Meeting men's contraceptive needs, orally administered retinoic acid receptor antagonists represent new lead molecules in developing non-hormonal, reversible male contraceptives without adverse side effects.
Abstract
Here we investigated a pharmacological approach to inhibit spermatogenesis in the mouse model by manipulating retinoid signaling using low doses of the pan-retinoic acid receptor (RAR) antagonist BMS-189453. Spermatogenesis was disrupted, with a failure of spermatid alignment and sperm release and loss of germ cells into lumen, abnormalities that resembled those in vitamin A-deficient and RARα-knockout testes. Importantly, the induced sterility was reversible. Enhanced efficacy and a lengthened infertility period with full recovery of spermatogenesis were observed using systematically modified dosing regimens. Hematology, serum chemistry, and hormonal and pathological evaluations revealed no detectable abnormalities or adverse side effects except the distinct testicular pathology. Our results suggest that testes are exquisitely sensitive to disruption of retinoid signaling and that RAR antagonists may represent new lead molecules in developing nonsteroidal male contraceptives.
The need for dietary retinol (vitamin A) and its physiologically active metabolite all-trans-retinoic acid (ATRA) for normal spermatogenesis has been recognized for decades, initially from classic studies on male sterility in rats that resulted from vitamin A deficiency (VAD) (1, 2; reviewed in Refs. 3–5). Most of the physiological effects of retinoids are due to their binding as ligands to the retinoic acid receptors (RAR) or retinoid X receptors (6). This mechanism of retinoid action is distinct from the specialized function in vision, in which dietary retinol is used for non-receptor-mediated production of rhodopsin.
Some insight into the molecular basis for retinoid signaling during spermatogenesis has been obtained using targeted mutagenesis in mouse models. Ablation of the mouse Rara gene caused male sterility and yielded defects in spermatogenesis that were similar to those in VAD testes (7–9). In particular, step 16 spermatids in stage VIII tubules failed to align at the lumen for spermiation, and earlier stage germ cells were shed into the lumen (8, 9). A crucial role during spermiogenesis of RARα-mediated retinoid signaling in the germline is further supported by the partial rescue of these defects by targeted expression of Rara cDNA specifically in haploid spermatids of otherwise RARα-deficient mice (10).
Although targeted mutagenesis in mouse models has contributed much to our understanding of retinoid function, genetic manipulations are permanent. Medicinal chemistry has been exploited to produce various synthetic retinoids that can separate pleiotropic cellular responses to retinoid signaling (11–20). However, such pharmacological approaches for perturbing spermatogenesis, potentially reversibly, have not been explored. A series of low-molecular-weight arotinoid compounds that function as RAR antagonists, blocking ATRA binding and activation of transcription of RAR target genes, were developed by Bristol-Myers Squibb (BMS) (Fig. 1, A–C) (18, 21). Among them, BMS-189453 exhibited good oral bioavailability (82–98%) (22) and a relatively short half-life (10 and 6 h in monkeys and rodents, respectively) (23). It is metabolized in the liver into excretable, water-soluble components, which are presumably nontoxic. There was a single study published some years ago reporting that oral administration of BMS-189453 to rats at doses from 12.5 to 100 mg/kg for 1 wk resulted in marked testicular degeneration but with only minimal other physiological effects (22). However, neither the etiology of the testicular degeneration nor the fertility status was determined, and importantly, the question of reversibility of the testicular degeneration after the cessation of drug treatment was not addressed.
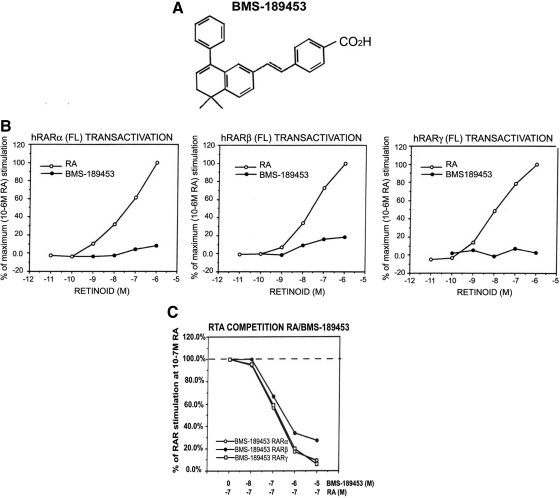
Fig. 1.
Characterization of a retinoid receptor antagonist. A, Chemical structure of the synthetic retinoid. BMS-189453. B, Characterization of the synthetic retinoid, BMS-189453, using transactivation. Increasing ATRA concentrations (open circles) stimulated transactivation of the reporter construct as indicated. Closed circles represent the normalized transactivation for each test compound in the same concentration range. Each data point represents the mean of three independent measurements. C, Transactivation competition assay for assessment of antagonist activity. CAT reporter activity was measured in the presence of 10−7 m ATRA and increasing concentrations of each retinoid. BMS-189453 (C) competes the activity of ATRA for RARα (open circle), RARβ (closed circle), and RARγ (open square) and is thus a pan-antagonist. RA, Retinoic acid; RTA, retinoid-induced transcriptional activation.
We therefore wished to explore the use of oral administration of even lower doses of this RAR antagonist as a pharmacological approach to inhibit spermatogenesis and fertility and, in particular, to assess its reversibility. We chose to use the genetically tractable mouse model for these studies to compare the effects of this drug on spermatogenesis to those observed in VAD and in RARα-deficient mice. We demonstrated that BMS-189453 inhibited spermatogenesis at even lower dose regimens. Notably, the effects of BMS-189453 were reversible. Detailed histological analysis revealed that the cellular processes involved in spermiogenesis and completion of spermiation are extremely sensitive to changes in retinoid signaling by this pharmacological intervention. We then systematically explored various dosing regimens to fine tune the inhibition of RAR-mediated signaling to yield enhanced efficacy and longer duration of sterility, again, in a reversible manner. Our results suggest that compounds that inhibit RAR function, such as BMS-189453, may thus provide a novel approach in the development of nonsteroidal male contraceptives.
Materials and Methods
Animal source and tissues
CD1 mice (8 wk, 30 g body weight) were obtained from Charles River Laboratories (Wilmington, MA). The use of animals was approved by Columbia University Medical Center Animal Care and Use Committee. At the specified time points, testes were dissected and weighed from anesthetized mice, which were then euthanized. One testis was fixed with 4% paraformaldehyde in PBS and the second was fixed with Bouin's fixative overnight at 4 C. Fixed tissues were embedded in paraffin, sectioned, and mounted as previously described (10). For staging of testicular tubules (24), sections were stained for Periodic acid-Schiff reaction before hematoxylin counterstaining as previously reported (8, 9) and examined by bright-field microscopy. Testicular weight within the same treatment group was assessed by statistical analysis using Student's paired t test using GraphPad Analysis Software (GraphPad Software, Inc., San Diego, CA).
