Abstract
Understanding which species are most vulnerable to human impacts is a prerequisite for designing effective conservation strategies. Surveys of terrestrial species have suggested that large-bodied species and top predators are the most at risk, and it is commonly assumed that such patterns also apply in the ocean. However, there has been no global test of this hypothesis in the sea. We analyzed two fisheries datasets (stock assessments and landings) to determine the life-history traits of species that have suffered dramatic population collapses. Contrary to expectations, our data suggest that up to twice as many fisheries for small, low trophic-level species have collapsed compared with those for large predators. These patterns contrast with those on land, suggesting fundamental differences in the ways that industrial fisheries and land conversion affect natural communities. Even temporary collapses of small, low trophic-level fishes can have ecosystem-wide impacts by reducing food supply to larger fish, seabirds, and marine mammals.
Keywords: body size, ecosystem-based management, food webs, life-history theory, marine conservation
Overfishing is one of the most serious conservation concerns in marine ecosystems (1), but understanding which species are most at risk remains a challenge. On land, life-history traits are strong predictors of extinction risk (2), and vulnerable species often have large body size and high trophic level (2, 3). In marine ecosystems, the well-publicized declines of large predatory fishes (4, 5) suggest that similar trends may also be common in the sea. However, research to date has found or proposed a wide range of life-history characteristics that cause high vulnerability, including large body size (6–9), late maturity (6, 9), long lifespan (6, 8–11), low fecundity and high parental investment in offspring (11, 12), or high trophic level (2, 13). Understanding which traits, or combinations of traits, are most useful for predicting vulnerability has been difficult because analyses have been limited to regional comparisons or narrow species groups, and because reliable global data have not been available to more broadly test which types of fishes are most likely to suffer fisheries collapse.
In addition, there are reasons to believe that regional or terrestrial life-history trends might not apply globally in the ocean. For example, fishery biologists often recommend higher harvest rates for fast-growing, highly productive species, and lower harvest rates for species with lower productivity (14). Where implemented, these adjustments might reduce the resilience of fast-growing species and put all harvested species at similar risks of decline. In addition, economic forces or management regime may be more important than life history in determining whether fishing effort is successfully controlled (15, 16). Small pelagic species, although often possessing a rapid growth rate, are also highly catchable, and therefore susceptible to overfishing (17). Finally, the conflict with human development that is particularly acute for large, terrestrial mammals (3) may be smaller in open-ocean ecosystems far from coastlines.
In this article, we used two independent fisheries databases to determine which stocks have collapsed to low population abundance. Our first database contained 223 scientific stock assessments for 120 species. For these assessments, a stock was defined as collapsed if its minimum annual biomass (BMIN) fell to < 20% of the biomass necessary to support maximum sustainable yield (BMSY) (1). In addition, we examined global landings reported by the Food and Agriculture Organization (FAO) for 1950 to 2006. We treated each FAO statistical area as a stock, for a total of 891 stocks across 458 species. For landings data, a stock was defined as collapsed if landings remained below 10% of the average of the five highest landings recorded for more than 2 y. We found this definition to have the lowest misclassification rate (18%) when we evaluated it against stocks for which we had both biomass and catch timeseries (Materials and Methods). In most misclassifications (14 of 24), we failed to detect collapses that had occurred, suggesting that our landings definition is relatively conservative. Finally, we assessed the prevalence of collapse across a broad range of life-history traits, including lifespan, age of maturity, body size, trophic level, growth rate, fecundity, and parental investment in offspring (egg size) (18).
Results
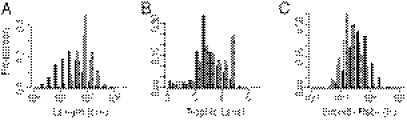
We first examined life-history traits of species targeted by global fisheries. Although fisheries caught the entire range of trophic levels and growth rates seen among marine fish, fisheries tended to catch larger, higher trophic level, and slower-growing species (P < 10−7) (Fig. 1). In addition, the very smallest species (< 8 cm) did not appear in global, industrial fisheries. There were no substantial size or trophic-level differences between the species that appear in the global landings database and those that appear in the scientific stock assessments, although the latter are on average somewhat slower growing (P = 10−10).
Fig. 1.
Life history patterns of fished species. Histograms for all marine fish (black), species in the landings database (gray), and species in the assessment database (white) for (A) length (n = 16,548/457/120 for all/landings/assessments), (B) trophic level (n = 16,548/457/120), and (C) growth rate (n = 14,118/447/120).
