Abstract
The hallmarks of Alzheimer's disease are the aggregates of amyloid-β (Αβ) peptide and tau protein. Autophagy is one major cellular pathway leading to the removal of aggregated proteins. We examined the possibility of inducing autophagy to reduce Aβ peptide and the amyloid precursor protein (APP)-derived fragment APP-CTF levels in cell lines and primary neuronal cultures. We found that induction of autophagy either by small-molecule enhancers of rapamycin (SMER)28, a small-molecule enhancer of autophagy, or following starvation greatly decreased the levels of Aβ peptide (apparent EC50 of ∼10 μM) and APP-CTF (apparent EC50 of ∼20 μM) in a γ-secretase-independent manner. Pharmacological inhibition of autophagy led to a significant accumulation of Aβ peptide and APP-CTF and diminished the effect of SMER28. Three essential components of the autophagic pathway, autophagy-related protein (Atg)5, Beclin1, and Ulk1, were shown to be involved in the degradation of Aβ and APP-CTF, and Atg5 was necessary for the effect of SMER28. In addition, the autophagic marker light chain 3-II cocompartmentalized with APP-CTF. These results support the involvement of autophagy in the clearance of Aβ and APP-CTF. We therefore propose that small molecule enhancers of autophagy, such as SMER28, may have therapeutic potential for the treatment of Alzheimer's disease and other proteinopathies.—Tian, Y., Bustos, V., Flajolet, M., Greengard, P. A small-molecule enhancer of autophagy decreases levels of Aβ and APP-CTF via Atg5-dependent autophagy pathway.
Keywords: Alzheimer's disease, SMER28, Beclin1, Ulk1
Alzheimer's disease (AD) is the most common neurodegenerative disorder. The hallmarks of the disease are the tau tangles and amyloid-β (Αβ) plaques. Aβ is generated via sequential proteolysis of amyloid precursor protein (APP; refs. 1, 2). ΑPP can be cleaved by β-secretase to produce a C-terminal fragment (βCTF), which is then further processed by γ-secretase to release Aβ peptides. Alternatively, APP can be successively cleaved by α-secretase and then γ-secretase to produce αCTF and P3 peptides. Although altered proteolytic processing of APP plays a central role in the production and accumulation of Aβ, failure of Aβ clearance can contribute to the pathogenesis in sporadic AD (3). It has been reported that Aβ accumulates within autophagic vacuoles in swollen dystrophic neurites in human AD brain, suggesting the involvement of autophagy in AD pathogenesis (4).
Macroautophagy, hereafter referred to as autophagy, is the major cellular pathway for degradation of long-lived and aggregated proteins, as well as cytoplasmic organelles (5, 6). Since most aggregate-prone proteins are high-molecular-weight complexes and they are too large to enter the narrow pore of the proteasome barrel, such proteins or complexes can only be cleared by the autophagy pathway (7). Morphologically, autophagy is initiated when a cup-shaped “isolation” membrane (a phagophore) is formed. The membrane of phagophore undergoes elongation and sequestrates cytosolic components and organelles into a double membrane-bound autophagic vacuole or autophagosome (8–10). Subsequently, autophagosomes fuse with lysosomes for content degradation (11).
Autophagy can be induced under physiological stress, such as starvation. Indeed, under nutrient-limiting conditions, the activity of the mammalian target of rapamycin (mTOR) kinase, a central sensor of nutrient signal, is inhibited. The inhibition of mTOR leads to dephosphorylation of autophagy-related protein 13 (Atg13) and Ulk1, resulting in the activation of the Ulk1-Atg13-FIP200 complex to trigger autophagy (12). During initiation of autophagosome formation, Vps34, a class III PI3K, can recruit other Atg proteins to form an autophagy-regulating macromolecular complex (13–15). This complex, together with the UlK1-Atg13-FIP200 complex, plays an important role in the initiation of autphagosome formation. Furthermore, the activity of Vsp34 is enhanced by binding to Beclin1 (7, 14). Two ubiquitin-like conjugation reactions are essential for the elongation process of the phagophore membrane. These reactions consist of the conjugation of Atg12 to Atg5, which requires Atg7 as E1 and Atg10 as E2, and the conjugation of LC3 to phosphatidylethanolamine to form LC3-II, sharing the same E1 (Atg7) but a different E2 (Atg3) (16). Once the autophagosomes form, they fuse with lysosomes for content degradation. However, the mechanism of autophagosome-lysosome fusion in mammalian autophagy is not clear. In addition to mTOR-dependent autophagy, mTOR-independent autophagy was discovered when autophagy was found to be induced by lowering intracellular inositol or inositol 1,4,5-trisphosphate levels independently of mTOR (17).
Age-dependent decrease in autophagy was suggested to be responsible for the accumulation of abnormal proteins during aging (18). Impairment of the autophagy pathway is suggested to be involved in neurodegeneration and a variety of neurodegenerative diseases, including Parkinson disease, Huntington disease, and AD (7, 19–21). Enhancing autophagy may be a possible therapeutic strategy for neurodegenerative disorders. Therefore, considerable effort has been made to identify autophagy-inducing molecules (7, 22). Small-molecule enhancers of rapamycin (SMERs), identified by chemical screens, were discovered to induce mTOR-independent autophagy and reduce mutant huntintin aggregates and A53T α-synuclein in Huntington and Parkinson disease cellular models (23). Nevertheless, it is not known if those compounds are able to remove aggregated proteins in other neurodegenerative diseases, such as AD.
