Abstract
Objectives:
If neuroplastic changes in aphasia are consistent across studies, this would imply relatively stereotyped mechanisms of recovery which could guide the design of more efficient noninvasive brain stimulation treatments. To address this question, we performed a meta-analysis of functional neuroimaging studies of chronic aphasia after stroke.
Methods:
Functional neuroimaging articles using language tasks in patients with chronic aphasia after stroke (n = 105) and control subjects (n = 129) were collected. Activation likelihood estimation meta-analysis determined areas of consistent activity in each group. Functional homology between areas recruited by aphasic patients and controls was assessed by determining whether they activated under the same experimental conditions.
Results:
Controls consistently activated a network of left hemisphere language areas. Aphasic patients consistently activated some spared left hemisphere language nodes, new left hemisphere areas, and right hemisphere areas homotopic to the control subjects' language network. Patients with left inferior frontal lesions recruited right inferior frontal gyrus more reliably than those without. Some areas, including right dorsal pars opercularis, were functionally homologous with corresponding control areas, while others, including right pars triangularis, were not.
Conclusions:
The network of brain areas aphasic patients recruit for language functions is largely consistent across studies. Several recruitment mechanisms occur, including persistent function in spared nodes, compensatory recruitment of alternate nodes, and recruitment of areas that may hinder recovery. These findings may guide development of brain stimulation protocols that can be applied across populations of aphasic patients who share common attributes.
In studies investigating transcranial magnetic stimulation (TMS) or transcranial direct current stimulation as treatments for aphasia, stimulation targets are typically selected by examining each subject individually using fMRI or TMS.1–3 These approaches create barriers to efficient treatment, and may be unnecessary if mechanisms of residual language function and recovery in aphasia are consistent across patients.
Aphasic patients activate both hemispheres during language tasks in functional neuroimaging studies. Activity in the left hemisphere (LH) is thought to support residual function and recovery.4–8 The role of the right hemisphere (RH) is less clear. RH activity may support recovery if homotopic areas take over functions of lesioned LH nodes,5,9–12 even if they are computationally less efficient.8,13 Alternatively, the RH may limit recovery if its processing is dysfunctional,14 or if transcallosal projections from the RH inhibit the LH.6,15–17 It is likely that some RH areas support recovery while others interfere,2,18 and others play no causal role.
To evaluate the consistency of neuroplastic mechanisms across patients, it is important to localize stable activity across methodologically diverse studies. If aphasic patients activate new brain areas in the same experimental contexts as control subjects activate normal language nodes, this would imply a homologous role in language processing for these new areas. We used activation likelihood estimation (ALE), a validated, quantitative neuroimaging meta-analysis method,19–21 to assess mechanisms of adaptation in aphasia by comparing brain activity of patients with chronic aphasia to that of normal control subjects across a variety of experimental contexts.
METHODS
Identification and selection of articles.
We searched PubMed, PsychINFO, and references of recent articles for fMRI or PET studies employing language tasks in patients with chronic aphasia after stroke and healthy controls. For inclusion, articles must have reported 1) results specifically in stroke-induced chronic aphasia; 2) activity for aphasic subjects and controls separately using the same tasks/analyses; 3) standard 3-dimensional coordinates. The ALE analyses only included direct task-vs-baseline contrasts with the lowest level control condition reported (e.g., verb generation vs rest, rather than verb generation vs repetition). The functional homology analysis included all available contrasts. Coordinates were converted to Montreal Neurological Institute space.22
Datasets.
