The selective actions of thyroid hormone receptor isoforms in vivo are directed by a subset of nuclear regulatory proteins.
Abstract
Studies using mice deficient in thyroid hormone receptors (TR) indicate that the two TR isoforms, TRα1 and TRβ1, in addition to mediating overlapping biological activities of the thyroid hormone, T3, also mediate distinct functions. Mice harboring an identical dominant negative mutation (denoted PV) at the C terminus of TRα1 (Thra1PV mice) or β1 (ThrbPV mice) also exhibit distinct phenotypes. These knockin mutant mice provide an opportunity to understand the molecular basis of isoform-dependent functions in vivo. Here we tested the hypothesis that the distinct functions of TR mutant isoforms are directed by a subset of nuclear regulatory proteins. Tandem-affinity chromatography of HeLa nuclear extracts showed that distinct 33 nuclear proteins including nuclear receptor corepressor (NCoR1) and six other proteins preferentially associated with TRα1PV or TRβ1PV, respectively. These results indicate that recruitment of nuclear regulatory proteins by TR mutants is subtype dependent. The involvement of NCoR1 in mediating the distinct liver phenotype of Thra1PV and ThrbPV mice was further explored. NCoR1 preferentially interacted with TRα1PV rather than with TRβ1PV. NCoR1 was recruited more avidly to the thyroid hormone response element-bound TRα1PV than to TRβ1PV in the promoter of the CCAAT/enhancer-binding protein α gene to repress its expression in the liver of Thra1PV mice, but not in ThrbPV mice. This preferential recruitment of NCoR1 by mutant isoforms could contribute, at least in part, to the distinct liver lipid phenotype of these mutant mice. The present study highlights a novel mechanism by which TR isoforms direct their selective functions via preferential recruitment of a subset of nuclear coregulatory proteins.
Thyroid hormone receptors (TR) are ligand-dependent transcription factors that regulate growth, differentiation, and maintenance of metabolic homeostasis. Human TRα1 and TRβ1 are encoded by the THRA and THRB genes, respectively. These two TR subtypes share extensive sequence similarities in the DNA- and ligand-binding domains but differ in the length and sequences of the amino-terminal A/B domains. The expression of these two TR isoforms is tissue dependent and developmentally regulated (1). A longstanding question in the understanding the biology of TR has been whether these TR isoforms can substitute for each other or whether they exert subtype-specific functions in target tissues. Targeted deletion of the Thra or Thrb gene in mice has shown that there are specific functions for each TR isoform (2–4). Combined deletion of both the Thra and Thrb genes in mice has further shown that TR isoforms could play selective roles and have overlapping functions (5, 6). However, how the selective role of TR isoforms is achieved remains unclear.
Since the first report of TR-interacting proteins (7), many nuclear receptor coregulatory proteins (coactivators and corepressors) have been reported that function to modulate the transcription activity of TR and other members of receptor superfamily (8). These nuclear receptor coregulators exhibit diverse regulatory roles to affect transcriptional initiation, elongation, splicing, and translation. Coactivators, after recruitment by TR, act to enhance the TR-mediated gene expression (9). Corepressors are molecules that function to repress gene expression, mainly through the interaction with unliganded TR (10). The expression of many nuclear receptor coregulatory proteins is tissue dependent. In view of the critical regulatory role of the widely distributed nuclear receptor coregulatory proteins in TR transcriptional activity, we hypothesized that one of the mechanisms by which TR isoforms achieve their selective cellular functions could be mediated by preferential recruitment of a subset of nuclear receptor coregulatory proteins.
To test this hypothesis in vivo, we turned to the use of two knockin mutant mice that harbor an identical mutation (denoted as PV) in the corresponding C terminus of TRβ1 (TRβ1PV; ThrbPV mice) or TRα1 (TRα1PV; Thra1PV mice). PV mutation was identified in a patient with resistance to thyroid hormone (RTH) (11). It has a frame-shift mutation in the C-terminal 14 amino acids, resulting in the complete loss of T3 binding activity and transcription capacity (12). ThrbPV mice faithfully recapitulate human RTH, exhibiting the hallmarks of dysregulation of the thyroid-pituitary axis and reduced sensitivity to T3 in other peripheral target tissues (13). In contrast, Thra1PV mice do not display the RTH phenotype; instead, they manifest dwarfism and other abnormalities distinct from those of ThrbPV mice (14). Moreover, in the same target tissues, such as in the liver, the abnormal regulation of T3-target genes in the Thra1PV mice and Thrb1PV mice are clearly distinct (15, 16). The contrasting phenotypes in these two mutant mice, harboring an identical PV mutation in the respective TR gene, provide us with an opportunity to test the hypothesis that recruitment of a subset of coregulatory proteins could underlie the distinct phenotypic expression of TR mutant isoforms.
In the present study, using tandem-affinity chromatography, we showed that TRβ1PV and TRα1PV recruited not only common nuclear proteins but also a subset of distinct nuclear proteins in HeLa cells. Interestingly, 5.5-fold more distinct nuclear proteins (33 and six distinct associated proteins for TRα1PV and TRβ1PV, respectively) were preferentially recruited by TRα1PV than by TRβ1PV, supporting the hypothesis that the action of TR mutant isoforms could be mediated, at least in part, by a subset of nuclear regulatory proteins. Moreover, we showed that nuclear receptor corepressor (NCoR1), a corepressor that was one of the nuclear proteins preferentially associated with TRα1PV, was more avidly recruited by TRα1PV than by TRβ1PV to the promoter of the CCAAT/enhancer-binding protein α gene (C/ebpα). C/EBPα is a master regulator of adipogenesis in the liver. Its mRNA expression was repressed in the liver of Thra1PV, but not ThrbPV, mice. Taken together, the present study has uncovered one mechanism by which the TR isoform achieves its selective cellular function via specific regulation by a subset of nuclear regulatory proteins.
Results
Recruitment of nuclear interacting proteins by TR mutants is isoform dependent
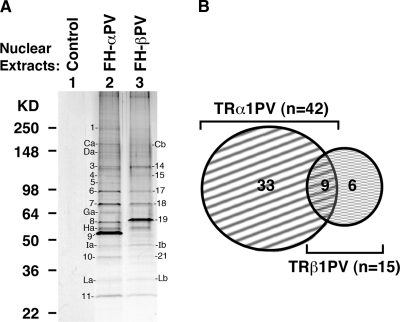
To test the hypothesis that selective actions of mutant TR isoforms could be directed by interaction with a specific subset of coregulator proteins, we used the epitope-tagging strategy [Flag-hemagglutinin (FH)-tagged mutant receptors] to identify TR mutant-interacting proteins from the nuclear extracts derived from HeLa cells stably expressing TRβ1PV [Flag-HA (FH)-βPV cells] or TRα1PV (FH-αPV cells). TRβ1PV- or TRα1PV-associated proteins were enriched first by Flag-tagged immunochromatography followed by HA-tagged affinity chromatography. This tandem purification to identify nuclear interaction proteins of mutant TR receptors was effective. As indicated in lane 1 (Fig. 1), virtually no nonspecific bands were detected from nuclear extracts of control cells (harboring vector only), supporting the specificity of the nuclear proteins associated with TRα1PV in lane 2 or with TRβ1PV in lane 3. Comparing the patterns of the associated proteins in lanes 2 and 3 shows that more visible protein bands were observed from nuclear extracts of FH-αPV cells (∼24 protein bands) than that from FH-βPV cells (∼18 protein bands). Comparison of the intensities of protein bands with apparent similar molecular weights under identical experimental conditions shows stronger staining for proteins from nuclear extracts of FH-αPV cells (e.g. bands Ca vs. Cb; no. 6 vs. no. 17; no. 7 vs. no. 18; no. 10 vs. no. 21, for FH-αPV cells and FH-βPV cells, respectively). Moreover, several protein bands were visible only in nuclear extracts from FH-αPV cells and were not detectable in the nuclear extracts from FH-βPV cells at the corresponding electrophoretic migration positions (e.g. band no. Ga from FH-αPV cells). These observations suggest that TRα1PV and TRβ1PV preferentially recruit different subsets of nuclear proteins. The protein bands that were visible by staining were analyzed by NanoLC-tandem mass spectrometry (MS/MS) peptide sequencing technology. The major proteins that could be identified by proteomic analysis were designated by numbers as shown in Fig. 1A. A total of 48 proteins were identified, the gene symbols and protein identities of which are listed in Table 1. Among the 48 proteins identified, 33 were found to preferentially associate with TRα1PV, and six were preferentially associated with TRβ1PV (Fig. 1B). Nine proteins were found to associate commonly with both mutant TR isoforms (Fig. 1B). These proteins could form complexes to regulate the activities of these TR isoforms via direct contact with TR mutant isoforms or via being part of large complexes (secondary coregulators) (17). Analysis of the functions of these TR mutant-associated proteins shows that these proteins were involved in chromatin remodeling, transcription regulation, RNA splicing/modification, protein translation, DNA repair, ubiquitination/proteasome, cell cycle/apoptosis, chaperone, enzymes, and protein trafficking (Tables 2 and 3). These data suggest that the actions of mutant TR isoforms could be regulated by common nuclear coregulatory proteins but also could be directed by an isoform-specific subset of nuclear regulatory proteins. For example, both TR mutant isoforms could be involved in chromatin-remodeling functions via the commonly associated proteins, PRMT5 (protein arginine methyltransferase 5), XRCC5 (ATP-dependent DNA helicase II, 80-kDa subunit), and XRCC6 (X-ray repair complementing defective repair in Chinese hamster 6), whereas the chromatin-remodeling activity of TRα1PV could be mediated preferentially by complexing with CHD4 (chromodomain-helicase-DNA-binding protein 4) and TRβ1PV by ACTB (actin β) (Table 2). Likewise, the transcription activity of both TR mutant isoforms could be regulated via association with the common nuclear protein (cell cycle- and apoptosis-regulatory protein 1), whereas that of TRα1PV could be preferentially regulated via complexing with the NCoR1 and/or with 10 other proteins (primary and/or secondary coregulators) listed in column 3, row 3 of Table 2. All together, these data clearly show that the actions of TR mutant isoforms could be preferentially directed by a subset of nuclear coregulatory proteins.
