Abstract
Hematopoietic cell transplantation (HCT) offers potentially curative therapy for Chronic Myelomonocytic Leukemia (CMML). We evaluated HCT outcomes in 85 patients with CMML, 1.0–69.1 (median 51.7) years of age, with follow-up extending to 19 years. CMML was considered de novo in 71 and secondary in 14 patients. Conditioning regimens were of various intensities. Thirty-eight patients had related (34 HLA identical), and 47 (39 HLA matched) unrelated donors. The source of stem cells was marrow in 32 and peripheral blood progenitor cells in 53 patients. Acute GVHD grades II–IV occurred in 72% and chronic GVHD in 26% of patients. Relapse incidence was 27% at 10 years. Relapse correlated with increasing scores by the MD Anderson prognostic score (p=0.01). The major causes of death were relapse and infections ±GVHD. Progression-free survival was 38% at 10 years. Mortality was negatively correlated with pre-HCT hematocrit (p=0.007), and increased with high-risk cytogenetics (p=0.02), higher HCT Comorbidity Index (p=0.0008), and increased age (p=.02). WHO classification did not statistically significantly affect outcome. Thus, a proportion of patients with CMML have lasting remissions following allogeneic HCT and appear to be cured of their disease.
Keywords: CMML, Cytogenetics, Co-morbidity, Hematopoietic cell transplantation
INTRODUCTION
Chronic myelomonocytic leukemia (CMML), currently characterized as a myelodysplastic/myeloproliferative disorder by World Health Organization (WHO) criteria, is a heterogeneous disease with variable course, generally ending in progression to acute myeloid leukemia. Various classification systems have been described [1]. The WHO distinguishes CMML-1 (<10% marrow blasts) and CMML-2 (10–20% blasts), for which median survivals of 20 and 15 months, respectively, have been reported [2]. The International Prognostic Scoring System (IPSS),recognized dysplastic and proliferative forms of CMML [3], with Jak2 mutations present in approximately 10% of patients with proliferative CMML [4–6]. Investigators at M.D. Anderson Cancer Center proposed a 4-stage classification on the basis of circulating immature cells, hemoglobin levels, lymphocyte counts, and marrow blasts [1]. Based on the presence of these risk factors, they divided patients into four groups with median life expectancies ranging from 5 to 24 months. Additional studies suggest that younger age at the time of diagnosis, splenomegaly, lymphadenopathy, elevated lactate dehydrogenase (LDH) levels and clonal cytogenetic abnormalities are associated with a more rapid progression [7,8]. While occasional patients have prolonged remissions with aggressive chemotherapy, the only current therapy with proven curative potential is hematopoietic cell transplantation (HCT) [9–12]. We reported previously results in 43 patients transplanted at the Fred Hutchinson Cancer Research Center (FHCRC) [11]. Here, we present results in 42 new patients and provide long-term follow-up extending to 19 years for previously reported patients.
PATIENTS AND METHODS
Patients and Disease Characteristics
Between May 1986 and December 2008, 85 patients with CMML had HCT at the FHCRC, 42 of these since our initial report in 2005 [11]. All provided informed consent for enrollment in investigational protocols and for long-term follow-up as required by the Institutional Review Board of the FHCRC. Patient and disease characteristics are summarized in Table 1. Patients were 1.0–69.1 (median 51.7) years old. By WHO criteria [13], 57 patients (67%) had CMML-1 and 26 (31%) had CMML-2; in two patients, the staging was inconclusive. In 54 patients (64%) the WBC was <13,000 at HCT, thus qualifying as dysplastic CMML. Among these 54 patients 8 had low-risk, 23 intermediate-1, 15 intermediate-2, and 7 high-risk disease by IPSS criteria [3] (cytogenetic information was missing for one patient). Among 81 patients with cytogenetic data, 45 (53%) were considered good risk, 14 (16%) intermediate risk, and 22 (26%) poor risk according to IPSS criteria. Using the M.D. Anderson prognostic score (MDAPS), 32 patients had low-risk, 23 intermediate-1, 17 intermediate-2, and 8 high-risk disease (data incomplete in 5 patients).
Table 1.
Patient and Disease Characteristics
| Variable | Number of Patients |
|
|---|---|---|
| Number of patients | 85 | |
| Age (years), range (median) | 1–69.1 (51.7) | |
| Sex (male/female) | 52/33 | |
| Diagnosis | ||
| FAB | ||
| Proliferative | 28 | |
| Non-proliferative | 54 | |
| WHO | ||
| CMML 1 | 57 | |
| CMML 2 | 26 | |
| IPSS risk | ||
| Low | 8 | |
| Intermediate-1 | 23 | |
| Intermediate-2 | 15 | |
| High | 7 | |
| MDAPS | ||
| Low | 32 | |
| Intermediate-1 | 23 | |
| Intermediate-2 | 17 | |
| High | 8 | |
| Hematology Parameters median (range) | ||
| WBC (× 109/L) | 7.38 (0.08–85.5) | |
| Lymphocytes (× 109/L) | 1.55 (0–12.83) | |
| Platelets (× 109/L) | 63 (7–882) | |
| Hemoglobin (gm/dL) | 10.5 (7.2–15.7) | |
| Cytogenetics risk (by IPSS) | ||
| Good | 45 | |
| Intermediate | 14 | |
| Poor | 22 | |
| Pre-transplantation therapy | ||
| None or Transfusion only | 13 | |
| Cytoreductive with or without HU | 49 | |
| Differentiating agents* | 10 | |
| Splenectomy with or without other treatment modalities | 15 | |
| Other modalities | 9 | |
Abbreviations:
HU=hydroxyurea; FAB = French-American-British classification; WHO = World Health Organization
IPSS = International Prognostic Scoring System; MDAPS = MD Anderson Prognostic Score; WBC = white blood cell count (see text)
In 14 patients CMML was thought to be “secondary”, following treatment for non- Hodgkin or Hodgkin lymphoma in 4, aplastic anemia in 2, breast cancer in 2, and one each for idiopathic thrombocytopenic purpura, chronic lymphocytic leukemia, Wegener’s granulomatosis, rhabdomyosarcoma, acute myeloid leukemia and liver transplantation.
