Abstract
The Janus kinase/signal transducer and activator of transcription (JAK–STAT) pathway mediates important responses in immune cells. Activation of any of the four JAK family members leads to phosphorylation of one or more of seven STAT family members. Phosphorylation of STAT family members leads to their dimerization and translocation into the nucleus, in which they bind specific DNA sequences to activate gene transcription. Regulation of JAKs and STATs therefore has a significant effect on signal transduction and subsequent cellular responses. Mast cells are important mediators of allergic disease and asthma. These cells have the ability to cause profound inflammation and vasodilation upon the release of preformed mediators, as well as subsequent synthesis of new inflammatory mediators. The regulation of mast cells is therefore of intense interest for the treatment of allergic disease. An important regulator of mast cells, STAT5, is activated downstream of the receptors for immunoglobulin E, interleukin-3 and stem cell factor. STAT5 contributes to mast cell homeostasis, by mediating proliferation, survival, and mediator release. Regulators of the JAK–STAT pathway, such as the suppressors of cytokine signaling (SOCS) and protein inhibitor of activated STAT (PIAS) proteins, are required to fine tune the immune response and maintain homeostasis. A better understanding of the role and regulation of JAKs and STATs in mast cells is vital for the development of new therapeutics.
Keywords: mast cell, JAK, STAT, allergy, IgE
Introduction
Mast cells are critical mediators of allergic disease, hypersensitivity reactions and asthma. The cost of asthma alone in the United States is estimated to be 19.7 billion dollars a year. (http://www.lungusa.org/assets/documents/ASTHMA-JAN-2009.pdf) The release of preformed mediators such as histamine, leukotrienes and prostaglandins from mast cells upon cross-linking of the immunoglobulin E (IgE) receptor (FcεRI) with specific antigen causes early-phase allergic reactions characterized by vasodilation, smooth muscle contraction and increased mucus production. Although signaling downstream of the IgE receptor is well characterized and is known to involve activation of the phosphatidylinositol 3-OH kinase pathway, the mitogen-activated protein kinase pathway, sphingosine kinases and transcription factors such as nuclear factor-κB and Jnk,1 less is known about the role of the Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway in mast cells. STAT proteins were first discovered in 1988 as proteins binding the interferon (IFN)-stimulated response elements of DNA sequences subsequent to stimulation with type I IFNs, and were referred to as IFN-1-stimulated gene factors.2,3 JAKs were subsequently identified in 1992 by three separate labs and the JAK–STAT pathway was born.4–6 The JAK–STAT pathway is known to have a critical role in mast cell proliferation, homeostasis and gene regulation subsequent to cytokine binding to specific receptors.7–10 This review, therefore, focuses on recent strides made in understanding the importance of the JAK–STAT pathway in mast cells.
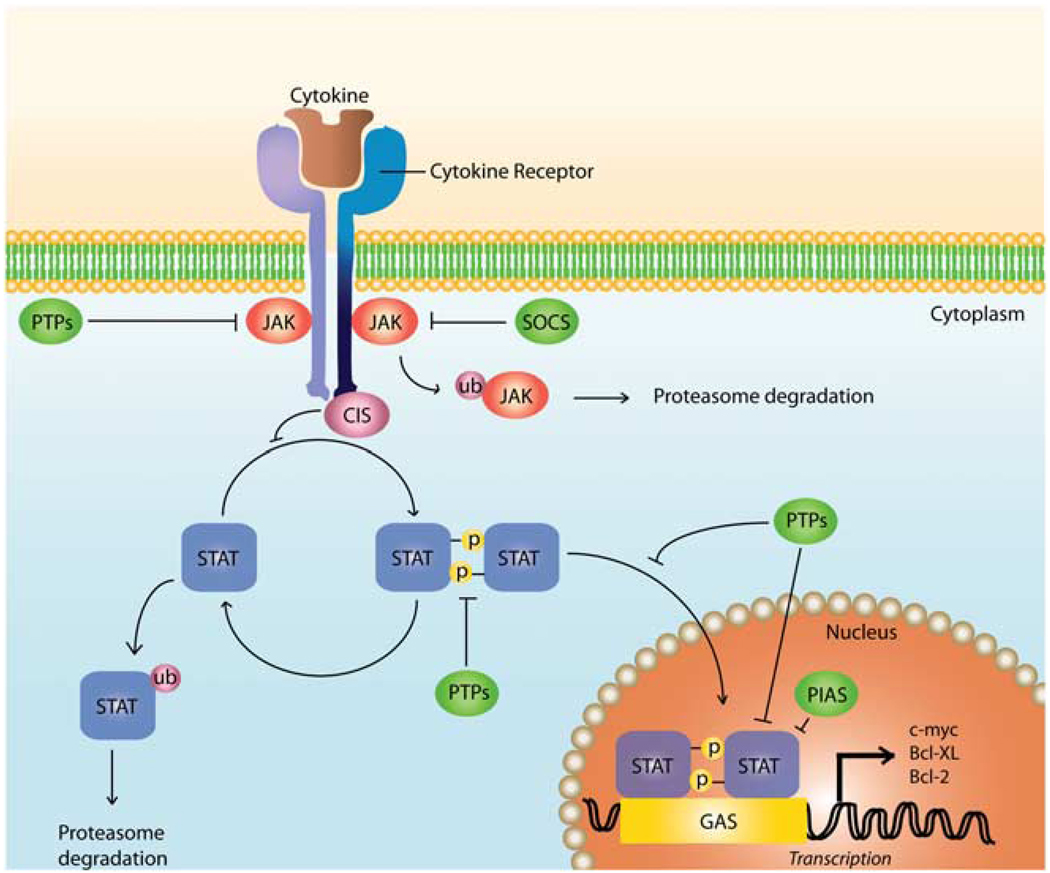
JAK tyrosine kinases are constitutively associated with the intracellular portion of cytokine receptors. The binding of a cytokine to the extracellular portion of its receptor on the cell surface induces the dimerization of the receptor, which subsequently results in the intracellular activation of JAKs. As a consequence of their activation, JAKs phosphorylate specific tyrosine residues of the intracytoplasmic portion of the cytokine receptor, transforming them to docking points for STATs. STATs are cytoplasmic transcription factors activated subsequent to JAK activation. The tyrosine phosphorylation of STATs by JAKs induces their dimerization and later their translocation into the nucleus, in which they function as gene regulators (Figure 1).11,12
Figure 1.
