Abstract
The sequences surrounding the first 5'GUC3' in the mRNA encoding the Alzheimer amyloid peptide precursor (beta APP) were used to construct a pair of transacting hammerhead ribozymes. Each ribozyme contained the conserved core bases of the hammerhead motif found in the positive strand of satellite RNA of tobacco ringspot virus [(+)sTRSV] and two stems, 7 and 8 bases long, complementary to the target, beta APP mRNA. However, one of the ribozyme cleaving strands was lengthened at its 3' end to include the early splicing and polyadenylation signal sequences of SV40 viral RNA. This RNA, therefore, more closely mimics transcripts produced by RNA polymerase II from eucaryotic expression vectors in vivo. RNA, prepared by run-off transcription of cDNA oligonucleotide or plasmid constructs containing a T7 RNA polymerase promoter was used to characterize several properties of the cleavage reaction. In the presence of both ribozyme cleaving strands magnesium-ion dependent cleavage of a model 26 base beta APP substrate RNA or full-length beta APP-751 mRNA was observed at the hammerhead consensus cleavage site. Neither ribozyme was active against non-message homologs of beta APP mRNA, nor was cleavage detected when point mutations were made in the conserved core sequences. However, the kcat/Km at 37 degrees C in 10 mM Mg+2 of the longer ribozyme was reduced twenty-fold when model and full-length substrates were compared. The use of short deoxyoligonucleotides (13-17 mers) that bind upstream of the ribozyme was found to enhance the rate of cleavage of the full-length but not beta APP model substrate RNAs. The rate of enhancement depended on both the length of the deoxyoligonucleotide used as well as its site of binding with respect to the ribozyme. These data demonstrate the utility of ribozymes to cleave target RNAs in a catalytic, site-specific fashion in vitro. Direct comparison of the efficiency of different ribozyme constructs and different modulating activities provide an experimental strategy for designing more effective ribozymes for therapeutic purposes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Been M. D., Perrotta A. T., Rosenstein S. P. Secondary structure of the self-cleaving RNA of hepatitis delta virus: applications to catalytic RNA design. Biochemistry. 1992 Dec 1;31(47):11843–11852. doi: 10.1021/bi00162a024. [DOI] [PubMed] [Google Scholar]
- Belinsky M. G., Dinter-Gottlieb G. Non-ribozyme sequences enhance self-cleavage of ribozymes derived from Hepatitis delta virus. Nucleic Acids Res. 1991 Feb 11;19(3):559–564. doi: 10.1093/nar/19.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier-Harlin M. C., Crawford F., Houlden H., Warren A., Hughes D., Fidani L., Goate A., Rossor M., Roques P., Hardy J. Early-onset Alzheimer's disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991 Oct 31;353(6347):844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Banerjea A. C., Harmison G. G., Haglund K., Schubert M. Multitarget-ribozyme directed to cleave at up to nine highly conserved HIV-1 env RNA regions inhibits HIV-1 replication--potential effectiveness against most presently sequenced HIV-1 isolates. Nucleic Acids Res. 1992 Sep 11;20(17):4581–4589. doi: 10.1093/nar/20.17.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Cotten M., Birnstiel M. L. Ribozyme mediated destruction of RNA in vivo. EMBO J. 1989 Dec 1;8(12):3861–3866. doi: 10.1002/j.1460-2075.1989.tb08564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman R. B., Miller D. L. Use of photobiotinylated deoxyoligonucleotides to detect cloned DNA. Biotechniques. 1989 Feb;7(2):138–141. [PubMed] [Google Scholar]
