Abstract
Objective:
To evaluate whether vitamin D is associated with multiple sclerosis (MS) status and disease severity in African Americans.
Methods:
Serum 25-hydroxyvitamin D was compared in a cross-sectional sample of 339 African Americans with MS and 342 African American controls. Correlations between disease severity (Multiple Sclerosis Severity Score [MSSS]) and 25-hydroxyvitamin D levels were sought.
Results:
A total of 71% of controls and 77% of patients with MS were vitamin D deficient (<50 nmol/L; <20 ng/mL), and 93% of controls and 94% of patients with MS were vitamin D insufficient (<75 nmol/L; <30 ng/mL). Median unadjusted (29.7 vs 36.6 nmol/L, p = 0.0001) and deseasonalized (p = 0.0013) 25-hydroxyvitamin D levels were lower in the MS group. Multivariable analysis revealed that differences in latitude and ultraviolet index accounted for much of this association. The median (interquartile range) MSSS was 6.1 (4.8–8.1). There was no apparent association between the MSSS and vitamin D status. A greater proportion of European genetic ancestry, a measure of genetic admixture, was positively correlated with 25-hydroxyvitamin D (p = 0.007).
Conclusions:
Levels of 25-hydroxyvitamin D were lower in African Americans with MS than controls, an observation primarily explained by differences in climate and geography. There was no apparent association between vitamin D status and disease severity. These results are consistent with observations in other populations that lower 25-hydroxyvitamin D is associated with having MS, but also highlight the importance of climate and ancestry in determining vitamin D status.
Multiple sclerosis (MS) is less prevalent in African Americans than whites,1,2 but the disease course tends to be more severe, with a greater risk for secondary progression and ambulatory disability.3,4
Low vitamin D status is reported to be a major risk factor for MS susceptibility5,6 and severity.7–11 The mechanism likely relates to effects of vitamin D on multiple pathways of the innate and adaptive immune systems.12–14 Vitamin D may also influence HLA-DRB1*15 expression, the main MS susceptibility allele in whites.15
Most of the vitamin D supply in humans comes from cutaneous synthesis following sun exposure.16 In national surveys, African Americans have a lower vitamin D status than non-Hispanic whites and Mexican Americans.17,18 The most likely explanation for this disparity is that melanin, the primary determinant of skin pigmentation, functions as an optical filter of ultraviolet (UV) light, limiting cutaneous vitamin D synthesis.19–23 Darker pigmented individuals require longer UV exposure times than lighter pigmented individuals to synthesize equivalent amounts of previtamin D3,21,22 although vitamin D status may be more important than skin pigmentation in determining absolute amounts of vitamin D production following UV exposure.24
A nested-case control study reported an association between vitamin D status and MS susceptibility in whites, but not in Hispanics or African Americans.5 Subsequent studies examining vitamin D and MS disease severity were conducted in populations that were predominantly white.7–9 Using a large, cross-sectional African American dataset, we asked whether vitamin D status is associated with having MS and whether lower vitamin D status correlates with MS disease severity.
METHODS
Study sample.
The African American Multiple Sclerosis Genetics Project is a study of self-identified African Americans with MS and unaffected African American controls, some of whom were spouses or friends of affected participants.25 Participants were referred through a nationwide network of MS centers. Blood and medical records were collected from subjects upon entry into the study. The collection as a whole is estimated to account for 3.6%–5.4% of all African Americans with MS in the United States.26
A subset of 345 African Americans with MS and 345 unaffected African American controls were selected from the African American Multiple Sclerosis Genetics Project for whom detailed clinical information, DNA, and serum samples were available. Six cases and 3 controls were excluded from this initial sample due to technical limitations or missing data, for a final sample of 339 cases and 342 controls. Blood samples were collected between 1998 and 2009.
All MS cases met International Panel diagnostic criteria27 as confirmed by systematic medical record review (medical records were reviewed by B.A.C.C.).3 Disability at the time of blood draw was measured by the Expanded Disability Status Scale (EDSS),28 and disease severity was measured by the Multiple Sclerosis Severity Score (MSSS),29 which incorporates disease duration and EDSS. A single investigator (B.A.C.C.) calculated the EDSS and MSSS. Genotyping of the HLA-DRB1 locus was performed as described previously.25 To account for possible influences of genetic heterogeneity on vitamin D status, the proportion of European genetic ancestry, a measure of genetic admixture, was measured by genotyping reference and variant single nucleotide polymorphisms from representative African American and European populations, as described previously.30
Standard protocol approvals, registrations, and patient consents.
