Abstract
Cholesterol circulates in the blood in association with triglycerides and other lipids, and elevated blood low-density lipoprotein cholesterol carries a risk for metabolic and cardiovascular disorders, whereas high-density lipoprotein (HDL) cholesterol in the blood is thought to be beneficial. Circulating cholesterol is the balance among dietary cholesterol absorption, hepatic synthesis and secretion, and the metabolism of lipoproteins by various tissues. We found that the CNS is also an important regulator of cholesterol in rodents. Inhibiting the brain’s melanocortin system by pharmacological, genetic or endocrine mechanisms increased circulating HDL cholesterol by reducing its uptake by the liver independent of food intake or body weight. Our data suggest that a neural circuit in the brain is directly involved in the control of cholesterol metabolism by the liver.
Dyslipidemia, obesity, hypertension and impaired glucose metabolism are hallmarks of the metabolic syndrome1, but limited information about the common molecular underpinnings of this syndrome has hampered efforts to treat it in its entirety. Efforts to find pharmacological therapeutics have resulted in an increasingly sophisticated model of molecular energy balance regulation. As an important part of that model, the gut hormone ghrelin is believed to inform the brain about energy availability and has been shown to increase adiposity, raise blood pressure and promote hyperglycemia2. The overwhelming majority of ghrelin’s effects on metabolism are mediated via CNS circuits, with the hypothalamic melanocortin system arguably being its most important direct target3. In turn, the melanocortin system is an essential and potent regulator of body adiposity, glucose metabolism and blood pressure4. Furthermore, mutations of melanocortin receptors are strongly correlated with human obesity5 and alterations in cholesterol transport are a common occurrence in obesity and the metabolic syndrome6. We hypothesized that a gut-brain axis integrates all of the primary physiological components known to be affected in the metabolic syndrome, that, in addition to regulating glucose homeostasis, blood pressure, food intake and body weight, it also likely controls cholesterol metabolism. We found that a gut-brain axis including ghrelin, glucagon-like peptide 1 (GLP-1) and the central melanocortin system directly regulates the hepatic synthesis and re-uptake of cholesterol.
RESULTS
Gut-brain signaling controls systemic cholesterol
Daily subcutaneous administration of ghrelin in wild-type mice for 1 week not only caused the expected increase in body fat7 (3.3 ± 0.1 versus 1.9 ± 0.3 g, P < 0.001), but also significantly increased total plasma cholesterol levels in these mice relative to vehicle-infused control mice (132.0 ± 4.7 versus 116.5 ± 1.4 mg dl−1, P < 0.05). Plasma triglyceride (101.1 ± 7.8 versus 82.6 ± 4.4 mg dl−1, P = 0.058) and plasma glucose levels (97.6 ± 1.1 versus 96.2 ± 0.9 mg dl−1) remained unchanged. As most of ghrelin’s effects on metabolism are believed to be mediated via its primary CNS target, the hypothalamic melanocortin system, we asked whether blockade or activation of such CNS circuitry would affect circulating cholesterol.
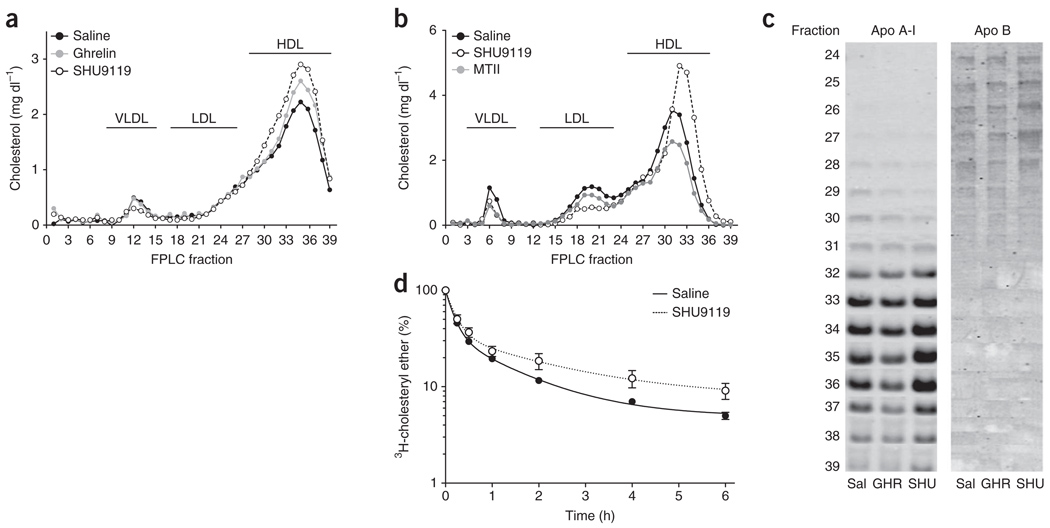
To determine whether the central melanocortin system could mediate the effects of ghrelin on cholesterol, we inhibited melanocortin signaling indirectly and directly by chronic intracerebroventricular (icv) administration of ghrelin or the melanocortin receptor antagonist SHU9119. To prevent ghrelin/SHU9119-induced hyperphagia (Supplementary Fig. 1) as a confounding factor, we pair-fed rats, limiting them to the amount of calories consumed by the icv vehicle–infused control group (Supplementary Fig. 1). Chronic icv infusion of ghrelin or SHU9119 increased total plasma cholesterol (Supplementary Fig. 2). This increase in cholesterol was a result of higher HDL cholesterol (HDL-C; Fig. 1a), implying that CNS melanocortin receptor activity regulates circulating HDL-C independently of food intake. Notably, icv-administered SHU9119 increased circulating HDL-C to a greater extent than icv ghrelin (Fig. 1a). Leptin and adiponectin levels also increased in response to CNS ghrelin or SHU9119 infusion, whereas several inflammatory markers remained unchanged (Supplementary Fig. 2). To determine whether HDL-C levels can be regulated in both directions by signaling changes in the CNS, we next administered the melanocortin receptor agonist MTII or the melanocortin antagonist SHU9119 directly into the lateral ventricle. Although chronic icv infusion of the melanocortin agonist MTII decreased plasma HDL-C levels, SHU9119 potently increased HDL-C (Fig. 1b). Notably, changes in HDL-C induced by the blockade of the central melanocortin system were observed under conditions in which no change in body mass occurred (Supplementary Fig. 1).