Overall, we found that 17.0 or 25.1% of the stocks in each species had collapsed, on average, in the assessment (n = 52) or landings data (n = 223), respectively. In addition, 23.3 or 34.9% of species had experienced at least one stock collapse (assessments n = 28, or landings n = 160, respectively) (Table S1).
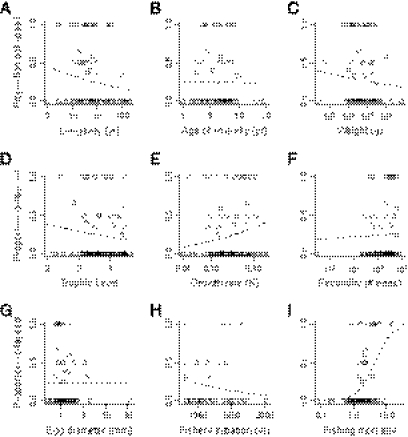
One hypothesis was that large, high trophic-level species would show a higher incidence of collapse than small, low trophic-level species. Instead, the assessment data revealed fisheries collapses across the range of life-history traits (Fig. 2 A–E). Among top predators [trophic level (TL) > 4.2], 12% of stocks had collapsed, but twice the percentage (25%) had collapsed among low trophic-level fishes (TL < 3.3). Among large species (> 16 kg), 16% of stocks had collapsed, but 29% had collapsed in small species (< 2.5 kg).
Fig. 2.
Collapses in the assessment database in relation to life history traits. Traits include (A) lifespan (n = 97), (B) age of maturity (n = 96), (C) weight (n = 93), (D) trophic level (n = 120), (E) growth rate (n = 120), (F) fecundity (n = 93), (G) investment in offspring (egg diameter, n = 97), (H) year of fishery initiation (n = 46), and (I) relative fishing mortality (n = 99). Each dot represents the proportion of stocks collapsed within a species. All x axes are log-transformed except those for trophic level and fishery initiation. Dashed line is the best fit from a generalized linear model.
Although the above comparisons suggested more collapses in small, low trophic-level fishes, generalized linear models fit to the assessment data showed that the proportion of stock collapses was not significantly related to trophic level (P = 0.15), weight (P = 0.26), longevity (P = 0.10), age of maturity (P = 0.92), fecundity (P = 0.77), or investment in offspring (P = 0.99) (Table S2). The incidence of collapse, however, was somewhat higher for fast growing species (P = 0.019).
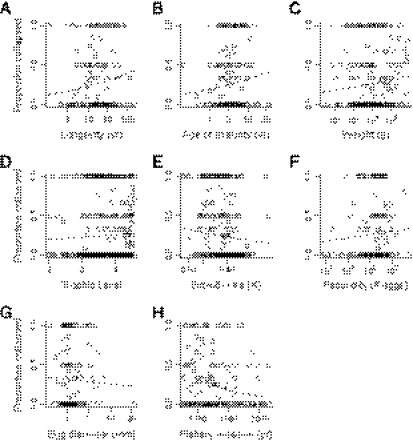
The landings data generally supported the conclusion that large, top predators are not more vulnerable than small, low trophic-level fishes (Fig. 3 A–E). Among top predators (TL > 4.2), 26% of stocks had collapsed, but a similar percentage (21%) had collapsed in low trophic-level species (TL < 3.4). Similarly, 36% of stocks had collapsed in large species (> 30 kg), but 31% had collapsed in small species (< 2.4 kg). Models suggested that the proportion of stocks collapsed did not change with trophic level (P = 0.22), fecundity (P = 0.23), or investment in offspring (P = 0.52). Trends were weakly toward more collapses among long-lived species (P = 0.012), those with later maturity (P = 0.036), heavier species (P = 0.014), and those with slower growth (P = 0.048), opposite to the trends in the assessment data. Trends were not significant after correction for multiple comparisons.
Fig. 3.
Collapses in the landings database in relation to life history traits. Traits include (A) lifespan (n = 206), (B) age of maturity (n = 216), (C) weight (n = 267), (D) trophic level (n = 457), (E) growth rate (n = 447), (F) fecundity (n = 172), (G) investment in offspring (egg diameter, n = 155), and (H) year of fishery initiation (n = 208). Also see notes for Fig. 2.
