Abstract
The decision of a neural precursor to stop dividing and begin its terminal differentiation at the correct place, and at the right time, is a crucial step in the generation of cell diversity in the nervous system. Here, we show that the Down's syndrome candidate gene (Mnb/Dyrk1a) is transiently expressed in prospective neurons of vertebrate CNS neuroepithelia. The gain of function (GoF) of Mnb/Dyrk1a induced proliferation arrest. Conversely, its loss of function (LoF) caused over proliferation and cell death. We found that MNB/DYRK1A is both necessary and sufficient to upregulate, at transcriptional level, the expression of the cyclin-dependent kinase inhibitor p27KIP1 in the embryonic chick spinal cord and mouse telencephalon, supporting a regulatory role for MNB/DYRK1A in cell cycle exit of vertebrate CNS neurons. All these actions required the kinase activity of MNB/DYRK1A. We also observed that MNB/DYRK1A is co-expressed with the NOTCH ligand Delta1 in single neuronal precursors. Furthermore, we found that MNB/DYRK1A suppressed NOTCH signaling, counteracted the pro-proliferative action of the NOTCH intracellular domain (NICD), stimulated Delta1 expression and was required for the neuronal differentiation induced by the decrease in NOTCH signaling. Nevertheless, although Mnb/Dyrk1a GoF led to extensive withdrawal of neuronal precursors from the cell cycle, it was insufficient to elicit their differentiation. Remarkably, a transient (ON/OFF) Mnb/Dyrk1a GoF efficiently induced neuronal differentiation. We propose that the transient expression of MNB/DYRK1A in neuronal precursors acts as a binary switch, coupling the end of proliferation and the initiation of neuronal differentiation by upregulating p27KIP1 expression and suppressing NOTCH signaling.
Keywords: Neurogenesis, Neural proliferation, Neuronal differentiation, Down's syndrome, Mouse, Chick
INTRODUCTION
Cell proliferation, cell specification and cell differentiation must be precisely coordinated during nervous system development in order to ensure that the proper number of the diverse cell types is generated in the correct place and at the right time. Accordingly, the developmental programs that control cell lineage specification must be tightly coordinated with the mechanisms that regulate cell cycle progression and terminal differentiation (reviewed by Pituello, 1997; Hollyday, 2001; Bally-Cuif and Hammerschmidt, 2003; Caviness et al., 2003; Cremisi et al., 2003; Ohnuma and Harris, 2003; Guillemot, 2005; Nguyen et al., 2006a; Guillemot, 2007; Agathocleous and Harris, 2009; Okano and Temple, 2009; Pitto and Cremisi, 2010). Overproliferation or premature differentiation of a given cell type or in a specific brain region will alter the balance in cell number between different populations. Such disequilibria will ultimately affect connectivity and cause malfunctions that can lead to tumorigenesis, neuropathologies and mental disorders.
Down's syndrome (DS; trisomy 21) is the most common genetic cause of mental retardation. Brains of individuals with DS are characterized by their reduced size, decreased neuron density in specific regions, dendritic atrophy and spine dysgenesis (for reviews, see Becker et al., 1991; Coyle et al., 1986). The fact that the neuronal deficit is detected in fetuses and children with DS (Wisniewski et al., 1984; Schmidt-Sidor et al., 1990; Larsen et al., 2008) indicates that this pathology originates through alterations in the processes of neurogenesis during development. Interestingly, several studies have identified alterations in neural proliferation and neurogenesis in the forebrain of fetuses with DS and in trisomic DS mouse models (Chakrabarti et al., 2007; Contestabile et al., 2007; Guidi et al., 2008).
MNB/DYRK1A (Minibrain, dual-specificity tyrosine-Y-regulated kinase 1A) is one of the genes harbored within the DS Critical Region (Guimerá et al., 1996; Song et al., 1996), the minimal region of Chromosome 21 that, when present in triplicate, generates most DS phenotypes, including the severe mental retardation (Delabar et al., 1993). Mnb/Dyrk1a is expressed in mouse brain regions that correspond to those in the human brain that are affected in DS. Moreover, MNB/DYRK1A is overexpressed in the brain of fetuses with DS (Guimerá et al., 1999). Numerous studies in humans and experimental models have implicated MNB/DYRK1A overexpression in developmental, cognitive and neurodegenerative phenotypes of DS (for reviews, see Hämmerle et al., 2003a; Dierssen and de Lagrán, 2006; Tejedor and Hämmerle, 2011; Wegiel et al., 2011). Furthermore, truncation of MNB/DYRK1A causes microcephaly in humans (Moeller et al., 2008).
The Mnb/Dyrk1a gene encodes a highly conserved protein kinase (Becker et al., 1998; Galceran et al., 2003). Mutations in the orthologous minibrain (mnb) gene of Drosophila reduce adult brain size, particularly in the optic lobes. This is due to altered proliferation in the neuroepithelial primordia of the larval optic lobes (Tejedor et al., 1995). These phenotypes suggest that MNB regulates neural proliferation and neurogenesis. Expression studies performed in the developing chick and mouse CNS predict sequential functions of Mnb/Dyrk1a in neural progenitors, nascent neurons and differentiating neurons (Hämmerle et al., 2002; Hämmerle et al., 2003b; Hämmerle et al., 2008). Thus, like Drosophila mutants, the brains of Dyrk1a+/− mice are decreased in size in a region-specific manner (Fotaki et al., 2002). Furthermore, inhibition of the MNB/DYRK1A protein kinase interferes with neurite formation (Göckler et al., 2009), an early process in neuronal differentiation. Together, these findings strongly suggest that Mnb/Dyrk1a fulfils several sequential roles in the transition from neural proliferation to neuronal differentiation.
Here, we have used three experimental systems, the prospective spinal cord of the chick, the developing telencephalon in the mouse and cultured PC12 cells, to gain insight into the mechanisms underlying some of these sequential functions fulfilled by MNB/DYRK1A. Interestingly, we found that the transient expression of Mnb/Dyrk1a promotes cell cycle exit by upregulating p27KIP1 (Cdkn1b – Mouse Genome Informatics) transcription and neuronal differentiation by suppressing NOTCH signaling. We discuss how these activities influence the coordination of neural proliferation and neuronal differentiation during vertebrate CNS development, and their possible implications for DS.
MATERIALS AND METHODS
In ovo electroporation of chick embryos
In ovo electroporation of chick embryos was performed essentially as described previously (Hämmerle and Tejedor, 2007), varying only the embryonic stage and the area of the neural tube under study. In brief, cDNAs containing the full coding sequence of Mnb/Dyrk1a (accession number NM_101395), Mnb/Dyrk1a(K188R) (a dead kinase mutant) (Wiechmann et al., 2003), cDelta-1 (accession number NM_204973) or a truncated version (DeltaDN) lacking all but 13 amino acids in the intracellular region (Henrique et al., 1997; Chitnis et al., 1995), were cloned into pCIG, a bicistronic vector that co-expresses nuclear GFP (Megason and McMahon, 2002). The intracellular domain of NOTCH (NICD) was cloned into the pEVRF vector (Matthias et al., 1989) and, in this case, the plasmid was co-transfected with the GFP containing EGFPN1 vector (Clontech).
Normal fertilized chicken eggs (Gallus domesticus) were incubated at 38°C until they had reached HH stage 11-12 (Hamburger and Hamilton, 1951). Plasmid DNA (1-3 μg/μl) was injected into the neural tube and two platinum electrodes were placed in parallel on either side of the neural tube at a distance of 4 mm, at the level of most rostral part of the prospective spinal cord. Five consecutive pulses (40-50 V/50 ms) were then applied to the embryos using an Intrasept TSS10 pulse stimulator (Intracell). DNA concentration and pulse voltage were adjusted in function of the desired transfection efficiency. After electroporation, embryos were incubated at 38°C. Transfection efficiency was tested by in vivo observation of GFP fluorescence under a stereomicroscope. After an adequate incubation period, the embryos were either labeled with BrdU, and/or fixed and processed for immunocytochemistry or fluorescent in situ hybridization as described below.
Ex vivo electroporation of mouse embryos
Mice (Parkes strain) were housed, bred and treated according to the guidelines approved by the Home Office under the Animals (Scientific procedures) Act 1986. Wild-type embryos were obtained from timed matings. Ex vivo electroporation of E14.5 mouse embryos was performed essentially as described previously (Nguyen et al., 2006b). DNA (2 μg/μl) mixed with 0.05% Fast Green (Sigma) was injected into the telencephalic vesicle, and five electrical pulses (50V/50ms) were then applied at 1-second intervals using 5 mm platinum tweezers electrodes (CUY650P5, Nepagene) and an ECM-830 BTX square wave electroporator (BTX, Gentronic). Following electroporation, the brains were dissected out in L15 (Invitrogen) and transferred into liquid 3% low melting agarose (Sigma) at 38°C. After embedding, coronal brain vibratome (250 μm) sections were obtained, and they were transferred onto sterile culture plate inserts (0.4 μm pore size; Millicell-CM, MilliporO) and cultured for 24 hours in semi-dry conditions in wells containing supplemented Neurobasal medium. After fixation and cryoprotection, they were embedded in OCT Compound (VWR) and sectioned coronally (10 μm) on a cryostat. For GoF experiments, we used a full-length MNB/DYRK1A cDNA (Guimerá et al., 1996) inserted into the pCIG-vector. For LoF experiments, we transfected a short Silencer Pre-designed siRNA (Ambion) containing the following 5′-3′Sequence: sense strand GGAUGUAUCUUGGUUGAAAtt, antisense strand UUUCAACCAAGAUACAUCCaa along with pCIG plasmid. As negative controls, samples were transfected with Silencer Select Negative Control siRNAs (Ambion) and no apparent effects were observed.
The efficiency of the Mnb/Dyrk1a constructs and siRNA to modify Mnb/Dyrk1a expression was tested by fluorescent in situ hybridization after transfection of chick and mouse embryos (see Fig. S1 in the supplementary material).