Fig. 1.
Identification of mutant TR isoform-associated proteins from HeLa cells by tandem-affinity chromatography and proteomic analysis. A, Nuclear extracts prepared from control (lane 1), FH-αPV (lane 2), or FH-βPV (lane 3) were purified by tandem affinity chromatography as described in Materials and Methods. B, After peptide sequencing and proteomic analysis, the number of nuclear proteins associated with TR mutant isoforms is shown.
Table 1.
Proteins identified from tandem-affinity chromatography and proteomic analysis
| Band number | Symbol | Protein name | Protein coverage (%) | Accession no. | Bound to |
|---|---|---|---|---|---|
| 1 | SPTAN1 | Spectrin, α, nonerythrocytic 1 (α-fodrin) isoform 2 | 39.04 | NP_003118.2 | α1PV |
| SPTBN1 | Spectrin, β, nonerythrocytic 1, isoform | 25.20 | EAX00138.1 | α1PV | |
| CHD4 | Chromodomain-helicase-DNA-binding Protein 4 | 9.94 | Q14839.1 | α1PV | |
| FLNA | Filamin A, α isoform 2 | 7.97 | NP_001104026.1 | α1PV | |
| RIF1 | RAP1 interacting factor 1 | 1.54 | NP_060621.3 | α1PV | |
| 3 | KIF11 | Kinesin family member 11 | 8.05 | NP_004514.2 | α1PV |
| NCoR1 | Nuclear receptor co-repressor 1 | 2.39 | BAA75814.1 | α1PV | |
| SF3B3 | Splicing factor 3b, subunit 3, 130 kDa | 4.19 | CAB56791.1 | α1PV | |
| 4 | NCL | nucleolin | 11.97 | NP_005372.2 | α1PV |
| NCoR1 | Nuclear receptor corepressor | 3.20 | BAA75814.1 | α1PV | |
| SND1 | Staphylococcal nuclease domain containing 1 | 4.07 | NP_055205.2 | α1PV | |
| KIF11 | Kinesin family member 11 | 2.37 | NP_004514.2 | α1PV | |
| CCAR1 | Cell cycle and apoptosis-regulatory protein 1 | 2.26 | NP_060707.2 | α1PV | |
| TRIM28 | Tripartite motif-containing 28 protein | 3.83 | NP_005753.1 | α1PV | |
| 5 | VCP | Valosin-containing protein | 7.69 | NP_009057.1 | α1PV |
| SFPQ | Splicing factor proline/glutamine-rich | 7.21 | NP_005057.1 | α1PV | |
| 6 | XRCC5 | x-Ray repair complementing defective repair in Chinese hamster cells 5 | 20.31 | EAW70563.1 | α1PV |
| NCoR1 | Nuclear receptor co-repressor 1 | 1.57 | BAA75814.1 | α1PV | |
| DNAJC10 | DnaJ (Hsp40) homolog, subfamily C, member 10 | 3.40 | NP_061854.1 | α1PV | |
| PLOD1 | Procollagen-lysine 1, 2-oxoglutarate 5-dioxygenase 1 | 3.16 | AAH16657.1 | α1PV | |
| ILF3 | M-phase phosphoprotein 4 | 6.06 | CAA66918.1 | α1PV | |
| 7 | HSPA1B | Heat shock 70-kDa protein 1B | 20.59 | NP_005337.2 | α1PV |
| XRCC6 | x-Ray repair complementing defective repair in Chinese hamster cells 6 | 24.79 | CAG47015.1 | α1PV | |
| PRMT5 | Protein arginine methyltransferase 5 | 4.71 | AAB66581.1 | α1PV | |
| RXRB | Retinoid X receptor, β | 4.69 | NP_068811.1 | α1PV | |
| 8 | STK38 | Serine/threonine kinase 38 | 13.33 | NP_009202.1 | α1PV |
| TBLXR1 | Transducin (β)-like 1 X-linked receptor 1 | 10.31 | NP_078941.2 | α1PV | |
| RXRB | retinoid X receptor, β | 8.44 | NP_068811.1 | α1PV | |
| PRPF31 | pre-mRNA processing factor 31 homolog | 6.81 | NP_056444.2 | α1PV | |
| NONO | non-POU domain-containing, octamer-binding | 6.37 | NP_031389.3 | α1PV | |
| 9 | THRA1 | Thyroid hormone receptor α 1 | 15.85 | NP_955366.1 | α1PV |
| 10 | ANXA2 | Annexin A2 isoform 2 | 13.57 | NP_004030.1 | α1PV |
| NPM1 | Nucleophosmin 1 isoform 1 | 18.71 | NP_002511.1 | α1PV | |
| HNRNPA2B1 | Heterogeneous nuclear ribonucleoprotein A2/B1 isoform A2 | 9.09 | NP_002128.1 | α1PV | |
| 11 | ADNP | Activity-dependent neuroprotector | 4.45 | NP_056154.1 | α1PV |
| SET | SET translocation (myeloid leukemia-associated) isoform 1 | 14.48 | NP_001116293.1 | α1PV | |
| 14 | KIF11 | Kinesin family member 11 | 13.92 | NP_004514.2 | βPV |
| 15 | KIF11 | Kinesin family member 11 | 8.24 | NP_004514.2 | βPV |
| CCAR1 | Cell-cycle and apoptosis regulatory protein 1 | 2.35 | NP_060707.2 | βPV | |
| 17 | EIF4B | Eukaryotic translation initiation factor 4B (eIF-4B) | 18.66 | P23588 | βPV |
| XRCC5 | ATP-dependent DNA helicase II | 4.78 | NP_066964.1 | βPV | |
| 18 | HSPA1B | Heat shock 70 kDa protein 1B | 25.43 | NP_005337.2 | βPV |
| PRMT5 | Protein arginine methyltransferase 5 isoform b | 11.77 | NP_001034708.1 | βPV | |
| RBM10 | RNA binding motif protein 10 isoform 2 | 3.05 | NP_690595.1 | βPV | |
| XRCC6 | x-Ray repair complementing defective repair in Chinese hamster cells 6 | 6.34 | EAW60448.1 | βPV | |
| 19 | THRB | Thyroid hormone receptor, β | 17.79 | NP_000452.2 | βPV |
| EIF4B | Eukaryotic translation initiation factor 4B41 | 6.71 | AAH73154.1 | βPV | |
| 21 | ANXA2 | Annexin A2 | 18.29 | AAH23990.1 | βPV |
| NPM1 | Nucleophosmin 1 isoform 3 | 7.72 | NP_001032827.1 | βPV | |
| Ca | RIF1 | RAP1-interacting factor | 6.07 | NP_060621.3 | α1PV |
| CHD4 | Chromodomain-helicase-DNA-binding protein 4 | 2.04 | Q14839.1 | α1PV | |
| SPTBN1 | Spectrin, β, nonerythrocytic 1, isoform | 1.76 | EAX00138.1 | α1PV | |
| Da | RIF1 | RAP1-interacting factor 1 | 5.87 | NP_060621.3 | α1PV |
| SPTAN1 | Spectrin, α, nonerythrocytic 1 (α-fodrin) isoform 2 | 1.58 | NP_003118.2 | α1PV | |
| Ga | PUF60 | poly-U-binding splicing factor 60-kDa isoform a | 9.66 | NP_510965.1 | α1PV |
| HNRNPK | Heterogeneous nuclear ribonucleoprotein K isoform a | 12.28 | NP_112553.1 | α1PV | |
| CDC23 | Anaphase-promoting complex subunit 8 | 4.06 | AAF05755.1 | α1PV | |
| SYNCRIP | Synaptotagmin binding, cytoplasmic RNA interacting protein | 5.52 | AAD38198.1 | α1PV | |
| LMNA | Lamin A/C | 5.38 | AAH33088.1 | α1PV | |
| Ha | HNRNPH1 | Heterogeneous nuclear ribonucleoprotein H1 | 7.35 | CAG33059.1 | α1PV |
| Ia | EEF1G | Eukaryotic translation elongation factor 1 γ | 7.55 | AAH13918.1 | α1PV |
| TUFM | Elongation factor Tu, mitochondrial precursor (EF-Tu) (P43) | 10.40 | P49411 | α1PV | |
| THRA1 | Thyroid hormone receptor α 1 | 7.80 | NP_955366.1 | α1PV | |
| La | NPM1 | Nucleophosmin 1 isoform 3 | 10.81 | NP_001032827.1 | α1PV |
| SPIN1 | Spindlin 1 | 16.88 | AAG38112.1 | α1PV | |
| ANP32B | Acidic (leucine-rich) nuclear phosphoprotein 32 family, member B | 9.56 | NP_006392.1 | α1PV | |
| Cb | RIF1 | RAP1-interacting factor 1 | 4.25 | NP_060621.3 | βPV |
| Ib | ACTB | Actin, β | 18.21 | AAH08633.1 | βPV |
| Lb | SPIN2B | Spindlin-like protein 2 | 17.44 | NP_001006682.1 | βPV |
| NPM1 | Nucleophosmin 1 isoform 3 | 16.22 | NP_001032827.1 | βPV | |
| KCTD5 | Potassium channel tetramerisation domain containing 5 | 15.81 | NP_061865.1 | βPV |
Table 2.