In addition, we examined whether combinations of life-history traits might predict vulnerability, because multiple traits are often necessary for defining a life-history strategy (11). However, combining life-history traits in multiple regression models could only predict 14 or 8% of the deviance in the data (P = 0.046 or P = 0.15, assessments and landings, respectively) (Table S2), suggesting that collapse incidence was not strongly related to life history in any combination. For the models based on assessment data, higher growth rate was the most frequently included trait, and these models suggested that species with higher growth rates experienced a higher incidence of fisheries collapse. Previous authors have proposed that fishes with low fecundity and high investment in offspring (large egg diameter) may be particularly vulnerable to fishing (11), but we did not find evidence that this combination could explain the incidence of fishery collapses (0.8% or 1.1% of deviance explained, P = 0.79 or 0.59 within the assessment and landings data, respectively).
We also explored the relationship between life history and alternative measures of vulnerability, including incidence of overfished stocks (BMIN < 50% BMSY), maximum depletion (BMIN/BMSY), and a less strict definition of landings collapse (landings below 10% of the single highest landings recorded for a single year). All trends were similar to those reported above (Figs. S1, S2, and S3). Removing small pelagic species (families Engraulidae and Clupeidae) known to fluctuate strongly with climate (19) also did not change our results.
As we would expect, incidence of collapse was higher for species that experienced greater relative fishing mortality (P = 1 × 10−5, 22% of deviance explained among assessment data) and a longer history of developed fisheries (P = 5 × 10−5, 5.8% of deviance explained among landings data) (Figs. 2 F and G, and 3F). However, correcting for fisheries characteristics did not change the relationships between collapse and life history (Fig. S4).
We also corrected for evolutionary relationships because phylogenetic history can reduce the independence of species-level data (20). However, using phylogenetically independent contrasts only revealed weaker relationships between life history and incidence of collapse. These relationships reduced the discrepancy in model results between assessment and landings data (Figs. S5 and S6).
Finally, we examined whether collapses last longer for certain life histories. In the assessment data, collapses are longer (18.5 y) for long-lived species (lifespan > 44 y) than for short-lived species (5.1 y, lifespan < 14 y, P = 0.028). Other life history comparisons were not significant (P > 0.21).
Discussion
Small, short-lived species have what is sometimes called a “fast” life-history strategy that is presumed to make them less vulnerable to fisheries (6, 9). In contrast, our review of global fisheries revealed that these fast species collapse just as often as species with slower life histories. We found collapsed stocks in short-lived species, such as summer flounder (Paralichthys dentatus) and Spanish mackerel (Scomberomorus maculatus) and among small, fast-growing species like capelin (Mallotus villosus) and herring (Clupea harengus and Clupea pallasii). Species low in the food chain had also collapsed, including winter flounder (Pseudopleuronectes americanus) and chub mackerel (Scomber japonicus). Although these collapses are well known to local fishermen and managers, the general prevalence of collapse among these types of species has not been recognized.
Our data suggest that species with fast life histories have at least as high a probability (per stock) of declining to low abundance as larger, slower species, which is dramatically different from the pattern among terrestrial species (2, 3). Why might fast species be more vulnerable in the ocean than we would expect? One explanation may be that fisheries management often recommends higher exploitation rates for species with faster life histories and greater productivity. For example, our assessment database revealed that the fishing mortality predicted to supply maximum sustainable yield (FMSY) for long-lived rockfishes (Sebastes spp.) is 0.07 (average of 20 stocks), but FMSY for short-lived skipjack tuna is 0.42 (average of three stocks). Managing with these or similar reference points can thus equalize the impact of fishing across species, a process with no widespread equivalent on land. When fishing rate is correctly determined relative to species’ biology, life history should not be an important determinant of fish collapse.
This rationale may also explain differences between the assessment and landings data. Many of the species in the landings database are not managed with scientific stock assessments and, presumably, fishing mortality is less closely matched to stock productivity. Under such conditions, we might expect life history to be more important for determining fisheries collapse and slow species to collapse more often. In fact, collapses were slightly more common among long-lived species in the landings database (Fig. 3A). Among the more closely managed stocks in the assessment database, this trend was absent.
