Mucus is a highly viscoelastic and adhesive substance that protects against infection and injury at nearly all entry points to the body not covered by skin. However, mucus also traps potentially life-saving drugs and nucleic acids delivered via synthetic nanoparticles, including those composed of poly(lactic-co-glycolic acid) (PLGA) and poly(ε-caprolactone) (PCL), two FDA-approved polymers commonly used in drug delivery applications.[1] Trapped particles, with diffusivities in mucus several thousand-fold lower than in water, do not efficiently reach the deeper mucus layers that are cleared much more slowly, or the underlying epithelium, and are thus eliminated by mucus clearance mechanisms (on the order of seconds to a few hours depending on anatomical site[2]). For sustained or targeted drug delivery to mucosal surfaces, nanoparticles must quickly penetrate mucus gels, a longstanding challenge in drug delivery.[2c]
We recently demonstrated that covalently coating particles with a high density of low MW poly(ethylene glycol) (PEG), a hydrophilic and uncharged polymer widely used in pharmaceuticals, can reduce particle affinity to mucus constituents.[3] Densely coated particles were able to rapidly penetrate fresh, undiluted human mucus, with speeds only a few-fold lower than in water, by diffusing within the low viscosity interstitial fluid between mucin fibers without experiencing the bulk viscosity of mucus.[4] However, current methods of producing mucus-penetrating particles (MPP) require covalent conjugation of PEG to polymers or pre-fabricated particles[3], resulting in new chemical entities (NCE), which are subject to a lengthy and expensive FDA regulatory process. We sought to develop a simple non-covalent coating process to produce MPP composed entirely of Generally Recognized As Safe (GRAS) materials. Uncharged amphiphilic GRAS materials, such as triblock copolymers of poly(ethylene glycol)-poly(propylene oxide)-poly(ethylene glycol) (PEG-PPO-PEG; known as Pluronics®), may coat hydrophobic particle surfaces by adsorption via the hydrophobic PPO segments, leaving a dense brush of uncharged, hydrophilic PEG segments protruding from the particle surface.[5] Here, we show that a number of Pluronics® molecules, containing PPO segments with MW ≥ 3 kDa, can effectively coat PLGA, PCL and latex nanoparticles, thereby enabling the formulation of MPP composed entirely of GRAS materials, with no NCE generated. Synthetic MPP composed entirely of GRAS materials will likely facilitate rapid translation of nanomaterials-based products into humans for the treatment of numerous diseases and conditions that affect mucosal tissues.
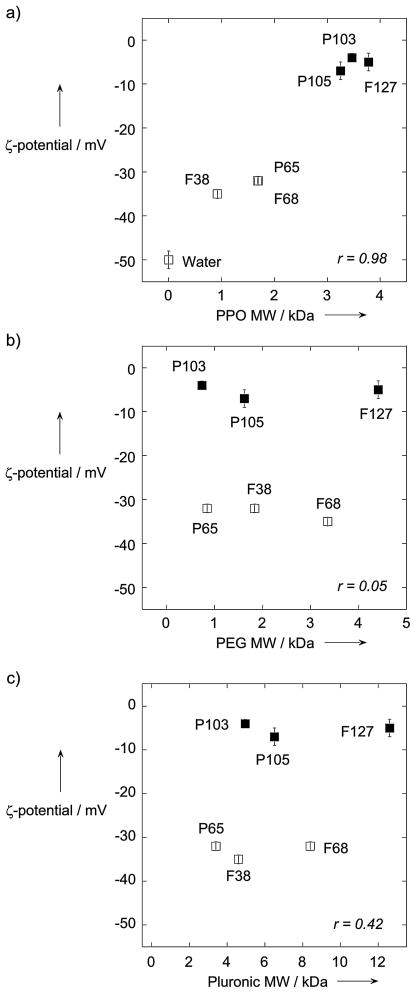
Pluronics of different MW and PPO/PEG ratios have been adopted for various biomedical applications.[6] We first sought to identify which Pluronics may coat normally mucoadhesive polymeric nanoparticles sufficiently to transform them into MPP. As a proof-of-concept, we formulated fluorescently-labeled PLGA nanoparticles, and incubated separate batches with Pluronic P65, F38, P103, P105, F68 or F127 (listed in order of increasing MW) followed by purification. We then observed nanoparticle transport dynamics in freshly obtained, undiluted human cervicovaginal mucus (CVM). Uncoated PLGA nanoparticles were extensively immobilized in CVM (Figure 1a). Three of the Pluronics (F38, P65 and F68) tested did not enhance the transport of PLGA particles, as evident by the highly constrained, non-Brownian time-lapse traces of the particles in mucus (Figure 1b). In contrast, coating PLGA particles with P103, P105 or F127 allowed them to readily penetrate CVM, as evident by their diffusive, Brownian trajectories that covered large distances over the course of 20 s movies (Figure 1c). The effectiveness of the Pluronic coatings was critically dependent on the MW of the PPO segment (Figure 1d), perhaps because hydrophobic adhesive interactions between short PPO segments and PLGA are inadequate to anchor a dense brush of Pluronic molecules (and consequently PEG) onto the particle surface. Indeed, P103, P105 and F127, all with PPO MW ≥ 3 kDa, produced coated particles with a ζ-potential between −8 mV and 0 mV (Figure 2a), compared to −50 mV for uncoated particles; we previously found that PEG-coatings that effectively shield latex particles from mucoadhesion exhibited a near-neutral particle ζ-potential (within −10 mV of neutral).