In situ hybridization and immunocytochemistry
Chicken embryos were fixed in 4% paraformaldehyde for 3 hours at room temperature. Whole-mount fluorescent in situ hybridization with RNA probes for cDelta and cHes5 was performed essentially as described previously (Hämmerle and Tejedor, 2007). To detect the probes, an anti-digoxigenin-POD antibody (Roche Molecular Biochemicals) was used in combination with the TSA Plus Fluorescence System (Perkin Elmer). For in situ hybridization of chick p27KIP1, two DIG labeled probes corresponding to positions 660-929 and 942-1232 of the cDNA sequence (Accession NM_204256) were synthesized by PCR. Dual fluorescent in situ hybridization for chick Mnb/Dyrk1a and Delta1 was performed with DIG- and fluorescein-labeled DNA probes (Hämmerle et al., 2002; Hämmerle and Tejedor, 2007), which were detected using a monoclonal anti-digoxigenin antibody (Roche Molecular Biochemicals) in combination with an anti-mouse-Cy3, and with a goat biotin-conjugated anti-fluorescein antibody (Vector Labs) along with Streptavidin Cy2. Appropriate negative controls without either probe but using all the antisera were run in parallel.
For mouse embryos, fluorescent in situ hybridization was carried out in forebrain cryosections as described previously (Sitz et al., 2008), using mouse Mnb/Dyrk1a (Hämmerle et al., 2008; Sitz et al., 2008) and mouse Hes5 (Akazawa et al., 1992) RNA probes.
The conditions were optimized for the use of the antisera against GFP (Invitrogen), phosphorylated-histone H3 (PH3: Upstate Biotechnology), p27KIP1 (clone 57: BD-Transduction Laboratories), neuronal class III β-tubulin (TUJ1: Covance) and activated caspase 3 (Cell Signaling Technology). The Cy2-, Cy3- and Cy5-conjugated secondary antibodies were used as recommended by the supplier (Jackson Immunochemicals). Counterstaining of nuclei was performed with DAPI.
BrdU labeling
Proliferating cells were detected in chick embryos by in ovo incorporation of BrdU as described previously (Hämmerle et al., 2002). Accordingly, 50 μl of a 5 mg/ml solution of BrdU in PBS were applied to the top of the embryo after opening a window in the eggshell. After incubation for 1 hour, the embryos were fixed as described above and cells that had incorporated BrdU were labeled in 40 μm vibratome sections with an antibody against BrdU (Becton Dickinson) and a Cy3-conjugated secondary antibody. Nuclei were visualized with DAPI. Images were acquired on a Leica TCS-SL spectral confocal microscope.
Whole-mount chicken embryo culture and MNB/DYRK1A kinase inhibition
Whole-mount chick embryos were cultured as described previously (Hämmerle and Tejedor, 2002). Briefly, after removing the embryos from the yolk and separating them from the area pellucida, they were immediately transferred into the culture chamber where they were incubated in supplemented neurobasal medium at 38°C with permanent oxygenation. Harmine and Epigallocatechin gallate (EGCG) treatments were performed by adding each drug to the culture medium at a final concentration of 2-5 and 20 μM, respectively, or equivalent volumes of the vehicle solutions to control embryos.
Phenotype and statistical analysis of transfected embryos
Each experiment was performed at least twice. For each series of experiments, the corresponding phenotype was assessed in a minimum of five transfected (or drug treated) embryos that were sectioned by vibratome and processed for immunohistological analysis. The cells labeled by the different markers (BrdU, PH3, p27KIP1, etc.) were counted in serial confocal sections in a minimum of five randomly selected vibratome sections taken from the area of interest from four or five embryos (in most experiments), although only three embryos were required in some experiments showing a highly penetrant phenotype to obtain significant data. The mean proportion of cells expressing specific markers was obtained and the error was calculated as the standard deviation. The statistical significance (P value) between experimental and control samples was determined using the double sided, unpaired Student's t-test.
Transient (ON/OFF) MNB/DYRK1A GoF in PC12 cells
PC12 cells were plated on poly-D-lysine coated coverslips in 24-well plates at a density of 26×104 cells/ml. The cells were transfected with 0.2 μg DNA/well of either the pCIG-MNB/DYRK1A plasmid (experiment) or the empty pCIG vector (control). Transient transfections were carried out with a combination of lipofection (Fugene HD, Roche) and Magnet Assisted Transfection (MA Lipofection Enhancer, Stratech) methods. Harmine was added at a final concentration of 2 μM, either immediately or 24 hours after transfection, and the cells were cultured for an additional 48-hour period. GFP- and TUJ1-labeled cells were counted in a minimum of three dishes.
RESULTS
Gain and loss-of-function of Mnb/Dyrk1a affects proliferation in the chick spinal cord
The influence of Mnb/Dyrk1a on the transition from neural proliferation to neuronal differentiation during vertebrate development was examined by studying the effects of its loss of function (LoF) and gain of function (GoF) in the prospective spinal cord of chick embryos. Owing to the well-defined rostrocaudal gradient of neurogenesis, there is a gradual separation of the cellular processes of proliferation and neurogenesis along the rostrocaudal axis of this tissue (for reviews, see Ericson et al., 1992; Hollyday, 2001; Diez del Corral and Storey, 2004). We focused on the rostral part of the developing spinal cord in HH12 embryos, between the first and fifth somite pair (a region that will be referred to as the neurogenic zone, NZ) where neurons are being generated (see Table S1 in the supplementary material for a summary of experimental results).
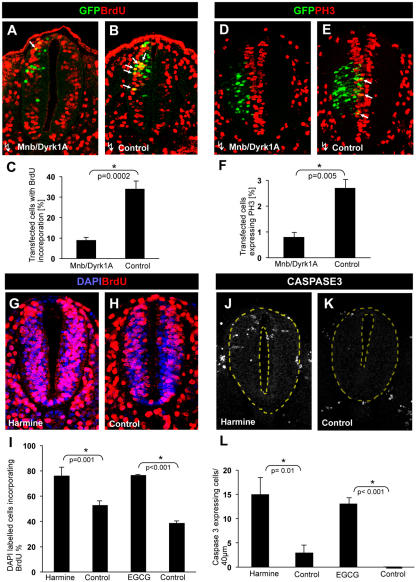
We first tested the effects of transfecting either pCIG-Mnb/Dyrk1a or the empty pCIG vector (control) on the incorporation of BrdU in this area of the neural tube. Ectopic expression of MNB/DYRK1A induced a strong decrease in the number of BrdU-labeled cells 18 hours after transfection (9% versus 34% in control transfected embryos: Fig. 1A-C). Similarly, transfection with Mnb/Dyrk1a induced a significant reduction in the mitotic cells labeled with phosphorylated Histone 3 (PH3) (0.8% versus 2.7% in controls: Fig. 1D-F). Therefore, we concluded that Mnb/Dyrk1a GoF inhibits the proliferation of neuronal progenitors.
Fig. 1.
Effect of the gain and loss of MNB/DYRK1A function on cell proliferation in the chick spinal cord. All images were collected from transverse vibratome sections taken at the level of the rostral spinal cord (NZ) of HH13-16 chick embryos. (A,B) Single confocal sections of embryos transfected with pCIG-Mnb/Dyrk1a or pCIG (controls), and immunostained for BrdU and GFP. Arrows indicate BrdU/GFP double-labeled cells. (D,E) Confocal projections (50 μm) of embryos transfected as above showing immunolabeling for PH3 and GFP. Arrows indicate PH3/GFP double-labeled cells. (G,H,J,K) Confocal projections (9 μm) of embryos cultured in the presence or absence (controls) of harmine (as indicated) showing BrdU (G,H) and activated caspase 3 (J,K) immunostaining. Nuclei were counterstained with DAPI. (C,F,I,L) Statistical analysis of the experiments as indicated. Data are mean±s.e.m.
In order to determine whether this antiproliferative effect was caused by the kinase activity of MNB/DYRK1A, we carried out similar electroporation of chick embryos with a MNB/DYRK1A(K188R) kinase dead mutant construct (Wiechmann et al., 2003). We found that this mutant kinase does not alter proliferation, as assayed by BrdU labeling (see Fig. S2 in the supplementary material), indicating that the antiproliferative effect of the Mnb/Dyrk1a GoF is kinase dependent, as formerly tested in other systems (Funakoshi et al., 2003; Yabut et al., 2010).
Mnb/Dyrk1a LoF experiments in the chick spinal cord were performed using two specific MNB/DYRK1A kinase inhibitors: harmine (Bain et al., 2007; Sitz et al., 2008; Göckler et al., 2009) and EGCG (Bain et al., 2003; Adayev et al., 2006; Guedj et al., 2009) (for a review, see Becker and Sippl, 2011). Culturing chick embryos for 4 hours in the presence of harmine resulted in a strong increase in BrdU incorporation in the NZ (76% versus 52% in untreated embryos: Fig. 1G-I). The same treatment also resulted in an increase in the number of mitotic cells (not shown). EGCG produced comparative results to harmine (Fig. 1I). Thus, the Mnb/Dyrk1a LoF augmented the number of proliferating cells, suggesting that neuronal precursors with decreased MNB/DYRK1A kinase activity were unable to withdraw from the cell cycle.
As altered cell proliferation can lead to apoptosis of neural cells (Becker and Bonni, 2004), we tested the incidence of apoptotic cell death in the chick spinal cord after Mnb/Dyrk1a LoF and GoF using activated caspase 3 as a marker. Reducing MNB/DYRK1A kinase activity with harmine or EGCG resulted in an important increase of apoptosis in the NZ (Fig. 1J-L), whereas the expression of MNB/DYRK1A or MNB/DYRK1A(K188R) did not alter the incidence of apoptosis in the NZ (see Fig. S3 in the supplementary material).
