Abstract
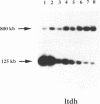
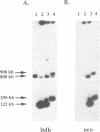
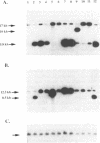
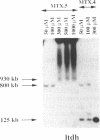
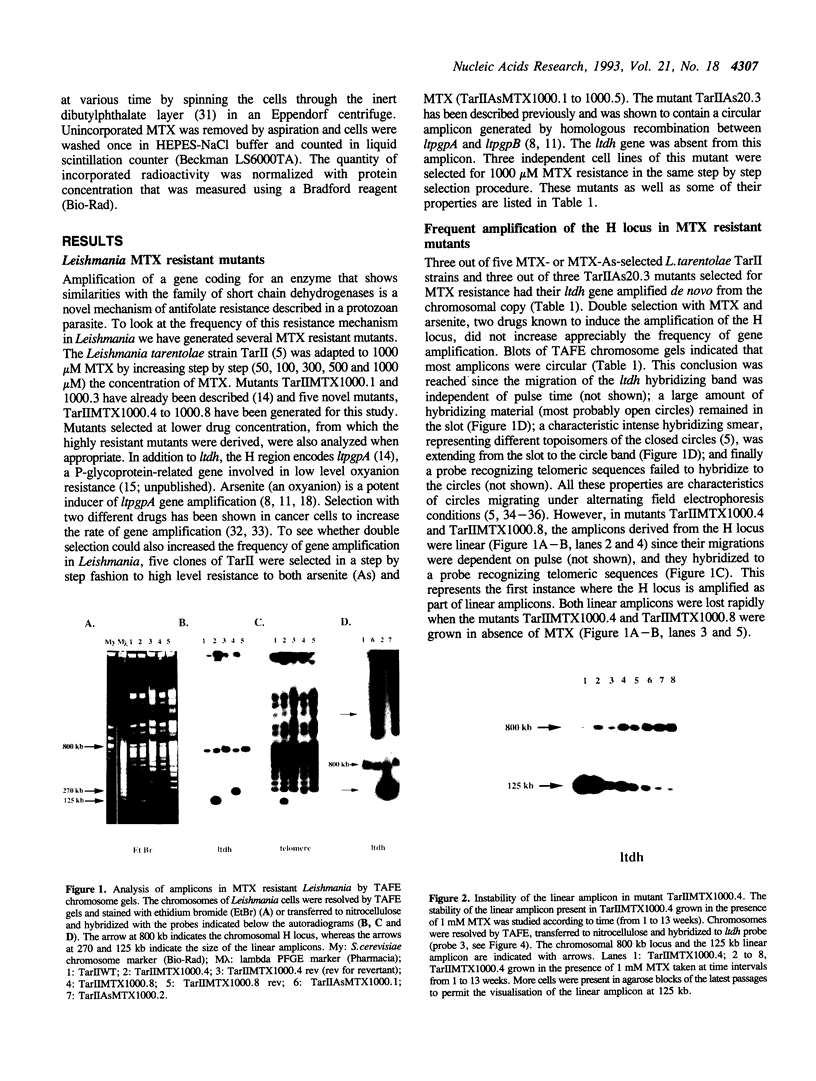
The H locus of Leishmania codes for a short chain dehydrogenase gene (ltdh) that is involved in antifolate resistance. Leishmania tarentolae cells, selected in a step by step fashion for resistance to the antifolate methotrexate (MTX), frequently amplified ltdh in response to drug selection. Both circular and linear extrachromosomal amplicons were generated de novo from the chromosomal H locus and several contained inverted duplications. At least four different rearrangement points were used during the formation of amplicons, with one of them used preferentially. All mutants highly resistant to MTX, whether or not they have the H locus amplified, showed a decreased steady-state accumulation of MTX. Nevertheless, two types of transport mutants were clearly discernable. In the first type, accumulation was reduced four to five-fold, whereas in the second class of mutants, accumulation was reduced more than 50-fold. The ltdh gene was amplified in all the mutants with the transport mutation of the first type, but not in all the mutants with a more pronounced decrease in the steady-state accumulation of MTX. Both types of transport mutation, leading to the reduction in MTX accumulation, arose early during the selection process and were stable even when cells were grown in absence of the drug for prolonged period.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronow B., Kaur K., McCartan K., Ullman B. Two high affinity nucleoside transporters in Leishmania donovani. Mol Biochem Parasitol. 1987 Jan 2;22(1):29–37. doi: 10.1016/0166-6851(87)90066-1. [DOI] [PubMed] [Google Scholar]
- Assaraf Y. G., Feder J. N., Sharma R. C., Wright J. E., Rosowsky A., Shane B., Schimke R. T. Characterization of the coexisting multiple mechanisms of methotrexate resistance in mouse 3T6 R50 fibroblasts. J Biol Chem. 1992 Mar 25;267(9):5776–5784. [PubMed] [Google Scholar]
- Bernards A., Van der Ploeg L. H., Frasch A. C., Borst P., Boothroyd J. C., Coleman S., Cross G. A. Activation of trypanosome surface glycoprotein genes involves a duplication-transposition leading to an altered 3' end. Cell. 1981 Dec;27(3 Pt 2):497–505. doi: 10.1016/0092-8674(81)90391-3. [DOI] [PubMed] [Google Scholar]
- Beverley S. M. Characterization of the 'unusual' mobility of large circular DNAs in pulsed field-gradient electrophoresis. Nucleic Acids Res. 1988 Feb 11;16(3):925–939. doi: 10.1093/nar/16.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M., Coderre J. A., Santi D. V., Schimke R. T. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984 Sep;38(2):431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Beverley S. M. Gene amplification in Leishmania. Annu Rev Microbiol. 1991;45:417–444. doi: 10.1146/annurev.mi.45.100191.002221. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Beverley S. M. A member of the aldoketo reductase family confers methotrexate resistance in Leishmania. J Biol Chem. 1992 Dec 5;267(34):24165–24168. [PubMed] [Google Scholar]