Functional categories of TRα1PV- and/or TRβ1PV-associated nuclear proteins
| TRα1PV and TRβ1PV | TRα1PV | TRβ1PV | |
|---|---|---|---|
| Chromatin-modeling | PRMT5; XRCC5; XRCC6 | CHD4 | ACTB |
| Transcription regulators | CCAR1 | ADNP; ILF3; NCOR1; NONO; RXRB; SFPQ; SET; SND1; TBL1XR1; THRA; TRIM28 | THRB |
| RNA splicing/modification | PRMT5 | HNRNPA2B1; HNRNPH1; HNRNPK; NONO; PRPF31; PUF60; SF3B3; SFPQ; SND1; SYNCRIP | RBM10 |
| Protein translation | EEF1G; NCL; TUFM | EIF4B | |
| DNA repair | RIF1; XRCC5; XRCC6 | ||
| Ubiquitination/proteasome-related | VCP | KCTD5 | |
| Cell cycle/apoptosis-related | CCAR1; KIF11 | CDC23; NUMA1; SPIN1; STK38 | SPIN2B |
| Cellular/nuclear structure-related | ANXA2 | FLNA; LMNA; SPTAN1; SPTBN1; | ACTB |
| Chaperone/chaperone-related | HSPA1B; NPM1 | ANP32B; DNAJC10; SET | |
| Enzyme | PLOD1; STK38 | ||
| Protein trafficking | VCP |
Table 3.
Nuclear TR mutant-associated proteins are in large protein complexes
| Symbol | Protein name | Functions |
|---|---|---|
| CCAR1 | Cell cycle- and apoptosis- regulatory protein 1; death inducer with SAP domain, DIS | Associates with components of the Mediator and p160 coactivator complexes and is a key regulator of mediator complex recruitment to NR target genes in response to the appropriate hormone; a novel component of Wnt/β-catenin signaling that plays an important role in transcriptional regulation by β-catenin. |
| CHD4 | Chromodomain-helicase-DNA-binding protein 4 (CHD-4) (ATP-dependent helicase CHD4) (Mi-2 autoantigen 218-kDa protein) (Mi2-β) | Belongs to the SNF2/RAD54 helicase family; it represents the main component of the nucleosome remodeling and deacetylase complex and plays an important role in epigenetic transcriptional repression; interacts with RORγ and represses RORγ-mediated transcriptional activation. |
| FLNA | Filamin A, α isoform 2 (Endothelial actin-binding protein) (actin-binding protein 280) (ABP-280) (nonmuscle filamin) | An actin-binding protein; a 280-kDa protein that crosslinks actin filaments into orthogonal networks in cortical cytoplasm; participates in the anchoring of membrane proteins for the actin cytoskeleton; interacts with AR. |
| KIF11 | Kinesin family member 11, kinesin-like protein KIF11 (Thyroid receptor-interacting protein 5) (TRIP) | A motor protein that belongs to the kinesin-like protein family; may be involved in various kinds of spindle dynamics including chromosome positioning, centrosome separation and establishing a bipolar spindle during cell mitosis; interacts with NCoR1 (Affinity-MS) and Thrb (yeast-two hybrid). |
| NCL | Nucleolin | A eukaryotic nucleolar phosphoprotein; involved in the synthesis and maturation of ribosomes; interacts with GR. |
| NONO | Non-POU domain- containing, octamer-binding | aka p54nrb (54 kDa RNA binding protein); plays various roles in the nucleus, including transcriptional regulation and RNA splicing; PSF and p54nrb (co-regulators) have also been reported to inhibit transcription by recruiting HDACs through mSin3A to other NRs including SF1, TRs, AR, and RXR; interacts with PR and acts as a corepressor. |
| SFPQ | Splicing factor proline/glutamine rich | aka PSF (polypyrimidine tract-binding protein-associated splicing factor); complexed with p54nrb; involved in transcription and RNA processing; directly interacts with PR and GR and specific co-regulatory proteins. |
| TBL1XR1 | Transducin (β)-like 1 X-linked receptor 1 | aka TBLR1; has sequence similarity with members of the WD40 repeat-containing protein family; may be involved in signal transduction, RNA processing, gene regulation, vesicular trafficking, cytoskeletal assembly and may play a role in the control of cytotypic differentiation; interacts with unliganded TRs. |
| TRIM28 | Tripartite motif-containing 28 protein, transcription intermediary factor 1-β (TIF1-β) (nuclear corepressor KAP-1) | Mediates transcriptional control by interaction with the Kruppel-associated box repression domain found in many transcription factors; interacts with ESR1 and GR; coactivator of NUR77 (NR4A1); exists in NCoR corepressor complex. |
AR, Androgen receptor; GR, glucocorticoid receptor; HDAC, histone deacetylase; PR, progesterone receptor; ROR, retinoid-related orphan receptor.
Differential interaction of NCoR1 with TR mutant isoforms in HeLa cells
The above findings prompted us to functionally validate the notion that the activity of TR mutant isoforms could be directed by preferential association with a subset of nuclear coregulatory proteins. Because it is beyond the scope of the present work to analyze functionally all proteins listed in Table 1, we chose to focus on NCoR1, which has become one of the best characterized corepressors since it was first reported by Hörlein et al. (18). Moreover, it is known that aberrant interaction of NCoR1 with TRβ mutants leads to its impaired dissociation from TRβ by T3. Impaired dissociation of NCoR1 from TRβ mutants has been implicated in the pathogenesis of RTH (19, 20). Even though very little is known about interaction of TRα mutants with NCoR1, the finding that NCoR1 was preferentially associated with TRα1PV (see Fig. 1A and Table 1) led us to focus on NCoR1 to test the hypothesis that differential recruitment of corepressors could direct the activities of TR mutant isoforms.
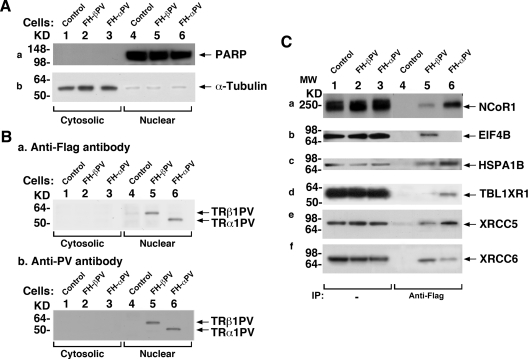
We first subfractionated cellular extracts from control cells, FH-βPV cells, and FH-αPV cells into cytoplasmic and nuclear fractions. Figure 2A shows that the cytoplasmic fractions, confirmed by using α-tubulin as marker, were not contaminated with nuclear proteins (lanes 1, 2, and 3 of Fig. 2A for control, FH-βPV, and FH-αPV cells, respectively) as indicated by the absence of the nuclear protein marker, poly(ADP-ribose) polymerase (PARP). Lanes 4, 5, and 6 show nuclear fractions were highly enriched as indicated by the detection of abundant PARP. When anti-Flag antibodies were used, Western blot analysis shows that both TR mutant isoforms were mainly localized in the nuclear fraction (lanes 5 and 6, Fig. 2B-a). A similar nuclear distribution for both TR mutant isoforms was further confirmed by using monoclonal antibody specifically against PV (lanes 5 and 6, Fig. 2B-b).
Fig. 2.
Physical interaction of NCoR1 with TR mutant isoforms in FH-βPV and FH-αPV cells. A, FH (controls) (lanes 1 and 4), FH-βPV (lanes 2 and 5), and FH-αPV (lanes 3 and 6) were cultured as described in Materials and Methods. Nuclear and cytoplasmic fractions were analyzed by Western blotting for the expression of markers for nuclear (PARP) (a) and cytoplasmic (α-tubulin) (b) fractions. (B-a) TRβ1PV and TRα1PV proteins were detected by monoclonal anti-Flag antibody, M2 (0.5 μg/ml), or by monoclonal anti-PV-specific antibody (2 μg/ml). B-b, Lanes 1 and 4 were from control cells as negative controls, indicating the specific bands detected in lanes 5 and 6. C, Association of NCoR1 and other nuclear proteins with TR mutants in FH-βPV and FH-αPV cells shown by coimmunoprecipitation. Nuclear extracts (1 mg) were immunoprecipitated with mouse anti-Flag M2 affinity gel followed by Western blot analysis with anti-NCoR1 antibody (C-a), anti-EIF4B antibody (C-b), anti-HSPA1B antibody (C-c), anti-TBL1XR1 antibody (C-d), anti-XRCC5 antibody (C-e), or anti-XRCC6 antibody (C-f). Lanes 5 and 6 were from nuclear extracts of FH-βPV and FH-αPV, respectively. Direct Western blot analysis (25 μg of nuclear extracts) is shown in lanes 1–3 as marked. IP, Immunoprecipitation.
To further confirm the preferential association of TRα1PV with NCoR1, we carried out coimmunoprecipitation assays. Consistent with findings using tandem-affinity chromatography, Fig. 2C shows that more NCoR1 was associated with TRα1PV (lane 6, Fig. 2C-a) than with TRβ1PV (lane 5, Fig. 2C-a). Lanes 1–3 were direct Western blot analysis, representing controls to indicate that the NCoR1 abundance was similar in the nuclear fractions of control, FH-βPV, and FH-αPV cells. For additional validation of the proteomic analysis shown in Fig. 1A (see also Table 2), we also showed that EIF4B was preferentially recruited to TRβ1PV (compare lanes 5 with 6, Fig. 2C-b), TBL1XR1 was preferentially recruited to TRα1PV (compare lanes 6 with 5, Fig. 2C-d), and that HSPA1B (Fig. 2C-c), XRCC5 (Fig. 2C-e), and XRCC6 (Fig. 2C-f) were commonly associated with both TR mutant isoforms (compare lanes 5 and 6 in panels c, e, and f). Taken together, these results show TR mutant isoforms recruit not only common coregulatory proteins but also preferentially recruit a subset of coregulatory proteins.
