemia the BBB is temporally disrupted, which leads to drugs penetrating to the brain. To further investigate whether systemic injections of SCF and G-CSF will be of benefit to chronic stroke, in the present study, we will determine whether SCF and G-CSF can cross the BBB in intact animals.
Materials and Methods
The experiment for using animals in this study was approved by The Animal Care and Use Committee at Northwestern University. All procedures were performed in accordance with the standards of NIH guidelines for the care and use of laboratory animals.
Recombinant rat SCF and recombinant human G-CSF were provided by Amgen (Thousand Oaks, CA, USA); and I125-labeling was performed by MP Biochemicals, (Irvine, CA, USA). Radiochemical purity of [125I]-SCF (2.47 µCi/µg) and [125I]-GCSF (2.47 MBq/mg) was examined for purity by TCA precipitation, which was greater than 99% before use.
Thirty Sprague-Dawley rats (2 months old) were intraperitoneally anesthetized with ketamine (40 mg/kg) and xylazine (5 mg/kg). Polyethylene catheters (PE-10) were inserted into the femoral artery and vein for collecting blood samples and administering the radioisotopes, respectively. Arterial blood pressure, blood gases, and body temperature were monitored throughout the experiment. Heat lamps were used to maintain body temperature close to 37 °C.
Twenty to thirty µCi of [125I]-SCF (n=15) or [125I]-GCSF (n=15) was injected into the femoral vein as an intravenous bolus. Arterial blood samples were collected at 10, 30, 60, and 120 min (n=3–4/time point/cytokine) after drug injection. Plasma was used for [125I] radioactivity counting by liquid scintillation counting with appropriate quench correction. After collecting blood samples, the rats were sacrificed at 10, 30, 60, and 120 min (n=3–4/time point for each cytokine). The rats were then decapitated, and the brains were removed and stored at − 40°C. The brains were homogenized, and mixed with 3 times their weight in saline after thaw. Aliquots of the brain samples were used for determination of tissue radioactivity by liquid scintillation counting. The plasma and brain tissue radioactivity counts were divided by the sample weights to give tissue radioactivity concentrations in nCi/g. The plasma (Cp) and brain (Am) radioactivity concentrations were used to determine the influx constant according to a graphical method of analysis described elsewhere (Patlak et al., 1983). With this method, the values of the integrated plasma activity divided by the final plasma value are plotted against the tissue radioactivity value divided by the final plasma value. An apparent K1 for each animal was also calculated using the equation . Vi presents the tissue plasma space and any rapidly equilibrating space, with units of ml/g. Cp (T) is the final plasma value. K1, the blood-to-brain tissue transfer constant, was presented with a value of ml/g/min.
Immunofluorescent staining, which has been described elsewhere (Zhao, et al., 2003), was used for determining SCF and G-CSF receptor expression in the capillaries of rat brain. Brain sections (cut by a cryostat, 10µm thick) were collected from Sprague-Dawley rats (3 month old). The sections were fixed with 4% formaldehyde in phosphate-buffered saline (PBS). After blocking nonspecific binding with goat normal serum, sections were incubated with primary antibodies: rabbit anti-cKit or rabbit anti-GCSFR (1:50, Santa Cruz Biotechnology, Santa Cruz, CA, USA) over night at 4°C. After 2-hrs incubation with Cy3-conjucted goat anti-rabbit (1:400, Jackson ImmunoResearch Lab, West Grove, PA, USA) in dark, counterstaining was performed with DAPI (1:5000, Sigma, St. Louis, MO, USA). Immunofluorescent staining was photographed with a fluorescent microscope (Nikon, Eclipse TE 2000-S).
For western blots, adult rat brain microvascular endothelial cells (SV-40 cell line, gift from Institute for Biological Sciences, Canada) were grown in M199 media contain 10% FBS. The cells were harvested and lysed with RIPA buffer containing a protease inhibitor cocktail (Pierce, Rockford, IL). Twenty micrograms proteins were loaded to 8% or 12% SDS-PAGE polyacrylamide gel and transferred to a PVDF membrane (Bio-Rad, Hercules, CA). Membranes were blocked with 5% non-fat milk and incubated with rabbit anti-c-Kit or rabbit anti-G-CSFR polyclonal antibodies (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubation with a peroxidase conjugated secondary antibody (Jackson Immunoresearch, West Brove, PA). Bands were visualized by chemiluminescent substrate (Pierce, Rockford, IL) according to the manufacturer's instructions. Immunoblotting with a mouse anti-β-actin (Sigma, Saint Louis, MO) was used as internal control.