Twelve articles met the inclusion criteria, with 319 activation foci from 105 aphasic patients and 267 foci from 129 control subjects (e-references and table e-1 on the Neurology® Web site at www.neurology.org). A total of 16 unique tasks were used, with 22 unique analyses of these tasks. The ALE analyses included 224 task-vs-baseline foci from aphasic subjects and 197 foci from control subjects. Approximately 70 patients were nonfluent based on heterogeneous classification methods across studies. Severity of aphasia varied, but all subjects were able to participate in language-related imaging tasks. To examine the impact of lesion location on activity, we split the aphasia dataset into a group of patients with lesions involving inferior frontal cortex (IF+; n = 65; approximately 62 nonfluent), and a group with lesions sparing it (IF−; n = 40; approximately 32 fluent). Inferior frontal lesion status was extrapolated from published text, figures, and tables. Case series data were split subject-by-subject. Groups with a majority of inferior frontal lesions were included in the IF+ list, and groups with a minority were included in the IF− list. This method assigned 90/105 patients correctly. The small proportion of misassignments should not impact results because significance required agreement across multiple studies.
As an illustrative example, study 1 compared 3 patients with nonfluent aphasia due to inferior frontal strokes to 4 age-matched controls using fMRI.14 Brain activity when subjects named pictures in the scanner (task) was compared to activity during visual fixation (baseline). Controls activated classic LH language areas. In contrast, aphasic subjects activated perilesional LH areas plus RH equivalents of the lesioned language areas. This activity was included in the main ALE analysis. Correlations between naming activity and item variables (e.g., age at acquisition) were reported for both groups, and included in the functional homology analysis.
ALE analyses.
ALE is an objective, quantitative, validated meta-analysis method that identifies brain areas at which colocalization of activity occurs across studies beyond what is predicted by chance.21 Coordinates of peak activity in published articles (“foci”) serve as the data. The uncertainty in localization of foci is modeled as Gaussian probability fields.21 The union of probabilities across studies yields voxel-wise activation likelihoods (ALE values), which indicate the level of agreement in location of activity across studies. Gaussian widths are calculated from sample sizes based on the relationship between N and localization uncertainty.19,23 We used an ALE algorithm unbiased by differences between studies in the number of foci or experiments (GingerALE2.04, BrainMap.org).23 Significance was tested as described by Eickhoff and colleagues19 against the null hypothesis that localization of activity is independent between studies, with a false discovery rate (FDR) of 0.01, and a cluster extent threshold of 100 mm3. Clusters and peaks were considered significant only if 3 or more studies activated them in task-vs-baseline analyses.24 Studies that reported a focus within 2 SD of localization uncertainty from an ALE peak were considered to have activated that location. These 2 SD kernels capture 95% of foci corresponding to each ALE peak. AAL atlas anatomic labels were assigned.25
Functional homology analysis.
For each peak in the aphasia ALE maps, we identified corresponding control ALE peaks, which we hypothesized the aphasic areas would function like. For each LH aphasia peak, we chose the nearest control peak with the same anatomic label. If a differently labeled control peak was closer by Euclidean distance, we selected it also. For each RH aphasia peak, homotopic control peaks were identified by left-right reversing coordinates and following the same selection rule as above. Next, we determined which analyses activated each ALE peak location using the kernel method described above.
The functional homology assessment compared an aphasic ALE peak to a corresponding control peak, determined the agreement between them in terms of the unique analyses that activated both or failed to activate both, and calculated the probability that the observed agreement occurred by chance. For each control ALE peak, we tallied a list of analyses that activated that location and a list of analyses that did not. For a corresponding aphasia ALE peak, we tallied the agreement in terms of analyses that activated the area and those that did not. The probability of equal or greater agreement between an aphasia list and a control list was calculated as the product of 2 cumulative binomial probabilities, one giving the probability of agreement among the analyses active for controls, and one giving the probability of agreement among analyses not active for controls. To confirm these calculations, we performed 5 simulations, each comparing 5 million random aphasic activation lists to a different control ALE site. The binomial probabilities matched the simulated probabilities to within 0.00025. Probabilities were converted to Z scores. Significance was determined using an FDR q of 0.05 multiple comparisons correction.
RESULTS
Controls.
