The large-pore ion channels pannexin 1 and 2 are expressed in secretory and nonsecretory pituitary cells and are probably coupled to purinergic signaling indirectly by releasing ATP.
Abstract
Pannexins are a newly discovered three-member family of proteins expressed in the brain and peripheral tissues that belong to the superfamily of gap junction proteins. However, in mammals pannexins do not form gap junctions, and their expression and function in the pituitary gland have not been studied. Here we show that the rat pituitary gland expresses mRNA and protein transcripts of pannexins 1 and 2 but not pannexin 3. Pannexin 1 was more abundantly expressed in the anterior lobe, whereas pannexin 2 was more abundantly expressed in the intermediate and posterior pituitary. Pannexin 1 was identified in corticotrophs and a fraction of somatotrophs, the S100-positive pituicytes of the posterior pituitary and AtT-20 (mouse pituitary adrenocorticotropin-secreting cells) and rat immortalized pituitary cells secreting prolactin, whereas pannexin 2 was detected in the S100-positive folliculostellate cells of the anterior pituitary, melanotrophs of the intermediate lobe, and vasopressin-containing axons and nerve endings in the posterior lobe. Overexpression of pannexins 1 and 2 in AtT-20 pituitary cells enhanced the release of ATP in the extracellular medium, which was blocked by the gap junction inhibitor carbenoxolone. Basal ATP release in At-T20 cells was also suppressed by down-regulating the expression of endogenous pannexin 1 but not pannexin 2 with their short interfering RNAs. These results indicate that pannexins may provide a pathway for delivery of ATP, which is a native agonist for numerous P2X cationic channels and G protein-coupled P2Y receptors endogenously expressed in the pituitary gland.
ATP is frequently released by cells and acts as an agonist for G protein-coupled purinergic P2 receptors (P2YR) and purinergic P2 receptor channel (P2XR), which are expressed in numerous tissues. The breakdown of ATP by ectonucleotidases not only terminates its extracellular messenger functions but also provides a pathway for the generation of two additional agonists: adenosine 5′-diphosphate, which acts via some P2YR, and adenosine, a native agonist for G protein-coupled adenosine receptors (AR) (1). The pituitary gland also expresses several members of each family of purinergic receptors. P2XR and AR are coexpressed in the somata and nerve terminals of vasopressin-releasing neurons and contribute to the control of vasopressin, but not oxytocin, release (2). Secretory anterior pituitary (AP) cells also express both P2XRs and ARs; activated P2XRs stimulate electrical activity and voltage-gated Ca2+ influx, modulate Ca2+ release from intracellular stores, and enhance hormone release, whereas AR terminate electrical activity, Ca2+ signaling and secretion. Calcium-mobilizing P2YR are predominantly expressed in nonsecretory cells of the AP and posterior pituitary (PP) (3, 4).
The physiological sources of the extracellular nucleotides required for activation of purinergic receptors in the pituitary gland remain largely uncharacterized. Neurons, neuroendocrine cells, and platelets release ATP by Ca2+-controlled exocytosis of nucleotides stored within synaptic vesicles or dense core granules (5). The secretory vesicles of the magnocellular neurons of the hypothalamus that control release of vasopressin and oxytocin also contain ATP (6), but no conclusive evidence was obtained to clarify the mechanism of ATP release by nerve endings in the PP. ATP is also released by normal and immortalized AP cells at resting conditions. Such basal ATP releases were enhanced in cells treated with ARL67156, an inhibitor of ectonucleotidases. However, basal ATP secretion was not enhanced by facilitation of prolactin release in perifused pituitary cells, suggesting that vesicular exocytosis does not account for ATP release (7).
Two members of the gap junction superfamily of proteins, connexins and pannexins (Panx), have been suggested to account for nonvesicular ATP release. These proteins show identical membrane topology: four transmembrane domains connected by two extracellular loops and one intracellular loop with both N and C termini in the cytosol. This structure is essential for the formation of hexameric pore complexes termed hemichannels, which are large, nonselective ion channels expressed in the plasma membrane before their assembly into gap junctions (8). Connexin hemichannels have been proposed as a conduit for ATP release in different type of cells (9). These proteins are also expressed in the pituitary gland, but their function is more consistent with formation of gap junction (10–16). Panx are a three-member family of channels, termed Panx1, Panx2, and Panx3. Unlike connexins, homomeric Panx1 hexamers do not form gap junctions when expressed in mammalian cells and, instead, operate as plasma membrane channels (17, 18). They are activated by mechanical stress (19), membrane depolarization (20), and in a receptor-dependent manner (21). Panx are permeable to ions, small molecules, and metabolites up to 1 kDa, including nicotinamide adenine dinucleotide, cyclic nucleotides, and inositol 1, 4, 5-triphosphate (22, 23). More recent reports have also indicated the potential role of these channels in ATP release in numerous cell types (24), including erythrocytes (25), taste buds (26), T cells (27), airway epithelia (28), astrocytes (29), and chondrocyte (30). The expression and the role of these proteins in the pituitary gland have not been studied previously.
Here we show for the first time that Panx1 and Panx2, but not Panx3, are also expressed at the mRNA and protein levels in all the three lobes of the pituitary gland. We analyzed the expression pattern of Panx1 and Panx2 in secretory and nonsecretory cells of AP, intermediate lobe (IL), and PP and immortalized secretory pituitary cells. We also studied the functional role of native Panx1 proteins in ATP release in immortalized pituitary cells. To confirm the specificity of Panx in ATP release, both pharmacological and molecular biology approaches were also used. These experiments revealed different distributions of Panx in three pituitary lobes and their roles in ATP release.
Materials and Methods
Chemicals and antibodies
Rabbit anti-Panx1 and -Panx2 antibodies (against antigen peptides located at the C terminal region of mouse Panx1 and Panx2 proteins), donkey antimouse AlexaFluor 555 IgG, donkey antirabbit AlexaFluor 555 IgG, goat antirabbit AlexaFluor 488 IgG, and anti-V5 monoclonal antibodies were purchased from Invitrogen (Carlsbad, CA). Anti-α-tubulin monoclonal and anti-FLAG M2 monoclonal peroxidase conjugated antibodies were obtained from Sigma (St. Louis, MO). Rabbit antirat GH, prolactin, βLH, βTSH, and ACTH were obtained from Dr. A. F. Parlow (National Institute of Diabetes and Digestive and Kidney Diseases, National Hormone and Peptide Program, Torrance, CA). Mouse monoclonal anti-S100 antibodies were obtained from Chemicon (Billerica, MA). Rabbit polyclonal antiserum to vasopressin was obtained from Abcam (Cambridge, MA). Peroxidase-conjugated goat antimouse IgG and goat antirabbit IgG were purchased from Kirkegaard & Perry Laboratories (Gaithersburg, MD). Unless stated otherwise, all other chemicals were obtained from Sigma.
Cell culture
AP cells from normal postpubertal female Sprague Dawley rats were obtained from Taconic Farm (Germantown, MD). Experiments were approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Animal Care and Use Committee. Pituitary cells were dispersed and cultured as mixed cells in medium 199 (Invitrogen) containing Earle's salts, sodium bicarbonate, 10% heat-inactivated horse serum, and penicillin (100 U/ml). Rat immortalized pituitary cells secreting prolactin (GH3) and AtT-20 (mouse pituitary adrenocorticotropin-secreting cells) were cultured in Ham's F12K medium supplemented with 2 mm l-glutamine, 1.5 g/liter sodium bicarbonate, 15% heat-inactivated horse serum, 2.5% fetal bovine serum, and gentamicin (100 μg/ml). Human embryonic kidney 293 (HEK293) cells were routinely maintained in DMEM containing 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin liquid (Invitrogen).