Effect of differential interaction of NCoR1 with TR mutant isoforms on the expression of hepatic genes
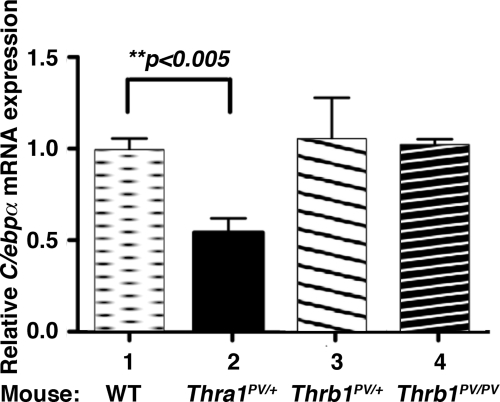
We have previously shown that mutations of the Thra gene (Thra1PV mice) or the Thrb gene (ThrbPV mice) lead to the manifestation of distinct phenotypes in these two mutant mice (13, 14). One prominent contrasting feature is the abnormalities in the liver. The liver mass of Thra1PV mice is decreased with lipid scarcity, whereas the liver mass of ThrbPV mice is increased with an excess depot of lipids (21–23). These observations provided us with an opportunity to test the hypothesis that NCoR1 could be one of the key regulators in directing the actions of mutant TR isoforms in lipid metabolism. To ascertain how NCoR1 could differentially modulate the effect of TR mutant isoforms at the transcription level, we focused on the CCAAT/enhancer binding protein α (C/ebpα) gene, a known direct T3-target gene (24), critical in the lipid metabolism in the liver. Figure 3 shows that, consistent with the distinct phenotype in the liver of these two mutant mice, the expression of the C/ebpα gene was repressed by nearly 50% in Thra1PV/+ mice as compared with that of wild-type liver (bar 2 vs. bar 1), whereas its expression was affected neither in the liver of the heterozygous (bar 3) nor in homozygous ThrbPV mice (bar 4).
Fig. 3.
Differential repression of mRNA expression of the C/ebpα gene in the liver of Thra1PV and ThrbPV mice. Quantitative real-time RT-PCR was performed as described in Materials and Methods to determine the mRNA expression of the C/ebpα gene in the liver of wild-type mice (bar 1), Thra1PV/+ mice (bar 2), ThrbPV/+ (bar 3), and ThrbPV/PV mice (bar 4). The data are expressed as mean ± ]scap]sem (n = 6; the P value is indicated). WT, wild type.
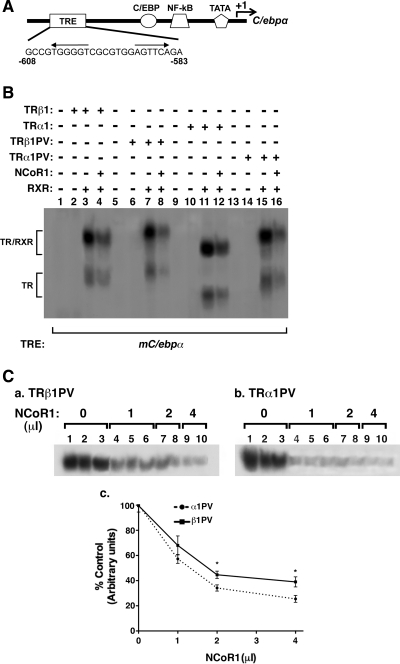
The effect of TR/T3 on the expression of the rat C/ebpα gene is mediated via thyroid response elements (TRE) located in the proximal promoter of the C/ebpα gene between nucleotide (nt) −611 and −586 (Supplemental Fig. IA-a published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org and Ref. 24). Because it was not clear whether there were species differences in TRE functioning to mediate T3 effect, we sought to identify the TRE in the mouse C/ebpα gene (mC/ebpα). Examination of the proximal promoter region of the mouse C/ebpα gene (Fig. 4A) shows a potential TRE with an everted repeat in the region of nt −608 and −583 (Fig. 4A) which was conserved between the rat and the mouse sequences (Supplemental Fig. IA-a). Analysis by EMSA shows that similar to the everted-repeat TRE in the chicken lysozyme gene (F2, lane 3, Supplemental Fig. IA-b), nt −608 and −583 (mC/epbα TRE) bound to TRβ1 as a heterodimer with the retinoid X receptor (RXR; lane 10, Supplemental Fig. IA-b). In the presence of anti-TRβ1 antibody C4, the bound TRβ1/RXR band was supershifted to a more retarded position (lane 11 for mC/epbα TRE; supershifted band in lane 5 for F2 TRE as a positive control), but not in the presence of an irrelevant antibody mineral oil-induced plasmacytoma (MOPC) (lane 12 for mC/epbα TRE; lane 6 for F2 TRE) as a negative control, indicating the specific binding of TRβ1 to mC/epbα TRE. RXR alone bound neither to F2 (lane 4), nor to mC/epbα TRE (lane 9). Lanes 1 and 7 were the controls in which only the labeled probes were used in EMSA.
Fig. 4.
A, A schematic diagram of the proximal promoter of the mouse C/ebpα gene. This proximal promoter region is conserved between mouse and rat. It contains several cis-regulatory elements for several transcription factors as indicated. The binding sites of cis-regulatory elements are shown according to the relative position to the transcription start site. A TRE is located between nt −608 and −583. B, Binding of wild-type (lanes 1–4 for TRβ1; lanes 9–12 for TRα1) and mutant TR isoforms (lanes 5–8 for TRβ1PV and lanes 13–16 for TRα1PV) in the presence (lanes 4, 8, 12, and 16) or absence of NCoR1 3RIDs (lanes 3, 7, 11, and 15). Equal amounts of in vitro translated wild-type and mutant TR isoforms were used in the EMSA. C-a and C-b, More TRα1PV than TRβ1PV complexed with NCoR1RIDs on TRE. EMSA was carried out with increasing amounts of in vitro translated NCoR1RIDs with an equal amount of TRβ1PV (C-a) or TRα1PV (C-b). C-c, Comparison of the extent of remainder TR mutant binding as TRβ1PV/RXR and TRα1PV/RXR on mC/ebpα TRE. The band intensities in C-a and C-b were quantified by using ImageJ software and then graphed. NF-kB, Nuclear factor-κB.
We further confirmed that indeed nt 5′-TGGGGT CGCGTGG AGTTCA GA contained an everted-repeat TRE by mutating the sequences (Mut 1 and Mut 2) as indicated in Supplemental Fig. IB-a. Although the wild-type mC/epbα TRE bound to TRβ1/RXR heterodimer (lanes 3–5, Supplemental Fig. IB-b), no binding was detected when 5′-TGGGGT was mutated to TGGATA (Mut 1, lanes 8–10) or AGTTCA-3′ mutated to ACATGA-3′ (Mut 2, lanes 13–15). Lanes 1, 6, and 11 were negative controls in which only labeled probes were present. Taken together, these results show that mC/epbα TRE bound specifically to TRβ1.
We have previously shown that PV, although failing to bind to T3, retains its binding activity to several TREs (25). To ascertain whether the binding of TR mutants to mC/epbα TRE was isoform dependent, we compared the binding of TRβ1, TRα1, TRβ1PV, and TRα1PV to the mC/epbα TRE (Fig. 4B). Similar levels of binding of these TR to mC/epbα TRE were observed under the experimental conditions (lanes 3, 7, 11, and 15, Fig. 4B). However, it is important to note that in the presence of in vitro translated NCoR1 [amino acids (aa) 1873–2453, containing three receptor interaction domains (RID): N1, N2, and N3; (26)], the mC/epbα TRE bound to TRβ1/RXR (compare lanes 3 and 4), to TRβ1PV/RXR (compare lanes 7 and 8), to TRα1/RXR (compare lanes 11 and 12), or to TRα1PV/RXR (compare lanes 15 and 16) was decreased, suggesting the formation of the ternary complex NCoR1/TR/RXR with a more retarded migration. However, when we attempted to use anti-NCoR1 antibody, which recognizes aa 2413–2431 (C-terminal region of NCoR1) or C4, which recognizes the C-terminal common epitope in TRβ1 and TRα1, we could not detect the supershifted mC/epbα TRE-bound NCoR1/TR/RXR bands, suggesting that the epitopes were not accessible to these antibodies in the ternary complexes. Nonetheless, the reduction in the less retarded mC/epbα TRE-bound TRβ1PV/RXR and TRα1PV/RXR in the presence of NCoR1 provided us with a tool to compare the avidity in the association of NCoR1 with the TRE-bound TRβ1PV or TRE-bound TRα1PV. We therefore carried out EMSA in the increasing concentrations of NCoR1. A stronger concentration-dependent decrease in TRE-bound TRα1PV/RXR (Fig. 4C-b) than TRβ1PV/RXR (Fig. 4C-a) was detected. The band intensities were quantified and graphed in Fig. 4C-c, which clearly shows that in the presence of increasing concentrations of NCoR1, less TRα1PV/RXR remained bound to the mC/epbα TRE (P < 0.05). These results indicate that TRα1PV formed a more stabilized complex with NCoR1 than did TRβ1PV.