Treatment before transplantation included transfusions alone in 13 patients; 49 patients received hydroxyurea or cytoreductive chemotherapy or both; ten received erythropoietin, prednisone or differentiating agents alone or in combination. Fifteen underwent splenectomy with or without other therapeutic modalities. Nine received other treatment including azacytidine or decitabine in 5, imatinib in two, thalidomide and lenalidomide in two.
The HCT comorbidity index (HCT-CI) score was 0 in 19, 1–2 in 23, 3 in 19, and 4–11 in 18 patients; the score could not be calculated in 8 patients due to missing data [14].
Donor and Transplant Characteristics
Donor and transplant characteristics are summarized in Table 2.
Table 2.
Donor and Transplant Characteristics
| Variable | Number of Patients | |
|---|---|---|
| Donor age (yrs), range(median) 3.4 – 69.1 (40.1) | ||
| Sex, male/female | 51/34 | |
| Donor/patient CMV status* | ||
| −/− | 21 | |
| −/+ | 26 | |
| +/+ | 25 | |
| +/− | 11 | |
| Donor patient relationship | ||
| –Related | ||
| HLA-identical sibling | 32 | |
| HLA-matched relative other than sibling | 2 | |
| HLA-mismatched relative | 4 | |
| –Unrelated | ||
| HLA-matched | 39 | |
| HLA-mismatched | 8 | |
| Donor / Patient sex | ||
| F / F | 13 | |
| F / M | 21 | |
| M / F | 20 | |
| M / M | 31 | |
| Conditioning regimen | ||
| BU (7 mg/kg)/CY (50 mg/kg)/TBI (12 Gy) | 10 | |
| BU (7 mg/kg)/TBI (12 Gy) | 11 | |
| BU (16 mg/kg)/CY (120 mg/kg)/THY (4.5 mg/kg) | 29 | |
| CY (120 mg/kg)/TBI (14.4 or 13.2 Gy) | 8 | |
| TBI (2–3 Gy) ± FLU (90 mg/m2) | 6 | |
| FLU (120 mg/m2)/BU (16 mg/kg) | 12 | |
| TBI (2 Gy)/iodine 131-anti-CD45 antibody | 6 | |
| FLU (150 mg/m2)/Treosulfan (3×14 g/m2) | 3 | |
| GVHD prophylaxis regimen | ||
| CSP/MTX | 44 | |
| CSP/MMF | 12 | |
| CSP/Other combinations | 6 | |
| FK506/MTX | 20 | |
| FK506/MMF | 3 | |
| Source of Stem Cells | ||
| Marrow | 32 | |
| PBPC | 53 | |
| Cell dose, range (median) | ||
| Marrow | 0.7–7.8 (2.9) × 108/kg | |
| PBPC (CD34+) | 4.0–30.0 (10.9) × 106/kg | |
Data missing for two donors.
Eight patients (4 conditioned with targeted BU/CY, 4 conditioned with FLU/targeted BU, and one conditioned with CY/TBI) also received anti-thymocyte globuline [ATG]. One patient conditioned with BU/CY was also given amifostine, 340 mg/m2 [29].
Abbreviations: Bu = busulfan; CMV = cytomegalovirus; CSP = cyclosporine; HLA = Human leukocyte antigen; F= female; FK506 = tacrolimus; Flu = fludarabine; M= male; MMF = mycophenolate mofetil; MTX = methotrexate; PBPC = peripheral blood progenitor cells; TBI = total body irradiation
Donor Selection
HLA typing of related donors involved intermediate resolution molecular typing for HLA-A, -B,-C and DQB1, and high resolution typing for DRB1 [15]. Unrelated donors were typed for HLA-A, - B, -C, and -DRB1 by high resolution and for DQB1 by intermediate resolution typing [15]. Thirty-eight patients (45%) had related donors – 32 were genotypically HLA-identical siblings, two were HLA-matched family members other than siblings, four were HLA non-identical family members (parent differing for HLA-A; sibling differing for HLA-A, -B, and –DR; child differing for HLA-A, and –DR; in one the donor information was incomplete), and 47 (55%) had unrelated donors, 39 were HLA matched, and 8 were HLA non-identical (four differing for HLA-A, three for HLA-DR, and one with an undetermined mismatch).
Source of Stem Cells
The stem cell source was bone marrow in 32 (38%), and G-CSF-mobilized peripheral blood progenitor cells (PBPC) in 53 patients (62%).
Conditioning Regimen
Conditioning regimens were determined by sequential protocols active at the time of HCT (Table 2). Ten patients were conditioned with busulfan (BU) 7 mg/kg orally (po), cyclophosphamide (CY) 50 mg/kg intravenously (iv), and total body irradiation (TBI) 6 × 200 cGy over 3 days for a total of 12 Gy. Eleven patients received BU, 7 mg/kg po and TBI, 6 × 200 cGy over three days for a total of 12 Gy. Twelve patients received fludarabine 120 mg/m2 iv over 3 days and BU 16 mg/kg po over 4 days (targeted to plasma levels of 800–900 ng/mL). Twenty-nine patients received BU 16 mg/kg po (targeted to plasma levels of 800–900 ng/mL) plus CY 120 mg/kg iv; 8 patients received CY 120 mg/kg iv and fractionated TBI 14.4/13.2 Gy over 3 to 4 days; six patients received 131I conjugated anti-CD45 antibody iv combined with TBI, 200 cGy and fludarabine 3 × 30 mg/m2 iv; six received TBI 2 or 3 Gy, with or without the addition of fludarabine, 3 × 30 mg/m2 iv; and three patients received fludarabine 5 × 30 mg/m2 iv plus treosulfan 14 g/m2 iv [16].
