Introduction
Pain associated with inflammation is an important clinical issue and can persist long after resolution of inflammation [20;34]. Peripheral inflammation increases the response to painful stimuli via direct actions of inflammatory mediators such as interleukin-1β (IL-1β) on primary sensory neurons [1;12;34]. This peripheral sensitization rapidly but transiently increases excitability of primary nociceptors and leads to a transient increase in the response to noxious stimulation [12]. Modulation of inflammatory hyperalgesia also takes place at the level of the spinal cord, and glial cells have been implicated in this process [6;23]. However, the neurobiological mechanisms underlying inflammation-associated pain have only begun to be unraveled. Very little is currently known about the intra- and intercellular pathways that contribute to resolution of inflammatory hyperalgesia. G protein coupled receptor kinase 2 (GRK2) belongs to a family of seven G protein coupled receptor kinases [28;32]. GRK2 phosphorylates agonist-activated G-protein-coupled receptors (GPCRs), leading to receptor desensitization and internalization [32;39]. GRK2 can also interact with elements of several intracellular signalling pathways, including Akt, MEK1/2, phosphoinositide-3 kinase (PI3-kinase) and p38 MAPK, as well as various cytoskeletal elements [27;33]. These two pathways allow GRK2 to regulate intracellular signaling not only in response to GPCR activation but also independently of GPCRs. An example of this last mechanism is the enhanced in vitro cytokine response of macrophages or microglia from GRK2+/− mice following Toll-like receptor (TLR)4 stimulation [25;27]. During the past several years, we have developed evidence for a role of GRK2 in regulating chronic pain. At the correlational level, we established that GRK2 in neurons of the spinal cord dorsal horn is reduced in two models of neuropathic pain, i.e. chronic constriction injury in rats and partial spinal nerve transection in mice [15;16]. In addition, GRK2 levels are reduced in microglia/macrophages isolated from the lumbar spinal cord in the L5 spinal transection model of neuropathic pain in rats [7]. Moreover, thermal hyperalgesia induced by transient inflammatory stimuli, such as carrageenan, the GPCR-binding chemokine CCL3 or the pro-inflammatory cytokine IL-1β, is markedly prolonged in GRK2+/− mice that express only 40–60% of normal GRK2 levels [7]. These data strongly suggest GRK2 to be a crucial molecule in actively suppressing events that are associated with thermal prolonged hyperalgesia.
The aim of experiments reported here was to test the hypothesis that reduced GRK2 in microglia/macrophages is critical for prolonging the duration of inflammatory pain and to investigate the underlying mechanisms. We first determined whether ongoing inflammation changes GRK2 expression in spinal cord microglia/macrophages. Subsequently, we investigated whether and how reduced GRK2 in microglia/macrophages regulates the duration of hyperalgesia induced by a single peripheral injection of IL-1β. For this purpose, we compared mice heterozygous for deletion of GRK2 only in microglia/macrophage generated using CRE-Lox technology and littermate controls. As a control, we used mice with low GRK2 specifically in primary sensory neurons or in astrocytes.
Materials and Methods
Animals
Female C57Bl/6 mice (12–14 weeks) heterozygous for targeted deletion of the GRK2 gene (GRK2+/−) and their WT littermates were used [7;14]. In addition, mice with cell-specific reduction of GRK2 were generated using CRE-Lox technology; GFAP-GRK2f/+, LysM-GRK2f/+, Nav1.8-GRK2f/+ and control GFAP-GRK2+/+, LysMGRK2+/+ or Nav1.8-GRK2+/+ offspring were generated by breeding heterozygous GFAP-CRE (Jackson laboratories, Bar Harbor, ME, USA), homozygous LysM-CRE (Jackson laboratories) or homozygous Nav1.8-CRE transgenic mice [35] with heterozygous GRK2-Lox mice (GRK2f/+) [21]. Heterozygous floxed GRK2 mice were used to obtain a similar reduction in GRK2 levels as in GRK2+/− mice. LysM-GRK2f/f mice were generated by breeding LysM-GRK2f/+ with homozygous GRK2-Lox mice. Mice were genotyped by PCR analysis on genomic DNA. Mice were bred and maintained in the animal facility of the University of Utrecht (The Netherlands). All experiments were performed in accordance with international guidelines and approved by the experimental animal committee of University Medical Center Utrecht.
Carrageenan paw inflammation, spinal microglia/macrophage isolation and immunohistochemical staining
Mice received an intraplantar injection of 20 μl λ-carrageenan (2 % (w/v), Sigma-Aldrich, St. Louis, MO, USA) in saline in both hind paws. At day 6 after carrageenan, mice were sacrificed and spinal cord lumbar enlargements of 4 mice were pooled. Spinal cords were ground briefly using a glass Potter in 4 ml ice-cold Hanks’ balanced salt solution (HBSS, Gibco, Carlsbad, CA, USA), containing 15 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Gibco) and 0.5% glucose (Sigma-Aldrich). Suspensions were passed through a 70-μm cell strainer (BD Biosciences, Alphen aan de Rijn, The Netherlands), cells were centrifuged at 400g for 7 min. at 10°C and resuspended in 75% Percoll (GE Healthcare, Uppsala, Sweden) in HBSS. A density gradient consisting of 4 ml cells in 75% Percoll, 3 ml 50% Percoll, 3 ml 35% Percoll, and 2 ml dPBS (Gibco) was centrifuged (1000g for 20 min. at 10°C). Cells at the 50/75% interface were collected, washed in ice-cold DPBS, and resuspended in DPBS containing 1% Bovine Serum Albumin. Cells were rapidly centrifuged onto a microscope slide using a cytospin centrifuge.
Cells were fixed in acetone at −20°C for 5 minutes. Slides were blocked in PBS containing 0.1% saponin and 2% BSA and 2% normal goat serum and incubated with rabbit-anti-GRK2 (1:100, Santa Cruz Biotechnology, Santa Cruz, CA) and rat-anti-CD11b (1:200, BD Biosciences). The specificity of GRK2 staining was controlled by using primary anti-GRK2 antibody blocked with a GRK2 blocking peptide (Santa Cruz Biotechnology). Specificity of CD11b staining was controlled by using isotype control antibody. Staining was visualized using alexa488-conjugated goat-anti-rabbit antibody and alexa594-conjugated goat-anti-mouse (1:200, Invitrogen, Paisley, UK) and slides were stained with Dapi (Sigma-Aldrich). Using this procedure, more than 95% of the isolated cells were CD11b positive. Cells were photographed with a Zeiss Apotome Microscope (Zeiss, Oberkochen, Germany) and GRK2 levels in CD11b+ cells were analysed with ImageJ software.
IL-1β-induced hyperalgesia
Mice received an intraplantar injection in the hind paw of 5 μl recombinant mice IL-1β (200 ng/ml or 20 ng/ml; Peprotech, Rocky Hill, NJ, USA) diluted in saline. Heat withdrawal latency times were determined using the Hargreaves (IITC Life Science, Woodland Hills, CA) test as described [10]. Intensity of the light beam was chosen to induce a heat withdrawal latency time of approximately 8 s at baseline. Mechanical allodynia was measured with von Frey hairs (Stoelting, Wood Dale, USA) and the 50% paw withdrawal thresholds were calculated as described [3]. Baseline withdrawal latencies were determined on three consecutive days. On the third day, mice received an intraplantar injection of IL-1β. Intraplantar injection of saline did not induce detectable hyperalgesia in any of the genotypes. All behavioral experiments were performed by an experimentator blinded to treatment and in a randomised set up.
Drug administration
Intrathecal (i.t) injections were performed as described by Hylden and Wilcox [13] under light isoflurane/O2 anesthesia. The following drugs were injected i.t. in a volume of 5 μl: minocycline (30 μg in PBS [7;36]; Sigma-Aldrich), mouse recombinant fractalkine (1 ng in saline [5]; R&D systems, Minneapolis, MN, USA), rabbit ant-rat CX3CR1 (1 μg in saline [5]; Torrey Pines Biolabs, NJ, USA) or normal rabbit IgG (1 μg in saline; Upstate, Millipore, MA, USA), p38 inhibitor SB239063 (5 μg in 10% DMSO in saline [2]; Sigma-Aldrich), PI3kinase inhibitor LY294002 (5 μg in saline [40]; Sigma-Aldrich), IL-1ra (10 ng in saline [17]; R&D systems).