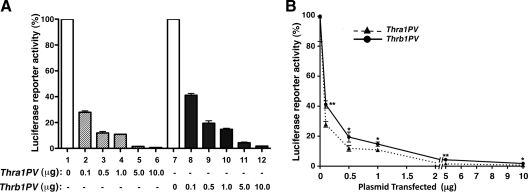
These in vitro EMSA findings would suggest that TRα1PV should be a stronger repressor than TRβ1PV in suppressing of the transcriptional activity of wild type TRs. To test this hypothesis, we cotransfected luciferase reporter containing the human C/EBPα promoter construct with increasing concentrations of expression plasmids of TRβ1PV or TRα1PV into hepatoma HepG2 cells stably expressing wild-type TRα1. As shown in Fig. 5A, the T3-activated transcriptional activity of C/EBPα promoter mediated by TRα1 was more repressed by TRα1PV than by TRβ1PV in a concentration-dependent manner. The stronger repression by TRα1PV than by TRβ1PV can be readily seen in Fig. 5B. The differences in degree of repression between TRα1PV and TRβ1PV were statistically significant (*, P < 0.05; **, P < 0.01). These cell-based results are consistent with the in vitro EMSA studies and suggest that TRα1PV recruits NCoR1 more avidly than TRβ1PV does and acts as a stronger repressor of the C/EBPα promoter activity.
Fig. 5.
The T3-dependent C/EBPα promoter activity was more inhibited by TRα1PV than by TRβ1PV in HepG2 cells stably expressing TRα1. HepG2TRα1 cells were cotransfected with 1 μg of the reporter plasmid (human C/EBPα promoter luciferase), 0.5 μg of RXRβ, and increasing concentrations of expression plasmid for TRα1PV (pcDNA3.1TRαPV) or TRβ1PV (pcDNA3.1TRβPV) as marked. Cells were treated without or with T3 (100 nm) for 24 h. Data were normalized against the protein concentration of the lysates. The percentage of luciferase activity after T3 treatment was calculated and defined as 100%. Assays were performed in triplicate and the data are expressed as mean ± ]scap]sem (n = 6). The data are expressed as bar graph (A) and x-y graph (B). The differences in the transcriptional activity were significant (*, P < 0.05; **, P < 0.01).
NCoR1 is more avidly recruited to the mouse C/ebpα promoter by TRα1PV than by TRβ1PV in hepatocytes
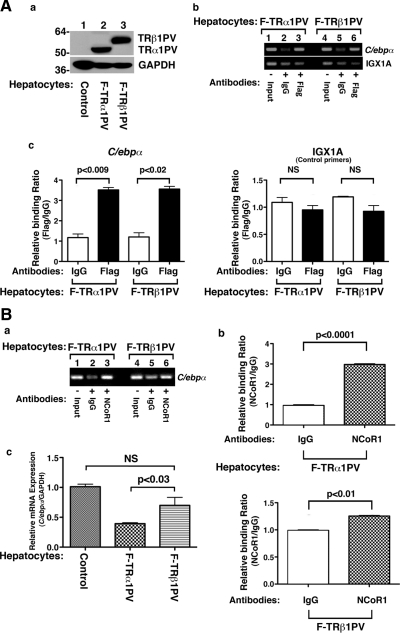
To further ascertain the preferential recruitment of NCoR1 by TRα1PV to the promoter of the mC/epbα gene, we carried out chromatin immunoprecipitation (ChIP) assays. TRα1PV or TRβ1PV was overexpressed in primary mouse hepatocytes isolated from wild-type mice via infection with adenoviral vectors containing cDNA for TR mutant isoforms. Figure 6A-a shows that an identical amount of TRα1PV or TRβ1PV was expressed in the hepatocytes in cells infected with respective TR mutant isoforms. The recruitment of TRα1PV or TRβ1PV to the promoter of the C/ebpα gene was illustrated in Fig. 6A-b. Consistent with results of EMSA (Fig. 4A) that show no apparent differences in the binding of TR mutant isoforms to the mC/ebpα TRE, the ChIP assay shows that both mutant TR isoforms bound to the promoter of the C/ebpα gene with similar avidity (Fig. 6A-b, upper panel, and Fig 6A-c, left panel). We also used irrelevant primers, IGX1A, in which no TRE was used as negative controls (Fig. 6A-b, lower panel, and Fig. 6A-c, right panel). We further analyzed the recruitment of NCoR1 associated with mutant isoforms bound to the promoter of the C/ebpα gene. Figure 6B-a shows that NCoR1 was recruited more to the C/ebpα gene (compare lanes 3 vs. 2 and 6 vs. 5 in Fig. 6B-a). Lanes 2 and 5 were the negative controls in which the IgGs were used in the ChIP assays. A quantitative comparison is shown in Fig. 6B-b to indicate a more avid recruitment of NCoR1 by TRα1PV (left panel of Fig. 6B-b) than by TRβ1PV (right panel of Fig. 6B-b) to the promoter of the C/ebpα gene. Consistent with these findings, Fig. 6B-c further shows that the expression of the endogenous C/ebpα gene was more repressed by TRα1PV than by TRβ1PV in hepatocytes. Thus, the different avidity in the recruitment of NCoR1 to the promoter of the C/ebpα gene by two mutant isoforms could underline, at least in part, the phenotypic distinction in the lipid profile in the liver between the TR mutants. Altogether, these results show that the differential interaction of NCoR1 with mutant TR isoforms plays a critical role in the actions of TR mutants.
Fig. 6.
NCoR1 is more avidly recruited by TRα1PV than by TRβ1PV to the promoter of the C/ebpα gene. Primary mouse hepatocytes were prepared as described in Materials and Methods and infected with adenovirus encoding F-TRα1PV (lane 2, A-a) and F-TRβ1PV (lane 3, A-a), respectively. A-a, Identical amount of TRα1PV and TRβ1PV were expressed in hepatocytes. A-b, ChIP assay was carried out using anti-Flag antibody (A-b, lanes 3 and 6) or anti-NCoR1 antibody (PHQQ) (B-a, lanes 3 and 6) or IgG as controls (A-b and B-a, lanes 2 and 5) as described in Materials and Methods. Lanes 1 and 4 in A-b and B-a are input control. Shown is a representative result of three experiments. A-c, The intensities of bands shown in panels b of A and panel a of B were quantified by densitometry analysis, and data are shown as reference to the intensity of IgG as 1. The negative controls of using IGX1A (control primers) are also shown. B-c, The expression of C/ebpα mRNA was more repressed in hepatocytes expressing TRα1PV than expressing TRβ1PV (lanes are as marked). Mouse primary hepatocytes were tranduced with adenovirus encoding Flag-tagged TRα1PV, TRβ1PV, or control virus as shown in Fig. 6A-a. Total RNA (200 ng) prepared as described in Materials and Methods was used in real-time quantitative PCR analysis. Glyceraldedyde 3-phosphate dehydrogenase primer was used as an internal control. Data are expressed as mean ± sem (n = 4). NS, Nonsignificant (P = 0.3094).
Discussion
One fundamental question in the understanding of the biology of TR and their molecular actions is whether TR isoforms exert overlapping functions or isoform-selective actions. Specific targeted Thra or Thrb gene deletion shows that each TR isoform has specific functions in vivo (2–5). Combined deletion of the Thra and Thrb genes has further shown that these two TR isoforms exert subtype-selective functions, but they also have overlapping functions (6). That these TR isoforms have selective functions in vivo is further supported by the distinct phenotypic manifestation of knockin mice that harbor an identical mutation at the corresponding C terminus of the Thra gene (Thra1PV mice) or Thrb gene (ThrbPV mouse). ThrbPV mice exhibit RTH similar to the human syndrome (13); however, Thra1PV mice do not have RTH but exhibit an array of distinct metabolic and growth abnormalities (14). We hypothesized that these distinct phenotypes could be mediated by recruitment of a subset of coregulatory proteins by TRβ1PV and TRα1PV, leading to distinct functional consequences. Indeed, tandem affinity chromatography of HeLa nuclear extracts identified nine nuclear proteins commonly associated with both TR mutant isoforms to mediate common categories of cellular functions (Table 2). Strikingly, 5.5-fold more nuclear proteins were preferentially associated with TRα1PV than with TRβ1PV (Fig. 1) to mediate diverse cellular functions. Among the various categories of cellular functions, the highest number of distinct proteins recruited more by TRα1PV than by TRβ1PV were those that function in transcription regulation (11:1 for TRα1PV vs. TRβ1PV; Table 2) as well in RNA splicing and modification (9:1 for TRα1PV vs. TRβ1PV; Table 2). The significance of more nuclear regulatory proteins being preferentially recruited by TRα1PV is not clear at present. One could speculate, however, that TRα1PV could require more regulatory proteins to fine tune and/or tightly control its cellular actions. It could also imply that the TRα1PV-signaling network could be more extensive and complex than that mediated by TRβ1PV. To distinguish these and other possibilities, additional functional characterization of the associated proteins would be required in future studies. However, it is important to point out that the distinct number and different categories of proteins complexed with these two TR mutant isoforms lend support to the notion that preferential recruitment of a subset of nuclear proteins could underlie the TR isoform-dependent actions.