MNB/DYRK1A regulates p27KIP1 expression in embryonic vertebrate neuroepithelia
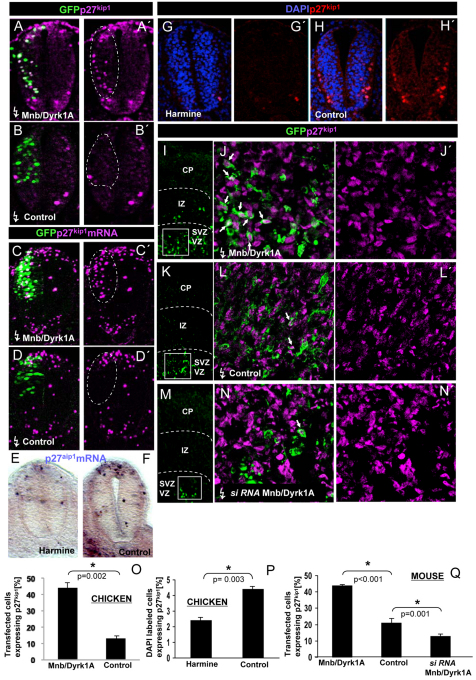
The effects of Mnb/Dyrk1a GoF and LoF on proliferation could reflect its involvement in the regulation of cell cycle exit of neurons. Thus, we analyzed the relationship of MNB/DYRK1A with cyclin-dependent kinase inhibitors (CKIs), the main negative effectors of the cell cycle. In particular, we focused our analysis on p27KIP1, a member of the Cip/Kip family of CKIs that plays an important role in regulating cell cycle exit in the vertebrate CNS (Sherr and Roberts, 1999; Nguyen et al., 2006a), as we have previously observed extensive co-expression with MNB/DYRK1A in the ventricular zone (VZ) of embryonic mouse, where neurons are born (Hämmerle et al., 2008). We initially tested the possible alterations of p27KIP1 expression in the chick spinal cord, where its ectopic expression has been previously found to induce cell cycle arrest of neural progenitors (Gui et al., 2007). We found that the ectopic expression of Mnb/Dyrk1a in the NZ resulted in a strong increase in the proportion of cells expressing p27KIP1 as early as 12 hours after transfection (see Fig. S4A in the supplementary material), although at 18 hours it reaches its maximum induction (44% versus 13% in controls: Fig. 2A,B,O). This is a very rapid response if we consider that Mnb/Dyrk1a expression is first detected by fluorescent in situ hybridization 8 hours after transfection (not shown). Conversely, culturing chick embryos for 6 hours in the presence of harmine significantly reduced the ratio of p27KIP1-positive cells in the NZ (Fig. 2G,H,P). This action of MNB/DYRK1A on p27KIP1 expression seems to happen at the transcriptional level as the GoF of Mnb/Dyrk1a induced an increase in the number of p27KIP1 mRNA-expressing cells 12 hours after transfection, as detected by in situ hybridization (Fig. 2C,D). Conversely, harmine induced a substantial decrease in the number of p27KIP1 mRNA-expressing cells (Fig. 2E,F).
Fig. 2.
Effects of loss and gain of MNB/DYRK1A function on p27KIP1 expression in the chick spinal cord and mouse telencephalon. (A-D′) All images were collected from transverse vibratome sections taken at the level of the rostral spinal cord (NZ) of HH13-16 chick embryos. (A-B′) Confocal projections (10 μm) of embryos transfected with pCIG-Mnb/Dyrk1a or pCIG (controls), and immunolabeled for p27KIP1 and GFP 18 hours after electroporation. Note the appearance of p27KIP1-positive cells in the transfected zone (outlined) in A. (C-D′) Confocal projections (15 μm) of embryos transfected with pCIG-Mnb/Dyrk1a or pCIG (controls), and immunolabeled for GFP after fluorescent in situ hybridization for p27KIP1, 12 hours after electroporation. (E-H′) Embryos cultured in the presence or absence (controls) of harmine for 6 hours and analyzed for the expression of p27KIP1 mRNA by in situ hybridization (E,F) or for protein (G-H′). Note the decrease in the number of p27KIP1-positive cells in harmine-treated embryos. (I,K,M) Coronal confocal sections showing GFP-positive cells at the frontal area of developing cerebral cortex of E14.5 embryos electroporated with pCIG-Mnb/Dyrk1a (Mnb/Dyrk1a GoF), pCIG (control) or Mnb/Dyrk1a siRNA + pCIG (Mnb/Dyrk1a LoF) and cultured afterwards for 24 hours. (J,J′,L,L′,N,N′) High-magnification images taken from the VZ in the boxed areas indicated in I,K,M showing p27KIP1 and GFP immunolabeling. Double GFP/p27KIP1-labeled cells are indicated with arrows. CP, cortical plate; IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone. (O-Q) Statistical analysis of the experiments as indicated. Data are mean±s.e.m.
To explore whether these findings in the chicken spinal cord also apply to other vertebrate neuroepithelia, we performed a similar analysis in the embryonic mouse telencephalon where p27KIP1 is thought to be the main CKI (Nguyen et al., 2006a). Forebrains of E14.5 embryos were electroporated ex vivo with either pCIG-Mnb/Dyrk1a, pCIG or Mnb/Dyrk1a siRNAs + pCIG, and then left in culture. In the case of Mnb/Dyrk1a GoF, we observed a strong increase in the number of transfected cells expressing p27KIP1 when compared with the controls (44% vs 21%: Fig. 2I-L,Q). Conversely, transfecting Mnb/Dyrk1a siRNAs produced a significant decrease in the proportion of p27KIP1 positive cells (13%: Fig. 2K-N,Q). Thus, MNB/DYRK1A seems to be necessary and sufficient to upregulate the expression of p27KIP1 in both the chick spinal cord and in the mouse telencephalon. These findings support a role of MNB/DYRK1A in regulating cell cycle exit of vertebrate neurons during CNS development.
Mnb/Dyrk1a co-expresses with Delta1 and inhibits NOTCH signaling in neurogenic vertebrate neuroepithelia
Given its role in promoting the cell cycle exit of neurons, we wondered whether MNB/DYRK1A might also be involved in the initiation of neuronal differentiation. Accordingly, we explored the possible interaction of MNB/DYRK1A with signaling pathways that control neuronal differentiation.
There is compelling evidence that NOTCH-mediated lateral inhibition is involved in the regulation of neuronal differentiation in the vertebrate CNS (for reviews, see Lewis, 1998; Yoon and Gaiano, 2005; Louvi and Artavanis-Tsakonas, 2006; Kageyama et al., 2009). Following the upregulation of Delta1 expression in individual cells, DELTA1 binds to NOTCH in neighboring cells, which leads to the cleavage of the intracellular domain of NOTCH (NICD) and its translocation to the nucleus. In the nucleus, NICD upregulates the expression of Hes family transcription factors, leading to the maintenance of proliferation and the inhibition of Delta1 expression. The downregulation of Delta1 provides a feedback to the neighboring cells to decrease NOTCH signaling in the Delta1-expressing cell. Thus, the NOTCH-activated cells remain as progenitors while the Delta1-expressing cell that has diminished NOTCH activity differentiates into a neuron.
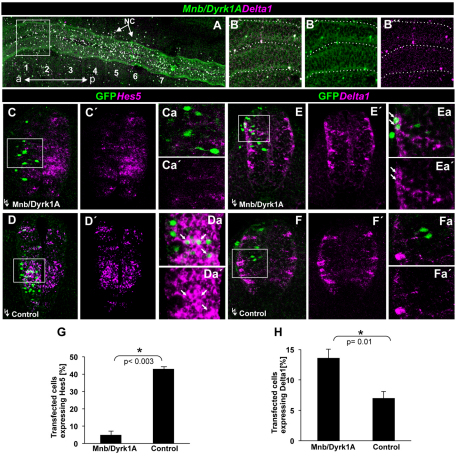
We previously reported that Mnb/Dyrk1a mRNA is expressed in single scattered progenitor cells in the so-called proliferation to neurogenesis transition zone (PNTZ) of the prospective chick spinal cord prior to neurogenesis (approximately at the level of the fourth to seventh somitic pairs in HH10 embryos) (Hämmele et al., 2002). Nevertheless, as we have observed before (Hämmele et al., 2002) and show here in more detail in Fig. 3A, there are also single scattered Mnb/Dyrk1a-expressing cells in the NZ (around the 1st-2nd somite pairs) and, interestingly, more than 80% of these cells co-express mRNA encoding the NOTCH ligand Delta1 (Fig. 3B).
Fig. 3.
Effect of MNB/DYRK1A on DELTA-NOTCH signaling. (A) Confocal projection of the prospective spinal cord of a HH10 whole-mount chick embryo (dorsal view) showing double fluorescent in situ hybridization labeling of Mnb/Dyrk1a and Delta1 in the neural tube, as well as some in migrating neural crest (NC) cells (arrows). (B-B″) Single confocal image at higher magnification of the boxed region at the level of somites 1-2 showing cellular co-labeling in the rostral spinal cord. (C-F′) Confocal projections (10 μM) of HH15-16 chick embryos transfected with pCIG-Mnb/Dyrk1a or pCIG (controls) showing fluorescent in situ hybridization labeling for either HES5 or DELTA1 and GFP inmmunolabeling 15 hours after electroporation. (Ca-Fa′) Single confocal image at higher magnification of the boxed regions showing double-labeled cells (arrows). (G,H) Statistical analysis of the corresponding experiments as indicated. Data are mean±s.e.m.
According to the DELTA-NOTCH model of lateral inhibition, the co-expression of Mnb/Dyrk1a with Delta1 in individual scattered cells of the NZ strongly suggests that it is transiently expressed in prospective neurons. As it has been recently reported that Mnb/Dyrk1a GoF attenuates NOTCH signaling (Fernandez-Martinez et al., 2009), we wondered whether Mnb/Dyrk1a could somehow be involved in the suppression of NOTCH signaling during neurogenesis. If this were the case, the ectopic expression of Mnb/Dyrk1a in the NZ should suppress in a cell-autonomous manner the expression of Hes5, a mediator of NOTCH signaling in the spinal cord at this stage (Fior and Henrique, 2005; Hämmerle and Tejedor, 2007). Indeed, 15 hours after transfection with pCIG-Mnb/Dyrk1a, we observed a strong decrease in the proportion of Hes5-expressing cells (5% versus 42% in pCIG transfected controls: Fig. 3C,D,G). Furthermore, MNB/DYRK1A induced a significant increase of Delta1-expressing cells (14% versus 7% in controls: Fig. 3E,F,H).
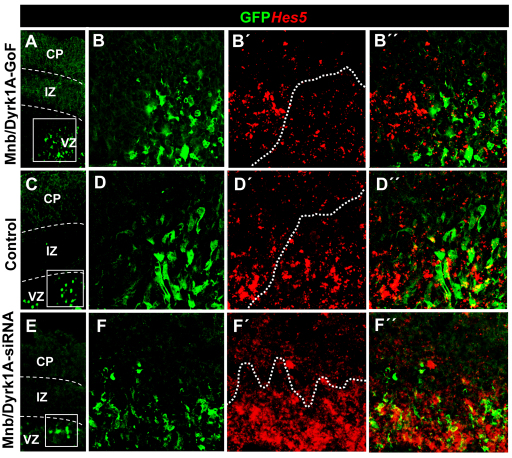
To determine whether Mnb/Dyrk1a could also suppress NOTCH signaling in other vertebrate neuroepithelia, we studied the effects of Mnb/Dyrk1a GoF in the embryonic mouse telencephalon by ex vivo electroporation. As in the chick spinal cord, the ectopic expression of Mnb/Dyrk1a led to a pronounced decrease in Hes5 expression in the VZ (Fig. 4A-D), whereas transfection of mouse embryos with Mnb/Dyrk1a siRNAs produced an increase in Hes5 expression (Fig. 4C-F). Together, these findings strongly suggest that Mnb/Dyrk1a suppresses NOTCH signaling during vertebrate CNS neurogenesis.
