Abstract
To evaluate the stability and heterogeneity of cytokine and chemokine profiles in 80 youth with and without HIV-1 infection, we tested plasma samples at repeated visits without antiretroviral therapy. Among nine analytes that were quantified using multiplexing assays, interleukin 10 (IL-10), IL-18, and soluble CD30 persistently showed a positive correlation with HIV-1 viral load (Spearman ρ = 0.40–0.59, p < 0.01 for all). A negative correlation with CD4+ T cell counts (ρ = −0.40 to −0.60, p < 0.01 for all) was also persistent for the three analytes. Analyses restricted to 48 AIDS-free youth (96 visits) yielded similar findings, as did multivariable models in which race, sex, age, body mass index, and time interval between visits were treated as covariates. These relationships reflected two novel features observed for all three analytes. First, their presence in plasma was relatively stable between visits (ρ = 0.50–0.90, p < 0.03), regardless of HIV-1 infection status. Second, pairwise correlation was strong and persistent in HIV-1-seropositive youth (ρ = 0.40–0.59, p < 0.01), but not in HIV-1, seronegatives (p > 0.13). Additional analytes, especially eotaxin/CCL11 and SDF-1β/CXCL12, had no correlation with HIV-1-related outcomes despite their stability between visits. Overall, circulating IL-10, IL-18, and soluble CD30 could partially track unfavorable responses to HIV-1 infection in youth. These markers of persistent immune activation are individually and collectively indicative of HIV-1 pathogenesis.
Introduction
Pattern-specific recognition of pathogen invasion (innate immunity) and antigen-specific T cell or B cell responses (acquired immunity) are closely regulated by cytokines and chemokines, which mediate the differentiation, migration, and function of immune cells. In the majority of individuals infected with HIV type 1 (HIV-1), persistent immune activation of CD4+ and CD8+ T cells1–4 is often accompanied by defective cytokine responses.5,6 A better understanding of variability (heterogeneity) in cytokine and chemokine responses to HIV-1 infection should benefit their use as therapeutic supplements7 or topical microbicides.8,9
Earlier studies have demonstrated that various cytokines can have a persistent and reproducible correlation with both virologic and immunologic outcomes following HIV-1 infection. In particular, an elevated interleukin (IL)-4:interferon (IFN)-γ ratio has been correlated with high plasma viral load and rapid loss of CD4+ T cells,6 while increases in IL-4 and IL-4-induced IgE production appear to coincide with the emergence of syncytium-inducing viruses.10,11 Emerging consensus findings point to circulating IL-7, IL-10, IL-15, IL-18, and tumor growth factor (TGF)-β1 as probable predictors or biomarkers of HIV-1-related outcomes that range from infection to disease progression and response to therapy.12–19 Analyses of other immune activation markers such as soluble CD30 (tumor necrosis factor receptor superfamily, member 8) can be equally informative.20–22 In addition, detection of intracellular cytokines, especially IFN-γ, IL-2, and TNF-α, is increasingly used to gauge the functionality of HIV-specific T cells.23,24 However, with few exceptions,25 past research has rarely yielded longitudinal data that can elucidate the timing and trajectory of cytokine or chemokine profile after HIV-1 infection.
The work described here follows our earlier observations that serum interleukin 18 (IL-18) is likely a universal and reliable predictor of unfavorable outcomes in HIV-1-infected adolescents and adults26 and that plasma is probably more suitable than serum when chemokines are studied in parallel.27 By focusing on cytokines and chemokines with a relatively stable presence in plasma, our study has produced clear evidence that at least three immunologic markers strongly correlate with variability in HIV-1 pathogenesis.
Materials and Methods
Study subjects
Subjects selected for this study came from the Reaching for Excellence in Adolescent Care and Health (REACH) project (1994–2001).28,29 Briefly, youth receiving active health care were recruited and enrolled from 13 U.S. cities for longitudinal evaluation and testing, at a 2:1 ratio between HIV-1 seropositive (seroprevalent) and seronegative subjects. The final cohort had close to 370 youth who were HIV-1 seropositive and they acquired infection primarily through high-risk sexual activities and occasionally through injection drug use, as reflected by the low rate of coinfection with hepatitis C virus (1.7% for HIV-1 seropositives and 0% for high-risk seronegatives).30 The 60 HIV-1-seropositive (HIV-1+) youth chosen for this retrospective study all had (1) two treatment-free visits within the first 2 years of follow-up (duration of infection unknown but should be less than 5 years and more than 6 months), (2) adequate plasma samples stored at −80°C and never thawed before use, (3) HIV-1 viral load (VL) and CD4+ T cell (CD4) counts measured at both visits, (4) no more than 12 months between visits, and (5) unlikely complication by hepatitis C virus infection. Representative of the entire REACH cohort in terms of age, sex ratio, ethnic backgrounds, and other characteristics (Table 1), the HIV-1+ youth consisted of 25 controllers (VL <1000 copies/ml and CD4 count >450 cells/μl) and 35 noncontrollers (VL >16,000 copies/ml and CD4 count <450 cells/μl) defined earlier in immunogenetic studies.31,32 For this study, the emphasis was on VL because of its dual impact on disease progression (pathogenesis) and transmission to exposed individuals. CD4 decline (expressed as visit 2 to visit 1 ratio) within the sampling period was also tested as a proxy for CD4 slope. Selection of 20 HIV-1-seronegative (HIV-1−) youth emphasized a relatively equal number of African-Americans and non-African-Americans. These research activities conformed to the U.S. Department of Health and Human Services guidelines for protection of human subjects. The protocols for obtaining written informed consent, blood sample, clinical information, data management, and data analysis were approved by institutional review boards (IRBs) at each clinical site, with further approval by the IRB at the University of Alabama at Birmingham (under Protocol X070405012).
Table 1.