Results and Discussion
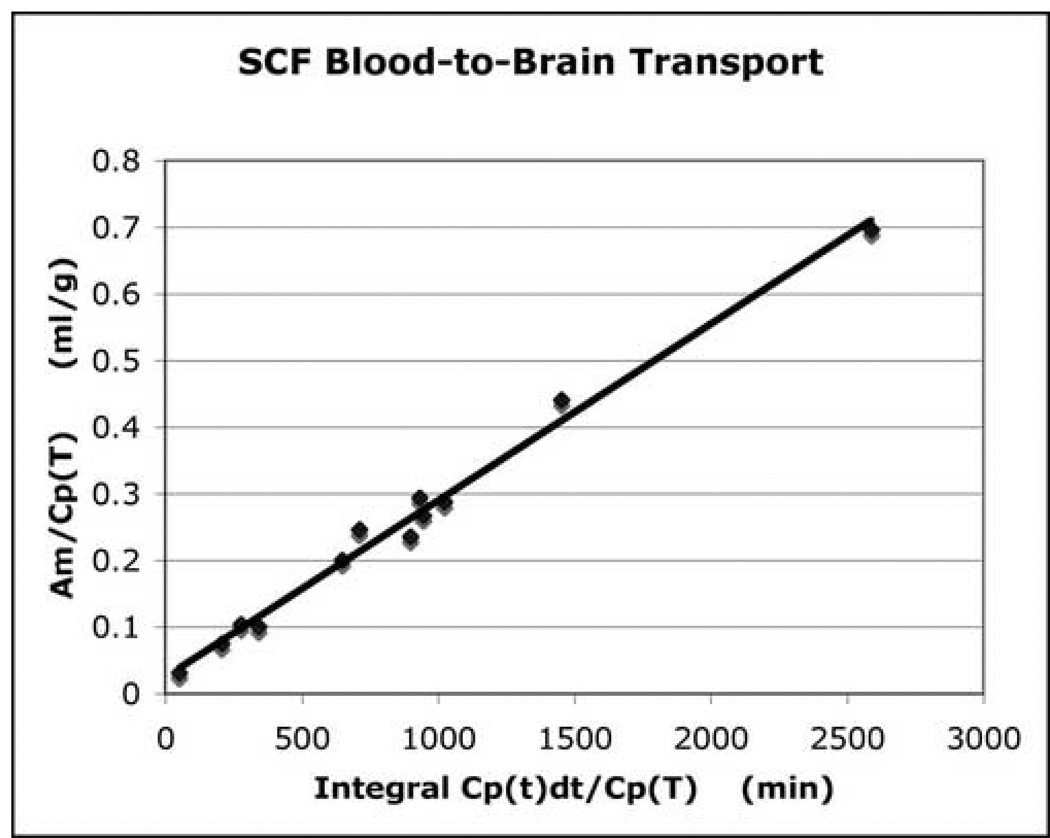
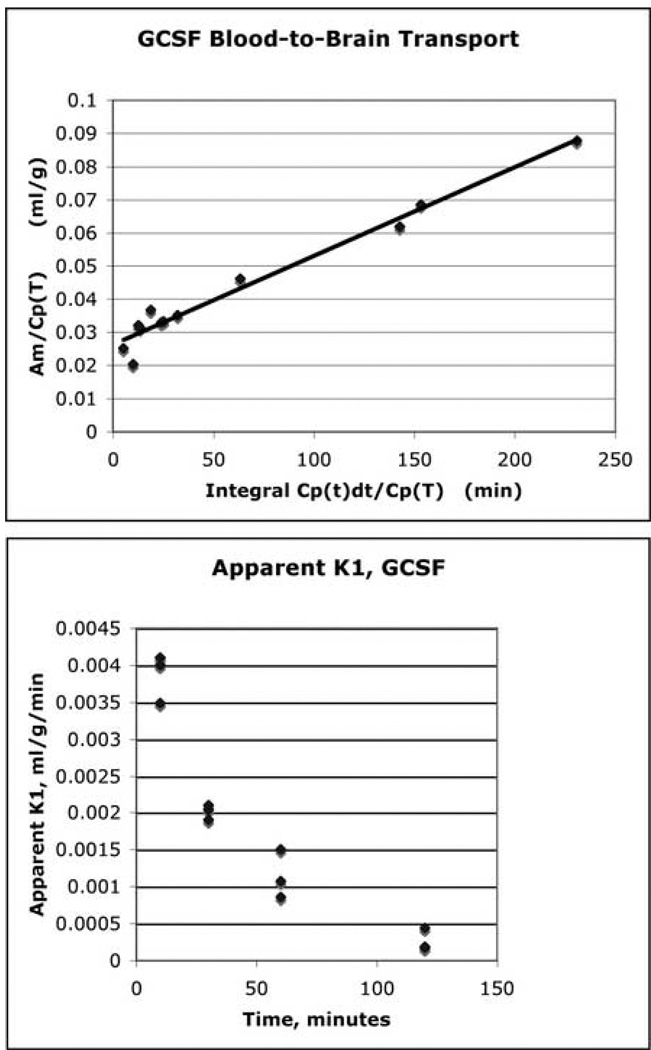
The present study demonstrated that both SCF and G-CSF crossed the BBB into the brain. The rate of penetration for both SCF and G-CSF was similar and expressed in a slow and constant manner. The influx constant (K1) of each growth factor was presented as line graphs (Fig. 1 and Fig. 2-upper panel). For SCF, the data presented in Figure 1 was fit by an equation with a slope of 0.000259 and an intercept of 0.021 (R2 = 0.9695). For G-CSF, the data presented in Figure 2 was fit by an equation with a slope of 0.000260, and an intercept of 0.0262 (R2 = 0.9889). When blood-to-brain transport of the growth factors was calculated as the “apparent” K1 for individual animal (Fig. 2-lower panel), the influx constant (K1) showed decrease over time, indicating the concentration of the growth factors in the blood was the highest at the beginning of the experiments and decreased following time. Since similar transport pattern was observed in both SCF and G-CSF, here only representatively showed “apparent” K1 data for G-CSF (Fig. 2-lower panel).
Fig. 1.
Transportation of SCF from the blood to the brain. The linear slope of the line presents the influx constant (K1). Y-axe presents tissue plasma space (ml/g). X-axe shows a measure of the plasma arterial integral over time (min). The data indicates that SCF is slowly and continuously transported from the blood to the brain.
Fig. 2.