Fig. 4.
Effects of the loss and gain of Mnb/Dyrk1a function on Hes5 expression in the mouse telencephalon. (A,C,E) Coronal confocal sections showing GFP-expressing cells at the frontal area of the developing cerebral cortex of E14.5 embryos electroporated with pCIG-Mnb/Dyrk1a (Mnb/Dyrk1a GoF), pCIG (control) or Mnb/Dyrk1a siRNA + pCIG (Mnb/Dyrk1a LoF), and cultured for 24 hours afterwards. (B-B″,D-D″,F-F″) High-magnification images taken from the boxed areas indicated in A,C,E showing fluorescent in situ hybridization labeling for Hes5 and GFP immunolabeling. Note the decrease in the number of Hes5-positive cells within the transfected region (dotted line) after Mnb/Dyrk1a GoF (B-B″), and the increase of Hes5 expression after Mnb/Dyrk1a LoF (F-F″), when compared with the control (D-D″) and with the neighboring non-transfected areas. CP, cortical plate; IZ, intermediate zone; VZ, ventricular zone.
Interaction of MNB/DYRK1A with NOTCH signaling in neural proliferation and neuronal differentiation
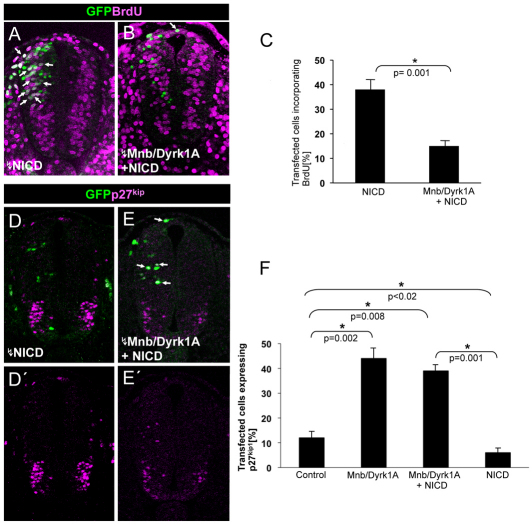
In order to gain insight into the mechanism by which Mnb/Dyrk1a suppresses NOTCH signaling during neurogenesis, we investigated whether MNB/DYRK1A acts upstream or downstream of NICD, as it has been reported that MNB/DYRK1A phosphorylates the NICD in cell lines (Fernandez-Martinez et al., 2009). To this end, we transfected embryonic chick spinal cord cells with Mnb/Dyrk1a + NICD or NICD alone, and we analyzed the capacity of the transfected cells to proliferate. As mentioned previously, NOTCH signaling serves to maintain neural progenitors in a state of proliferation, thereby suppressing neurogenesis, whereas, as shown here, the expression of MNB/DYRK1A promotes cell cycle exit of neuronal precursors. If MNB/DYRK1A were to act upstream of NICD, it should not inhibit proliferation in the presence of exogenous NICD, whereas if it acts downstream, it should be able to suppress proliferation even in the presence of NICD. Not only did MNB/DYRK1A suppress BrdU incorporation in the presence of NICD (Fig. 5A-C: 15% versus 38%) but MNB/DYRK1A was sufficient to revert the inhibitory effect that NICD exerted on the expression of p27KIP1 (Fig. 5D-F: 39% of MNB/DYRK1A+ NICD transfected cells compared with 13% of controls and 6% of NICD transfected cells). These results strongly suggest that MNB/DYRK1A suppresses NOTCH signaling by acting downstream of NICD.
Fig. 5.
The effects of the interaction of MNB/DYRK1A with NOTCH signaling on neural proliferation. (A,B) Confocal projections (10 μm) showing immunolabeling for BrdU and GFP at the level of the spinal cord NZ of HH15-16 chick embryos that were transfected with pEVRF-NICD or pCIG-Mnb/Dyrk1a + pEVRF-NICD. (C) Statistical analysis of the results from this experiment. Although NICD strongly suppresses neuronal differentiation in the spinal cord (Hämmerle and Tejedor, 2007), the proportion of control (pCIG) transfected cells that differentiate at this stage is rather low (7%, control in Fig. 6J) and, accordingly, NICD produced only a slight increase of BrdU incorporation over control transfected cells (compare with control in Fig. 1C). Data are mean±s.e.m. (D,E) Confocal projections (10 μm) showing immunolabeling for p27KIP1 and GFP at the level of the spinal cord NZ of HH15-16 chick embryos that were transfected with pEVRF-NICD or pCIG-Mnb/Dyrk1a + pEVRF-NICD. (F) Statistical analysis of the effect on p27KIP1 expression in the spinal cord NZ of HH15-16 chick embryos transfected with: pCIG (control); pCIG-Mnb/Dyrk1a; pCIG-Mnb/Dyrk1a + pEVRF-NICD; pEVRF-NICD. Both MNB/DYRK1A alone or with NICD greatly increases the proportion of p27KIP1-positive cells, whereas NICD alone significantly decreases the number of cells expressing p27KIP1 compared with the control. Data are mean±s.e.m.
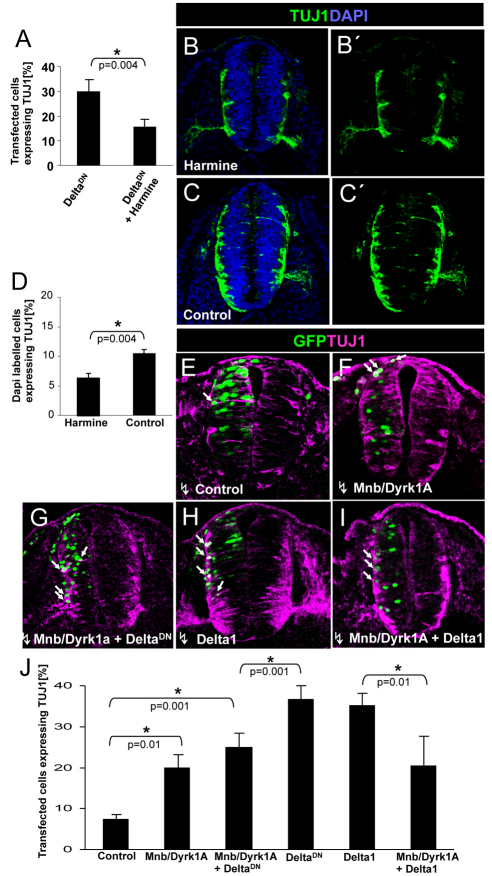
We previously found that transfection with DeltaDN, a truncated form of DELTA that acts as a dominant-negative suppressor of NOTCH signaling in a cell-autonomous manner (Sun and Artavanis-Tsakonas, 1996), was sufficient to induce neuronal differentiation in the NZ (Hämmerle and Tejedor, 2007). Given the capacity of MNB/DYRK1A to suppress NOTCH signaling, we hypothesized that MNB/DYRK1A could be required for the neuronal differentiation induced by inhibiting NOTCH signaling. Accordingly, we tested whether harmine could inhibit the neuronal differentiation induced by DeltaDN. We found that exposure to harmine of DeltaDN transfected embryos strongly precluded the expression of the neuronal marker TUJ1 (15% versus 30% in controls: Fig. 6A). Thus, we concluded that the differentiation induced by the decrease of NOTCH signaling requires MNB/DYRK1A kinase function. Furthermore, the incubation of non-transfected embryos with harmine significantly reduced the proportion of endogenous TUJ1-expressing cells (6% versus 10% in untreated: Fig. 6B-D) indicating that MNB/DYRK1A kinase function is required for neuronal differentiation.
Fig. 6.
Effects of MNB/DYRK1A LoF or GoF on neuronal differentiation induced by the suppression of NOTCH signaling. (A) HH11-12 embryos were transfected with pCIG-DeltaDN in the NZ and, after 3 hours in ovo, embryos were cultured in the presence or absence of harmine for 15 hours. Data are mean±s.e.m. (B-C′) Representative images showing TUJ1 immunolabeling and DAPI counterstaining in sections of the rostral spinal cord from HH11-12 embryos that were cultured for 12 hours in the presence or absence of harmine. (D) Quantitative analysis of this experiment. Data are mean±s.e.m. (E-I) Representative images showing TUJ1 immunolabeling and GFP in sections of the rostral spinal cord from HH11-12 embryos that were transfected with the pCIG vector carrying the cDNAs indicated, and analyzed 18 hours afterwards. Arrows indicate the transfected cells expressing TUJ1. (J) Statistical analysis of the effect of the indicated constructs on TUJ1 expression in the NZ. Data are mean±s.e.m.
Transient but not maintained GoF of MNB/DYRK1A induces neuronal differentiation
As MNB/DYRK1A induces cell cycle exit and suppresses NOTCH signaling, we tested whether MNB/DYRK1A expression might be sufficient to induce neuronal differentiation. The ectopic expression of MNB/DYRK1A in the NZ moderately increased TUJ1 expression (20% versus 7% in controls: Fig. 6E,F,J). However, the proportion of TUJ1-expressing cells (20%) among MNB/DYRK1A transfected cells was clearly lower than that of p27KIP1 expressing cells (44%; compare Fig. 2O with Fig. 6J), indicating that only a small part of the cells that exited the cell cycle due to MNB/DYRK1A expression began to differentiate. As the time required for TUJ1 expression (i.e. the beginning of neuronal differentiation) must be longer than that for p27KIP1 expression (i.e. cell cycle exit), we repeated the experiment but analyzing the cells 36 hours after transfection (instead of 18 hours). After this longer time period, the ratio of TUJ1-positive cells did not increase and the ratio of p27KIP1 positive cells did not decrease (see Fig. S4A,B in the supplementary material). Furthermore, we did not find indications of increased cell death in the MNB/DYRK1A-transfected cells (see Fig. S4C in the supplementary material). Together, these results indicate that only a small proportion of the cells that stop proliferating in response to the GoF of Mnb/Dyrk1a by upregulating p27KIP1 expression, subsequently differentiate.