Figure 1.
Ghrelin and melanocortin action in the CNS control of plasma HDL-C. (a,b) Effect on cholesterol distribution of different lipoproteins after a 7-d icv infusion of ghrelin (2.5 nmol d−1) and SHU9119 (24 nmol d−1) (a) and after a 7-d icv infusion of SHU9119 (24 nmol d−1) and MTII (1 nmol d−1) in pair-fed rats (b). VLDL, very low density lipoproteins. (c) Apo B and A-I content in FPLC fractions from pooled plasma (n = 6–8) after a 7-d icv infusion of saline (Sal), ghrelin (GHR, 2.5 nmol d−1) and SHU9119 (SHU, 24 nmol d−1) in pair-fed Wistar rats. (d) Effect on HDL–3H-cholesterol ether plasma clearance of 4-d infusion of SHU9119 (10 nmol d−1) in rats. Icv infusion of SHU9119 significantly reduced HDL-C plasma clearance (P < 0.05, extra sum of squares F test, mean ± s.e.m., n = 9–10).
To determine whether the hunger-inducing hormone ghrelin is the only gut hormone that controls cholesterol metabolism via the CNS, we evaluated the satiating hormone GLP-1, which has recently been shown to oppose ghrelin in the hypothalamic melanocortin system8. Icv infusion of GLP-1 in mice for 1 week decreased circulating cholesterol compared with ad libitum and pair-fed vehicle-infused control mice (Supplementary Fig. 2). These data indicate that circulating cholesterol levels can be both positively and negatively regulated in response to the action of peripheral gastrointestinal hormones. We concluded that a neuroendocrine circuit involving the gut-brain axis controls HDL-C independently of changes in food intake or body weight. We then investigated whether CNS signaling–induced modulation of HDL-C involves changes in the size of the HDL particles. Using ultracentrifugation, we purified HDL particles from pair-fed rats infused icv with ghrelin or SHU9119. The migration in a nondenaturing gel of purified HDL particles was not substantially affected by the icv treatment of ghrelin or SHU9119 (Supplementary Fig. 3), similar to the fast protein liquid chromatography (FPLC) elution pattern that we found in pair-fed rats. Thus, these findings indicate that the treatment did not change the size of the HDL particles. For additional verification of the lipoprotein separation by FPLC, we asked whether the increase in HDL-C was associated with an increase in apolipoprotein (Apo) A-I levels. Total plasma Apo A-I levels were similar among groups (Supplementary Fig. 3), but immunoblots from FPLC fractions revealed that icv-administered SHU9119 increased both HDL-C and Apo A-I levels in HDL fractions (Fig. 1c). Apo B measurements confirmed the absence of low-density lipoprotein (LDL) particles in these fractions (Fig. 1c). This increase in HDL-C and Apo A-I levels induced by the blockade of the central melanocortin system occurred in spite of a considerable reduction in hepatic Apoa1 and Abca1 gene expression (Supplementary Fig. 4).
To determine whether modulation of CNS melanocortin 4 receptor (MC4-R) signaling affects cholesterol fluxes in vivo, we asked whether blockade of the central melanocortin receptors changed the plasma clearance of injected radio-labeled HDL-C. We intravenously injected HDL particles labeled with 3H-cholesterol into rats that were simultaneously icv infused with SHU9119 for 4 d (implanted osmotic mini-pumps). Icv SHU9119 infusion significantly decreased the plasma clearance of HDL-C (P < 0.05; Fig. 1d). These findings suggest that changes in circulating HDL-C in response to altered CNS melanocortin signaling is a result of decreased HDL-C reuptake rather than being a consequence of increased hepatic synthesis.
Because hypothalamic neuronal circuitries control hepatic glucose metabolism via the autonomic nervous system9, we hypothesized that the efferent vagus nerve may be a crucial pathway mediating the CNS modulation of plasma HDL-C levels. We found that hepatic vagotomy did not affect the HDL-C levels in icv-administered saline control pair-fed rats, but abolished SHU9119-induced increases of HDL-C (Supplementary Fig. 5).