The nuclear proteins identified to associate with these two mutant isoforms were from proteins that are in direct contact with TR mutants and also from the indirect secondary contact via protein-protein interaction in large protein complexes. For example, among the nine proteins commonly associated with both TR mutant isoforms (see Table 2), two are known to be in large complexes (Table 3). Cell cycle and apoptosis-regulatory protein 1 was reported to associate with components of the mediator and p160 coactivator complexes and is a key regulator of the mediator complex recruited to nuclear receptor target genes in response to hormones (27). It is also a novel component of Wnt/β-catenin signaling that plays an important role in transcriptional regulation by β-catenin (28). KIF11 (kinesin family number 11) is a motor protein that belongs to the kinesin-like protein family involved in various kinds of spindle dynamics including chromosome positioning, centrosome separation, and establishment of a bipolar spindle during cell mitosis. It is known to interact with NCoR1 (29). In contrast, for the associated proteins involved in the common functions of chromatin modeling, CHD4 was preferred by TRα1PV, whereas CHD4 was not shown to associate with TRβ1PV under the present experimental conditions (see Table 2). The structural basis for the preferential recruitment was not apparent because, except for the amino-terminal A/B domains, there is extensive sequence similarity between these two mutant isoforms. It is reasonable to postulate that in the tertiary structure of intact proteins, the A/B domain of each TR mutant isoform could influence how these two mutants contact the associated proteins involved in direct interaction. Further, there would be a rippling effect in that associated proteins that are in direct contact could affect how they influence the interaction with the secondary associated proteins to form the large protein complexes. This question of how the A/B domain could dictate the interaction of TR isoform with associate proteins could be answered when x-ray crystallographic structures of intact TRs become available to discern whether the interaction surfaces differ between the two TR isoforms.
The present studies have advanced understanding, at least in part, of the molecular basis of the distinct phenotypic expression in the liver of Thra1PV and ThrbPV mice. The former exhibit scarcity in lipids, and the latter display excess lipid accumulation. Because the C/ebpα gene is a T3 target gene with TREs in its promoter (24), we determined whether it could contribute to the different phenotypic manifestations observed in the liver of two TR mutant mice. C/EBP proteins are a family of transcription factors that contain a basic region-leucine zipper motif required for dimer formation. The C/ebpα gene is the first member of the family to be cloned. It is expressed at high levels in the adipose tissue as well as in the liver. It plays an important role in controlling differentiation of hepatocytes and energy metabolism. Recently, we observed that the expression of a panel of lipogenic genes in the liver is isoform dependent (21), being more repressed in the liver of Thra1PV mice than in ThrbPV mice. In the present study, we demonstrated that the C/ebpα mRNA expression in the liver was repressed in Thra1PV mice, but not in ThrbPV mice. Altogether, the present data could explain the scarcity of lipids in the liver of Thra1PV mice (Fig. 3). Transcription studies using reporters and ChIP assays show that TRE-bound TRα1PV more avidly recruited NCoR1 than did TRE-bound TRβ1PV to the promoter of the C/ebpα gene. These findings lend further support to the notion that preferential interaction of TRα1PV with a nuclear corepressor could partly explain the distinct phenotypes observed in the liver of ThrbPV and Thra1PV mice. Whereas the present studies focused on using NCoR1 and a relevant liver gene (the C/ebpα gene) to functionally validate the proteomic findings shown in Fig. 1 and Table 1, further in-depth functional analyses of other coregulatory proteins listed in Table 1 would certainly reveal new insights in understanding how recruitment of a subset of coregulatory proteins could mediate the TR mutant isoforms actions in vivo.
The distinct phenotypes of ThrbPV and Thra1PV mice are not limited to the liver. ThrbPV mice exhibit dysregulation of the pituitary-thyroid axis (13), whereas no such abnormalities are apparent in Thra1PV/+ mice (14). Thra1PV/+, but not ThrbPV/+, mice are dwarfs (13, 14). Glucose utilization in the brain and heart is significantly reduced in Thra1PV/+ mice, but no changes or increase in the brain and heart of ThrbPV/+ mice, respectively (30–32). Bone development is delayed in Thra1PV/+ mice (33, 34), whereas it is accelerated in ThrbPV/+ mice, reflecting thyrotoxicosis in the latter (35). The profiles of abnormal regulation of T3 target genes between ThrbPV/+ and Thra1PV/+ mice are clearly distinct (13, 14, 30–32). Because the expression of TR mutant-associated proteins is expected to be tissue dependent, one could envision that the nuclear proteins that act to direct these arrays of distinct phenotypes between ThrbPV and Thra1PV mice would be target tissue dependent. Further identification of the nuclear proteins that preferentially associated with mutant TR isoforms in other target tissues would certainly lead to a better understanding of the pathogenesis of the abnormalities of these TR mutants. Future studies using other TR isoform mutants could reveal further whether preferential recruitment of other nuclear regulators could underlie their distinct actions. Furthermore, it would also be important to test whether the distinct actions (not-shared functions) of wild-type TR isoforms are also mediated by preferential recruitment of nuclear coregulatory proteins.
Materials and Methods
Mouse strains
All aspects of the care and handling of animals used in this study were approved by the National Cancer Institute Animal Care and Use Committee. Generation and genotyping of ThrbPV and Thra1PV mice were described previously (13, 14). Wild-type littermates were used for the comparison of phenotypes with those of mutant mice.
Identification of mutant TR isoform-associated proteins from HeLa cells by tandem affinity chromatography and proteomic analysis
The establishment of HeLa cells stably expressing Flag-hemagglutinin-tagged TRα1PV (FH-αPV cells) or TRβ1PV (FH-βPV cells) and FH (control cells) was as previously reported (23, 36).
The FH-tagging strategy to isolate TR-interacting proteins from HeLa cells was performed essentially as previously described (36) with modifications. Briefly, the cells were grown in DMEM with 10% fetal bovine serum and harvested at near confluence. The cell pellet was then suspended in buffer A [10 mm HEPES (pH 7.9), 10 mm KCl, 0.1 mm EDTA, 1 mm dithiothreitol (DTT), and protease inhibitor mixture]. The cells were allowed to swell on ice for 15 min, after which 10% NP-40 was added to a final concentration of 0.6%. The tube was vigorously vortexed for 1 min. The homogenate was centrifuged for 10 min at 3000 rpm. The nuclear pellet was resuspended in ice-cold buffer C [20 mm HEPES (pH 7.9), 0.4 m NaCl, 1 mm EDTA, 1 mm DTT, and protease inhibitor mixture], and the tube was placed on ice for 15 min and intermittently vortexed. Nuclear extract (50–100 mg) was ultracentrifuged at 30,000 rpm for 2 h at 4 C, and the supernatants were diluted with buffer D (20 mm HEPES (pH 7.9), 1 mm EDTA] to the 100 mm final NaCl concentration, and used as nuclear extracts for immunochromatography by anti-Flag antibody-conjugated M2 agarose (Sigma Chemical Co., St. Louis, MO). The bound polypeptides eluted with the 200 μg/ml of Flag peptide were further affinity purified by EZview red anti-HA affinity gel (Sigma). The final elutes from the HA-beads with HA peptides (400 μg/ml) were resolved by SDS-PAGE on a 4%–20% gradient gel for silver staining or colloidal blue staining (SilverQuest silver staining kit, or SimplyBlue SafeStain; Invitrogen, Carlsbad, CA) to visualize the protein bands.
The protein identification work was carried out at ProtTech, Inc. (Norristown, PA) by using the NanoLC- electron spray ionization-MS/MS peptide sequencing technology. In brief, each protein gel band was destained, disulfide linkage was reduced by DTT, and all cysteine residues were alkylated by iodoacetamid. The sample was cleaned by washing with water and digested in gel with sequencing grade modified trypsin (Promega Corp., Madison, WI) in a digestion buffer of 100 mm ammonium bicarbonate (pH 8.5). The digested peptides were extracted by an acetonitrile/water mixture solution, completely dried down in a vacuum (SpeedVac). The samples were redissolved in 2% acetonitrile, 97.5%water, 0.5% formic acid (HPLC Solvent A). The dissolved peptide samples were then injected in to a HPLC system (Beckman Coulter, Inc., Brea, CA) with a 75-μm inner diameter reverse-phase C18 column. HPLC Solvent B was 9.5% water, 90% acetonitrile, 0.5% formic acid. The gradation time was 60 min from 2% solvent B to 90% solvent B, plus 20 min for sample loading and 20 min for column washing. The column flow rate is around 800 nl/min. The HPLC system was coupled with an ion trap mass spectrometer (Thermo Scientific, Rockford, IL) in a way a sample eluted from HPLC column was ionized by an electron spray ionization process and enter into the mass spectrometry. The mass spectrometer was set at data-dependent mode to acquire MS/MS data via a low-energy collision-induced dissociation process. The mass spectrometric data acquired were used to search the most recent nonredundant protein database with ProtTech's proprietary software suite. The output from the database search was manually validated before reporting.
Western blot analysis
The preparation of nuclear and cytoplasmic extracts of FH-βPV, FH-αPV, and control cells was carried out similarly as described previously by Furuya et al. (37). The Western blot analysis was performed as described by Furumoto et al. (38) using the appropriate antibodies: monoclonal anti-PV antibody (2 μg /ml) (37), monoclonal anti-Flag antibody (M2) (F-3165, 5 μg/ml; Sigma, Inc.), rabbit anti-PARP antibody (1:1,000 dilution) (sc-7150; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), mouse anti-α-tubulin antibody (1:6000 dilution) (T6199, Sigma), mouse monoclonal antitransducin (β)-like 1 X-linked receptor 1 antibody (TBL1XR1) (1:1000 dilution) (sc-100908), rabbit polyclonal antieukaryotic initiation factor 4B antibody (eIF4B) (1:1000 dilution) (catalog no. 3592; Cell Signaling Technology, Danvers, MA), mouse monoclonal anti-HSPA1B (1:1000 dilution) (BD Transduction Laboratories, Lexington, KY), mouse monoclonal anti-XRCC5 (p80) Ab2 (1:200 dilution) (catalog no. MS-285-PO; Thermo Scientific). Polyclonal affinity-purified anti-NCoR1 antibody, PHQQ (2 μg/ml) was generously provided by Anthony Hollenberg (Harvard Medical School, Boston, MA).