It was recently reported that the Beclin1 complex regulates APP processing and plays an important role in AD pathology (24). In our study, we have demonstrated that basal Aβ and APP-CTF can be degraded by autophagy and that the autophagic proteins Atg5, Beclin1, and Ulk1 are all involved in the process. SMER28, a small-molecule enhancer of autophagy, can promote the Atg5-dependent degradation of Aβ and APP-CTF. Therefore, we propose that small molecule autophagy enhancers, such as SMER28, have significant therapeutic potential for the treatment of AD and possibly other proteinopathies.
MATERIALS AND METHODS
Reagents
The following antibodies were used at 1:1,000 dilutions: RU-369, a rabbit polyclonal antibody that recognizes the C-terminal of APP695 (25); Ab14 antiserum targeting residues 1–25 of presenilin 1 (PS1)-NTF (26); 6E10 antibody against Aβ1–16 (Convance, Princeton, NJ, USA); anti-LC3 (Sigma, St. Louis, MO, USA); anti-APLP1 (Calbiochem, Gibbstown, NJ, USA); anti-Beclin1 (BD Biosciences, San Jose, CA, USA); anti-PS1-CTF (Millipore, Billerica, MA, USA); anti-γ-Adaptin (BD Biosciences); and anti-Bip (Abcam, Cambridge, MA, USA). Compound SMER28 was purchased from EMD Chemicals (Gibbstown, NJ, USA). LDH assay kit was purchased from Roche (Nutley, NJ, USA).
Cell culture and siRNA
Mouse embryonic fibroblast (MEF) cells generated from WT and Atg5−/− embryos (5) were obtained from the RIKEN BRC cell bank (Tsukuba, Ibaraki, Japan) and maintained in DMEM with 10% FBS. Starvation was carried out in DMEM without amino acids and FBS for 2 h. Mouse neuroblastoma (N2a) cells were maintained in medium containing 50% DMEM and 50% Opti-MEM, supplemented with 5% FBS (Invitrogen, Carlsbad, CA, USA). The siRNAs for Beclin1 and Ulk1 were purchased from Dharmacon (Lafayette, CO, USA; On-TARGETplus set of 4 siRNAs, J-055895-05; On-TARGETplus SMARTpool, L-040155-00-0005). The control siRNA was purchased from Dharmacon (On-TARGET plus GAPD Control siRNA, D-001830-02-05).
Primary neuronal cultures
Cerebral cortices or hippocampi were dissected from embryonic day 18 (E18) rat embryos. Cells were dissociated with trypsin and grown in Neurobasal medium (Gibco-BRL, Carlsbad, CA, USA) supplemented with B27, N2, and 0.5 mM glutamine (Gibco-BRL). Cortical neurons were plated at a density of 8 × 104/cm2 on 24-well cell culture plates coated with poly-l-lysine (Sigma).
Immunofluorescence and confocal microscopy
Cells were grown in 4-well slide chambers (Lab Tek; Nalge Nunc, Rochester, NY, USA) and fixed with 4% paraformaldehyde. Cells were then permeabilized in 0.1% Triton X-100 and stained with primary antibodies, followed by FITC-conjugated secondary antibodies. The coverslips were mounted by Prolong Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA, USA). The images were aquired using a confocal microscope (LSM510 META; Carl Zeiss MicroImaging, Thornwood, NY, USA) and the LSM5 3.2 software.
Subcellular fractionation
For sucrose density gradient fractionation, cells were prepared as described previously (27, 28). Briefly, cells were homogenized by using a stainless steel ball-bearing homogenizer in 0.25 M sucrose, 10 mM Tris-HCl (pH 7.4), 1 mM MgAc2, and a protease inhibitor cocktail. The homogenate was loaded on top of a step gradient comprised of 1 ml of 2 M sucrose, 4 ml of 1.3 M sucrose, 3.5 ml of 1.16 M sucrose, and 2.0 ml of 0.8 M sucrose. The gradients were centrifuged for 2.5 h at 390,000 g in a Beckman SW41Ti rotor (Beckman Instruments, Fullerton, CA, USA). Fractions (1 ml) were collected from the top of each gradient and assayed by Western blot using γ-adaptin, BIP, LC3, Rab9, and Ru-369 antibodies.