RNA preparation and cDNA synthesis
Total RNA was extracted from rat pituitary tissue, primary culture of rat AP cells, and different cell lines by the TRIzol regent (Invitrogen). The integrity of total RNA was examined by electrophoresis, and the quantity of RNA was determined by measuring OD260 with a UV spectrophotometer. After treatment with deoxyribonuclease I (Invitrogen), total RNA (5 μg) was used to synthesize single-strand cDNA using SuperScript III ribonuclease H− reverse transcriptase (Invitrogen) and an oligodeoxythymidine adaptor primer (5′-TCGAATTCGGATCCGAGCTCVT17-3′) according to the manufacturer's instructions.
Isolation of Panx1 and Panx2 cDNA
Full-length cDNA encoding Panx1 and Panx2 were amplified by RT-PCR from rat pituitary cells using Herculase Hotstart PCR master mix (Stratagene, La Jolla, CA) and primers covering their coding regions (Table 1). To increase the specificity of the amplification, nested PCR was applied for cloning Panx1 cDNA. After the first round of PCR using Panx1F and Panx1R as primers, the PCR products were diluted 100 times and PCR was performed again using the primer pair PanxF1/PanxR1 (Table 1) at 62 C. The PCR products of the expected size were then excised and purified with the QIAquick gel extraction kit (QIAGEN, Gaithersburg, MD). The purified PCR products were then subcloned into pGEM-T Easy vector (Promega, Madison, WI) and sequenced.
Table 1.
Primers used for cloning and detection of Panx mRNA transcripts
| Primers | Sequence (5′-3′) |
|---|---|
| Panx1F | CTG CGA GGT AGG CGC AGC GAC TG |
| Panx1R | AGC ACT GCC AGT CCA GAA CGG TG |
| Panx1F1 | GTC GTT GAC GGC GCG GAC TC |
| Panx1R1 | CAC AGG AGT CAC AGG CTT GA |
| Panx1F2 | GAA AGC CAC TTC AAG TAC CCA A |
| Panx1R2 | AGG TTT GTC AGG AGT AGC AT |
| Panx2F | CAT GCA CCA CCT CCT GGA GCA |
| Panx2R | TCT ACT CAT GCC TAG GCT CAG CTG |
| Panx2F1 | CAT CTT CCG CAA GAG CAA C |
| Panx2R1 | GTG GGG TAT GGG ATT TCC TT |
| Panx3F | ACT CAC TGG CTC ACT ATA AAC |
| Panx3R | GGT AGG TGG CCA CTA GCC AAT G |
Expression profile analysis of Panx mRNA transcripts
The presence of Panx1, Panx2, and Panx3 mRNA transcripts in rat pituitary tissue, immortalized GH3, AtT-20, and HEK293 cells was analyzed by RT-PCR. Total RNA was prepared as mentioned above, and 2-μg aliquots from rat pituitary tissue and different cell lines were transcribed into cDNA in 20 μl reaction mixture using SuperScript III ribonuclease H− reverse transcriptase (Invitrogen). The generated cDNA were used as a template for PCR, which was performed with 1.5 mm MgCl2, 200 μm deoxynucleotide triphosphate, 1.5 U Taq DNA polymerase, and 25 pm of each primer (Panx1F2/Panx1R2 for Panx1 detection; Panx2F1/Panx2R1 for Panx2 detection; and Panx3F/Panx3R for Panx3 detection; Table 1), with plasmids containing the cDNA of Panx1, Panx2, and Panx3 serving as positive controls. After amplification, the PCR product identities were confirmed by DNA sequencing. PCR products were resolved on a 1.2% agarose gel and stained with ethidium bromide. To confirm that samples were not contaminated with genomic DNA, negative control reactions for RT-PCR were performed in absence of cDNA template (without transcription). Real-time PCR was performed using predesigned TaqMan gene expression assays (catalog no. for TaqMan probes of rat and mouse Panx1, Panx2, and glyceraldehyde-3-phosphate dehydrogenase are Rn01447979_m1, Rn01308054_m1, Rn01462662_g1, Mm00450900_m1, Mm01308054_m1, and Mm99999915_g1, respectively) from Applied Biosystems with LightCycler TaqMan master mix (Roche, Indianapolis, IN) and LightCycler 2.0 real-time PCR system (Roche). Total RNA from cultured AP cells, intermediate/posterior tissue, and cell lines were prepared as described above. Expression levels of the Panx genes were determined by the comparative 2(-δ δ cycle threshold [C(T)]) quantification method using glyceraldehyde-3-phosphate dehydrogenase as a reference gene where [δ δ C(T)] = (CT, target − CT, reference) sample − (CT, target − CT, reference) control (31). To compare the relative expression level of individual Panx genes, the levels were calibrated against Panx1 (set to 100%).
DNA constructs, cell transfection, and small interfering RNA (siRNA) silencing
Panx1 and Panx2 were amplified by PCR with PfuUltra II fusion HS DNA polymerase (Stratagene) using full-length plasmids as the template. The PCR products were purified, digested, and cloned into the expression vector pcDNA3.1 (Invitrogen) containing a V5 epitope at the C terminus and p3XFLAG-CMV-7.1 expression vector (Sigma) containing a FLAG epitope at the N terminus. The correct sequences of all recombinant plasmids were confirmed by DNA sequencing. HEK293, GH3, and AtT-20 cells were transfected with the different plasmids using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's recommendations. Transfection was conducted 24 h after plating the cells. After 4.5 h of incubation, the transfection mixture was replaced with normal culture medium, and cells were cultured for an additional 24–48 h before being assayed. To silence endogenous Panx1 and Panx2 expression, 1 million AtT-20 pituitary cells were transfected with 2.5 μg prescreened Silencer Select predesigned siRNA (Ambion, Austin, TX) for mouse Panx1 (sense: 5′GCUCGAGAUUUGGACCUAAtt3′; antisense: 5′UUAGGUCCAAAUCUCGAGCac3′) or siGENOME SMARTpool siRNA (Dharmacon, Lafayette, CO) for mouse Panx2 using Nucleofection kit L and program T-005 (Amaxa, Gaithersburg, MD). The same amount of Silencer negative siRNA no. 1 (Ambion) or siGENOME nontargeting siRNA pool no. 1 (Dharmacon) was also transfected into AtT-20 cells to serve as negative control. The silencing efficiency was examined by real-time PCR (48 h after transfection) and Western blot (72 h after transfection).