Lack of Ghrl/Ghsr or Mc4r directly affects plasma cholesterol
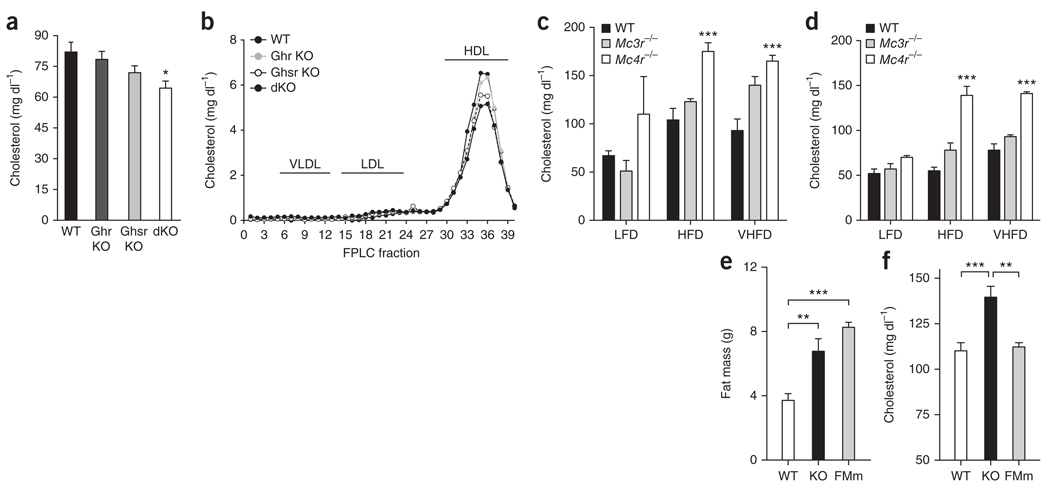
To determine whether the endogenous ghrelin and melanocortin systems have a physiological role in the regulation of cholesterol, we quantified circulating cholesterol in mice lacking ghrelin (Ghrl−/− mice), ghrelin receptor (Ghsr−/−mice) or both (Ghrl−/−; Ghsr−/−), and in mice deficient for MC3-R (Mc3r–/–) and MC4-R (Mc4r–/–). Ghrl−/− and Ghsr−/−mice had modestly reduced circulating cholesterol (Fig. 2a), whereas Ghrl−/−; Ghsr−/− mice had significantly lower (P < 0.05) circulating cholesterol than wild-type controls. Lipoprotein profiles of these Ghrl−/−; Ghsr−/−mice revealed that differences in plasma cholesterol were predominantly a consequence of decreased HDL-C fractions in mice with deficient ghrelin signaling (Fig. 2b).
Figure 2.
Altered plasma cholesterol in mutant mice deficient for ghrelin or melanocortin signaling. (a,b) Plasma cholesterol (a) and cholesterol content in FPLC fractions from pooled plasma (n = 6–8, b) of wild-type (WT), Ghrl−/− (Ghr KO), Ghsr−/− (Ghsr KO) and Ghrl−/−; Ghsr−/− (dKO) mice after an overnight fasting (*P < 0.05 versus wild type, one-way ANOVA, mean ± s.e.m., n = 6–8). (c,d) Plasma cholesterol in male (c) and female (d) wild-type, Mc3r−/− and Mc4r−/− overnight fasted mice maintained on diets with low (10% kJ from fat, LFD), moderate (45% kJ from fat, HFD) or very high (60% kJ from fat, VHFD) fat content (***P < 0.001 Mc4r−/− versus Mc3r−/− and wild type, **P < 0.01 Mc3r−/− versus wild type, two-way ANOVA, mean ± s.e.m., n = 3–10). (e,f) Fat mass (e) and plasma cholesterol (f) of Mc4r−/− mice (KO) compared with wild-type littermates and fat mass–matched (FMm) C57BL/6 mice (one-way ANOVA, mean ± s.e.m., n = 6–7).
Conversely, cholesterol levels were increased in mice deficient for MC4-R, especially on a high-fat diet (Fig. 2c,d). Mice deficient for MC3-R developed obesity10, but did not exhibit a clear increase in circulating cholesterol11. MC4-R knockout mice also had significantly higher cholesterol levels than fat mass– and sex-matched C57BL/6 mice (P < 0.01) or lean wild-type mice (P < 0.001) (Fig. 2e,f). These data confirm the physiological relevance of neuroendocrine cholesterol regulation by ghrelin and the melanocortin system independent of body fat and indicate that MC4-R is a crucial hypothalamic receptor subtype for the neuroendocrine control of HDL-C.