RESULTS
SMER28 induces a marked decrease in Aβ and APP-CTF levels
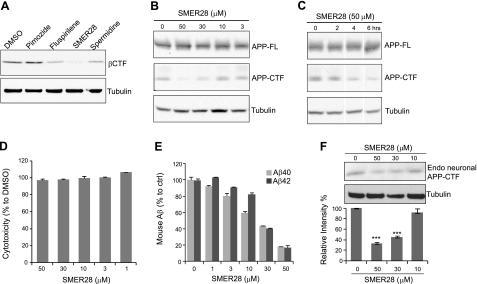
Previous reports (7, 29) described the identification of small-molecule compounds capable of inducing autophagy. Based on safety profiles and therapeutic potentials, we tested 4 of those compounds, pimozide, fluspirilene, SMER28, and spermidine, for their ability to reduce Aβ40 peptide and APP-βCTF levels in cultured cells overexpressing βCTF. SMER28 induced a significant decrease in secreted Aβ40 peptide, and it had a greater effect on reducing APP-βCTF levels (Fig. 1A). For these reasons, and the absence of cytotoxicity under the conditions tested, we chose to focus our efforts on SMER28. N2a cells stably expressing APP-695 (N2a-APP) were treated with various concentrations of SMER28 for 16 h (Fig. 1B) or with a fixed concentration (50 μM) for various periods of time (Fig. 1C). SMER28 induced both a dose- and time-dependent reduction of APP-CTF (Fig. 1B, C) with apparent EC50 of ∼20 μM. With the concentrations of SMER28 tested, no cytotoxicity by LDH assay was observed (Fig. 1D). N2a cells stably expressing βCTF were also treated with various concentrations of SMER28 for 16 h. The levels of βCTF, detected using the 6E10 antibody, were decreased by SMER28 in a dose-dependent manner (Supplemental Fig. S1). We next tested the effect of SMER28 on primary neuronal cultures. Mixed cultures of cortical-hippocampal neurons were prepared from rat E18 embryos, cultured for 8 d, and exposed to SMER28 for 16 h. The secreted conditioned media were assayed for Aβ40 and Aβ42 by ELISA, and cell lysates were assayed for APP-CTF by Western blotting. SMER28 induced a significant decrease of secreted Aβ40 and Aβ42 (EC50 ∼10 μM) and of endogenous APP-CTF (EC50 of ∼20 μM) in a dose-dependent manner in the primary neuronal cultures (Fig. 1E, F).
Figure 1.
Effect of SMER28 on levels of Aβ peptide and APP-CTF. A) MEF cells overexpressing βCTF were treated with autophagy-enhancing compounds (10 μM) for 16 h. Cell lysates were analyzed by SDS-PAGE and Western blotting using anti-βCTF (6E10) and anti-tubulin antibodies. B, C) N2a-APP cells were treated with increasing concentrations of SMER28 for 16 h (B) or with a fixed concentration of SMER28 (50 μM) for various periods of time (C). Cell lysates were analyzed by SDS-PAGE and Western blotting for the presence of APP-FL and APP-CTF using RU-369 antibody. D) N2a-APP cells were treated with various concentrations of SMER28 for 16 h, and the cells were analyzed using an LDH assay (Roche) according to the manufacturer's protocol. E) Rat primary neuronal cultures were treated with various concentrations of SMER28 for 16 h. Conditioned media were analyzed for the presence of soluble Aβ40 and Aβ42 peptides by ELISA. F) Top panels: cell lysates were analyzed by SDS-PAGE and Western blotting for endogenous APP-CTF. Bottom panel: quantification of APP-CTF (n≥3). Error bars = se. ***P < 0.001.
SMER28 and starvation enhance the degradation of Aβ and APP-CTF via autophagy
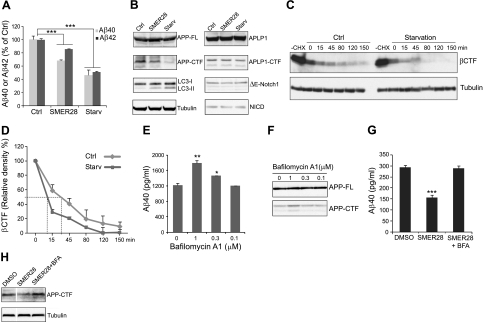
We next investigated whether autophagy was involved in the mechanism by which SMER28 induces down-regulation of Aβ40/Aβ42 and APP-CTF. To this end, N2a-APP cells were treated with SMER28 for 6 h or starved (medium depleted in amino acids and serum) for 2 h. The increase in LC3-II, a classical autophagy marker, in the SMER28- and starvation-treated samples indicated the occurrence of autophagy (Fig. 2B). As with SMER28, starvation induced a marked decrease of both Aβ40/Aβ42 and APP-CTF levels but only slightly affected full-length APP expression level (Fig. 2A, B). APLP1 and Notch are two other proteins that are targeted by γ-secretase and undergo proteolysis. Therefore, we tested the specificity of the effects by investigating whether APLP1 and Notch signaling are regulated by SMER28 or by starvation. As shown in Fig. 2B, there was no change in N2a cells in the levels of expression of either endogenous full-length APLP1 or, more important, the processed form APLP1-CTF. Furthermore, by introducing an engineered form of Notch capable of being cleaved directly by γ-secretase (ΔE-Notch1) into the same cellular system, we were able to show that ΔE-Notch1 and NICD, a fragment generated after γ-secretase cleavage, were not affected on SMER28 or starvation treatment. To further characterize the effect of autophagy on APP-CTF, we used cycloheximide to inhibit protein synthesis and measured the half-life of βCTF on starvation. The disappearance of βCTF in the starved cells was more rapid than in control cells (no starvation), with a half-time of 10 min compared with 32 min for the control cells (Fig. 2C, D). Taken together, our results demonstrate that SMER28 and starvation specifically stimulate degradation of Aβ40/Aβ42 and APP-CTF. Fusion to lysosomes is required for the degradation of proteins sequestered in autophagosomes, and this fusion can be blocked by bafilomycin A1 (BFA). When N2a-APP cells were treated with 1 μM BFA, a significant accumulation of Aβ40 peptide and APP-CTF fragment was observed (Fig. 2E, F). Although 0.1 μM BFA had no effect on Aβ40 and APP-CTF basal levels (Fig. 2E, F), it was able to prevent the SMER28 induced decrease of Aβ40 and APP-CTF (Fig. 2G, H). These results indicate that the autophagic-lysosomal degradation pathway is required for basal or SMER28-induced removal of Aβ and APP-CTF.