For coimmunoprecipitation to show the physical interaction of NCoR1 with TRβ1PV or TRα1PV, nuclear extracts prepared from control cells, FH-βPV, and FH-αPV cells were first incubated with mouse anti-Flag M2 affinity gel (30 μl) (A-2220, Sigma) for 3 h at 4 C to immunoprecipitate FH-TRβ1PV or FH-TRα1PV. Beads were washed three times with BC100 buffer (20 mm HEPES, 1 mm EDTA, 100 mm KCl, 0.1% NP-40). Bound proteins were separated by SDS-PAGE for Western blot analysis.
EMSA
Complementary oligonucleotides containing the mouse C/ebpα TRE (C/ebpα −5′-GCCGTGGGGTCGCGTGGAGTTCAGA and C/ebpα −3′-CCCCAGCGCACCTCAAGTCTCTTTT) or F2-Lys (chicken lysozyme F2 silencer TRE, inverted palindrome) probes were annealed, and the recess 3′-end was filled with DNA polymerase (Klenow fragment) in the presence of [α-32P] deoxy-CTP to generate double-stranded DNA probes, and the EMSA was carried out as described by Ying et al. (39). TRβ1, TRα1, TRβ1PV, and TRα1PV proteins were prepared by in vitro transcription/translation (TNT-quick-couple transcription/translation system; Promega) using plasmids pcDNA3.1TRβ1, pcDNA3.1TRα1, pcDNA3.1TRβPV, and pcDNA3.1TRαPV, respectively. For in vitro transcription/translation of 3RID (aa 1873–2453), the plasmid pCMV3RIDNCoR was used (a generous gift of Brian West, Plexxikon Inc.,). To be certain that an equal amount of in vitro translated TR protein isoforms was used in EMSAs, [35S]methionine was used in the in vitro transcription/translation. [35S]methionine-labeled proteins were analyzed by SDS-PAGE, followed by autoradiography. The intensities of the protein bands were analyzed, and protein concentrations were adjusted to be certain that an equal amount of proteins was used in EMSA for accurate comparison. Approximately 0.2 ng of labeled probe (100,000 cpm) was incubated with an equal amount of in vitro translated TR proteins, in the presence or absence of retinoid X receptor β (RXRβ) (2 μl) in binding buffer for 60 min at room temperature (39). The resulting complexes were resolved on 5% native polyacrylamide gel for 2.5–3 h at 250 V. At the end of the run the gel was fixed, dried, and subjected to autoradiography. For supershift experiments, monoclonal anti-TR antibody, C4, or an irrelevant mouse monoclonal control, MOPC, was used (MOPC141, Sigma).
Quantitative real-time RT-PCR
Liver RNA was prepared by using Trizol reagent (Invitrogen) according to the manufacturer's instructions. Quantitative real-time RT-PCR was performed with a QuantiTect SYBR Green RT-PCR kit (QIAGEN, Chatsworth, CA) using a LightCycler thermal cycler (Roche Diagnostics, Mannheim, Germany). The determination of mRNA by real-time RT-PCR was carried out as described previously (21) by using total liver RNA (200 ng). The primer sequences for C/ebpα are forward, 5′-TTACAACAGGCCAGGTTTCC-3′; and reverse 5′-CTCTGGGATGGATCGATTGT-3′ (22).
Mouse primary hepatocytes were seeded at a density of 2 × 106 (60-mm dish). After culturing for 24 h, cells were infected with adenovirus encoding Flag-tagged TRα1PV, TRβ1PV, or control virus at a multiplicity of infection of 10, and cells were further incubated for an additional 24 h. The preparation of total RNA and quantitative PCR were carried out similarly as described above.
Transient transfection assay
The transient transfection assays were carried out similarly as described previously by Ying et al. (39). Briefly, HepG2 cells stably expressing TRα1 (a generous gift of K. H Lin, Chang Gung University, Tao-Yuan, Taiwan) were plated at a density of 1.8 × 105 cells per well in six-well plates. After culturing cells for an additional 24 h, cells were transfected with the reporter plasmid, C/EBPα luciferase (1 μg) (a generous gift from Dr. G. Darlington, Baylor College of Medicine, Houston, TX), pCMV-RXRβ1 (0.5 μg) and with or without PV expression plasmids (pcDNA3.1TRβPV or pcDNA3.1TRαPV; 0.1, 0.5, 1, 5.0, and 10.0 μg). The transfection was conducted using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Cells were cultured 24 h after transfection with or without 100 nm T3 for an additional 24 h. Cells were lysed in reporter lysis buffer (3× cell lysis buffer; BD Bioscience Pharmingen, San Diego, CA). Lysates were assayed for luciferase activity and normalized to total protein concentration. All experiments were performed in triplicate.
Hepatocyte isolation and ChIP assays
The perfusion of the liver and isolation of primary hepatocytes from wild-type mice were carried out similarly as described by Kao et al. (40). After isolation of primary hepatocytes, cell number was determined by using a cell counter (Beckman).
The ChIP assay was performed using the commercially available ChIP-IT kit (Active Motif). In brief, 5 × 106 of primary mouse hepatocytes were seeded in 100-mm dishes in DMEM supplemented with 10% FBS. After 24 h, cells were infected with adenovirus encoding Flag-tagged TRβ1PV (F-TRβ1PV) or Flag-tagged TRα1PV (F-TRα1PV) and cultured for an additional 24 h. Hepatocytes were cross-linked with 10 ml of formaldehyde (270 μl/ml growth medium) and gently swirled to mix for 10 min. Unreacted formaldehyde was quenched by incubation with 5 ml of 125 mm glycine for 5 min, after which samples were rinsed twice with ice-cold PBS containing protease inhibitors. Cells were scraped and spun down at 2000 × g for 5 min. The cell pellet was resuspended in 1 ml sonication buffer and then sonicated with five sets of 20-sec pulses and 30-sec rest on wet ice (Misonix XL2000). The concentration of sheared chromosomal DNA was measured, and 50 μg of chromosomal DNA were used per antibody reaction. To preclear the cell lysate, 150 μl protein G agarose were added, and the mixture was incubated at 4 C for 1 h and then centrifuged at 2000 × g for 2 min. of precleared chromosomal DNA (50 μg) was incubated with IgG (control), NCoR1 antibody (PHQQ), or monoclonal anti-Flag antibody (F-3165, Sigma) at 4 C overnight. Protein G agarose was added, after which the mixture was incubated for 1 h and centrifuged at 2000 × g for 2 min. Protein G agarose and chromatin complexes were washed and recovered with elution buffer containing 1 m NaHCO3 and 0.1% sodium dodecyl sulfate. Eluted protein-DNA complexes were reversed and purified using spin columns. For the determination of NCoR1 associated with DNA bound to TRβ1PV or TRα1PV, PCR was conducted using the following gene-specific primers: C/ebpα forward, 5′-TAGAGAAGCTGGGCGAAAAGA-3′; and reverse, 5′-AGGTTGGAGACTGCTTTGGA-3′. For negative controls, we used IGX1A control primers (SABioscience, Frederick, MD) that detect a sequence within a 900-kb open reading frame-free intergenic region containing no known or predicted transcription start sites.
Supplementary Material
Acknowledgments
We thank Dr. Osamu Araki (Kamimoku Spa Hospital, Japan) for the use of tandem-affinity chromatography to identify PV mutant-associated proteins in the early phase of the present work and Dr. C. Guigon (Universite Paris Diderot-Paris 7, Paris, France) for her technical assistance with EMSA and valuable discussion. We are grateful for the generous gifts of the reporter plasmid provided by Dr. G. Darlington (Baylor College of Medicine), anti-NCoR1 antibodies provided by Dr. A. Hollenberg (Harvard Medical School), the expression plasmid, pCMV3RIDNCoR, from Dr. Brian West (Plexxikon, Inc., Berkeley, CA), and HepG2 cells stably expressing TRα1 from Dr. K. H. Lin (Chang Gung University, Taiwan).
This work was supported by the Intramural Research Program of National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages:
Nuclear Receptors: TR-α | TR-β | RXR-β;
Coregulators: NCOR;
Ligands: Thyroid hormone.
Footnotes
- aa
- Amino acids
- C/EBPα
- CCAAT/enhancer-binding protein α
- CHD4
- chromodomain-helicase-DNA-binding protein 4
- ChIP
- chromatin immunoprecipitation
- DTT
- dithiothreitol
- FH
- Flag-hemagglutinin
- HA
- hemagglutinin
- nt
- nucleotide
- MOPC
- mineral oil-induced plasmacytoma
- MS/MS
- tandem mass spectrometry
- NCoR1
- nuclear receptor corepressor
- PARP
- poly(ADP-ribose) polymerase
- RID
- receptor interaction domain
- RTH
- resistance to thyroid hormone
- RXR
- retinoid X receptor
- TR
- thyroid hormone receptors
- TRE
- thyroid response elements.