All participants provided written informed consent, and the UCSF Committee on Human Research approved the study protocol.
Vitamin D status.
Blood samples were drawn upon enrollment into the study and sent to the UCSF MS Genetics laboratory for processing and storage (at −80°C). Samples selected for this study were then sent to a reference laboratory at UC Davis Medical Center to measure 25-hydroxyvitamin D using the Diasorin Liaison Total Vitamin D assay.31 The reference laboratory was blinded to case/control status. Interassay coefficients of variation for this assay were 8.3% for low (mean 15.9 ng/mL) and 6.3% for high (mean 55.0 ng/mL) control samples. Levels below the assay limit (<10 nmol/L) were assigned a value of 5 nmol/L for analysis.
Levels of 25-hydroxyvitamin D are reported in nmol/L (to convert to ng/mL, divide by 2.496).
An analysis of variance was performed to examine whether seasonal variation in vitamin D status in the control population differed between broad latitude bands (table e-1 on the Neurology® Web site at www.neurology.org).5 Deseasonalized vitamin D levels were calculated by regressing the measured 25-hydroxyvitamin D level (in nmol/L) on the periodic function: sin(2π[day of year drawn/365]) × cos(2π[day of year drawn/365]) × sin(2π[day of year drawn/183]) × cos(2π[day of year drawn/183]), and then adding the residuals to the seasonal average to create a deseasonalized vitamin D level for each individual.5,8 This provides a way to adjust for the seasonal variation of 25-hydroxyvitamin D given that blood samples were collected at nonuniform times throughout the year. “January 1” deseasonalized values were arbitrarily chosen for analysis, though in a periodic function any date would be expected to have been equally informative (and subject to the same limitations). Given the assumptions and limitations inherent in deseasonalized analysis, we also performed and report parallel analyses using unadjusted 25-hydroxyvitamin D values.
Geographic location, latitude, and UV index.
Geographic location at the time of blood draw was determined by the US Postal Service zip code of the participant's home address; for participants with no listed home address, the zip code of the referring MS center was substituted. The corresponding latitude for each zip code was obtained from a commercially available dataset (ZIPList5 Geocode Database, Zipinfo.com, Woodlands, TX). The average UV index for the month and nearest of 58 forecast cities to the location and time of blood draw was assigned based on 1997 monthly means published by the National Weather Service (http://www.cpc.noaa.gov/products/stratosphere/uv_index/uv_meanmax.shtml). The UV index is an ordinal scale from 0 (minimal UV exposure) to 11+ (extremely high) of the strength of UV solar radiation.
Statistical analysis.
For univariate analyses (table 1 and figure), the Wilcoxon rank sum test was used to test for differences in 25-hydroxyvitamin D status between the MS and control groups. The χ2 test was used for categorical variables.
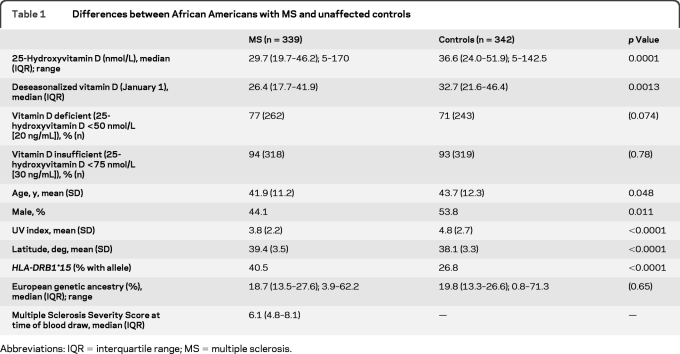
Table 1.
Differences between African Americans with MS and unaffected controls
Abbreviations: IQR = interquartile range; MS = multiple sclerosis.
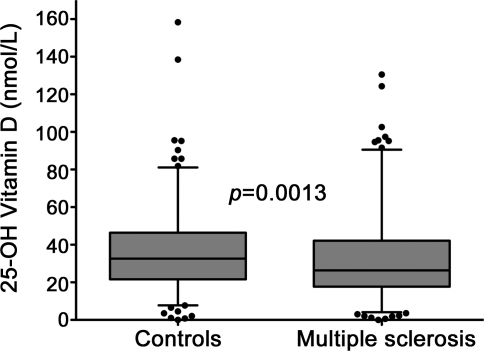
Figure. 25-Hydroxyvitamin D levels are lower in African Americans with multiple sclerosis than controls.