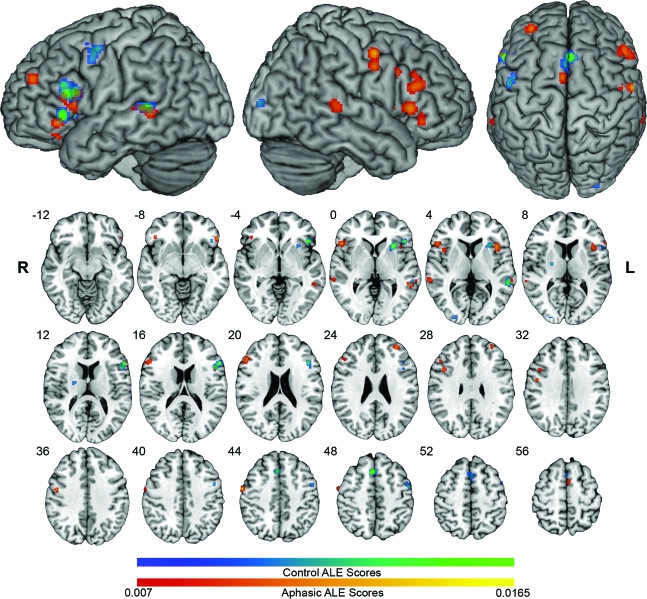
The ALE analysis of the control dataset revealed an expected network of LH cortical activation likelihoods associated with various language and motor processes (table e-2; figure 1). Corresponding to the preponderance of speech production tasks, much of the activation likelihood was in the left inferior frontal gyrus (IFG) with peaks in the pars opercularis (POp), triangularis (PTr), and orbitalis (POrb). The left posterior middle temporal gyrus (pMTG) also demonstrated significant activation likelihood.
Figure 1. Activation likelihood estimation (ALE) maps in all aphasic patients and control subjects.
Control ALE clusters are in blue-green scale, and show left hemisphere language and motor activity. ALE clusters for the group of all aphasic patients are in red-yellow scale. Significant areas in ALE maps represent locations at which peak activity is highly likely to occur in functional imaging experiments, not functional activity per se. ALE maps are overlaid on the standard Colin brain in Montreal Neurological Institute (MNI) space, using a false discovery rate q = 0.01 critical threshold, and minimum cluster size of 100 mm3. Slices are in radiologic orientation, with the corresponding MNI Z coordinate.
Aphasic subjects.
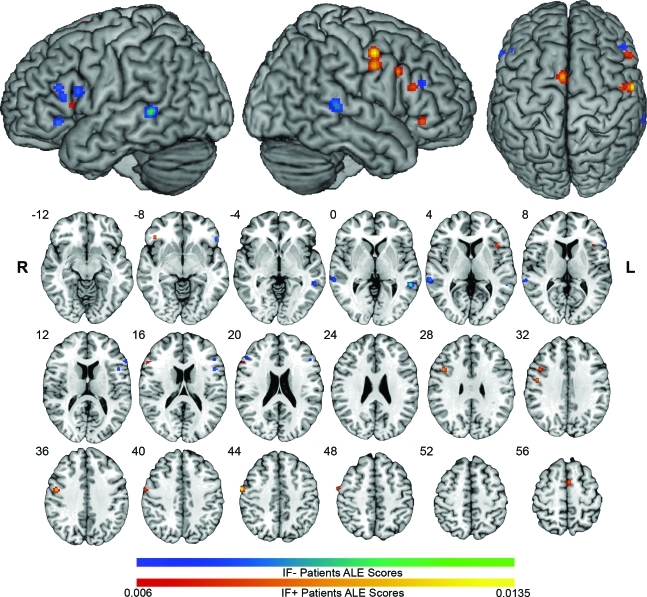
The ALE analysis of all patients with aphasia demonstrated a bilateral distribution of activation likelihoods representing areas of consistent activity across multiple studies. This network included spared areas of the normal LH language network, LH areas outside the normal network, and RH areas that mirrored the LH network in controls (table e-2 and figure 1). Like controls, most activation likelihood was in the IFG, although bilaterally for aphasic subjects. For this reason, we focus our attention on the IFG below. Subanalyses of the IF+ and IF− groups demonstrated that left inferior frontal lesions resulted in greater likelihood of right IFG activation (figure 2).