Retinoid transactivation and transactivation competition assays
BMS-189453 was obtained from BMS and used in a transactivation assay as described previously (16, 20, 25). Briefly, HeLa cells were transfected with cDNA constructs encoding each of the receptors (RARα, -β, and -γ) along with a retinoic acid response element (RARE)-chloramphenicol acetyl transferase (CAT) reporter construct and a tk-β-galactosidase construct to normalize transfection efficiency (26). ATRA or the synthetic compounds were incubated with transfected cells for 24 h to induce CAT expression, which was measured using a CAT-ELISA kit (5-Prime→3 Prime, Inc., Boulder, CO).
Antagonist activity of the compounds was determined by a competition assay. Using the same constructs described above, HeLa cells were incubated with a submaximal dose of ATRA (10−7 m) and with increasing concentrations of the compound, and CAT expression was measured after 24 h. Data points for each compound were normalized for RAR stimulation at 10−7 m ATRA. These experiments were performed in triplicate, and the data points are means of three measurements. Less than 10% variation from day-to-day measurements was routinely obtained. A compound was considered an antagonist if it inhibited ATRA-induced CAT reporter expression but did not, itself, induce CAT reporter expression.
Treatment of mice with BMS-189453
BMS-189453 was suspended in a vehicle of aqueous 1.5% Avicel (CL-611; FMC BioPolymer, Rockland, ME) to obtain the desired concentrations. In the initial series of experiments, BMS-189453 was administered to CD1 males (n = 10) via oral gavage at daily doses of 0.1, 1, and 5 mg/kg for 7 d. Control groups (n = 5) received vehicle alone for 7 d. Four time points were selected for morphological assessment of the effect of the compound on spermatogenesis: 1 wk (end of treatment) and 1-, 3- and 6 months after dose. During the period of treatment, the mice were observed daily for changes in overall health and behavior. Body weight was recorded, and physical examinations were performed weekly.
To further fine tune the inhibition of RAR-mediated signaling by BMS-189453, a modified chronic treatment regimen was developed as follows. Two groups of mice (n = 30 each) with dosing regimens of 5.0 mg/kg for 2 wk (group I) and 2.5 mg/kg for 4 wk (group II) were used. For both groups, one third of the mice (n = 10) were euthanized at the end of 4 wk after the initial dose. A second cohort of mice (n = 5) was euthanized during the infertile/recovering period for histological assessment. The remaining mice (n = 15) were assessed for fertility as described below.
Hormone measurement
Serum testosterone levels were measured in blood samples obtained by retroorbital collection at the time of euthanasia. The samples were clotted at room temperature for 90 min and centrifuged at 2000 × g for 15 min at room temperature. The unextracted serum samples were assayed using a commercially available competitive RIA (Coat-A-Count; Siemens Healthcare Diagnostics, Deerfield, IL). The assay has been validated for rodent samples (rat and mouse) by spiking mouse or rat serum pools with various concentrations of testosterone across the assay range and determining recovery (results showed recovery within 10% of expected values across the assay range for both rat and mouse). The assays were performed by The Center for Research in Reproduction Ligand Assay and Analysis Core at the University of Virginia (Charlottesville, VA). The sensitivity of the RIA for testosterone was 10 ng/dl. The reportable range of the testosterone assay was 15.6–1084.6 ng/dl, and the intra- and interassay coefficients of variation were less than 10% and 20%. The package insert for the assay describes cross-reactivity for several steroids. Cross-reactivity is very low (<1%) for all endogenous androgens tested, including androstenedione (0.05%), dehydroepiandrosterone (0.002%), and dehydroepiandrosterone sulfate (0.006%), with the exception of dihydrotestosterone (3.3% cross-reactivity). However, because total testosterone levels in the blood are 10-fold higher than dihydrotestosterone levels, that level of cross-reactivity is not a concern.
To further verify the quality of the testosterone measurements, part of the experiment was repeated (dosing regimen of 5 mg for 7 d with two time points: immediately and 1 month after drug treatment), and diethyl ether extraction was performed with the serum samples according to published procedures (27). Equal volume of diethyl ether (Sigma-Aldrich, St. Louis, MO) was added to each of the serum samples. The tubes were mixed completely by vortex briefly for 20 sec and centrifuged at 12,500 rpm for 10 min at 4 C. The top layer was carefully transferred to a new tube. The extraction was repeated twice, and the combined ether extracts were then evaporated to dryness in a vacuum fume hood. The samples were reconstituted in 350–500 μl of charcoal-stripped fetal bovine serum (Invitrogen, Carlsbad, CA) by gentle shaking for 2 h at 4 C and stored at −20 C.
Sperm counts
Sperm were collected into modified Whitten's medium at 37 C from both cauda epididymides of selected animals as described previously (28). Briefly, caudae were minced with small scissors to release the sperm, which were further extruded by round forceps. Large cells, tissue fragments, and debris were filtered via a 100-μm filter. A 1:10 dilution was made, and sperm were counted manually using a hemocytometer. The caudal epididymal sperm, if present, were observed for motility according to protocols of The Jackson Laboratory (Bar Harbor, ME) for male infertility evaluation (http://reprogenomics.jax.org/maleprotocol.html). The motility of at least 100 spermatozoa was determined. A spermatozoon is considered motile when sperm progression or regular movement of the flagellum is observed.
Fertility assessment
Fertility was assessed according to published procedures (29). Briefly, drug-treated males were placed with two untreated virgin females for 14 d (three estrous cycles should have elapsed). Females were checked daily for postcoital vaginal plugs. At 14 d, they were euthanized and numbers of pregnant females, implantation sites, viable fetuses, resorptions, and females with resorptions were recorded. Another two females were replaced until either fertility was restored (a minimum of seven consecutive pregnancies were assessed) or the mice reached 6 months after dose. The mice were then euthanized and the testes were prepared for morphological examination.
Chemical synthesis of BMS-189453 (designated compound 9)
Our original source of the compound was archived samples from BMS, which no longer manufactures it. We therefore sought alternative sources of the compound. Compound 9/BMS-189453 was synthesized by AsisChem, Inc. (Watertown, MA). The procedures for the nine-step synthesis are shown in scheme 1 of Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. To verify the activity of this newly synthesized compound, dosing regimens of 5 mg/kg for 7 d and 2.5 mg/kg for 4 wk were used to evaluate the effects on spermatogenesis and fertility. Because tailoring the dose-to-weight change on a daily basis would not be an ideal regimen for eventual application to humans, we used a modified daily dosing approach for the 4-wk study as follows. The amount of compound to be administered each day was calculated based on the average weight of all animals in a group at the same age on the first day of each week of treatment. In this study, the weekly weights for tailoring the dose were: wk 1, 29.23 g; wk 2, 30.33 g; wk 3, 32.50 g; wk 4, 32.21 g.