These data suggest that MNB/DYRK1A causes the precursors to withdraw from the cell cycle while keeping them in a quiescent state prior to their differentiation. Indeed, co-transfection with Mnb/Dyrk1a induced a significant decrease in the proportion of TUJ1-positive cells generated by DeltaDN (Fig. 6G,J), without altering the proportion of p27KIP-positive cells induced by MNB/DYRK1A (not shown). Interestingly, TUJ1 expression was also strongly suppressed when we co-transfected Mnb/Dyrk1a with Delta1 (Fig. 6H-J), indicating that MNB/DYRK1A blocked the transition from cell cycle exit to differentiation when this was induced by both lateral inhibition (DELTA1) and cell-autonomous suppression of NOTCH signaling (DeltaDN). These findings strongly suggest that MNB/DYRK1A may block the transition from cell cycle exit to differentiation by maintaining the cells in a quiescent state. This could explain the weak effect that the Mnb/Dyrk1a GoF alone had on neuronal differentiation (Fig. 6E,J), although it does not fit readily with the clear requirement of the MNB/DYRK1A kinase for neuronal differentiation (Fig. 6A-D). Although intriguing, this finding is not completely surprising as previous studies on neural cell lines have found that the transient expression or activation of MNB/DYRK1A induced neuronal differentiation (Yang et al., 2001; Kelly and Rahmani, 2005), whereas its stable overexpression impaired differentiation (Park et al., 2007). The pattern of MNB/DYRK1A expression in the embryonic telencephalon of mice may provide some clues to resolve this apparent contradiction. We have previously shown that MNB/DYRK1A was frequently co-expressed with p27KIP1 in cells located in apical positions of the VZ, whereas it was mostly absent from TUJ1-positive cells in basal positions (Hämmerle et al., 2008). This suggests that MNB/DYRK1A is expressed in prospective neurons as they exit the cell cycle, and that it is downregulated as these newborn neurons begin to differentiate and migrate out of the VZ. Accordingly, we hypothesized that the upregulation of Mnb/Dyrk1a expression drives the neuronal precursors out of the cell cycle and that Mnb/Dyrk1a expression is then down-regulated to allow these cells to differentiate. Thus, when Mnb/Dyrk1a was expressed under the control of an exogenous promoter, there was no downregulation of Mnb/Dyrk1a and the transition to differentiation was blocked after cell cycle exit.
To test this hypothesis, we designed a transient (ON/OFF) GoF experiment aimed at inhibiting MNB/DYRK1A kinase activity some time after inducing Mnb/Dyrk1a expression by transfection. We first assessed the effects of this protocol in PC12 cells, a suitable model for neuronal differentiation. Thus, PC12 cells were transfected with Mnb/Dyrk1a and the effects of inhibiting MNB/DYRK1A activity with harmine on TUJ1 expression were analyzed at different times after transfection. Significantly, the addition of harmine 24 hours after transfection of MNB/DYRK1A strongly enhanced TUJ1 labeling (see Fig. S5 in the supplementary material), in contrast to the lack of effect when harmine was added immediately after transfection (not shown). These results stimulated us to employ the same approach in the embryonic chick spinal cord. Accordingly, chick embryos were transfected with Mnb/Dyrk1a and, after 24 hours, the transfected embryos were cultured in the presence or absence of harmine for 6 hours before testing TUJ1 expression. We found that exposure to harmine induced a strong increase of TUJ1 expression in Mnb/Dyrk1a transfected cells (39% versus 20% in untreated/transfected embryos: Fig. 7). Thus, we concluded that the transient (ON/OFF) GoF of Mnb/Dyrk1a is sufficient to promote cell cycle exit and the onset of neuronal differentiation.
Fig. 7.
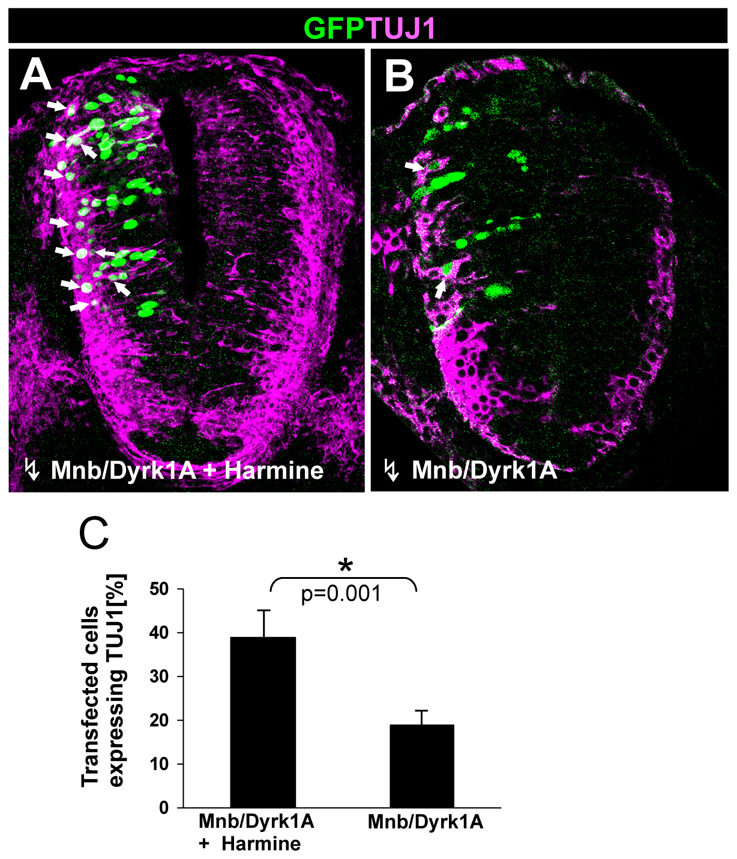
Effect of the transient (ON/OFF) MNB/DYRK1A GoF on neuronal differentiation. (A,B) Confocal projections (10 μm) of transverse sections from the spinal cord NZ in HH11-12 chick embryos that were transfected with pCIG-Mnb/Dyrk1a and, after 24 hours in ovo, cultured for 6 hours in the presence or absence of harmine as indicated. Arrows indicate GFP/TUJ1 double-labeled cells. (C) Statistical analysis of the data from this experiment. Note the important increase in the ratio TUJ1-positive cells that was achieved when MNB/DYRK1A transfection was followed by harmine treatment. Data are mean±s.e.m.
DISCUSSION
MNB/DYRK1A promotes cell cycle exit of neuronal precursors by upregulating p27KIP1 expression
Regulation of the cell cycle exit of neural precursor appears to be crucial for correct neurogenesis. Thus, it is well known that during nervous system development, several key factors that regulate cell cycle progression influence neural cell fate and conversely, several cell determination factors regulate the cell cycle. Furthermore, important cell fate decisions appear to be taken by precursor cells during their last cell cycle (for reviews, see Bally-Cuif and Hammerschmidt, 2003; Cremisi et al., 2003; Ohnuma and Harris, 2003; Nguyen et al., 2006a).
We have shown here that MNB/DYRK1A is necessary and sufficient to induce the expression of p27KIP1 in nascent neurons. p27KIP1 belongs to the Cip/Kip family of CKIs that bind to and inhibit the cyclin/cyclin-dependent kinases (CDKs) complex that control G1/S transition, playing a crucial role in regulating cell cycle exit (for a review, see Sherr and Roberts, 1999). Thus, p27Kip1 expression has been associated with controlling the timing of the birth of mammalian cortical neurons (Caviness et al., 2003; Lukaszewicz et al., 2005). Indeed, p27KIP1 LoF increases cell proliferation and causes brain hyperplasia (Fero et al., 1996), as well as the increased production of late-born neurons (Goto et al., 2004). Thus, the induction of p27KIP1 together with the effects of the Mnb/Dyrk1a LoF and GoF in proliferation, fit with the idea that MNB/DYRK1A promotes the cell cycle exit of CNS neurons by upregulating the expression of p27KIP1. The fact that harmine inhibits p27KIP1 expression strongly suggests that the protein kinase activity of MNB/DYRK1A is required for this function. Post-translational phosphorylation of p27KIP1 by different kinases is crucial for cell cycle regulation (Sherr and Roberts, 1999). For example, MIRK/DYRK1B, the closest MNB/DYRK1A homologue, phosphorylates p27KIP1, which regulates its stability/activity in tumor cells (Deng et al., 2004). By contrast, MNB/DYRK1A regulates the expression of p27KIP1 at transcriptional level. The known capacity of the MNB/DYRK1A kinase to translocate to the nucleus and to phosphorylate several transcription factors (for reviews, see Galceran et al., 2003; Tejedor and Hämmerle, 2011) can explain this action. Nevertheless, the mechanism downstream of the MNB/DYRK1A kinase involved in this regulation remains to be elucidated.
It has been recently reported that the overexpression of MNB/DYRK1A inhibits proliferation of neural progenitors derived from human embryonic stem cells (Park et al., 2010) and in the developing mouse cerebral cortex (Yabut et al., 2010) through p53 phosphorylation followed by induction of p53 target genes (e.g. p21CIP1) (Park et al., 2010) and by the nuclear export and degradation of Cyclin D1 (Yabut et al., 2010). Although the biological relevance of these two mechanisms for the regulation of the end of progenitor proliferation remain to be demonstrated by LoF experiments, it is possible that they can co-exist with the mechanism that we have here shown (i.e. p27KIP1 expression). For example, CyclinD1 and p27KIP1 (and p21CIP1) are positive and negative regulators of the G1/S phase transition, respectively. Thus, MNB/DYRK1A may act bi-directionally to ensure the precise timing of cell cycle exit, promoting p27KIP1 (and p21CIP1) expression and downregulating cyclin D1. Nevertheless, the fact that MNB/DYRK1A induced p27KIP1 expression in only a subset of neural precursors, indicates that different mechanisms might operate in different neural progenitor populations.
MNB/DYRK1A suppresses NOTCH signaling for neuronal differentiation
As mentioned above, the canonical NOTCH signaling (i.e. DELTA-NOTCH lateral inhibition) is used in vertebrate neuroepithelia to maintain a pool of progenitors (those cells receiving NOTCH signal) and to select a subset of cells (those upregulating Delta1 expression) that will differentiate into neurons. In this manner, NOTCH signaling controls the balance between neurogenic progenitors and neuronal precursors, permitting the stepwise specification of distinct neuronal populations during development. Thus, the loss of NOTCH signaling results in precocious neurogenesis at the expense of maintaining progenitor cells and, conversely, activation of NOTCH signaling (with NICD) inhibits neurogenesis and maintains progenitor proliferation (for reviews, see Lewis, 1998, Yoon and Gaiano, 2005; Louvi and Artavanis-Tsakonas, 2006; Kageyama et al., 2009).