[3b] In contrast, PLGA nanoparticles incubated in F38, P65 and F68, each with PPO MW < 3 kDa, exhibited surface charges between −30 to −35 mV, indicating partial, but inadequate, surface PEG coverage. There was no correlation between Pluronic coating density and either the MW of the PEG segments or total Pluronic MW (Figure 2b and 2c). It is possible that the Pluronic coating may desorb from particles over time. However, we have observed that the surface charges for P103-, P105- and F127-coated particles remain neutral at 4°C in buffer 24 hr after particle synthesis, suggesting the coatings are stable at least over that duration (data not shown).
Figure 1.
Transport behavior of uncoated and Pluronic-coated PLGA particles in fresh human CVM. Representative trajectories of a) uncoated PLGA particles, b) particles coated with low PPO MW Pluronic (F68, F38 or P65) and c) particles coated with high PPO MW Pluronic (F127, P103 or P105). d) Phase diagram correlating muco-inert vs. mucoadhesive particle behavior to Pluronic PPO and PEG segment MW. Filled symbols indicate MPP formulations, while open symbols indicate mucoadhesive formulations.
Figure 2.
Muco-inert vs. mucoadhesive behavior of PLGA particles coated with various Pluronics (F38, P65, P103, P105, F68 and F127) in fresh human CVM. a–c) Correlation between the zeta potential of Pluronic-coated PLGA particles and the MW of the a) PPO segment, b) PEG segment or c) entire Pluronic molecule. “Water” indicates the zeta potential of uncoated PLGA particles made in water. Filled symbols indicate MPP formulations, while open symbols indicate mucoadhesive formulations. r represents the correlation coefficient.
Pluronic F127 is one of the most commonly used Pluronics for pharmaceutical applications;[6b, 6d, 7] we thus focused subsequent investigations on F127-coated particles. To quantify the speeds of F127-coated PLGA nanoparticles (PLGA/F127 NP) in mucus, we analyzed the motions of PLGA/F127 NP using multiple particle tracking, a technique that allows quantitative measurements of hundreds of individual particles.[2c, 8] The time-scale dependent ensemble mean squared displacement (<MSD>) of PLGA/F127 NP was 280-fold higher than that for uncoated PLGA nanoparticles (PLGA NP) at a time scale of 1 s (Figure 3a). Few, if any, PLGA/F127 NP were trapped in mucus compared to PLGA NP (Figure 3b). Importantly, PLGA/F127 NP were slowed only ~10-fold in CVM compared to their theoretical speeds in water, whereas PLGA NP were slowed ~4000-fold (Table S1 in Supporting Information). The similar speeds of PLGA/F127 NP and nanoparticles with covalently conjugated low MW PEG[3] suggest that non-covalent coating with Pluronic F127, as well as other Pluronics with PPO MW ≥ 3 kDa, shields adhesive particle surfaces as efficiently as does a covalent PEG coating. We also tested particles composed of other mucoadhesive polymers, including the widely used PCL polymer and a generic hydrophobic polymer, polystyrene (PS; also known as latex). For both, we observed extensive immobilization for uncoated particles and rapid mucus penetration for F127-coated particles, with effective diffusivities similar to those measured for PLGA NP and PLGA/F127 NP (Figure S1 in Supporting Information). The majority of F127-coated nanoparticles (~60–80%), regardless of the core material, are expected to penetrate physiologically-thick mucus layers within 30 minutes, whereas < 1% of uncoated particles will do so over the same duration (Figure 3c, S1g and S1h).