- Denman R. B., Purow B., Rubenstein R., Miller D. L. Hammerhead ribozyme cleavage of hamster prion pre-mRNA in complex cell-free model systems. Biochem Biophys Res Commun. 1992 Jul 31;186(2):1171–1177. doi: 10.1016/0006-291x(92)90870-q. [DOI] [PubMed] [Google Scholar]
- Denman R., Colgan J., Nurse K., Ofengand J. Crosslinking of the anticodon of P site bound tRNA to C-1400 of E.coli 16S RNA does not require the participation of the 50S subunit. Nucleic Acids Res. 1988 Jan 11;16(1):165–178. doi: 10.1093/nar/16.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman R., Potempska A., Wolfe G., Ramakrishna N., Miller D. L. Distribution and activity of alternatively spliced Alzheimer amyloid peptide precursor and scrapie PrP mRNAs on rat brain polysomes. Arch Biochem Biophys. 1991 Jul;288(1):29–38. doi: 10.1016/0003-9861(91)90161-b. [DOI] [PubMed] [Google Scholar]
- Denman R., Weitzmann C., Cunningham P. R., Nègre D., Nurse K., Colgan J., Pan Y. C., Miedel M., Ofengand J. In vitro assembly of 30S and 70S bacterial ribosomes from 16S RNA containing single base substitutions, insertions, and deletions around the decoding site (C1400). Biochemistry. 1989 Feb 7;28(3):1002–1011. doi: 10.1021/bi00429a013. [DOI] [PubMed] [Google Scholar]
- Fedor M. J., Uhlenbeck O. C. Kinetics of intermolecular cleavage by hammerhead ribozymes. Biochemistry. 1992 Dec 8;31(48):12042–12054. doi: 10.1021/bi00163a012. [DOI] [PubMed] [Google Scholar]
- Fedor M. J., Uhlenbeck O. C. Substrate sequence effects on "hammerhead" RNA catalytic efficiency. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1668–1672. doi: 10.1073/pnas.87.5.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goate A., Chartier-Harlin M. C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991 Feb 21;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Goodchild J. Enhancement of ribozyme catalytic activity by a contiguous oligodeoxynucleotide (facilitator) and by 2'-O-methylation. Nucleic Acids Res. 1992 Sep 11;20(17):4607–4612. doi: 10.1093/nar/20.17.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Heidenreich O., Eckstein F. Hammerhead ribozyme-mediated cleavage of the long terminal repeat RNA of human immunodeficiency virus type 1. J Biol Chem. 1992 Jan 25;267(3):1904–1909. [PubMed] [Google Scholar]
- Hendry P., McCall M. J., Santiago F. S., Jennings P. A. A ribozyme with DNA in the hybridising arms displays enhanced cleavage ability. Nucleic Acids Res. 1992 Nov 11;20(21):5737–5741. doi: 10.1093/nar/20.21.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachim C. L., Selkoe D. J. The seminal role of beta-amyloid in the pathogenesis of Alzheimer disease. Alzheimer Dis Assoc Disord. 1992 Spring;6(1):7–34. doi: 10.1097/00002093-199205000-00003. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Koizumi M., Ohtsuka E. Effects of phosphorothioate and 2-amino groups in hammerhead ribozymes on cleavage rates and Mg2+ binding. Biochemistry. 1991 May 28;30(21):5145–5150. doi: 10.1021/bi00235a005. [DOI] [PubMed] [Google Scholar]