Levels of 25-hydroxyvitamin D (deseasonalized) were lower in African Americans with multiple sclerosis than controls in this cross-sectional dataset (p = 0.0013). The box plot indicates the median and interquartile range, the whiskers designate 1.5 times the interquartile distance, and the dots denote outlying values. The p value was calculated by the Wilcoxon rank sum test.
To examine whether vitamin D status is associated with having MS, logistic regression was performed, adjusting for age, sex, and HLA-DRB1*15 status (tables 2 and e-2). Models adjusting for latitude (for deseasonalized 25-hydroxyvitamin D) and UV index (for unadjusted 25-hydroxyvitamin D) were also analyzed to examine the influence of baseline geographic and climatic differences.
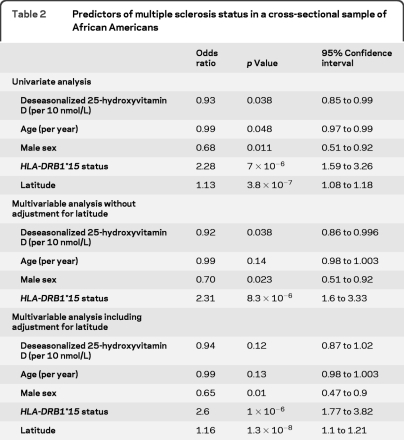
Table 2.
Predictors of multiple sclerosis status in a cross-sectional sample of African Americans
To examine predictors of vitamin D status in both the control group and study sample as a whole, multiple linear regression was performed, adjusting for age, sex, HLA-DRB1*15 status, proportion European genetic ancestry, MS status, latitude (for deseasonalized 25-hydroxyvitamin D values), and UV index (for unadjusted 25-hydroxyvitamin D values).
To analyze the association between vitamin D status and disease severity (MSSS) in the MS group, multiple linear regression was performed, adjusting for age, sex, HLA-DRB1*15 status, and proportion European genetic ancestry. Ordinal logistic regression (categorizing by MSSS 0 to ≤2, >2 to ≤4, >4 to ≤6, >6 to ≤8, >8 to ≤10) and logistic regression (low [MSSS ≤6] vs high [MSSS >6] disability) models were also examined.
Variables for 25-hydroxyvitamin D (unadjusted and deseasonalized), MSSS, and proportion European genetic ancestry were transformed as required to ensure normality. Statistical analysis was performed using Stata 10.1 (Stata Corp., College Station, TX).
RESULTS
The median (interquartile range) unadjusted 25-hydroxyvitamin D level was 29.7 (19.7–46.2) nmol/L in the MS group and 36.6 (24.0–51.9) nmol/L in controls (p = 0.0001) (table 1). Seventy-seven percent of the MS group and 71% of controls had 25-hydroxyvitamin D levels in a range commonly considered to be vitamin D deficient (<50 nmol/L or <20 ng/mL), and 94% of the MS group and 93% of controls had levels in a range commonly considered to be vitamin D insufficient (<75 nmol/L or <30 ng/mL).16
The distribution of 25-hydroxyvitamin D levels was modestly left skewed with a long right tail. Differences in the seasonal variation of unadjusted 25-hydroxyvitamin D levels were examined in the control population across 3 latitude bands (<37 degrees, ≥37 to <41 degrees, and ≥41 degrees) (table e-1). Season was highly associated with vitamin D status across all latitude bands, but differences in amounts of seasonal variation were not significant between latitude bands (analysis of variance, p = 0.11); similar results were obtained using the square root of unadjusted 25-hydroxyvitamin D, which was normally distributed (p = 0.08). Because season was highly correlated with vitamin D status and there were no significant differences in seasonal variation between latitude bands, we elected to use deseasonalized vitamin D levels for subsequent analysis. Entirely separate analyses by latitude band were not possible based on sample size. A parallel analysis using unadjusted 25-hydroxyvitamin D values is also reported.