In addition, a fast life history may actually increase vulnerability to collapse. Populations of short-lived species can grow or decline quickly in response to climatic shifts (19), and a rapid decline in productivity often requires similarly rapid reductions in fishing effort (21). If fisheries management lags behind these biophysical changes, a population can be driven to collapse (21, 22). The high harvest rates on many short-lived species also mean that errors in setting harvest rates can have particularly severe consequences. In addition, we note that environmental variability alone can drive variation in fish abundance (19, 23), and when of sufficiently large magnitude, this variation may be detected as a collapse by our methods. Short-lived species may be particularly sensitive to such environmental variability because of their fast growth rates and short generation times (11). Long-lived species respond more slowly to changes in climatic conditions because they store more biomass in older age groups and are less dependent on recent recruitment success (11).
Our findings contrast with previous studies suggesting that population declines in marine species are correlated with large size, late age of maturity, slow growth rate, and high trophic level (7, 9, 24–28). However, previous analyses focused primarily on the North Atlantic (9, 25, 28) or on bycatch and artisanally fished species (24–27). The biased decline of large, high trophic-level species appears to be a unique pattern of the North Atlantic that does not apply globally (29, 30). Bycatch and artisanally fished species are less likely to appear in the stock assessments or global landings that we analyzed. The adjustment of fishing pressure as a function of species’ productivity, as explained above, is also unlikely to occur for these weakly or unmanaged species. In addition, the small proportion of marine fishes that have been assessed under the World Conservation Union's (IUCN's) Red List of Threatened Species (31) have tended to be larger species, perhaps helping to explain why listed, threatened species tend to be larger than unlisted species (7).
Although our article has focused on testing whether lifespan, age of maturity, size, trophic level, growth rates, fecundity, and offspring investment are useful predictors of fisheries collapse, life histories are multifaceted strategies that include traits we could not examine. However, many life-history traits among fishes are strongly correlated, including body size, natural mortality, size at maturity, and population growth rate at low abundance (10, 32–34). In addition, our use of multivariate models allowed us to test whether combinations of traits might be useful predictors of collapse. We note that life-history evolution often reflects a complex adaptive response to the scales of environmental variation (11, 35), and life histories often diverge from a simple slow vs. fast dichotomy (11).
The high incidence of collapse that we uncover among small, short-lived, low trophic-level species has important implications for ecosystem structure and function, especially in “wasp-waisted” ecosystems where a few species play a large role in transferring food energy to higher trophic-level fishes, birds, and marine mammals (36). For example, sandeels (Ammodytes marinus) are targeted by the largest single-species fishery in the North Sea, and declining sandeel abundance can cause severely reduced breeding success in seabirds (37). Other studies suggest similar sensitivity to a few small fish species across a range of seabirds and pinnipeds (38–40). Even though short-lived species may recover more quickly from collapse than other fishes (41), collapses in small, low trophic-level species can last from years to decades (17). These durations are long enough to have substantial impacts on the food web (37, 39, 40).
In summary, analysis of stock assessment and global landings databases revealed that patterns of vulnerability in the ocean are dramatically different from those on land, and that both small and large fishes are vulnerable to collapse. A major driver of differences between marine and terrestrial vulnerabilities may be the importance of harvest versus habitat loss in these different ecosystems. A halt to overfishing is needed across the full spectrum of life histories, not just for top predators, to reduce the incidence of fishery collapses and to avoid the ecological, economic, and social disruption that they cause.
Materials and Methods
Data Sources.
We downloaded stock assessments on June 9, 2010 from the RAM Legacy database (1). The database was compiled in 2009 and 2010 from countries around the world, and assessments were the most current available at that time. In particular, we extracted time series of catch, model-estimated biomass, and fishing mortality rates from 1950 to 2008. The final year included in the time series was on average 2006 ± 1.5 (SD) (range: 2000–2008) and duration was on average 39.6 ± 12.4 (SD) y (range: 10–59 y). We also extracted reference points for BMSY and FMSY (the biomass and instantaneous fishing mortality rate, respectively, which result in maximum sustainable yield). We only used assessments for fishes (not invertebrates).
Our landings database contained statistics reported to the FAO of the United Nations (downloaded from http://www.fao.org/fishery/statistics/software/fishstat/en, December 2009). Only species-level records for fish with cumulative landings > 1,000 tons were retained; invertebrates and records for species groups (e.g., “Cods” or “Flatfishes”) were removed. We removed records with low cumulative catches to avoid minor and experimental fisheries, and results were similar when we only retained records with total landings > 10,000 tons. Data were reported in one of 19 major statistical areas, and one species in one area was considered a stock.