Figure 2. Activation likelihood estimation (ALE) maps of inferior frontal cortex (IF)+ and IF− groups.
IF− ALE clusters are in blue-green scale. IF+ ALE clusters are in red-yellow scale. ALE maps are overlaid on the standard Colin brain in Montreal Neurological Institute (MNI) space, using a false discovery rate q = 0.01 critical threshold, and minimum cluster size of 100 mm3. Slices are in radiologic orientation, with the corresponding MNI Z coordinate.
In the LH, ALE clusters that overlapped spatially with clusters in the control map (i.e., normal language nodes spared by lesions) were also functionally homologous to them (PTr, POp, and pMTG). Among clusters that did not overlap spatially with control clusters, and hence represented recruitment of areas outside the normal language network activated by these tasks, some were functionally homologous to nearby control areas (POrb, anterior insula) and others were not (middle frontal gyrus).
RH clusters in the aphasia maps were homotopic to LH control clusters to within 10 mm, except in the insula (12 mm). Different patterns of localization and homology were identified in different subregions of the right IFG. A right ventral POp cluster in the overall group (52, 20, 2) was active regardless of lesion site (3 studies in each group, nonsignificant in each group alone) and was functionally homologous to the control subjects' left POp. The dorsal right POp and the right POrb were present in the IF+ map, but not the IF− map, and were homotopic and homologous with their LH counterparts in the control map. A right PTr cluster in the IF+ map was closely homotopic with a LH control site (4.9 mm), but was not functionally homologous to it. The IF− group produced only one small right anterior PTr cluster, which was homotopic to a secondary IF− left PTr peak (6 mm) and coactivated in the same studies.
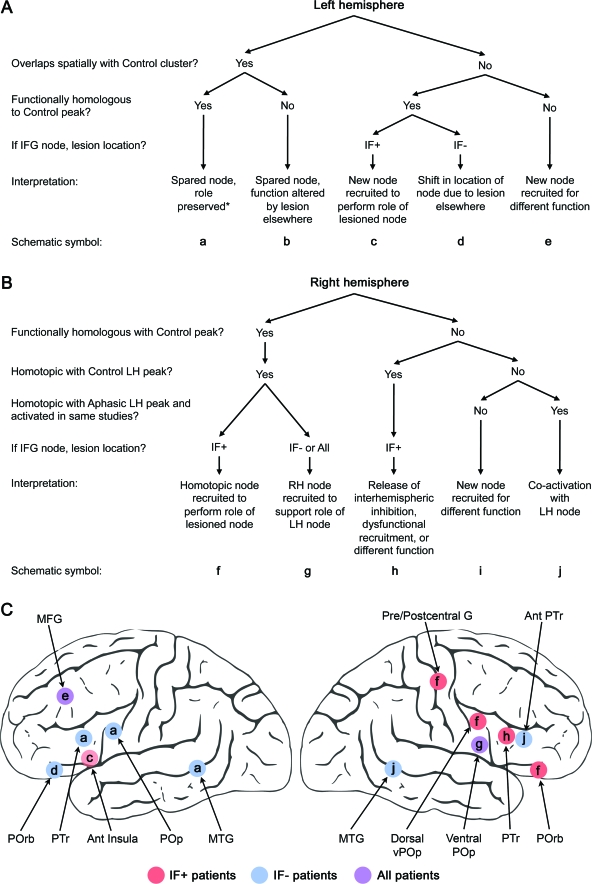
We developed interpretative algorithms to infer putative recruitment mechanisms for areas identified in the aphasia ALE maps (figure 3). Based on these algorithms, different compensatory mechanisms accounted for different areas consistently recruited by aphasic subjects. The summary schematics in figure 3 illustrate the main results.
Figure 3. Algorithms used for interpretation and schematics of main results.