Figure 2.
SMER28 and starvation lead to reduced levels of Aβ peptide and APP-CTF through autophagy pathway. A, B) Effect of SMER28 and starvation on APP- and autophagy-related protein levels. A) N2a-APP cells were treated with SMER28 at 50 μM for 16 h or starved for 2 h, and conditioned media were analyzed for soluble Aβ40 or Aβ42 peptides by ELISA. ELISA signals were normalized to controls (n≥3). ctrl, control condition; starv, starvation condition. B) Cell lysates were analyzed by SDS-PAGE and Western blotting. C) N2a cells stably expressing βCTF were treated with or without cycloheximide (CHX; 50 μg/ml) for 1 h in the presence of γ-secretase inhibitor L-685,458 (1 μM). CHX-treated cells were then starved for different amounts of time (as indicated), and cell lysates were analyzed by SDS-PAGE and Western blotting for βCTF using 6E10 antibody. D) Kinetics of the disappearance of βCTF in control and starved cells. Dashed lines indicate half-time for disappearance. E, F) Effect of bafilomycin A (BFA), a blocker of the fusion of autophagosomes to lysosomes, on Aβ40 peptide (E) and APP-CTF levels (F) in N2a-APP cells (n≥3). G, H) BFA (0.1 μM) blocked the effect of SMER28 (50 μM) on Aβ40 (G) and APP-CTF (H) in N2a-APP cells (n≥3). Error bars = se. *P < 0.05; **P < 0.01; ***P < 0.005.
SMER28- and starvation-induced degradation of Aβ and APP-CTF are γ-secretase independent
To clarify whether the decrease of Aβ peptides is due to a decrease in γ-secretase activity on autophagy induction, we analyzed the levels of PS1-NTF and PS1-CTF, the catalytic core of γ-secretase (26, 30), (31), on SMER28 and starvation treatment. Their levels of expression were similar to the ones observed in control conditions (Supplemental Fig. S2A). Furthermore, we used compound L-685,458 to block the activity of γ-secretase. As expected, γ-secretase inhibitor treatment led to accumulation of APP-CTF (Supplemental Fig. S2B, C). Starvation and SMER28 treatment dramatically reduced the amount of accumulated APP-CTF in the presence of γ-secretase inhibitor (Supplemental Fig. S2B, C). Taken together, these results demonstrated that the reductions of Aβ peptides and APP-CTF by starvation or SMER28 were not dependent on γ-secretase.
Atg5 plays an essential role in degradation of Aβ and APP-CTF, induced by starvation or SMER28
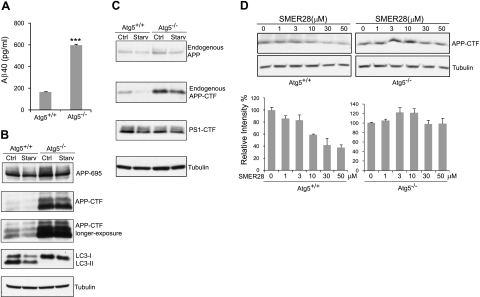
The biological functions of autophagy have been examined by generating mice lacking several critical genes such as: Atg5 (5), beclin 1 (32, 33), and Atg7 (34). All showed severe defects in autophagy. To further investigate the involvement of autophagy in the degradation of Aβ40 and APP-CTF, we used Atg5−/− MEF and control Atg5+/+ MEF cells (5). In MEF cells overexpressing APP-695, Aβ40 levels increased 3.7-fold in the Atg5−/− cells compared with wild-type Atg5+/+ cells (Fig. 3A). APP-CTF levels were increased 9.4-fold in the Atg5−/− control cells compared with Atg5+/+ controls, while APP695 was increased by 38% (Fig. 3B, top 3 panels). Furthermore, endogenous APP/APP-CTF levels were analyzed in Atg5−/− and Atg5+/+ MEF cells. In this context, endogenous APP-CTF also showed a significant accumulation in Atg5−/− cells (increase 4.4-fold) in comparison to Atg5+/+ cells, while full-length APP level increased only by 25% (Fig. 3C, top 2 panels). These results suggested that Atg5 was required for the housekeeping clearance of Aβ and APP-CTF, in addition to the partial removal of APP full length. Moreover, starvation of the cells reduced 74% of endogenous APP-CTF (Fig. 3C, 2nd panel) and 43% of the overexpressed APP-CTF (Fig. 3B, middle 2 panels) in Atg5+/+ cells, but it only induced a reduction of 20 and 4.6%, respectively, in Atg5−/− cells. These results indicated that the degradation of APP-CTF triggered by starvation also required Atg5. It is worth noting that there was a modest reduction of APP-CTF in Atg5−/− cells following starvation. To determine whether the effect of Atg5 deletion on Aβ40 and APP-CTF degradation was specific, we analyzed the levels of PS1-CTF and tubulin. No significant difference was detected among Atg5+/+ and Atg5−/− cells, with or without starvation (Fig. 3C). Taken together, these results suggested that Atg5 is required for the clearance of Aβ and APP-CTF via autophagy.
Figure 3.