Fishery Collapses.
Assessment data.
For the assessments, we analyzed biomass relative to BMSY. Stocks that fell below 20 or 50% of BMSY were defined as collapsed or overfished, respectively. We also recorded the maximum depletion as BMIN/ BMSY, where BMIN is the minimum biomass in the time series. The length of collapse was the maximum number of consecutive years that a stock was below 20% of BMSY.
In cases where neither BMSY nor a proxy used in place of BMSY was reported in the stock assessment, we followed (1) and estimated BMSY from Schaefer surplus-production models fit to the assessment time series of annual total biomass and total catch or landings. Surplus-production models are commonly used in fisheries science and allow calculation of both carrying capacity and maximum sustainable yield (1). Models were fit in AD Model Builder (http://admb-project.org) assuming normally distributed errors. We only used time series greater than 20 y. We used surplus-production models to find reference points for 92 of 223 stocks.
To examine sensitivity to our choice of model form, we also fit a Fox surplus-production model. Compared with the Schaefer model, the Fox model assumes that BMSY is a smaller fraction of unfished biomass (37% instead of 50%). Only five stocks (2.2%) were reclassified using this approach, and in all cases this was from collapsed to not collapsed. This small change did not affect our results.
Landings data.
The choice of appropriate definitions for fisheries collapse in landings data has been contentious because some apparent collapses may result from stochasticity or changes in reporting, management, or fishing practices rather than population status (42, 43). By focusing only on true species (rather than species groups), we avoid false collapses that would otherwise appear when reporting improves and species groups begin to be reported as individual species. However, if management measures severely restrict landings to allow rebuilding of an overfished stock, a stock's landings could appear collapsed even if biomass was overfished but not fully collapsed. Changes in fleet capacity or fishing efficiency could also reduce landings independently from changes to population abundance.
To avoid false detection of collapses in landings to the extent possible, we evaluated a range of potential collapse definitions. For each definition, a stock was defined as collapsed when annual landings fell below 10% of a reference level for a specified window of time. The reference level was the maximum annual landings averaged over 1 or 5 y. Although 1 y has been used before (44), the 5-y average was used here to avoid false collapses triggered by a single, spuriously high year of landings (43). As our time window, we used either 1 (44), 2, or 4 consecutive years (45). We only looked for collapses in the years following the maximum annual landings.
We tested the landings-based definitions against the stock assessments for which we had both biomass and catch or landings data (n = 131). Taking the assessment-based collapse definitions to be accurate, the 5-y reference/2-y window collapse definition for landings data had a somewhat lower error rate than the others and misclassified 24 stocks (18%). Of these, 14 collapsed stocks were not detected by the landings definition, but 10 uncollapsed stocks were falsely detected. Falsely detected collapses tended to be for species with longer lifespans than those that we failed to detect (P = 0.006). In comparison, the lax 1-y maximum/1-y window collapse definition misclassified 28 stocks (21%). Other combinations of threshold and time window produced intermediate numbers of misclassified stocks. Therefore, we used the 5-y maximum/2-y window definition of collapse for landings data in our article.
Fisheries Characteristics.
We defined fishery initiation as the year in which landings reached 10% of the maximum annual landings within a stock. Relative fishing mortality for a stock in the assessment database was defined as the maximum instantaneous fishing mortality rate (maximum F) divided by the fishing mortality predicted to produce maximum sustainable yield (FMSY). Where FMSY was not available, we estimated FMSY from a Schaefer surplus-production model, as above (1). We used a 5-y running mean to average out noise, producing maximum F5-y/FMSY. Both metrics were averaged across all stocks within a species.
Life-History Traits.
We extracted information on each species’ maximum total length (centimeter), maximum weight (kilogram), lifespan (year), age of maturity (year), trophic level, growth rate, fecundity (eggs per individual), and parental investment in offspring from ref. 18. Growth rate was measured as the exponent (K) in the von Bertalanffy growth function (46). Offspring investment was measured as egg diameter (millimeter). We supplemented fecundity and egg diameter data with literature searches because sample size was initially low for these traits (Table S3). We used the average if multiples values were available. For the one stock assessment conducted on a species group (“Redfish species” on the Newfoundland-Labrador Shelf), we averaged the life history characteristics for the two species targeted by this fishery (Sebastes mentella and Sebastes fasciatus).