Intraperitoneal administrations of minocycline (50 mg/kg [30]) were performed at day 1 and day 2 after intraplantar IL-1β injection.
Immunohistochemistry spinal cord slices and paw biopsies
Two days after intraplantar IL-1β mice were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.), and perfused intracardially with saline followed by 4% paraformaldehyde in PBS. Spinal cords were removed, post-fixed in 4% paraformaldehyde for 6 hours at 4°C, equilibrated in 30% sucrose, and mounted in OCT compound (Optimal Cutting Temperature compound; Sakura, Zoeterwoude, The Netherlands). Transverse spinal cord sections (10 μm) of thoracic segments T8–T10 and lumbar segments L2–L4 were cut using a cryostat. Sections were blocked in 5% normal goat serum (NGS) and 0.1% Triton X-100 in PBS. Sections were then incubated overnight at 4°C with 1:200 rabbit anti-Iba-1 (Wako Pure Chemical Industries, Japan) and binding was visualized using alexa488-conjugated goat-anti-rabbit antibody (1:200; Invitrogen). Slides were analysed for Iba-1+ cells with a Leica TCS-NT confocal microscope.
Paw biopsies were fixed in 4% paraformaldehyde, embedded in paraffin, blocked with 5% NGS in PBS/0.5% TritonX. Sections were incubated with 1:200 rabbit anti-Iba-1 (Wako) monoclonal antibody followed by incubation with 1:200 goat-anti-rabbit biotin (Vector Laboratories, Burlingame, CA, USA). Staining was visualized using Vectastatin ABC kit (Vector Laboratories) and diaminobenzamidine (DAB) (Sigma-Aldrich).
Primary microglia and macrophage isolation
To control for effective cell-specific deletion or reduction of GRK2 expression, we analysed GRK2 protein levels in peritoneal macrophages and primary microglia. Macrophages were collected from the peritoneal cavity after intraperitoneal injection of 3 ml RPMI-1640 (Gibco) using CD11b micro beads and a MACS column (Miltenyi Biotec, Bergisch Gladbach, Germany). Mixed primary cultures of cortical astrocytes and microglia were obtained from one day-old mice. Cortices were dissected after removal of meninges and blood vessels, minced and incubated with 0.25% trypsin for 15 min. in Grey’s balanced salt solution containing 100 U/ml penicillin, 100 μg/ml streptomycin and 30 mM D(+)-glucose. Cells were dissociated and cultured in poly-L-ornithine (15 μg/ml) coated culture flasks in DMEM/Ham’s F10 (1:1) supplemented with 10% FCS, 2 mM glutamine and antibiotics as stated above. After 10–12 days, flasks were shaken for 3 h at 37°C to collect microglia.
Total cell lysates were prepared in RIPA buffer (20 mM HEPES pH 7.5, 1% Triton X-100, 150 mM NaCl, 10 mM EDTA and protease inhibitors (Sigma-Aldrich)) for 30 min. at 4°C followed by 15 min. centrifugation at 13,000 rpm.
Western Blotting
Protein concentrations of the total cell lysates were determined using a protein assay (Bradford assay, Bio-Rad, Alphen a/d Rijn, The Netherlands). Protein samples (20 μg) were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes (Hybond-C, Amersham Biosciences, Roosendaal, The Netherlands). Membranes were stained with 1:300 rabbit anti-GRK2 or 1:2000 goat anti-β-actin (both Santa Cruz Biotechnology), followed by incubation with 1:5000 donkey anti rabbit-HRP (Amersham Biosciences) or 1:5000 donkey-anti-goat-HRP (Santa Cruz Biotechnology). Specific bands were visualised by chemiluminescence (ECL, Amersham Biosciences) with X-ray film exposure. Films were scanned with a GS-700 Imaging Densitometer and analyzed with Quantity One Software (Bio-Rad).
Real-time RT-PCR
Total RNA was isolated from thoracic and lumbar spinal cord or paw biopsies with Trizol (Invitrogen). One μg of total RNA was used to synthesize cDNA with SuperScript Reverse Transcriptase (Invitrogen). The real-time PCR reaction was performed with iQ5 Real-Time PCR Detection System (Bio-Rad) using the following primers: CatS forward: CggCgTCTCATCTgggAAAAg, reverse: gAgCACCCATCCgACACAAg, Fractalkine forward: gCgAgTgACTACTAggAg, reverse: gATTCgTgAggTCATCTTg, CX3CR1 forward: TgTCCACCTCCTTCCCTgAA, reverse: TCgCCCAAATAACAggCC, IL-1β forward: CAACCAACAAgTgATATTCTCCATg, reverse: gATCCACACTCTCCAgCTgCA. Data were normalized for GAPDH expression. GAPDH forward: TgAAgCAggCATCTgAggg, reverse CgAAggTggAAgAgTgggAg.
MPO-activity
MPO-activity in paw biopsies was determined as described before [8]. Briefly, frozen paw biopsies were homogenized in 50 mM HEPES buffer (pH 8.0) using a Potter homogenizer. Homogenates were centrifuged for 30 min at 10,000 g at 4°C, pellets were used to determine myeloperoxidase (MPO) activity.
Statistical Analysis
All data are presented as mean ± SEM. Two-way ANOVA with Bonferroni post-tests was used to analyze the effect of time or location and genotype.
Results
GRK2 levels are decreased in spinal cord microglia/macrophages during inflammatory hyperalgesia
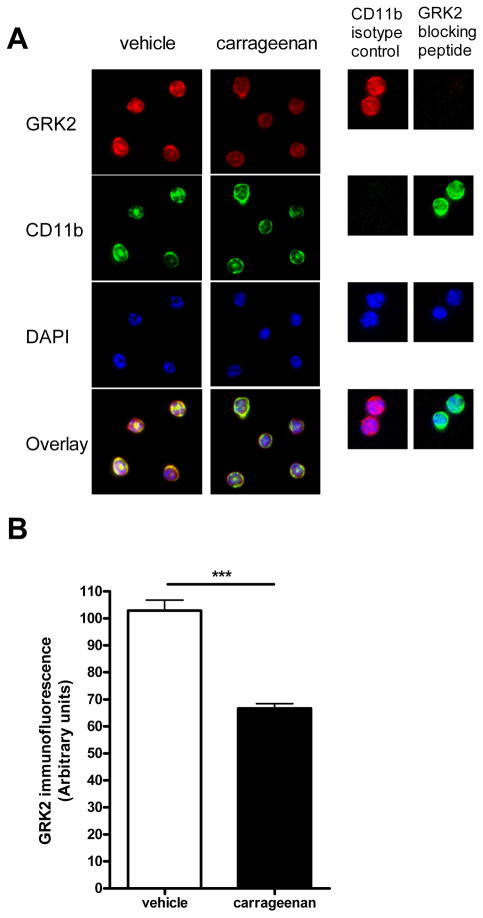
The first question we addressed is whether ongoing peripheral inflammation is associated with changes in GRK2 expression in spinal cord microglia/macrophages. The rationale to perform these experiments was to determine whether GRK2 in spinal cord microglia/macrophages is reduced under pathophysiological conditions. To answer this question, we used the high dose carrageenan model of paw inflammation [12]. WT mice received an intraplantar injection of carrageenan at a dose that induces prolonged inflammation and hyperalgesia (20 μl, 2% [11]). At 6 days after intraplantar carrageenan, thermal sensitivity (heat withdrawal latency at baseline 8.5 ± 0.2 s) was increased indicating ongoing hyperalgesia (decrease in heat withdrawal latency; vehicle 1.34 ± 2.14%; carrageenan: 39.33 ± 2.92%, n = 12 per group; p<0.01) and paw thickness (baseline 1.47 ± 0.02 mm) was increased (vehicle: 0.94 ± 5.72%, carrageenan: 48.69 ± 8.37%, n = 4 per group; p<0.001) indicative of ongoing inflammatory activity. At this time point, mice were sacrificed and microglia/macrophages were isolated from lumbar spinal cord to determine the level of GRK2 protein by immunohistochemistry. We used this approach because our earlier studies showed that GRK2 levels in microglia/macrophages cannot be determined reliably in tissue sections due the high level of expression of GRK2 in spinal cord neurons that masks the microglial/macrophage signal [15;16]. As is shown in figure 1, GRK2 protein level in microglia/macrophages isolated from lumbar spinal cord of carrageenan-treated mice was reduced by approximately 40% as compared to microglia/macrophages from spinal cord of saline-treated controls. These findings indicate that ongoing peripheral inflammation and the associated ongoing hyperalgesia co-occur with a reduction in GRK2 protein levels in spinal cord microglia/macrophages. In the next set of experiments we focussed on the effect of reduced GRK2 in microglia/macrophages on hyperalgesia induced by the cytokine IL-1β.