Hematology, serum chemistry, and pathological analysis
Possible adverse side effects of BMS-189453/compound 9, toxicity assessments were performed on mice treated with 2.5 mg/kg for 4 wk (adjusted weekly). Hematology, serum chemistry, and histopathological analyses were performed blinded at the Comparative Pathology Laboratory, University of California Davis for standard toxicological parameters. The tissues examined and the serum chemistry assessments are described in Supplemental Tables 1 and 2.
Quantification of defects in spermatogenesis at the histological level
We further systematically examined the morphology and association of cell types within testicular tubules in mice at specific stages after BMS-189453 treatment. In brief, 8-wk-old males were administered 5 mg/kg of BMS-189453 for 7 d and euthanized at 0 (BMS-189453 + 0), 4 (BMS-189453 + 4), 12 (BMS-189453 + 12), or 14 (BMS-189453 + 14) wk after drug treatment. To guide our analysis, we first took advantage of a well-defined classification index used in other studies examining the abnormalities in testes of mice in the absence of vitamin A, which showed similar types of spermatogenic defects as in our current drug-treated model (30). Three to five males were used at each time point, and at least 200 tubules per testis were analyzed. Quantification of the cellular abnormalities was done essentially as described by Ghyselinck et al. (30). Tubules containing normal germ cell associations are designated “Normal”; T1 indicates tubular sections displaying a failure of sperm alignment and sperm release or abnormal round spermatids with crescent-like chromatin condensation with juxtanuclear clear spaces; T2 indicates tubular sections lacking a given germ cell layer; T3 indicates tubular sections displaying a single type of spermatocytes or round spermatids (steps 1–8) or both as the most advanced germ cell types; and T4 indicates tubular sections containing only Sertoli cells and spermatogonia.
We next quantified the changes in specific cell types associated with the induction of sterility after drug treatment and the subsequent recovery of spermatogenesis. At least 40 tubules of each testis from three to five different animals were analyzed, and all germ cell types including total spermatogonia, preleptotene/leptotene/zygotene spermatocytes, pachytene and diplotene spermatocytes, step 1–8 round spermatids, step 9–12 elongating spermatids, step 13–16 elongated spermatids, and Sertoli cells were counted per group as described previously (8–10, 31–33). Only those sections cut perpendicularly through the diameter of a tubule, as judged by their approximation to a circle and by regularity of cell layers, were analyzed (32). Significant differences were assessed by statistical analysis using Student's paired t test using GraphPad Analysis software.
Results
Characterization of BMS-189453 as a pan-RAR antagonist
BMS-189453 inhibited ATRA-induced RARE-CAT reporter expression by concomitantly transfected RAR but did not, by itself, induce reporter expression (Fig. 1A). It further exhibited minimal transactivation of a RARE-CAT reporter for RARα (Fig. 1B, left panel) and RARγ (Fig. 1B, right panel) and only a small but reproducible transactivation for RARβ (Fig. 1B, middle panel). Its antagonist function for all three RAR was demonstrated in competition assays by its ability to inhibit the activation of the reporter construct by ATRA (Fig. 1C).
Cellular and temporal specificity of dose-dependent disruption of spermatogenesis by treatment with BMS-189453
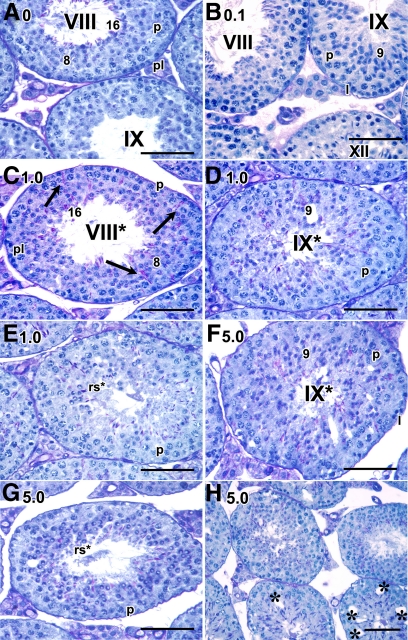
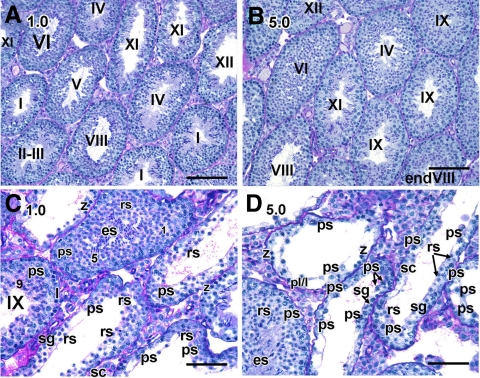
Other than the mention of marked testicular degeneration in rats treated with BMS-189453 for 1 wk at doses from 12.5 to 100 mg/kg (22), the effects of this drug on spermatogenesis at the cellular level and the question of reversibility of the degeneration had not been addressed. We therefore examined the testicular morphology in detail, extending the previous observations in the rat to the mouse model and using regimens with even lower doses (0.1, 1.0, and 5.0 mg/kg). Detailed morphological analysis at the histological level of testes obtained immediately after the 7-d dosing period revealed striking abnormalities. Specifically, there was a failure of spermatid alignment of step 16 spermatids at the lumen in stage VIII tubules and sperm release at stage IX into the lumen in testes from mice treated with both the 1 mg (Fig. 2, C and D) and 5 mg (Fig. 2F) dose, but not with the vehicle alone (Fig. 2A) or with 0.1 mg (Fig. 2B), where normal spermatid alignment and sperm release were observed. Interestingly, these defects in stage VIII and IX tubules are also consistently found in VAD and Rara−/− testes (4, 5, 8, 9). In addition, the round spermatids displayed crescent-like chromatin condensation with juxtanuclear clear spaces (Fig. 2, E and G). Vacuolar-like spaces were particularly prominent in tubules of the 5-mg group (Fig. 2H). No abnormalities in epididymal spermatozoa were observed (data not shown).
Fig. 2.
Acute disruptive effect of BMS-189453 on spermatid alignment and release in testicular tubules immediately after dosing. A–F, Histological sections of mice treated with vehicle alone (A) or 0.1 (B), 1.0 (C–E), and 5.0 (F–H) mg/kg of BMS-189453. Magnification: A–G, ×60; H, ×40. pl, Preleptotene spermatocytes; l, leptotene spermatocytes; p, pachytene spermatocytes; rs, round spermatids. Arabic numerals indicate the step of spermatid differentiation; Roman numerals indicate the stage of the tubules. Although abnormal cell associations complicate staging, an attempt was made to stage the drug-treated tubules using acrosomal system, and tubules are labeled with a Roman numeral followed by an asterisk (e.g. stage IX*). Scale bar, 50 μm.