SMER28-induced clearance of Aβ40 and APP-CTF is dependent on Atg5. A, B) Atg5+/+ and Atg5−/− MEF cells were transfected with a plasmid encoding APP695. A) At 16 h post-transfection, conditioned media were collected and subjected to Aβ40 peptide analysis by ELISA (n≥3). ***P < 0.005. B) Cell lysates were analyzed by SDS-PAGE and Western blotting. C) Atg5+/+ and Atg5−/−MEF cells were starved for 2 h, lysed, and analyzed for endogenous APP-FL, APP-CTF, PS1-CTF, and tubulin using SDS-PAGE and Western blotting. D) Top panels: Atg5+/+ and Atg5−/−MEF cells were treated with increasing concentrations of SMER28 for 6 h. Endogenous APP-CTF and tubulin expression levels were analyzed by SDS-PAGE and Western blotting. Bottom panels: results of APP-CTF quantifications from Western blots. Error bars = se.
Next, we sought to determine whether Atg5 is necessary for the effect of SMER28 on Aβ and APP-CTF degradation. Atg5+/+ and Atg5−/− MEF cells were treated with SMER28 for 6 h, and endogenous APP-CTF fragment was analyzed by SDS-PAGE and Western blotting. We observed a dose-dependent decrease of APP-CTF in Atg5+/+ MEF cells but not in Atg5−/− MEF cells (Fig. 3D). These results suggest that SMER28 induced APP-CTF degradation is through an Atg5-dependent pathway.
Beclin1 and Ulk1 regulate basal, but not SMER28-induced, clearance of Aβ and APP-CTF
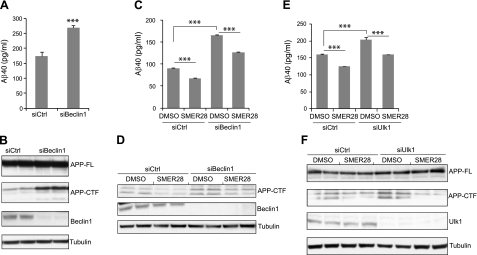
To further confirm the role of autophagy in clearing Aβ and APP-CTF, we examined whether Beclin1 or Ulk1, two other components of the autophagy pathway, were involved. siRNA was used to knock down either Beclin1 or Ulk1 in N2a-APP cells. As shown in Fig. 4B, D, F, we obtained high Beclin1 or Ulk1 silencing efficiency. We found that Aβ40 (Fig. 4A) and APP-CTF (Fig. 4B) significantly increased after Beclin1 silencing. Similarly, silencing of Ulk1 also caused significant accumulation of both Aβ40 and APP-CTF (Fig. 4E, F). Subsequently, Beclin1- or Ulk1-silenced cells were treated with SMER28 for 6 h. We observed a 25% decrease of Aβ40 and a 49% decrease of APP-CTF in both control and Beclin1-silenced cells (Fig. 4C, D), suggesting that knocking down of Beclin1 had no inhibitory effect on SMER28-induced Aβ40 and APP-CTF degradation. Likewise, Ulk1 silencing had no obvious inhibitory effect on SMER28 treatment (Fig. 4E, F). These results suggested that Beclin1 and Ulk1 are required for the degradation of Aβ40 and APP-CTF through autophagy and that SMER28 may act downstream of Beclin1 and Ulk1 for the reduction of Aβ40 and APP-CTF levels. However, we can not rule out the possibility that residual levels of Beclin1 and Ulk1after siRNA knockdown are sufficient to carry out its function in autophagy on SMER28 treatment.
Figure 4.
Beclin1 and Ulk1 regulate basal, but not SMER28-induced, clearance of Aβ40 and APP-CTF. A, B) N2a-APP cells were transfected with control siRNAs or with a mixture of 4 Beclin1 siRNAs for 24 h. A) Conditioned media were recovered, and the presence of soluble Aβ40 was measured by ELISA (n≥3). B) Cell lysates were prepared and analyzed by SDS-PAGE and Western blotting for APP-FL, APP-CTF, Beclin1, and tubulin. C, D) N2a-APP cells were transfected with control or Beclin1 siRNAs for 24 h and then treated with SMER28 (50 μM) for 6 h. Conditioned media (C) and cell lysates (D) were harvested for analysis as described above (n≥3). E, F) N2a-APP cells were transfected with control siRNA or a mixture of Ulk1 siRNAs for 24 h h and then treated with SMER28 (50 μM) for 6 h. Conditioned media (E) and cell lysates (F) were harvested for analysis as described above (n≥3). Error bars = se. ***P < 0.005.