Neuroendocrine control of hepatic HDL-C re-uptake
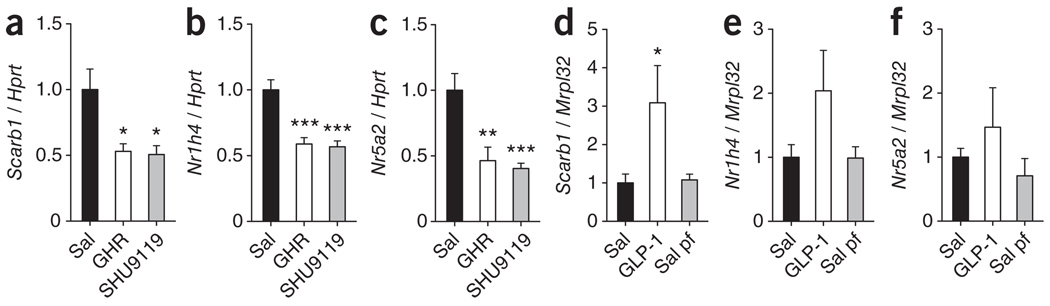
On the basis of our finding in rats with blocked CNS melanocortin receptors, which showed increased Apo A-I in FPLC fractions containing HDL particles in spite of reduced hepatic Apo A-I synthesis, we reasoned that higher levels of HDL-C in plasma may be primarily a result of decreased hepatic cholesterol re-uptake. We assessed this hypothesis by analyzing mRNA expression of several components of hepatic cholesterol reuptake following direct (SHU9119) and indirect (ghrelin) inhibition of the central melanocortin system. Icv administration of ghrelin or SHU9119 under pair-fed conditions potently downregulated gene expression of one major hepatic receptor responsible for HDL-C re-uptake, the scavenger receptor class B type 1 Scarb 1 (Fig. 3a). Components of separate pathways involved in the regulation of hepatic cholesterol uptake, such as the protein convertases Pcsk9 and Pcsk5, were not significantly changed (P > 0.05; Supplementary Fig. 4). Transcription factors that are known to control Scarb1 expression12,13, such as farnesoid X receptor (FXR, Nr1h4) and liver receptor homolog 1 (LRH-1, Nr5a2), were downregulated in rat livers following icv-administered ghrelin or SHU9119 independent from food intake (Fig. 3b,c). To assess physiological importance, we used Ghsr−/− mice, as MC4-R–deficient mice are morbidly obese and Ghsr−/− mice have normal body weight and food intake. Hepatic expression levels of Scarb1 (5.1 ± 1.0, P < 0.01), FXR (2.5 ± 0.2, P < 0.001) and LRH-1 (2.6 ± 0.3, P < 0.001) were all increased in Ghsr−/− mice (fold relative to Mrpl32 expression), indicating that our pharmacological findings are physiologically relevant. Icv infusion of GLP-1, a satiety-inducing gut hormone that has been reported to modulate hypothalamic melanocortin signaling8, induced a significant increase (P < 0.05) in the hepatic expression levels of Scarb1 (Fig. 3d), with hepatic levels of Nr1h4 and Nr5a2 mRNA tending toward increased expression (Fig. 3e,f), suggesting that gut-brain interactions can regulate Scarb1 gene transcription in both directions. In summary, these data suggest a gut-brain control system, which regulates cholesterol metabolism in response to changes in gastrointestinal nutrient availabilities.
Figure 3.
Gut hormones and melanocortin action in the CNS modulates hepatic HDL-C re-uptake pathways independent of food intake. (a–f) Changes in Scarb1 (a,d), Nr1h4 (FXR, b,e) and Nr2h5 (LRH-1, c,f) hepatic mRNA levels. Ghrelin (2.5 nmol d−1) or SHU9119 (24 nmol d−1) were icv infused for 7 d in pair-fed (pf) Wistar rats (mean ± s.e.m., n = 8, a–c). GLP-1 (2.5 nmol d−1) was icv infused for 48 h in C57BL/6 male mice (mean ± s.e.m., n = 6–8, d–f). *P < 0.05, **P < 0.01 and ***P < 0.001 versus saline (one-way ANOVA).
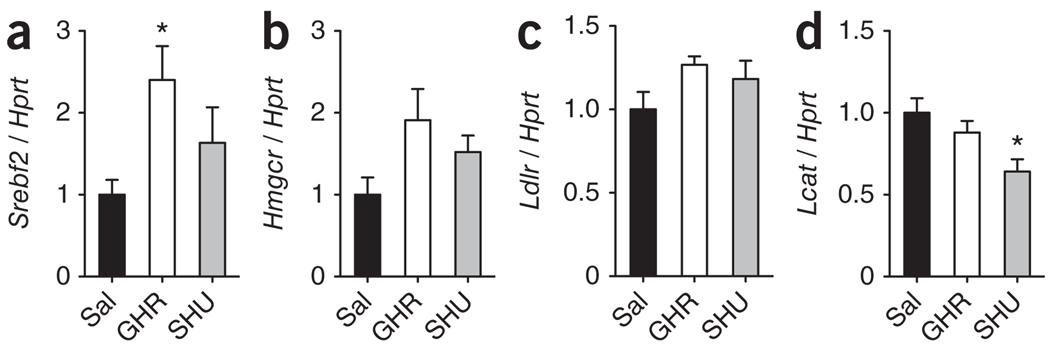
We then asked whether direct and indirect inhibition of the central melanocortin system would, in addition to decreasing hepatic cholesterol re-uptake, stimulate the hepatic synthesis of cholesterol. We measured mRNA expression of several signaling components involved in hepatic cholesterol synthesis and uptake following icv infusion of ghrelin or SHU9119. Indirect (ghrelin) or direct (SHU9119) blockade of hypothalamic melanocortin receptors potently increased hepatic levels of sterol response element binding protein 2 (Srebf2), hydroxymethyl glutaryl Co-A reductase (Hmgcr), LDL receptor (Ldlr) and lecithin-cholesterol acetyl transferase (Lcat) (Supplementary Fig. 4). However, the neuroendocrine control of hepatic cholesterol synthesis and uptake pathways could also be a consequence of parallel changes in body weight or food intake. We therefore asked whether acute icv injections of ghrelin or SHU9119 would elicit rapid changes of hepatic cholesterol synthesis pathways; that is, before body weight changes could occur. Significant increases (P < 0.05) in the gene expression of factors related with hepatic cholesterol synthesis and uptake (Hmgcr and Ldlr) were apparent 4 h after ghrelin and SHU9119 administration (Supplementary Fig. 4). Under pair-feeding conditions, however, the effects of chronically changed neuroendocrine signaling on pathways involved in cholesterol synthesis were much less impressive (Fig. 4a–d and Supplementary Fig. 4). We therefore concluded that, although it may represent one contributing component, a CNS control of hepatic cholesterol synthesis pathways could not fully explain the powerful neuroendocrine control of circulating HDL-C.