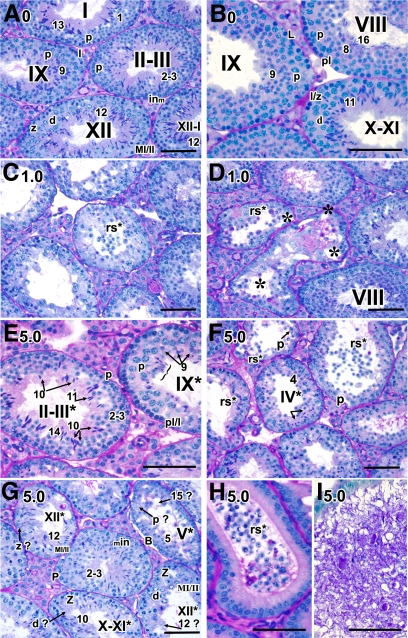
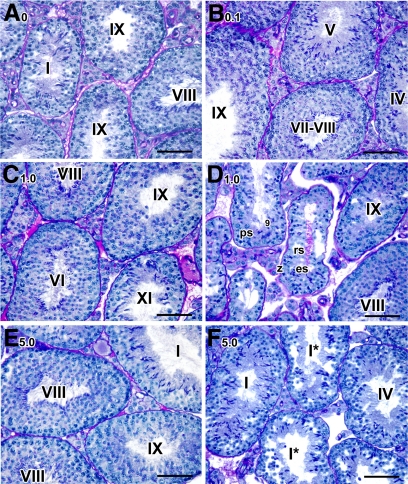
By 1 month after treatment, spermatogenesis was severely disrupted in the 5-mg group. In virtually all testicular tubules that contained round spermatids (62%), spermatids were being sloughed into the tubular lumen, and tubular diameter was reduced by 35%. Any elongated spermatids that developed failed to align and be released at spermiation (bracket, Fig. 3E). Some tubules contained two to three different steps of spermatids (left tubule, Fig. 3E) whereas others were missing entire layers of specific types of spermatogenic cells (Fig. 3G, the missing layer of specific cell types is indicated with an arrow and question mark). For example, the entire layer of zygotene spermatocytes (z ?) and step 12 spermatids (12 ?) were missing in tubules at stage XII* (upper left and bottom right tubule, respectively, Fig. 3G). Abnormal round spermatids being sloughed into the lumen were consistently observed in both 1-mg (Fig. 3C) and 5-mg (Fig. 3F) samples. Such abnormal round spermatids were also detected in caput epididymides whereas abnormal spermatozoa with massive cellular debris and giant multinucleated germ cells were found in caudal epididymides (Fig. 3, H and I, respectively). Vacuolar-like spaces were also more prominent in the 1-mg samples (Fig. 3D); however, there was still a large proportion of normal-appearing tubules (70%, Fig. 3D).
Fig. 3.
Temporal, cell-specific, and dose-responsive disruption of spermatogenesis in testes at 1 month after treatment. A–F, Histological sections of mice treated with 0 (A and B), 1.0 (C and D), and 5.0 (E–l) mg/kg of BMS-189453. A–G, The testes; H-I, the epididymides. Magnification: A, C–D, F, and G, ×40; B, E, H, and I, ×60. in, Intermediate spermatogonia; z, zygotene spermatocytes; d, diplotene spermatocytes; p, pachytene spermatocytes; MI/II, meiosis I/II; rs, round spermatids. At stage XII* (G, right bottom tubule): MI/MII spermatocytes with chromosomes aligned at metaphase plate, and zygotene and diplotene spermatocytes were readily seen. However, step 1 or 12 spermatids were missing. In stage XII* tubule (G, left upper tubule), zygotene spermatocytes were missing. The missing layer of specific cell types is indicated with an arrow and question mark. Scale bar, 50 μm.
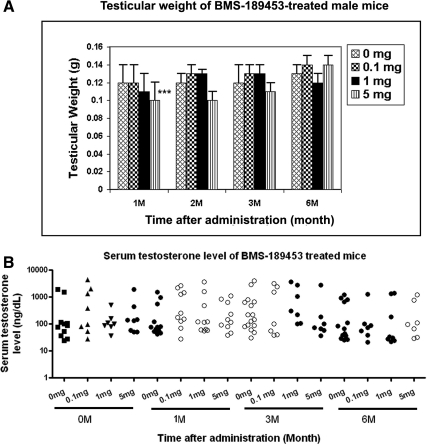
No changes in body weight of the drug-treated mice compared with vehicle-alone controls were observed throughout the study at any of the dosing regimens (data not shown). Testicular weights of the 5-mg (but not 1 mg or 0.1 mg) samples were slightly but significantly reduced at 1 month (Fig. 4A), but not at 3 and 6 months after dosing. None of the dosing regimens changed testosterone levels as measured in total blood serum samples (Fig. 4B) and verified in measurements of selected ether-extracted samples (Supplemental Fig. 3), suggesting that the drug would not affect male libido or sexuality. The variation in testosterone levels that we observed is typically seen in mice (34) compared with relatively consistent levels measured in both rats and human and did not differ between drug-treated and control samples. In fact, the normal mating behaviors of the mice observed (see below) are the most cogent biological data of normal testosterone action.
Fig. 4.
Gonad weight and serum testosterone. A, The testicular weight of BMS-189453-treated male mice at different post-dose time points. The bars represent the mean ± sd of at least seven mice for each time point. ***, Significant differences within the same age group as assessed by paired Student's t test. P < 0.0005. B, Male mice were treated with BMS-189453 at three different doses, and sera were obtained for determination of testosterone level. Data points from individual mice are presented.
Kinetics of recovery of spermatogenesis after cessation of treatment with BMS-189453
The usefulness of compounds such as BMS-189453 as contraceptives depends on the reversibility of inhibition of spermatogenesis upon cessation of treatment (35–39). We were therefore most interested to observe that, at 3 months after dosing, an apparent recovery of spermatogenesis was evidenced at the histological level by the presence of normal-appearing tubules in testes from both the 1-mg (n = 5, Fig. 5A) and 5-mg (n = 6, Fig. 5B) samples, although some testes still had a proportion of abnormal tubules. That is, two mice from the 1-mg group and one from the 5-mg group exhibited severe germ cell depletion in 13.4% and 18.2% (1-mg group) and 21.6% (5-mg group) of their tubules (Fig. 5, panels C and D, respectively). Neither abnormal spermiation nor defective alignment of spermatids was found in any of the samples (stage IX in Fig. 5C), suggesting that effects of BMS-189453 on spermiation and spermatid alignment were acute responses. There were, however, some abnormally shaped round spermatids consistently observed in those few still aberrant tubules in both groups (data not shown).
Fig. 5.
Assessment of spermatogenesis in testes 3 months after treatment with BMS-189453. A–D, Histological sections of mice treated with 1 (A and C) and 5 (B and D) mg/kg of BMS-189453. Magnification: A and B, ×20; C and D, ×40. sg, Spermatogonia; pl/liter, preleptotene/leptotene spermatocytes; ps, pachytene spermatocytes; z, zygotene spermatocytes; rs, round spermatids; es, elongated spermatids. Arabic numerals indicate the step of spermatid differentiation. Roman numerals indicate the stage of the tubules. Scale bar, 50 μm.