Immunohistochemistry
Adult rat pituitaries were carefully removed and fixed in Bouin's fix solution for 48 h, dehydrated in a series of alcohol washes, immersed in xylene, and embedded in paraffin. Five-micrometer coronal sections were cut on a microtome and mounted on gelatin-coated slides. Sections were deparaffinized with xylene, rehydrated, and antigens retrieved in citrate buffer (pH 6.0) by a microwave treatment. Panx1 and Panx2 immunofluorescence staining was performed using a Tyramide signal amplification kit with horseradish peroxidase-goat antirabbit IgG and Alexa fluor 488 tyramide, according to the manufacturer's instructions (Invitrogen). Sections were incubated with polyclonal rabbit anti-Panx1 (2.5 μg/ml) and anti-Panx2 (5 μg/ml) against the epitopes located in the C terminus of rat Panx1 and Panx2, respectively, overnight at 4 C. For double-immunofluorescence labeling, after immunostaining of Panx1 or Panx2, sections were rinsed extensively in PBS (pH 7.4) and blocked in PBS with 1% BSA for 1 h, followed by incubation with anti-S100 antibodies for 1 h at room temperature (5 μg/ml; Chemicon). The sections were then incubated with donkey antimouse AlexaFluor 555 (1:200 dilution) for 2 h at room temperature. For double-immunohistochemical labeling using two rabbit antisera/antibodies, to avoid false colocalization, we used the microwave treatment (32). Briefly, after Panx1 or Panx2 immunofluorescence staining, as described above, sections were immersed in citrate buffer (pH 6.0), brought to the boiling point in the microwave oven at maximum power and then microwaved for 5 min more at half-power. After cooling down, Panx1-labeled sections were stained for ACTH (1:1000 dilution) and GH (1:2000 dilution). Panx2-labeled sections were stained for vasopressin (1:200 dilution). Finally, the sections were mounted in Mowiol (Calbiochem) and examined under the Zeiss Axiovert optical fluorescent microscope with EC Plan-Neofluar ×63 objective. The images were collected using the Apotome system for optical sectioning. Negative controls of labeling were performed by the omission of the primary antibodies, which did not result in specific labeling.
Immunocytochemistry
To study the colocalization of different hormones and Panx in AP cells, rat anterior pituitary cells were grown on poly-l-lysine-coated (0.01%), 25-mm coverslips overnight before double-immunocytochemistry labeling assay. For double immunolabeling of hormones (prolactin, ACTH, LH, TSH, and GH) and Panx1 and Panx2, the hormone IgGs were directly labeled with Fluor 633 using the DyLight 633-microscale antibody labeling kit (Pierce, Rockford, IL) according to the manufacturer's recommendations. Cells were washed with PBS two times, fixed with 4% paraformaldehyde for 20 min, washed with PBS twice, and permeabilized with 0.1% Triton X-100-containing PBS for 15 min at room temperature. Coverslips were then washed three times with PBS, blocked with PBS containing 10% normal goat serum and 5% bovine serum albumin for 2 h at room temperature, and incubated with 1.25 μg/ml diluted anti-Panx1 or anti-Panx2 antibodies at 4 C overnight. This was followed by washing three times with PBS containing 0.05% Tween 20, incubation with 1:600 diluted goat antirabbit secondary IgG labeled with AlexaFluor 488 for 1 h at room temperature, and again washing three times with PBS and Tween 20. The cells were blocked again with 10% normal rabbit serum for 2 h at room temperature, incubated with diluted fluorochrome-labeled antihormone IgG (1:500 dilution for antiprolactin and anti-ACTH and 1:250 dilution for anti-LH, TSH, and GH) for 2 h at room temperature and followed by washing three times. Coverslips were further treated with ProLong Gold antifade reagent and 4′,6′-diamino-2-phenylindole to label nuclei (Invitrogen) and finally mounted on an inverted confocal laser-scanning microscope equipped with a ×63 oil immersion objective (LSM 510; Carl Zeiss GmbH, Jena, Germany).
Native-PAGE analysis of Panx oligomeric complexes
Native-PAGE analyses were performed on transfected HEK293 cells and pituitary tissue. HEK293 cells were transfected with FLAG-tagged Panx1 or V5-tagged Panx1 and Panx2 constructs. Freshly prepared rat pituitary tissue was washed three times with cold PBS to remove blood residue and homogenized on ice using a glass homogenizer. Proteins were extracted from pituitary tissue and transfected HEK293 cells with PBS plus 1% digitonin containing a protease inhibitor cocktail (Calbiochem). Cell lysates were kept on ice for 30 min and centrifuged at 50,000 rpm for 35 min at 4 C. The supernatant was collected and subjected to electrophoresis immediately. To determine the molecular identity of oligomeric complexes of Panx1, lysates from transfected HEK293 cells and pituitary tissue were treated with different concentrations of sodium dodecyl sulfate (SDS) for 30 min at room temperature. The samples were supplemented with 2× Tris-glycine native sample buffer (Invitrogen) and separated on 4–12 or 4–20% Tris-glycine native PAGE gels using the high-molecular-weight calibration kit for native electrophoresis (GE Healthcare, Buckinghamshire, UK) to estimate protein molecular size. Proteins were transferred onto a polyvinyl difluoride membrane, and the Panx1 and Panx2 oligomers were detected by immunoblotting using anti-FLAG, anti-V5, anti-Panx1, or anti-Panx2 antibodies.
Western blot analysis
Protein samples were separated by Tris-glycine SDS-PAGE or Tris-glycine native PAGE and transferred onto polyvinyl difluoride membranes. The membrane was blocked for 1 h at room temperature with PBS supplemented with 0.1% Tween 20 and 5% nonfat milk and then incubated overnight at 4 C with the following diluted primary antibodies: anti-V5 monoclonal antibodies (1:5000), anti-Panx1 and anti-Panx2 antibodies (0.5 μg/ml), anti-FLAG M2 monoclonal peroxidase conjugated antibodies (1:4000), or anti-α-tubulin monoclonal antibodies (1:6000). After washing four times with PBS containing Tween 20, positive signals of individual blots were visualized. This was done directly using anti-FLAG M2 monoclonal peroxidase-conjugated antibodies or by incubating the membrane with peroxidase-conjugated goat antirabbit or goat antimouse secondary antibodies (1:10000; Kirkegaard & Perry Laboratories).
Measurements of extracellular ATP
ATP release was measured in AtT-20 cells. The culture medium was replaced with Krebs solution (in millimoles: 145 NaCl; 4.5 KCl; 10 HEPES; 10 glucose; 1 MgCl2; and 2 CaCl2, pH 7.4) with or without 100 μm carbenoxolone (CBX) for 10 min to reestablish basal conditions. The cells were then incubated with normal ATP release buffer (in millimoles: 145 NaCl; 4.5 KCl; 10 HEPES; 10 glucose; and 1 EDTA, pH 7.4) or a 50% dilution of this buffer (hypotonic buffer), with or without 100 μm CBX for 10 min at room temperature. The supernatant was then collected, followed by a brief 13,000 rpm spin, and the ATP levels were subsequently measured with an ATP bioluminescent assay kit (Sigma) in Mithras LB 940 instrument (Berthold, Wildbad, Germany). The ATP release was presented as normalized values.
Results
Expression profile of Panx transcripts in pituitary cells
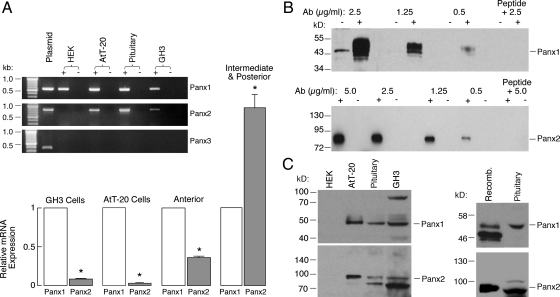
The presence of mRNA transcripts for Panx in rat pituitary tissue, immortalized pituitary cells (AtT-20 and GH3), and HEK293 cells was analyzed by RT-PCR using primers listed in Table 1. The RT-PCR analysis revealed that Panx1 mRNA transcripts were present in all tested cells (Fig. 1A, inset). The Panx2 mRNA was also expressed in normal and immortalized pituitary cells but not in HEK293 cells. On the other hand, Panx3 mRNA transcripts were not observed in any of cell types tested. No PCR products were detected from controls containing all components except reverse transcriptase, ruling out the possibility of genomic DNA contamination. Real-time PCR analysis further revealed that Panx1 was expressed at levels about 3 times higher than those of Panx2 in AP tissue. A similar predominance in expression of Panx1 was also observed in GH3 and AtT-20 pituitary cells. In contrast, the expression of Panx2 was higher than Panx1 in IL/PP tissues (Fig. 1A, main panel).