We have shown here that MNB/DYRY1A suppresses NOTCH signaling in a cell-autonomous manner and that the MNB/DYRK1A kinase is required for the neuronal differentiation induced by the suppression of NOTCH signaling. These findings strongly suggest that the MNB/DYRK1A kinase plays a key role in neuronal differentiation by negatively regulating NOTCH signaling in CNS neuronal precursors. Interestingly, MNB/DYRK1A upregulates Delta1 expression, although we do not know whether this is a direct effect of MNB/DYRK1A or if it is caused indirectly through the suppression of NOTCH signaling. In any case, the consequence is that MNB/DYRK1A could enhance the differences in the levels of Delta1 expression among neighboring cells, thereby amplifying the lateral inhibition feedback process and facilitating neuronal specification of the DELTA1-positive/NOTCH-signal-suppressed cell.
In addition, our finding that MNB/DYRK1A precludes the pro-proliferative effects of NICD in the chick spinal cord is, in principle, compatible with the mechanism proposed by Fernandez-Martinez et al. that MNB/DYRK1A attenuates NOTCH signaling by phosphorylating the NICD in cell lines. Nevertheless, further studies must be performed in the CNS, to define the molecular mechanisms underlying the inhibition of NOTCH signaling by the MNB/DYRK1A kinase.
MNB/DYRK1A couples cell cycle exit to neuronal differentiation
A complete account of the process of neurogenesis comprises several sequential cellular steps. Initially, neural progenitors (NPs) proliferate (self-replicate) through proliferative divisions. Afterwards, they begin to divide in a neurogenic manner, giving rise to a new NP and a precursor cell that exits the cycle and differentiates as a neuron (for reviews, see Caviness et al., 2003; Götz and Huttner, 2005; Agathocleous and Harris, 2009; Okano and Temple, 2009). These sequential steps need to be coordinated in order to generate the correct number and type of neurons at the right place and time. Thus, cell cycle exit must be precisely coupled to terminal differentiation (Caviness et al., 2003; Cremisi et al., 2003; Ohnuma and Harris, 2003; Nguyen et al., 2006a; Pitto and Cremisi, 2010). Several studies have shown that cell cycle exit is insufficient to trigger neuronal differentiation of neuronal progenitors (Garcia-Dominguez et al., 2003; Gui et al., 2007; Bel-Vialar et al., 2007; Hämmerle and Tejedor, 2007). For example, we previously found that suppressing NOTCH signaling in preneurogenic progenitors in the so called transition zone of the prospective spinal cord of early chick embryos arrested proliferation but it did not elicit neuronal differentiation. By contrast, similar suppression in the NZ efficiently induced neuronal differentiation once the rostrocaudal wave of differentiation had reached that region (Hämmerle and Tejedor, 2007). Similarly, it was shown that Pax6 upregulation is sufficient to push neural progenitors toward cell cycle exit. Nevertheless, these neuronal precursors fail to perform neuronal differentiation until Pax6 is turned off (Bel-Vialar et al., 2007).
Evidence from several experimental systems points to various sequential functions of MNB/DYRK1A in CNS development at the transition from proliferation to neuronal differentiation (for a review, see Tejedor and Hämmerle, 2011). Thus, we have previously shown that Mnb/Dyrk1a is transiently expressed in preneurogenic NPs of the chick spinal cord at the transition from proliferative to neurogenic divisions and it is asymmetrically segregated during cell division into one of the daughter cells (Hämmerle et al., 2002). These data suggest that MNB/DYRK1A may act as a cell determinant of neurogenesis. Accordingly, neural stem cells from Dyrk1a+/− mice exhibit defects in self-renewal (Ferrón et al., 2010).
We have shown here that subsequent to the mentioned expression in preneurogenic NPs, Mnb/Dyrk1a is again transiently expressed in prospective neurons. We propose that this second transient (ON/OFF) expression of MNB/DYRK1A functions as a binary switch to couple the end of proliferation, through the upregulation of p27KIP1 expression, with the initiation of neuronal differentiation by negatively regulating NOTCH signaling (see Fig. 8 for a schematic model). Furthermore, our experiments support the hypothesis that this transient expression of Mnb/Dyrk1a is required in neuronal precursors for the transition from cell cycle exit to neuronal differentiation. Thus, in contrast to the LoF of p27KIP1 that produces brain hyperplasia in the mouse (Fero et al., 1996) and Drosophila (Wallace et al., 2000), Mnb/Dyrk1a LoF decreases the number of neurons in both organisms (Tejedor et al., 1995; Fotaki et al., 2002). This can be explained because, as shown here, the Mnb/Dyrk1a LoF primarily causes overproliferation during CNS neurogenesis but it also induces extensive apoptosis that eliminates the supernumerary cells, leading to neuronal deficit. This cell death is functionally different from that caused by the LoF of MNB/DYRK1A during retina development, which is mediated by phosphorylation of caspase 9 without effects on proliferation or specification (Laguna et al., 2008). Moreover, the fact that the LoF of MNB/DYRK1A kinase does not induce cell death in differentiating mouse brain neurons (Göckler et al., 2009) suggests that the apoptotic cell death is induced on CNS neuronal precursors when cell cycle exit and neuronal differentiation are uncoupled by the LoF of MNB/DYRK1A.
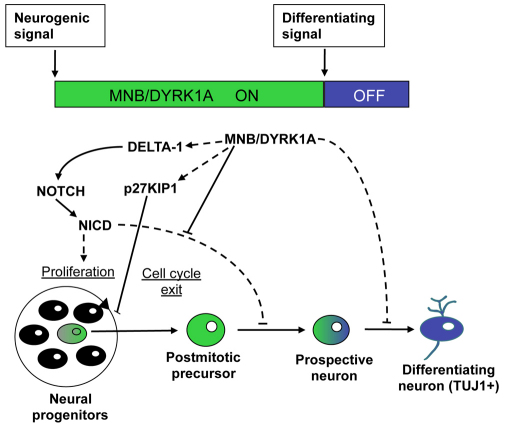
Fig. 8.
A working model: MNB/DYRK1A functions as a binary switch in the coupling of cell cycle exit and neuronal differentiation. During neurogenesis, NOTCH signaling maintains neural progenitors in proliferation and inhibits neuronal differentiation. MNB/DYRK1A is transiently (ON/OFF) expressed in single neuronal precursors. The upregulation of MNB/DYRK1A expression (ON phase, presumably caused by neurogenic signals) promotes cell cycle exit by upregulating p27KIP1 expression. In addition, MNB/DYRK1A suppresses NOTCH signaling and upregulates Delta1 expression, which increases NOTCH signaling in the neighboring cells and reinforces the feedback loop of lateral inhibition, thereby facilitating the generation of prospective neurons that remain in a quiescent state while MNB/DYRK1A expression level is high. Subsequently, MNB/DYRK1A is downregulated (OFF phase, presumably caused by differentiating signals), allowing the prospective neuron to differentiate.
We hypothesize that the transient (ON/OFF) expression of Mnb/Dyrk1a may withdraw neuronal precursors from the cell cycle and keep them in a quiescent state, opening a time window in which they could receive other signals, thereby providing spatiotemporal specificity to the differentiation process. The capacity of MNB/DYRK1A to respond to different signaling pathways (for reviews, see Galceran et al., 2003; Park et al., 2009) makes this scenario possible. It will therefore be important to study in detail the mechanisms regulating the ON/OFF of Mnb/Dyrk1a expression and the signaling pathways that interact with MNB/DYRK1A during neurogenesis.
Possible implications for Down's syndrome
Based on its function in neural proliferation/neurogenesis and its overexpression in the brain of fetuses with DS, MNB/DYRK1A has been widely proposed to be involved in the neuronal deficits of DS (for reviews, see Hämmerle et al., 2003a; Dierssen and de Lagrán, 2006; Tejedor and Hämmerle, 2011). Given the function of MNB/DYRK1A in the coupling of cell cycle exit and neuronal differentiation shown here, the overexpression of MNB/DYRK1A in the DS brain could predictably cause premature neurogenesis and depletion of the neural progenitor pool, contributing to neuronal deficit. The overexpression of MNB/DYRK1A could also alter neurogenesis by increasing p27KIP1 levels in progenitor cells as the overexpression of p27KIP1 can extend the G1 phase in mouse cortical progenitors (Mitsuhashi et al., 2001). Indeed, such lengthening was found to be sufficient to induce the switch from proliferative to neurogenic divisions (Calegari et al., 2005). Strikingly, a brain hyperplasia phenotype has been reported for a (YAC) transgenic mouse model of partial trisomy 21 overexpressing five human genes, one of them MnB/DYRK1A (Branchi et al., 2004; Sebrié et al., 2008), However, transgenic mice overexpressing only MNB/DYRK1A do not exhibit such brain alterations (Altafaj et al., 2001). Thus, further work with other DS experimental models will be required to assess the involvement of MNB/DYRK1A in the neuronal deficit of DS.