At 6 months after treatment, there was an apparent full recovery of spermatogenesis in testes of all but two of the 1-mg group (n = 5, Fig. 6C). One male from this group still had some level of germ cell depletion (usually missing one layer of cells) in 25% of the tubules, and the second showed 35% aberrant tubules containing a mixture of cell types (middle and left tubules in Fig. 6D). In the 5-mg group, full recovery of spermatogenesis was found in all but one mouse (n = 6, Fig. 6E), and in that mouse, 10% of all the tubules examined (out of 150) displayed abnormal but recovering tubules (Fig. 6F). For example, recovering-stage I tubules (labeled with asterisk, Fig. 6F) exhibited correct cellular associations, but with fewer cells and some vacuoles (Fig. 6F).
Fig. 6.
Full recovery of spermatogenesis in testes 6 months after treatment. A–F, Histological sections of mice treated with vehicle alone (A), 0.1 (B), 1 (C and D), and 5 (E and F) mg/kg of BMS-189453. Magnification: A–F: ×40. ps, Pachytene spermatocytes; z, zygotene spermatocytes; rs, round spermatids; es, elongated spermatids. Arabic numerals indicate the step of spermatid differentiation. Roman numerals indicate the stage of the tubules. Scale bar, 50 μm.
Induction of sterility and recovery of fertility with treatment of BMS-189453
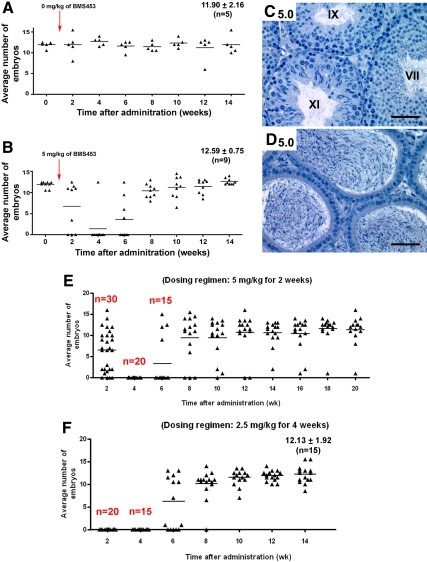
We next monitored when infertility appeared in the mice and asked whether the apparent recovery of spermatogenesis evidenced histologically in testes from both the 1-mg and 5-mg groups indeed restored fertility by performing mating studies with the 5-mg group. Interestingly, immediately after dosing (during 1–2 wk after treatment), three males failed to yield pregnancies, indicating BMS-189453 has immediate effects on sperm function in some males, and by 4 wk after treatment, all but one mouse (n = 8) became infertile (Fig. 7B). Importantly, fertility was restored by 14 wk in these males, with comparable embryonic litter sizes vs. controls (Fig. 7B). There were no obvious differences in testicular weight (drug-treated, 0.11 g ± 0.01; n = 9, vs. control, 0.12 g ± 0.01; n = 5), tubular sizes (Fig. 7C, n = 9) and morphology of epididymal sperm (Fig. 7D, n = 9). Further, caudal epididymal sperm counts were comparable to control (35.96 × 106 ± 6.42; n = 9 vs. control, 37.60 × 106 ± 2.51; n = 5).
Fig. 7.
Effects on spermatogenesis and fertility upon systematic modification of dosing regimens to fine tune the inhibition of spermatogenesis. A–D, Induction and reversibility of sterility by BMS-189453. The average numbers of embryos obtained at each assessed time point during the mating period of 14 wk in mice treated with 5 mg/kg of BMS-189453 for 7 d (B, n = 9), compared with control (A, n = 5). Histological sections of 5-mg group (C and D) showed full recovery of spermatogenesis in testes and epididymides from six of nine mice at 3.5 months after treatment. Magnification: C and D, ×40. Roman numerals indicate the stage of the tubules. T=0 is the time the administration stopped, i.e. end of dosing period. E and F, Timely induction and lengthened period of infertility with a full recovery of spermatogenesis with modified dosing regimens. The average numbers of embryos obtained after each assessment time point during the mating period in group I mice treated with 5 mg/kg of BMS-189453 for 2 wk (E), compared with group II mice (F) treated with an even lower dose of 2.5 mg/kg for 4 wk. Two other experiments using different groups of mice and similar dosing regimens (n = 20) yielded similar results.
To assess the effects of the drug on caudal epididymal sperm counts during the experimental period, mice were treated with the same dosing regimen (5 mg/kg for 7 d) and terminated either at the end of the dosing period or 4 wk after treatment. The caudal epididymal sperm counts immediately after drug treatment were reduced relative to control (1.97 × 106 ± 0.75; n = 5, vs. control, 47.60 × 106 + 5.82; n = 5) and were further reduced at 4 wk after treatment (0.65 × 106 ± 0.73; n = 5). The reduced numbers of caudal epididymal sperm collected from mice immediately after cessation of treatment nonetheless exhibited normal forward motility as did the normal numbers of sperm collected from mice that had fully recovered from the induced infertility. In contrast, caudal epididymal sperm collected from mice at 4 wk after treatment moved more slowly (n = 5 out of five males) and some exhibited circular motion (30%, n = 3 of five males).
We next examined the testes at the histological level to identify and quantify the cellular abnormalities associated with the induction of sterility and the subsequent recovery of spermatogenesis. We systematically examined the morphology and association of cell types within testicular tubules in mice at specific stages after BMS-189453 treatment. To guide our analysis, we took advantage of a well-defined classification index used in other studies examining the abnormalities in testes of mice in the absence of vitamin A, which showed similar types of spermatogenic defects as in our current drug-treated model (30). Quantification of the tubules containing specific defects showed that the progression from T1 to T4 abnormal tubules correlated nicely with the induction of sterility (Table 1). That is, the analysis revealed striking changes in testicular cell associations immediately after drug treatment and at 4 wk after treatment that coincided with the infertility. Detailed quantitative analysis of specific spermatogenic cell types in tubules revealed that there was a significant accumulation of steps 9–12 elongating spermatids immediately after drug treatment (Supplemental Fig. 4), resembling the temporary arrest of steps 8 and 9 spermatids at 4 wk of age seen in RARα-deficient mice (8). A drop in total spermatogonia and steps 1–8 round spermatids were also found, compared with control (Supplemental Fig. 4). At 4 wk after treatment, there was an overall decrease in most of the germ cell types including preletotene/leptotene/zygotene spermatocytes, pachytene and diplotene spermatocytes, round and elongated spermatids (Supplemental Fig. 4). Interestingly, 12 wk after cessation of treatment, most of the tubules recovered completely with correct germ cell association (Table 1 and Supplemental Fig. 4), in accordance with the recovery of fertility.
Table 1.