Fig. 1.
Identification of Panx transcripts in rat pituitary cells and cell lines. The primer pairs Panx1F2/Panx1R2, Panx2F1/Panx2R1, and Panx3F/Panx3R were used for detection of Panx1, Panx2, and Panx3 mRNAs, respectively (Table 1). A, inset, The examination of Panx1, Panx2, and Panx3 mRNA transcripts in pituitary tissue and various cell lines by RT-PCR. The sizes of PCR products were as follows: 548 bp for rat Panx1, 786 bp for rat Panx2, and 372 bp for rat Panx3. PCR was performed using first-strand cDNA samples with (+) and without (−) reverse transcriptase, and agarose gel was performed to analyze the PCR products. The identity of all PCR products was confirmed by DNA sequencing. No PCR products were detected in controls containing all components except the reverse transcriptase. A, main panel, Real-time RT-PCR analysis of Panx gene expression in rat anterior and intermediate-posterior pituitary tissues, GH3, and AtT-20 pituitary cell lines. Panx1 gene expression was set as 100% and Panx2 gene expression was therefore presented as a normalized value. Panx1 and Panx2 genes show significantly different levels of expression in different tissues and cells. *, P < 0.01, estimated by Student's t test. B, Characterization of Panx antibodies by Western blot analysis. HEK293 cells were transfected (+) or untransfected (−) with Panx1 or Panx2 plasmid, and 5 μg (transfected) or 50 μg (untransfected) cell lysates were applied onto each well of gels. The blot membranes were incubated with different dilutions of anti-Panx antibodies, developed, and exposed under the same conditions. The specific bands for both Panx1 and Panx2 were completely eliminated when the anti-Panx antibodies were preadsorbed with their respectively cognate peptides by 1:1 weight ratio. C, Expression of Panx1 and Panx2 proteins in rat pituitary cells. Approximately 50 μg total proteins from each cell type was loaded onto each well of gels and Western blots were performed using rabbit anti-Panx1 and -Panx2 antibodies (0.5 μg/ml). Panx1 and Panx2 proteins were expressed in cultured pituitary cells, AtT-20, and GH3 immortalized AP cells but not in HEK293 cells (left panel). The upper band for Panx1 (∼100 kDa) in GH3 cells represents a dimer. Panx2 shows a single band in AtT-20 pituitary cells but displays two bands in pituitary cells as well as in GH3 pituitary cells. Recombinant V5-tagged Panx1 or Panx2 protein serves as a control to confirm the identities of endogenous expressed Panx (right panels). Note that the ectopically expressed Panx1 protein shows three bands that represent different glycosylated forms, whereas Panx1 protein from rat pituitary cells presents only one glycosylated form. In this and the following figures, triplicate experiments were performed and similar results were obtained.
The presence of Panx1 and Panx2 proteins in rat pituitary cells was also studied by Western blot analysis (Fig. 1). In the initial phase of our experiments, we tested all commercially available Panx antibodies from four different companies. In our hands, only Invitrogen antibodies gave satisfactory results. The specificity of anti-Panx1 and anti-Panx2 antibodies was proved by peptide-absorption assays, in which the antibodies were preincubated with their respectively conjugate peptides at a 1:1 weight ratio at room temperature for 2 h. This peptide preabsorption completely eliminated the blotting band (Fig. 1B). We also tested different dilutions of both antibodies (0.5–2.5 μg/ml for Panx1 and 0.5–5 μg/ml for Panx2) and found that 0.5 μg/ml anti-Panx1 and anti-Panx2 is a minimum concentration that could be used in Western blot analysis (Fig. 1B).
Panx1 and Panx2 were detected in pituitary cells, prolactin-secreting GH3 cells, and ACTH-secreting AtT-20 pituitary cells (Fig. 1C, left). The Panx1 protein in HEK293 cells could be detected with a weak signal only when high concentrations of anti-Panx1 antibodies were applied, which indicated low expression level in our experimental conditions (Fig. 1B). Panx2 protein was not observed in HEK293 cells when anti-Panx2 antibodies were used in 0.5- to 5-μg/ml concentrations, which is consistent with the absence of Panx2 mRNA in this type of cell and the specificity of anti-Panx2 antibodies used in these experiments at higher concentrations. The identities of the endogenously expressed Panx proteins were further confirmed by the parallel blotting of recombinant and native Panx1 and Panx2 proteins in the same blot (Fig. 1C, right).
Using the primer pairs Panx1F1/Panx1R1 and Panx2F/Panx2R (Table 1), which cover the coding regions of rat Panx1 and Panx2 cDNA, respectively, we isolated their mRNA transcripts from primary cultures of rat AP cells. The deduced amino acid sequences of Panx1 and Panx2 were identical to the reference sequences from the National Center for Biotechnology Information (Bethesda, MD) database (GenBank accession no: NM_199397.1 and NM_199409.2, respectively). In addition to the expected size of the Panx1 cDNA, we also observed two shorter cDNA products, which were similar in sequence to Panx1. The two new short isoforms had not been previously reported and have been deposited in the GenBank with the accession numbers GQ499839 and GQ499840, respectively.
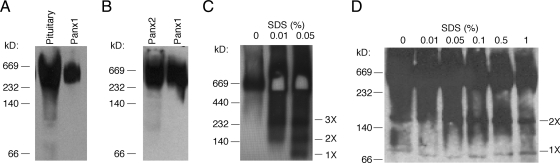
Oligomeric organization of Panx in pituitary gland
To determine the assembly pattern of Panx oligomeric complexes, HEK293 cells were transfected with recombinant plasmids to express FLAG-tagged or V5-tagged Panx proteins, and membrane proteins from transfected cells or pituitary tissue were solubilized with the mild nonionic detergent digitonin. The proteins were then separated by native Tris-glycine PAGE and analyzed by Western blot. Figure 2A shows that endogenously and heterologously expressed Panx1 assembled into similar homomeric oligomeric complexes that were shown by anti-Panx1 antibodies. The heterologously expressed Panx2 also formed homooligomeric complexes similar to those formed by Panx1, as identified by anti-V5 antibodies (Fig. 2B). The identity of Panx1 protein oligomeric complexes was further confirmed by treatment with SDS to induce partial dissociation. The heterologously expressed Panx1 protein oligomeric complex was dissociated into three additional bands by SDS treatment. These bands probably represented trimeric, dimeric, and monomeric forms of Panx 1, respectively (Fig. 2C), whereas the Panx1 protein oligomeric complex from pituitary tissue dissociated into two bands that probably represented dimeric and monomeric forms (Fig. 2D).
Fig. 2.