Characteristics of HIV-1 Seropositive and Seronegative Youth Selected from the REACH Cohort
| Characteristics | HIV+youth | HIV−youth |
|---|---|---|
| No. of subjects | 60a | 20b |
| Sex ratio (M/F) | 0.33 (15/45) | 0.67 (8/12) |
| Ethnicity | ||
| African-American (AA) | 44 | 11 |
| Otherc | 16 | 9 |
| Age at visit 1d | 17.6 ± 1.3 | 17.6 ± 0.8 |
| Age at visit 2d | 18.4 ± 1.4 | 17.9 ± 1.0 |
| Interval (in days) between visits | 237 ± 107 | 170 ± 25 |
| BMI at visit 1 | 27.6 ± 7.3 | 28.3 ± 9.5 |
| BMI at visit 2 | 27.5 ± 7.5 | 28.4 ± 9.7 |
| Earliest visit date | March 1996 | March 1996 |
| Latest visit date | October 2000 | November 1999 |
| Plasma HIV-1 viral load (log10 copies/ml) | ||
| Visit 1 | 3.68 ± 1.57 | NA |
| Visit 2 | 3.69 ± 1.68 | NA |
| CD4+ T cell counts (cells per μl) | ||
| Visit 1 | 502 ± 342 | 1016 ± 345 |
| Visit 2 | 480 ± 378 | 870 ± 368 |
| Change (visit 2 to visit 1 ratio) | 0.93 ± 0.30 | 0.86 ± 0.24 |
Representative of HIV-1-seropositive youth in the REACH cohort (duration of infection unknown).
With an emphasis on relatively equal representation of African-Americans and others. NA, not applicable.
Mostly Hispanic Americans in this group.
These selected visits correspond to the first two eligible for analyses of HIV-1-related outcomes in the absence of antiretroviral therapy. Summary data as shown correspond to mean ± standard deviation.
HIV+, HIV-1 seropositive; HIV−, HIV-1 seronegative.
Multiplexing enzyme-linked immunosorbent assay (SearchLight)
Highly sensitive SearchLight proteome array (Aushon BioSystems, Inc., Billerica, MA; formerly Thermo Fisher Scientific, Rockford, IL) was used to quantify nine analytes in plasma samples collected with EDTA-coated tubes. Capture antibodies (in array format) spotted on the bottom of 96-well polystyrene microtiter plates were specific for nine analytes, including two cytokines (IL-10 and IL-18), five chemokines (eotaxin/CCL11, MIP-1α, MIP-1β, RANTES/CCL5, and SDF-1β/CXCL12) and two inflammatory markers (soluble CD30 and C-reactive protein). EDTA-plasma samples were tested in duplicate at three dilutions (1:2, 1:50, and 1:1000). The bound protein analytes were incubated with biotinylated detection antibodies. After addition of streptavidin-horseradish peroxidase (HRP) and SuperSignal ELISA Femto Chemiluminescent substrate, the light signal produced from the HRP-catalyzed oxidation of substrate was captured by digital scanning (SearchLight Imaging System). The scanned image was processed using ArrayVision customized software. Product concentrations at individual spots were extrapolated off a standard curve. The lower limits of detection ranged from 0.2 pg/ml for IL-10 to 6.2 pg/ml for MIP-1α. The results were highly reproducible (coefficients of variation <20% in >80% of test samples). Testing of the first eight analytes was based on (1) initial screening of 42 analytes (representing TH1 and TH2 cytokines, C-X-C and C-C motif chemokines, growth factors, as well as markers of inflammation and angiogenesis) using the Human Cytokine Array III (RayBiotech Inc., Norcross GA),33 (2) dismissal of IFN-γ, IL-2, IL-4, IL-6, and tumor necrosis factor alpha (TNF-α) as informative markers of HIV-1 pathogenesis in the REACH cohort,34 (3) analysis of serum samples from the REACH cohort,26 and (4) successful quantification in over 90% of samples from HIV-1+ youth. Inclusion of C-reactive protein (CRP) was based on its frequent recognition as a reliable marker of chronic inflammation related to various immune disorders.35,36
Statistical analyses
Several routine statistical procedures in the Statistical Analysis Software (SAS) package, version 9.2 (SAS Institute, Cary, NC) were used to determine (1) intervisit correlation for all analytes and two major HIV-1-related outcomes (i.e., VL and CD4 count), (2) visit-specific pairwise correlation among all analytes measured on the same day, and (3) visit-specific correlation between individual analytes and HIV-1-related outcomes, including CD4 T cell change or decline (visit 2 to visit 1 ratio). To emphasize the strength and persistence of correlations, the Spearman method was used throughout this work; estimates of correlation coefficients (rho = ρ) were highlighted if the p values reached the minimal statistical significance level (0.05) at both visits (results from visit 2 tested the hypothesis derived from visit 1). Whenever possible, statistical adjustments were made for race, sex, age, BMI, and the time interval between visits.
Results
Analyses based on 60 HIV-1-seropositive youth
Among HIV-1+ youth (Table 1), 25 controllers and 35 noncontrollers differed starkly in VL and CD4 count (p < 0.0001 for all comparisons). In controllers, VL was 2.04 ± 1.03 log10 (mean ± standard deviation) and 1.99 ± 0.91 at visit 1 and visit 2, respectively, with corresponding CD4 counts of 834 ± 257 and 849 ± 290 cells/μl. In noncontrollers, VL was 4.80 ± 0.54 log10 at visit 1 and 4.91 ± 0.79 log10 at visit 2, with corresponding CD4 counts of 274 ± 150 and 217 ± 127. Declines in CD4 within the sampling intervals also differed between these two subgroups (the visit 2 to visit 1 ratio was 1.07 ± 0.28 for controllers and 0.82 ± 0.27 for noncontrollers, p < 0.0001 by Student's t-test).