Activation and regulation of the JAK–STAT pathway. Cytokine binding to specific surface receptors causes receptor dimerization and results in JAK activation and subsequent phosphorylation of specific tyrosine residues on the intracytoplasmic region of the cytokine receptor. Phosphorylated tyrosine residues serve as docking sites for STATs, which bind these sites through their Src homology 2 (SH2) domains. Once recruited, STATs are phosphorylated on specific tyrosine residues by JAKs, which allows for their dimerization. STAT homodimers or heterodimers translocate to the nucleus, in which they recognize specific sequences such as the γ-activating sequence. Along with transcriptional co-activators and enhancers, STATs direct the transcription of their target genes. Many layers of control exist in the JAK– STAT pathway, such as protein inhibitors of activated STATs and protein tyrosine phosphatases. Although tyrosine phosphatases (PTPs) function to dephosphorylate JAKs and STATs, SOCS proteins can work in a variety of mechanisms, including direct inhibition of tyrosine activity and by competing with STATs for phosphotyrosine binding sites. PIAS proteins, on the other hand, can interfere with STAT DNA binding or may act as transcriptional corepressors through their interaction with histone deacetylases.10
The JAK family kinases include four members (JAK1, JAK2, JAK3 and TYK2 (tyrosine kinase 2)) in mammals, whereas there are seven STATs (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B and STAT6). JAKs contain seven homology motifs, including a pseudokinase domain (JH2) in addition to their tyrosine kinase domain (JH1).13–15 STATs are characterized by the high similarities in their DNA-binding region and in their SH2 (Src homology 2) domain, responsible for activation and dimerization of STATs.16,17 The carboxy terminus of STATs, known as the transactivation domain, contains highly conserved residues and mediates the recruitment of molecules such as histone deacetylases, DNA polymerase II, as well as co-activators of transcription.18 The amino terminus, on the other hand, is involved in STAT regulation such as dephosphorylation or tetramer formation.19–22
Mast cells are the only fully differentiated hematopoietic-derived cells to highly express the stem cell factor (SCF) receptor c-kit (CD117) on their surface. Binding of SCF to c-kit on the mast cell surface induces JAK2 activation. JAK2 activation can result in phosphorylation of several STATs in various cell types, including STAT1a, STAT3, STAT5A, STAT5B and STAT6.9,23–27 Specifically, SCF-induced activation of JAK2 in human mast cells has been shown to activate STAT5 and STAT6.27 STAT5 activation in mast cells promotes their development, survival and proliferation.8,24,28 Another molecule particularly important for mast cell expansion is interleukin-3 (IL-3), which activates JAK229 as well as STAT3 and STAT5 in mast cells and is critical in regulating the mast cell response against intestinal pathogens.30 It has been suggested that IL-3 and SCF may share overlapping or synergistic functions in mast cells, possibly due to overlapping activation of STAT5.8,30,31
Regulation of JAKs
JAKs undergo a variety of specific post-translational modifications directly affecting their activation status. In fact, once activated by phosphorylation, JAKs represent a target for suppressors of cytokine signaling (SOCS) and protein tyrosine phosphatases. SOCS are extensively studied inhibitors of the JAK–STAT pathway in mast cells. It has been shown that the SOCS family includes eight members (SOCS1 to SOCS7 and CIS (cytokine-induced SH2 domain)). SOCS1–3 and CIS are present in unstimulated mast cells at very low levels and are rapidly upregulated after stimulation, forming a negative feedback loop resulting in inhibition of the JAK–STAT signaling pathway.32,33
SOCS1 directly binds to the SH2 domain of activated JAKs, inhibiting JAK activation, whereas SOCS3, after activation by JAKs on its Tyr203 and Tyr221 residues, binds to the intracellular portion of the activated cytokine receptor, preventing recruitment of JAKs (Figure 1).34–38 On the other hand, CIS proteins have been shown to act as competitors to the activated receptor docking sites for STATs.39 Some studies have shown that both SOCS1 and SOCS3 were activated by IL-6 and IFNγ and as a result, they were able to inhibit IL-6 and IFNγ-induced JAK–STAT signaling.10 SOCS proteins have also been reported to be involved in the degradation of signaling molecules such as JAKs and STATs through the ubiquitin-proteasome pathway. In fact, it has been suggested that the SOCS box, a domain of conserved amino acids in the carboxy terminus of over 30 proteins but first identified in SOCS proteins, binds to Elongins B and C. Elongins B and C are parts of the ubiquitin E3 ligase, which catalyzes the transfer of ubiquitin to target molecules, marking them for degradation.34,39–41 As an example, many studies reported that JAK2 is degraded through the SOCS1-mediated ubiquitin-proteasome pathway.10
Regulation of STATs
Similar to JAKs, STATs are post-translationally modified by tyrosine and/or serine phosphorylation, methylation, acetylation, attachment of small ubiquitin-related modifier proteins (SUMOylation), ISGylation and ubiquitylation.42 After cytokine binding and JAK activation, STATs are recruited to the docking sites of the intracellular portion of the cytokine receptor and phosphorylated. Generally, STATs are activated by phosphorylation on specific tyrosine residues, which is required for their dimerization and translocation to the nucleus. Although tyrosine phosphorylation of STATs is well studied and documented, less is known about the importance of serine phosphorylation of STATs. It has been reported that serine phosphorylation of STATs is independent of their tyrosine phosphorylation.43,44 Furthermore, several studies have shown that serine phosphorylation of STAT1 seems to enhance its transcriptional activity,39,45 whereas serine phosphorylation of STAT3 has been shown to negatively regulate the tyrosine phosphorylation of this molecule.46 Numerous serine kinases, such as Erk, p38 and JNK, have been shown to be involved in the serine phosphorylation of STATs.41,46,47
STAT activity can be cytoplasmically and nuclearly inhibited by protein tyrosine phosphatases such as SHP2, which can remove the phosphate group present on the tyrosine residue of the activated STAT, resulting in the inhibition of STAT and a return to its cytoplasmic and inactive form (Figure 1). In addition, similar to JAKs, STAT proteins can be inhibited by specific interactions with suppressor molecules of the protein inhibitor of activated STAT (PIAS) family. There are five members of the PIAS family in mammals that, unlike members of the SOCS family, are constitutively expressed.42 These proteins can inhibit STATs by blocking their DNA binding sites, or by acting as ligases in facilitating SUMOylation, a process similar to ubiquitination.42,48 Several studies have also reported cross-talk between the JAK-STAT pathway and many other intracellular signaling pathways such as the nuclear factor-KB pathway.49 In fact, SOCS1 is known to interact with IRAK (IL-1R–associated-kinase), a key molecule in the nuclear factor-KB pathway. This interaction might be responsible for the inhibitory effect of SOCS1 on lipopolysaccharide-induced activation of the nuclear factor-KB pathway.50 In addition, similar to JAKs, STATs can be regulated through ubiquitination and subsequent degradation. The ubiquitin E3 ligase, SLIM, has been reported to not only target STATs, namely STAT1 and STAT4, but also to inhibit tyrosine phosphorylation of STATs.51
An additional layer of regulation of STATs is the production of different isoforms through alternative splicing and proteolytic processing. This is of particular interest in mast cells as there has been shown to be a mast cell-specific isoform of STAT6 that is truncated at the C-terminal end and lacks a distinct transactivation domain.52 Interestingly, it is this truncated isoform, not the full-length STAT6, also present in mast cells, that is the main respondent to IL-4 signaling, perhaps mediating specific effects of this cytokine in this highly specialized cell type.52 The mast cell-specific STAT6 isoform is generated through proteolytic cleavage, rather than through alternative splicing, and the protease has been shown to be in the elastase family of proteases.53,54
Although the JAK-STAT pathway is known to be one of the major regulatory pathways downstream of cytokine receptors and despite the fact that the general mechanisms by which this pathway is regulated have been cited, more challenging work is left to be performed to fully understand its importance in mast cell homeostasis. A better understanding of the JAK-STAT regulatory pathway may lead to the identification of key therapeutic targets and would ultimately lead to better drugs designed specifically to inhibit these molecules.
Targets of STATs
STATs have been implicated in the transcriptional activation of target genes involved in cancer, including c-myc and (Bcl-xL),55,56 as well as cytokine signaling in inflammatory diseases.57 Mast cells have an important role in allergic responses and are major factors in mastocytosis, mast cell leukemia and mast cell sarcoma. There is likely a link between STAT-mediated activation of oncogenes and mast cell-related cancers. However, much remains to be done in the area of STAT target genes, specifically in mast cells.