Oligomeric organization of Panx channels. HEK293 cells were transfected with FLAG-tagged Panx1 (A and C) or V5-tagged Panx1 and Panx2 constructs (B). Proteins from the pituitary tissue and transfected HEK293 cells were extracted with PBS containing 1% digitonin, and Panx1 and Panx2 oligomers were evaluated under native conditions (for details see Materials and Methods). A, Assembly of Panx1 homomeric oligomers in HEK293 cells and pituitary tissue. B, Assembly of Panx2 homomeric oligomers in HEK293 cells. C and D, Characterization of Panx1 protein oligomeric complexes in HEK293 cells (C) and pituitary tissue (D) by SDS treatment. Proteins were immunoblotted with anti-Panx1 (A and D), anti-V5 antibody (B), or anti-FLAG horseradish peroxidase-labeled antibody (C). Note that SDS-treated Panx1 oligomeric complexes from HEK293 cells can partially dissociate into three additional bands, probably representing trimeric (three times), dimeric (two times), and monomeric (one time) forms, whereas Panx1 oligomeric complexes from pituitary tissue can only partially dissociate into dimeric and monomeric forms through SDS treatment. The molecular-weight protein markers are shown on the left sides of the panels, which may not represent the actual size of the proteins (52).
Distribution of Panx1 and Panx2 proteins in pituitary gland
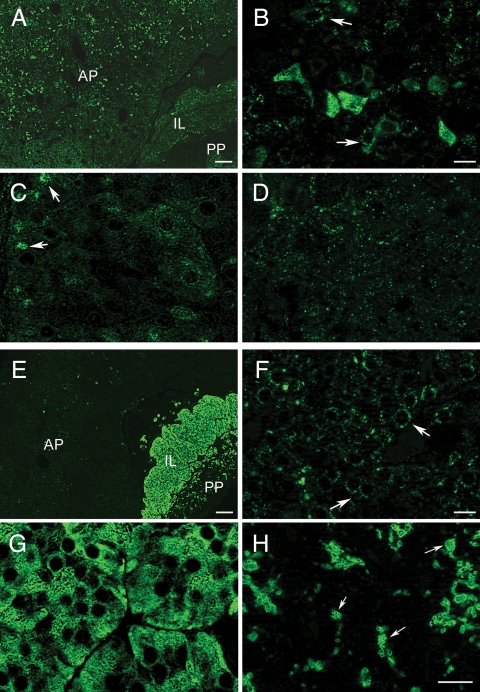
In further experiments, we used immunohistochemical staining to study the presence and distribution of Panx1 and Panx2 proteins in the rat pituitary gland using 2.5 μg/ml anti-Panx1 and 5 μg/ml anti-Panx2 antibodies. Panx1 was detected in all three lobes (Fig. 3A). Large numbers of cells that were intensely immunolabeled for Panx1 were scattered throughout the AP gland (Fig. 3B). The extensively labeled cells were elongated with an angular morphology and resembled corticotrophs. Other cells in the AP were not very intensely stained by anti-Panx1 but displayed a punctate, cytoplasmatic, and membranous distribution (Fig. 3B, arrows). The expression of Panx1 in the endocrine cells of the IL (melanotrophs) was lower and less conspicuous (Fig. 3C) and slightly higher in the epithelial cells lining the pituitary cleft (Fig. 3C, arrows). Additionally, Panx1 showed a punctate, diffuse distribution throughout the PP (Fig. 3D).
Fig. 3.
Localization of Panx1 and Panx2 proteins in the rat pituitary gland. Immunofluorescence labeling for Panx1 (A–D) and Panx2 (E–H) in different lobes of the pituitary gland. A and E, Low-magnification immunofluorescence images showing the overall distribution of Panx1 (A) and Panx2 (E) in the AP, IL, and PP, respectively. B, In the AP, anti-Panx1 strongly labeled cells with irregular shapes that morphologically resembled corticotrophs. Panx1 also displayed a dot-like cytoplasmic and membranous distribution in other cells of the AP (arrows). C, In the IL, the expression of Panx1 was low, with the exception of the epithelial cells bordering the pituitary cleft, which were moderately immunolabeled (arrows). D, In the PP, Panx1 displayed punctate and diffuse distribution, without distinguishable cellular structures. F, In the AP, Panx2 showed punctate, cytoplasmatic, and membranous distribution pattern (arrows). G, Panx2 was strongly and intensely expressed in endocrine cells throughout the IL (melanotrophs). H, In the PP, Panx2 showed intensive immunostaining in round or ovoid-shaped presumably neuronal structures with variable dimensions (arrows). Scale bars (A and E), 100 μm; (B–D, F, and G), 10 μm; (H), 50 μm.
Panx2 was also detected in all three lobes of the rat pituitary but predominantly in the IL and PP (Fig. 3E). In the AP, Panx2 exhibited scattered immunostaining with a pronounced dot-like distribution around the nuclei (Fig. 3F). Strong Panx2 immunoreactivity was observed in the cell bodies and cytoplasmic processes of melanotrophs in the IL (Fig. 3G). In the PP, Panx2 was strongly localized in round and ovoid shaped structures (Fig. 3H, arrows), which were up to 15 μm in diameter. This immunostaining pattern indicated the presence of Panx2 in neuronal structures, such as Herring bodies (the terminal ends of axons from hypothalamus), whereas pituicytes could not be distinguished (Fig. 3H).
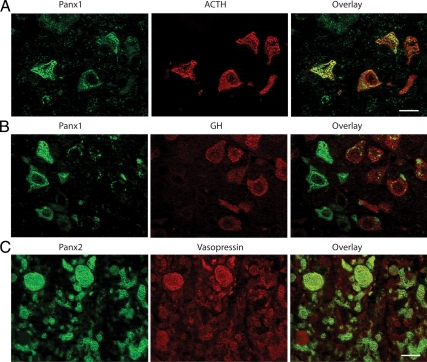
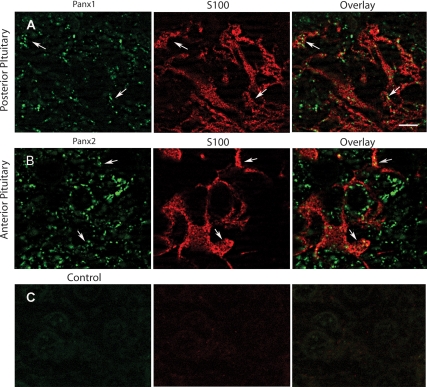
To determine which cell subpopulations in the AP express Panx1, we performed double-immunofluorescence labeling with pituitary hormones. Double labeling with ACTH revealed that Panx1 is abundantly expressed by corticotrophs (Fig. 4A). Only punctate staining of Panx1 could be detected in a fraction of GH-producing cells (Fig. 4B), whereas cells producing prolactin, βLH, or TSH subunits were almost completely devoid of staining (data not shown). Because the IL almost exclusively contains melanotrophs, double immunostaining was not performed. The localization of Panx2 in PP was addressed by means of double-immunofluorescent labeling with vasopressin. In our hands, labeling of vasopressin positive axons and nerve endings, also known as Herring bodies, in PP was similar to that observed by others (33). Most of vasopressin-positive axons and nerve endings were also clearly labeled for Panx2 (Fig. 4C). Double-immunofluorescence labeling of Panx with the folliculostellate cell marker protein S100 indicated that Panx1 could be detected in S100-labeled pituicytes in the PP (Fig. 5A) but was not expressed in S100 immunopositive folliculostellate cells in the AP (data not shown). In contrast, Panx2 was detected in folliculostellate cells in the AP (Fig. 5B) but was not observed in the pituicytes of the PP (data not shown).
Fig. 4.