- Krzyzosiak W., Denman R., Nurse K., Hellmann W., Boublik M., Gehrke C. W., Agris P. F., Ofengand J. In vitro synthesis of 16S ribosomal RNA containing single base changes and assembly into a functional 30S ribosome. Biochemistry. 1987 Apr 21;26(8):2353–2364. doi: 10.1021/bi00382a042. [DOI] [PubMed] [Google Scholar]
- L'Huillier P. J., Davis S. R., Bellamy A. R. Cytoplasmic delivery of ribozymes leads to efficient reduction in alpha-lactalbumin mRNA levels in C127I mouse cells. EMBO J. 1992 Dec;11(12):4411–4418. doi: 10.1002/j.1460-2075.1992.tb05541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E., Carman M. D., Fernandez-Madrid I. J., Power M. D., Lieberburg I., van Duinen S. G., Bots G. T., Luyendijk W., Frangione B. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990 Jun 1;248(4959):1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Dolnick B. J. Specific hybridization arrest of dihydrofolate reductase mRNA in vitro using anti-sense RNA or anti-sense oligonucleotides. Arch Biochem Biophys. 1987 Feb 15;253(1):214–220. doi: 10.1016/0003-9861(87)90654-0. [DOI] [PubMed] [Google Scholar]
- Miller W. A., Silver S. L. Alternative tertiary structure attenuates self-cleavage of the ribozyme in the satellite RNA of barley yellow dwarf virus. Nucleic Acids Res. 1991 Oct 11;19(19):5313–5320. doi: 10.1093/nar/19.19.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Mullan M., Crawford F., Axelman K., Houlden H., Lilius L., Winblad B., Lannfelt L. A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992 Aug;1(5):345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- Murrell J., Farlow M., Ghetti B., Benson M. D. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science. 1991 Oct 4;254(5028):97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
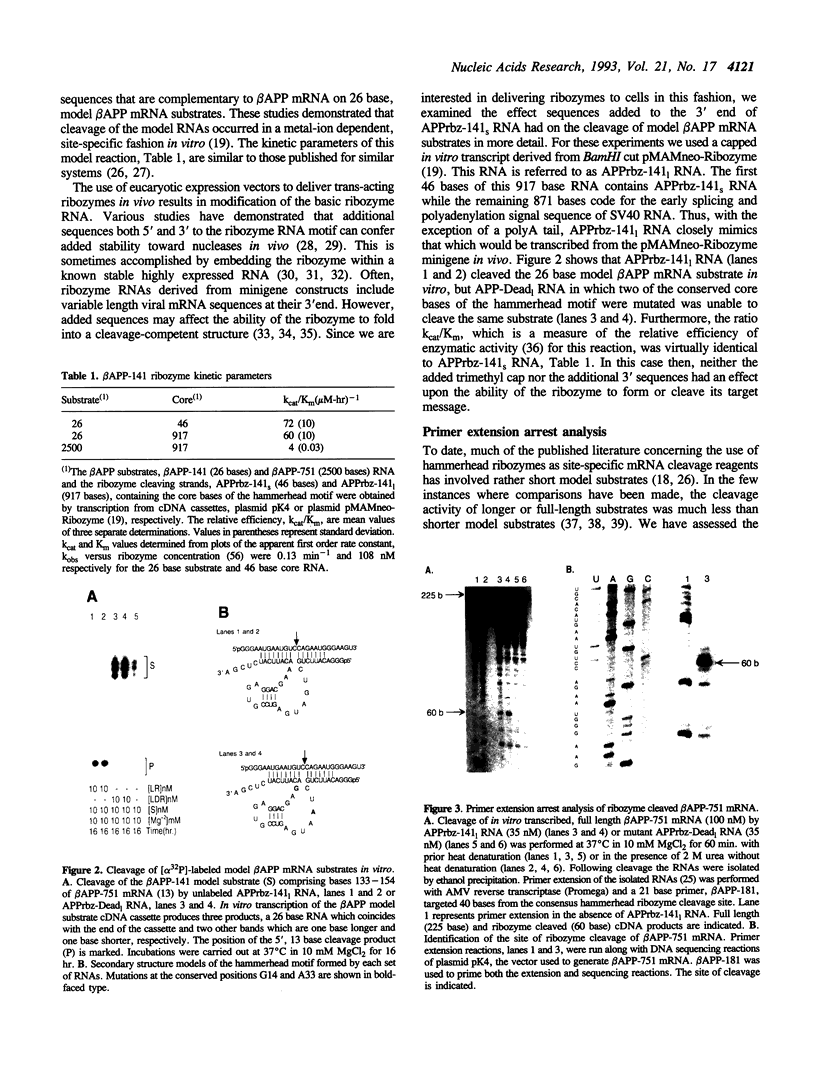
- Nurse K., Colgan J., Denman R., Wilhelm J., Ofengand J. Covalent cross-linking of AcVal-tRNA to Tetrahymena thermophila cytoplasmic ribosomes and two of its 17S rRNA mutants. Biochimie. 1987 Oct;69(10):1105–1112. doi: 10.1016/0300-9084(87)90010-1. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Fritz L. C., Schenk D. B., Lieberburg I., Johnson-Wood K. L., Beattie E. C., Ward P. J., Blacher R. W., Dovey H. F., Sinha S. The secreted form of the Alzheimer's amyloid precursor protein with the Kunitz domain is protease nexin-II. Nature. 1989 Sep 14;341(6238):144–147. doi: 10.1038/341144a0. [DOI] [PubMed] [Google Scholar]
- Pabón-Peña L. M., Zhang Y., Epstein L. M. Newt satellite 2 transcripts self-cleave by using an extended hammerhead structure. Mol Cell Biol. 1991 Dec;11(12):6109–6115. doi: 10.1128/mcb.11.12.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace U., Branch A. D., Robertson H. D. Generation of viroid conformational isomers that are stable to incubation with magnesium ions and in a nuclear extract from tomato plants. Nucleic Acids Res. 1992 Dec 25;20(24):6681–6686. doi: 10.1093/nar/20.24.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirilli J. A., McConnell T. S., Zaug A. J., Noller H. F., Cech T. R. Aminoacyl esterase activity of the Tetrahymena ribozyme. Science. 1992 Jun 5;256(5062):1420–1424. doi: 10.1126/science.1604316. [DOI] [PubMed] [Google Scholar]