STATs are activated through phosphorylation at their tyrosine residue, not only by cytokine-associated kinases such as the JAKs, but also by oncogenic non-receptor tyrosine kinases such as the translocation responsible for the Philadelphia chromosome, BCR-ABL.58 Upon activation, STATs hetero- or homodimerize, enter the nucleus and bind a specific DNA sequence in the promoter of target genes to activate gene transcription. STAT homo-dimers bind to members of the GAS (γ-activating sequence) family enhancer motif with the sequence TTTCCNGGAAA, in which N is any nucleotide.12 The GAS consensus sequence can be used as an indicator of potential STAT-regulated genes. For example, a STAT5-binding element in the SLP-76 (SH2 domain-containing leukocyte protein of 76 kDa)-related adaptor protein MIST (mast cell immunoreceptor signal transducer, also termed Clnk) promoter was identified. Under the control of a constitutively active form of STAT5a, endogenous MIST mRNA expression was enhanced.59 Interactions with other transcription cofactors and/or co-activators also contribute to STAT-mediated expression of target genes. Some proteins involved in this include Sp1,60 c-Jun61 and CBP/p300.62–64
Studies regarding the regulation of proliferation and apoptosis in a variety of cell types have identified a major role for STATs in targeting apoptotic genes including those of the Bcl-2 family (Figure 1). STATs 1, 3 and 5 are known to bind the bcl-x gene promoter and activate Bcl-xL in erythropoietin progenitor cells, red blood cell progenitors, bone marrow cells, chronic myelogenous leukemia cells and myeloma cells.55,65–68 The cytokines IL-3, which signals through both STAT3 and STAT5, and SCF, which signals through STAT5 and STAT6 in mast cells, prevent apoptosis by upregulating Bcl-2 expression in mast cells.69,70 In a study by Shelburne et al.,7 cultured murine bone marrow-derived mast cells (BMMCs) deficient in STAT5 were unable to proliferate and died in the presence of IL-3 or SCF alone, suggesting that STAT5 has a role in cell survival. Upon further examination, expression of Bcl-2 and Bcl-xL mRNA and protein levels were nearly absent in STAT5-deficient cultures. In addition, STAT5-deficient cultures expressed increased active caspases 9 and 3 in comparison to wild-type BMMCs when cultured with either IL-3 or SCF. This study identified STAT5 as having a major role in mast cell survival and proliferation as well as implicating STAT5 in the transcription of Bcl-2 or Bcl-xL.7 Ikeda et al.71 further studied the role of STAT5 in murine mast cell development in vivo. Although BMMCs from STAT5a−/− mice develop normally, expressing normal levels of c-kit, FcγR II/III and the IgE receptor, these mice show significantly decreased mast cell numbers in the peritoneal cavity when compared with wild-type mice. Survival of peritoneal mast cells ex vivo, as well as BMMCs, is also decreased in STAT5a-deficient mice, which is evident by an increase in the number of apoptotic cells, and decrease in Bcl-xL mRNA expression. These results further suggest a role for STAT5a in vivo in the suppression of apoptosis through regulation of the antiapoptotic gene Bcl-xL.71
Human mast cell survival also seems to be regulated by Bcl-2 and Bcl-xL. When SCF is removed from the culture media, apoptosis is induced and the levels of Bcl-2 and Bcl-xL proteins are decreased. In the SCF-independent human mast cell lines HMC-1.1 and HMC-1.2, Bcl-xL and Bcl-2 protein levels are significantly elevated, possibly explaining the ability of these cells to persist in the absence of signaling elicited by SCF.69 The similarity between the decreases observed in human mast cell Bcl-xL expression upon removal of SCF69 and murine mast cell Bcl-xL expression71 suggests a similar mechanism regulating the Bcl-xL gene, namely STAT5.
Examination of IL-15 in the mouse mast cell line MC/9 and BMMCs identified a role for STAT6 in regulating Bcl-xL expression and apoptosis. With activation by IL-15, STAT6 is able to bind the bcl-xl gene promoter and activate Bcl-xL expression, as identified through electrophoretic mobility shift assay and transfection of a dominant-negative STAT6, respectively. When expressed in MC/9 cells, the dominant-negative STAT6 suppressed Bcl-xL mRNA expression and induced apoptosis,72 thus implicating STAT6 as another regulator of Bcl-xL expression in mast cells.
The role that STAT5 and STAT6 seem to have in regulating the antiapoptotic Bcl-2 family in mast cells has potential implications in cancer development. STATs have been identified to be constitutively active in a number of cancers, promoting cell survival, angiogenesis and proliferation.73,74 Although little has been studied as to the specific STAT target genes in mast cells, complementary DNA array data and identification of potential binding sites of STATs in promoters of genes related to survival and proliferation suggest that there are additional targets for STATs. Other potential genes include VEGF (vascular endothelial growth factor), pim-1, and cyclin D3 (JJ Ryan and KD Bunting, unpublished data;75). It is interesting that although STATs have been implicated as having significant roles in mast cell survival and proliferation, little has been uncovered with regard to specific target genes, and it is an area that should be addressed.
STATs in allergic disease
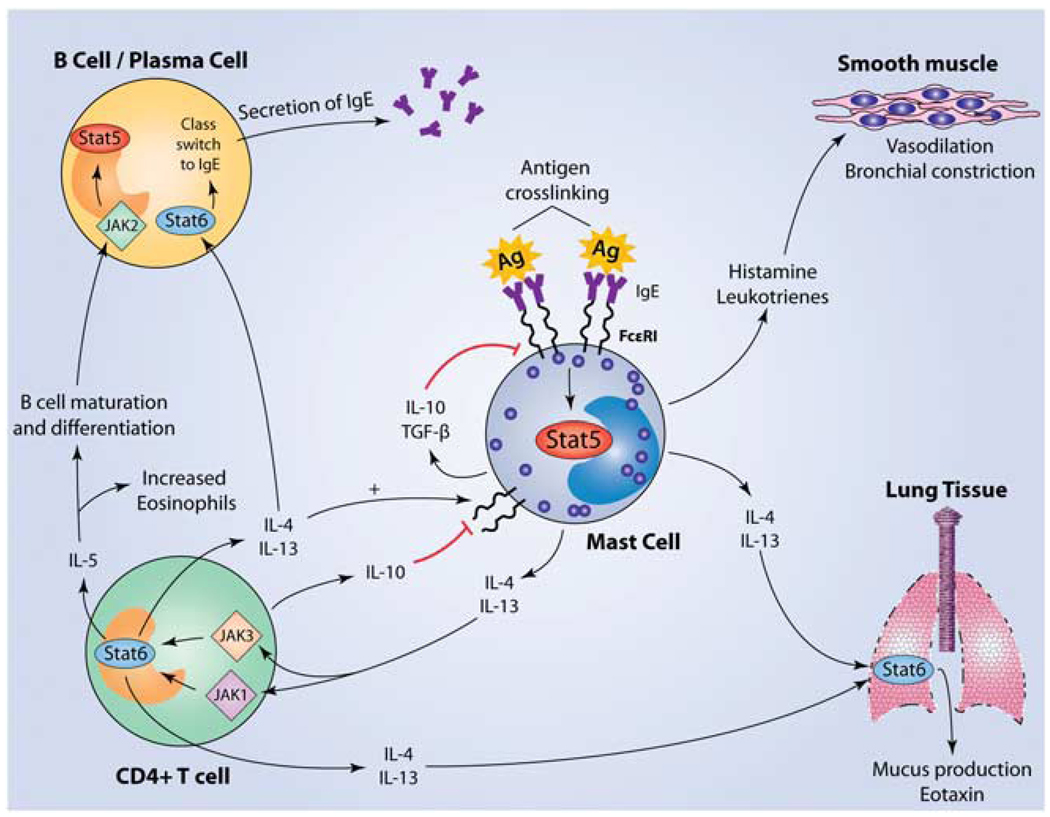
Given the fact that the JAK-STAT pathway has a significant role in maintaining the growth and survival of mast cells, which are the principal mediators of allergic disease, it is reasonable to hypothesize that alterations in JAK-STAT signaling in mast cells can affect allergic disease and asthma. Allergic disease is mediated through antigen-specific IgE molecules that are constitutively associated with FcεRI on the surface of mast cells. Crosslinking of these IgE molecules by specific antigen induces the release of inflammatory mediators and vasodilators such as histamine, leukotrienes and prostaglandins within minutes of exposure, resulting in a T helper 2 (Th2)-mediated allergic response (Figure 2).76 With 50 to 60 million Americans suffering from allergies and asthma every year, the elucidation of the role of JAK-STAT signaling in mast cells is of great importance in the development of therapeutic targets.7,8,77–79
Figure 2.