Double-immunofluorescence labeling of Panx in AP and PP. A, Panx1 (green fluorescence) was highly expressed by ACTH-containing cells (red fluorescence). B, Panx1 (green fluorescence) showed punctate staining in GH-positive cells (red fluorescence). C, Panx2 (green fluorescence) is expressed by most of the vasopressin-containing axonal elements (red fluorescence). Scale bar (applies to all images), 10 μm.
Fig. 5.
Double-immunofluorescence labeling of Panx and S100 protein in the AP and PP. A, Panx1 (green fluorescence) was associated with S100-positive pituicytes (red florescence) in the PP (arrows). B, Panx2 (green fluorescence) was observed in S100-positive (red florescence) foliculostellate cells in the AP (arrows). C, Negative controls of immunolabeling did not result in specific labeling. Scale bar (applies to all images), 5 μm.
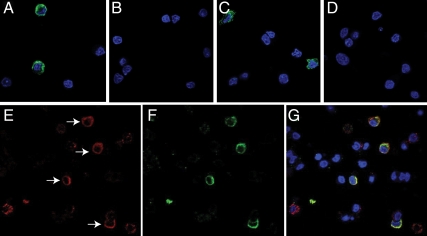
In further experiments, we used immunocytochemical staining to study the presence and distribution of Panx1 and Panx2 proteins in cultured AP cells. The initial dilution study indicated that 1.25 μg/ml anti-Panx1 and anti-Panx2 antibodies was the minimum concentration needed to observe stained cells. Figure 6 shows that only a fraction of cells were positive for Panx 1 (Fig. 6A) and Panx 2 (Fig. 6C). The specificity of staining was confirmed by peptide preabsorption assays, which completely eliminated the Panx1 and Panx2 immunolabeling when the anti-Panx antibodies were preadsorbed with their respectively cognate peptides (Fig. 6, B and D).
Fig. 6.
Immunofluorescence labeling of Panx in cultured AP cells. The presence of Panx1 (A) and Panx2 (C) in a fraction of cells is shown. The immunolableing for both Panx1 (B) and Panx2 (D) was completely eliminated when the anti-Panx antibodies were preadsorbed with their respectively cognate peptides by 1:1 weight ratio. E–G, Double immunocytochemistry of ACTH and Panx1 in primary AP cells. Representative images show ACTH-labeled cells (E, red fluorescence, arrows), Panx1 immunopositive cells (F, green fluorescence), and merged image (G). 4′,6′-Diamino-2-phenylindole (blue fluorescence) denotes the nuclei of cells in all panels.
To further identify cell populations that are positive for Panx1 and Panx2, we performed double immunolabeling of Panx with five different pituitary hormones in cultured AP cells. These experiments confirmed the endogenous Panx1 expression in pituitary cells and that Panx1 was present in corticotrophs (Fig. 6, E–G). Other hormone positive cells had no obvious Panx1 labeling. The lack of Panx1 in immunopositive somatotrophs in immunocytochemistry is consistent with low expression of this protein observed in immunohistochemistry using 2.5 μg/ml anti-Panx1 antibodies. In cultured pituitary cells, none of five secretory cell types express Panx2 (data not shown), indicating that the positively stained cells (Fig. 6C) belong to nonsecretory cell types.
Panx1-mediated ATP release in pituitary cells
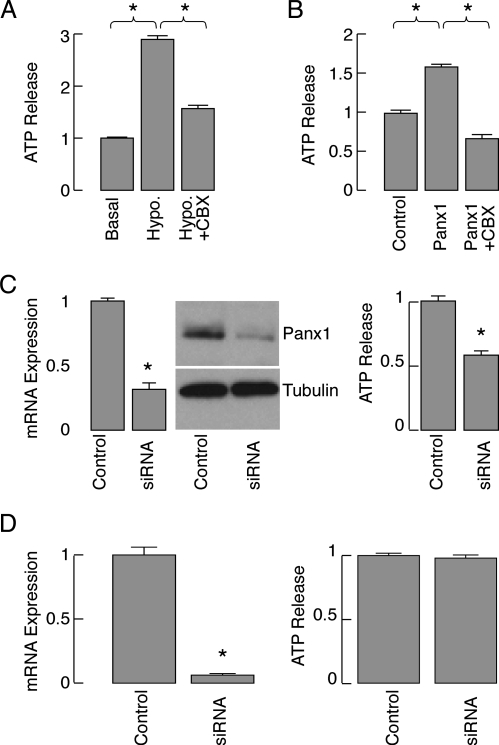
To examine the potential role of Panx in ATP release, we selected the AtT-20 pituitary cell line as an endocrine pituitary cell model because these cells express both Panx1 and Panx2 endogenously. We initially tested the potential role of endogenous Panx1 for ATP release in AtT-20 pituitary cells. As shown in Fig. 7A, an elevated ATP release was observed when the cells were exposed to hypotonic stress, and this increased ATP release could be partially inhibited by coincubation with CBX, which blocks Panx1 channels with higher efficiency than connexin channels (34). Overexpression of Panx1 in these cells also resulted in a significantly increased ATP release compared with vector-plasmid transfected cells when activated by hypotonic stress. This increase was also reduced by CBX treatment (Fig. 7B), confirming the specificity of this compound for Panx1. Similar results were also observed in GH3 cells (data not shown).
Fig. 7.
Panx1-mediated extracellular ATP release in AtT-20 pituitary cells. A, Basal and hypotonic (hypo)-induced ATP release in the presence and absence of 100 μm CBX. Results were normalized with respect to basal ATP release. B, Effects of overexpression of Panx1 on hypotonic-induced ATP release in the presence and absence of 100 μm CBX. Results were normalized with respect to hypotonically induced ATP release by cells transfected with control plasmid. C, left panels, Down-regulation of endogenous Panx1 expression by Panx1 siRNA in AtT-20 cells. These cells were transfected with control siRNA or Panx1 siRNA and the knockdown of endogenous Panx1 expression was examined by real-time PCR (left panels, 48 h after transfection) and Western blot (right panels, 72 h after transfection). C, right panels, Inhibition of ATP release by silencing the endogenously expressed Panx1 with Panx1 siRNA (after 72 h incubation). D, The lack of effect of silencing Panx2 expression on ATP release. Data shown are normalized with respect to controls and are shown as mean ± sem values. *, P < 0.001, estimated by Student's t test.
To further assess the contribution of Panx1 for ATP release in these pituitary cells, endogenous expression of Panx1 in AtT-20 pituitary cells was down-regulated by Panx1 siRNA. After a 48- to 72-h incubation, the expression of Panx1 was substantially reduced at both the RNA and protein levels (Fig. 7C, left panel) and was accompanied with attenuation of ATP release for about 40% (Fig. 7C, right panel). Overexpression of Panx2 in AtT-20 cells also facilitated ATP release (controls cells = 100 ± 2.3%, Panx2 overexpressing cells 135 ± 2%, n = 5). In contrast, silencing Panx2 expression in At-T20 cells for 3 d (Fig. 7D, left panel) did not affect ATP release (Fig. 7D, right panel). These results indicate that Panx contribute to ATP release when expressed at sufficient level.
Discussion
Here we report for the first time that Panx1 and Panx2 mRNA and protein transcripts are endogenously expressed in the rat pituitary gland. These two proteins are also expressed in other regions of the brain (35). In agreement with the finding that Panx3 is predominantly expressed in skin and connective tissue (36, 37), the presence of this protein in pituitary cells was not observed. Our results also indicated that Panx1 and Panx2 were able to form homooligomeric complexes in pituitary tissue, and the Panx1 homomeric oligomers could dissociate into different oligomeric forms by SDS treatment. Immunofluorescence-labeling studies revealed that Panx1 and Panx2 were expressed in the anterior, intermediate, and posterior lobes of rat pituitary. Both immunofluorescence labeling and quantitative RT-PCR studies indicated higher expression of Panx1 in the anterior pituitary, whereas Panx2 was more highly expressed in the IL and PP.