Percentages of tubules containing specific defects after BMS-189453 treatmenta
| Drug treatment + weeks after treatment | Normal | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| Control | 100% | 0 | 0 | 0 | 0 |
| BMS-189453 + 0 | 42 ± 9% | 46 ± 2% | 13 ± 10% | 0 | 0 |
| BMS-189453 + 4 | 9 ± 8% | 6 ± 4% | 29 ± 7% | 45 ± 10% | 11 ± 7% |
| BMS-189453 + 12 | 94 ± 10% | 0 | 2 ± 4% | 4 ± 7% | 1 ± 1% |
| BMS-189453 + 14 | 99 ± 3% | 0 | 1 ± 2% | 0 | 0 |
Males (8 wk old) were administered 5 mg/kg of BMS-189453 for 7 d and euthanized at 0 (BMS-189453 + 0), 4 (BMS-189453 + 4), 12 (BMS-189453 + 12), or 14 (BMS-189453 + 14) wk after drug treatment. Three to five males were used at each time point, and at least 200 tubules per testis were analyzed. Quantification of the cellular abnormalities was done essentially as described by Ghyselinck et al. (30). Tubules containing normal germ cell associations are designated “Normal”; T1 indicates tubular sections displaying a failure of sperm alignment and sperm release or abnormal round spermatids with crescent-like chromatin condensation with juxtanuclear clear spaces; T2, tubular sections lacking a given germ cell layer; T3, tubular sections displaying a single type of spermatocytes or round spermatids (steps 1–8) or both as the most advanced germ cell types; and T4, tubular sections containing only Sertoli cells and spermatogonia.
Finally, to ensure that there were no adverse effects on progeny and their gametes, the resulting progeny of two recovered males (four litters, 22 males and 22 females) were allowed to grow to adulthood. These progeny were healthy; males exhibited normal testicular weight, spermatogenesis, and serum testosterone levels (n = 22; Supplemental Fig. 2); and both male and female progeny yielded a similar number of embryos (12.52 ± 2.51 and 12.41 ± 3.02, respectively) upon mating.
Systematic modification of dosing regimens to fine tune the effects on spermatogenesis by inhibiting retinoid signaling through RAR
For eventual human application, the lowest possible dose levels are always desirable, but this must be balanced with efficacy of treatment (inhibition of spermatogenesis and 100% sterility by the end of treatment), length of time with desired outcome (the to-be-determined infertility duration), and ultimate reversibility of compound-induced sterility. We therefore designed experiments to assess whether fertility could be restored after a longer, chronic treatment regimen and whether a lower dose (2.5 mg/kg) but for longer dosing period (4 wk) induced infertility and restoration of fertility while maintaining the same compound burden (group I, 5.0 mg/kg for 2 wk, vs. group II, 2.5 mg/kg for 4 wk).
By 4 wk after treatment, all group I mice (n = 20) became infertile and stayed infertile for 2 wk after cessation of treatment (Fig. 7E) (five mice were euthanized for morphological examination; data not shown). Within 8 wk, 10 of the remaining 15 mice regained fertility. By 20 wk, fertility resumed completely in all males but one. However, the sperm counts on this male were normal (51.5 × 106), and subsequent histological analysis of the testes revealed intact testicular morphology, suggesting that spermatogenesis had recovered normally and the infertility was idiopathic. In group II, all males (n = 20) became infertile by the end of treatment (five mice were euthanized for morphological examination; data not shown), and interestingly, infertility lasted for 4 wk after cessation of treatment (Fig. 7F). By 12 wk, fertility resumed completely in all males, demonstrating that sterility could be induced in a timely manner at even lower doses, and with a lengthened period that was still reversible. Further, all recovered males generated normal embryonic litter sizes (Fig. 7F). As expected, none of the dosing regimens changed testosterone levels (data not shown), and all recovered males showed normal testicular weight and proper spermatogenic cell layers in the seminiferous tubules (data not shown). Identical results were obtained with treatment with compound 9 (a commercially synthesized version of BMS-189453) and when we used a modified standard daily dosing regimen in which the daily dose is adjusted weekly (see Materials and Methods).
Hematology, serum chemistry, and pathological evaluation
Given that there are variations in metabolism and toxicity responses among species, we wanted to rule out any possible toxicity of BMS-189453/compound 9 in the mouse model. Hematological and serum chemistry analysis and pathological evaluation of all physiological organs/tissues (Supplemental Tables 1 and 2) were performed on mice treated with compound 9 on a 2.5 mg/kg for 4 wk dosing regimen (adjusted weekly) (n = 5). Gross necropsy was performed, and the tissue/organ samples were collected for histopathological analysis of various physiological systems (Supplemental Tables 1 and 2). With the exception of the testes, no gross or histopathological abnormalities were found in any of the tissues/organs from control (n = 5) or drug-treated (n = 5) mice. Hematology and serum chemistry analysis did not show any significant differences from normal reference ranges or between groups.
Discussion
As the world's population continues to soar, contraceptive methods for men are an essential component of worldwide reproductive health (35–39). However, the two most common methods, mechanical devices and chemical intervention, are problematic. Steroid hormone-based methods, analogous to birth control pills for women, have been plagued by problems with effectiveness and side effects. For example, although chronic administration of testosterone suppresses sperm production, albeit not completely, there are ethnic differences in its effectiveness, and there is a long delay between initiation of treatment and induction of infertility (37, 40). Further, testosterone is reduced to dihydrotestosterone, which can overstimulate prostate growth, potentially leading to complications such as benign prostatic hyperplasia (36, 41). Supplementation with progestogens enhances the suppression of sperm production at lower doses of testosterone, but with greater weight gain and decreases in high-density lipoprotein cholesterol (37).
Nonhormonal strategies using gossypol and tripterygium have been complicated by low efficacy and/or toxicity (38). In a recent study of the small indazole carboxylic acid analog Gamendazole, infertility was achieved in male rats 3 wk after a single oral dose of 6 mg/kg: however, fertility returned only in four of seven animals (42). The antispermatogenic effect of l-CDB-4022 in primates was encouraging, but only oligozoospermia resulted (38). Intraperitoneal administration of adjudin conjugated to a FSH mutant caused male sterility in rats that was reversible (38). Nevertheless, long-term ip injection of a drug in humans is not an acceptable method of administration; rather, other options such as an oral route are preferable. Thus, a need exists for an effective, nonhormonal male contraceptive that exhibits few if any side effects or health risks.
In the present study, we demonstrated that even low levels of pan-RAR antagonist BMS-189453 inhibited spermatogenesis without any other adverse side effects in all physiological organs examined. We further showed that male sterility was achieved completely by 4 wk after treatment at a dosing regimen of 5 mg/kg for 2 wk and by the end of the treatment in a regimen of 2.5 mg/kg for 4 wk. Importantly, sterility was readily reversed even in the extended dosing regimen (2.5 mg/kg for 4 wk). Our morphological data revealed two possible reasons at the cellular level for the infertility: 1) acute disruptive effects on spermatid alignment at the lumen in stage VIII tubules and release into the tubular lumen at stage IX; and 2) temporal, cell-specific disruption of spermatogenesis with sloughing of germ cell layers in testes.