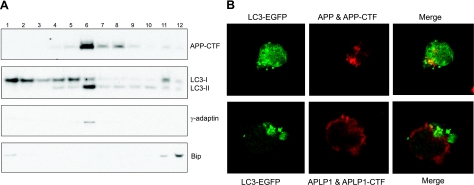
APP-CTF and LC3-II are cocompartmentalized on SMER28 treatment
To investigate whether the trafficking pathway of APP-CTF overlaps with the autophagy pathway, we examined, first by subcellular fractionation, whether APP-CTF cocompartmentalizes with the autophagosome marker LC3-II. N2a-APP cells were treated with SMER28 for 6 h, and cell lysates were fractioned by sucrose centrifugation. Twelve fractions from top to bottom were collected and analyzed by SDS-PAGE and Western blotting. The majority of APP-CTF was localized in fraction 6, where γ-adaptin, a Golgi marker protein, is also localized (Fig. 5A). Interestingly, the autophagy marker LC3-II was also enriched in fraction 6. In contrast, LC3-I showed a more diffuse pattern, with enrichment in lower-density fractions. Bip, an ER protein marker, mostly localized to fractions 11 and 12. This result suggested APP-CTF cocompartmentalized with autophagosome. We further assessed the colocalization of APP-CTF and LC3 by immunoflurescence. N2a-APP cells were transfected with LC3-EGFP and treated with SMER28 for 6 h. APP and APP-CTF were stained with an antibody recognizing the C-terminal of APP. After SMER28 treatment, typical punctuate structures of LC3-EGFP were seen (Fig. 5B, top left panel) and APP/APP-CTF were localized at Golgi and trans-Golgi compartments (35) as expected (Fig. 5B, top middle panel). After merging LC3 and APP staining, a certain degree of colocalization of APP-CTF with the LC3-marked autophagosome (shown as green dots) was indeed observed (Fig. 5B, top right panel; Supplemental Fig. S3). We used APLP1 staining as a negative control and showed that it was mostly located at the cell surface (Fig. 5B, bottom middle panel), which is consistent with a previous report (36), and that it does not colocalize with LC-3 puncta (Fig. 5B, bottom right panel). Taken together, the above results using both the sucrose fractionation and immunoflurescence staining methods support the concept that the trafficking pathway of APP/APP-CTF can merge, at least partially, with the autophagosome-lysosomal pathway.
Figure 5.
SMER28 induced cocompartmentalization of APP-CTF and LC3-II. A) N2a-APP cells were treated for 6 h with SMER28 (50 μM), and whole-cell lysates were fractionated by sucrose gradient. An aliquot of each of the 12 fractions recovered was analyzed by SDS-PAGE and Western blotting for APP-CTF, LC3, γ-adaptin, and Bip. B) N2a-APP cells were transfected with an LC3-EGFP-containing plasmid; at 16 h post-transfection, cells were treated for 6 h with SMER28 (50 μM). Cells were fixed and immunostained with RU-369, an APP C-terminal antibody, or APLP1 antibody and imaged by confocal microscopy.
DISCUSSION
The Aβ peptides, which aggregate and accumulate in the brains of patients with AD, are prime targets for AD therapies. In the present study, we have shown that the autophagic proteins Atg5, Beclin1, and Ulk1 are involved in the housekeeping clearance of Aβ and APP-CTF. Furthermore, a small molecule, SMER28, reduced the levels of Aβ and APP-CTF in an Atg5-dependent fashion. We propose that low-molecular-weight autophagy enhancers have significant therapeutic potential for AD treatment.
It has been thought that basal autophagic activity is low in the healthy brain. However, a recent study (37) has shown that autophagy in primary cortical neurons is highly efficient and that newly formed autophagosomes are rapidly removed by fusion with lysosomes. Consistent with those results, we observed that in Atg5-knockout MEF cells (Atg5−/−), as well as in Beclin1 and Ulk1 knocked-down cells, Aβ and APP-CTF levels were dramatically increased, suggesting that under physiological conditions, Aβ and APP-CTF are rapidly cleared by autophagy. Therefore, autophagy might be a housekeeping mechanism for the removal of Aβ and APP-CTF. We think that in parallel to the decline of autophagy in aging, the efficiency of the housekeeping clearance decreases, so that Aβ, normally rapidly degraded, will gradually accumulate until reaching concentrations compatible with aggregate formation. Therefore, enhancing the degradation of the Aβ and APP-CTF in aging brain might be beneficial to prevent and/or treat AD.
Recent studies (19, 20, 38), demonstrating that loss of basal autophagy in mouse neuronal cells resulted in neurodegeneration, suggest that autophagy may have a protective role against the development of various neurodegenerative diseases. In addition, it has been shown that pharmacological stimulation of autophagy can increase life span in yeast (39, 40), C. elegans (41, 42), D. melanogaster (43), and mice (44) and is beneficial for Aβ induced toxicity in vivo (45, 46) in AD. Furthermore, it was reported that in Parkinson disease and Hungtington disease, misfolded proteins, α-synuclein and huntingtin, respectively, were targeted by autophagy (7, 23, 47). Therefore, the use of pharmacological boosters of autophagy could represent an effective therapeutic approach to prevent or treat age-related neurodegenerative symptoms and progression (48). Theoretically, one can reduce Aβ accumulation in AD either through inhibiting its production or through enhancing its degradation. Enormous effort has been made to inhibit its production, but progress has been slow due to lack of specificity and possibly the side-effects of inhibiting a multitasking protease. Therefore, activating autophagy to enhance the degradation of Aβ is an alternative approach for AD therapy. Our present studies showed that SMER28, a small-molecule enhancer of autophagy, can promote Aβ and APP-CTF degradation without affecting related proteins, such as Notch and APLP1. Since APP-βCTF was implicated in endosome dysfunction in Down syndrome (49), pharmacological removal of APP-βCTF and Aβ might be beneficial not only to patients with AD but also to patients with Down syndrome.
Supplementary Material
Acknowledgments
The authors thank Drs. Wenjie Luo and William Netzer for sharing reagents and for helpful discussion, Dr. Zhenyu Yue (Mount Sinai Medical College, New York, NY, USA) for providing reagents, and Dr. Noboru Mizushima (Department of Physiology and Cell Biology, Tokyo Medical and Dental University, Tokyo, Japan) for the use of Atg5+/+ and Atg5−/− MEF cells.