In the anterior lobe, Panx1 was identified in corticotrophs and a fraction of somatotrophs. Immortalized ACTH-producing AtT-20 cells and prolactin-producing GH3 lactosomatotrophs also express Panx1. No expression of Panx1 was observed in lactotrophs, gonadotrophs, and thyrotrophs. We were also unable to detect Panx2 expression in any of five major secretory AP cells, but the nonsecretory folliculostellate cells appear to express this protein, in addition to connexin 43 expression (38). Here we also show the abundant expression of Panx2 in the IL, which is predominantly populated by melanotrophs, as indicated by high immunofluorescence labeling.
The high expression of Panx2 and its presence in neuronal elements in the PP are consistent with the relatively high abundance of this protein in the supraoptic nucleus (39). However, we did not observe significant immunostaining of Panx1 in neuronal elements of the PP, whereas its presence was reported in the supraoptic nucleus (40). In contrast, pituicytes of the posterior lobe express the low level of Panx1. The expression of Panx1 in these glia-type cells is not unique for the PP; Panx1 was previously shown to be expressed in the specialized Bergman glia cells in the cerebellum, and Panx1 and Panx2 transcripts were detected in primary astrocyte cultures (41).
What is the physiological role of Panx in the pituitary gland? In general, the role of these channels in mammals has not been fully characterized. The term used frequently describing these proteins as hemichannels is misleading for mammalian tissues because they do not form the gap junctions. It is more appropriate to call them nonselective channels or megachannels (24). Two functions for these channels have been suggested and both are related to purinergic signaling. Initially, it was suggested that Panx1 is the megapore of P2X7R (42–44). Recent studies, however, questioned this hypothesis and supported the original hypothesis that P2X2R and P2X7R pore dilate (45–47). The functional P2X7R have not been identified in the pituitary gland (3), suggesting that Panx should play a different role in pituitary cells positive for expression of these channels.
More recent reports have suggested the role of these nonselective channels in ATP release in erythrocytes (25), taste buds (26), T cells (27), airway epithelia (28), astrocytes (29), and chondrocytes (30). To test the potential role of these channels in ATP release, we purposely selected AtT-20 immortalized corticotrophs. Like native corticotrophs, these cells express Panx1, but they also express Panx2 endogenously. Silencing the expression of Panx1 but not Panx2 by siRNA reduced the hypotonic-induced ATP release in these cells, which was attenuated by CBX, a specific inhibitor of these channels. Overexpression of both Panx1 and Panx2 in AtT-20 cells significantly elevated ATP release in a CBX-sensitive manner. These results indicate that both Panx can conduct ATP and suggest that in native corticotrophs these channels could provide a pathway for activation of purinergic receptors, which are endogenously expressed in these cells (48). In AtT-20 cells, ATP up-regulates the expression of proopiomelanocortin (49), further indicating the potential physiological relevance of this signaling pathway.
The most puzzling finding shown here is the differential expression of two Panx in corticotrophs and melanotrophs; although of the same origin (50), these cells express different Panx subtypes. Furthermore, the level of expression of Panx2 in IL appears to be higher than in any other part of the pituitary gland. Because the recombinant Panx2 also releases ATP, it is reasonable to suggest that they provide a pathway for release of ATP by melanotrophs. The expression and role of P2XR and P2YR in melanotrophs has not been studied previously, but it is known that these cells express AR (51).
Endogenously released ATP was shown to regulate vasopressin release from neurohypophyseal terminals, but its source and pathways involved in release have not yet been identified (2). Here we have shown that Panx2 is abundantly expressed on vasopressin-containing axons and Herring bodies, suggesting that Panx2 could be involved in ATP release and therefore in control of vasopressin release. On the other hand, the low level expression of Panx in folliculostellate cells and pituicytes, combined with finding that silencing of lowly expressed Panx2 in AtT-20 cells does not affect the background ATP release, argues against the hypothesis that they contribute to the control of ATP release in these two nonendocrine pituitary cells. However, because of their larger conductance (500 pS), only a few channels are required to have a functional impact in ion fluxes (24).
Acknowledgments
Some of the microscopy imaging was performed at the Microscopy and Imaging Core (Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health) with the assistance of Dr. Vincent Schram.
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, and the Serbian Ministry of Science and Technology, project no. 41014.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- AP
- Anterior pituitary
- AR
- adenosine receptor
- AtT-20
- mouse pituitary adrenocorticotropin-secreting cell
- CBX
- carbenoxolone
- C(T)
- cycle threshold
- GH3
- rat immortalized pituitary cell secreting prolactin
- HEK293
- human embryonic kidney 293
- IL
- intermediate lobe
- Panx
- pannexin
- PP
- posterior pituitary
- P2XR
- purinergic P2 receptor channel
- P2YR
- G protein-coupled purinergic P2 receptor
- SDS
- sodium dodecyl sulfate
- siRNA
- small interfering RNA.