Support for the ability of inhibiting retinoid signaling as a novel approach to male contraception is supported by observations on another pan-RAR antagonist, AGN-194310. AGN-194310 is in phase III clinical trials for use in treating psoriasis and phase II trials for treating acne (12) and has also been suggested for treatment of prostate cancer (43). AGN-194310 has also been used in a clinical trial to treat hyperlipidemia (European Patent no. 07022682.4). Although there are no published data, a preliminary fertility study by E. S. Klein, Y. Yuan, and R. A. Chandraratna (US Patent no. 10304,665) reported that rats treated orally for 4 wk with 0.075 mg/kg of AGN-194310 displayed spermatogenic arrest after 2 and 4 wk of treatment and regained fertility after 23 wk.
In summary, we have developed a pharmacological model for manipulating retinoid signaling to inhibit spermatogenesis in a reversible manner. We have demonstrated that even low levels of BMS-189453/compound 9 delivered orally disrupted spermatogenesis, with testicular defects similar to those seen in VAD rats and RARα-deficient mice. A dosing regimen of 2.5 mg/kg for 4 wk resulted in induction of male sterility in all treated animals that was completely reversible (two separate experiments and n = 15 in each), importantly, with no detectable adverse effects in all physiological organs examined. The kinetics of this recovery suggests that the initiation of recovery occurred rapidly after cessation of treatment.
The common findings of defects in spermiogenesis in VAD rat testes, in the RARα-deficient mouse model and in response to treatment with pan-RAR antagonist BMS-189453/compound 9, suggest that the cellular processes leading to spermiogenesis and completion of spermiation are extremely sensitive to changes in retinoid signaling, whether through genetic, nutritional, or pharmacological intervention. Given the impaired spermiogenesis in RARα-deficient and drug-treated mice, the defects might result from the down-regulation of retinoid receptor-mediated target genes in round spermatids. Our genetic model in which restoration of RARα expression in round spermatids partially rescued spermatogenesis in Rara−/− testes further supports the critical role the receptor and retinoid signaling play in spermiogenesis. Our pharmacological model that reversibly disrupts germ cell differentiation during spermiogenesis will provide a useful tool to dissect the retinoid-signaling pathways involved in germ cell differentiation by, e.g. genome-wide microarray expression analysis (44). Such analyses ultimately will expand our understanding of the transcriptional network regulating spermatogenesis and the unique role of RARα in this differentiation.
Supplementary Material
Acknowledgments
We thank Chris Zusi (BMS) for his support, communication of unpublished data, and for facilitating compound acquisition; we thank BMS for the kind gift of BMS-189453.
This work was supported by CONRAD Foundation CIG-05-105 and CIG-05-107 (to D.J.W. and P.R.R.); National Institutes of Health Grant U01-HD060479 (to D.J.W. and S.S.W.C.). The testosterone assays were performed by the Ligand Assay and Analysis Core at University of Virginia Center for Research in Reproduction, which is supported by National Institute of Child Health and Human Development (SSCPRR) grant U54-HD28934.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATRA
- All-trans-retinoic acid
- BMS
- Bristol-Myers Squibb
- CAT
- chloramphenicol acetyltransferase
- RAR
- retinoic acid receptor
- RARE
- retinoic acid response element
- VAD
- vitamin A deficiency.
References
- 1. Howell JM, Thompson JN, Pitt GA. 1963. Histology of the lesions produced in the reproductive tract of animals fed a diet deficient in vitamin A alcohol but containing vitamin A acid. I. The male rat. J Reprod Fertil 5:159–167 [DOI] [PubMed] [Google Scholar]
- 2. Wolbach SB, Howe PR. 1925. Tissue changes following deprivation of fat-soluble A vitamin. J Exp Med 42:753–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eskild W, Hansson V. 1994. Vitamin A functions in the reproductive organs. In: Blomhoff R. ed. Vitamin A in health and disease. New York: Dekker; 531–559 [Google Scholar]
- 4. Chung SS, Wolgemuth DJ. 2004. Role of retinoid signaling in the regulation of spermatogenesis. Cytogenet Genome Res 105:189–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolgemuth DJ, Chung SS. 2007. Retinoid signaling during spermatogenesis as revealed by genetic and metabolic manipulations of retinoic acid receptor α. Soc Reprod Fertil Suppl 63:11–23 [PMC free article] [PubMed] [Google Scholar]
- 6. Chambon P. 1996. A decade of molecular biology of retinoic acid receptors. FASEB J 10:940–954 [PubMed] [Google Scholar]
- 7. Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, LeMeur M, Chambon P. 1993. High postnatal lethality and testis degeneration in retinoic acid receptor α mutant mice. Proc Natl Acad Sci U S A 90:7225–7229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung SS, Sung W, Wang X, Wolgemuth DJ. 2004. Retinoic acid receptor α is required for synchronization of spermatogenic cycles and its absence results in progressive breakdown of the spermatogenic process. Dev Dyn 230:754–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chung SS, Wang X, Wolgemuth DJ. 2005. Male sterility in mice lacking retinoic acid receptor α involves specific abnormalities in spermiogenesis. Differentiation 73:188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung SS, Wang X, Wolgemuth DJ. 2009. Expression of retinoic acid receptor α in the germline is essential for proper cellular association and spermiogenesis during spermatogenesis. Development 136:2091–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Lera AR, Bourguet W, Altucci L, Gronemeyer H. 2007. Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nat Rev Drug Discov 6:811–820 [DOI] [PubMed] [Google Scholar]
- 12. Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. 2007. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov 6:793–810 [DOI] [PubMed] [Google Scholar]
- 13. Altucci L, Gronemeyer H. 2001. The promise of retinoids to fight against cancer. Nat Rev Cancer 1:181–193 [DOI] [PubMed] [Google Scholar]
- 14. Zusi FC, Lorenzi MV, Vivat-Hannah V. 2002. Selective retinoids and rexinoids in cancer therapy and chemoprevention. Drug Discov Today 7:1165–1174 [DOI] [PubMed] [Google Scholar]
- 15. Kagechika H. 2002. Novel synthetic retinoids and separation of the pleiotropic retinoidal activities. Curr Med Chem 9:591–608 [DOI] [PubMed] [Google Scholar]