Figure 1. GRK2 levels in spinal microglia/macrophages during inflammatory hyperalgesia.
(a) GRK2 expression in microglia/macrophages isolated from lumbar spinal cord at 6 days after intraplantar carrageenan or saline administration was compared by immunofluorescence analysis. Representative pictures of GRK2, CD-11b and DAPI-staining of isolated microglia/macrophages. (b) GRK2 expression was quantified in approximately 60 cells per group on three different slides each containing microglia/macrophages from spinal cord of 4 mice per group. Data are expressed as mean SEM. *** p < 0.001.
IL-1β-induced hyperalgesia and mechanical allodynia are prolonged in GRK2-deficient mice
Homozygous GRK2 knockout mice die in utero and therefore we used GRK2+/− mice and WT littermate controls to determine the contribution of GRK2 to the regulation of hyperalgesia. We have shown before that the level of GRK2 in tissues from \heterozygous GRK2+/− mice is reduced by 40–60% [7;15;25]. The cytokine interleukin-1β was used to induce hyperalgesia because this cytokine is known to induce transient hyperalgesia in WT animals [1].
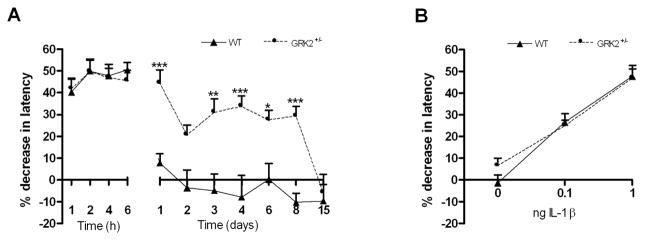
As expected, IL-1β-induced hyperalgesia was transient in WT mice and completely disappeared within one day (Fig. 2a). In GRK2+/− mice, the acute phase of hyperalgesia was similar to that observed in control mice until 6 h after IL-1β. However, the hyperalgesic response of GRK2+/− mice lasted significantly longer than that of WT mice. Baseline thermal sensitivity was not affected by reduced GRK2 [7]. To investigate whether the lack of effect of low GRK2 on acute hyperalgesia during the first 6 h after intraplantar IL-1β was due to a ceiling effect, a lower dose of IL-1β (0.1 ng/paw) was injected intraplantarly. Also at this lower dose, the magnitude of acute hyperalgesia was similar in WT and GRK2+/− mice (Fig. 2b).
Figure 2. GRK2 regulates duration of peripheral IL-1β-induced hyperalgesia.
Percentage decrease in heat withdrawal latency in WT and GRK2+/− mice (a) after intraplantar IL-1β at a dose of 1 ng (n = 8) (b) 4 hours after intraplantar injection of 0 (saline), 0.1 or 1 ng IL-1β (n = 8). Data are expressed as mean ± SEM. * p<0.05, ** p<0.01, *** p<0.001.
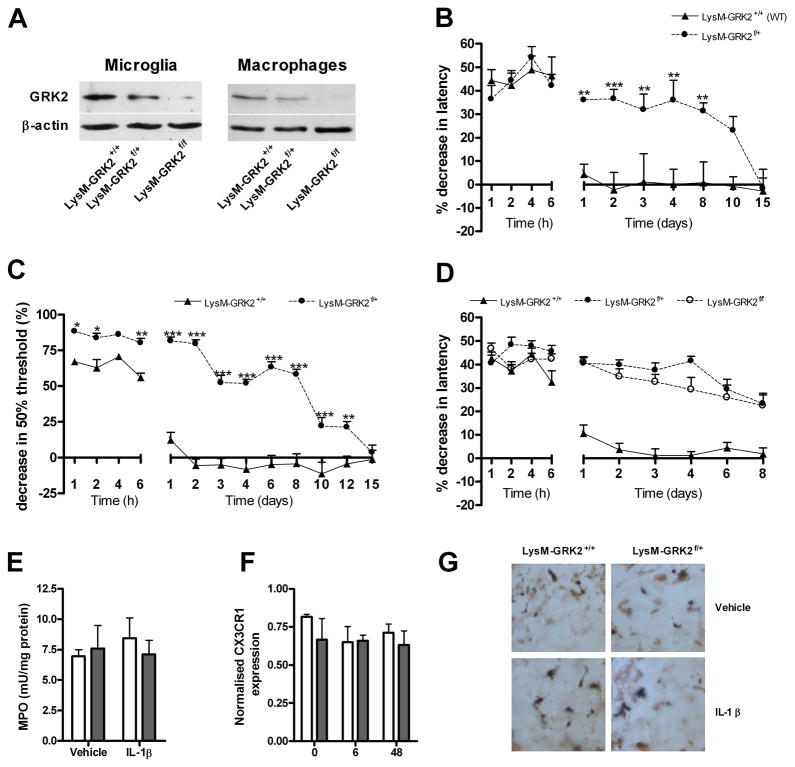
Next, we compared IL-1β-induced hyperalgesia in LysM-GRK2f/+ mice with a ~40 % reduction in GRK2 only in microglia/macrophages (Fig 3a) and littermate control LysM-GRK2+/+ mice. The course of IL-1β-induced hyperalgesia was similar in mice with reduced GRK2 only in microglia/macrophages (Fig. 3b) and in mice with reduced GRK2 in all cells (Fig. 2a), indicating that GRK2 in microglia/macrophages is critical for determining the duration of hyperalgesia in this model. Baseline thermal sensitivity was not affected by low GRK2 in microglia/macrophages (heat withdrawal latency LysM-GRK2f/+ mice 8.1 ± 0.1 s vs. control LysM-GRK2+/+ mice 8.2 ± 0.2 s; n = 22). To determine whether a gene dosage effect of GRK2 exists, we generated homozygous LysM-GRK2f/f mice. GRK2 expression in microglia or peritoneal macrophages from LysM-GRK2f/f mice was decreased by >95% compared to LysM-GRK2+/+ controls (Fig. 3a). The residual GRK2 expression in LysM-GRK2f/f that was observed in Western blot analysis might be due to incomplete removal of the floxed genes in some cells. Residual GRK2 expression may also be derived from contaminating astrocytes in the microglial preparation or lymphocytes in the macrophage enriched fraction. The data in figure 3c demonstrate that the magnitude and duration of IL-1β-induced hyperalgesia was similar in mice heterozygous or homozygous for deletion of GRK2 in LysM-positive cells. Low GRK2 in microglia/macrophages also affects mechanical allodynia as determined using von Frey hairs. In control LysM-GRK2+/+ mice, intraplantar injection of IL-1β induces a transient increase in sensitivity to mechanical stimulation (Fig. 3d). At one day after intraplantar IL-1β, mechanical sensitivity in control mice has returned to baseline levels (Fig. 3d), whereas LysM-GRK2f/+ mice continue to display mechanical allodynia until at least 12 days after the intraplantar injection of IL-1β.