Activation of mast cells initiates a cascade of events resulting in Th2 polarization and both positive and negative feedback regulatory loops. Mast cells are activated when antigen binds to specific IgE molecules bound to FcεRI receptors on the surface of the mast cell. Subsequent crosslinking results in signaling through the IgE receptor and activation of multiple pathways, including the JAK–STAT pathway. STAT5 has been shown to be required for IgE-mediated cytokine production as well as histamine and leukotriene release.82 Although histamine and leukotrienes cause the early-phase response through vasodilation and bronchial constriction, cytokines such as IL-4 and IL-13 mediate the late-phase response. IL-4 and IL-13 share the ability to signal through the IL-4Rα, which is associated with JAKs 1 and 3, leading to the activation of STAT6. STAT6 activation leads to polarization toward a T helper 2 phenotype, resulting in the release of cytokines such as IL-10, IL-6, IL-5, IL-9, IL-4 and IL-13. IL-4 and IL-13 can both signal through STAT6 in B cells, inducing isotype switching and subsequent production of IgE, which can bind FcεRI on mast cells.113–115 Unlike the allergy-promoting effects of IL-4 on B cells, IL-10 has an inhibitory effect on mast cells, leading to the suppression of both c-kit and FcεRI expression, accompanied by the induction of apoptosis.116–122 Mast cell-derived transforming growth factor-β (TGF-β) also acts as a negative regulator, working in an autocrine manner on mast cells.123–126 IL-5, on the other hand, supports the differentiation, survival and proliferation of both B cells and eosinophils, partly mediated through JAK2–STAT5 signaling, in conjunction with other pathways.127,128 Further recruitment of eosinophils occurs through production of the chemokine eotaxin, largely by cells of the airway epithelia. Eotaxin can be induced by the cytokines IL-4, IL-13 and tumor necrosis factor-α (TNF-α), which are derived from Th2 cells and possibly directly from mast cells themselves.129–132
Of the family of STAT proteins, STAT4 and STAT6 are particularly important in the differentiation and subsequent modulation of T helper cell responses. T helper cells differentiate into Th1 and Th2 cells in response to the cytokine milieu. A Th1 response is elicited by the cytokines IL-12 and IL-23, which signal through JAK2 and TYK2 that, in turn, phosphorylate STAT4.80 In contrast, Th2 cells are elicited by IL-4 and IL-13 activation of JAK1 and JAK3, which subsequently phosphorylate STAT6 (Figure 2).81 Although STAT4 and STAT6 have importance in the development of the Th1 and Th2 lineages, STAT5 is critical for mast cell function. Barnstein et al.82 have shown that BMMCs lacking STAT5 have a 50% reduction in the release of histamine and leukotriene B4 after antigen crosslinking of FcεRI. STAT5 was also shown to be necessary for the production of IL-6, IL-13 and tumor necrosis factor-α in the late phase of mast cell activation, with STAT5 knockout (KO) BMMCs producing only 5–10% of the wild-type levels of these cytokines.82 Therefore, JAK-STAT signaling is capable of contributing to allergic disease directly by facilitating the release of mast cell mediators, and indirectly through skewing immunity toward a Th2 response.
The implication of STAT6 in Th2 cell responses has been best studied by generating STAT6 KO mice, which fail to mount a Th2 response in vivo as well as in vitro. STAT6 has a pivotal role in the differentiation, memory, development and survival of Th2 cells.83–85 It has a unique role in the production of IgE and mucus in the airway hyper-responsiveness (AHR) model. The development of airway hyper-responsiveness in STAT6 KO mice shows varying responses in different strains of mice. In Balb/c mice, eosinophils are decreased by 50%, whereas in C57BL/6 mice, they are completely blocked.85 Mathew et al.83 report that the development of allergic pulmonary inflammation requires the activation of STAT6, not only in T cells, but also in the parenchymal cells of the lungs because of the fact that transfer of antigen-specific Th2 cells from STAT6 wild-type mice to STAT6 KO mice did not effectively elicit airway hyper-responsiveness. This inadequate response is attributed to a defect in the production of eotaxin, resulting in impaired Th2 cell chemotaxis to the lungs (Figure 2). STAT6 is also required in mucus production, as STAT6 KO mice did not manifest goblet cell hyperplasia and have decreased mucus secretion in response to the ovalbumin challenge asthma model.86 Again, the transfer of antigen-specific STAT6 + / + T cells failed to compensate for this defect, implying STAT6 within the lung as a key mediator of mucus production.83 Thus, STAT6 has a central role in the pathogenesis of asthma.
JAKs and STATs in mastocytosis
Mastocytosis is a broad term used to define abnormalities caused by increased proliferation and subsequent accumulation of mast cells. Mastocytosis is one of several types of chronic myeloproliferative neoplasms (MPNs), or clonal disorders of hematopoietic cells. According to the newly revised World Health Organization classification, mastocytosis is now classified as a MPN, as opposed to a chronic myeloproliferative disease under the previous nomenclature. Other MPNs include diseases such as chronic neutrophilic leukemia, polycythemia vera and primary myelofibrosis.87 There are two main types of mastocytosis: cutaneous mastocytosis and systemic mastocytosis (SM). Cases of cutaneous mastocytosis most often involve urticaria pigmentosa and have an overall favorable prognosis. Cases of SM can be further subdivided into indolent SM (of which there are two types: smoldering and isolated bone marrow mastocytosis), SM with an associated hematologic disorder, aggressive SM and mast cell leukemia.87–89 In a large retrospective study conducted using 342 SM patients spanning 31 years at the Mayo Clinic, it was found that the median survival rate of individuals with mast cell leukemia was only 2 months, which was substantially lower than that of patients with SM with an associated hematologic disorder and aggressive SM, who had median survival rates of 24 and 41 months, respectively. Overall, there is approximately a 6% rate of leukemic (acute and myeloid) transformation among patients with mastocytosis.89 Interestingly, the median survival of patients with indolent SM was not substantially different from that of the normal age- and sex-matched population.89
Identifying specific mutations that may lead to abnormal mast cell accumulation is critical for the development of therapeutics to treat these diseases. One of the most common mutations occurring in over 90% of MPNs is the substitution of a valine for a phenylalanine at position 617 of the JAK2 molecule,90 which was first noted by James et al.91 in 2005. This mutation lies within the JAK2 pseudokinase (JH2) domain, which is required for the inhibition of basal kinase activity,90,92 thus leading to constitutive tyrosine kinase activity and cytokine hypersensitivity.91 Patients with MPNs who were homozygous for the JAK2 mutation had a longer symptomatic duration of disease than those who were heterozygous for the mutation, whereas MPN patients lacking this mutation had the shortest duration of disease.93 STAT5 is a major target of JAK2 in hematopoietic cells, and accordingly, activating mutations in JAK2 have been shown to lead to constitutive phosphorylation of STAT5.90,94 Increased phospho-STAT5 has also been shown to be concurrent with increased levels of phosphorylated AKT.90,95 AKT is a serine threonine kinase whose activation leads to increases in cellular growth and survival. AKT is a major target of the phosphoinositide 3-kinase pathway and is aberrantly activated in a wide variety of malignancies.96 In fact, immunoprecipitation studies have shown co-precipitation of STAT5 with the p85 subunit of AKT, suggesting a cross-talk between the JAK–STAT pathway and the phosphoinositide 3-kinasepathway.95,97 STAT5 activation as a result of JAK2 mutation has also been found to influence cell cycle transition, given that constitutively active STAT5 leads to a reduction of the cell cycle inhibitor p27kip with a concomitant increase in cylcin D2. Inhibition of JAK2 causes reduced STAT5 phosphorylation and cell cycle arrest in the G1 phase, presumably because of an observed increase in p27kip and decrease in cyclin D2.94