- 16. Yu KL, Spinazze P, Ostrowski J, Currier SJ, Pack EJ, Hammer L, Roalsvig T, Honeyman JA, Tortolani DR, Reczek PR, Mansuri MM, Starrett JE., Jr 1996. Retinoic acid receptor β, γ-selective ligands: synthesis and biological activity of 6-substituted 2-naphthoic acid retinoids. J Med Chem 39:2411–2421 [DOI] [PubMed] [Google Scholar]
- 17. Dawson MI. 2004. Synthetic retinoids and their nuclear receptors. Curr Med Chem Anticancer Agents 4:199–230 [DOI] [PubMed] [Google Scholar]
- 18. Chen JY, Penco S, Ostrowski J, Balaguer P, Pons M, Starrett JE, Reczek P, Chambon P, Gronemeyer H. 1995. RAR-specific agonist/antagonists which dissociate transactivation and AP1 transrepression inhibit anchorage-independent cell proliferation. EMBO J 14:1187–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen JY, Clifford J, Zusi C, Starrett J, Tortolani D, Ostrowski J, Reczek PR, Chambon P, Gronemeyer H. 1996. Two distinct actions of retinoid-receptor ligands. Nature 382:819–822 [DOI] [PubMed] [Google Scholar]
- 20. Chen S, Ostrowski J, Whiting G, Roalsvig T, Hammer L, Currier SJ, Honeyman J, Kwasniewski B, Yu KL, Sterzycki R, Kim CU, Starrett J, Mansuri M, Reczek P. 1995. Retinoic acid receptor γ mediates topical retinoid efficacy and irritation in animal models. J Invest Dermatol 104:779–783 [DOI] [PubMed] [Google Scholar]
- 21. Loeliger P, Bollag W, Mayer H. 1980. Arotinoids, a new class of highly active retinoids. Eur J Med Chem-Chem Ther 15:9–15 [Google Scholar]
- 22. Schulze GE, Clay RJ, Mezza LE, Bregman CL, Buroker RA, Frantz JD. 2001. BMS-189453, a novel retinoid receptor antagonist, is a potent testicular toxin. Toxicol Sci 59:297–308 [DOI] [PubMed] [Google Scholar]
- 23. Cipollone D, Amati F, Carsetti R, Placidi S, Biancolella M, D'Amati G, Novelli G, Siracusa G, Marino B. 2006. A multiple retinoic acid antagonist induces conotruncal anomalies, including transposition of the great arteries, in mice. Cardiovasc Pathol 15:194–202 [DOI] [PubMed] [Google Scholar]
- 24. Russell LD, Ettlin RA, SinhaHikim AP, Clegg ED. 1990. Histological and histopathological evaluation of the testis. Clearwater, FL: Cache River Press [Google Scholar]
- 25. Ostrowski J, Hammer L, Roalsvig T, Pokornowski K, Reczek PR. 1995. The N-terminal portion of domain E of retinoic acid receptors α and β is essential for the recognition of retinoic acid and various analogs. Proc Natl Acad Sci USA 92:1812–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vasios GW, Gold JD, Petkovich M, Chambon P, Gudas LJ. 1989. A retinoic acid-responsive element is present in the 5′ flanking region of the laminin B1 gene. Proc Natl Acad Sci USA 86:9099–9103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raivio T, Palvimo JJ, Dunkel L, Wickman S, Jänne OA. 2001. Novel assay for determination of androgen bioactivity in human serum. J Clin Endocrinol Metab 86:1539–1544 [DOI] [PubMed] [Google Scholar]
- 28. Travis AJ, Jorgez CJ, Merdiushev T, Jones BH, Dess DM, Diaz-Cueto L, Storey BT, Kopf GS, Moss SB. 2001. Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J Biol Chem 276:7630–7636 [DOI] [PubMed] [Google Scholar]
- 29. Al-Thani RK, Al-Thani AS, Elbetieha A, Darmani H. 2003. Assessment of reproductive and fertility effects of amitraz pesticide in male mice. Toxicol Lett 138:253–260 [DOI] [PubMed] [Google Scholar]
- 30. Ghyselinck NB, Vernet N, Dennefeld C, Giese N, Nau H, Chambon P, Viville S, Mark M. 2006. Retinoids and spermatogenesis: lessons from mutant mice lacking the plasma retinol binding protein. Dev Dyn 235:1608–1622 [DOI] [PubMed] [Google Scholar]
- 31. Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P. 1997. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J 16:2262–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oakberg EF. 1956. A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am J Anat 99:391–413 [DOI] [PubMed] [Google Scholar]
- 33. Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. 2004. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet 36:653–659 [DOI] [PubMed] [Google Scholar]
- 34. van der Spoel AC, Jeyakumar M, Butters TD, Charlton HM, Moore HD, Dwek RA, Platt FM. 2002. Reversible infertility in male mice after oral administration of alkylated imino sugars: a nonhormonal approach to male contraception. Proc Natl Acad Sci USA 99:17173–17178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mruk DD. 2008. New perspectives in non-hormonal male contraception. Trends Endocrinol Metab 19:57–64 [DOI] [PubMed] [Google Scholar]
- 36. Anderson RA, Baird DT. 2002. Male contraception. Endocr Rev 23:735–762 [DOI] [PubMed] [Google Scholar]
- 37. Amory JK, Page ST, Bremner WJ. 2006. Drug insight: Recent advances in male hormonal contraception. Nat Clin Pract Endocrinol Metab 2:32–41 [DOI] [PubMed] [Google Scholar]
- 38. Page ST, Amory JK, Bremner WJ. 2008. Advances in male contraception. Endocr Rev 29:465–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aitken RJ, Baker MA, Doncel GF, Matzuk MM, Mauck CK, Harper MJ. 2008. As the world grows: contraception in the 21st century. J Clin Invest 118:1330–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu PY, Swerdloff RS, Anawalt BD, Anderson RA, Bremner WJ, Elliesen J, Gu YQ, Kersemaekers WM, McLachlan RI, Meriggiola MC, Nieschlag E, Sitruk-Ware R, Vogelsong K, Wang XH, Wu FC, Zitzmann M, Handelsman DJ, Wang C. 2008. Determinants of the rate and extent of spermatogenic suppression during hormonal male contraception: an integrated analysis. J Clin Endocrinol Metab 93:1774–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Emberton M, Zinner N, Michel MC, Gittelman M, Chung MK, Madersbacher S. 2007. Managing the progression of lower urinary tract symptoms/benign prostatic hyperplasia: therapeutic options for the man at risk. BJU Int 100:249–253 [DOI] [PubMed] [Google Scholar]
- 42. Tash JS, Attardi B, Hild SA, Chakrasali R, Jakkaraj SR, Georg GI. 2008. A novel potent indazole carboxylic acid derivative blocks spermatogenesis and is contraceptive in rats after a single oral dose. Biol Reprod 78:1127–1138 [DOI] [PubMed] [Google Scholar]
- 43. Keedwell RG, Zhao Y, Hammond LA, Wen K, Qin S, Atangan LI, Shurland DL, Wallace DM, Bird R, Reitmair A, Chandraratna RA, Brown G. 2004. An antagonist of retinoic acid receptors more effectively inhibits growth of human prostate cancer cells than normal prostate epithelium. Br J Cancer 91:580–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chalmel F, Rolland AD, Niederhauser-Wiederkehr C, Chung SS, Demougin P, Gattiker A, Moore J, Patard JJ, Wolgemuth DJ, Jégou B, Primig M. 2007. The conserved transcriptome in human and rodent male gametogenesis. Proc Natl Acad Sci USA 104:8346–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.