References
- 1. Ralevic V, Burnstock G. 1998. Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492 [PubMed] [Google Scholar]
- 2. Knott TK, Marrero HG, Custer EE, Lemos JR. 2008. Endogenous ATP potentiates only vasopressin secretion from neurohypophysial terminals. J Cell Physiol 217:155–161 [DOI] [PubMed] [Google Scholar]
- 3. Stojilkovic SS. 2009. Purinergic regulation of hypothalamopituitary functions. Trends Endocrinol Metab 20:460–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stojilkovic SS, He ML, Koshimizu TA, Balik A, Zemkova H. 2010. Signaling by purinergic receptors and channels in the pituitary gland. Mol Cell Endocrinol 314:184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burnstock G, Knight GE. 2004. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304 [DOI] [PubMed] [Google Scholar]
- 6. Troadec JD, Thirion S. 2002. Multifaceted purinergic regulation of stimulus-secretion coupling in the neurohypophysis. Neuro Endocrinol Lett 23:273–280 [PubMed] [Google Scholar]
- 7. He ML, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. 2005. Release and extracellular metabolism of ATP by ecto-nucleotidase eNTPDase 1–3 in hypothalamic and pituitary cells. Purinergic Signal 1:135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barbe MT, Monyer H, Bruzzone R. 2006. Cell-cell communication beyond connexins: the pannexin channels. Physiology (Bethesda, Md) 21:103–114 [DOI] [PubMed] [Google Scholar]
- 9. Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. 1998. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA 95:15735–15740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morand I, Fonlupt P, Guerrier A, Trouillas J, Calle A, Remy C, Rousset B, Munari-Silem Y. 1996. Cell-to-cell communication in the anterior pituitary: evidence for gap junction-mediated exchanges between endocrine cells and folliculostellate cells. Endocrinology 137:3356–3367 [DOI] [PubMed] [Google Scholar]
- 11. Guérineau NC, Bonnefont X, Stoeckel L, Mollard P. 1998. Synchronized spontaneous Ca2+ transients in acute anterior pituitary slices. J Biol Chem 273:10389–10395 [DOI] [PubMed] [Google Scholar]
- 12. Fauquier T, Guérineau NC, McKinney RA, Bauer K, Mollard P. 2001. Folliculostellate cell network: a route for long-distance communication in the anterior pituitary. Proc Natl Acad Sci USA 98:8891–8896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis BM, Pexa A, Francis K, Verma V, McNicol AM, Scanlon M, Deussen A, Evans WH, Rees DA, Ham J. 2006. Adenosine stimulates connexin 43 expression and gap junctional communication in pituitary folliculostellate cells. FASEB J 20:2585–2587 [DOI] [PubMed] [Google Scholar]
- 14. Meda P, Pepper MS, Traub O, Willecke K, Gros D, Beyer E, Nicholson B, Paul D, Orci L. 1993. Differential expression of gap junction connexins in endocrine and exocrine glands. Endocrinology 133:2371–2378 [DOI] [PubMed] [Google Scholar]
- 15. Belluardo N, Mudò G, Trovato-Salinaro A, Le Gurun S, Charollais A, Serre-Beinier V, Amato G, Haefliger JA, Meda P, Condorelli DF. 2000. Expression of connexin36 in the adult and developing rat brain. Brain Res 865:121–138 [DOI] [PubMed] [Google Scholar]
- 16. Yamamoto T, Hossain MZ, Hertzberg EL, Uemura H, Murphy LJ, Nagy JI. 1993. Connexin43 in rat pituitary: localization at pituicyte and stellate cell gap junctions and within gonadotrophs. Histochemistry 100:53–64 [DOI] [PubMed] [Google Scholar]
- 17. Huang Y, Grinspan JB, Abrams CK, Scherer SS. 2007. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia 55:46–56 [DOI] [PubMed] [Google Scholar]
- 18. Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. 2007. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem 282:31733–31743 [DOI] [PubMed] [Google Scholar]
- 19. Bao L, Locovei S, Dahl G. 2004. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572:65–68 [DOI] [PubMed] [Google Scholar]
- 20. Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. 2009. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem 284:18143–18151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. 2008. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 322:1555–1559 [DOI] [PubMed] [Google Scholar]
- 22. D'hondt C, Ponsaerts R, De Smedt H, Bultynck G, Himpens B. 2009. Pannexins, distant relatives of the connexin family with specific cellular functions? Bioessays 31:953–974 [DOI] [PubMed] [Google Scholar]
- 23. Dubyak GR. 2009. Both sides now: multiple interactions of ATP with pannexin-1 hemichannels. Focus on “A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP.” Am J Physiol Cell Physiol 296:C235–C241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacVicar BA, Thompson RJ. 2010. Non-junction functions of pannexin-1 channels. Trends Neurosci 33:93–102 [DOI] [PubMed] [Google Scholar]
- 25. Locovei S, Bao L, Dahl G. 2006. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA 103:7655–7659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. 2007. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA 104:6436–6441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F. 2008. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal 1:ra6. [DOI] [PubMed] [Google Scholar]
- 28. Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. 2009. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol 41:525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. 2009. Pannexin 1: the molecular substrate of astrocyte “hemichannels.” J Neurosci 29:7092–7097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iwamoto T, Nakamura T, Doyle A, Ishikawa M, de Vega S, Fukumoto S, Yamada Y. 2010. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J Biol Chem 285:18948–18958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-ΔΔC(T)] method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 32. Tóth ZE, Mezey E. 2007. Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J Histochem Cytochem 55:545–554 [DOI] [PubMed] [Google Scholar]
- 33. Morita S, Oohira A, Miyata S. 2010. Activity-dependent remodeling of chondroitin sulfate proteoglycans extracellular matrix in the hypothalamo-neurohypophysial system. Neuroscience 166:1068–1082 [DOI] [PubMed] [Google Scholar]
- 34. Bruzzone R, Barbe MT, Jakob NJ, Monyer H. 2005. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem 92:1033–1043 [DOI] [PubMed] [Google Scholar]
- 35. Litvin O, Tiunova A, Connell-Alberts Y, Panchin Y, Baranova A. 2006. What is hidden in the pannexin treasure trove: the sneak peek and the guesswork. J Cell Mol Med 10:613–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. 2007. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci 120:3772–3783 [DOI] [PubMed] [Google Scholar]
- 37. Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. 2004. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 83:706–716 [DOI] [PubMed] [Google Scholar]
- 38. Horiguchi K, Fujiwara K, Kouki T, Kikuchi M, Yashiro T. 2008. Immunohistochemistry of connexin 43 throughout anterior pituitary gland in a transgenic rat with green fluorescent protein-expressing folliculo-stellate cells. Anat Sci Int 83:256–260 [DOI] [PubMed] [Google Scholar]
- 39. Zappalà A, Li Volti G, Serapide MF, Pellitteri R, Falchi M, La Delia F, Cicirata V, Cicirata F. 2007. Expression of pannexin2 protein in healthy and ischemized brain of adult rats. Neuroscience 148:653–667 [DOI] [PubMed] [Google Scholar]
- 40. Zappalà A, Cicero D, Serapide MF, Paz C, Catania MV, Falchi M, Parenti R, Pantò MR, La Delia F, Cicirata F. 2006. Expression of pannexin1 in the CNS of adult mouse: cellular localization and effect of 4-aminopyridine-induced seizures. Neuroscience 141:167–178 [DOI] [PubMed] [Google Scholar]
- 41. Lai CP, Bechberger JF, Thompson RJ, MacVicar BA, Bruzzone R, Naus CC. 2007. Tumor-suppressive effects of pannexin 1 in C6 glioma cells. Cancer Res 67:1545–1554 [DOI] [PubMed] [Google Scholar]
- 42. Pelegrin P, Surprenant A. 2006. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pelegrin P, Surprenant A. 2009. The P2X(7) receptor-pannexin connection to dye uptake and IL-1β release. Purinergic Signal 5:129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. 2007. Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett 581:483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yan Z, Khadra A, Li S, Tomic M, Sherman A, Stojilkovic SS. 2010. Experimental characterization and mathematical modeling of P2X7 receptor channel gating. J Neurosci 30:14213–14224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yan Z, Li S, Liang Z, Tomić M, Stojilkovic SS. 2008. The P2X7 receptor channel pore dilates under physiological ion conditions. J Gen Physiol 132:563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chaumont S, Khakh BS. 2008. Patch-clamp coordinated spectroscopy shows P2X2 receptor permeability dynamics require cytosolic domain rearrangements but not Panx-1 channels. Proc Natl Acad Sci USA 105:12063–12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Villalobos C, Alonso-Torre SR, Núñez L, García-Sancho J. 1997. Functional ATP receptors in rat anterior pituitary cells. Am J Physiol 273:C1963–C1971 [DOI] [PubMed] [Google Scholar]
- 49. Zhao LF, Iwasaki Y, Oki Y, Tsugita M, Taguchi T, Nishiyama M, Takao T, Kambayashi M, Hashimoto K. 2006. Purinergic receptor ligands stimulate pro-opiomelanocortin gene expression in AtT-20 pituitary corticotroph cells. J Neuroendocrinol 18:273–278 [DOI] [PubMed] [Google Scholar]
- 50. Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT. 2009. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev 30:790–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mei YA, Soriani O, Castel H, Vaudry H, Cazin L. 1998. Adenosine potentiates the delayed-rectifier potassium conductance but has no effect on the hyperpolarization-activated Ih current in frog melanotrophs. Brain Res 793:271–278 [DOI] [PubMed] [Google Scholar]
- 52. Nicke A, Bäumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G. 1998. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J 17:3016–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]