GVHD Prophylaxis
Graft versus host disease (GVHD) prophylaxis consisted of cyclosporine (CSP) and methotrexate (MTX) in 44 patients, tacrolimus and MTX in 20, CSP and mycophenolate mofetil (MMF) in 12, tacrolimus and MMF in 3, and CSP plus other combinations [17] in six patients.
Evaluation
Engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) ≥ 0.5 × 109/L, and platelet engraftment as the first of three days with platelet counts of greater than 20 × 109/L, without transfusion support. Acute and chronic GVHD severity were assessed and treated as described previously [18–20]. We did not reclassify chronic GVHD according to the more recently developed NIH consensus criteria [21,22].
Relapse/disease progression was defined as re-appearance/ persistence of host cells with the morphologic, cytogenetic, molecular or immunophenotypic markers of the disease pre-transplant.
Statistical Analysis
Survival was defined as the time from transplant to death or date of last contact. Relapse-free survival was defined as the time from transplant to relapse or death by causes other than relapse. Non-relapse mortality was defined as death without prior relapse. Estimates of the probability of overall and relapse-free survival were obtained by the Kaplan-Meier method, and estimates of the probability of relapse, non-relapse mortality, and chronic GVHD were summarized using cumulative incidence estimates. Death without relapse was considered a competing risk for NRM, NRM a competing risk for relapse, and death without chronic GVHD a competing risk for chronic GVHD. Association of various factors with the cause-specific hazard of failure for each of these endpoints was assessed using Cox regression. The factors assessed, along with univariate regression results, are contained in Table 3. All 2-sided pvalues from regression models were estimated using the Wald test, and no adjustments were made for multiple comparisons. Data were analyzed as of 1/27/10.
Table 3.
Univariate Regression Results
| Factor | Overall Mortality | Mortality or Relapse | NRM | Relapse |
|---|---|---|---|---|
| MD Anderson | 1.11 (0.87–1.42, p=.40) | 1.14 (0.90–1.45, p=.29) | 0.88 (0.63–1.21, p=.42) | 1.63 (1.11–2.39, p=.01) |
| Prognostic Score* | ||||
| WBC > 13 × 109/l | 1 | 1 | 1 | 1 |
| WBC < 13 × 109/l | 1.11 (0.60–2.05, p=.74) | 0.99 (0.55–1.78, p=.96) | 0.88 (0.40–1.93, p=.75) | 1.14 (0.46–2.79, p=.78) |
| Patient/Donor Sex | ||||
| M/M | 1 | 1 | 1 | 1 |
| F/F | 0.90 (0.41–1.99, p=.79) | 1.07 (0.50–2.31, p=.85) | 0.41 (0.12–1.42, p=.16) | 3.55 (1.04–12.13, p=.04) |
| F/M | 0.58 (0.26–1.28, p=.18) | 0.63 (0.29–1.36, p=.24) | 0.33 (0.11–0.99, p=.05) | 1.75 (0.49–6.21, p=.39) |
| M/F | 0.81 (0.39–1.66, p=.56) | 0.85 (0.41–1.74, p=.65) | 0.62 (0.25–1.53, p=.30) | 1.68 (0.45–6.25, p=.44) |
| global p=.60 | global p=.61 | global p=.16 | global p=.22 | |
| Patient/Donor CMV status | ||||
| −/− | 1 | 1 | 1 | 1 |
| +/+ | 1.37 (0.62–3.02, p=.43) | 1.32 (0.60–2.90, p=.50) | 1.38 (0.50–3.80, p=.53) | 1.22 (0.34–4.32, p=.76) |
| +/− | 1.45 (0.66–3.21, p=.35) | 1.68 (0.78–3.65, p=.19) | 1.55 (0.56–4.26, p=.40 | 1.89 (0.57–6.27, p=.30) |
| −/+ | 1.03 (0.37–2.84, p=.95) | 1.07 (0.39–2.94, p=.90) | 0.62 (0.13–3.07, p=.56) | 1.69 (0.42–6.76, p=.46) |
| global p=.75 | global p=.55 | global p=.61 | global p=.71 | |
| Hemoglobin* | 0.86 (0.73–2.36, p=.37) | 0.87 (0.75–1.02, p=.09) | 0.87 (0.70–1.08, p=.21) | 0.87 (0.69–1.10, p=.24) |
| Hematocrit* | 0.92 (0.87–0.98, p=.007) | 0.94 (0.89–0.99, p=.02) | 0.92 (0.85–0.99, p=.03) | 0.96 (0.89–1.04, p=.34) |
| Lymphocytes* | 1.00 (0.90–1.12, p=.96) | 1.02 (0.91–1.13, p=.77) | 0.95 (0.80–1.13, p=.55) | 1.08 (0.94–1.23, p=.29) |
| Platelets* | 1.00 (0.99–1.00, p=.05) | 1.00 (0.99–1.00, p=.07) | 1.00 (0.99–1.00, p=.15) | 1.00 (0.99–1.00, p=.27) |
| Donor | ||||
| Matched Sibling | 1 | 1 | 1 | 1 |
| Non-sibling Relative | 0.45 (0.10–1.96, p=.29) | 0.44 (0.10–1.93, p=.28) | 0.45 (0.06–3.63, p=.46) | 0.43 (0.05–3.46, p=.43) |
| Unrelated | 1.03 (0.56–1.91, p=.92) | 1.11 (0.60–2.04, p=.74) | 1.26 (0.55–2.91, p=.58) | 0.95 (0.39–2.33, p=.91) |
| Cytogenetics | ||||