Compared with the control group, the MS group was slightly younger, more likely to be female, tended to live in higher latitudes, and had a lower average monthly UV index at the time of blood draw (table 1). Proportion of European genetic ancestry was similar between the MS group and controls.
Deseasonalized 25-hydroxyvitamin D was slightly but significantly lower in the MS group compared to controls (p = 0.001) (table 1 and figure), as were unadjusted 25-hydroxyvitamin D levels. On multivariable analysis (table 2), lower deseasonalized 25-hydroxyvitamin D remained associated with MS status after adjustment for age, sex, and HLA-DRB1*15 (which was highly associated with MS status). Differences in latitude between groups accounted for much of this apparent association. Similar results were observed using unadjusted 25-hydroxyvitamin D levels and adjusting for UV index (table e-2).
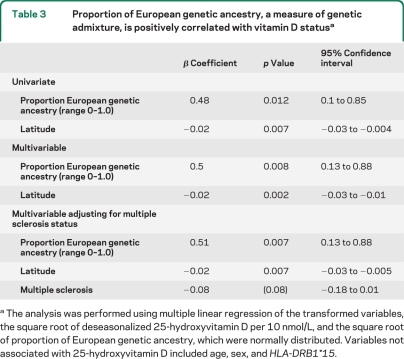
A greater proportion of European genetic ancestry was positively correlated with 25-hydroxyvitamin D status in this African American dataset, an association that retained statistical significance even after adjusting for differences in latitude (table 3) or UV index (table e-3). The association between European genetic admixture and vitamin D status was also present in the control population alone (p = 0.012 univariate, p = 0.01 adjusting for latitude). As expected, UV index and latitude were highly correlated with 25-hydroxyvitamin D status. A possible interaction between MS status and proportion of European ancestry was examined for by adding a multiplicative term to the model and not found. Additional factors that were not predictive of vitamin D status were age, sex, HLA-DRB*15 status, and length of blood storage time.
Table 3.
Proportion of European genetic ancestry, a measure of genetic admixture, is positively correlated with vitamin D statusa
The analysis was performed using multiple linear regression of the transformed variables, the square root of deseasonalized 25-hydroxyvitamin D per 10 nmol/L, and the square root of proportion of European genetic ancestry, which were normally distributed. Variables not associated with 25-hydroxyvitamin D included age, sex, and HLA-DRB1*15.
The median (interquartile range) MSSS of the MS group was 6.1 (4.8–8.1), indicating a high rate of ambulatory disability in this study sample. There was no association between MSSS and vitamin D status using linear regression. Per 10 nmol/L deseasonalized 25-hydroxyvitamin D, the β coefficient was −0.034, 95% confidence interval (CI) −0.15 to 0.08, p = 0.57. Per 10 nmol/L unadjusted 25-hydroxyvitamin D, the β coefficient was −0.034, 95% CI −0.15 to 0.08, p = 0.56. There was no association between low vs high MSSS (MSSS ≤6 vs MSSS >6) and vitamin D status using logistic regression for deseasonalized vitamin D (odds ratio [OR] 0.97, 95% CI 0.65 to 1.43, p = 0.86) or unadjusted 25-hydroxyvitamin D (OR 1.009, 95% CI 0.66 to 1.5, p = 0.97), adjusting for age and sex. There was also no association between MSSS and vitamin D status using ordinal logistic regression (categorizing by MSSS 0 to ≤2, >2 to ≤4, >4 to ≤6, >6 to ≤8, >8 to ≤10). Adjusting for HLA-DRB1*15 status and proportion of European genetic ancestry in the above regression models did not alter this result. There was no association between MSSS and age, HLA-DRB1*15 status, proportion of European genetic ancestry, or latitude (for deseasonalized) or UV index (for unadjusted 25-hydroxyvitamin D). There was no apparent association between EDSS and unadjusted or deseasonalized 25-hydroxyvitamin D.