Transportation of G-CSF from the blood to the brain. Upper panel: the linear slope of the line presents the influx constant (K1) showing that G-CSF slowly and constantly enters into the brain. Lower panel: G-CSF blood-to-brain transport is presented as an apparent K1. Note that K1 appears to decrease over time, indicating the amount of the isotope in the blood plasma is highest at the beginning of the experiments and decreases as time goes on.
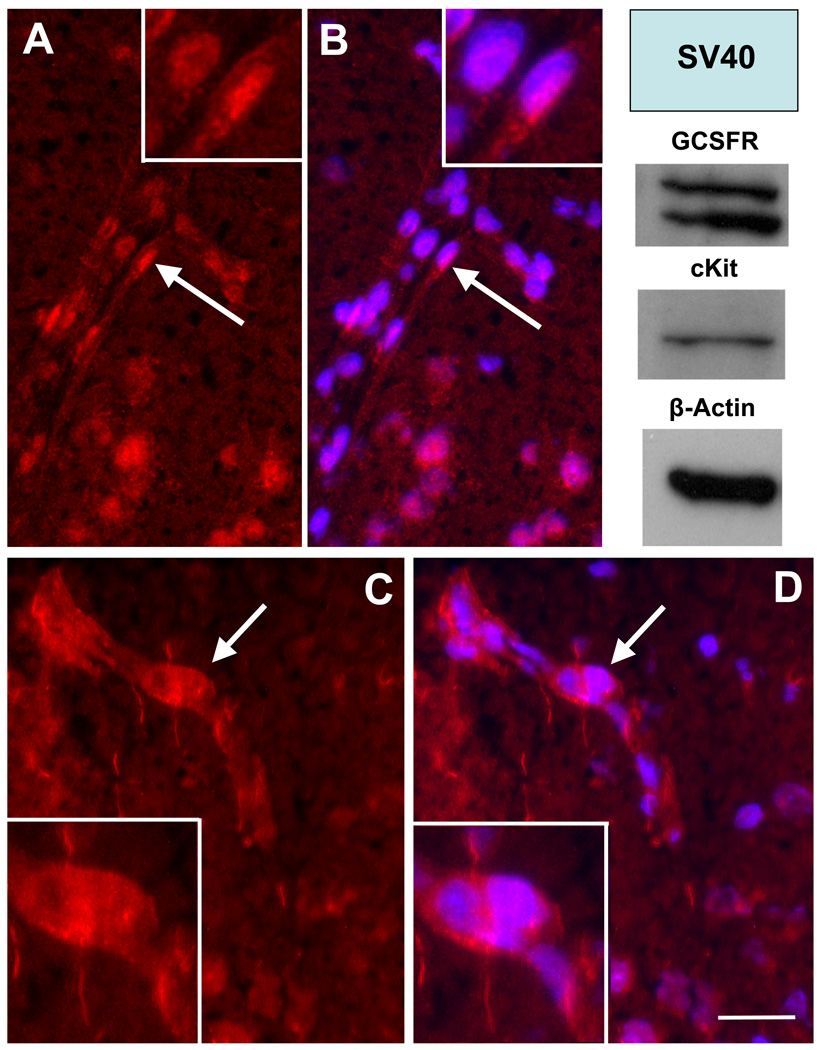
Expressions of SCF and G-CSF receptors in the capillaries of brain were also observed in adult rats. Immunofluorescent positive staining for cKit, receptor for SCF, was seen in the cell membrane and/or cytoplasma of the cells in the capillary wall (Fig. 3C & D). GCSFR, receptor for G-CSF, was expressed in the nuclei of the endothelial cells, and the staining was colabeled with nuclear dye (DAPI) (Fig.3 A–B). Using Western blots, we also noted that both cKit and GCSFR protein were expressed in adult rat brain microvasculer endothelial cells (SV-40 cell line) (Fig. 3).
Fig. 3.
Receptors for SCF and G-CSF on the wall of brain capillaries. Representative brain sections show that the walls of brain capillaries in the superficial layer of cortex express receptors for G-CSF (A & B) and SCF (C & D). GCSFR, the receptor for G-CSF (red, in A & B), was expressed in the nuclei (blue) of endothelial cells. Ckit, the receptor for SCF (C & D), was detected in the cell surface and/or cytoplasma of the cells in the capillary wall. Upright panel shows the results of western blots. Note: both cKit and GCFSR are expressed in adult rat brain microvascular endothelial cells (SV-40 cell line). Inserts: the high power images of indicated areas (arrows) in A–D. Bar in F (for A–D) = 20µm.