| Good/Intermediate | 1 | 1 | 1 | 1 |
| Poor | 2.12 (1.15–3.90, p=.02) | 2.20 (1.21–3.99, p=.009) | 2.20 (1.00–4.82, p=.05) | 2.20 (0.89–5.47, p=.09) |
| Source of Stem Cells | ||||
| PBPC | 1 | 1 | 1 | 1 |
| Marrow | 1.40 (0.80–2.47, p=.24) | 1.31 (0.75–2.30, p=.34) | 1.52 (0.73–3.15, p=.26) | 1.08 (0.45–2.57, p=.87) |
| No Excess Blasts | 1 | 1 | 1 | 1 |
| Excess Blasts | 0.97 (0.55–1.71, p=.92) | 1.00 (0.57–1.74, p=.99) | 0.83 (0.40–1.73, p=.62) | 1.29 (0.54–3.07, p=.57) |
| CMML2 | 1 | 1 | 1 | 1 |
| CMML1 | 1.10 (0.59–2.02, p=.77) | 1.07 (0.59–1.94, p=.83) | 1.57 (0.67–3.67, p=.30) | 0.68 (0.28–1.61, p=.38) |
| Cytogenetics (Spanish)* | 2.07 (1.13–3,82, p=.02) | 2.11 (1.17–3.83, p=.01) | 2.83 (1.32–6.07, p=.008) | 1.36 (0.50–3.71, p=.55) |
| IPSS* | 1.28 (0.89–1.86, p=.19) | 1.27 (0.88–1.82, p=.21) | 1.22 (0.76–1.98, p=.41) | 1.33 (0.76–2.32, p=.32) |
| HCT-CI 0–2 | 1 | 1 | 1 | 1 |
| HCT-CI > 2 | 2.80 (1.53–5.14, p=.0008) | 2.53 (1.41–4.55, p=.002) | 4.31 (1.79–10.39, p=.001) | 1.47 (0.62–3.47, p=.38) |
| Age* | 1.02 (1.00–1.04, p=.06) | 1.02 (1.00–1.04, p=.06) | 1.03 (0.99–1.06, p=.06) | 1.01 (0.98–1.04, p=.47) |
| Disease duration* | 1.02 (0.90–1.17, p=.75) | 1.02 (0.90–1.17, p=.73) | 0.88 (0.63–1.20, p=.43) | 1.10 (0.96–1.24, p=.18) |
| Chronic GVHD** | 1.15 (0.55–2.40, p=.70) | 1.19 (0.56–2.54, p=.66) | 2.33 (0.62–8.68, p=.21) | 0.82 (0.31–2.13, p=.68) |
| Year of transplant* | 0.99 (0.94–1.04, p=.69) | 1.00 (0.95–1.05, p=.85) | 0.99 (0.93–1.06, p=.81) | 1.00 (0.93–1.08, p=.99) |
Modeled as continuous linear variables; HR (hazard ratio) reflects increase in hazard associated with increase in one unit. For age, year of transplant and disease duration, one unit corresponds to 1 year. For pre-transplant hematologic parameters the units were as follows; hemoglobin – g/l; hematocrit – percent ; lymphocytes and platelets – 10 9/l.
modeled as time-dependent covariate
Abbreviations: M= male, F = female; CMV = cytomegalo virus; PBPC = peripheral blood progenitor cells; IPSS = International Prognostic Scoring System; HCT-CI _ hematopoietic cell transplantation comorbidity index; GVHD = graft versus host disease;
RESULTS
Engraftment
Seventy-seven patients (91%) achieved sustained engraftment, as defined by neutrophil counts of 0.5 × 105/L, at 9 to 31 (median 18) days, including one patient, prepared with a reduced intensity regimen) in whom the ANC never declined below 0.5 × 105/L. Seven of the remaining 8 patients died between day 11 and day 80 without achieving 0.5 × 105/L. One patient showed 100% donor cells (CD3+ and CD33+) initially, but never achieved a neutrophil count of ≥ 0.5 × 109/L and died on day 438 with recurrent CMML. A transfusion-independent platelet count of 20 × 109/L or greater was reached at 8 to 97 (median 14.5) days, by 67 patients (79%); 18 patients died between days 11 and 115 without platelet reconstitution.
GVHD
Acute GVHD of grades II–IV developed in 58 (72%), and grades III–IV in 21 (26%) of the 81 patients who were assigned a grade. Chronic GVHD occurred in 37 patients by 2 years for a cumulative incidence estimate of 44%. In addition, one patient was diagnosed with chronic GVHD nearly 8 years following transplantation.
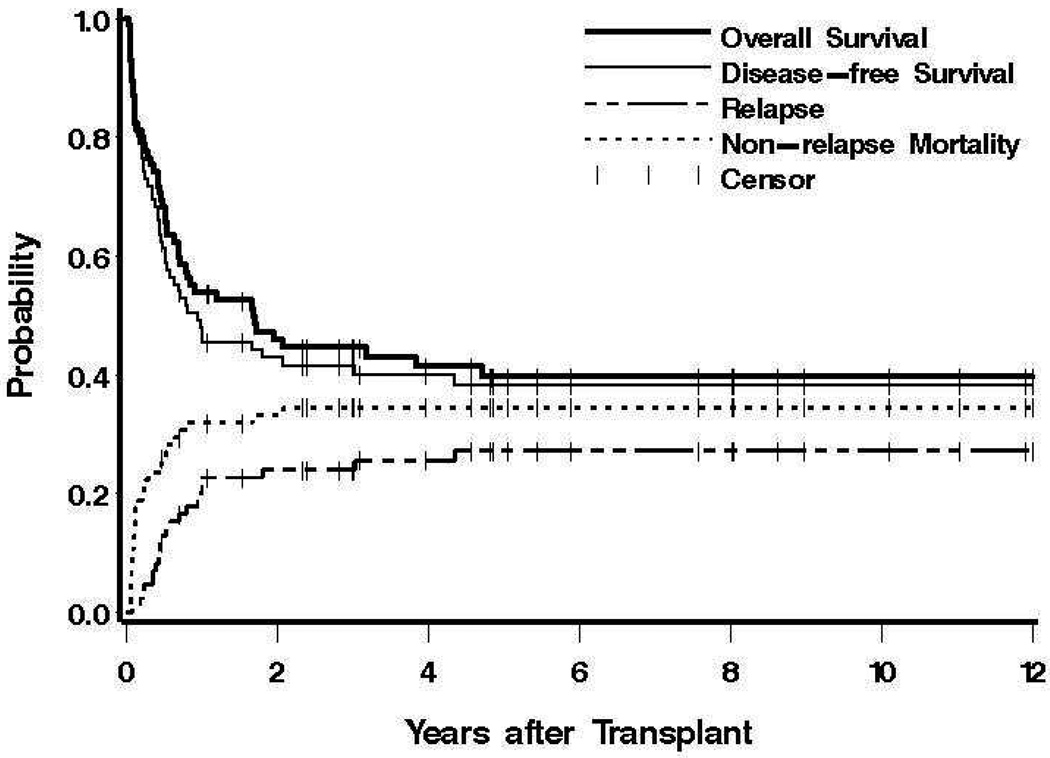
Relapse and Non-relapse Mortality
Relapse or progression of CMML occurred in 22 patients between 28 and 1585 (median 183) days after transplantation (Figure 1). The estimated probability of relapsed disease progression was 24% at 2 years and 27% at 10 years. Univariate regression models are summarized in Table 3. MDAPS was statistically significantly associated with the risk of relapse, while poor-risk cytogenetics were suggestively associated with the risk of relapse. Female patients with a female donor had a higher risk of relapse compared to male patients transplanted from a male donor, although the univariate global p-value for patient/donor gender was p=0.22. In a multivariable regression model (Table 4), MDAPS showed a similar magnitude of association as in the univariate model, while patient/donor gender showed a suggestive association (in particular, F/F compared to M/M).