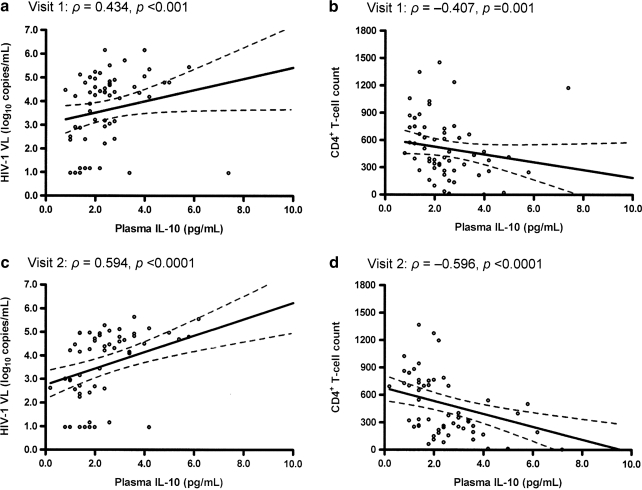
For the nine analytes (eotaxin/CCL11, MIP-1α, MIP-1β, RANTES/CCL5, SDF-1β/CXCL12, IL-10, IL-18, soluble CD30, and CRP) tested in EDTA-plasma samples, IL-10, IL-18, and soluble CD30 were positively correlated with HIV-1 VL and inversely correlated with CD4 counts at both visits (Table 2 and Fig. 1), with ρ values ranging from 0.26 (p < 0.05) to 0.59 (p < 0.0001) in tests of VL. Statistical adjustments for race, sex, age, BMI, and visit interval did not substantially alter these relationships (Supplementary Table S1; Supplementary Data are available online at www.liebertonline.com/aid). The other six analytes (CRP, eotaxin, MIP-1α, MIP-1β, RANTES/CCL5, and SDF-1β/CXCL12) had no clear correlation with HIV-1 VL or CD4 counts at any visit (results not shown).
Table 2.
Pairwise Correlation among Three Plasma Analytes and Two HIV-1-Related Outcomes in 60 Youth from the REACH Cohorta
| Parameter | IL-10 | IL-18 | Soluble CD30 | HIV-1 viral load | CD4+T cell |
|---|---|---|---|---|---|
| IL-10 | 0.749 (<0.0001) | 0.353 (<0.01) | 0.397 (<0.01) | 0.434 (<0.001) | −0.407 (0.001) |
| IL-18 | 0.428 (<0.001) | 0.771 (<0.0001) | 0.259 (<0.05) | 0.402 (<0.01) | −0.449 (<0.001) |
| Soluble CD30 | 0.499 (<0.0001) | 0.334 (0.009) | 0.852 (<0.0001) | 0.523 (<0.0001) | −0.395 (<0.01) |
| HIV-1 viral load | 0.594 (<0.0001) | 0.404 (0.001) | 0.568 (<0.0001) | 0.869 (<0.0001) | −0.797 (<0.0001) |
| CD4+ T cell | −0.594 (<0.0001) | −0.430 (0.001) | −0.510 (<0.0001) | −0.823 (<0.0001) | 0.913 (<0.0001) |
Each individual is analyzed for two treatment-free visits within the first 3 years of longitudinal follow-up study. Results (Spearman ρ and p values in parentheses) from visit 1 are over the diagonal; results from visit 2 are below the diagonal; comparison between visit 1 and visit 2 are in italics. The best predictor of HIV-1 viral load within each visit is shown in bold.
FIG. 1.
Persistent correlation of plasma IL-10 with HIV-1 viral load (a, c) and CD4+ T cell counts (b, d) at two separate visits without antiretroviral therapy. Each panel represents univariate correlation analysis of data from 60 individuals using the Spearman method. Projected slope and its 95% confidence intervals at each visit are shown in solid and broken lines, respectively. Similar findings are seen with IL-18 and soluble CD30 (Table 2).
The three analytes (IL-10, IL-18, and soluble CD30) associated with HIV-1-related outcomes all had relatively stable concentrations between visits (Table 2), with soluble CD30 being most stable (intervisit ρ = 0.85 and p < 0.0001) and IL-10 the least (ρ = 0.75 and p < 0.0001). On the basis of assay quality (coefficient of variation < 5% for duplicate samples), intervisit stability, and magnitude of correlation at both visits, soluble CD30 was the best predictor of HIV-1 VL, which was in turn a strong predictor of CD4 counts (ρ = 0.87 and 0.91 for visit 1 and visit 2, respectively; p < 0.0001 for both).
When 12 patients with severe immunodeficiency (i.e., CD4 count <200 cells/μl) were excluded, the key findings remained similar (Supplementary Table S2). Again, soluble CD30 was the best predictor of HIV-1 viral load (ρ = 0.76 for visit 1 and 0.74 for visits 2, p < 0.0001 for both visits). Further (exploratory) analyses using the 12 youth with severe immunodeficiency (<200 CD4 count per μl of blood) revealed that eotaxin/CCL11 at any visit strongly correlated with visit 2 viral load (ρ = 0.73 and 0.86, p < 0.01 and <0.001 for visit 1 and visit 2 eotaxin, respectively). However, plasma eotaxin/CCL11 did not correlate with CD4 counts at any visit (p > 0.13 in all tests involving the 12 patients). Similar tests for other analytes were not informative (results not shown).
In alternative analyses, both IL-18 and soluble CD30 had a persistent inverse correlation with CD4 change/decline (ρ ≤−0.25 and p ≤ 0.05) (Table 3), while IL-10 showed a correlation at visit 2 (ρ = −0.28 and p = 0.02) and not at visit 1 (ρ = −0.13 and p = 0.32). Relative changes in these three analytes (visit 2 to visit 1 ratios) were not informative in any of the statistical models (p ≥ 0.19).
Table 3.
Alternative Analysis of CD4+ T Cell Change in 60 Youth from the REACH Cohorta
| |
Visit 1 |
Visit 2 |
Visit 2 to visit 1 ratio |
|||
|---|---|---|---|---|---|---|
| Plasma analyte | ρ | p | ρ | p | ρ | p |
| IL-10 | −0.13 | 0.32 | −0.28 | 0.03 | −0.17 | 0.19 |
| IL-18 | −0.27 | 0.04 | −0.25 | 0.05 | 0.04 | 0.75 |
| Soluble CD30 | −0.40 | <0.01 | −0.35 | <0.01 | 0.09 | 0.51 |
CD4+ T cell counts at two separate visits are converted to a ratio (visit 2 to visit 1) before being treated as an alternative outcome measure. Spearman correlation (ρ) and associated p value are shown for each of the three analytes at both visits and for the ratio of these analytes (also visit 2 to visit 1).