Figure 3. Gene dosage effect and cellular specificity of the effect of low GRK2 on thermal hyperalgesia and mechanical allodynia.
(a) GRK2 expression in primary microglia and in CD11b+ peritoneal macrophages from LysM-GRK2+/+ (WT), LysM-GRK2f/+ and LysM-GRK2f/f mice was determined by Western blot analysis. (b) Percentage decrease in heat withdrawal latency after intraplantar IL-1β (1 ng) injection in control LysM-GRK2+/+ (WT) and LysM-GRK2f/+ mice (n = 8) (c) Change in heat withdrawal latency in control LysM-GRK2+/+ (WT) (n = 18), heterozygous LysM-GRK2f/+ (n = 12) and homozygous LysM-GRK2f/f mice (n = 14) after intraplantar injection of 1 ng IL-1β. (d) Decrease in 50% threshold for withdrawal in response to mechanical stimulation after intraplantar IL-1β (1 ng) in LysM-GRK2+/+ (WT) and LysM-GRK2f/+ mice (n = 8). (e) MPO-content of paw biopsies as a measure of neutrophil infiltration at 2 days after intraplantar IL-1β injection (n = 8). (f–g) As a measure of macrophage infiltration after intraplantar IL-1β adminstration, samples were analyzed for (f) CX3CR1 mRNA expression in paw biopsies at 0, 6 and 48 hours after injection of IL-1β (n = 6) (g) Iba-1 staining in paw biopsies at 2 days after injection of IL-1β. Data are expressed as mean ± SEM. * p<0.05, ** p<0.01, *** p<0.001.
Neutrophil influx into the paw was determined by measuring MPO activity (Fig. 3e) and macrophage influx was determined by expression of CX3CR1 mRNA (Fig. 3f) and Iba-1-staining (Fig. 3g) in paw biopsies of LysM-GRK2f/+ and LysM-GRK2+/+ mice. The findings indicate that the genotype-related differences in IL-1β-induced thermal hyperalgesia and allodynia are not caused by genotype-related differences in the influx of neutrophils and macrophages into the paw (Fig 3e–g).
Contribution of GRK2 in astrocytes and primary sensory neurons to IL-1β-induced hyperalgesia
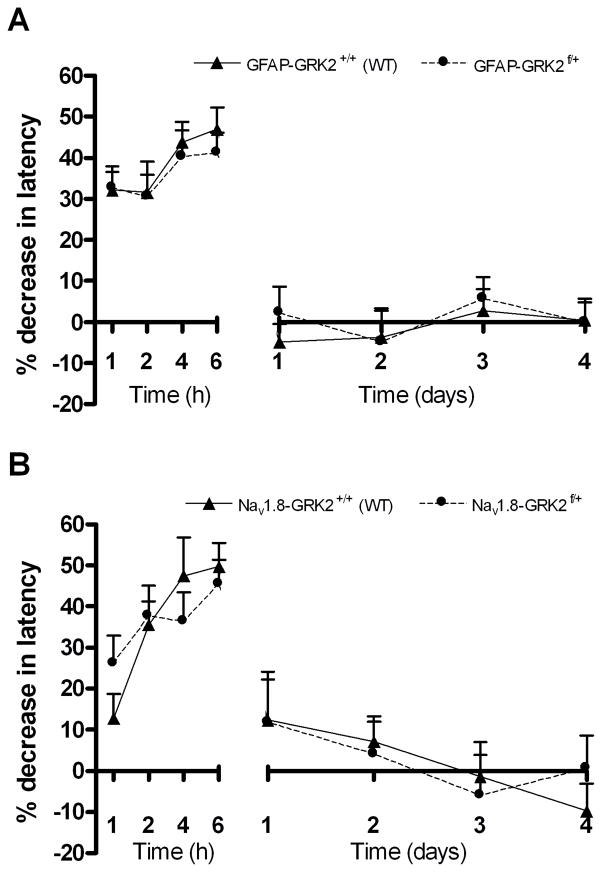
We also examined the effect of reduction of GRK2 in GFAP-positive astrocytes. Previously, we showed that the level of GRK2 in astrocytes from GFAP-GRK2f/+ mice was approximately 60% lower than in WT control mice [7] The data in figure 4a demonstrate that changes in astrocyte GRK2 did not affect the course of intraplantar IL-1β-induced hyperalgesia. Similarly, the reduction of GRK2 in Nav1.8-positive peripheral sensory neurons that occurs in Nav1.8-GRK2f/+ mice [7] had no effect on IL-1β-induced hyperalgesia (Fig. 4b).
Figure 4. Contribution of GRK2 in astrocytes and primary sensory neurons to IL-1β-induced hyperalgesia.
Percentage decrease in heat withdrawal latency after intraplantar IL-1β (1 ng) in (a) control GFAP-GRK2+/+ (WT) and GFAP-GRK2f/+ mice (n = 8) (b) control Nav1.8-GRK2+/+ (WT) and Nav1.8-GRK2f/+ mice (n = 8).
Spinal cord microglial/macrophage activation plays a key role in IL-1β-induced chronic hyperalgesia in GRK2-deficient mice
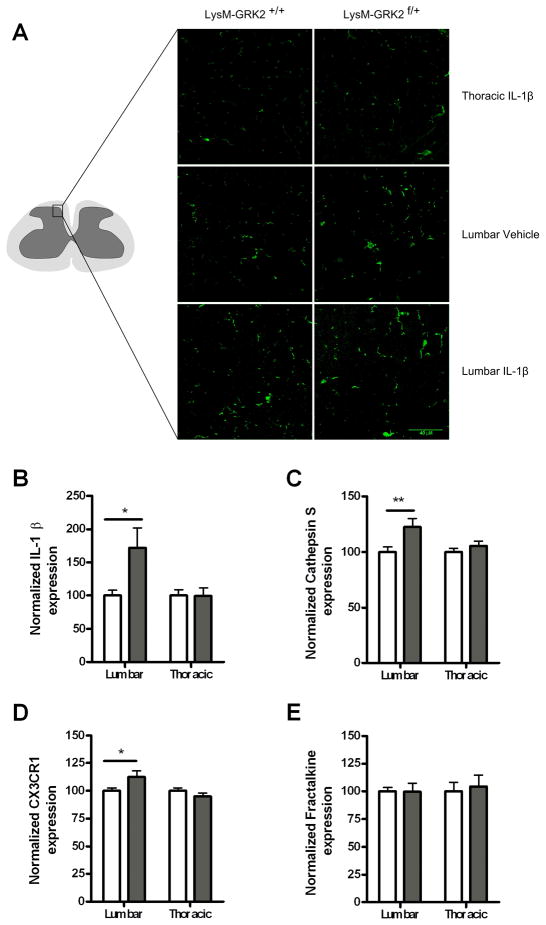
Next, we determined whether ongoing hyperalgesia in mice with low GRK2 in microglia/macrophages was associated with signs of ongoing activation of these cells in the spinal cord. We observed an increased number of lumbar but not thoracic spinal cord Iba-1+ microglia/macrophages with an activated phenotype in LysM-GRK2f/+ mice compared to control LysM-GRK2+/+ mice at day two after intraplantar IL-1β (Fig. 5a). As a measure of spinal cord microglial/macrophage activity, we also analyzed mRNA expression of the fractalkine receptor CX3CR1, the fractalkine releasing enzyme cathepsin S [5] and IL-1β that are all expressed by microglia/macrophages. The data in figure 5b–e indicate that IL-1β, cathepsin S and CX3CR1 mRNA expression were all significantly increased in lumbar spinal cord of GRK2+/− mice as compared to WT mice. No changes were observed in thoracic spinal cord that was analyzed as a control. In contrast, mRNA expression for fractalkine that is produced by neurons was not affected by low GRK2.
Figure 5. Spinal cord microglia/macrophage activity.