Figure 1. Survival, relapse and non-relapse mortality.
Shown for all patients are overall and disease (relapse)-free survival, and the probabilities of relapse and non-relapse mortality. Tickmarks indicate censored patients.
Table 4.
Multivariable Analysis of Outcomes (Hazard Ratio [95% confidence limits, p-value))
| Relapse | NRM | Mortality or Relapse | Overall Mortality | |
|---|---|---|---|---|
| Patient/Donor Sex | ||||
| M/M | 1 | |||
| F/F | 4.64 (1.20–18.01, p=.03) | |||
| F/M | 1.86 (0.45–7.69, p=.39) | |||
| M/F | 1.60 (0.35–7.19, p=.54) | |||
| Global p=.10 | ||||
| MDAPS | 1.65 (1.11–2.45, p=.01) | |||
| Hematocrit | 0.92 (0.84–1.00, p=.06) | 0.94 (0.88–1.00, p=.04) | ||
| HCT-CI | ||||
| 0–2 | 1 | 1 | 1 | 1 |
| >2 | 3.97 (1.54–10.23, p=.004) | 2.46 (1.33–4.54, p=.004) | 2.62 (1.36–5.05, p=.004) | |
| Cytogenetics (IPSS) | ||||
| Good/Interm. | 1 | 1 | 1 | 1 |
| Poor | 3.09 (1.21–7.88, p=.02) | 3.35 (1.73–6.48, p=.0003) | 2.73 (1.37–5.44, p=.004) | |
| Age | 1.04 (1.00–1.07, p=.06) | 1.03 (1.01–1.06, p=.009) | 1.03 (1.00–1.05, p=.02) | |
Abbreviations: CI = confidence interval; F = female; HCT-CI = Hematopoietic cell transplantation co-morbidity index; HR = hazard ratio; IPSS = International Prognostic Scoring System; M = male; NRM = Non-relapse mortality. MDAPS = MD Anderson Prognostic Score.
Overall, 29 deaths without a prior relapse had occurred by the time of last contact, for a 2-year estimate of NRM of 33%, and a 10-year estimate of NRM of 34%. As indicated in Table 3, in univariate regression models pre-transplant hematocrit, cytogenetics, Spanish classification, HCT-CI, and age showed statistically significant or suggestive associations with the risk of NRM. In a multivariable regression model, each of these covariates showed a statistically significant association with NRM, although cytogenetics and Spanish classification failed to show an association when both were included in the model, due to the strong correlation between these two factors.
Causes of Death
At the time of last contact (database locked on 01/27/2010), 49 patients had died, 20 with progression or relapse of CMML and 29 from non-relapse causes. These included multi-organ failure (n= 13, associated with GVHD in 2), viral or fungal infections (n=7), central nervous system or pulmonary hemorrhage (n=2), respiratory failure (n=2), and GVHD (n=2); in 3 patients the cause of death was not determined.