DISCUSSION
This is the largest study to date examining vitamin D status in African Americans with MS. Levels of 25-hydroxyvitamin D were low in both the MS and control groups. Three-quarters of all participants were vitamin D deficient (<50 nmol/L [20 ng/mL]) and 94% were vitamin D insufficient (<75 nmol/L [30 ng/L]). This degree of vitamin D insufficiency may have important implications for bone health, cardiovascular, inflammatory, and neoplastic disease, in addition to its possible role in modulating MS.16
Levels of 25-hydroxyvitamin D were lower in African Americans with MS than controls. This association was weakened, but still present, when controlling for differences in climate and geography between groups. The cross-sectional study design does not allow for a determination of causality, and possible explanations include an influence of vitamin D on MS susceptibility or the reverse causality of an influence of having MS on vitamin D status. UV radiation, which was very strongly correlated with both MS and vitamin D status in this dataset, may also have direct immunosuppressant effects32 and was recently shown to suppress disease activity in a murine model of experimental autoimmune encephalomyelitis independently of 25-hydroxyvitamin D production.33 The cross-sectional nature of our dataset does not allow for a determination of the relative contributions of UV radiation and vitamin D status in this kind of analysis. Longitudinal studies with larger samples of African Americans will be needed to establish cause-effect relationships between vitamin D status and MS in this population.
There was no apparent association between 25-hydroxyvitamin D and MS disease severity (MSSS) in this dataset. The lack of an association between vitamin D status and disease severity could be a consequence of sample size limitations, smaller effect sizes in darker pigmented populations, or the use of the MSSS, and not relapse rate, as a marker of disease severity.7,8 Relapse rate was not determined in this dataset, as clinical variables were ascertained by retrospective chart review. It is possible that vitamin D status might influence the relapse rate independently of cumulative disability. It is also possible that the biological consequences of a lower vitamin D status in darker pigmented populations might be different than in lighter pigmented populations.34 In rheumatoid arthritis, an analogous association between lower vitamin D status and worsened disease severity that was seen in a predominantly white population35 was not observed in a predominantly African American population.36
There is substantial genetic heterogeneity within African American populations,37 and in this dataset, European genetic ancestry ranged from 1% to 71% (median 19%). The proportion of European genetic ancestry was one of the strongest predictors of vitamin D status (but not MS status) in this African American study population. The positive correlation between European genetic ancestry and vitamin D status might simply reflect unmeasured differences in skin pigmentation, but genetically influenced differences in vitamin D utilization cannot be excluded.38
Because of the genetic heterogeneity that is masked and diluted by traditional definitions of race, there is ongoing debate in the medical and scientific community about whether race should still be used to characterize populations for biological investigation.39 Percent African genetic ancestry was recently reported to be more informative than race in defining normative values for pulmonary function testing.40 The results from our study similarly illustrate how a measure of genetic heterogeneity was able to reveal a biological association with vitamin D status that might have been obscured using racial classification alone. This observation highlights the potential importance of controlling for genetic admixture in future studies of vitamin D in addition to, or in place of, race.
There are a number of limitations to this analysis. The study sample was not population-based and may not be reflective of all African American MS populations. The high degree of ambulatory disability in this dataset, however, is typical of other study populations of African Americans with MS. (The median MSSS was 6.0 in African Americans in the New York State Multiple Sclerosis Consortium dataset,4 which is very similar to the median MSSS of 6.1 in our study.) Data on other possible confounders of MS susceptibility, such as Epstein-Barr virus serology, infectious mononucleosis history, and smoking history, were not collected as part of this study. The average UV index at the time of blood draw is also only a rough estimate of ambient UV radiation and does not measure actual sunlight exposure, which could lead to residual confounding. Finally, genetic polymorphisms of vitamin D pathway proteins were not analyzed. Whether such differences might influence MS susceptibility and severity remains an important question.
Levels of 25-hydroxyvitamin D were lower in African Americans with MS than controls in this cross-sectional dataset. Differences in climate and geography accounted for much, but not all, of this association. There was no association between vitamin D status and MS disease severity (MSSS or EDSS). Greater European genetic ancestry was positively correlated with vitamin D status. Larger, prospective studies of diverse populations are needed to explore the relationship of MS and vitamin D further.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Cuquita Gomez, Rosa Guerrero, and Robin Lincoln for sample processing and database management; Soraya Foutouhi, Teri Cook, and John Tsushima from the Research and Reference Laboratories of the UC Davis Department of Medical Pathology and Laboratory Medicine for sample processing; and the patients and controls who participate in the African American Multiple Sclerosis Genetics Project.