(a) LysM-GRK2+/+ (WT) and LysM-GRK2f/+ mice received an intraplantar injection of IL-1β or saline. At two days after injection, spinal cord was collected and frozen sections of lumbar spinal cord (L2–L4) and as a control thoracic spinal cord (T8–T10) were stained with Iba-1 to visualize microglia/macrophages. Representative example of morphology of Iba-1-positive cells in the dorsal horn (see drawing for exact location) of one out of three mice per group is displayed. (b–d) WT (white bars) and GRK2+/− mice (grey bars) received an intraplantar injection of IL-1β (1ng) and lumbar and as a control thoracic spinal cord was collected two days later. Samples were analysed for mRNA encoding (b) IL-1β; (c) Cathepsine S; (d) CX3CR1; and (e) fractalkine. n = 10 per group. * p<0.05
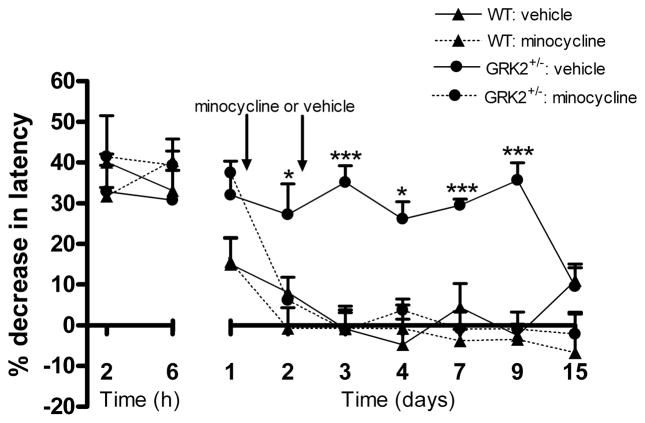
To determine whether ongoing microglial/macrophage activity was required for maintaining hyperalgesia in GRK2+/− mice, we used the inhibitor of microglial/macrophage activation minocycline [30;36]. We examined whether we could reverse ongoing thermal hyperalgesia in mice with reduced GRK2 by treatment with minocycline at day one and two after intraplantar IL-1β. The data in figure 6 demonstrate that this treatment reversed the ongoing hyperalgesia in GRK2+/− mice. Minocycline treatment at these time points did not have any effect on thermal sensitivity in WT mice that had returned already to baseline. These data indicate that ongoing spinal cord microglial/macrophage activity is required to maintain the hyperalgesic state that developed in GRK2+/− mice after a single intraplantar injection of IL-1β.
Figure 6. Effect of minocycline treatment at day one and two after intraplantar IL-1β on hyperalgesia.
WT and GRK2+/− mice received an intraplantar injection of IL-1β (1 ng) and the percentage decrease in heat withdrawal latency was determined. At one and two days after IL-1β, minocycline (50 mg/kg) or vehicle was administered (n = 4 per group). Data are expressed as mean ± SEM. * p<0.05, ** p<0.01, *** p<0.001 vs. vehicle.
Spinal cord pathways contributing to ongoing IL-1β-hyperalgesia in GRK2- deficient mice
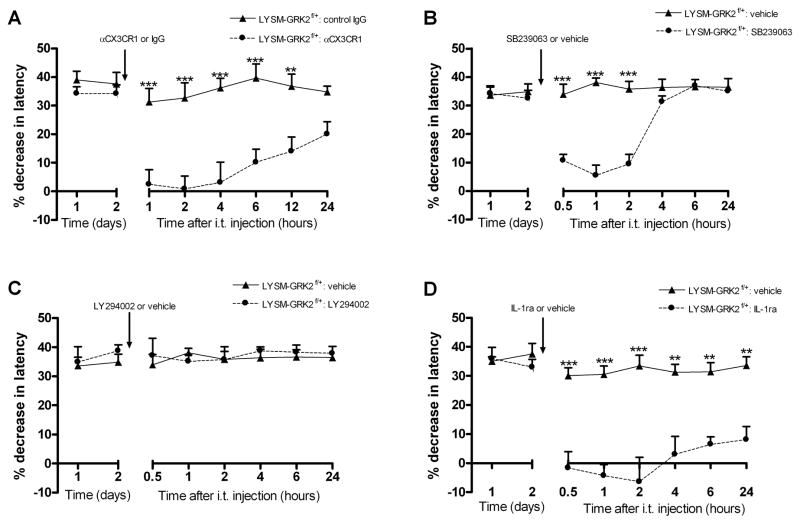
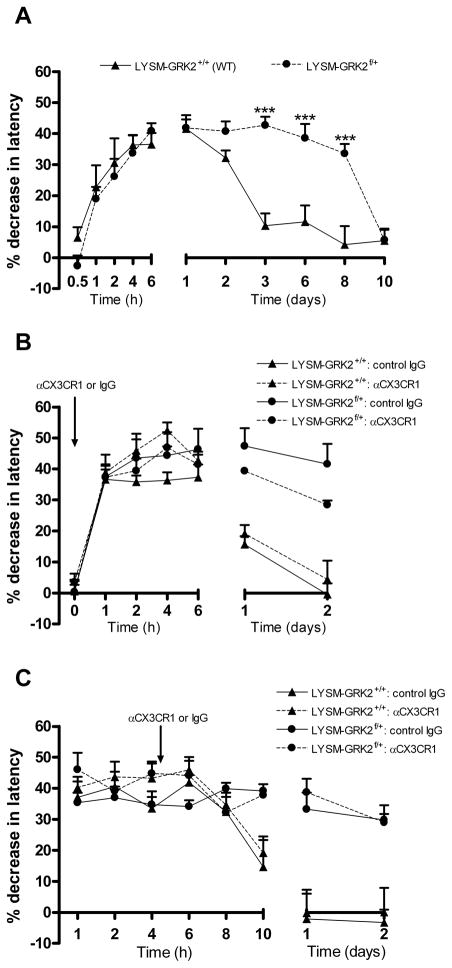
The data in figure 5 indicate that ongoing hyperalgesia in GRK2-deficient mice was associated with increased spinal cord expression of the fractalkine releasing enzyme cathepsin S and upregulation of the fractalkine receptor CX3CR1 that was detectable at two days after IL-1β. To determine the contribution of fractalkine signaling to ongoing hyperalgesia in LysM-GRK2f/+ mice, mice were treated with a neutralizing antibody against CX3CR1 at day two after intraplantar IL-1β. Intrathecal injection of anti-CX3CR1 (1 μg/mouse) during the prolonged phase of IL-1β hyperalgesia blocked ongoing hyperalgesia in LysM-GRK2f/+ mice as determined from 1 h after anti-CX3CR1 injection. The same dose of normal rabbit IgG did not have any effect and anti-CX3CR1 did not affect thermal sensitivity in control LysM-GRK2+/+ mice (Fig. 7a).
Figure 7. Factors contributing to ongoing hyperalgesia in GRK2-deficient mice.
LysM-GRK2f/+ mice received an intraplantar injection of IL-1β and the percentage decrease in heat withdrawal latency was determined. At day 2 after intraplantar IL-1β, mice received an intrathecal injection of (a) αCX3CR1 (1 ug/mouse) or rabbit IgG (1 μg/mouse) (n = 6 per group) (b) p38 inhibitor SB239063 (5 μg/mouse; n = 8) or vehicle (n = 4) (c) PI3 kinase inhibitor LY294002 (5 μg/mouse) or vehicle (n = 4 per group) (d) IL-1ra (10 ng/mouse; n = 6) or vehicle (n = 4). Heat sensitivity in IL-1β-treated LysM-GRK2+/+ (WT) mice had already returned to baseline at day 2 after injection and was not affected by any of the treatments (data not shown). The inhibitors did not affect heat sensitivity in naïve mice of either genotype. Data are expressed as mean ± SEM. * p<0.05, ** p<0.01, *** p<0.001 vs. vehicle or control IgG.