Increased STAT5 was also shown to be associated with a different mutation, the substitution of an aspartic acid to a valine residue at codon 816 in the c-kit receptor itself, an alteration found in over 90% of cases of systemic mastocytosis.95,97 This mutation was first characterized by Furitsu et al.98 in the HMC-1 cells line and later noted in patients by Nagata et al.99 in 1995. It was later shown that this c-kit mutation leads to low, but constitutive, levels of phosphorylated tyrosine residues at positions 568, 703, 721, 823 and 936 on c-kit.97,100 These levels of autophosphorylation of the c-kit receptor ultimately lead to constitutive phosphorylation of key downstream mediators, including AKT, extracellular signal-regulated kinase, and the adaptor molecules Shc and Gab2. In addition, the p85 subunit of AKT, which associates with the phosphorylated tyrosine at residue 721, was shown to be constitutively bound to the mutated form of c-kit, independent of ligand activation with SCF.97 Similar to STAT5 activation resulting from gain-of-function JAK2 mutations, phosphorylated STAT5 resulting from the D816V mutation was also found to physically associate with AKT in the cytoplasm.95 Clinically, neoplastic mast cells in SM patients have been shown to unanimously express elevated levels of phosphorylated STAT5 found in both the cytoplasm and the nucleus of mast cells, an effect that was attributed to D816V c-kit signaling and blunted by inhibition of D816V kinase activity.101
The identification of specific mutations leading to mastocytosis and other myeloproliferative disorders has resulted in emerging therapies to precisely target the causes of these disorders. Currently, imatinib is a widely used tyrosine kinase inhibitor that has shown considerable success in the treatment of chronic myeloid leukemia, characterized by the presence of the Philadelphia chromosome, resulting in the bcr-abl fusion protein. Imatinib is a competitive inhibitor that functions at the ATP binding site of BCR-ABL102 and also works by binding to the inactive form of the receptor tyrosine kinase, c-kit, which is found on hematopoietic progenitor cells as well as other cells such as mast cells and melanocytes.88,97 As imatinib binds to the c-kit activation loop when it is in its inactive form, mutations in the activation loop, such as the D816V mutation, alter the binding site for imatinib, rendering it less effective.103,104 A newer tyrosine kinase inhibitor, dasatinib, which was approved for use in chronic myeloid leukemia patients in 2006, is also an ATP competitive inhibitor. Unlike imatinib, dasatinib has dual specificity for both the SRC and ABL kinases and has shown efficacy against the mutated forms of c-kit in cases of mastocytosis harboring the D816V c-kit mutation.102–104 This drug has been used in phase II trials for the treatment of SM with beneficial, but not curative, responses.105 When combined with chemotherapy, this drug seems promising for the treatment of D816V + SM patients with AML. Dasatinib may also be used in combination with other tyrosine kinase inhibitors such as PKC412 for the treatment of D816V + neoplastic mast cells.106,107 PKC412 is a broad-spectrum inhibitor of serine, threonine and tyrosine kinases and is being investigated for use in a variety of malignancies, including use in SM and related hematological disorders.107–110 Although tyrosine kinase inhibitors effective against the D816V mutation are making progress, therapeutics against the more recently discovered JAK2 V617F mutation are still in the early stages of drug approval. A particular compound from Incyte INCB018424 has shown promising results, having recently completed phase II clinical trials. Although compounds targeting this mutation have shown promise in alleviating symptoms, no curative effects have been observed thus far.111,112 Whether or not inhibition of JAK2 alone may be effective in reducing disease in individuals harboring this mutation is currently the topic of much debate, as several companies find themselves in a race toward approval by the Food and Drug Administration.
Conclusion
The JAK–STAT pathway is central to mediating the responses of a variety of immune cells, including mast cells. STAT5, in particular, has been shown by our group to be of critical importance in mast cells, as it is the downstream target of both FcεRI and the c-kit receptor. The importance of JAKs and STATs in mast cells is highlighted by the consequences of mutations in these molecules, such as those that occur in mastocytosis. Although the JAK–STAT pathway has been well characterized in other immune cells, there is much work to be done in identifying specific targets of this pathway in mast cells. Particular alterations in JAKs and STATs and their regulators that lead to allergic disease and asthma require further investigation so that they may be exploited for therapeutic purposes.
Acknowledgements
This study was supported by grants from the National Institutes of Health (1R01AI59638 and U19AI077435).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy DE, Kessler DS, Pine R, Reich N, Darnell JE., Jr Interferon-induced nuclear factors that bind a shared promoter element correlate with positive and negative transcriptional control. Genes Dev. 1988;2:383–393. doi: 10.1101/gad.2.4.383. [DOI] [PubMed] [Google Scholar]
- 3.Rutherford MN, Hannigan GE, Williams BR. Interferon-induced binding of nuclear factors to promoter elements of the 2-5A synthetase gene. EMBO J. 1988;7:751–759. doi: 10.1002/j.1460-2075.1988.tb02872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schindler C, Shuai K, Prezioso VR, Darnell JE., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992;257:809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- 5.Shuai K, Schindler C, Prezioso VR, Darnell JE., Jr Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992;258:1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- 6.Velazquez L, Fellous M, Stark GR, Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992;70:313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- 7.Shelburne CP, McCoy ME, Piekorz R, Sexl V, Roh KH, Jacobs-Helber SM, et al. Stat5 expression is critical for mast cell development and survival. Blood. 2003;102:1290–1297. doi: 10.1182/blood-2002-11-3490. [DOI] [PubMed] [Google Scholar]
- 8.Shelburne CP, McCoy ME, Piekorz R, Sexl VV, Gillespie SR, Bailey DP, et al. Stat5: an essential regulator of mast cell biology. Mol Immunol. 2002;38:1187–1191. doi: 10.1016/s0161-5890(02)00061-5. [DOI] [PubMed] [Google Scholar]
- 9.Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 10.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3:900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- 11.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 12.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 13.Harpur AG, Andres AC, Ziemiecki A, Aston RR, Wilks AF. JAK2, a third member of the JAK family of protein tyrosine kinases. Oncogene. 1992;7:1347–1353. [PubMed] [Google Scholar]
- 14.Wilks AF, Harpur AG, Kurban RR, Ralph SJ, Zurcher G, Ziemiecki A. Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol Cell Biol. 1991;11:2057–2065. doi: 10.1128/mcb.11.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giordanetto F, Kroemer RT. Prediction of the structure of human Janus kinase 2 (JAK2) comprising JAK homology domains 1 through 7. Protein Eng. 2002;15:727–737. doi: 10.1093/protein/15.9.727. [DOI] [PubMed] [Google Scholar]