- Callahan H. L., Beverley S. M. Heavy metal resistance: a new role for P-glycoproteins in Leishmania. J Biol Chem. 1991 Oct 5;266(28):18427–18430. [PubMed] [Google Scholar]
- Coderre J. A., Beverley S. M., Schimke R. T., Santi D. V. Overproduction of a bifunctional thymidylate synthetase-dihydrofolate reductase and DNA amplification in methotrexate-resistant Leishmania tropica. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2132–2136. doi: 10.1073/pnas.80.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A., Beverley S. M. Gene replacement in parasitic protozoa. Nature. 1990 Nov 8;348(6297):171–173. doi: 10.1038/348171a0. [DOI] [PubMed] [Google Scholar]
- Detke S., Katakura K., Chang K. P. DNA amplification in arsenite-resistant Leishmania. Exp Cell Res. 1989 Jan;180(1):161–170. doi: 10.1016/0014-4827(89)90220-6. [DOI] [PubMed] [Google Scholar]
- Dewes H., Ostergaard H. L., Simpson L. Impaired drug uptake in methotrexate resistant Crithidia fasciculata without changes in dihydrofolate reductase activity or gene amplification. Mol Biochem Parasitol. 1986 May;19(2):149–161. doi: 10.1016/0166-6851(86)90120-9. [DOI] [PubMed] [Google Scholar]
- Eid J., Sollner-Webb B. Stable integrative transformation of Trypanosoma brucei that occurs exclusively by homologous recombination. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2118–2121. doi: 10.1073/pnas.88.6.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Biochemistry and regulation of folate and methotrexate transport in Leishmania major. J Biol Chem. 1987 Jul 25;262(21):10053–10058. [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Multiple drug resistance and conservative amplification of the H region in Leishmania major. J Biol Chem. 1989 Sep 5;264(25):15094–15103. [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Reductions in methotrexate and folate influx in methotrexate-resistant lines of Leishmania major are independent of R or H region amplification. J Biol Chem. 1987 Oct 5;262(28):13501–13506. [PubMed] [Google Scholar]
- Ellenberger T. E., Wright J. E., Rosowsky A., Beverley S. M. Wild-type and drug-resistant Leishmania major hydrolyze methotrexate to N-10-methyl-4-deoxy-4-aminopteroate without accumulation of methotrexate polyglutamates. J Biol Chem. 1989 Sep 25;264(27):15960–15966. [PubMed] [Google Scholar]
- Giulotto E., Knights C., Stark G. R. Hamster cells with increased rates of DNA amplification, a new phenotype. Cell. 1987 Mar 13;48(5):837–845. doi: 10.1016/0092-8674(87)90080-8. [DOI] [PubMed] [Google Scholar]
- Gomez-Eichelmann M. C., Holz G., Jr, Beach D., Simpson A. M., Simpson L. Comparison of several lizard Leishmania species and strains in terms of kinetoplast minicircle and maxicircle DNA sequences, nuclear chromosomes, and membrane lipids. Mol Biochem Parasitol. 1988 Jan 15;27(2-3):143–158. doi: 10.1016/0166-6851(88)90034-5. [DOI] [PubMed] [Google Scholar]