Activation of CX3CR1 on microglia is thought to activate p38 leading to increased production of pro-inflammatory cytokines such as IL-1β and subsequent central sensitization via a direct effect on sensory neurons. The possible contribution of this pathway was tested by intrathecal injection of the p38 inhibitor SB239063 at day two after intraplantar IL-1β. This treatment reversed ongoing thermal hyperalgesia in mice with low GRK2 in microglia/macrophages (Fig. 7b). Intrathecal SB239063 at day 2 after IL-1β did not affect thermal sensitivity in control LysM-GRK2+/+ mice.
The phosphatidylinostitol-3 kinase (PI3kinase) pathway can also be triggered by soluble and membrane-bound fractalkine, leading to modulation of microglial/macrophage activity [19;31]. However, intrathecal administration of the PI3kinase inhibitor LY294002 at day two after intraplantar IL-1β injection did not have any effect on ongoing hyperalgesia in LysM-GRK2f/+ mice (Fig. 7c).
As mentioned above, fractalkine signaling to microglial p38 is thought to enhance IL-1- release leading to neuronal sensitization and hyperalgesia. To examine the contribution of ongoing spinal cord IL-1-signaling to the prolonged hyperalgesia that we observed in LysM-GRK2f/+ after intraplantar IL-1β, we used the IL-1 antagonist IL-1ra. Intrathecal administration of IL-1ra at day two after intraplantar IL-1β reversed ongoing hyperalgesia in LysM-GRK2f/+ mice (Fig. 7d) within 1 h after administration.
No effect of intrathecal IL-1ra was observed in control LysM-GRK2+/+ mice.
Contribution of early spinal cord microglial/macrophage activity
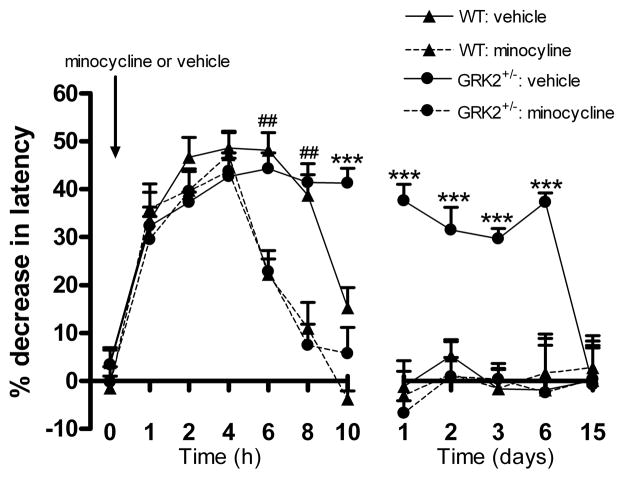
To address the question whether early spinal cord microglial activity contributed to the transition from acute to prolonged IL-1β hyperalgesia in GRK2-deficient mice, we now administered minocycline (30 μg) intrathecally at 60 minutes before intraplantar IL-1β. This pre-treatment did not have any effect on acute IL-1β hyperalgesia at 1 to 4 h after intraplantar IL-1β injection in either genotype. However, hyperalgesia at 6 h and 8 h after IL-1β injection was significantly reduced by pre-emptive minocycline treatment to the same extent in WT and GRK2+/− mice. Moreover, in GRK2+/− mice the development of the chronic phase of hyperalgesia was completely prevented by minocycline pre-treatment (Fig. 8).
Figure 8. Contribution of early spinal cord microglial/macrophage activity to IL-1β-induced hyperalgesia.
WT and GRK2+/− mice (n = 8 per group) received an intrathecal injection of minocycline (30 μg) or vehicle 60 min. prior to intraplantar IL-1β and the percentage change in heat withdrawal latency was determined. Data are expressed as mean ± SEM. * p<0.05, ** p<0.01, *** p<0.001 for GRK2+/− mice/minocycline vs. GRK2+/− mice/vehicle. ## p<0.01 for both genotypes with minocycline vs. saline.
Contribution of fractalkine signalling to prolonged hyperalgesia in response to intraplantar IL-1β
The fractalkine receptor CX3CR1 is a member of the G protein-coupled receptor family and in the spinal cord CX3CR1 is mainly expressed on microglia/macrophages. GRK2 regulates a wide array of GPCRs via desensitization of these receptors. Although it is not known whether CX3CR1 is a substrate for this kinase, low GRK2 may contribute to transition from acute to prolonged IL-1β-hyperalgesia by facilitating fractalkine signalling as a result of impaired CX3CR1 desensitization. To determine whether mice with low GRK2 in microglia/macrophages are more sensitive to fractalkine-induced hyperalgesia, we compared the effect of intrathecal administration of fractalkine on thermal sensitivity in LysM-GRK2f/+ and control LysM-GRK2+/+ mice. In line with data in the literature [5;24], intrathecal injection of fractalkine (1 ng/mouse) in WT mice induced hyperalgesia that was first detectable at 2 h after fractalkine injection (Fig. 9a). During the first 6 h after injection, fractalkine-induced hyperalgesia was identical in LysM-GRK2f/+ and control LysM-GRK2+/+ mice. Also at lower doses of fractalkine (10–100 pg/mouse), acute hyperalgesia in control and LysM-GRK2f/+ mice was indistinguishable (data not shown). Notably, however, fractalkine-induced hyperalgesia did become chronic in mice with reduced GRK2 in microglia/macrophages, whereas it was only transient in control LysM-GRK2+/+ littermates (Fig. 9a).
Figure 9. Contribution of fractalkine to prolonged IL-1β-induced hyperalgesia in GRK2-deficient mice.
(a) Mice received an intrathecal injection of fractalkine (1 ng) and the percentage decrease in heat withdrawal latency was determined in LysM-GRK2+/+ (WT) and in LysM-GRK2f/+ mice (n = 8 per group). (b–c) Mice received and intraplantar injection of IL-1β. The effect of intrathecal administration of anti-CX3CR1 (1 μg/mouse) or rabbit IgG (1 μg/mouse) (b) before or (c) 4 hours after intraplantar IL-1β administration on hyperalgesia in LysM-GRK2+/+ (WT) and LysM-GRK2f/+ mice (n = 4 per group) was determined. Data are expressed as mean ± SEM. *** p<0.001.
Next, we investigated whether the transition from acute to prolonged peripheral IL-1β-induced hyperalgesia is dependent on spinal cord fractalkine signaling. Mice received an intraplantar injection of IL-1β and we examined the effect of intrathecal injection of anti-CX3CR1 before or at 4 h after intraplantar IL-1β. As is shown in figure 8b/c, this early administration of anti-CX3CR1 did not have any effect on IL-1β-induced hyperalgesia in LysM-GRK2+/+ mice or LysM-GRK2f/+ mice (Fig 9b, c), suggesting that fractalkine signalling is not involved in the transition from acute to chronic hyperalgesia.
Discussion
The present study assessed the contribution of GRK2 in microglia/macrophages to the neurobiology of inflammation-induced hyperalgesia using mice with low GRK2 only in microglia/macrophages that were generated using CRE-Lox technology. The level of microglia/macrophage GRK2 appeared to play a crucial role in determining duration of hyperalgesia. Thermal hyperalgesia or mechanical allodynia induced by one single intraplantar injection of IL-1β lasted at least 8 days in LysM-GRK2f/+ mice whereas in WT LysM-GRK2+/+ mice IL-1β hyperalgesia and allodynia resolved within 24 h. These genotype-related differences in the duration of hyperalgesia and allodynia were not associated with genotype differences in the influx of neutrophils or macrophages. Prolonged thermal hyperalgesia in GRK2-deficient mice was dependent on ongoing microglial/macrophage activity, fractalkine signaling, p38 activity and IL-1β signaling in the spinal cord. We propose that normal levels of microglial/macrophage GRK2 are important to ensure adequate termination of the activity of spinal cord microglia/macrophages and resolution of hyperalgesia. The pathophysiological relevance of these findings is strengthened by the recent finding that spinal cord microglia/macrophage GRK2 is reduced by ~35% in a rat model of chronic neuropathic pain [7] and in a model of ongoing inflammatory pain as is shown here.