Comparisons between HIV-1- seropositive and -seronegative youth
For visit 1, the three plasma analytes (IL-10, IL-18, and soluble CD30) associated with HIV-1-related outcomes were all present at higher concentrations in HIV-1+ than HIV-1− youth (p < 0.001 for all) (Table 4). The analytes with the clearest (>10-fold) difference between HIV-1+ and HIV-1− youth were MIP-1α and CRP (p < 0.0001), with the former being undetectable (<6.2 pg/ml) in 18 out of 20 HIV-1− youth. Two other analytes, eotaxin and SDF-1β/CXCL12, had ∼4-fold higher concentrations in HIV-1+ than HIV-1− youth (p < 0.0001 for both), while MIP-1β and RANTES/CCL5 had a slight trend for higher concentrations in HIV-1− than HIV-1+ youth, although the difference did not reach statistical significance (p > 0.20 for both). Results for visit 2 mostly mirrored those for visit 1 (Table 4), except that IL-10 concentrations were no longer different between HIV-1+ and HIV-1− youth (p = 0.48 by Wilcoxon U-test).
Table 4.
Overall Comparison of Nine Plasma Products (Analytes) Between 60 HIV-1-Seropositive and 20 Seronegative Youth
| |
Visit 1 resultsa |
Visit 2 resultsa |
||||
|---|---|---|---|---|---|---|
| Analytes | HIV+youth | HIV−youth | p value | HIV+youth | HIV−youth | p value |
| IL-10 | 2.4 (1.6–2.8) | 1.3 (0.8–1.9) | <0.001 | 2.1 (1.4–3.3) | 2.0 (1.3–2.7) | 0.48 |
| IL-18 | 332 (261–403) | 135 (101–167) | <0.0001 | 329 (251–428) | 145 (110–192) | <0.0001 |
| Soluble CD30 | 2.7 (1.8–3.8) | 1.0 (0.8–1.3) | <0.0001 | 2.1 (1.6–4.0) | 1.1 (0.9–1.6) | <0.0001 |
| Eotaxin/CCL11 | 88 (61–132) | 20 (15–48) | <0.0001 | 86 (61–133) | 23 (12–40) | <0.0001 |
| MIP-1α | 61.5 (52.3–68.9) | <6.2 | NA | 66.2 (54.5–72.4) | <6.2 | NA |
| MIP-1β | 48 (38–62) | 38 (29–68) | 0.21 | 47 (40–56) | 39 (31–78) | 0.27 |
| RANTES/CCL5 | 14.3 (5.2–39.9) | 26.5 (4.8–57.9) | 0.29 | 14.8 (5.6–37.4) | 14.9 (7.0–50.9) | 0.61 |
| SDF-1β/CXCL12 | 1220 (916–1847) | 310 (142–360) | <0.0001 | 1277 (924–1808) | 253 (151–402) | <0.0001 |
| C-reactive protein (CRP) | 1421 (540–3368) | 117 (23–190) | <0.0001 | 943 (482–3440) | 127 (27–1340) | <0.001 |
Concentrations are shown in pg/ml for all analytes except soluble CD30, RANTES/CCL5, and CRP (in ng/ml). The median and interquartile range (IQR) are shown for each analyte and p values are based on Wilcoxon U-tests (NA, not applicable when most values are below the lower limit of detection).
As in HIV-1+ youth (Table 2), IL-10, IL-18, and soluble CD30 also had a stable presence in the plasma of HIV-1− youth, although the ranking differed slightly among the three analytes: IL-18 was most stable in the absence of HIV-1 infection (ρ = 0.90 and p < 0.0001) and IL-10 was least stable (ρ = 0.50 and p < 0.03). However, there was no pairwise correlation among these analytes at any visit in the HIV-1− youth (p > 0.13 for all), which contrasted with findings based on HIV-1+ youth (Table 2).
Stability ranking for the other analytes in the absence of HIV-1 infection was as follows: eotaxin/CCL11 (ρ = 0.94 and p < 0.0001) > SDF-1β/CXCL12 (ρ = 0.72 and p < 0.001) > CRP (ρ = 0.71 and p < 0.001) > MIP-1β (ρ = 0.63 and p < 0.01) > RANTES/CCL5 (ρ = 0.49 and p = 0.03). For HIV-1+ youth, the ranking was slightly different: eotaxin/CCL11 (ρ = 0.86) > SDF-1β (ρ = 0.73) > RANTES/CCL5 (ρ = 0.67) > MIP-1β (ρ = 0.60) > CRP (ρ = 0.57) (p < 0.0001 for all intervisit relationships). Overall, eotaxin/CCL11 and SDF-1β/CXCL12 were quite stable regardless of HIV-1 infection status. The relative ranking for MIP-1α could not be compared because most plasma samples from HIV-1− youth had undetectable levels.
Discussion
With attention to adequate and repeated sampling of HIV-1+ and HIV-1− youth, our analysis produced clear evidence that multiple cytokines, chemokines, and related products have a relatively stable presence in EDTA-plasma samples and that three analytes are highly informative in terms of their persistent correlation with two HIV-1-related outcomes, especially VL. These findings support the notion that circulating immunologic markers may closely track viral pathogenesis. Of note, the three products (IL-10, IL-18, and soluble CD30) positively correlated with HIV-1 VL and inversely so with CD4 counts are all markers of immune activation, which is consistent with evidence from previous studies featuring different demographic characteristics.13,17,20–22,37,38 Among the three analytes, IL-10 has the best defined function properties in viral infection or clearance.39–41 Compared with IL-18 and soluble CD30, use of plasma IL-10 as a biomarker for HIV-1 pathogenesis per se is somewhat compromised by its lack of persistent correlation with CD4 decline during HIV-1 infection (Table 3) and by its apparent fluctuation even in healthy subjects (Table 4).
Immune activation in HIV-1-infected individuals is driven by viral antigens as well as endotoxins (lipopolysaccharides, LPS) derived from translocated bacteria,42–44 even in patients with minimal viral replication.45,46 Circulating LPS is a prominent marker of HIV-1 pathogenesis,47 as is the soluble form of the endotoxin receptor CD14.48,49 The three immunologic markers identified in our study population may also imply the importance of LPS and soluble CD14 through various immune pathways associated with HIV-1 infection in youth. While activated CD4+ (mostly TH2) and CD8+ (cytotoxic) T cells are the major source of IL-10 production in humans,50 IL-18 and CD30 can be released by a variety of cells.50,51 Persistent alteration in these products is expected to have a broad impact on innate and adaptive immune responses. In study populations in which frequent coinfections with other pathogens (e.g., hepatitis C virus and mycobacteria) may further complicate systemic immune responses, it remains to be seen if IL-10, IL-18, and soluble CD30 can maintain their respective correlation with established markers of HIV-1 pathogenesis. Data from seroconverter cohorts (rare for adolescents and youth) should be particularly informative.