Supplementary Material
Acknowledgements
We are very grateful to Emilie Pacary for the help with ex vivo electroporation, to C. Papadopoulos for the help with PC12 cell culture and to members of our labs for interesting discussions. We thank E. Llorens for expert technical assistance. This work was supported by Deutsche Forschungsgemeinschaft grants (BE 1967/2-1) to W.B. and (SFB-870) J.G., by a grant in aid from the Medical Research Council (U117570528) to F.G., by grants from the SpanishMinistry of Science and Innovation, the Generalitat Valenciana and the Fundación Inocente Inocente to F.J.T., and by a join grant from the Fondation Jerôme Lejeune to W.B. and F.J.T. B.H. was recipient of two Travel-Fellowships of the Generalitat Valenciana. We are indebted to M. L. Montesinos for critically reading the manuscript. Deposited in PMC for release after 6 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.066167/-/DC1
References
- Adayev T., Chen-Hwang M. C., Murakami N., Wegiel J., Hwang Y. W. (2006). Kinetic properties of a MNB⁄DYRK1A mutant suitable for the elucidation of biochemical pathways. Biochemistry 45, 12011-12019. [DOI] [PubMed] [Google Scholar]
- Agathocleous M., Harris W. A.(2009). From progenitors to differentiated cells in the vertebrate retina. Annu. Rev. Cell Dev. Biol. 25, 45-69. [DOI] [PubMed] [Google Scholar]
- Akazawa C., Sasai Y., Nakanishi S., Kageyama R. (1992). Molecular characterization of a rat negative regulator with a basic helix-loop-helix structure predominantly expressed in the developing nervous system. J. Biol. Chem. 267, 21879-21885. [PubMed] [Google Scholar]
- Altafaj X., Dierssen M., Baamonde C., Martí E., Visa J., Guimerà J., Oset M., González J. R., Flórez J., Fillat C., et al. (2001) Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A (minibrain), a murine model of Down's syndrome. Hum. Mol. Genet. 10, 1915-1923 [DOI] [PubMed] [Google Scholar]
- Bain J., McLauchlan H., Elliott M., Cohen P. (2003). The specificities of protein kinase inhibitors: an update. Biochem. J. 371, 199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J., Plater L., Elliott M., Shpiro N., Hastie C. J., McLauchlan H., Klevernic I., Arthur J. S., Alessi D. R., Cohen P. (2007). The selectivity of protein kinase inhibitors: a further update. Biochem. J. 408, 297-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally-Cuif L., Hammerschmidt M. (2003). Induction and patterning of neuronal development, and its connection to cell cycle control. Curr. Opin. Neurobiol. 13, 16-25. [DOI] [PubMed] [Google Scholar]
- Becker E. B., Bonni A. (2004). Cell cycle regulation of neuronal apoptosis in development and disease. Prog. Neurobiol. 72, 1-25. [DOI] [PubMed] [Google Scholar]
- Becker L., Mito T., Takashima S., Onodera K. (1991). Growth and development of the brain in Down Syndrome. Prog. Clin. Biol. Res. 373, 133-152. [PubMed] [Google Scholar]
- Becker W., Sippl W. (2011). Activation, regulation, and inhibition of DYRK1A. FEBS J. 278, 246-256. [DOI] [PubMed] [Google Scholar]
- Becker W., Weber Y., Wetzel K., Eirmbter K., Tejedor F. J., Joost H. G. (1998). Sequence characteristics, subcellular localization, and substrate specificity of DYRK-related kinases, a novel family of dual specificity protein kinases. J. Biol. Chem. 273, 25893-25902. [DOI] [PubMed] [Google Scholar]
- Bel-Vialar S., Medevielle F., Pituello F. (2007). The on/off of Pax6 controls the tempo of neuronal differentiation in the developing spinal cord. Dev. Biol. 305, 659-673. [DOI] [PubMed] [Google Scholar]
- Branchi I., Bichler Z., Minghetti L., Delabar J. M., Malchiodi-Albedi F., Gonzalez M. C., Chettouh Z., Nicolini A., Chabert C., Smith D. J., et al. (2004). Transgenic mouse in vivo library of human Down syndrome critical region 1, association between DYRK1A overexpression, brain development abnormalities, and cell cycle protein alteration. J. Neuropathol. Exp. Neurol. 63, 429-440. [DOI] [PubMed] [Google Scholar]
- Calegari F., Haubensak W., Haffner C., Huttner W. B. (2005). Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development. J. Neurosci. 25, 6533-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness V. S., Jr, Goto T., Tarui T., Takahashi T., Bhide P. G., Nowakowski R. S. (2003) Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cereb. Cortex. 13, 592-598. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L., Galdzicki Z., Haydar T. F. (2007). Defects in embryonic neurogenesis and initial synapse formation in the forebrain of the Ts65Dn mouse model of Down syndrome. J. Neurosci. 27, 11483-11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis A., Henrique D., Lewis J., Ish-Horowicz D., Kintner C. (1995). Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature 375, 761-766. [DOI] [PubMed] [Google Scholar]
- Contestabile A., Fila T., Ceccarelli C., Bonasoni P., Bonapace L., Santini D., Bartesaghi R., Ciani E. (2007). Cell cycle alteration and decreased cell proliferation in the hippocampal dentate gyrus and in the neocortical germinal matrix of fetuses with Down syndrome and in Ts65Dn mice. Hippocampus 17, 665-678. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Oster-Granite M. L., Gearhart J. D. (1986). The neurobiologic consequences of Down syndrome. Brain Res. Bull. 16, 773-787. [DOI] [PubMed] [Google Scholar]
- Cremisi F., Philpott A., Ohnuma S. (2003). Cell cycle and cell fate interactions in neural development. Curr. Opin. Neurobiol. 13, 26-33. [DOI] [PubMed] [Google Scholar]
- Delabar J. M., Theophile D., Rahmani Z., Chettouh Z., Blouin J. L., Prieur M., Noel B., Sinet P. M. (1993). Molecular mapping of twenty-four features of Down syndrome on chromosome 21. Eur. J. Hum. Genet. 1, 114-124. [DOI] [PubMed] [Google Scholar]
- Deng X., Mercer S. E., Shah S., Ewton D. Z., Friedman E. (2004). The cyclin-dependent kinase inhibitor p27Kip1 is stabilized in G(0) by Mirk/dyrk1B kinase. J. Biol. Chem. 279, 22498-22504. [DOI] [PubMed] [Google Scholar]
- Dierssen M., de Lagrán M. M. (2006). DYRK1A (dual-specificity tyrosine-phosphorylated and -regulated kinase 1A): a gene with dosage effect during development and neurogenesis. Scientific World Journal 6, 1911-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez del Corral R., Storey K. G. (2004). Opposing FGF and retinoid pathways: a signalling switch that controls differentiation and patterning onset in the extending vertebrate body axis. Bioessays 26, 857-869. [DOI] [PubMed] [Google Scholar]
- Ericson J., Thor S., Edlund T., Jessell T. M., Yamada T. (1992). Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science 256, 1555-1560. [DOI] [PubMed] [Google Scholar]
- Fernandez-Martinez J., Vela E. M., Tora-Ponsioen M., Ocaña O. H., Nieto M. A., Galceran J. (2009). Attenuation of Notch signalling by the Down-syndrome-associated kinase DYRK1A. J. Cell Sci. 122, 1574-1583. [DOI] [PubMed] [Google Scholar]
- Fero M. L., Rivkin M., Tasch M., Porter P., Carow C. E., Firpo E., Polyak K., Tsai L. H., Broudy V., Perlmutter R. M., et al. (1996). A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1) deficient mice. Cell 85, 733-744. [DOI] [PubMed] [Google Scholar]
- Ferrón S. R., Pozo N., Laguna A., Aranda S., Porlan E., Moreno M., Fillat C., de la Luna S., Sánchez P., Arbonés M. L., et al. (2010). Regulated segregation of Kinase Dyrk1A during asymmetric neural stem cell division is critical for EGFR-mediated biased signaling. Cell Stem Cell 7, 367-379. [DOI] [PubMed] [Google Scholar]
- Fior R., Henrique D. (2005). A novel hes5/hes6 circuitry of negative regulation controls Notch activity during neurogenesis. Dev. Biol. 281, 318-333. [DOI] [PubMed] [Google Scholar]
- Fotaki V., Dierssen M., Alcantara S., Martinez S., Marti E., Casas C., Visa J., Soriano E., Estivill X., Arbones M. L. (2002). Dyrk1A haploinsufficiency affects viability and causes developmental delay and abnormal brain morphology in mice. Mol. Cell. Biol. 22, 6636-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi E., Hori T., Haraguchi T., Hiraoka Y., Kudoh J., Shimizu N., Ito F. (2003). Overexpression of the human MNB/DYRK1A gene induces formation of multinucleate cells through overduplication of the centrosome. BMC Cell Biol. 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galceran J., de Graaf K., Tejedor F. J., Becker W. (2003). The MNB/DYRK1A protein kinase: genetic and biochemical properties. J. Neural Transm. 67 Suppl., 139-148. [DOI] [PubMed] [Google Scholar]
- Garcia-Dominguez M., Poquet C., Garel S., Charnay P. (2003). Ebf gene function is required for coupling neuronal differentiation and cell cycle exit. Development 130, 6013-6025. [DOI] [PubMed] [Google Scholar]
- Göckler N., Jofre G., Papadopoulos C., Soppa U., Tejedor F. J., Becker W. (2009). Harmine specifically inhibits protein kinase DYRK1A and interferes with neurite formation. FEBS J. 276, 6324-6337. [DOI] [PubMed] [Google Scholar]
- Goto T., Mitsuhashi T., Takahashi T. (2004). Altered patterns of neuron production in the p27 knockout mouse. Dev. Neurosci. 26, 208-217. [DOI] [PubMed] [Google Scholar]
- Götz M., Huttner W. B. (2005) The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6, 777-788. [DOI] [PubMed] [Google Scholar]
- Guedj F., Sébrié C., Rivals I., Ledru A., Paly E., Bizot J. C., Smith D., Rubin E., Gillet B., Arbones M., et al. (2009). Green tea polyphenols rescue of brain defects induced by overexpression of DYRK1A. PLoS ONE 4, e4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui H., i S., Matise M. P. (2007). A cell-autonomous requirement for Cip/Kip cyclin-kinase inhibitors in regulating neuronal cell cycle exit but not differentiation in the developing spinal cord. Dev. Biol. 301, 14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi S., Bonasoni P., Ceccarelli C., Santini D., Gualtieri F., Ciani E., Bartesaghi R. (2008). Neurogenesis impairment and increased cell death reduce total neuron number in the hippocampal region of fetuses with Down syndrome. Brain Pathol. 18, 180-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F. (2005). Cellular and molecular control of neurogenesis in the mammalian telencephalon. Curr. Opin. Cell Biol. 17, 639-647. [DOI] [PubMed] [Google Scholar]
- Guillemot F. (2007). Spatial and temporal specification of neural fates by transcription factor codes. Development 134, 3771-3780. [DOI] [PubMed] [Google Scholar]