Figure 4.
Effect of the gut hormone ghrelin and melanocortin action in the CNS on the hepatic gene expression of components of cholesterol synthesis pathway. (a–d) Effect of 7-day icv infusion of ghrelin (2.5 nmol d−1) and SHU9119 (24 nmol d−1) on hepatic mRNA expression of Srebf2 (a), Hmgcr (b), Ldlr (c) and Lcat (d) in pair-fed Wistar rats. *P < 0.05 versus saline (one-way ANOVA, mean ± s.e.m., n = 8).
Increased HDL-C in rats prone to diet-induced obesity
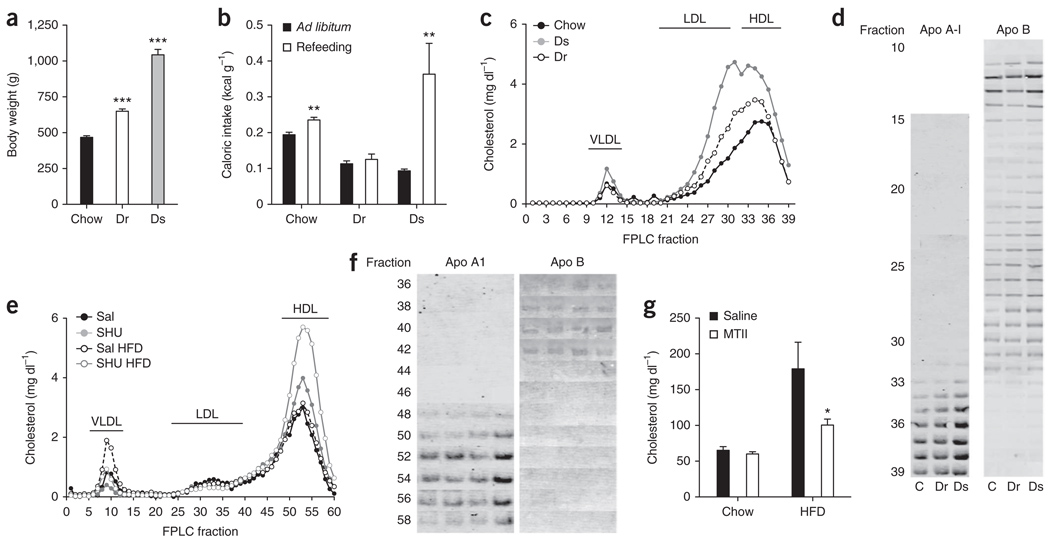
Several reports have indicated that obesity does not abolish central sensitivity to melanocortin receptor agonists14,15. Rodents with increased susceptibility to diet-induced obesity (diet-induced obese (DIO) sensitive) have an increased ratio of endogenous melanocortin antagonist to agonist ligands (agouti-related protein/melanocyte-stimulating hormone) and higher expression of MC4-R. This results in decreased hypothalamic melanocortin signaling when compared with animals that are resistant to diet-induced obesity (DIO resistant)16. We identified DIO-sensitive (1,031 ± 35 g) and DIO-resistant (650 ± 16 g) rats from a large pool of rats that were maintained on a high-fat diet for 10 months (Fig. 5a). As expected16, DIO-sensitive rats exhibited enhanced orexigenic drive after food deprivation (Fig. 5b). A detailed analysis of lipoprotein cholesterol distribution indicated that DIO-sensitive rats had higher cholesterol in Apo B–containing lipoproteins than their DIO-resistant counterparts or chow-fed controls (Fig. 5c,d). This is not surprising considering the extreme obesity and the diet of these rats. Notably, DIO-sensitive rats also exhibited increased HDL-C and Apo A-I levels (Fig. 5c,d) and decreased hepatic Scarb1 protein levels (Supplementary Fig. 6); Scarb1 gene expression tended to be reduced as well (Supplementary Fig. 6).
Figure 5.
Animal models with increased susceptibility to diet-induced obesity and decreased hypothalamic melanocortin signaling have increased HDL-C. (a–d) Body weight (a), feeding response (b), cholesterol (c) and Apo B and A-I content in FPLC fractions (d) from pooled plasma (n = 8) of chow-fed rats, DIO-resistant (Dr) and DIO-sensitive (Ds) rats (***P < 0.001 versus chow fed group, one-way ANOVA; **P < 0.01, t test; mean ± s.e.m., n = 8). (e,f) Effect on cholesterol (e) and Apo B and A-I content in FPLC fractions from pooled plasma (n = 8) (f) of 7-d icv infusion of SHU9119 (24 nmol d−1) in rats fed ad libitum with chow or HFD. (g) Effect on plasma cholesterol of two acute icv double injection of MTII (7.5 nmol every 12 h) in rats fed with chow or HFD (*P < 0.05, t test, mean ± s.e.m., n = 5–6).