This work was supported by U.S. National Institutes of Health grant AG-09464 and the Fisher Center for Alzheimer's Research Foundation.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. (1988) Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature 331, 528–530 [DOI] [PubMed] [Google Scholar]
- 2. Sisodia S. S., Price D. L. (1995) Role of the beta-amyloid protein in Alzheimer's disease. FASEB J. 9, 366–370 [DOI] [PubMed] [Google Scholar]
- 3. Selkoe D. J. (2000) Toward a comprehensive theory for Alzheimer's disease. Hypothesis: Alzheimer's disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann. N. Y. Acad. Sci. 924, 17–25 [DOI] [PubMed] [Google Scholar]
- 4. Nixon R. A., Wegiel J., Kumar A., Yu W. H., Peterhoff C., Cataldo A., Cuervo A. M. (2005) Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J. Neuropathol. Exp. Neurol. 64, 113–122 [DOI] [PubMed] [Google Scholar]
- 5. Kuma A., Hatano M., Matsui M., Yamamoto A., Nakaya H., Yoshimori T., Ohsumi Y., Tokuhisa T., Mizushima N. (2004) The role of autophagy during the early neonatal starvation period. Nature 432, 1032–1036 [DOI] [PubMed] [Google Scholar]
- 6. Klionsky D. J., Emr S. D. (2000) Autophagy as a regulated pathway of cellular degradation. Science 290, 1717–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia-Arencibia M., Hochfeld W. E., Toh P. P., Rubinsztein D. C. (2010) Autophagy, a guardian against neurodegeneration. Semin. Cell Dev. Biol. 21, 691–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mizushima N., Ohsumi Y., Yoshimori T. (2002) Autophagosome formation in mammalian cells. Cell Struct. Funct. 27, 421–429 [DOI] [PubMed] [Google Scholar]
- 9. Wang C. W., Klionsky D. J. (2003) The molecular mechanism of autophagy. Mol. Med. 9, 65–76 [PMC free article] [PubMed] [Google Scholar]
- 10. Mizushima N., Yamamoto A., Matsui M., Yoshimori T., Ohsumi Y. (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell 15, 1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jahreiss L., Menzies F. M., Rubinsztein D. C. (2008) The itinerary of autophagosomes: from peripheral formation to kiss-and-run fusion with lysosomes. Traffic 9, 574–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 22, 132–139 [DOI] [PubMed] [Google Scholar]
- 13. Itakura E., Kishi C., Inoue K., Mizushima N. (2008) Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol. Biol. Cell 19, 5360–5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Funderburk S. F., Wang Q. J., Yue Z. The Beclin 1-VPS34 complex–at the crossroads of autophagy and beyond. Trends Cell Biol. 20, 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhong Y., Wang Q. J., Li X., Yan Y., Backer J. M., Chait B. T., Heintz N., Yue Z. (2009) Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat. Cell Biol. 11, 468–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanida I., Ueno T., Kominami E. (2004) LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 36, 2503–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sarkar S., Floto R. A., Berger Z., Imarisio S., Cordenier A., Pasco M., Cook L. J., Rubinsztein D. C. (2005) Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 170, 1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cuervo A. M., Bergamini E., Brunk U. T., Droge W., French M., Terman A. (2005) Autophagy and aging: the importance of maintaining “clean” cells. Autophagy 1, 131–140 [DOI] [PubMed] [Google Scholar]
- 19. Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., Yokoyama M., Mishima K., Saito I., Okano H., Mizushima N. (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 [DOI] [PubMed] [Google Scholar]
- 20. Komatsu M., Waguri S., Chiba T., Murata S., Iwata J., Tanida I., Ueno T., Koike M., Uchiyama Y., Kominami E., Tanaka K. (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441, 880–884 [DOI] [PubMed] [Google Scholar]
- 21. Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. (2008) Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang L., Yu J., Pan H., Hu P., Hao Y., Cai W., Zhu H., Yu A. D., Xie X., Ma D., Yuan J. (2007) Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc. Natl. Acad. Sci. U. S. A. 104, 19023–19028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sarkar S., Perlstein E. O., Imarisio S., Pineau S., Cordenier A., Maglathlin R. L., Webster J. A., Lewis T. A., O'Kane C. J., Schreiber S. L., Rubinsztein D. C. (2007) Small molecules enhance autophagy and reduce toxicity in Huntington's disease models. Nat. Chem. Biol. 3, 331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jaeger P. A., Pickford F., Sun C. H., Lucin K. M., Masliah E., Wyss-Coray T. Regulation of amyloid precursor protein processing by the Beclin 1 complex. PLoS One 5, e11102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Netzer W. J., Powell C., Nong Y., Blundell J., Wong L., Duff K., Flajolet M., Greengard P. Lowering beta-amyloid levels rescues learning and memory in a Down syndrome mouse model. PLoS One 5, e10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thinakaran G., Borchelt D. R., Lee M. K., Slunt H. H., Spitzer L., Kim G., Ratovitsky T., Davenport F., Nordstedt C., Seeger M., Hardy J., Levey A. I., Gandy S. E., Jenkins N. A., Copeland N. G., Price D. L., Sisodia S. S. (1996) Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 17, 181–190 [DOI] [PubMed] [Google Scholar]
- 27. Wang H., Luo W. J., Zhang Y. W., Li Y. M., Thinakaran G., Greengard P., Xu H. (2004) Presenilins and gamma-secretase inhibitors affect intracellular trafficking and cell surface localization of the gamma-secretase complex components. J. Biol. Chem. 279, 40560–40566 [DOI] [PubMed] [Google Scholar]