Figure 3.
Transport of uncoated and F127-coated PLGA particles in human CVM. a) Ensemble-averaged geometric mean square displacements (<MSD>) as a function of time scale. b) Distributions of the logarithms of individual particle effective diffusivities (Deff) at a time scale of 1 s. * denotes statistically significant difference across all time scales (p < 0.05). c) Estimated fraction of particles predicted to be capable of penetrating a 30 μm thick mucus layer over time.
Our findings highlight numerous potential advantages of using Pluronic-coated particles for drug delivery applications. First, Pluronics have an extensive safety profile and have been used since the 1950s[6a] in many commercially available products, including drug delivery devices.[9] Combining Pluronic with other GRAS materials may, therefore, produce mucus-penetrating drug delivery platforms that are likely to be safe in humans, and also greatly simplify manufacturing while reducing the time and costs for clinical development. Second, since this method involves only a short incubation of pre-fabricated particles with Pluronic, the formulation process of the drug-loaded particle core remains unchanged. The simplicity of the coating process may accelerate economical and scalable translational development of the MPP technology. Third, tailored release profiles and high encapsulation efficiencies may be achieved for a wide array of cargo therapeutics simply by selecting an appropriate GRAS material, with optimal degradation kinetics and polymer-drug affinity, for the particle core.[10] Using an optimal core material may also help minimize the potential buildup of unwanted polymers in the body, as can occur with repeated administration of carriers that release drug quickly but are composed of slowly degrading polymers.[11] Fourth, we expect Pluronic coatings to facilitate rapid particle penetration at other mucosal surfaces, since human CVM possesses biochemical content and rheological properties similar to those of mucus fluids derived from the eyes, nose, lungs, gastrointestinal tract and more.[3a] Indeed, we have found that a Pluronic F127 coating markedly improves the transport of polymeric particles in both sputum expectorated by cystic fibrosis patients as well as mucus collected via surgery from the nasal cavity of patients with chronic sinusitis (unpublished observations).
We show here that otherwise mucoadhesive polymeric particles can be sufficiently coated with specific Pluronic molecules to allow rapid nanoparticle penetration of human mucus secretions without introducing any NCE. Enhanced mucus penetration is expected to facilitate prolonged retention and more uniform distribution of drug carriers at mucosal surfaces, leading to improved pharmacokinetics and therapeutic efficacy.[2c] While Pluronic was investigated here, we expect other molecules may similarly reduce particle mucoadhesion by forming non-covalent coatings that block adhesive interactions. The continual development of alternative, non-covalent coatings for biodegradable polymer nanoparticles will further expand the diversity of mucosal delivery systems for the treatment of mucosal diseases, including infections, cancer and inflammation in the eyes, sinuses, female reproductive tract, respiratory tract, and gastrointestinal tract.
Experimental Section
The general experimental methods were as follows (details are available in Supporting Information): Pluronic-coated biodegradable nanoparticles were synthesized in water, followed by collection and simple incubation in 1% w/v Pluronic solution, and were purified via size exclusion chromatography. Particles were characterized for size and surface charge. The displacements of uncoated and Pluronic-coated particles were tracked in fresh, undiluted human CVM using multiple particle tracking.[3, 12]
Supplementary Material
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
We thank the Integrated Imaging Center at Johns Hopkins University. This work was supported in part by the NIH R21AI079740, R01CA140746, R21HL089816 and U54CA151838, a Croucher Foundation Fellowship to S.K.L. and a National Science Foundation Graduate Research Fellowship to Y.-Y.W.. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Cancer Institute.
Contributor Information
Ming Yang, Department of Biomedical Engineering, Johns Hopkins University.
Dr. Samuel K. Lai, Department of Chemical & Biomolecular Engineering, Johns Hopkins University.
Ying-Ying Wang, Department of Biomedical Engineering, Johns Hopkins University.
Weixi Zhong, Department of Biomedical Engineering, Johns Hopkins University.
Christina Happe, Department of Chemical & Biomolecular Engineering, Johns Hopkins University.
Michael Zhang, Department of Chemical & Biomolecular Engineering, Johns Hopkins University.