To determine whether decreasing CNS melanocortin receptor activity would enhance the high fat diet (HFD)-induced accumulation of HDL-C, we icv-infused SHU9119 into rats whose diet was switched from chow to HFD for the duration of the experimental period. Chronic icv infusion of SHU9119 more potently increased plasma cholesterol, HDL-C and Apo A-I levels in rats on the high-fat diet than in rats infused with saline, whereas LDL cholesterol and Apo B levels remained unchanged (Fig. 5e,f and Supplementary Figs. 2 and 3). This difference in cholesterol response to SHU9119 icv infusion between DIO-sensitive and DIO-resistant occurred despite icv-administered SHU9119 having similar effects on food intake and body weight (Supplementary Fig. 1) and was accompanied by decreased hepatic Scarb1 protein levels in icv SHU9119–treated rats independent of the diet (Supplementary Fig. 6). Again, these results suggest a decreased reverse cholesterol transport induced by blocked CNS melanocortin signaling. To further test CNS MC4-R hypersensitivity on high-fat diet16, we asked whether acute icv treatment with MTII at a dose that does not affect plasma cholesterol in rats on standard chow diet could rescue hypercholesterolemia in rats on a high-fat diet. Acute icv injection of MTII significantly decreased (P < 0.05) plasma cholesterol in DIO rats on a high-fat diet, but not on standard chow (Fig. 5g). This finding confirms the metabolically relevant hypersensitivity of the hypothalamic melanocortin system16 and suggests that acute CNS signaling changes can potently decrease plasma cholesterol without changing body fat mass.
DISCUSSION
We found that circulating levels of HDL-C can be modulated in the CNS. Specifically, we found that the hypothalamic melanocortin system and its only known circulating inhibitor, ghrelin, regulate plasma HDL-C levels through vagally mediated modulation of hepatic pathways controlling cholesterol synthesis and re-uptake. The satiating gut hormone GLP-1, however, activates the CNS melanocortin system and opposes ghrelin’s actions on hepatic cholesterol metabolism. Our results strongly suggest that the same CNS circuits that promote food intake and facilitate energy storage also have a specific role as a remote control system for hepatic cholesterol synthesis. Our data further indicate that, in addition to facilitating hepatic cholesterol synthesis, the central melanocortin system influences cholesterol transport by modulating HDL cholesterol levels.
Reduced CNS melanocortin receptor signaling reduced hepatic Scarb1 expression, suggesting that Scarb1 has an important mechanistic role in the CNS control of plasma HDL-C. Genetically modified mice lacking Scarb1 (refs. 17,18) have high HDL-C, indicating that Scarb1 is important for selective cholesterol uptake from HDL particles. Indeed, mice exhibiting a complete lack of Scarb1 expression exhibit accumulation of large HDL particles17. Others have reported that a mouse model with a mutated Scarb1 promoter that decreases its hepatic expression by 50% had a proportional reduction in HDL-C removal from plasma18. Notably, when the cholesterol distribution in these mice was studied by FPLC, there was a slight increase of cholesterol in fractions containing large HDL particles and an increase of more than 50% in cholesterol levels in the HDL region. Furthermore, these mice had higher Apo A-I lipoprotein content than wild-type mice. These findings are consistent with our results, which does not exclude the possibility that multiple mechanisms involved in the control of lipoprotein cholesterol transport are modulated by the central melanocortin system. However, our data suggest that the decrease in hepatic Scarb1 levels could explain the increase in HDL-C and Apo A-I levels induced by the blockade of the central melanocortin system. Conversely, the activation of the melanocortin system decreases plasma cholesterol levels and increases hepatic Scarb1 expression. We found that this mechanism was indirectly used by afferent gastrointestinal hormones such as ghrelin or GLP-1, which are known to have opposite effects on the central melanocortin system and food intake. In summary, neuroendocrine gut-brain pathways known to promote intake and storage of calories also appear to prevent the elimination of a metabolite that is essential for many anabolic processes, and vice versa.