As in most studies to date, correlates of protective (favorable) immune responses to HIV-1 infection continue to be elusive. Lack of close relationships between circulating beta chemokines (i.e., MIP-1α and MIP-1β and RANTES/CCL5) and HIV-1-related outcomes was especially disappointing, because these small molecules have well-recognized anti-HIV-1 activities, mostly through binding to CCR5, the major HIV-1 coreceptor. High concentrations of β-chemokines in plasma are expected to interfere with HIV-1 dissemination, as demonstrated by in vitro assays.52,53 Yet even high concentrations of RANTES/CCL5 had no differential impact on VL or CD4 counts. As we pointed out earlier,27 quantitative assays that can distinguish various β-chemokine isoforms and cleavage products33,54 may provide new insights: it is possible that certain isoforms, including nonallelic products encoded by closely related genes, are more potent than others in their antiviral properties and these can be differentially distributed among tissue compartments. Judging from preliminary findings on eotaxin/CCL11 in patients with severe immunodeficiency, timing of the chemokine response can be another critical issue. The specific role of eotaxin/CXCL11 in late stage of HIV-1 infection may deserve some further investigation.
Unfavorable outcomes following HIV-1 infection are also associated with cellular factors, including CD38 and PD-1 on CD8+ T cells.1,3,4,55–58 Work based on the REACH cohort and other youth populations has produced confirmatory findings about the prognostic value of CD8+CD38+ T cell percentage,59–62 suggesting that hyperimmune activation is also a common feature in youth and children. Use of soluble instead of cellular factor as correlates or predictors of HIV-1 pathogenesis is usually advantageous because quantification of cellular factors requires an ample amount of viable cells. Nonetheless, assessment of normal and abnormal ranges of specific cytokines, chemokines, and related products in extracellular compartments will require close attention to standardized procedures for sample processing, followed by vigorous testing in different study populations. Our data from HIV-1+ and HIV-1− youth should serve this purpose well.
The search for clinically and epidemiologically useful biomarkers in human proteomes has yielded some success before.63 High concentrations of three soluble products of cellular receptors (CD27, CD40L, and CD120a/TNFR1) and plasma IL-6 in HIV-1 patients before combination therapy have been shown to predict AIDS-defining illnesses after treatment.64 In patients with viral hepatitis, two commercially available serum marker panels (FibroSURE and FIBROSpect II) appear to be equally reliable in gauging the success of interferon-based therapy in patients with hepatic C virus infection.65 A combination of several CXCR3-related chemokines (CXCL9, CXCL10, and CXCL11) may provide noninvasive markers of hepatic fibrosis.66,67 Soluble immunologic markers highlighted in studies of hepatitis B and C bear no close similarity to markers derived from studies of HIV-1 infection. Such disease- or pathway-specific findings clearly imply distinct underlying mechanisms and/or sites of viral pathogenesis. Efforts to identify and confirm immunologic markers specific for HIV-1 infection should eventually benefit the design of targeted intervention.
HIV-1-specific questions aside, our work has also indicated that cytokine and chemokine concentrations in plasma can remain relatively stable in youth regardless of HIV-1 infection status. Differences in stability ranking, as seen occasionally between HIV-1+ and HIV-1− youth, may hint at the disruption of certain interactive pathways following HIV-1 infection. Stability ranking as a novel measure of plasma analytes may become useful in distinguishing HIV-1 controllers from noncontrollers. Thus, an analysis of stability alone may lead to new findings important to HIV-1 pathogenesis.
Supplementary Material
Acknowledgments
We thank REACH investigators, staff [listed in J Adolesc Health 2001;29(Suppl):5–6] and participants for their valuable contributions. We are also indebted to Dr. Richard A. Kaslow for programmatic support and to Dr. HanSoo Kim for technical advice. Funding for this work came from the National Institute of Allergy and Infectious Diseases (NIAID) through an R01 grant (AI051173) and an independent scientist award (AI076123). The REACH study (1994–2001) was sponsored by the National Institute of Child Health and Human Development, with supplemental funding from NIAID, the National Institute on Drug Abuse, and the National Institute of Mental Health (U01-HD32830).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Giorgi JV. Detels R. T-cell subset alterations in HIV-infected homosexual men: NIAID Multicenter AIDS cohort study. Clin Immunol Immunopathol. 1989;52:10–18. doi: 10.1016/0090-1229(89)90188-8. [DOI] [PubMed] [Google Scholar]
- 2.Ramzaoui S. Jouen-Beades F. Gilbert D. Borsa-Lebas F. Michel Y. Humbert G. Tron F. During HIV infection, CD4+ CD38+ T-cells are the predominant circulating CD4+ subset whose HLA-DR positivity increases with disease progression and whose V beta repertoire is similar to that of CD4+ CD38− T-cells. Clin Immunol Immunopathol. 1995;77:33–41. doi: 10.1016/0090-1229(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 3.Savarino A. Bottarel F. Malavasi F. Dianzani U. Role of CD38 in HIV-1 infection: An epiphenomenon of T-cell activation or an active player in virus/host interactions? AIDS. 2000;14:1079–1089. doi: 10.1097/00002030-200006160-00004. [DOI] [PubMed] [Google Scholar]