- 16.Shuai K, Horvath CM, Huang LH, Qureshi SA, Cowburn D, Darnell JE., Jr Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell. 1994;76:821–828. doi: 10.1016/0092-8674(94)90357-3. [DOI] [PubMed] [Google Scholar]
- 17.Fu XY, Zhang JJ. Transcription factor p91 interacts with the epidermal growth factor receptor and mediates activation of the c-fos gene promoter. Cell. 1993;74:1135–1145. doi: 10.1016/0092-8674(93)90734-8. [DOI] [PubMed] [Google Scholar]
- 18.Schindler C, Plumlee C. Inteferons pen the JAK-STAT pathway. Semin Cell Dev Biol. 2008;19:311–318. doi: 10.1016/j.semcdb.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shuai K, Stark GR, Kerr IM, Darnell JE., Jr A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science. 1993;261:1744–1746. doi: 10.1126/science.7690989. [DOI] [PubMed] [Google Scholar]
- 20.Shuai K, Liao J, Song MM. Enhancement of antiproliferative activity of gamma interferon by the specific inhibition of tyrosine dephosphorylation of Stat1. Mol Cell Biol. 1996;16:4932–4941. doi: 10.1128/mcb.16.9.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, Sun YL, Hoey T. Cooperative DNA binding and sequence-selective recognition conferred by the STAT amino-terminal domain. Science. 1996;273:794–797. doi: 10.1126/science.273.5276.794. [DOI] [PubMed] [Google Scholar]
- 22.Muller M, Laxton C, Briscoe J, Schindler C, Improta T, Darnell JE, et al. Complementation of a mutant cell line: central role of the 91 kDa polypeptide of ISGF3 in the interferon-alpha and -gamma signal transduction pathways. EMBO J. 1993;12:4221–4228. doi: 10.1002/j.1460-2075.1993.tb06106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brizzi MF, Dentelli P, Gambino R, Cabodi S, Cassader M, Castelli A, et al. STAT5 activation induced by diabetic LDL depends on LDL glycation and occurs via src kinase activity. Diabetes. 2002;51:3311–3317. doi: 10.2337/diabetes.51.11.3311. [DOI] [PubMed] [Google Scholar]
- 24.Ryan JJ, Huang H, McReynolds LJ, Shelburne C, Hu-Li J, Huff TF, et al. Stem cell factor activates STAT-5 DNA binding in IL-3-derived bone marrow mast cells. Exp Hematol. 1997;25:357–362. [PubMed] [Google Scholar]
- 25.Gotoh A, Takahira H, Mantel C, Litz-Jackson S, Boswell HS, Broxmeyer HE. Steel factor induces serine phosphorylation of Stat3 in human growth factor-dependent myeloid cell lines. Blood. 1996;88:138–145. [PubMed] [Google Scholar]
- 26.Brizzi MF, Dentelli P, Rosso A, Yarden Y, Pegoraro L. STAT protein recruitment and activation in c-Kit deletion mutants. J Biol Chem. 1999;274:16965–16972. doi: 10.1074/jbc.274.24.16965. [DOI] [PubMed] [Google Scholar]
- 27.Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FcepsilonRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104:2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- 28.Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leonard WJ. Type I cytokines and interferons and their receptors. In: Paul WE, editor. Fundamental Immunology. 5th edn. Philadelphia: Lippincott Williams and Wilkins; 2003. p. 701. [Google Scholar]
- 30.Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 31.Shivakrupa R, Linnekin D. Lyn contributes to regulation of multiple Kit-dependent signaling pathways in murine bone marrow mast cells. Cell Signal. 2005;17:103–109. doi: 10.1016/j.cellsig.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Alexander WS. Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol. 2002;2:410–416. doi: 10.1038/nri818. [DOI] [PubMed] [Google Scholar]
- 33.Greenhalgh CJ, Hilton DJ. Negative regulation of cytokine signaling. J Leukoc Biol. 2001;70:348–356. [PubMed] [Google Scholar]
- 34.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 35.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, et al. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 36.Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki A, Yasukawa H, Shouda T, Kitamura T, Dikic I, Yoshimura A. CIS3/SOCS-3 suppresses erythropoietin (EPO) signaling by binding the EPO receptor and JAK2. J Biol Chem. 2000;275:29338–29347. doi: 10.1074/jbc.M003456200. [DOI] [PubMed] [Google Scholar]
- 38.Nicholson SE, Willson TA, Farley A, Starr R, Zhang JG, Baca M, et al. Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. EMBO J. 1999;18:375–385. doi: 10.1093/emboj/18.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 40.Zhang JG, Metcalf D, Rakar S, Asimakis M, Greenhalgh CJ, Willson TA, et al. The SOCS box of suppressor of cytokine signaling-1 is important for inhibition of cytokine action in vivo. Proc Natl Acad Sci USA. 2001;98:13261–13265. doi: 10.1073/pnas.231486498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 42.Lim CP, Cao X. Structure, function, and regulation of STAT proteins. Mol Biosyst. 2006;2:536–550. doi: 10.1039/b606246f. [DOI] [PubMed] [Google Scholar]
- 43.Zhu X, Wen Z, Xu LZ, Darnell JE., Jr Stat1 serine phosphorylation occurs independently of tyrosine phosphorylation and requires an activated Jak2 kinase. Mol Cell Biol. 1997;17:6618–6623. doi: 10.1128/mcb.17.11.6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovarik P, Stoiber D, Novy M, Decker T. Stat1 combines signals derived from IFN-gamma and LPS receptors during macrophage activation. EMBO J. 1998;17:3660–3668. doi: 10.1093/emboj/17.13.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horvath CM, Darnell JE., Jr The antiviral state induced by alpha interferon and gamma interferon requires transcriptionally active Stat1 protein. J Virol. 1996;70:647–650. doi: 10.1128/jvi.70.1.647-650.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung J, Uchida E, Grammer TC, Blenis J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol. 1997;17:6508–6516. doi: 10.1128/mcb.17.11.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuringa JJ, Jonk LJ, Dokter WH, Vellenga E, Kruijer W. Interleukin-6-induced STAT3 transactivation and Ser727 phosphorylation involves Vav, Rac-1 and the kinase SEK-1/ MKK-4 as signal transduction components. Biochem J. 2000;347(Part 1):89–96. [PMC free article] [PubMed] [Google Scholar]
- 48.Kotaja N, Karvonen U, Janne OA, Palvimo JJ. PIAS proteins modulate transcription factors by functioning as SUMO-1 ligases. Mol Cell Biol. 2002;22:5222–5234. doi: 10.1128/MCB.22.14.5222-5234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 50.Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, et al. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677–687. doi: 10.1016/s1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka T, Soriano MA, Grusby MJ. SLIM is a nuclear ubiquitin E3 ligase that negatively regulates STAT signaling. Immunity. 2005;22:729–736. doi: 10.1016/j.immuni.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Sherman MA, Secor VH, Brown MA. IL-4 preferentially activates a novel STAT6 isoform in mast cells. J Immunol. 1999;162:2703–2708. [PubMed] [Google Scholar]
- 53.Suzuki K, Nakajima H, Ikeda K, Tamachi T, Hiwasa T, Saito Y, et al. Stat6-protease but not Stat5-protease is inhibited by an elastase inhibitor ONO-5046. Biochem Biophys Res Commun. 2003;309:768–773. doi: 10.1016/j.bbrc.2003.08.067. [DOI] [PubMed] [Google Scholar]
- 54.Shelburne CP, Piekorz RP, Bouton LA, Chong HJ, Ryan JJ. Mast cell-restricted p70 Stat6 isoform is a product of selective proteolysis. Cytokine. 2002;19:218–227. [PubMed] [Google Scholar]
- 55.Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a−/−5b−/− mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 56.Xie S, Lin H, Sun T, Arlinghaus RB. Jak2 is involved in c-Myc induction by Bcr-Abl. Oncogene. 2002;21:7137–7146. doi: 10.1038/sj.onc.1205942. [DOI] [PubMed] [Google Scholar]
- 57.Chen W, Khurana Hershey GK. Signal transducer and activator of transcription signals in allergic disease. J Allergy Clin Immunol. 2007;119:529–541. doi: 10.1016/j.jaci.2007.01.004. quiz 542–543. [DOI] [PubMed] [Google Scholar]