We found that the gut-brain control of cholesterol metabolism is independent of changes in food intake or body weight. In several short-term experiments, cholesterol metabolism changes preceded body weight changes (Fig. 5g and Supplementary Fig. 1). Cholesterol changes were observed in genetic and pharmacological experiments using lean rodent models or during normal food intake (Figs. 2a,b and 5b). In chronic studies, cholesterol metabolism was changed, whereas body adiposity remained stable (Supplementary Fig. 1). It has been reported that insulin facilitates the translocation of Scarb1 to the membrane in adipocytes19 and hepatocytes20. Previous studies have found that this translocation of Scarb1 to the plasma membrane induced by insulin is mediated by phosphatidylinositol 3-kinase activity, suggesting that the Scarb1 cholesterol uptake could be impaired in situations of insulin resistance20. However, we found that neuroendocrine modulation of plasma cholesterol (Supplementary Fig. 2) was achieved in the absence of impaired glucose homeostasis (Supplementary Figs. 2 and 4). Cholesteryl ester transfer protein (CETP) decreases the amount of cholesterol in HDL, facilitating the transfer of cholesteryl esters from HDL particles to more atherogenic triglyceride-containing lipoproteins21, and is increasingly active in obesity22. The absence of CETP in rodents indicates that it is not involved in the cholesterol regulation pathway that we examined, further supporting the idea that a gut-brain axis affects cholesterol metabolism by modulating hepatic synthesis and reuptake. Several studies have reported lower levels of the HDL receptor Scarb1 in obese mice lacking leptin signaling, such as ob/ob and db/db mice23,24. Furthermore, studies25,26 have indicated that leptin may be a molecular regulator of HDL cholesterol. Leptin action promotes hepatic Apoa1 gene expression, decreases HDL-C plasma levels and increases HDL-C uptake from hepatocytes in ob/ob mice25,26. From our data, we speculate that leptin may affect HDL-C metabolism via its main target in the brain, the CNS melanocortin system4. An integrated neuroendocrine control of food intake, body weight and glucose homeostasis, as well as cholesterol metabolism and cardiovascular lipid exposure, would connect all of the hallmarks of the metabolic syndrome. Therapies promoting the increase of HDL levels have been proposed for the prevention of atherosclerosis in humans27. However, in rodent models lacking CETP, increased HDL-C is frequently associated with the metabolic syndrome. Ob/ob mice, which have high HDL-C levels, exhibit massive atherosclerosis on an Ldlr−/− background, which is more relevant for human atherosclerosis28. In addition, rodent models with high HDL-C levels resulting from deficiency for Scarb1 are also prone to atherosclerosis29,30. We speculate that modulation of neuroendocrine circuits may offer therapeutic opportunities to prevent cardiovascular disease.
A sterol regulatory element binding protein–regulated pathway controls the de novo cholesterol synthesis31. When cholesterol is depleted in the liver, sterol regulatory element binding proteins increase cholesterol biosynthesis and upregulate LDL receptor expression to increase lipoprotein uptake from the plasma32. We propose that, in parallel with these canonical control mechanisms, liver cholesterol metabolism and blood cholesterol levels also are under the immediate control of specific CNS circuits. Such a conclusion supports the idea that the CNS directs a number of critical aspects of peripheral metabolism33 via the autonomic nervous system and the classic endocrine axes34. Such processes under neuroendocrine control include food intake35, adaptive thermogenesis36 and endocrine pancreas function37. More recent studies have integrated the CNS control of non-exercise activity thermogenesis38 or hepatic glucose production9. Recent reports on CNS control of lipid metabolism suggested a potentially direct neuroendocrine control of triglyceride metabolism in liver39 or adipose tissue40.
Our observations add a component to the existing model of neuroendocrine control of peripheral metabolism by showing for the first time, to the best of our knowledge, that circulating levels of cholesterol are under remote, but direct, control of specific neuroendocrine circuits in the CNS. Numerous synthetic agonists for CNS MC4-Rs have been described and potent ghrelin receptor antagonists are currently emerging. Direct or indirect pharmacological modulation of hypothalamic melanocortin tone may offer a potent way to treat hypercholesterolemia and to simultaneously target all major components of the metabolic syndrome.
Methods
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureneuroscience/.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by US National Institute of Health grants NIDDK56863 (S.C.W. and M.H.T.), 5R01DK077975 (M.H.T.), R01 DK076907 (D.Y.H.) and HL67093 (W.S.D.). S.M.H. is the recipient of a Scientist Development Award from the American Heart Association (#0635079N). S.M.H. is a recipient of a Basic Science Award (1-10-BS-72) from the American Diabetes Association.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
AUTHOR CONTRIBUTIONS
D.P.-T., S.M.H., J.B., R.N. and P.T.P. designed and performed most of the experiments and wrote the manuscript. J.L.T. performed experiments, J.T.P. synthesized receptor ligands and A.A.B., S.C.B. and M.W.S. generated mouse models. N.G. carried out FPLC analyses. W.S.D. performed lipoprotein electrophoresis, E.G. and H.W.-P. carried out in vivo experiments, gene expression, inmunoblots and inmunoassays, M.A. performed surgical procedures and S.C.W. interpreted data and co-wrote the manuscript. R.D.D., D.Y.H. and M.H.T. conceptualized, analyzed and interpreted all studies and co-wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at www.nature.com/natureneuroscience/.