- CI
- confidence interval
- EDSS
- Expanded Disability Status Scale
- MS
- multiple sclerosis
- MSSS
- Multiple Sclerosis Severity Score
- OR
- odds ratio
- UV
- ultraviolet
Supplemental data at www.neurology.org
DISCLOSURE
Dr. Gelfand receives research support from the NIH (training grant) and a UCSF CTSI Resident Research Grant and his wife is an editorial team member of Neurology®'s Resident & Fellow Section. Dr. Cree serves on a scientific advisory board for Biogen Idec; serves as a consultant for Biogen Idec, Elan Corporation, Genzyme Corporation, sanofi-aventis, and Teva Pharmaceutical Industries Ltd.; and receives research support from BioMS Medical, EMD Serono, Inc., Genentech, Inc., the NIH, and the National Multiple Sclerosis Society. Dr. McElroy reports no disclosures. Dr. Oksenberg serves an as Associate Editor for Annals of Neurology® and receives research support from the NIH and the National Multiple Sclerosis Society. Dr. R. Green serves on the editorial board of Nutrition and Dietary Supplements; serves as a consultant for Emisphere Technologies, Inc.; receives research support from the NIH (NIDDK, NCI), the US Department of Defense, and the American Egg Board; holds stock in Amgen, Merck Serono, and Onyx Pharmaceuticals; and has provided expert advice in medico-legal cases. Dr. Mowry receives research support from the NIH, the National Multiple Sclerosis Society Sylvia Lawry Fellowship Award, and the Partners MS Center Clinical Fellowship Award. Dr. Miller serves as a consultant for and has received funding for travel from PAR Pharmaceutical; and receives research support from the NIH and the US Department of Defense. Dr. Hauser has served as both a scientific advisory board member and consultant to BioMarin Pharmaceutical Inc. and Receptos Inc., and as a consultant to Novartis; has received travel accommodations from Roche; his institution (UCSF) has received speaker honoraria from Wyeth-Pfizer Inc; serves as Editor-in-Chief of Annals of Neurology; receives publishing royalties for Harrison's Principles of Internal Medicine (McGraw-Hill Publishers, 1994–present); and has received stock options from Receptos Inc., all of which were transferred to his institution (UCSF). Dr. A.J. Green serves on scientific advisory boards for Biogen Idec and Novartis; serves on the editorial board of Neurology®; receives research support from the NIH and the Howard Hughes Medical Institute; and has served as an expert witness in a legal proceeding.
REFERENCES
- 1. Kurtzke JF, Beebe GW, Norman JE., Jr Epidemiology of multiple sclerosis in U.S. veterans: 1: race, sex, and geographic distribution. Neurology 1979;29:1228–1235 [DOI] [PubMed] [Google Scholar]
- 2. Wallin MT, Page WF, Kurtzke JF. Multiple sclerosis in US veterans of the Vietnam era and later military service: race, sex, and geography. Ann Neurol 2004;55:65–71 [DOI] [PubMed] [Google Scholar]
- 3. Cree BA, Khan O, Bourdette D, et al. Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology 2004;63:2039–2045 [DOI] [PubMed] [Google Scholar]
- 4. Kister I, Chamot E, Bacon JH, et al. Rapid disease course in African Americans with multiple sclerosis. Neurology 2010;75:217–223 [DOI] [PubMed] [Google Scholar]
- 5. Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006;296:2832–2838 [DOI] [PubMed] [Google Scholar]
- 6. Islam T, Gauderman WJ, Cozen W, Mack TM. Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology 2007;69:381–388 [DOI] [PubMed] [Google Scholar]
- 7. Simpson S, Jr, Taylor B, Blizzard L, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol 2010;68:193–203 [DOI] [PubMed] [Google Scholar]
- 8. Mowry EM, Krupp LB, Milazzo M, et al. Vitamin D status is associated with relapse rate in pediatric-onset multiple sclerosis. Ann Neurol 2010;67:618–624 [DOI] [PubMed] [Google Scholar]
- 9. Soilu-Hanninen M, Laaksonen M, Laitinen I, Eralinna JP, Lilius EM, Mononen I. A longitudinal study of serum 25-hydroxyvitamin D and intact parathyroid hormone levels indicate the importance of vitamin D and calcium homeostasis regulation in multiple sclerosis. J Neurol Neurosurg Psychiatry 2008;79:152–157 [DOI] [PubMed] [Google Scholar]
- 10. Smolders J, Menheere P, Kessels A, Damoiseaux J, Hupperts R. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler 2008;14:1220–1224 [DOI] [PubMed] [Google Scholar]
- 11. van der Mei IA, Ponsonby AL, Dwyer T, et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia J Neurol 2007;254:581–590 [DOI] [PubMed] [Google Scholar]