Overall and Relapse-free Survival
Currently 36 patients are surviving (34 in remission) at 0.5 to 19.1 (median 5.2) years, with a probability of survival (relapse-free survival) at 10 years of 40% (38%) (Figure 1)
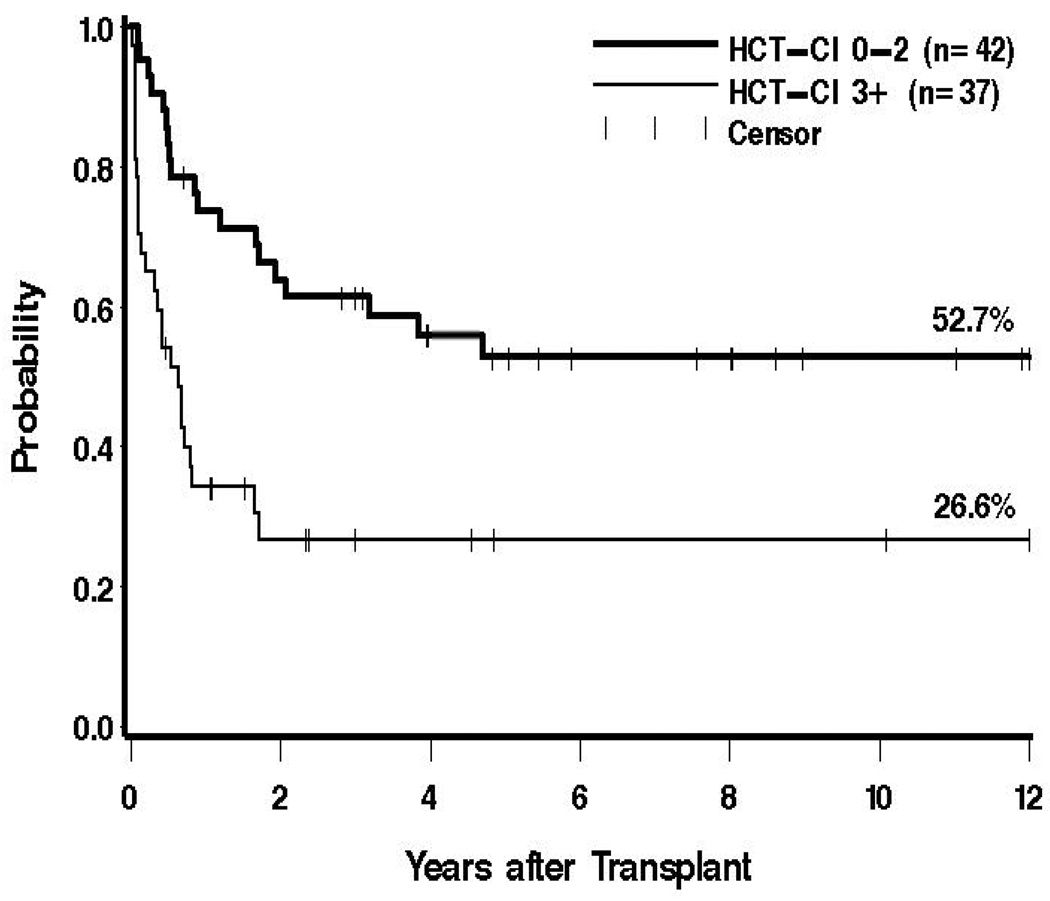
Univariate regression models are summarized in Table 3. For both overall survival and relapse-free survival, the same factors were either statistically significantly or suggestively associated with outcome (pre-transplant hemoglobin, hematocrit, and platelet counts; cytogenetics, HCT-CI, age, and Spanish classification. In a multivariable regression model, increasing pre-transplant hematocrit was associated with decreased mortality and increased relapse-free survival, and increasing age, higher HCT-CI (Figure 2), and poor-risk cytogenetics were each associated with increased mortality and reduced relapse-free survival (Table 4).
Figure 2. Survival dependent upon pre-transplant co-morbidities.
Shown are the probabilities of survival for 42 patients with HCT-CI scores 0–2 (52.7%), and for 37 patients with scores of 3 or greater (26.6%)
DISCUSSION
CMML is a hematopoietic malignancy with dysplastic and proliferative characteristics. While some patients have a relatively indolent disease course extending over several years, others progress rapidly to acute leukemia. Intensive chemotherapy as used for remission induction in patients with acute leukemia has met with limited success. The probability of achieving complete remissions is low, and remission duration has been short. A randomized trial comparing etoposide and hydroxyurea showed superior survival with hydroxyurea [23]. More recent trials using farnesyl transferase inhibitors [24] or hypomethylating agents [25] showed less early toxicity and mortality than observed with conventional chemotherapy, but typically resulted in only short-lasting responses.
Hematopoietic cell transplantation is currently the only treatment modality with proven curative potential, offering the chance of long-term survival. The present findings confirm our earlier results, showing an estimated post-transplant survival probability of 40% at 10 years with follow-up of surviving patients extending to two decades. The major factors determining long-term relapse-free survival as well as overall survival were pre-transplant hematocrit, cytogenetic risk category, co-morbidity index, and age. While disease classification by MDAPS criteria could predict the risk of recurrent malignancy, as already suggested by our initial report [11], neither this nor any other classification examined in the present analysis statistically significantly affected long-term survival or relapse-free survival. However, the parameters identified as significant were reminiscent of those described by Spanish investigators as determining survival in non-transplanted patients [7]. In an analysis of data on 419 patients who had been followed for a median of 33 months, these investigators identified in univariate analysis CMML-2 with the presence of two or three peripheral blood cytopenias, poor-risk cytogenetics (defined as trisomy-8 or complex karyotype), and red blood cell transfusion dependence as factors that were associated with shorter overall survival and higher risk of evolution to acute leukemia (p<.001) [7].