The maximal effect of low microglial/macrophage GRK2 on prolongation of IL-1β-induced hyperalgesia was already obtained in heterozygous LysM-GRK2f/+ mice with a 40–60% reduction in microglial/macrophage GRK2. The only previous study using mice homozygous for cell-specific deletion of GRK2 showed that neither partial nor total GRK deletion in cardiac myocytes had any consequences for the end point investigated (cardiac development) [29]. Therefore, we do not know whether the lack of gene dosage effect is specific for microglial/macrophage GRK2 or represents a general phenomenon. We previously described a similar lack of gene dosage effect for GRK6; experimental colitis was prolonged to the same extent in mice that were heterozygous or homozygous for deletion of GRK6 [8].
IL-1β acts directly on peripheral sensory neurons to increase excitability leading to a rapid increase in thermal sensitivity [1;22] via enhancement of TTX-resistant sodium currents [1]. Consistent with direct sensitization of sensory neurons by IL-1β, we show that intrathecal administration of the microglial/macrophage inhibitor minocycline did not affect hyperalgesia during the first 4 h after intraplantar IL-1β. However, this treatment reduced hyperalgesia as determined at 6–8 h after IL-1β and this effect was irrespective of genotype. These findings indicate that spinal cord microglial/macrophage activity contributes to the late phase of acute IL-1β-induced hyperalgesia, a process which is not altered by lowering GRK2. In addition, the acute phase of hyperalgesia after activation of spinal microglia by intrathecal administration of fractalkine was indistinguishable in LysM-GRK2+/+ and LysM-GRK2f/+ mice. Moreover, spinal cord fractalkine signaling was not required for the initial activation of microglia/macrophages in our model, since early inhibition of fractalkine signaling by administration of anti-CX3CR1 before or at 4 h after intraplantar IL-1β did not affect the course of hyperalgesia. These findings imply that although microglial/macrophage activity is contributing to hyperalgesia at 6–8 h after IL-1β, fractalkine signaling is not yet involved in microglial/macrophage activation. Moreover, GRK2-deficiency does not affect the initial hyperalgesic response to fractalkine stimulation of microglia/macrophages.
It is well established that spinal cord microglia/macrophage activity contributes to chronic hyperalgesia in response to complete Freund’s adjuvant or carrageenan models of chronic inflammation-induced hyperalgesia [4;6;23]. What is not known, however, is which factors contribute to terminating spinal cord microglial/macrophage activity after resolution of peripheral inflammation. In WT mice microglial/macrophage-dependent central sensitization is a self-limiting process as hyperalgesia completely resolves within 12–24 h after intraplantar IL-1β or 3 days after intrathecal fractalkine. In contrast, mice with low GRK2 in microglia/macrophages hyperalgesia did not resolve until 7–8 days after intraplantar IL-1β or 8–10 days after intrathecal fractalkine. The prolonged phase of hyperalgesia in GRK2-deficient mice was reversed by minocycline treatment at 24–48 h after intraplantar IL-1β, indicating that low GRK2 facilitates ongoing microglial/macrophage activity. The prolonged hyperalgesic state in GRK2-deficient mice is dependent on spinal cord fractalkine signaling, since intrathecal administration of anti-CX3CR1 at 2 days after intraplantar IL-1β attenuated ongoing hyperalgesia. Activation of CX3CR1 on microglia/macrophages is thought to lead to activation of p38 and release of pro-inflammatory cytokines including IL-1β [5]. We propose that in LysM-GRK2f/+ mice the prolonged hyperalgesia in response to peripheral IL-1β is maintained via a loop involving fractalkine signaling, p38 activation and IL-1β production. This hypothesis is based on our findings that inhibition of either fractalkine signaling, p38 activity or IL-1β signaling attenuated the ongoing hyperalgesic response in GRK2-deficient mice. The model of IL-1β-induced hyperalgesia that we used here is acute and transient in WT animals. However, we show that a ~50% reduction in GRK2 in microglia/macrophages is sufficient to markedly prolong IL-1β-hyperalgesia and allodynia. Thus, our data strongly underline the importance of microglia/macrophages in determination of duration of hyperalgesia and allodynia and identify a central role for GRK2 in this process. Fractalkine signaling not only contributes to microglial activity, but may also serve to keep cerebral microglia in a quiescent state via a PI3kinase dependent pathway [19]. Since inhibition of spinal cord PI3kinase activity did not have any effect in our model of peripheral inflammatory hyperalgesia, spinal cord fractalkine signaling probably does not serve to suppress spinal cord microglial activation in this model. This may be due to site specific differences in mechanisms operative in brain versus spinal cord microglia.
We previously showed that acute hyperalgesia induced by the GPCR binding chemokine CCL3 as determined within the first 3 h after intraplantar injection was more pronounced in GRK2+/− than in WT mice. Increased acute CCL3 hyperalgesia was also observed in mice deficient for GRK2 only in peripheral sensory neurons [7]. In contrast, our present data show that IL-1β hyperalgesia was completely normal in mice with low GRK2 only in peripheral sensory neurons. CCL3 signals via CCR1 and CCR5 and these receptors are sensitive to GRK2 dependent desensitization [7;26;37]. IL-1β signaling is mediated by receptors that are not GPCR. This might explain why IL-1β-hyperalgesia is not affected by low GRK2 in primary sensory neurons. However, IL-1β-induced sensitization of peripheral sensory neurons is mediated via a p38 dependent pathway [1] and GRK2 can regulate p38 activation. Direct interaction of GRK2 with p38 can result in phosphorylation of Thr-123 in the docking groove which reduces binding of upstream kinases like MKK6 as well as down stream targets [27]. GRK2 has been shown to reduce LPS-induced cytokine production by microglia/macrophages via this mechanism [7;25;27]. However, since a ~50% reduction in GRK2 was not sufficient to enhance IL-1β-induced acute hyperalgesia, a possible interaction between GRK2 and p38 in peripheral sensory neurons does not appear to contribute to regulation of acute IL-1β hyperalgesia.
Previously, we described that spinal cord neuronal GRK2 was reduced in rat or mouse models of neuropathic pain [15;16]. Moreover, our recent data showed that GRK2 levels in microglia/macrophages isolated from ispilateral lumbar spinal cord of rats at day 14 after L5 spinal nerve transection were decreased by ~35% as compared to cells from contralateral spinal cord or from sham control rats [7]. Here we show that the high dose carrageenan paw inflammation model of prolonged hyperalgesia is also associated with reduced spinal cord microglial/macrophage GRK2. Thus, in vivo, in models of chronic pain, spinal microglial/macrophage GRK2 levels are reduced. Our findings that reduced microglial/macrophage GRK2 prolongs hyperalgesia indicate that the reduced levels of GRK2 that occur in these cells may well contribute to the chronic pain that develops in these models of chronic inflammation. In vitro, culture of various cell types with inflammatory mediators including cytokines can decrease GRK2 levels [9;18]. Clinically, we have shown that GRK2 protein levels in peripheral blood mononuclear cells from patients with rheumatoid arthritis or multiple sclerosis was reduced as compared to healthy controls [18;38]. These observations indicate that in humans GRK2 can be down regulated in immune cells during chronic inflammation. It is unclear however, whether painful inflammatory conditions in humans are also associated with reduced GRK2 in spinal cord microglia/macrophages and this question is subject of our current investigations.
Collectively, our data indicate that inflammatory hyperalgesia is associated with reduced spinal cord microglial/macrophage GRK2. The pathophysiological significance of low GRK2 in these cells is attested by our finding that peripheral IL-1β-induced hyperalgesia was markedly prolonged in mice with reduced microglial/macrophage GRK2. We propose that the ongoing hyperalgesia observed in mice with low GRK2 is maintained via a positive feedback loop that involves fractalkine signaling, p38 activation, IL-1 release and ongoing microglial/macrophage activity in the spinal cord. Taken together our data indicate that microglial/macrophage GRK2 may be crucial for resolution of inflammatory hyperalgesia. Future studies should aim at determining the mechanism how spinal cord microglia/macrophage activity is terminated in the context of inflammatory pain.