- Grondin K., Papadopoulou B., Ouellette M. Homologous recombination between direct repeat sequences yields P-glycoprotein containing amplicons in arsenite resistant Leishmania. Nucleic Acids Res. 1993 Apr 25;21(8):1895–1901. doi: 10.1093/nar/21.8.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson S., Beverley S. M., Wagner W., Ullman B. Unstable amplification of two extrachromosomal elements in alpha-difluoromethylornithine-resistant Leishmania donovani. Mol Cell Biol. 1992 Dec;12(12):5499–5507. doi: 10.1128/mcb.12.12.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower R. C., Metge D. W., Santi D. V. Plasmid migration using orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1987 Oct 26;15(20):8387–8398. doi: 10.1093/nar/15.20.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower R. C., Ruiz-Perez L. M., Wong M. L., Santi D. V. Extrachromosomal elements in the lower eukaryote Leishmania. J Biol Chem. 1988 Nov 15;263(32):16970–16976. [PubMed] [Google Scholar]
- Iovannisci D. M., Ullman B. High efficiency plating method for Leishmania promastigotes in semidefined or completely-defined medium. J Parasitol. 1983 Aug;69(4):633–636. [PubMed] [Google Scholar]
- Kaur K., Coons T., Emmett K., Ullman B. Methotrexate-resistant Leishmania donovani genetically deficient in the folate-methotrexate transporter. J Biol Chem. 1988 May 25;263(15):7020–7028. [PubMed] [Google Scholar]
- Laban A., Tobin J. F., Curotto de Lafaille M. A., Wirth D. F. Stable expression of the bacterial neor gene in Leishmania enriettii. Nature. 1990 Feb 8;343(6258):572–574. doi: 10.1038/343572a0. [DOI] [PubMed] [Google Scholar]
- Li W. W., Lin J. T., Tong W. P., Trippett T. M., Brennan M. F., Bertino J. R. Mechanisms of natural resistance to antifolates in human soft tissue sarcomas. Cancer Res. 1992 Mar 15;52(6):1434–1438. [PubMed] [Google Scholar]
- Liu J., Salinas G., Gajendran N., Muthui D., Muyldermans S., Hamers R. DNA recombination associated with short direct repeats in Leishmania mexicana M379. Mol Biochem Parasitol. 1992 Feb;50(2):351–353. doi: 10.1016/0166-6851(92)90233-a. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Borst P. Drug resistance and P-glycoprotein gene amplification in the protozoan parasite Leishmania. Res Microbiol. 1991 Jul-Aug;142(6):737–746. doi: 10.1016/0923-2508(91)90089-s. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Fase-Fowler F., Borst P. The amplified H circle of methotrexate-resistant leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 1990 Apr;9(4):1027–1033. doi: 10.1002/j.1460-2075.1990.tb08206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M., Hettema E., Wüst D., Fase-Fowler F., Borst P. Direct and inverted DNA repeats associated with P-glycoprotein gene amplification in drug resistant Leishmania. EMBO J. 1991 Apr;10(4):1009–1016. doi: 10.1002/j.1460-2075.1991.tb08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M., Papadopoulou B. Mechanisms of drug resistance in Leishmania. Parasitol Today. 1993 May;9(5):150–153. doi: 10.1016/0169-4758(93)90135-3. [DOI] [PubMed] [Google Scholar]