Careful consideration of the response properties of areas identified in the aphasia activation likelihood estimation (ALE) analyses suggested reasonable hypotheses regarding the specific adaptive mechanisms to account for the activity. We interpreted the areas identified in the aphasia ALE maps algorithmically based on localization of activity relative to normal control activity, functional homology to corresponding normal control sites, and relationship of activity to lesion location. (A) The algorithm used for left hemisphere (LH) areas. (B) The algorithm used for right hemisphere (RH) areas. (C) Schematics of the main results. Since the right pre/postcentral gyrus activity occurred only in the inferior frontal cortex (IF)+ group, we assumed that the left pre/postcentral gyri were lesioned with the left inferior frontal gyrus (IFG) in most cases (this appeared to be true based on available figures in the papers). If this assumption were false, the correct interpretation would be g. *See Discussion for caveats. MTG=middle temporal gyrus; POp=pars opercularis; POrb=pars orbitalis; PTr=pars triangularis; MFG=middle frontal gyrus.
DISCUSSION
We quantitatively compared the brain activity of aphasic patients to that of controls across a variety of language-related tasks. This method allowed us to infer neuroplastic mechanisms in aphasia recovery, but not to identify specific language processes performed by particular areas. As such, we focus our discussion on general mechanisms of recovery rather than specific language functions.
The first main finding is that the network of brain regions recruited by chronic aphasic subjects is consistent across methodologically diverse imaging studies. This network is composed of spared areas of the normal LH language network, new LH nodes, and RH nodes that are homotopic to normal LH nodes. This consistency supports the notion that therapeutic brain stimulation targets might be selected based on group-level evidence. Still, patient-specific factors must result in differential recruitment from individual to individual. We were able to show that the recruitment pattern systematically varies based on lesion location, but further investigation is needed to identify other important patient-specific factors.
Second, the response properties of different areas activated by aphasic patients suggested different compensatory mechanisms. Regional differences in connectivity, inhibitory connections between nodes,26 and differences in the degree of lateralization between processes27,28 provide a basis for differences in mechanisms of recovery depending on which part of the network is disrupted.
In the LH, we identified consistent activity among aphasic subjects in both anterior and posterior language regions that overlapped spatially with those of controls, representing activity of patients whose lesions spared these sites. Although distant lesions can cause network disruption that alters activity in spared nodes,29,30 the spared sites identified here retained their normal role based on our homology measure.
Consistent aphasic activity was also identified in LH areas outside the normal network identified in control subjects. The aphasic POrb and anterior insula sites, which did not overlap spatially with control clusters, likely represent cortical sites in which activity was augmented to compensate for lesioned nodes. The anterior insula was recruited by patients with left inferior frontal lesions and was activated under the same experimental conditions as the normal POrb. This pattern implies that the anterior insula assumed functions of the POrb when it was lesioned. Note that the right POrb was also homologous to the normal left POrb in IF+ patients, suggesting that 2 alternate nodes were needed to compensate for the lost left POrb, possibly due to computational inefficiency of the alternate nodes. In contrast, activity in the left POrb site recruited by aphasic subjects, which was anatomically distinct from the control POrb site, only occurred in the patients without inferior frontal lesions, in whom it functioned like the normal control POrb site. Thus, lesions sparing the IFG entirely resulted in a shift of activity from the typically active part of POrb to an alternate site capable of serving the same role in language processing. This might occur due to deafferentation of the preferred POrb node by distant lesions or due to a subtle change in computational demands as new strategies for language processing are adopted by aphasic patients.
Aphasic clusters that did not share functional homology with control areas, like the left dorsolateral prefrontal (middle frontal gyrus) cluster, may reflect an increased reliance on supporting cognitive functions, like executive control of working memory, that are not heavily taxed during simple language tasks in normal control subjects.31
The meta-analytic findings in the RH demonstrate that across a variety of experimental conditions and patient characteristics, RH areas recruited by aphasic subjects are mirror images of LH areas recruited by normal healthy subjects for the same tasks. Although some studies report RH language activity in controls32,33 that may play a causal role in some language processes,34 none was consistent in controls across the studies examined here. Thus, the RH activity found here for aphasic subjects cannot be explained on the basis of normal recruitment of the RH.