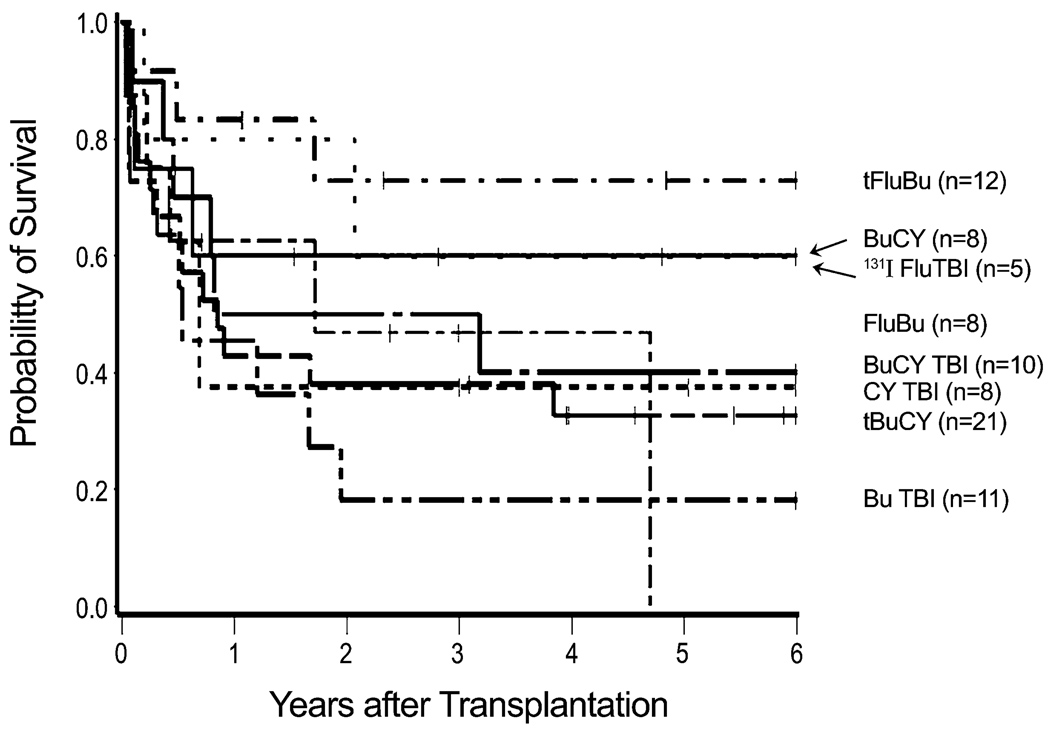
Of note, patient age, CMV status, source of stem cells, and type of donor did not statistically significantly affect overall or relapse-free survival, although female-to-female transplants were associated with a higher probability of relapse compared to male-to-male transplants in univariate analysis. Whether patient/donor gender is truly associated with relapse or is a product of multiple comparisons is not known. A global analysis of the four gender combinations was not statistically significant (p=0.10), and lacking a biological explanation for this association we acknowledge that the observed association may not be real. Similarly, the type of conditioning regimen did not statistically significantly affect outcome; it appeared, however, that patients conditioned with fludarabine and targeted busulfan had a low relapse incidence and the highest probability of survival. Many reports on transplant outcome in patients with myelodysplastic syndromes included patients with CMML [9,12,26], while few focused on this disease group exclusively [10,11,27]. A report from the Mayo Clinic on 17 patients with CMML, 26–60 years of age, showed a 41% NRM with 3 patients (18%) surviving in remission at a median follow-up of about 3 years. The incidence of relapse was 41%. A report from King’s College in London summarized results in 18 patients, 38 – 66 years of age, most of whom had received T cell depleted transplants following fludarabine/busulfan conditioning [28]. The 3-year overall survival was 31%, and the relapse incidence 47%. Similar to the present study, high risk cytogenetics were associated with mortality; however, small patient numbers prevented a strong statistical assessment. Mittal et al. included 7 patients with CMML in a report from the M.D. Anderson Cancer Center, showing a relapse-free survival of 37% with a median follow-up of 1.5 years [26]. Kroger et al. reported relapse-free survival of 18% at 40 months, in a study summarizing results from multiple institutions, some of which used T-cell depletion of the stem cell inoculum [27]. The present results in patients transplanted at a single institution compare favorably with those data.
All published reports, including the present one, have identified disease relapse as a major problem, occurring in 25% to more than 40% of patients. It was of note, however, that the cumulative incidence of relapse in the small cohort of patients conditioned with a low-intensity regimen of fludarabine and 200 cGy of TBI, 25%, was not significantly different from the incidence observed with higher-intensity regimens, consistent with a clinically relevant graft-versus-leukemia effect as also suggested by other investigators [10,27].
Even so, survival with this regimen was not improved relative to other conditioning strategies, conceivably related to higher rates of co-morbidities in patients included in this cohort. Although patient selection bias obviously could play a large role, the similar incidence rates of relapse with conditioning regimens of various intensities suggest that modalities other than cytotoxic therapy should be incorporated into transplant regimens for patients with CMML.
Overall and relapse-free survivals in this updated analysis were similar to results presented in our previous report [11]. High-risk karyotypes were correlated with high relapse rates, although the impact of karyotype decreased in multivariable analysis when other factors, including hematologic parameters pre-transplant were entered into the analysis. The effect of these factors indicates that the MDAPS or the Spanish classification identify parameters with significant impact on transplant outcome [1]. As in our initial study [11], co-morbidity scores were correlated with increasing non-relapse mortality. Thus, while currently used transplant regimens may be quite effective in patients without significant co-morbidities and with good-risk cytogenetics, new strategies are required for patients with high-risk features. Whether the use of radioactive isotope-conjugated antibodies to hematopoietic cells in the conditioning regimen can improve overall results remains to be determined. The subcohort of patients in the present study that was treated by this approach was too small to allow for firm conclusions.
Our observation that disease parameters identified as prognostically relevant in patients not undergoing HCT were also prognostically relevant for outcomes after HCT is of note. For one, this observation should allow to select high-risk patients for HCT. Secondly, it might be possible to identify patients with deteriorating parameters and possibly proceed with HCT earlier than would have otherwise have been the case. Clearly, the HCT-CI, hematologic parameters and cytogenetic findings are the factors with the most profound impact on post-transplant outcome, and novel regimens with low toxicity but greater efficacy in patients with high-risk cytogenetics must be developed.
Figure 3. Survival by conditioning regimen.
Shown are survivals with specific conditioning regimens of various dose intensities. (The group of 3 patients conditioned with fludarabine and treosulfan is not included.)
ACKNOWLEDGMENTS
We thank all referring physicians for their continued support and all patients for participating in these trials. We are greatful to Joanne Greene RN, Michelle Bouvier RN and Gary Schoch for data collection and management, and Helen Crawford and Bonnie Larson for help with manuscript preparation.
Grant Support: The authors are grateful for research funding from the National Institutes of Health, Bethesda, MD grants P01HL036444, P01CA018029, P30CA015704, and HL088021. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None.