For comparison of collapse rates among species with opposing traits, we compared the upper and lower quartiles for each trait. For example, we defined top predators as species in the upper quartile of all trophic levels, and low trophic-level species as those in the lowest quartile. Thresholds were chosen independently for species in the assessment and landings databases, and so are slightly different between the two.
We used log-transformed values for lifespan, age of maturity, weight, length, growth rate, fecundity, and egg diameter. Because length and weight are highly correlated (Pearson correlation: P < 0.0001, ρ = 0.89 on log-transformed variables), we only report results for weight. We did not consider reproductive lifespan (lifespan minus age of maturity) because it is highly correlated to lifespan (Pearson correlation: P < 0.0001, ρ = 0.99 on log-transformed variables). Other life-history traits remain somewhat correlated (e.g., lifespan and growth rate or length and trophic level), but our conclusions do not depend on multiple regressions that would be affected by this lack of independence.
Statistical Models.
We fit generalized linear models with binomial errors and a logit link (47) to predict the probability of a stock collapsing within each species (either assessment or landings data). In other words, the proportion of stocks collapsed within each species i was assumed to follow a binomial distribution with mean pi. The linear predictor of pi was
where β1,j and β2,j are the fitted coefficients for trait j, and xi,j is the value of trait j for species i. The traits (x) were life history or fisheries characteristics. The binomial error model accounted for the fact that variance changes with the number of stocks within each species. Fitting the models to species-level (rather than stock-level) data avoided pseudo-replication of species. We used the same format to fit models to the proportion of overfished stocks. For maximum depletion in the assessment data, we used standard linear models.
Next, we evaluated models with all possible combinations of life history variables:
where β is a vector of parameters and xi a vector of life history traits for species i. The models did not include interactions between variables because of the large number of potential combinations. We only tested these models on species for which we had complete life history data (n = 55 for assessment data, n = 67 for landings). We evaluated all models within a model-choice framework (48) and retained the minimal adequate models with an Akaike's Information Criterion within 2 of the lowest Akaike's Information Criterion. We also tested the specific hypothesis that species adapted to stable environments, as indicated by low fecundity and high parental investment in offspring (11), would be more vulnerable to fisheries collapse. We did this by fitting a model with fecundity, egg diameter, and their interaction. In all cases, we evaluated a model's significance with a χ2 test comparing the reduction in deviance between a null model (only the mean) and the focal model.
To test whether fishery characteristics affected our results, we used the assessment data to build a generalized linear model for the proportion of stocks collapsed as a function of relative fishing mortality. We then used the model residuals in linear regressions that included each of the life-history characteristics.
Phylogenetically Independent Contrasts.
To correct for shared evolutionary history among species, we fit linear regressions through the origin on phylogenetically independent contrasts generated with the Analyses of Phylogenetics and Evolution package (49) in R 2.12.1. We used a simple phylogeny based upon the taxonomic classification of each species and equal branch lengths. In all cases, the response variable was the proportion of stocks collapsed within each species. This approach cannot account for differences in variance driven by the number of stocks in each species.
Supplementary Material
Acknowledgments
This research was part of a National Center for Ecological Analysis and Synthesis Distributed Graduate Seminar. We thank T. Branch, R. Hilborn, and B. Worm for insightful feedback. J. Baum, C. Minto, R. Froese, and S. Tracey helped with database development. This work was supported in part by National Science Foundation and National Defense Science and Engineering Graduate fellowships (to M.L.P.), a David H. Smith Postdoctoral Fellowship (to O.P.J.), National Science Foundation Comparative Analysis of Marine Ecosystem Organizaton Grant 1041678 (to O.P.J.), and the Census of Marine Life/Future of Marine Animal Populations (D.R.); financial support for the assessment database was provided by Natural Sciences and Engineering Research Council grants to J.A. Hutchings and a Canadian Foundation for Innovation grant to H. Lotze.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.E.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015313108/-/DCSupplemental.