In general, the right IFG was more reliably recruited when the left inferior frontal cortex was lesioned, but this differed between subregions of the IFG. Two different areas of the right PTr were recruited dependent on lesion location, and the function in both was unlike that of the normal left PTr. In contrast, the right dorsal POp was recruited specifically by patients with inferior frontal lesions, and functioned like the normal left POp, implying a possible compensatory takeover of the function of the lesioned node. Overall, these findings suggest that different mechanisms likely account for functional activity in different parts of the RH aphasic language network.
The functional role of the right posterior IFG (including POp) in aphasia recovery was initially suggested by Barlow,35 who reported the case of a boy who developed aphasia after a LH stroke, recovered language function, and then had a recurrence of aphasia after a second small stroke to the right posterior IFG. Evidence that inhibitory TMS to the right POp disrupts naming in chronic nonfluent aphasic subjects supports the functional role of the right POp in aphasia recovery.2,18 Dorsal stream phonologic processes, in which the POp are involved,36,37 may be relatively less lateralized compared to word level semantic processes.27,28 Evidence suggests that the right POp plays a causal role in phonologic processing even in normal subjects.34 It may then be well suited to sustain recovery of phonologic language functions when the function of the left POp is impaired.
In contrast, inhibition of the right PTr with TMS enhances naming and propositional speech performance in some chronic nonfluent aphasic subjects,2,6,16–18 suggesting the right PTr impairs some language functions in these patients. The lack of a relationship between the function of the right PTr and that of the left in our analysis suggests that the right PTr is truly dysfunctional with respect to language processing14 or that it plays a role in a completely different cognitive process than the left PTr. For example, if injury to the left PTr causes increased activation of the right PTr through a release of interhemispheric inhibition,8,38 the overactive right PTr may impede language production through an exaggeration of its normal role in response inhibition.39 The current analysis is not able to discern the specific role of the right PTr, but confirms that its function is fundamentally different from that of the left in aphasic patients and warrants further investigation.
The functional homology analysis allowed us to infer reasonable hypotheses regarding why aphasic patients activated specific areas based on whether they were involved in similar functions to normal language areas. Still, the simple presence of activity for a given task does not necessarily imply normal computational efficiency, so homologous areas discussed above may not function exactly like normal language areas in healthy subjects, even if their basic role in language processing is similar. The interpretation of activity becomes simpler (although not unambiguous) if only correct responses are considered, but only one study here did so, resulting in typical bilateral activation patterns in the task-vs-baseline comparison.40 Another study directly compared correct and incorrect responses, finding that most right IFG activity occurred during incorrect trials.14
Other causes of aphasia might result in different adaptive mechanisms, so conclusions here are limited to stroke-induced aphasia. Since neural adaptation in aphasia changes over time,5 these findings also cannot be extrapolated to acute or subacute aphasia. We elected to include any aphasia type or language task, but due to the few studies available we were unable to determine whether activation patterns and adaptive mechanisms vary based on these factors, which they almost certainly do. Some studies were excluded due to restrictions of the ALE method (e.g., standardized coordinates were not reported). We also excluded unpublished data because readers would have no way to verify that these experiments were valid. Including these studies could have reduced the homogeneity between studies and changed our results.
The goal of this analysis was to gain a broad perspective on residual and recovered language functions in aphasia by considering the anatomic and functional relationships between brain activity of aphasic subjects and controls across a variety of experimental conditions. Collectively, the results suggest a pattern of adaptation after lesions to LH language networks that involves retention of function where possible, anatomic shifts in LH processors when needed, and regionally specific variation in the mechanisms of RH recruitment that depends on lesion location. Localization and recruitment patterns are consistent across studies, providing support for targeting brain stimulation across populations of aphasic patients who share similar attributes, like lesion location. Further research will be needed to confirm the efficacy of this strategy, and to define the relevant patient-specific factors that impact target selection.