- Ruffner D. E., Stormo G. D., Uhlenbeck O. C. Sequence requirements of the hammerhead RNA self-cleavage reaction. Biochemistry. 1990 Nov 27;29(47):10695–10702. doi: 10.1021/bi00499a018. [DOI] [PubMed] [Google Scholar]
- Saxena S. K., Ackerman E. J. Ribozymes correctly cleave a model substrate and endogenous RNA in vivo. J Biol Chem. 1990 Oct 5;265(28):17106–17109. [PubMed] [Google Scholar]
- Schubert D., Cole G., Saitoh T., Oltersdorf T. Amyloid beta protein precursor is a mitogen. Biochem Biophys Res Commun. 1989 Jul 14;162(1):83–88. doi: 10.1016/0006-291x(89)91965-7. [DOI] [PubMed] [Google Scholar]
- Schubert D., Jin L. W., Saitoh T., Cole G. The regulation of amyloid beta protein precursor secretion and its modulatory role in cell adhesion. Neuron. 1989 Dec;3(6):689–694. doi: 10.1016/0896-6273(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Schubert D., Schroeder R., LaCorbiere M., Saitoh T., Cole G. Amyloid beta protein precursor is possibly a heparan sulfate proteoglycan core protein. Science. 1988 Jul 8;241(4862):223–226. doi: 10.1126/science.2968652. [DOI] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Sioud M., Drlica K. Prevention of human immunodeficiency virus type 1 integrase expression in Escherichia coli by a ribozyme. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7303–7307. doi: 10.1073/pnas.88.16.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioud M., Natvig J. B., Førre O. Preformed ribozyme destroys tumour necrosis factor mRNA in human cells. J Mol Biol. 1992 Feb 20;223(4):831–835. doi: 10.1016/0022-2836(92)90244-e. [DOI] [PubMed] [Google Scholar]
- Stephenson P., Gibson I. In vitro cleavage of an N-ras messenger-like RNA by a ribozyme. Antisense Res Dev. 1991 Fall;1(3):261–268. [PubMed] [Google Scholar]
- Terry R. D., Katzman R. Senile dementia of the Alzheimer type. Ann Neurol. 1983 Nov;14(5):497–506. doi: 10.1002/ana.410140502. [DOI] [PubMed] [Google Scholar]
- Walstrum S. A., Uhlenbeck O. C. The self-splicing RNA of Tetrahymena is trapped in a less active conformation by gel purification. Biochemistry. 1990 Nov 20;29(46):10573–10576. doi: 10.1021/bi00498a022. [DOI] [PubMed] [Google Scholar]
- Wiśniewski H. M., Narang H. K., Terry R. D. Neurofibrillary tangles of paired helical filaments. J Neurol Sci. 1976 Feb;27(2):173–181. doi: 10.1016/0022-510x(76)90059-9. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Hearst J. E. A sequence from Drosophila melanogaster 18S rRNA bearing the conserved hypermodified nucleoside am psi: analysis by reverse transcription and high-performance liquid chromatography. Nucleic Acids Res. 1981 Apr 10;9(7):1723–1741. doi: 10.1093/nar/9.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Hearst J. E. Reverse transcriptase pauses at N2-methylguanine during in vitro transcription of Escherichia coli 16S ribosomal RNA. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3751–3754. doi: 10.1073/pnas.76.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama N., Ohkawa J., Inokuchi Y., Shirai M., Sato A., Nishikawa S., Taira K. Construction of a tRNA-embedded-ribozyme trimming plasmid. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1271–1279. doi: 10.1016/s0006-291x(05)81543-8. [DOI] [PubMed] [Google Scholar]