- 28. Greenfield J. P., Tsai J., Gouras G. K., Hai B., Thinakaran G., Checler F., Sisodia S. S., Greengard P., Xu H. (1999) Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer beta-amyloid peptides. Proc. Natl. Acad. Sci. U. S. A. 96, 742–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eisenberg T., Knauer H., Schauer A., Buttner S., Ruckenstuhl C., Carmona-Gutierrez D., Ring J., Schroeder S., Magnes C., Antonacci L., Fussi H., Deszcz L., Hartl R., Schraml E., Criollo A., Megalou E., Weiskopf D., Laun P., Heeren G., Breitenbach M., Grubeck-Loebenstein B., Herker E., Fahrenkrog B., Frohlich K. U., Sinner F., Tavernarakis N., Minois N., Kroemer G., Madeo F. (2009) Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11, 1305–1314 [DOI] [PubMed] [Google Scholar]
- 30. Li Y. M., Xu M., Lai M. T., Huang Q., Castro J. L., DiMuzio-Mower J., Harrison T., Lellis C., Nadin A., Neduvelil J. G., Register R. B., Sardana M. K., Shearman M. S., Smith A. L., Shi X. P., Yin K. C., Shafer J. A., Gardell S. J. (2000) Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature 405, 689–694 [DOI] [PubMed] [Google Scholar]
- 31. Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., Selkoe D. J. (1999) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature 398, 513–517 [DOI] [PubMed] [Google Scholar]
- 32. Yue Z., Jin S., Yang C., Levine A. J., Heintz N. (2003) Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. U. S. A. 100, 15077–15082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qu X., Yu J., Bhagat G., Furuya N., Hibshoosh H., Troxel A., Rosen J., Eskelinen E. L., Mizushima N., Ohsumi Y., Cattoretti G., Levine B. (2003) Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 112, 1809–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Komatsu M., Waguri S., Ueno T., Iwata J., Murata S., Tanida I., Ezaki J., Mizushima N., Ohsumi Y., Uchiyama Y., Kominami E., Tanaka K., Chiba T. (2005) Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 169, 425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thinakaran G., Koo E. H. (2008) Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 283, 29615–29619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaden D., Voigt P., Munter L. M., Bobowski K. D., Schaefer M., Multhaup G. (2009) Subcellular localization and dimerization of APLP1 are strikingly different from APP and APLP2. J. Cell Sci. 122, 368–377 [DOI] [PubMed] [Google Scholar]
- 37. Boland B., Kumar A., Lee S., Platt F. M., Wegiel J., Yu W. H., Nixon R. A. (2008) Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J. Neurosci. 28, 6926–6937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu H., Wang P., Song W., Sun X. (2009) Degradation of regulator of calcineurin 1 (RCAN1) is mediated by both chaperone-mediated autophagy and ubiquitin proteasome pathways. FASEB J. 23, 3383–3392 [DOI] [PubMed] [Google Scholar]
- 39. Kaeberlein M., Powers R. W., 3rd, Steffen K. K., Westman E. A., Hu D., Dang N., Kerr E. O., Kirkland K. T., Fields S., Kennedy B. K. (2005) Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310, 1193–1196 [DOI] [PubMed] [Google Scholar]
- 40. Powers R. W., 3rd, Kaeberlein M., Caldwell S. D., Kennedy B. K., Fields S. (2006) Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 20, 174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jia K., Chen D., Riddle D. L. (2004) The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development 131, 3897–3906 [DOI] [PubMed] [Google Scholar]
- 42. Vellai T., Takacs-Vellai K., Zhang Y., Kovacs A. L., Orosz L., Muller F. (2003) Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426, 620 [DOI] [PubMed] [Google Scholar]
- 43. Kapahi P., Zid B. M., Harper T., Koslover D., Sapin V., Benzer S. (2004) Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 14, 885–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harrison D. E., Strong R., Sharp Z. D., Nelson J. F., Astle C. M., Flurkey K., Nadon N. L., Wilkinson J. E., Frenkel K., Carter C. S., Pahor M., Javors M. A., Fernandez E., Miller R. A. (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Caccamo A., Majumder S., Richardson A., Strong R., Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J. Biol. Chem. 285, 13107–13120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spilman P., Podlutskaya N., Hart M. J., Debnath J., Gorostiza O., Bredesen D., Richardson A., Strong R., Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One 5, e9979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Michiorri S., Gelmetti V., Giarda E., Lombardi F., Romano F., Marongiu R., Nerini-Molteni S., Sale P., Vago R., Arena G., Torosantucci L., Cassina L., Russo M. A., Dallapiccola B., Valente E. M., Casari G. The Parkinson-associated protein PINK1 interacts with Beclin1 and promotes autophagy. Cell Death Differ. 17, 962–974 [DOI] [PubMed] [Google Scholar]
- 48. Rubinsztein D. C., Gestwicki J. E., Murphy L. O., Klionsky D. J. (2007) Potential therapeutic applications of autophagy. Nat, Rev. Drug Discov. 6, 304–312 [DOI] [PubMed] [Google Scholar]
- 49. Jiang Y., Mullaney K. A., Peterhoff C. M., Che S., Schmidt S. D., Boyer-Boiteau A., Ginsberg S. D., Cataldo A. M., Mathews P. M., Nixon R. A. Alzheimer's-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition. Proc. Natl. Acad. Sci. U. S. A. 107, 1630–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.