- 12. Rigby WF, Waugh M, Graziano RF. Regulation of human monocyte HLA-DR and CD4 antigen expression, and antigen presentation by 1,25-dihydroxyvitamin D3. Blood 1990;76:189–197 [PubMed] [Google Scholar]
- 13. Smolders J, Damoiseaux J, Menheere P, Hupperts R. Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol 2008;194:7–17 [DOI] [PubMed] [Google Scholar]
- 14. Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain 2009;132:1146–1160 [DOI] [PubMed] [Google Scholar]
- 15. Ramagopalan SV, Maugeri NJ, Handunnetthi L, et al. Expression of the multiple sclerosis-associated MHC class II Allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet 2009;5:e1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–281 [DOI] [PubMed] [Google Scholar]
- 17. Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr 2008;88:558S–564S [DOI] [PubMed] [Google Scholar]
- 18. Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population 1988–2004. Arch Intern Med 2009;169:626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaidbey KH, Agin PP, Sayre RM, Kligman AM. Photoprotection by melanin: a comparison of black and Caucasian skin. J Am Acad Dermatol 1979;1:249–260 [DOI] [PubMed] [Google Scholar]
- 20. Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol 2000;39:57–106 [DOI] [PubMed] [Google Scholar]
- 21. Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science 1981;211:590–593 [DOI] [PubMed] [Google Scholar]
- 22. Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982;1:74–76 [DOI] [PubMed] [Google Scholar]
- 23. Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci USA 2010;107(suppl 2):8962–8968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bogh MK, Schmedes AV, Philipsen PA, Thieden E, Wulf HC. Vitamin D production after UVB exposure depends on baseline vitamin D and total cholesterol but not on skin pigmentation. J Invest Dermatol 2010;130:546–553 [DOI] [PubMed] [Google Scholar]
- 25. Oksenberg JR, Barcellos LF, Cree BA, et al. Mapping multiple sclerosis susceptibility to the HLA-DR locus in African Americans. Am J Hum Genet 2004;74:160–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cree BA, Reich DE, Khan O, et al. Modification of multiple sclerosis phenotypes by African ancestry at HLA. Arch Neurol 2009;66:226–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–127 [DOI] [PubMed] [Google Scholar]
- 28. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452 [DOI] [PubMed] [Google Scholar]
- 29. Roxburgh RH, Seaman SR, Masterman T, et al. Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology 2005;64:1144–1151 [DOI] [PubMed] [Google Scholar]
- 30. Reich D, Patterson N, De Jager PL, et al. A whole-genome admixture scan finds a candidate locus for multiple sclerosis susceptibility. Nat Genet 2005;37:1113–1118 [DOI] [PubMed] [Google Scholar]
- 31. Ersfeld DL, Rao DS, Body JJ, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem 2004;37:867–874 [DOI] [PubMed] [Google Scholar]
- 32. Garssen J, Vandebriel RJ, De Gruijl FR, et al. UVB exposure-induced systemic modulation of Th1- and Th2-mediated immune responses. Immunology 1999;97:506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Becklund BR, Severson KS, Vang SV, DeLuca HF. UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc Natl Acad Sci USA 2010;107:6418–6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harris SS. Vitamin D and African Americans. J Nutr 2006;136:1126–1129 [DOI] [PubMed] [Google Scholar]
- 35. Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum 2004;50:72–77 [DOI] [PubMed] [Google Scholar]
- 36. Craig SM, Yu F, Curtis JR, et al. Vitamin D status and its associations with disease activity and severity in African Americans with recent-onset rheumatoid arthritis. J Rheumatol 2010;37:275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tishkoff SA, Reed FA, Friedlaender FR, et al. The genetic structure and history of Africans and African Americans. Science 2009;324:1035–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010;376:180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cooper RS, Kaufman JS, Ward R. Race and genomics. N Engl J Med 2003;348:1166–1170 [DOI] [PubMed] [Google Scholar]
- 40. Kumar R, Seibold MA, Aldrich MC, et al. Genetic ancestry in lung-function predictions. N Engl J Med 2010;363:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.