REFERENCES
References as of 09-17-2010
- 1.Onida F, Kantarjian HM, Smith TL, et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood. 2002;99:840–849. doi: 10.1182/blood.v99.3.840. [DOI] [PubMed] [Google Scholar]
- 2.Germing U, Strupp C, Knipp S, et al. Chronic myelomonocytic leukemia in the light of the WHO proposals. Haematologica. 2007;92:974–977. doi: 10.3324/haematol.11051. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [erratum appears in Blood 1998 Feb 1;91(3):1100] [PubMed] [Google Scholar]
- 4.Pich A, Riera L, Sismondi F, et al. JAK2V617F activating mutation is associated with the myeloproliferative type of chronic myelomonocytic leukaemia. J Clin Pathol. 2009;62:798–801. doi: 10.1136/jcp.2009.065904. [DOI] [PubMed] [Google Scholar]
- 5.Jelinek J, Oki Y, Gharibyan V, et al. JAK2 mutation 1849G>T is rare in acute leukemias but can be found in CMML, Philadelphia chromosome-negative CML, and megakaryocytic leukemia. Blood. 2005;106:3370–3373. doi: 10.1182/blood-2005-05-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gondek LP, Tiu R, O'Keefe CL, Sekeres MA, Theil KS, Maciejewski JP. Chromosomal lesions and uniparental disomy detected by SNP arrays in MDS, MDS/MPD, and MDS-derived AML. Blood. 2008;111:1534–1542. doi: 10.1182/blood-2007-05-092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Such E, Cervera J, Nomdedeu B, et al. A new prognostic scoring system including transfusion dependency and cytogenetic abnormalities for patients with chronic myelomonocytic leukemia. Blood. 2009;114:695–696. #1750 [abstr.] [Google Scholar]
- 8.Fukuhara T, Kakinoki Y. Clinical features of a new category, myelodysplastic/myeloproliferative diseases, defined by WHO classification. Rinsho Byori - Japanese Journal of Clinical Pathology. 2006;54:243–249. [Japanese]. [PubMed] [Google Scholar]
- 9.Laport GG, Sandmaier BM, Storer BE, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008;14:246–255. doi: 10.1016/j.bbmt.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott MA, Tefferi A, Hogan WJ, et al. Allogeneic stem cell transplantation and donor lymphocyte infusions for chronic myelomonocytic leukemia. Bone Marrow Transplant. 2006;37:1003–1008. doi: 10.1038/sj.bmt.1705369. [DOI] [PubMed] [Google Scholar]
- 11.Kerbauy DMB, Chyou F, Gooley T, et al. Allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia. Biol Blood Marrow Transplant. 2005;11:713–720. doi: 10.1016/j.bbmt.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Warlick ED, Cioc A, DeFor T, Dolan M, Weisdorf D. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant. 2009;15:30–38. doi: 10.1016/j.bbmt.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 14.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersdorf EW, Gooley TA, Anasetti C, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92:3515–3520. [PubMed] [Google Scholar]
- 16.Nemecek ER, Guthrie KA, Sorror ML, et al. Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant. doi: 10.1016/j.bbmt.2010.05.007. 9999;prepublished online May 25, 2010; doi:10.1016/j.bbmt.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin PJ, Pei J, Gooley T, et al. Evaluation of a CD25-specific immunotoxin for prevention of graft-versus-host disease after unrelated marrow transplantation. Biol Blood Marrow Transplant. 2004;10:552–560. doi: 10.1016/j.bbmt.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 19.Martin P, Nash R, Sanders J, et al. Reproducibility in retrospective grading of acute graft-versus-host disease after allogeneic marrow transplantation. Bone Marrow Transplant. 1998;21:273–279. doi: 10.1038/sj.bmt.1701083. [DOI] [PubMed] [Google Scholar]
- 20.Benesch M, Deeg HJ. Acute graft-versus-host disease. In: Soiffer RJ, editor. Hematopoietic Stem Cell Transplantation. Totowa, NJ: Humana Press; 2008. pp. 589–620. [Google Scholar]
- 21.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Flowers MED, Storer BE, Lee SJ, et al. Risk factors for the development of acute and National Institute of Health (NIH) chronic graft-versus-host disease (GVHD) Blood. 2009;114:146. #345[abstr.] [Google Scholar]
- 23.Wattel E, Guerci A, Hecquet B, et al. A randomized trial of hydroxyurea versus VP16 in adult chronic myelomonocytic leukemia. Blood. 1996;88:2480–2487. [PubMed] [Google Scholar]
- 24.Kurzrock R, Albitar M, Cortes JE, et al. Phase II study of R115777, a farnesyl transferase inhibitor, in myelodysplastic syndrome. J Clin Oncol. 2004;22:1287–1292. doi: 10.1200/JCO.2004.08.082. [DOI] [PubMed] [Google Scholar]
- 25.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mittal P, Saliba RM, Giralt SA, et al. Allogeneic transplantation: a therapeutic option for myelofibrosis, chronic myelomonocytic leukemia and Philadelphia-negative/BCR-ABL-negative chronic myelogenous leukemia. Bone Marrow Transplant. 2004;33:1005–1009. doi: 10.1038/sj.bmt.1704472. [DOI] [PubMed] [Google Scholar]
- 27.Kroger N, Zabelina T, Guardiola P, et al. Allogeneic stem cell transplantation of adult chronic myelomonocytic leukaemia. A report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 2002;118:67–73. doi: 10.1046/j.1365-2141.2002.03552.x. [DOI] [PubMed] [Google Scholar]
- 28.Krishnamurthy P, Lim ZY, Nagi W, et al. Allogeneic haematopoietic SCT for chonic myelomonocytic leukaemia: a single-centre experience. Bone Marrow Transplant. doi: 10.1038/bmt.2009.375. 9999;prepublished online January 25, 2010; doi:10.1038/bmt.2009.375- [DOI] [PubMed] [Google Scholar]
- 29.Benesch M, McDonald GB, Schubert M, Appelbaum FR, Deeg HJ. Lack of cytoprotective effect of amifostine following HLA-identical sibling transplantation for advanced myelodysplastic syndrome (MDS): a pilot study. Bone Marrow Transplant. 2003;32:1071–1075. doi: 10.1038/sj.bmt.1704277. [DOI] [PubMed] [Google Scholar]