References
- 1.Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J. Clin. Endocrinol. Metab. 2007;92:399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- 2.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol. Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Cowley MA, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 4.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr. Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 5.Vaisse C, et al. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J. Clin. Invest. 2000;106:253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. J. Am. Med. Assoc. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 7.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 8.Ma X, Bruning J, Ashcroft FM. Glucagon-like peptide 1 stimulates hypothalamic proopiomelanocortin neurons. J. Neurosci. 2007;27:7125–7129. doi: 10.1523/JNEUROSCI.1025-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005;1:53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Sutton GM, et al. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology. 2006;147:2183–2196. doi: 10.1210/en.2005-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen AS, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 12.Schoonjans K, et al. Liver receptor homolog 1 controls the expression of the scavenger receptor class B type I. EMBO Rep. 2002;3:1181–1187. doi: 10.1093/embo-reports/kvf238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert G, et al. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J. Biol. Chem. 2003;278:2563–2570. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- 14.Cettour-Rose P, Rohner-Jeanrenaud F. The leptin-like effects of 3-d peripheral administration of a melanocortin agonist are more marked in genetically obese Zucker (fa/fa) than in lean rats. Endocrinology. 2002;143:2277–2283. doi: 10.1210/endo.143.6.8871. [DOI] [PubMed] [Google Scholar]
- 15.Blüher S, et al. Responsiveness to peripherally administered melanocortins in lean and obese mice. Diabetes. 2004;53:82–90. doi: 10.2337/diabetes.53.1.82. [DOI] [PubMed] [Google Scholar]
- 16.Enriori PJ, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Rigotti A, et al. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc. Natl. Acad. Sci. USA. 1997;94:12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varban ML, et al. Targeted mutation reveals a central role for SR-BI in hepatic selective uptake of high density lipoprotein cholesterol. Proc. Natl. Acad. Sci. USA. 1998;95:4619–4624. doi: 10.1073/pnas.95.8.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tondu AL, et al. Insulin and angiotensin II induce the translocation of scavenger receptor class B, type I from intracellular sites to the plasma membrane of adipocytes. J. Biol. Chem. 2005;280:33536–33540. doi: 10.1074/jbc.M502392200. [DOI] [PubMed] [Google Scholar]
- 20.Shetty S, Eckhardt ER, Post SR, van der Westhuyzen DR. Phosphatidylinositol-3-kinase regulates scavenger receptor class B type I subcellular localization and selective lipid uptake in hepatocytes. Arterioscler. Thromb. Vasc. Biol. 2006;26:2125–2131. doi: 10.1161/01.ATV.0000233335.26362.37. [DOI] [PubMed] [Google Scholar]
- 21.Ginsberg HN. Insulin resistance and cardiovascular disease. J. Clin. Invest. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arai T, et al. Increased plasma cholesteryl ester transfer protein in obese subjects. A possible mechanism for the reduction of serum HDL cholesterol levels in obesity. Arterioscler. Thromb. 1994;14:1129–1136. doi: 10.1161/01.atv.14.7.1129. [DOI] [PubMed] [Google Scholar]
- 23.Gruen ML, et al. Persistence of high density lipoprotein particles in obese mice lacking apolipoprotein A-I. J. Lipid Res. 2005;46:2007–2014. doi: 10.1194/jlr.M500181-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Lundåsen T, Liao W, Angelin B, Rudling M. Leptin induces the hepatic high density lipoprotein receptor scavenger receptor B type I (SR-BI) but not cholesterol 7alpha-hydroxylase (Cyp7a1) in leptin-deficient (ob/ob) mice. J. Biol. Chem. 2003;278:43224–43228. doi: 10.1074/jbc.M302645200. [DOI] [PubMed] [Google Scholar]
- 25.Silver DL, Jiang XC, Tall AR. Increased high density lipoprotein (HDL), defective hepatic catabolism of ApoA-I and ApoA-II, and decreased ApoA-I mRNA in ob/ob mice. Possible role of leptin in stimulation of HDL turnover. J. Biol. Chem. 1999;274:4140–4146. doi: 10.1074/jbc.274.7.4140. [DOI] [PubMed] [Google Scholar]
- 26.Silver DL, Wang N, Tall AR. Defective HDL particle uptake in ob/ob hepatocytes causes decreased recycling, degradation, and selective lipid uptake. J. Clin. Invest. 2000;105:151–159. doi: 10.1172/JCI8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J. Clin. Invest. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasty AH, et al. Severe hypercholesterolemia, hypertriglyceridemia, and atherosclerosis in mice lacking both leptin and the low density lipoprotein receptor. J. Biol. Chem. 2001;276:37402–37408. doi: 10.1074/jbc.M010176200. [DOI] [PubMed] [Google Scholar]
- 29.Huby T, et al. Knockdown expression and hepatic deficiency reveal an atheroprotective role for SR-BI in liver and peripheral tissues. J. Clin. Invest. 2006;116:2767–2776. doi: 10.1172/JCI26893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, et al. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelking LJ, et al. Schoenheimer effect explained—feedback regulation of cholesterol synthesis in mice mediated by Insig proteins. J. Clin. Invest. 2005;115:2489–2498. doi: 10.1172/JCI25614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horton JD, Goldstein JL, Brown MS. SREBPs: transcriptional mediators of lipid homeostasis. Cold Spring Harb. Symp. Quant. Biol. 2002;67:491–498. doi: 10.1101/sqb.2002.67.491. [DOI] [PubMed] [Google Scholar]
- 33.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J. Clin. Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 35.Coll AP, Farooqi IS, O’Rahilly S. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 37.Ahrén B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 38.Castañeda TR, et al. Obesity and the neuroendocrine control of energy homeostasis: the role of spontaneous locomotor activity. J. Nutr. 2005;135:1314–1319. doi: 10.1093/jn/135.5.1314. [DOI] [PubMed] [Google Scholar]
- 39.Lam TK, et al. Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nat. Med. 2007;13:171–180. doi: 10.1038/nm1540. [DOI] [PubMed] [Google Scholar]
- 40.Nogueiras R, et al. The central melanocortin system directly controls peripheral lipid metabolism. J. Clin. Invest. 2007;117:3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.