Dr. Jie Fu, Department of Ophthalmology, Johns Hopkins University School of Medicine
Prof. Dr. Justin Hanes, Department of Ophthalmology, Biomedical Engineering, Chemical & Biomolecular Engineering and Oncology, Center for Cancer Nanotechnology Excellence, Institute for NanoBioTechnology, and Center for Nanomedicine, Johns Hopkins University School of Medicine, 400 N Broadway, Baltimore, MD 21287 (USA).
References
- 1.a) Hoffman AS. J Control Release. 2008;132:153. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]; b) Kumari A, Yadav SK, Yadav SC. Colloids Surf B Biointerfaces. 2010;75:1. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]; c) Mohamed F, van der Walle CF. J Pharm Sci. 2008;97:71. doi: 10.1002/jps.21082. [DOI] [PubMed] [Google Scholar]; d) Putnam D. Nat Mater. 2006;5:439. doi: 10.1038/nmat1645. [DOI] [PubMed] [Google Scholar]
- 2.a) Cone RA. Adv Drug Deliv Rev. 2009;61:75. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]; b) Knowles MR, Boucher RC. J Clin Invest. 2002;109:571. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lai SK, Wang YY, Hanes J. Adv Drug Deliv Rev. 2009;61:158. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Proc Natl Acad Sci U S A. 2007;104:1482. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang YY, Lai SK, Suk JS, Pace A, Cone R, Hanes J. Angew Chem Int Ed Engl. 2008;47:9726. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Lai SK, Wang YY, Hida K, Cone R, Hanes J. Proc Natl Acad Sci U S A. 2010;107:598. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lai SK, Wang YY, Wirtz D, Hanes J. Adv Drug Deliv Rev. 2009;61:86. doi: 10.1016/j.addr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Illum L, Davis SS. FEBS Lett. 1984;167:79. doi: 10.1016/0014-5793(84)80836-4. [DOI] [PubMed] [Google Scholar]
- 6.a) Emanuele RM. Expert Opin Investig Drugs. 1998;7:1193. doi: 10.1517/13543784.7.7.1193. [DOI] [PubMed] [Google Scholar]; b) Batrakova EV, Kabanov AV. J Control Release. 2008;130:98. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Rodeheaver GT, Kurtz L, Kircher BJ, Edlich RF. Ann Emerg Med. 1980;9:572. doi: 10.1016/s0196-0644(80)80228-9. [DOI] [PubMed] [Google Scholar]; d) Escobar-Chavez JJ, Lopez-Cervantes M, Naik A, Kalia YN, Quintanar-Guerrero D, Ganem-Quintanar A. J Pharm Pharm Sci. 2006;9:339. [PubMed] [Google Scholar]
- 7.Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. Pharm Res. 2006;23:2709. doi: 10.1007/s11095-006-9104-4. [DOI] [PubMed] [Google Scholar]
- 8.Suh J, Dawson M, Hanes J. Adv Drug Deliv Rev. 2005;57:63. doi: 10.1016/j.addr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 9.a) Donaldson D, Gelskey SC, Landry RG, Matthews DC, Sandhu HS. J Clin Periodontol. 2003;30:171. doi: 10.1034/j.1600-051x.2003.00017.x. [DOI] [PubMed] [Google Scholar]; b) Lo JB, Appel LE, Herbig SM, McCray SB, Thombre AG. Drug Dev Ind Pharm. 2009;35:1522. doi: 10.3109/03639040903037223. [DOI] [PubMed] [Google Scholar]; c) Pui CH. Expert Opin Pharmacother. 2002;3:433. doi: 10.1517/14656566.3.4.433. [DOI] [PubMed] [Google Scholar]
- 10.a) Tamada J, Langer R. J Biomater Sci Polym Ed. 1992;3:315. doi: 10.1163/156856292x00402. [DOI] [PubMed] [Google Scholar]; b) Yeo Y, Park K. Arch Pharm Res. 2004;27:1. doi: 10.1007/BF02980037. [DOI] [PubMed] [Google Scholar]
- 11.Fu J, Fiegel J, Krauland E, Hanes J. Biomaterials. 2002;23:4425. doi: 10.1016/s0142-9612(02)00182-5. [DOI] [PubMed] [Google Scholar]
- 12.Dawson M, Wirtz D, Hanes J. Journal of Biological Chemistry. 2003;278:50393. doi: 10.1074/jbc.M309026200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.