Supplementary Material
Supplemental data at www.neurology.org
- ALE
- activation likelihood estimation
- FDR
- false discovery rate
- IF
- inferior frontal cortex
- IFG
- inferior frontal gyrus
- LH
- left hemisphere
- pMTG
- posterior middle temporal gyrus
- POp
- pars opercularis
- POrb
- pars orbitalis
- PTr
- pars triangularis
- RH
- right hemisphere
- TMS
- transcranial magnetic stimulation
AUTHOR CONTRIBUTIONS
Dr. Turkeltaub contributed to the study conception and design, statistical analysis and interpretation, and drafting the manuscript. S. Messing contributed to the study conception and design and acquisition of data. C. Norise contributed to the analysis and interpretation and acquisition of data. Dr. Hamilton contributed to the study conception and design and study supervision and coordination.
DISCLOSURE
Dr. Turkeltaub is funded by the American Academy of Neurology Foundation (Clinical Research Training Fellowship) and the International Dyslexia Association (General Grant 2009). S. Messing owns stock in MEDITECH, Inc. C. Norise reports no disclosures. Dr. Hamilton receives research support from the NIH/NINDS and the Robert Wood Johnson Foundation/Harold Amos Medical Faculty Development Program.
REFERENCES
- 1. Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke 2010;41:1229–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin PI, Naeser MA, Ho M, et al. Research with transcranial magnetic stimulation in the treatment of aphasia. Curr Neurol Neurosci Rep 2009;9:451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Monti A, Cogiamanian F, Marceglia S, et al. Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry 2008;79:451–453 [DOI] [PubMed] [Google Scholar]
- 4. Karbe H, Kessler J, Herholz K, Fink GR, Heiss WD. Long-term prognosis of poststroke aphasia studied with positron emission tomography. Arch Neurol 1995;52:186–190 [DOI] [PubMed] [Google Scholar]
- 5. Saur D, Lange R, Baumgaertner A, et al. Dynamics of language reorganization after stroke. Brain 2006;129:1371–1384 [DOI] [PubMed] [Google Scholar]
- 6. Martin PI, Naeser MA, Ho M, et al. Overt naming fMRI pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post-TMS. Brain Lang 2009;111:20–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Winhuisen L, Thiel A, Schumacher B, et al. The right inferior frontal gyrus and poststroke aphasia: a follow-up investigation. Stroke 2007;38:1286–1292 [DOI] [PubMed] [Google Scholar]
- 8. Heiss WD, Thiel A, Kessler J, Herholz K. Disturbance and recovery of language function: correlates in PET activation studies. NeuroImage 2003;20(suppl 1):S42–S49 [DOI] [PubMed] [Google Scholar]
- 9. Kinsbourne M. The minor cerebral hemisphere as a source of aphasic speech. Arch Neurol 1971;25:302–306 [DOI] [PubMed] [Google Scholar]
- 10. Ohyama M, Senda M, Kitamura S, Ishii K, Mishina M, Terashi A. Role of the nondominant hemisphere and undamaged area during word repetition in poststroke aphasics: a PET activation study. Stroke 1996;27:897–903 [DOI] [PubMed] [Google Scholar]
- 11. Musso M, Weiller C, Kiebel S, Muller SP, Bulau P, Rijntjes M. Training-induced brain plasticity in aphasia. Brain 1999;122:1781–1790 [DOI] [PubMed] [Google Scholar]
- 12. Leff A, Crinion J, Scott S, Turkheimer F, Howard D, Wise R. A physiological change in the homotopic cortex following left posterior temporal lobe infarction. Ann Neurol 2002;51:553–558 [DOI] [PubMed] [Google Scholar]
- 13. Winhuisen L, Thiel A, Schumacher B, et al. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke 2005;36:1759–1763 [DOI] [PubMed] [Google Scholar]
- 14. Postman-Caucheteux WA, Birn RM, Pursley RH, et al. Single-trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. J Cogn Neurosci 2010;22:1299–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol 2007;3:383–393 [DOI] [PubMed] [Google Scholar]
- 16. Naeser MA, Martin PI, Nicholas M, et al. Improved naming after TMS treatments in a chronic, global aphasia patient: case report. Neurocase 2005;11:182–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naeser MA, Martin PI, Nicholas M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study. Brain Lang 2005;93:95–105 [DOI] [PubMed] [Google Scholar]