The BBB is a unique physical and enzymatic barrier that separates the brain from blood circulation. The physical barrier is composed by a complex network of endothelial cells with their tight junctions, astrocytes, pericytes, perivascular macrophages, and a basal lamina. The enzymatic barrier of the BBB consists of abundant enzymes, which are capable of metabolizing blood-borne drugs and nutrients (Witt et al., 2001, 2006). The BBB is dynamically acting that permits nutrients to enter to the brain and neurotoxics and waste products to exit. Generally, lipid solubility of small molecules with molecular weight less than 400 Daltons free diffuse to the brain through the BBB (Levin, 1980). Nutrients and larger molecules such as hormones, growth factors, enzymes, and plasma proteins need to be transported to the brain via endogenous BBB transport system. Receptor-mediated transport (RMT) is particularly directed to transporting endogenous peptides and proteins. Small molecules such as O2, CO2, NO, and H2O penetrate more rapidly. RMT is a highly specific type of energy dependent transport; therefore, growth factors and proteins cross the BBB that is slower than small molecules. In the present study, both receptors for SCF and G-CSF were observed in the capillary wall of the brain. We postulate that slow penetration of both SCF and G-CSF is associated with energy dependent RMT. However, precise mechanism underlying RMT dependency for SCF and G-CSF needs further studies to elucidate.