- 4.Chun TW. Justement JS. Sanford C. Hallahan CW. Planta MA. Loutfy M. Kottilil S. Moir S. Kovacs C. Fauci AS. Relationship between the frequency of HIV-specific CD8+ T cells and the level of CD38+ CD8+ T cells in untreated HIV-infected individuals. Proc Natl Acad Sci USA. 2004;101:2464–2469. doi: 10.1073/pnas.0307328101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clerici M. Shearer GM. A Th1 → Th2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 6.Altfeld M. Addo MM. Kreuzer KA. Rockstroh JK. Dumoulin FL. Schliefer K. Leifeld L. Sauerbruch T. Spengler U. TH1 to TH2 shift of cytokines in peripheral blood of HIV-infected patients is detectable by reverse transcriptase polymerase chain reaction but not by enzyme-linked immunosorbent assay under nonstimulated conditions. J Acquir Immune Defic Syndr. 2000;23:287–294. doi: 10.1097/00126334-200004010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Abrams D. Levy Y. Losso MH. Babiker A. Collins G. Cooper DA. Darbyshire J. Emery S. Fox L. Gordin F. Lane HC. Lundgren JD. Mitsuyasu R. Neaton JD. Phillips A. Routy JP. Tambussi G. Wentworth D. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009;361:1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lederman MM. Veazey RS. Offord R. Mosier DE. Dufour J. Mefford M. Piatak M., Jr Lifson JD. Salkowitz JR. Rodriguez B. Blauvelt A. Hartley O. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306:485–487. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 9.Vangelista L. Secchi M. Lusso P. Rational design of novel HIV-1 entry inhibitors by RANTES engineering. Vaccine. 2008;26:3008–3015. doi: 10.1016/j.vaccine.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama EE. Hoshino Y. Xin X. Liu H. Goto M. Watanabe N. Taguchi H. Hitani A. Kawana-Tachikawa A. Fukushima M. Yamada K. Sugiura W. Oka SI. Ajisawa A. Sato H. Takebe Y. Nakamura T. Nagai Y. Iwamoto A. Shioda T. Polymorphism in the interleukin-4 promoter affects acquisition of human immunodeficiency virus type 1 syncytium-inducing phenotype. J Virol. 2000;74:5452–5459. doi: 10.1128/jvi.74.12.5452-5459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li YG. Iwabu Y. Warachit J. Kinomoto M. Ibrahim MS. Tsuji S. Mukai T. Kameoka M. Tokunaga K. Sata T. Ikuta K. Interleukin-4 up-regulates T-tropic human immunodeficiency virus type 1 transcription in primary CD4+ CD38+ T-lymphocyte subset. Microbiol Immunol. 2005;49:155–165. doi: 10.1111/j.1348-0421.2005.tb03715.x. [DOI] [PubMed] [Google Scholar]
- 12.Napolitano LA. Grant RM. Deeks SG. Schmidt D. De Rosa SC. Herzenberg LA. Herndier BG. Andersson J. McCune JM. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: Implications for T-cell homeostasis. Nat Med. 2001;7:73–79. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 13.Boulassel MR. Young M. Routy JP. Sekaly RP. Tremblay C. Rouleau D. Circulating levels of IL-7 but not IL-15, IGF-1, and TGF-β are elevated during primary HIV-1 infection. HIV Clin Trials. 2004;5:357–359. doi: 10.1310/M0CV-R6BX-A9DP-JJV0. [DOI] [PubMed] [Google Scholar]
- 14.Stylianou E. Aukrust P. Kvale D. Muller F. Froland SS. IL-10 in HIV infection: Increasing serum IL-10 levels with disease progression–down-regulatory effect of potent anti-retroviral therapy. Clin Exp Immunol. 1999;116:115–120. doi: 10.1046/j.1365-2249.1999.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amicosante M. Poccia F. Gioia C. Montesano C. Topino S. Martini F. Narciso P. Pucillo LP. D'Offizi G. Levels of interleukin-15 in plasma may predict a favorable outcome of structured treatment interruption in patients with chronic human immunodeficiency virus infection. J Infect Dis. 2003;188:661–665. doi: 10.1086/377454. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad R. Sindhu ST. Toma E. Morisset R. Ahmad A. Studies on the production of IL-15 in HIV-infected/AIDS patients. J Clin Immunol. 2003;23:81–90. doi: 10.1023/a:1022568626500. [DOI] [PubMed] [Google Scholar]
- 17.Torre D. Speranza F. Martegani R. Pugliese A. Castelli F. Basilico C. Biondi G. Circulating levels of IL-18 in adult and paediatric patients with HIV-1 infection. AIDS. 2000;14:2211–2212. doi: 10.1097/00002030-200009290-00023. [DOI] [PubMed] [Google Scholar]
- 18.Iannello A. Samarani S. Debbeche O. Ahmad R. Boulassel MR. Tremblay C. Toma E. Routy JP. Ahmad A. Potential role of interleukin-18 in the immunopathogenesis of AIDS: Involvement in fratricidal killing of NK cells. J Virol. 2009;83:5999–6010. doi: 10.1128/JVI.02350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiercinska-Drapalo A. Flisiak R. Jaroszewicz J. Prokopowicz D. Increased plasma transforming growth factor-β1 is associated with disease progression in HIV-1-infected patients. Viral Immunol. 2004;17:109–113. doi: 10.1089/088282404322875502. [DOI] [PubMed] [Google Scholar]
- 20.Pizzolo G. Vinante F. Morosato L. Nadali G. Chilosi M. Gandini G. Sinicco A. Raiteri R. Semenzato G. Stein H, et al. High serum level of the soluble form of CD30 molecule in the early phase of HIV-1 infection as an independent predictor of progression to AIDS. AIDS. 1994;8:741–745. doi: 10.1097/00002030-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Rizzardi GP. Barcellini W. Tambussi G. Lillo F. Malnati M. Perrin L. Lazzarin A. Plasma levels of soluble CD30, tumour necrosis factor (TNF)-alpha and TNF receptors during primary HIV-1 infection: Correlation with HIV-1 RNA and the clinical outcome. AIDS. 1996;10:F45–50. doi: 10.1097/00002030-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Pizzolo G. Vinante F. Nadali G. Krampera M. Morosato L. Chilosi M. Raiteri R. Sinicco A. High serum level of soluble CD30 in acute primary HIV-1 infection. Clin Exp Immunol. 