- 58.Carlesso N, Frank DA, Griffin JD. Tyrosyl phosphorylation and DNA binding activity of signal transducers and activators of transcription (STAT) proteins in hematopoietic cell lines transformed by Bcr/Abl. J Exp Med. 1996;183:811–820. doi: 10.1084/jem.183.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sasanuma H, Tatsuno A, Tsuji K, Hidano S, Morita S, Kitamura T, et al. Transcriptional regulation of SLP-76 family hematopoietic cell adaptor MIST/Clnk by STAT5. Biochem Biophys Res Commun. 2004;321:145–153. doi: 10.1016/j.bbrc.2004.06.126. [DOI] [PubMed] [Google Scholar]
- 60.Look DC, Pelletier MR, Tidwell RM, Roswit WT, Holtzman MJ. Stat1 depends on transcriptional synergy with Sp1. J Biol Chem. 1995;270:30264–30267. doi: 10.1074/jbc.270.51.30264. [DOI] [PubMed] [Google Scholar]
- 61.Schaefer TS, Sanders LK, Nathans D. Cooperative transcriptional activity of Jun and Stat3 beta, a short form of Stat3. Proc Natl Acad Sci USA. 1995;92:9097–9101. doi: 10.1073/pnas.92.20.9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhattacharya S, Eckner R, Grossman S, Oldread E, Arany Z, D’Andrea A, et al. Cooperation of Stat2 and p300/CBP in signalling induced by interferon-alpha. Nature. 1996;383:344–347. doi: 10.1038/383344a0. [DOI] [PubMed] [Google Scholar]
- 63.Zhang JJ, Vinkemeier U, Gu W, Chakravarti D, Horvath CM, Darnell JE., Jr Two contact regions between Stat1 and CBP/ p300 in interferon gamma signaling. Proc Natl Acad Sci USA. 1996;93:15092–15096. doi: 10.1073/pnas.93.26.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gingras S, Simard J, Groner B, Pfitzner E. p300/CBP is required for transcriptional induction by interleukin-4 and interacts with Stat6. Nucleic Acids Res. 1999;27:2722–2729. doi: 10.1093/nar/27.13.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silva M, Benito A, Sanz C, Prosper F, Ekhterae D, Nunez G, et al. Erythropoietin can induce the expression of bcl-x(L) through Stat5 in erythropoietin-dependent progenitor cell lines. J Biol Chem. 1999;274:22165–22169. doi: 10.1074/jbc.274.32.22165. [DOI] [PubMed] [Google Scholar]
- 66.Dumon S, Santos SC, Debierre-Grockiego F, Gouilleux-Gruart V, Cocault L, Boucheron C, et al. IL-3 dependent regulation of Bcl-xL gene expression by STAT5 in a bone marrow derived cell line. Oncogene. 1999;18:4191–4199. doi: 10.1038/sj.onc.1202796. [DOI] [PubMed] [Google Scholar]
- 67.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 68.Horita M, Andreu EJ, Benito A, Arbona C, Sanz C, Benet I, et al. Blockade of the Bcr-Abl kinase activity induces apoptosis of chronic myelogenous leukemia cells by suppressing signal transducer and activator of transcription 5-dependent expression of Bcl-xL. J Exp Med. 2000;191:977–984. doi: 10.1084/jem.191.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mekori YA, Gilfillan AM, Akin C, Hartmann K, Metcalfe DD. Human mast cell apoptosis is regulated through Bcl-2 and Bcl-XL. J Clin Immunol. 2001;21:171–174. doi: 10.1023/a:1011083031272. [DOI] [PubMed] [Google Scholar]
- 70.Mekori YA, Oh CK, Dastych J, Goff JP, Adachi S, Bianchine PJ, et al. Characterization of a mast cell line that lacks the extracellular domain of membrane c-kit. Immunology. 1997;90:518–525. doi: 10.1046/j.1365-2567.1997.00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ikeda K, Nakajima H, Suzuki K, Watanabe N, Kagami S, Iwamoto I. Stat5a is essential for the proliferation and survival of murine mast cells. Int Arch Allergy Immunol. 2005;137 Suppl 1:45–50. doi: 10.1159/000085431. [DOI] [PubMed] [Google Scholar]
- 72.Masuda A, Matsuguchi T, Yamaki K, Hayakawa T, Yoshikai Y. Interleukin-15 prevents mouse mast cell apoptosis through STAT6-mediated Bcl-xL expression. J Biol Chem. 2001;276:26107–26113. doi: 10.1074/jbc.M011475200. [DOI] [PubMed] [Google Scholar]
- 73.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 74.Xi S, Zhang Q, Dyer KF, Lerner EC, Smithgall TE, Gooding WE, et al. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem. 2003;278:31574–31583. doi: 10.1074/jbc.M303499200. [DOI] [PubMed] [Google Scholar]
- 75.Stout BA, Bates ME, Liu LY, Farrington NN, Bertics PJ. IL-5 and granulocyte-macrophage colony-stimulating factor activate STAT3 and STAT5 and promote Pim-1 and cyclin D3 protein expression in human eosinophils. J Immunol. 2004;173:6409–6417. doi: 10.4049/jimmunol.173.10.6409. [DOI] [PubMed] [Google Scholar]
- 76.Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001;344:30–37. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 77.Pernis AB, Rothman PB. JAK-STAT signaling in asthma. J Clin Invest. 2002;109:1279–1283. doi: 10.1172/JCI15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- 79.Decker T, Stockinger S, Karaghiosoff M, Muller M, Kovarik P. IFNs and STATs in innate immunity to microorganisms. J Clin Invest. 2002;109:1271–1277. doi: 10.1172/JCI15770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 81.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, et al. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 82.Barnstein BO, Li G, Wang Z, Kennedy S, Chalfant C, Nakajima H, et al. Stat5 expression is required for IgE-mediated mast cell function. J Immunol. 2006;177:3421–3426. doi: 10.4049/jimmunol.177.5.3421. [DOI] [PubMed] [Google Scholar]
- 83.Mathew A, MacLean JA, DeHaan E, Tager AM, Green FH, Luster AD. Signal transducer and activator of transcription 6 controls chemokine production and T helper cell type 2 cell trafficking in allergic pulmonary inflammation. J Exp Med. 2001;193:1087–1096. doi: 10.1084/jem.193.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaplan MH, Grusby MJ. Regulation of T helper cell differentiation by STAT molecules. J Leukoc Biol. 1998;64:2–5. doi: 10.1002/jlb.64.1.2. [DOI] [PubMed] [Google Scholar]
- 85.Akimoto T, Numata F, Tamura M, Takata Y, Higashida N, Takashi T, et al. Abrogation of bronchial eosinophilic inflammation and airway hyperreactivity in signal transducers and activators of transcription (STAT)6-deficient mice. J Exp Med. 1998;187:1537–1542. doi: 10.1084/jem.187.9.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyata S, Matsuyama T, Kodama T, Nishioka Y, Kuribayashi K, Takeda K, et al. STAT6 deficiency in a mouse model of allergen-induced airways inflammation abolishes eosinophilia but induces infiltration of CD8+ T cells. Clin Exp Allergy. 1999;29:114–123. doi: 10.1046/j.1365-2222.1999.00405.x. [DOI] [PubMed] [Google Scholar]
- 87.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 88.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–956. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113:5727–5736. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 90.Grimwade LF, Happerfield L, Tristram C, McIntosh G, Rees M, Bench AJ, et al. Phospho-STAT5 and phospho-Akt expression in chronic myeloproliferative neoplasms. Br J Haematol. 2009;147:495–506. doi: 10.1111/j.1365-2141.2009.07870.x. [DOI] [PubMed] [Google Scholar]
- 91.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 92.Luo H, Rose P, Barber D, Hanratty WP, Lee S, Roberts TM, et al. Mutation in the Jak kinase JH2 domain hyperactivates Drosophila and mammalian Jak-Stat pathways. Mol Cell Biol. 1997;17:1562–1571. doi: 10.1128/mcb.17.3.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 94.Walz C, Crowley BJ, Hudon HE, Gramlich JL, Neuberg DS, Podar K, et al. Activated Jak2 with the V617F point mutation promotes G1/S phase transition. J Biol Chem. 2006;281:18177–18183. doi: 10.1074/jbc.M600064200. [DOI] [PubMed] [Google Scholar]