- Papadopoulou B., Roy G., Ouellette M. A novel antifolate resistance gene on the amplified H circle of Leishmania. EMBO J. 1992 Oct;11(10):3601–3608. doi: 10.1002/j.1460-2075.1992.tb05444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrillo-Peixoto M. L., Beverley S. M. Amplified DNAs in laboratory stocks of Leishmania tarentolae: extrachromosomal circles structurally and functionally similar to the inverted-H-region amplification of methotrexate-resistant Leishmania major. Mol Cell Biol. 1988 Dec;8(12):5188–5199. doi: 10.1128/mcb.8.12.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. C., Ling V., Schimke R. T. Frequencies of independent and simultaneous selection of Chinese hamster cells for methotrexate and doxorubicin (adriamycin) resistance. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9261–9264. doi: 10.1073/pnas.84.24.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Debatisse M., Giulotto E., Wahl G. M. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell. 1989 Jun 16;57(6):901–908. doi: 10.1016/0092-8674(89)90328-0. [DOI] [PubMed] [Google Scholar]
- Stuart K. D. Circular and linear multicopy DNAs in Leishmania. Parasitol Today. 1991 Jul;7(7):158–159. doi: 10.1016/0169-4758(91)90119-9. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Liu A. Y., Borst P. Structure of the growing telomeres of Trypanosomes. Cell. 1984 Feb;36(2):459–468. doi: 10.1016/0092-8674(84)90239-3. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Schwartz D. C., Cantor C. R., Borst P. Antigenic variation in Trypanosoma brucei analyzed by electrophoretic separation of chromosome-sized DNA molecules. Cell. 1984 May;37(1):77–84. doi: 10.1016/0092-8674(84)90302-7. [DOI] [PubMed] [Google Scholar]
- White T. C., Fase-Fowler F., van Luenen H., Calafat J., Borst P. The H circles of Leishmania tarentolae are a unique amplifiable system of oligomeric DNAs associated with drug resistance. J Biol Chem. 1988 Nov 15;263(32):16977–16983. [PubMed] [Google Scholar]
- Wilson K., Beverley S. M., Ullman B. Stable amplification of a linear extrachromosomal DNA in mycophenolic acid-resistant Leishmania donovani. Mol Biochem Parasitol. 1992 Oct;55(1-2):197–206. doi: 10.1016/0166-6851(92)90140-f. [DOI] [PubMed] [Google Scholar]
- ten Asbroek A. L., Ouellette M., Borst P. Targeted insertion of the neomycin phosphotransferase gene into the tubulin gene cluster of Trypanosoma brucei. Nature. 1990 Nov 8;348(6297):174–175. doi: 10.1038/348174a0. [DOI] [PubMed] [Google Scholar]
- van der Bliek A. M., Lincke C. R., Borst P. Circular DNA of 3T6R50 double minute chromosomes. Nucleic Acids Res. 1988 Jun 10;16(11):4841–4851. doi: 10.1093/nar/16.11.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]