1997;108:251–253. doi: 10.1046/j.1365-2249.1997.d01-1005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey JR. Williams TM. Siliciano RF. Blankson JN. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J Exp Med. 2006;203:1357–1369. doi: 10.1084/jem.20052319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Betts MR. Nason MC. West SM. De Rosa SC. Migueles SA. Abraham J. Lederman MM. Benito JM. Goepfert PA. Connors M. Roederer M. Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stacey AR. Norris PJ. Qin L. Haygreen EA. Taylor E. Heitman J. Lebedeva M. DeCamp A. Li D. Grove D. Self SG. Borrow P. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song W. Wilson CM. Allen S. Wang C. Li Y. Kaslow RA. Tang J. Interleukin 18 and human immunodeficiency virus type 1 infection in adolescents and adults. Clin Exp Immunol. 2006;144:117–124. doi: 10.1111/j.1365-2249.2006.03050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao W. Tang J. Song W. Wang C. Li Y. Wilson CM. Kaslow RA. CCL3L1 and CCL4L1: Variable gene copy number in adolescents with and without human immunodeficiency virus type 1 (HIV-1) infection. Genes Immun. 2007;8:224–231. doi: 10.1038/sj.gene.6364378. [DOI] [PubMed] [Google Scholar]
- 28.Rogers AS. Futterman DK. Mosciki AB. Wilson CM. Ellenberg J. Vermund SH. The REACH project of the adolescent medicine HIV/AIDS research network: Design, methods, and selected characteristics of participants. J Adolesc Health. 1998;22:300–311. doi: 10.1016/s1054-139x(97)00279-6. [DOI] [PubMed] [Google Scholar]
- 29.Wilson CM. Houser J. Partlow C. Rudy BJ. Futterman DC. Friedman LB. The REACH (Reaching for Excellence in Adolescent Care and Health) project: Study design, methods, and population profile. J Adolesc Health. 2001;29:8–18. doi: 10.1016/s1054-139x(01)00291-9. [DOI] [PubMed] [Google Scholar]
- 30.Holland CA. Ma Y. Moscicki B. Durako SJ. Levin L. Wilson CM. Seroprevalence and risk factors of hepatitis B, hepatitis C, and human cytomegalovirus among HIV-infected and high-risk uninfected adolescents: Findings of the REACH Study. Adolescent Medicine HIV/AIDS Research Network. Sex Transm Dis. 2000;27:296–303. doi: 10.1097/00007435-200005000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Tang J. Wilson CM. Meleth S. Myracle A. Lobashevsky E. Mulligan MJ. Douglas SD. Korber B. Vermund SH. Kaslow RA. Host genetic profiles predict virological and immunological control of HIV-1 infection in adolescents. AIDS. 2002;16:2275–2284. doi: 10.1097/00002030-200211220-00007. [DOI] [PubMed] [Google Scholar]
- 32.Shrestha S. Aissani B. Song W. Wilson CM. Kaslow RA. Tang J. Host genetics and HIV-1 viral load set-point in African-Americans. AIDS. 2009;23:673–677. doi: 10.1097/QAD.0b013e328325d414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang J. Chemokine receptor, ligand genes. In: Kaslow RA, editor; McNicholl JM, editor; Hill AVS, editor. Genetic Susceptibility to Infectious Diseases. Oxford University Press; New York: 2008. pp. 247–262. [Google Scholar]
- 34.Douglas SD. Durako S. Sullivan KE. Camarca M. Moscicki AB. Wilson CM. TH1 and TH2 cytokine mRNA and protein levels in human immunodeficiency virus (HIV)-seropositive and HIV-seronegative youths. Clin Diagn Lab Immunol. 2003;10:399–404. doi: 10.1128/CDLI.10.3.399-404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowe GD. The relationship between infection, inflammation, and cardiovascular disease: An overview. Ann Periodontol. 2001;6:1–8. doi: 10.1902/annals.2001.6.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Signorelli SS. Mazzarino MC. Spandidos DA. Malaponte G. Proinflammatory circulating molecules in peripheral arterial disease. Int J Mol Med. 2007;20:279–286. [PubMed] [Google Scholar]
- 37.Manetti R. Annunziato F. Biagiotti R. Giudizi MG. Piccinni MP. Giannarini L. Sampognaro S. Parronchi P. Vinante F. Pizzolo G. Maggi E. Romagnani S. CD30 expression by CD8+ T cells producing type 2 helper cytokines. Evidence for large numbers of CD8+ CD30+ T cell clones in human immunodeficiency virus infection. J Exp Med. 1994;180:2407–2411. doi: 10.1084/jem.180.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadeghi M. Susal C. Daniel V. Naujokat C. Zimmermann R. Huth-Kuhne A. Opelz G. Decreasing soluble CD30 and increasing IFN-γ plasma levels are indicators of effective highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2007;23:886–890. doi: 10.1089/aid.2006.0228. [DOI] [PubMed] [Google Scholar]
- 39.Ji J. Sahu GK. Braciale VL. Cloyd MW. HIV-1 induces IL-10 production in human monocytes via a CD4-independent pathway. Int Immunol. 2005;17:729–736. doi: 10.1093/intimm/dxh252. [DOI] [PubMed] [Google Scholar]
- 40.Brooks DG. Trifilo MJ. Edelmann KH. Teyton L. McGavern DB. Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alter G. Kavanagh D. Rihn S. Luteijn R. Brooks D. Oldstone M. van Lunzen J. Altfeld M. IL-10 induces aberrant deletion of dendritic cells by natural killer cells in the context of HIV infection. J Clin Invest. 2010;120:1905–1913. doi: 10.1172/JCI40913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jirillo E. Covelli V. Brandonisio O. Munno I. De Simone C. Mastroianni CM. Antonaci S. Riccio P. HIV-infection and in vivo lipopolysaccharide-induced release of cytokines. An amplified mechanism of damage to the host. Acta Neurol (Napoli) 1991;13:188–196. [PubMed] [Google Scholar]
- 43.Brenchley JM. Price DA. Schacker TW. Asher TE. Silvestri G. Rao S. Kazzaz Z. Bornstein E. Lambotte O. Altmann D. Blazar BR. Rodriguez B. Teixeira-Johnson L. Landay A. Martin JN. Hecht FM. Picker LJ. Lederman MM. Deeks SG. Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 44.Douek D. HIV disease progression: Immune activation, microbes, and a leaky gut. Top HIV Med. 2007;15:114–117. [PubMed] [Google Scholar]