SCF with molecular weight 45 kDa (Glaspy et al., 1996) and G-CSF with molecular weight 19.6 kDa (Hill et al., 1993) are important hematopoietic growth factors. G-CSF (Filgrastim) is a Food and Drug Administration-approved drug for treatment of neutropenia (Nemunaitis, 1997). SCFCSF has been showed to have a synergistic effect on mobilizing bone marrow stem cells in patients after chemotherapy (Facon et al., 1999; Stiff et al., 2000; To et al., 2003). In the present study, we observed that both receptors for SCF and G-CSF were expressed in the capillaries of the brain, and both of them crossed the BBB slowly at a similar rate. We postulate that SCF and G-CSF are transported to the brain by RMT. Others and we have previously demonstrated that receptors for SCF (cKit) and G-CSF (GCSFR) were expressed in the neurons and neurogenic zones (Jin et al., 2002; Schneider et al., 2005; Zhao et al., unpublished data). In vitro studies have shown that SCF (Dhandapani et al., 2005) and G-CSF (Schneider et al., 2005) protect cortical neurons against exitotoxic lesion. These findings suggested that endogenous SCF and G-CSF play a role in neurophysiology and neuropathology. In an early study, we observed that systemic administration of SCF and G-CSF in the acute-to-subacute phase of focal brain ischemia dramatically stimulated NPC proliferation, reduced infarction volume, and ameliorated ischemia-induced neurological deficits (Zhao et al., unpublished data). Many other studies have also shown that systemic injections of G-CSF protected neurons from acute brain ischemia (Six et al., 2003; Schabitz et al., 2003; Shyu et al., 2004; Zhao et al., unpublished data). Our current data showed that SCF and G-CSF crossed the BBB in the intact animals, which implies that systemic administration of SCF and G-CSF to chronic stroke may also contribute to functional restoration. SCF and G-CSF are naturally produced in the body to regulate hematopoiesis. The present study may also provide information to help in further investigating the physiological and pathological role of peripheral SCF and G-CSF in the central nervous system and may also assist in developing a novel therapy to neurological disorders and neurodegenerative diseases.
Acknowledgements
Authors appreciate Joseph Jones for their assistant in brain section preparation. We thank Amgen for supplying both SCF and G-CSF. This work was supported by NIH grants (to Dr. Kessler) R01 NS20778, R01 NS20013, and R01 NS34758; and by AHA grant (to Dr. Zhao) 0665522B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Dhandapani KM, Wade FM, Wakade C, Mahesh VB, Brann DW. Neuroprotection by stem cell factor in rat cortical neurons involves AKT and NFkappaB. J Neurochem. 2005;95:9–19. doi: 10.1111/j.1471-4159.2005.03319.x. [DOI] [PubMed] [Google Scholar]
- Facon T, Harousseau JL, Maloisel F, Attal M, Odriozola J, Alegre A, Schroyens W, Hulin C, Schots R, Marin P, Guilhot F, Granena A, De Waele M, Pigneux A, Meresse V, Clark P, Reiffers J. Stem cell factor in combination with filgrastim after chemotherapy improves peripheral blood progenitor cell yield and reduces apheresis requirements in multiple myeloma patients: a randomized, controlled trial. Blood. 1999;94:1218–1225. [PubMed] [Google Scholar]
- Galli SJ, Zsebo KM, Geissler EN. The kit ligand, stem cell factor. Adv Immunol. 1994;55:1–96. doi: 10.1016/s0065-2776(08)60508-8. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;25(287):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Glaspy J, Davis MW, Parker WR, Foote M, McNiece I. Biology and clinical potential of stem-cell factor. Cancer Chemother Pharmacol. 1996;38:S53–S57. doi: 10.1007/s002800051039. [DOI] [PubMed] [Google Scholar]
- Hartung T. Anti-inflammatory effects of granulocyte colony-stimulating factor. Curr Opin Hematol. 1998;5:221–225. doi: 10.1097/00062752-199805000-00013. [DOI] [PubMed] [Google Scholar]