References
- 1. Cheng SY. 2000. Multiple mechanisms for regulation of the transcriptional activity of thyroid hormone receptors. Rev Endocr Metab Disord 1:9–18 [DOI] [PubMed] [Google Scholar]
- 2. Forrest D, Erway LC, Ng L, Altschuler R, Curran T. 1996. Thyroid hormone receptor β is essential for development of auditory function. Nat Genet 13:354–357 [DOI] [PubMed] [Google Scholar]
- 3. Gauthier K, Plateroti M, Harvey CB, Williams GR, Weiss RE, Refetoff S, Willott JF, Sundin V, Roux JP, Malaval L, Hara M, Samarut J, Chassande O. 2001. Genetic analysis reveals different functions for the products of the thyroid hormone receptor α locus. Mol Cell Biol 21:4748–4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gauthier K, Chassande O, Plateroti M, Roux JP, Legrand C, Pain B, Rousset B, Weiss R, Trouillas J, Samarut J. 1999. Different functions for the thyroid hormone receptors TRα and TRβ in the control of thyroid hormone production and post-natal development. EMBO J 18:623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Göthe S, Wang Z, Ng L, Kindblom JM, Barros AC, Ohlsson C, Vennström B, Forrest D. 1999. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev 13:1329–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Forrest D, Vennström B. 2000. Functions of thyroid hormone receptors in mice. Thyroid 10:41–52 [DOI] [PubMed] [Google Scholar]
- 7. Lee JW, Choi HS, Gyuris J, Brent R, Moore DD. 1995. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol Endocrinol 9:243–254 [DOI] [PubMed] [Google Scholar]
- 8. Lonard DM, O'Malley BW. 2007. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell 27:691–700 [DOI] [PubMed] [Google Scholar]
- 9. Lonard DM, O'Malley BW. 2005. Expanding functional diversity of the coactivators. Trends Biochem Sci 30:126–132 [DOI] [PubMed] [Google Scholar]
- 10. Glass CK, Rosenfeld MG. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14:121–141 [PubMed] [Google Scholar]
- 11. Weiss RE, Refetoff S. 2000. Resistance to thyroid hormone. Rev Endocr Metab Disord 1:97–108 [DOI] [PubMed] [Google Scholar]
- 12. Parrilla R, Mixson AJ, McPherson JA, McClaskey JH, Weintraub BD. 1991. Characterization of seven novel mutations of the c-erbA beta gene in unrelated kindreds with generalized thyroid hormone resistance. Evidence for two “hot spot” regions of the ligand binding domain. J Clin Invest 88:2123–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaneshige M, Kaneshige K, Zhu X, Dace A, Garrett L, Carter TA, Kazlauskaite R, Pankratz DG, Wynshaw-Boris A, Refetoff S, Weintraub B, Willingham MC, Barlow C, Cheng S. 2000. Mice with a targeted mutation in the thyroid hormone β receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci USA 97:13209–13214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaneshige M, Suzuki H, Kaneshige K, Cheng J, Wimbrow H, Barlow C, Willingham MC, Cheng S. 2001. A targeted dominant negative mutation of the thyroid hormone α 1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc Natl Acad Sci USA 98:15095–15100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng SY. 2005. Thyroid hormone receptor mutations and disease: beyond thyroid hormone resistance. Trends Endocrinol Metab 16:176–182 [DOI] [PubMed] [Google Scholar]
- 16. Cheng SY. 2005. Isoform-dependent actions of thyroid hormone nuclear receptors: lessons from knockin mutant mice. Steroids 70:450–454 [DOI] [PubMed] [Google Scholar]
- 17. Stallcup MR, Kim JH, Teyssier C, Lee YH, Ma H, Chen D. 2003. The roles of protein-protein interactions and protein methylation in transcriptional activation by nuclear receptors and their coactivators. J Steroid Biochem Mol Biol 85:139–145 [DOI] [PubMed] [Google Scholar]
- 18. Hörlein AJ, Näär AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Söderström M, Glass CK, Rosenfeld MG. 1995. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377:397–404 [DOI] [PubMed] [Google Scholar]
- 19. Yoh SM, Chatterjee VK, Privalsky ML. 1997. Thyroid hormone resistance syndrome manifests as an aberrant interaction between mutant T3 receptors and transcriptional corepressors. Mol Endocrinol 11:470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoh SM, Privalsky ML. 2000. Resistance to thyroid hormone (RTH) syndrome reveals novel determinants regulating interaction of T3 receptor with corepressor. Mol Cell Endocrinol 159:109–124 [DOI] [PubMed] [Google Scholar]
- 21. Araki O, Ying H, Zhu XG, Willingham MC, Cheng SY. 2009. Distinct dysregulation of lipid metabolism by unliganded thyroid hormone receptor isoforms. Mol Endocrinol 23:308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mishra A, Zhu XG, Ge K, Cheng SY. 2010. Adipogenesis is differentially impaired by thyroid hormone receptor mutant isoforms. J Mol Endocrinol 44:247–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ying H, Araki O, Furuya F, Kato Y, Cheng SY. 2007. Impaired adipogenesis caused by a mutated thyroid hormone α1 receptor. Mol Cell Biol 27:2359–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Menéndez-Hurtado A, Santos A, Pérez-Castillo A. 2000. Characterization of the promoter region of the rat CCAAT/enhancer-binding protein α gene and regulation by thyroid hormone in rat immortalized brown adipocytes. Endocrinology 141:4164–4170 [DOI] [PubMed] [Google Scholar]
- 25. Zhu XG, McPhie P, Cheng SY. 1997. Differential sensitivity of thyroid hormone receptor isoform homodimers and mutant heterodimers to hormone-induced dissociation from deoxyribonucleic acid: its role in dominant negative action. Endocrinology 138:1456–1463 [DOI] [PubMed] [Google Scholar]
- 26. Makowski A, Brzostek S, Cohen RN, Hollenberg AN. 2003. Determination of nuclear receptor corepressor interactions with the thyroid hormone receptor. Mol Endocrinol 17:273–286 [DOI] [PubMed] [Google Scholar]
- 27. Kim JH, Yang CK, Heo K, Roeder RG, An W, Stallcup MR. 2008. CCAR1, a key regulator of mediator complex recruitment to nuclear receptor transcription complexes. Mol Cell 31:510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ou CY, Kim JH, Yang CK, Stallcup MR. 2009. Requirement of cell cycle and apoptosis regulator 1 for target gene activation by Wnt and β-catenin and for anchorage-independent growth of human colon carcinoma cells. J Biol Chem 284:20629–20637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoon HG, Chan DW, Reynolds AB, Qin J, Wong J. 2003. N-CoR mediates DNA methylation-dependent repression through a methyl CpG binding protein Kaiso. Mol Cell 12:723–734 [DOI] [PubMed] [Google Scholar]
- 30. Itoh Y, Esaki T, Kaneshige M, Suzuki H, Cook M, Sokoloff L, Cheng SY, Nunez J. 2001. Brain glucose utilization in mice with a targeted mutation in the thyroid hormone α or β receptor gene. Proc Natl Acad Sci USA 98:9913–9918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Esaki T, Suzuki H, Cook M, Shimoji K, Cheng SY, Sokoloff L, Nunez J. 2003. Functional activation of cerebral metabolism in mice with mutated thyroid hormone nuclear receptors. Endocrinology 144:4117–4122 [DOI] [PubMed] [Google Scholar]
- 32. Esaki T, Suzuki H, Cook M, Shimoji K, Cheng SY, Sokoloff L, Nunez J. 2004. Cardiac glucose utilization in mice with mutated α- and β-thyroid hormone receptors. Am J Physiol Endocrinol Metab 287:E1149–E1153 [DOI] [PubMed] [Google Scholar]
- 33. O'Shea PJ, Bassett JH, Sriskantharajah S, Ying H, Cheng SY, Williams GR. 2005. Contrasting skeletal phenotypes in mice with an identical mutation targeted to thyroid hormone receptor α1 or β. Mol Endocrinol 19:3045–3059 [DOI] [PubMed] [Google Scholar]
- 34. O'Shea PJ, Bassett JH, Cheng SY, Williams GR. 2006. Characterization of skeletal phenotypes of TRα1 and TRβ mutant mice: implications for tissue thyroid status and T3 target gene expression. Nucl Recept Signal 4:e011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weiss RE, Forrest D, Pohlenz J, Cua K, Curran T, Refetoff S. 1997. Thyrotropin regulation by thyroid hormone in thyroid hormone receptor β-deficient mice. Endocrinology 138:3624–3629 [DOI] [PubMed] [Google Scholar]
- 36. Ying H, Furuya F, Zhao L, Araki O, West BL, Hanover JA, Willingham MC, Cheng SY. 2006. Aberrant accumulation of PTTG1 induced by a mutated thyroid hormone β receptor inhibits mitotic progression. J Clin Invest 116:2972–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Furuya F, Hanover JA, Cheng SY. 2006. Activation of phosphatidylinositol 3-kinase signaling by a mutant thyroid hormone β receptor. Proc Natl Acad Sci USA 103:1780–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Furumoto H, Ying H, Chandramouli GV, Zhao L, Walker RL, Meltzer PS, Willingham MC, Cheng SY. 2005. An unliganded thyroid hormone β receptor activates the cyclin D1/cyclin-dependent kinase/retinoblastoma/E2F pathway and induces pituitary tumorigenesis. Mol Cell Biol 25:124–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ying H, Suzuki H, Zhao L, Willingham MC, Meltzer P, Cheng SY. 2003. Mutant thyroid hormone receptor β represses the expression and transcriptional activity of peroxisome proliferator-activated receptor γ during thyroid carcinogenesis. Cancer Res 63:5274–5280 [PubMed] [Google Scholar]
- 40. Kao CY, Factor VM, Thorgeirsson SS. 1996. Reduced growth capacity of hepatocytes from c-myc and c-myc/TGF-α transgenic mice in primary culture. Biochem Biophys Res Commun 222:64–70 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.