- Guimerá J., Casas C., Pucharcos C., Solans A., Domenech A., Planas A. M., Ashley J., Lovett M., Estivill X., Pritchard M. A. (1996). A human homologue of Drosophila minibrain (MNB) is expressed in the neuronal regions affected in Down syndrome and maps to the critical region. Hum. Mol. Genet. 5, 1305-1310. [DOI] [PubMed] [Google Scholar]
- Guimerá J., Casas C., Estivill X., Pritchard M. (1999). Human minibrain homologue (MNBH/DYRK1): characterization, alternative splicing, differential tissue expression, and overexpression in Down syndrome. Genomics 57, 407-418. [DOI] [PubMed] [Google Scholar]
- Hamburger V., Hamilton H. L. (1951). A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49-92. [PubMed] [Google Scholar]
- Hämmerle B., Tejedor F. J. (2002). A method for pulse and chase BrdU-labeling of early chick embryos. J. Neurosci. Methods 122, 59-64. [DOI] [PubMed] [Google Scholar]
- Hämmerle B., Tejedor F. J. (2007). A novel function of DELTA-NOTCH signalling mediates the transition from proliferation to neurogenesis in neural progenitor cells. PLoS ONE 2, e1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerle B., Vera-Samper E., Speicher S., Arencibia R., Martinez S., Tejedor F. J. (2002). Mnb/Dyrk1A is transiently expressed and asymmetrically segregated in neural progenitor cells at the transition to neurogenic divisions. Dev. Biol. 246, 259-273. [DOI] [PubMed] [Google Scholar]
- Hämmerle B., Elizalde C., Galceran J., Becker W., Tejedor F. J. (2003a). The MNB/DYRK1A protein kinase: neurobiological functions and Down syndrome implications. J. Neural Transm. 67 Suppl., 129-137. [DOI] [PubMed] [Google Scholar]
- Hämmerle B., Carnicero A., Elizalde C., Ceron J., Martínez S., Tejedor F. J. (2003b). Expression patterns and subcellular localization of the Down syndrome candidate protein MNB/DYRK1A suggest a role in late neuronal differentiation. Eur. J. Neurosci. 17, 2277-2286. [DOI] [PubMed] [Google Scholar]
- Hämmerle B., Elizalde C., Tejedor F. J. (2008). The spatio-temporal and subcellular expression of the candidate Down syndrome gene Mnb/Dyrk1A in the developing mouse brain suggests distinct sequential roles in neuronal development. Eur. J. Neurosci. 27, 1061-1074. [DOI] [PubMed] [Google Scholar]
- Henrique D., Hirsinger E., Adam J., Le Roux I., Pourquie O., Ish-Horowicz D., Lewis J. (1997). Maintenance of neuroepithelial progenitor cells by Delta-Notch signaling in the embryonic chick retina. Curr. Biol. 7, 661-670. [DOI] [PubMed] [Google Scholar]
- Hollyday M. (2001). Neurogenesis in the vertebrate neural tube. Int. J. Dev. Neurosci. 19, 161-173. [DOI] [PubMed] [Google Scholar]
- Kageyama R., Ohtsuka T., Shimojo H., Imayoshi I. (2009). Dynamic regulation of Notch signaling in neural progenitor cells. Curr. Opin. Cell Biol. 21, 733-740. [DOI] [PubMed] [Google Scholar]
- Kelly P. A., Rahmani Z. (2005). DYRK1A enhances the mitogen-activated protein kinase cascade in PC12 cells by forming a complex with Ras, B-Raf, and MEK1. Mol. Biol. Cell 16, 3562-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguna A., Aranda S., Barallobre M. J., Barhoum R., Fernández E., Fotaki V., Delabar J. M., de la Luna S., de la Villa P., Arbonés M. L. (2008) The protein kinase DYRK1A regulates caspase-9-mediated apoptosis during retina development. Dev. Cell. 15, 841-853. [DOI] [PubMed] [Google Scholar]
- Larsen K. B., Laursen H., Graem N., Samuelsen G. B., Bogdanovic N., Pakkenberg B. (2008). Reduced cell number in the neocortical part of the human fetal brain in Down syndrome. Ann. Anat. 190, 421-427. [DOI] [PubMed] [Google Scholar]
- Lewis J. (1998). Notch signalling and the control of cell fate choices in vertebrates. Semin. Cell Dev. Biol. 9, 583-589. [DOI] [PubMed] [Google Scholar]
- Louvi A., Artavanis-Tsakonas S. (2006). Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 7, 93-102. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz A., Savatier P., Cortay V., Giroud P., Huissoud C., Berland M., Kennedy H., Dehay C. (2005). G1 phase regulation, area‑specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron 47, 353-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias P., Müller M. M., Schreiber E., Rusconi S., Schaffner W. (1989). Eukaryotic expression vectors for the analysis of mutant proteins. Nucleic Acids Res. 17, 6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megason S. G., McMahon A. P. (2002). A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development 129, 2087-2098. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi T., Aoki Y., Eksioglu Y. Z., Takahashi T., Bhide P. G., Reeves S. A., Caviness V. S., Jr (2001). Overexpression of p27Kip1 lengthens the G1 phase in a mouse model that targets inducible gene expression to central nervous system progenitor cells. Proc. Natl. Acad. Sci. USA 98, 6435-6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller R. S., Kübart S., Hoeltzenbein M., Heye B., Vogel I., Hansen C. P., Menzel C., Ullmann R., Tommerup N., Ropers H. H., et al. (2008). Truncation of the Down syndrome candidate gene DYRK1A in two unrelated patients with microcephaly. Am. J. Hum. Genet. 82, 1165-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L., Besson A., Roberts J. M., Guillemot F. (2006a). Coupling cell cycle exit, neuronal differentiation and migration in cortical neurogenesis. Cell Cycle 20, 2314-2318. [DOI] [PubMed] [Google Scholar]
- Nguyen L., Besson A., Heng J. I., Schuurmans C., Teboul L., Parras C., Philpott A., Roberts J. M., Guillemot F. (2006b). p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 20, 1511-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma S., Harris W. A. (2003). Neurogenesis and the cell cycle. Neuron. 40, 199-208. [DOI] [PubMed] [Google Scholar]
- Okano H., Temple S. (2009). Cell types to order: temporal specification of CNS stem cells. Curr. Opin. Neurobiol. 19, 112-119. [DOI] [PubMed] [Google Scholar]
- Park J., Yang E. J., Yoon J. H., Chung K. C. (2007). Dyrk1A overexpression in immortalized hippocampal cells produces the neuropathological features of Down syndrome. Mol. Cell. Neurosci. 36, 270-279. [DOI] [PubMed] [Google Scholar]
- Park J., Song W. J., Chung K. C. (2009). Function and regulation of Dyrk1A: towards understanding Down syndrome. Cell. Mol. Life Sci. 66, 3235-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Oh Y., Yoo L., Jung M. S., Song W. J., Lee S. H., Seo H., Chung K. C. (2010). DYRK1A phosphorylates p53 and inhibits proliferation of embryonic neuronal cells. J. Biol. Chem. 285, 31895-31906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitto L., Cremisi F. (2010). Timing neurogenesis by cell cycle. Cell Cycle 9, 434-435. [DOI] [PubMed] [Google Scholar]
- Pituello F. (1997). Neuronal specification: generating diversity in the spinal cord. Curr. Biol. 7, R701-R704. [DOI] [PubMed] [Google Scholar]
- Schmidt-Sidor B., Wisniewski K. E., Shepard T. H., Sersen E. A. (1990). Brain growth in Down syndrome subjects 15 to 22 weeks of gestational age and birth to 60 months. Clin. Neuropathol. 9, 181-190. [PubMed] [Google Scholar]
- Sebrié C., Chabert C., Ledru A., Guedj F., Po C., Smith D. J., Rubin E., Rivals I., Beloeil J. C., Gillet B., et al. (2008). Increased dosage of DYRK1A and brain volumetric alterations in a YAC model of partial trisomy 21. Anat. Rec. (Hoboken). 291, 254-262. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. (1999). CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501-1512. [DOI] [PubMed] [Google Scholar]
- Sitz J. H., Baumgärtel K., Hämmerle B., Papadopoulos C., Hekerman P., Tejedor F. J., Becker W., Lutz B. (2008). The Down syndrome candidate dual-specificity tyrosine phosphorylation-regulated kinase 1A phosphorylates the neurodegeneration-related septin 4. Neuroscience 157, 596-605. [DOI] [PubMed] [Google Scholar]
- Song W. J., Sternberg L. R., Kasten-Sportes C., Keuren M. L., Chung S. H., Slack A. C., Miller D. E., Glover T. W., Chiang P. W., Lou L., et al. (1996). Isolation of human and murine homologues of the Drosophila minibrain gene: human homologue maps to 21q22.2 in the Down syndrome “critical region”. Genomics 38, 331-339. [DOI] [PubMed] [Google Scholar]
- Sun X., Artavanis-Tsakonas S. (1996). The intracellular deletions of DELTA and SERRATE define dominant negative forms of the Drosophila Notch ligands. Development 122, 2465-2474. [DOI] [PubMed] [Google Scholar]
- Tejedor F. J., Hämmerle B. (2011). MNB/DYRK1A as a multiple regulator of neuronal development. FEBS J. 278, 223-235. [DOI] [PubMed] [Google Scholar]
- Tejedor F., Zhu X. R., Kaltenbach E., Ackermann A., Baumann A., Canal I., Heisenberg M., Fischbach K. F., Pongs O. (1995). minibrain: a new protein kinase family involved in postembryonic neurogenesis in Drosophila. Neuron 14, 287-301. [DOI] [PubMed] [Google Scholar]
- Wallace K., Liu T. H., Vaessin H. (2000). The pan-neural bHLH proteins DEADPAN and ASENSE regulate mitotic activity and cdk inhibitor dacapo expression in the Drosophila larval optic lobes. Genesis 26, 77-85. [DOI] [PubMed] [Google Scholar]
- Wegiel J., Gong C. X., Hwang Y. W. (2011). The role of DYRK1A in neurodegenerative diseases. FEBS J. 278, 236-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiechmann S., Czajkowska H., de Graaf K., Grötzinger J., Joost H. G., Becker W. (2003). Unusual function of the activation loop in the protein kinase DYRK1A. Biochem. Biophys. Res. Commun. 302, 403-408. [DOI] [PubMed] [Google Scholar]
- Wisniewski K. E., Laure-Kamionowska M., Wisniewski H. M. (1984). Evidence of arrest of neurogenesis and synaptogenesis in brains of patients with Down's syndrome. N. Engl. J. Med. 311, 1187-1188. [DOI] [PubMed] [Google Scholar]
- Yabut O., Domogauer J., D'Arcangelo G. (2010). Dyrk1A overexpression inhibits proliferation and induces premature neuronal differentiation of neural progenitor cells. J. Neurosci. Mar. 30, 4004-4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E. J., Ahn Y. S., Chung K. C. (2001). Protein kinase Dyrk1 activates cAMP response element-binding protein during neuronal differentiation in hippocampal progenitor cells. J. Biol. Chem. 276, 39819-39824. [DOI] [PubMed] [Google Scholar]
- Yoon K., Gaiano N. (2005). Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat. Neurosci. 8, 709-715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.