Acknowledgments
The authors thank Dr. J. N. Wood, University College London, London, UK for sharing the Nav1.8-CRE mice and Mrs. Ilona den Hartog, UMC Utrecht, for excellent technical assistance.
Footnotes
We have no conflicts of interests related to this study.
Reference List
- 1.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao FL, Liu MG, Hao J, Li Z, Lu ZM, Chen J. Different roles of spinal p38 and c-Jun N-terminal kinase pathways in bee venom-induced multiple pain-related behaviors. Neurosci Lett. 2007;427:50–54. doi: 10.1016/j.neulet.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 4.Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur J Pain. 2007;11:223–230. doi: 10.1016/j.ejpain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Clark AK, Yip PK, Grist J, Gentry C, Staniland AA, Marchand F, Dehvari M, Wotherspoon G, Winter J, Ullah J, Bevan S, Malcangio M. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:10655–10660. doi: 10.1073/pnas.0610811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 7.Eijkelkamp N, Heijnen CJ, Willemen HLDM, Deumens R, Joosten EAJ, Kleibeuker W, den Hartog IJM, van Velthoven CTJ, Nijboer CH, Nassar MA, Dorn GW, II, Wood JN, Kavelaars A. GRK2: a Novel Cell Specific Regulator of Severity and Duration of Inflammatory Pain. J Neurosci. 2010 doi: 10.1523/JNEUROSCI.5752-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eijkelkamp N, Heijnen CJ, Lucas A, Premont RT, Elsenbruch S, Schedlowski M, Kavelaars A. G protein-coupled receptor kinase 6 controls chronicity and severity of dextran sodium sulphate-induced colitis in mice. Gut. 2007;56:847–854. doi: 10.1136/gut.2006.107094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan J, Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med. 2003;9:315–321. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 10.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 11.Honore P, Wade CL, Zhong C, Harris RR, Wu C, Ghayur T, Iwakura Y, Decker MW, Faltynek C, Sullivan J, Jarvis MF. Interleukin-1alphabeta gene-deficient mice show reduced nociceptive sensitivity in models of inflammatory and neuropathic pain but not post-operative pain. Behav Brain Res. 2006;167:355–364. doi: 10.1016/j.bbr.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 14.Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Jr, Lefkowitz RJ, Caron MG, Giros B. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci U S A. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleibeuker W, Gabay E, Kavelaars A, Zijlstra J, Wolf G, Ziv N, Yirmiya R, Shavit Y, Tal M, Heijnen CJ. IL-1 beta signaling is required for mechanical allodynia induced by nerve injury and for the ensuing reduction in spinal cord neuronal GRK2. Brain Behav Immun. 2008;22:200–208. doi: 10.1016/j.bbi.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Kleibeuker W, Ledeboer A, Eijkelkamp N, Watkins LR, Maier SF, Zijlstra J, Heijnen CJ, Kavelaars A. A role for G protein-coupled receptor kinase 2 in mechanical allodynia. Eur J Neurosci. 2007;25:1696–1704. doi: 10.1111/j.1460-9568.2007.05423.x. [DOI] [PubMed] [Google Scholar]
- 17.Laughlin TM, Bethea JR, Yezierski RP, Wilcox GL. Cytokine involvement in dynorphin-induced allodynia. Pain. 2000;84:159–167. doi: 10.1016/s0304-3959(99)00195-5. [DOI] [PubMed] [Google Scholar]
- 18.Lombardi MS, Kavelaars A, Schedlowski M, Bijlsma JW, Okihara KL, Van de PM, Ochsmann S, Pawlak C, Schmidt RE, Heijnen CJ. Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FASEB J. 1999;13:715–725. doi: 10.1096/fasebj.13.6.715. [DOI] [PubMed] [Google Scholar]
- 19.Lyons A, Lynch AM, Downer EJ, Hanley R, O’Sullivan JB, Smith A, Lynch MA. Fractalkine-induced activation of the phosphatidylinositol-3 kinase pathway attentuates microglial activation in vivo and in vitro. J Neurochem. 2009;110:1547–1556. doi: 10.1111/j.1471-4159.2009.06253.x. [DOI] [PubMed] [Google Scholar]
- 20.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 21.Matkovich SJ, Diwan A, Klanke JL, Hammer DJ, Marreez Y, Odley AM, Brunskill EW, Koch WJ, Schwartz RJ, Dorn GW. Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ Res. 2006;99:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 22.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009:417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O’Connor KA, Verge GM, Chapman G, Green P, Foster AC, Naeve GS, Maier SF, Watkins LR. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004;20:2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- 25.Nijboer CH, Heijnen CJ, Willemen HLDM, Groenendaal F, Dorn GW, II, van Bel F, Kavelaars A. Cell-specific roles of GRK2 in onset and severity of hypoxic-ischemic brain damage in neonatal mice. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oppermann M, Mack M, Proudfoot AE, Olbrich H. Differential effects of CC chemokines on CC chemokine receptor 5 (CCR5) phosphorylation and identification of phosphorylation sites on the CCR5 carboxyl terminus. J Biol Chem. 1999;274:8875–8885. doi: 10.1074/jbc.274.13.8875. [DOI] [PubMed] [Google Scholar]
- 27.Peregrin S, Jurado-Pueyo M, Campos PM, Sanz-Moreno V, Ruiz-Gomez A, Crespo P, Mayor F, Jr, Murga C. Phosphorylation of p38 by GRK2 at the docking groove unveils a novel mechanism for inactivating p38MAPK. Curr Biol. 2006;16:2042–2047. doi: 10.1016/j.cub.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 28.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 29.Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, DeGeorge BR, Jr, Matkovich S, Houser SR, Most P, Eckhart AD, Dorn GW, Koch WJ. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. 2008;103:413–422. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 31.Ré DB, Przedborski S. Fractalkine: moving from chemotaxis to neuroprotection. Nat Neurosci. 2006;9:859–861. doi: 10.1038/nn0706-859. [DOI] [PubMed] [Google Scholar]
- 32.Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F., Jr The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 35.Stirling LC, Forlani G, Baker MD, Wood JN, Matthews EA, Dickenson AH, Nassar MA. Nociceptor-specific gene deletion using heterozygous NaV1.8-Cre recombinase mice. Pain. 2005;113:27–36. doi: 10.1016/j.pain.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vroon A, Heijnen CJ, Lombardi MS, Cobelens PM, Mayor F, Jr, Caron MG, Kavelaars A. Reduced GRK2 level in T cells potentiates chemotaxis and signaling in response to CCL4. J Leukoc Biol. 2004;75:901–909. doi: 10.1189/jlb.0403136. [DOI] [PubMed] [Google Scholar]
- 38.Vroon A, Kavelaars A, Limmroth V, Lombardi MS, Goebel MU, Van Dam AM, Caron MG, Schedlowski M, Heijnen CJ. G protein-coupled receptor kinase 2 in multiple sclerosis and experimental autoimmune encephalomyelitis. J Immunol. 2005;174:4400–4406. doi: 10.4049/jimmunol.174.7.4400. [DOI] [PubMed] [Google Scholar]
- 39.Vroon A, Lombardi MS, Kavelaars A, Heijnen CJ. Taxol normalizes the impaired agonist-induced beta2-adrenoceptor internalization in splenocytes from GRK2+/− mice. Eur J Pharmacol. 2007;560:9–16. doi: 10.1016/j.ejphar.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Xu JT, Tu HY, Xin WJ, Liu XG, Zhang GH, Zhai CH. Activation of phosphatidylinositol 3-kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp Neurol. 2007;206:269–279. doi: 10.1016/j.expneurol.2007.05.029. [DOI] [PubMed] [Google Scholar]