- Hill CP, Osslund TD, Eisenberg D. The structure of granulocyte-colony-stimulating factor and its relationship to other growth factors. Proc Natl Acad Sci U S A. 1993;90:5167–5171. doi: 10.1073/pnas.90.11.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Mao XO, Sun Y, Xie L, Greenberg DA. Stem cell factor stimulates neurogenesis in vitro and in vivo. J Clin Invest. 2002;110:311–319. doi: 10.1172/JCI15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katafuchi T, Li AJ, Hirota S, Kitamura Y, Hori T. Impairment of spatial learning and hippocampal synaptic potentiation in c-kit mutant rats. Learn Mem. 2000;7:383–392. doi: 10.1101/lm.33900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Choi BH, Park HC, Park SR, Kim YS, Yoon SH, Park HS, Kim EY, Ha Y. Effects of GM-CSF on the neural progenitor cells. Neuroreport. 2004;15:2161–2165. doi: 10.1097/00001756-200410050-00003. [DOI] [PubMed] [Google Scholar]
- Levin VA. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J Med Chem. 1980;23:682–684. doi: 10.1021/jm00180a022. [DOI] [PubMed] [Google Scholar]
- Motro B, Wojtowicz JM, Bernstein A, van der Kooy D. Steel mutant mice are deficient in hippocampal learning but not long-term potentiation. Proc Natl Acad Sci U S A. 1996;93:1808–1813. doi: 10.1073/pnas.93.5.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemunaitis J. A comparative review of colony-stimulating factors. Drugs. 1997;54:709–729. doi: 10.2165/00003495-199754050-00004. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Scholzke MN, Sommer C, Schwab S. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003;34:745–751. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schabitz WR. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115:2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, Li H. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110:1847–1854. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- Six I, Gasan G, Mura E, Bordet R. Beneficial effect of pharmacological mobilization of bone marrow in experimental cerebral ischemia. Eur J Pharmacol. 2003;458:327–328. doi: 10.1016/s0014-2999(02)02785-1. [DOI] [PubMed] [Google Scholar]
- Stiff P, Gingrich R, Luger S, Wyres MR, Brown RA, LeMaistre CF, Perry J, Schenkein DP, List A, Mason JR, Bensinger W, Wheeler C, Freter C, Parker WRL, Emmanouilides C. A randomized phase 2 study of PBPC mobilization by stem cell factor and filgrastim in heavily pretreated patients with Hodgkin's disease or non-Hodgkin's lymphoma. Bone Marrow Transplant. 2000;26:471–481. doi: 10.1038/sj.bmt.1702531. [DOI] [PubMed] [Google Scholar]
- To LB, Bashford J, Durrant S, MacMillan J, Schwarer AP, Prince HM, Gibson J, Lewis I, Swart B, Marty J, Rawling T, Ashman L, Charles S, Cohen B. Successful mobilization of peripheral blood stem cells after addition of ancestim (stem cell factor) in patients who had failed a prior mobilization with filgrastim (granulocyte colony-stimulating factor) alone or with chemotherapy plus filgrastim. Bone Marrow Transplant. 2003;31:371–378. doi: 10.1038/sj.bmt.1703860. [DOI] [PubMed] [Google Scholar]
- Warlow C, Sudlow C, Dennis M, Wardlaw J, Sandercock P. Stroke. Lancet. 2003;362:1211–1224. doi: 10.1016/S0140-6736(03)14544-8. [DOI] [PubMed] [Google Scholar]
- Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G. Filgrastim (r-metHuG-CSF): the first 10 years. Blood. 1996;88:1907–1929. [PubMed] [Google Scholar]
- Williams DE, Lyman SD. Characterization of the gene-product of the Steel locus. Prog Growth Factor Res. 1991;3:235–242. doi: 10.1016/0955-2235(91)90002-l. [DOI] [PubMed] [Google Scholar]
- Witt KA, Gillespie TJ, Huber JD, Egleton RD, Davis TP. Peptide drug modifications to enhance bioavailability and blood-brain barrier permeability. Peptides. 2001;22:2329–2343. doi: 10.1016/s0196-9781(01)00537-x. [DOI] [PubMed] [Google Scholar]
- Witt KA, Davis TP. CNS drug delivery: opioid peptides and the blood-brain barrier. AAPS J. 2006;8:E76–E88. doi: 10.1208/aapsj080109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LR, Duan WM, Reyes M, Verfaillie CM, Low WC. Immunohistochemical identification of multipotent adult progenitor cells from human bone marrow after transplantation into the rat brain. Brain Res Protoc. 2003;11:38–45. doi: 10.1016/s1385-299x(03)00014-x. [DOI] [PubMed] [Google Scholar]