- 95.Harir N, Boudot C, Friedbichler K, Sonneck K, Kondo R, Martin-Lanneree S, et al. Oncogenic Kit controls neoplastic mast cell growth through a Stat5/PI3-kinase signaling cascade. Blood. 2008;112:2463–2473. doi: 10.1182/blood-2007-09-115477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 97.Pedersen M, Ronnstrand L, Sun J. The c-Kit/D816V mutation eliminates the differences in signal transduction and biological responses between two isoforms of c-Kit. Cell Signal. 2009;21:413–418. doi: 10.1016/j.cellsig.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 98.Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayama H, Koshimizu U, et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest. 1993;92:1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannen-baum S, Suzuki Y, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci USA. 1995;92:10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun J, Pedersen M, Ronnstrand L. The D816V mutation of c-Kit circumvents a requirement for Src family kinases in c-Kit signal transduction. J Biol Chem. 2009;284:11039–11047. doi: 10.1074/jbc.M808058200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baumgartner C, Cerny-Reiterer S, Sonneck K, Mayerhofer M, Gleixner KV, Fritz R, et al. Expression of activated STAT5 in neoplastic mast cells in systemic mastocytosis. Subcellular distribution and role of the transforming oncoprotein KIT D816V. Am J Pathol. 2009;175:2416–2429. doi: 10.2353/ajpath.2009.080953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Steinberg M. Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Clin Ther. 2007;29:2289–2308. doi: 10.1016/j.clinthera.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 103.Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. 2006;108:286–291. doi: 10.1182/blood-2005-10-3969. [DOI] [PubMed] [Google Scholar]
- 104.Schittenhelm MM, Shiraga S, Schroeder A, Corbin AS, Griffith D, Lee FY, et al. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66:473–481. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- 105.Verstovsek S, Tefferi A, Cortes J, O’Brien S, Garcia-Manero G, Pardanani A, et al. Phase II study of dasatinib in Philadelphia chromosome-negative acute and chronic myeloid diseases, including systemic mastocytosis. Clin Cancer Res. 2008;14:3906–3915. doi: 10.1158/1078-0432.CCR-08-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gleixner KV, Mayerhofer M, Aichberger KJ, Derdak S, Sonneck K, Bohm A, et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V–mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood. 2006;107:752–759. doi: 10.1182/blood-2005-07-3022. [DOI] [PubMed] [Google Scholar]
- 107.Gleixner KV, Mayerhofer M, Sonneck K, Gruze A, Samor-apoompichit P, Baumgartner C, et al. Synergistic growth-inhibitory effects of two tyrosine kinase inhibitors, dasatinib and PKC412, on neoplastic mast cells expressing the D816V–mutated oncogenic variant of KIT. Haematologica. 2007;92:1451–1459. doi: 10.3324/haematol.11339. [DOI] [PubMed] [Google Scholar]
- 108.El Fitori J, Su Y, Buchler P, Ludwig R, Giese NA, Buchler MW, et al. PKC 412 small-molecule tyrosine kinase inhibitor: single-compound therapy for pancreatic cancer. Cancer. 2007;110:1457–1468. doi: 10.1002/cncr.22931. [DOI] [PubMed] [Google Scholar]
- 109.Aichberger KJ, Mayerhofer M, Gleixner KV, Krauth MT, Gruze A, Pickl WF, et al. Identification of MCL1 as a novel target in neoplastic mast cells in systemic mastocytosis: inhibition of mast cell survival by MCL1 antisense oligonu-cleotides and synergism with PKC412. Blood. 2007;109:3031–3041. doi: 10.1182/blood-2006-07-032714. [DOI] [PubMed] [Google Scholar]
- 110.Aichberger KJ, Gleixner KV, Mirkina I, Cerny-Reiterer S, Peter B, Ferenc V, et al. Identification of pro-apoptotic Bim as a tumor suppressor in neoplastic mast cells: role of KIT D816V and effects of various targeted drugs. Blood. 2009;114:5342–5351. doi: 10.1182/blood-2008-08-175190. [DOI] [PubMed] [Google Scholar]
- 111.Garber K. JAK2 inhibitors: not the next imatinib but researchers see other possibilities. J Natl Cancer Inst. 2009;101:980–982. doi: 10.1093/jnci/djp216. [DOI] [PubMed] [Google Scholar]
- 112.Pesu M, Laurence A, Kishore N, Zwillich SH, Chan G, O’Shea JJ. Therapeutic targeting of Janus kinases. Immunol Rev. 2008;223:132–142. doi: 10.1111/j.1600-065X.2008.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Linehan LA, Warren WD, Thompson PA, Grusby MJ, Berton MT. STAT6 is required for IL-4-induced germline Ig gene transcription and switch recombination. J Immunol. 1998;161:302–310. [PubMed] [Google Scholar]
- 114.Punnonen J, de Vries JE. IL-13 induces proliferation, Ig isotype switching, and Ig synthesis by immature human fetal B cells. J Immunol. 1994;152:1094–1102. [PubMed] [Google Scholar]
- 115.Punnonen J, Aversa G, Cocks BG, McKenzie AN, Menon S, Zurawski G, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Speiran K, Bailey DP, Fernando J, Macey M, Barnstein B, Kolawole M, et al. Endogenous suppression of mast cell development and survival by IL-4 and IL-10. J Leukoc Biol. 2009;85:826–836. doi: 10.1189/jlb.0708448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mirmonsef P, Shelburne CP, Fitzhugh Yeatman C, Chong HJ, Ryan JJ. Inhibition of Kit expression by IL-4 and IL-10 in murine mast cells: role of STAT6 and phosphatidylinositol 3’-kinase. J Immunol. 1999;163:2530–2539. [PubMed] [Google Scholar]
- 118.Gillespie SR, DeMartino RR, Zhu J, Chong HJ, Ramirez C, Shelburne CP, et al. IL-10 inhibits Fc epsilon RI expression in mouse mast cells. J Immunol. 2004;172:3181–3188. doi: 10.4049/jimmunol.172.5.3181. [DOI] [PubMed] [Google Scholar]
- 119.Bailey DP, Kashyap M, Bouton LA, Murray PJ, Ryan JJ. Interleukin-10 induces apoptosis in developing mast cells and macrophages. J Leukoc Biol. 2006;80:581–589. doi: 10.1189/jlb.0405201. [DOI] [PubMed] [Google Scholar]
- 120.Yeatman CF, Jacobs-Helber SM, Mirmonsef P, Gillespie SR, Bouton LA, Collins HA, et al. Combined stimulation with the T helper cell type 2 cytokines interleukin (IL)-4 and IL-10 induces mouse mast cell apoptosis. J Exp Med. 2000;192:1093–1103. doi: 10.1084/jem.192.8.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu ZQ, Zhao WH, Shimamura T, Galli SJ. Interleukin-4-triggered, STAT6-dependent production of a factor that induces mouse mast cell apoptosis. Eur J Immunol. 2006;36:1275–1284. doi: 10.1002/eji.200526275. [DOI] [PubMed] [Google Scholar]
- 122.Kennedy Norton S, Barnstein B, Brenzovich J, Bailey DP, Kashyap M, Speiran K, et al. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J Immunol. 2008;180:2848–2854. doi: 10.4049/jimmunol.180.5.2848. [DOI] [PubMed] [Google Scholar]
- 123.Zhao W, Gomez G, Yu SH, Ryan JJ, Schwartz LB. TGF-beta1 attenuates mediator release and de novo Kit expression by human skin mast cells through a Smad-dependent pathway. J Immunol. 2008;181:7263–7272. doi: 10.4049/jimmunol.181.10.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gomez G, Ramirez CD, Rivera J, Patel M, Norozian F, Wright HV, et al. TGF-beta 1 inhibits mast cell Fc epsilon RI expression. J Immunol. 2005;174:5987–5993. doi: 10.4049/jimmunol.174.10.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ryan JJ, Kashyap M, Bailey D, Kennedy S, Speiran K, Brenzovich J, et al. Mast cell homeostasis: a fundamental aspect of allergic disease. Crit Rev Immunol. 2007;27:15–32. doi: 10.1615/critrevimmunol.v27.i1.20. [DOI] [PubMed] [Google Scholar]
- 126.Kashyap M, Bailey DP, Gomez G, Rivera J, Huff TF, Ryan JJ. TGF-beta1 inhibits late-stage mast cell maturation. Exp Hematol. 2005;33:1281–1291. doi: 10.1016/j.exphem.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 127.Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21:1303–1309. doi: 10.1093/intimm/dxp102. [DOI] [PubMed] [Google Scholar]
- 128.Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol. 2008;20:288–294. doi: 10.1016/j.coi.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 129.Paplinska M, Grubek-Jaworska H, Chazan R. Role of eotaxin in the pathophysiology of asthma. Pneumonol Alergol Pol. 2007;75:180–185. [PubMed] [Google Scholar]
- 130.Conroy DM, Williams TJ. Eotaxin and the attraction of eosinophils to the asthmatic lung. Respir Res. 2001;2:150–156. doi: 10.1186/rr52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brightling CE, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID, Bradding P. Interleukin-4 and −13 expression is co-localized to mast cells within the airway smooth muscle in asthma. Clin Exp Allergy. 2003;33:1711–1716. doi: 10.1111/j.1365-2222.2003.01827.x. [DOI] [PubMed] [Google Scholar]
- 132.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]