- 18. Hamilton RH, Sanders L, Benson J, et al. Stimulating conversation: enhancement of elicited propositional speech in a patient with chronic non-fluent aphasia following transcranial magnetic stimulation. Brain Lang 2010;113:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 2009;30:2907–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laird AR, Fox PM, Price CJ, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 2005;25:155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turkeltaub P, Eden G, Jones K, Zeffiro T. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage 2002;16:765–780 [DOI] [PubMed] [Google Scholar]
- 22. Lancaster JL, Tordesillas-Gutierrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 2007;28:1194–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Turkeltaub PE, Eickhoff SB, Laird AR, Fox PM, Wiener M, Fox PT. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp Epub 2011 Feb 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turkeltaub PE, Coslett HB. Localization of sublexical speech perception components. Brain Lang 2010;114:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002;15:273–289 [DOI] [PubMed] [Google Scholar]
- 26. Heim S, Eickhoff SB, Amunts K. Different roles of cytoarchitectonic BA 44 and BA 45 in phonological and semantic verbal fluency as revealed by dynamic causal modelling. NeuroImage 2009;48:616–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci 2007;8:393–402 [DOI] [PubMed] [Google Scholar]
- 28. Hickok G, Okada K, Barr W, et al. Bilateral capacity for speech sound processing in auditory comprehension: evidence from Wada procedures. Brain Lang 2008;107:179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Price CJ, Warburton EA, Moore CJ, Frackowiak RS, Friston KJ. Dynamic diaschisis: anatomically remote and context-sensitive human brain lesions. J Cogn Neurosci 2001;13:419–429 [DOI] [PubMed] [Google Scholar]
- 30. Grabowski TJ, Damasio H, Tranel D, et al. Residual naming after damage to the left temporal pole: a PET activation study. NeuroImage 2003;19:846–860 [DOI] [PubMed] [Google Scholar]
- 31. D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature 1995;378:279–281 [DOI] [PubMed] [Google Scholar]
- 32. Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage 1999;10:15–35 [DOI] [PubMed] [Google Scholar]
- 33. Raboyeau G, De Boissezon X, Marie N, et al. Right hemisphere activation in recovery from aphasia: lesion effect or function recruitment? Neurology 2008;70:290–298 [DOI] [PubMed] [Google Scholar]
- 34. Hartwigsen G, Price CJ, Baumgaertner A, et al. The right posterior inferior frontal gyrus contributes to phonological word decisions in the healthy brain: evidence from dual-site TMS. Neuropsychologia 2010;48:3155–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barlow T. On a case of double cerebral hemiplegia, with cerebral symmetrical lesions. BMJ 1877;2:103–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fiez JA. Phonology, semantics and the role of the left inferior prefrontal cortex. Hum Brain Mapp 1997;5:79–83 [PubMed] [Google Scholar]
- 37. Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci 2005;25:8010–8016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thiel A, Herholz K, Koyuncu A, et al. Plasticity of language networks in patients with brain tumors: a positron emission tomography activation study. Ann Neurol 2001;50:620–629 [DOI] [PubMed] [Google Scholar]
- 39. Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation asso-ciated with response inhibition is task-dependent. Neuropsychologia 2008;46:224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thompson CK, Bonakdarpour B, Fix SF. Neural mechanisms of verb argument structure processing in agrammatic aphasic and healthy age-matched listeners. J Cogn Neurosci 2010;22:1993–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.