References
- 1.Worm B, et al. Rebuilding global fisheries. Science. 2009;325:578–585. doi: 10.1126/science.1173146. [DOI] [PubMed] [Google Scholar]
- 2.Fisher DO, Owens IPF. The comparative method in conservation biology. Trends Ecol Evol. 2004;19:391–398. doi: 10.1016/j.tree.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Cardillo M, et al. Multiple causes of high extinction risk in large mammal species. Science. 2005;309:1239–1241. doi: 10.1126/science.1116030. [DOI] [PubMed] [Google Scholar]
- 4.Myers RA, Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423:280–283. doi: 10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- 5.Baum JK, et al. Collapse and conservation of shark populations in the Northwest Atlantic. Science. 2003;299:389–392. doi: 10.1126/science.1079777. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds JD, Dulvy NK, Goodwin NB, Hutchings JA. Biology of extinction risk in marine fishes. Proc Biol Sci. 2005;272:2337–2344. doi: 10.1098/rspb.2005.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olden JD, Hogan ZS, Zanden MJV. Small fish, big fish, red fish, blue fish: Size-biased extinction risk of the world's freshwater and marine fishes. Glob Ecol Biogeogr. 2007;16:694–701. [Google Scholar]
- 8.Dulvy NK, Sadovy Y, Reynolds JD. Extinction vulnerability in marine populations. Fish Fish. 2003;4:25–64. [Google Scholar]
- 9.Jennings S, Reynolds JD, Mills SC. Life history correlates of responses to fisheries exploitation. P Roy Soc Lond B Bio. 1998;265:333–339. [Google Scholar]
- 10.Denney NH, Jennings S, Reynolds JD. Life-history correlates of maximum population growth rates in marine fishes. Proc Biol Sci. 2002;269:2229–2237. doi: 10.1098/rspb.2002.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winemiller KO. Life history strategies, population regulation, and implications for fisheries management. Can J Fish Aquat Sci. 2005;62:872–885. [Google Scholar]
- 12.King JR, McFarlane GA. Marine fish life history strategies: Applications to fishery management. Fish Manag Ecol. 2003;10:249–264. [Google Scholar]
- 13.Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F., Jr Fishing down marine food webs. Science. 1998;279:860–863. doi: 10.1126/science.279.5352.860. [DOI] [PubMed] [Google Scholar]
- 14.Hilborn R, Walters CJ. Quantitative Fisheries Stock Assessment: Choice, Dynamics, and Uncertainty. Boston: Kluwer Academic Publishers; 1992. [Google Scholar]
- 15.Sethi SA, Branch TA, Watson R. Global fishery development patterns are driven by profit but not trophic level. Proc Natl Acad Sci USA. 2010;107:12163–12167. doi: 10.1073/pnas.1003236107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costello C, Gaines SD, Lynham J. Can catch shares prevent fisheries collapse? Science. 2008;321:1678–1681. doi: 10.1126/science.1159478. [DOI] [PubMed] [Google Scholar]
- 17.Beverton RJH. Small marine pelagic fish and the threat of fishing: Are they endangered? J Fish Biol. 1990;37(sA):5–16. [Google Scholar]
- 18.Froese R, Pauly D, editors. FishBase. 2010. www.fishbase.org. Accessed January 21, 2008.
- 19.Chavez FP, Ryan J, Lluch-Cota SE, Niquen C M. From anchovies to sardines and back: multidecadal change in the Pacific Ocean. Science. 2003;299:217–221. doi: 10.1126/science.1075880. [DOI] [PubMed] [Google Scholar]
- 20.Felsenstein J. Phylogenies and comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- 21.Bakun A, Broad K. Environmental “loopholes” and fish population dynamics: Comparative pattern recognition with focus on El Niño effects in the Pacific. Fish Oceanogr. 2003;12:458–473. [Google Scholar]
- 22.Fryxell JM, Packer C, McCann K, Solberg EJ, Saether BE. Resource management cycles and the sustainability of harvested wildlife populations. Science. 2010;328:903–906. doi: 10.1126/science.1185802. [DOI] [PubMed] [Google Scholar]
- 23.Baumgartner TR, Soutar A, Ferreira-Bartrina V. Reconstruction of the history of Pacific sardine and northern anchovy populations over the past two millenia from sediments of the Santa Barbara basin, California. CCOFI Rep. 1992;33:24–40. [Google Scholar]
- 24.Jennings S, Reynolds JD, Polunin NVC. Predicting the vulnerability of tropical reef fishes to exploitation with phylogenies and life histories. Conserv Biol. 1999;13:1466–1475. [Google Scholar]
- 25.Dulvy NK, Metcalfe JD, Glanville J, Pawson MG, Reynolds JD. Fishery stability, local extinctions, and shifts in community structure in skates. Conserv Biol. 2000;14:283–293. [Google Scholar]
- 26.Dulvy NK, Reynolds JD. Predicting extinction vulnerability in skates. Conserv Biol. 2002;16:440–450. [Google Scholar]
- 27.Byrnes JE, Reynolds PL, Stachowicz JJ. Invasions and extinctions reshape coastal marine food webs. PLoS ONE. 2007;2:e295. doi: 10.1371/journal.pone.0000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jennings S, Greenstreet SPR, Reynolds JD. Structural change in an exploited fish community: A consequence of differential fishing effects on species with contrasting life histories. J Anim Ecol. 1999;68:617–627. [Google Scholar]
- 29.Essington TE, Beaudreau AH, Wiedenmann J. Fishing through marine food webs. Proc Natl Acad Sci USA. 2006;103:3171–3175. doi: 10.1073/pnas.0510964103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher JAD, Frank KT, Leggett WC. Global variation in marine fish body size and its role in biodiversity–ecosystem functioning. Mar Ecol Prog Ser. 2010;405:1–13. [Google Scholar]
- 31.Baillie JEM, Hilton-Taylor C, Stuart S, editors. 2004 IUCN Red List of Threatened Species: A Global Species Assessment. Gland, Switzerland and Cambridge, United Kingdom: International Union for Conservation of Nature; 2004. [Google Scholar]
- 32.Charnov EL. Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology. Oxford: Oxford University Press; 1993. [Google Scholar]
- 33.Frisk MG, Miller TJ, Fogarty MJ. Estimation and analysis of biological parameters in elasmobranch fishes: A comparative life history study. Can J Fish Aquat Sci. 2001;58:969–981. [Google Scholar]
- 34.Roff DA. The evolution of life history parameters in teleosts. Can J Fish Aquat Sci. 1984;41:989–1000. [Google Scholar]
- 35.Roff DA. Life History Evolution. Sunderland, MA: Sinauer Associates; 2002. [Google Scholar]
- 36.Cury P, et al. Small pelagics in upwelling systems: Patterns of interaction and structural changes in “wasp-waist” ecosystems. ICES J Mar Sci. 2000;57:603–618. [Google Scholar]
- 37.Frederiksen M, Wanless S, Harris MP, Rothery P, Wilson LJ. The role of industrial fisheries and oceanographic change in the decline of North Sea black-legged kittiwakes. J Appl Ecol. 2004;41:1129–1139. [Google Scholar]
- 38.Guénette S, Heymans SJJ, Christensen V, Trites AW. Ecosystem models show combined effects of fishing, predation, competition, and ocean productivity on Steller sea lions (Eumetopias jubatus) in Alaska. Can J Fish Aquat Sci. 2006;63:2495–2517. [Google Scholar]
- 39.Duffy DC. Environmental uncertainty and commercial fishing: Effects on Peruvian guano birds. Biol Conserv. 1983;26:227–238. [Google Scholar]
- 40.Crawford RJM. Food, fishing and seabirds in the Benguela upwelling system. J Ornithol. 2007;148:253–260. [Google Scholar]
- 41.Hutchings JA. Collapse and recovery of marine fishes. Nature. 2000;406:882–885. doi: 10.1038/35022565. [DOI] [PubMed] [Google Scholar]
- 42.de Mutsert K, Cowan JH, Jr, Essington TE, Hilborn R. Reanalyses of Gulf of Mexico fisheries data: landings can be misleading in assessments of fisheries and fisheries ecosystems. Proc Natl Acad Sci USA. 2008;105:2740–2744. doi: 10.1073/pnas.0704354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilberg MJ, Miller TJ. Comment on “Impacts of biodiversity loss on ocean ecosystem services”. Science. 2007;316:1285. doi: 10.1126/science.1137946. author reply 1285. [DOI] [PubMed] [Google Scholar]
- 44.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 45.Mullon C, Fréon P, Cury P. The dynamics of collapse in world fisheries. Fish Fish. 2005;6:111–120. [Google Scholar]
- 46.von Bertalanffy L. A quantitative theory of organic growth (Inquiries on Growth Laws. II.) Hum Biol. 1938;10:181–213. [Google Scholar]
- 47.Dobson AJ. An Introduction to Generalized Linear Models. Boca Raton: Chapman & Hall; 2002. 2nd Ed. [Google Scholar]
- 48.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd Ed. New York: Springer; 2002. p. 488. [Google Scholar]
- 49.Paradis E, Claude J, Strimmer K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.