- 45.Hunt PW. Brenchley J. Sinclair E. McCune JM. Roland M. Page-Shafer K. Hsue P. Emu B. Krone M. Lampiris H. Douek D. Martin JN. Deeks SG. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baroncelli S. Galluzzo CM. Pirillo MF. Mancini MG. Weimer LE. Andreotti M. Amici R. Vella S. Giuliano M. Palmisano L. Microbial translocation is associated with residual viral replication in HAART-treated HIV+ subjects with <50 copies/ml HIV-1 RNA. J Clin Virol. 2009;46:367–370. doi: 10.1016/j.jcv.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 47.Papasavvas E. Pistilli M. Reynolds G. Bucki R. Azzoni L. Chehimi J. Janmey PA. DiNubile MJ. Ondercin J. Kostman JR. Mounzer KC. Montaner LJ. Delayed loss of control of plasma lipopolysaccharide levels after therapy interruption in chronically HIV-1-infected patients. AIDS. 2009;23:369–375. doi: 10.1097/QAD.0b013e32831e9c76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lien E. Aukrust P. Sundan A. Muller F. Froland SS. Espevik T. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 (HIV-1) infection: Correlation to disease progression and clinical events. Blood. 1998;92:2084–2092. [PubMed] [Google Scholar]
- 49.Ryan LA. Zheng J. Brester M. Bohac D. Hahn F. Anderson J. Ratanasuwan W. Gendelman HE. Swindells S. Plasma levels of soluble CD14 and tumor necrosis factor-alpha type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis. 2001;184:699–706. doi: 10.1086/323036. [DOI] [PubMed] [Google Scholar]
- 50.eBioscience: Cytokines––Master Regulators of the Immune System. www.ebioscience.com/ebioscience/whatsnew/pdf/Cytokines.pdf. [Aug 18;2010 ]. www.ebioscience.com/ebioscience/whatsnew/pdf/Cytokines.pdf
- 51.eBioscience: Human CD & Other Cellular Antigens. http://www.ebioscience.com/ebioscience/contact.asp. [Aug 18;2010 ]. http://www.ebioscience.com/ebioscience/contact.asp
- 52.Rubbert A. Weissman D. Combadiere C. Pettrone KA. Daucher JA. Murphy PM. Fauci AS. Multifactorial nature of noncytolytic CD8+ T cell-mediated suppression of HIV replication: Beta-chemokine-dependent and -independent effects. AIDS Res Hum Retroviruses. 1997;13:63–69. doi: 10.1089/aid.1997.13.63. [DOI] [PubMed] [Google Scholar]
- 53.Bernstein HB. Kinter AL. Jackson R. Fauci AS. Neonatal natural killer cells produce chemokines and suppress HIV replication in vitro. AIDS Res Hum Retroviruses. 2004;20:1189–1195. doi: 10.1089/aid.2004.20.1189. [DOI] [PubMed] [Google Scholar]
- 54.Chiravuri M. Agarraberes F. Mathieu SL. Lee H. Huber BT. Vesicular localization and characterization of a novel post-proline-cleaving aminodipeptidase, quiescent cell proline dipeptidase. J Immunol. 2000;165:5695–5702. doi: 10.4049/jimmunol.165.10.5695. [DOI] [PubMed] [Google Scholar]
- 55.Trautmann L. Janbazian L. Chomont N. Said EA. Wang G. Gimmig S. Bessette B. Boulassel MR. Delwart E. Sepulveda H. Balderas RS. Routy JP. Haddad EK. Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 56.Freeman GJ. Wherry EJ. Ahmed R. Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petrovas C. Casazza JP. Brenchley JM. Price DA. Gostick E. Adams WC. Precopio ML. Schacker T. Roederer M. Douek DC. Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Day CL. Kaufmann DE. Kiepiela P. Brown JA. Moodley ES. Reddy S. Mackey EW. Miller JD. Leslie AJ. DePierres C. Mncube Z. Duraiswamy J. Zhu B. Eichbaum Q. Altfeld M. Wherry EJ. Coovadia HM. Goulder PJ. Klenerman P. Ahmed R. Freeman GJ. Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 59.Paul ME. Shearer WT. Kozinetz CA. Lewis DE. Comparison of CD8(+) T-cell subsets in HIV-infected rapid progressor children versus non-rapid progressor children. J Allergy Clin Immunol. 2001;108:258–264. doi: 10.1067/mai.2001.117179. [DOI] [PubMed] [Google Scholar]
- 60.Resino S. Bellon JM. Gurbindo MD. Munoz-Fernandez MA. CD38 expression in CD8+ T cells predicts virological failure in HIV type 1-infected children receiving antiretroviral therapy. Clin Infect Dis. 2004;38:412–417. doi: 10.1086/380793. [DOI] [PubMed] [Google Scholar]
- 61.Wilson CM. Ellenberg JH. Douglas SD. Moscicki AB. Holland CA. CD8+ CD38+ T cells but not HIV type 1 RNA viral load predict CD4+ T cell loss in a predominantly minority female HIV+ adolescent population. AIDS Res Hum Retroviruses. 2004;20:263–269. doi: 10.1089/088922204322996482. [DOI] [PubMed] [Google Scholar]
- 62.Rudy BJ. Lindsey JC. Flynn PM. Bosch RJ. Wilson CM. Hughes ME. Douglas SD. Immune reconstitution and predictors of virologic failure in adolescents infected through risk behaviors and initiating HAART: Week 60 results from the PACTG 381 cohort. AIDS Res Hum Retroviruses. 2006;22:213–221. doi: 10.1089/aid.2006.22.213. [DOI] [PubMed] [Google Scholar]
- 63.List EO. Berryman DE. Bower B. Sackmann-Sala L. Gosney E. Ding J. Okada S. Kopchick JJ. The use of proteomics to study infectious diseases. Infect Disord Drug Targets. 2008;8:31–45. doi: 10.2174/187152608784139640. [DOI] [PubMed] [Google Scholar]
- 64.Kalayjian RC. Machekano RN. Rizk N. Robbins GK. Gandhi RT. Rodriguez BA. Pollard RB. Lederman MM. Landay A. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis. 2010;201:1796–1805. doi: 10.1086/652750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel K. Benhamou Y. Yoshida EM. Kaita KD. Zeuzem S. Torbenson M. Pulkstenis E. Subramanian GM. McHutchison JG. An independent and prospective comparison of two commercial fibrosis marker panels (HCV FibroSURE and FIBROSpect II) during albinterferon alfa-2b combination therapy for chronic hepatitis C. J Viral Hepat. 2009;16:178–186. doi: 10.1111/j.1365-2893.2008.01062.x. [DOI] [PubMed] [Google Scholar]
- 66.Helbig KJ. Ruszkiewicz A. Lanford RE. Berzsenyi MD. Harley HA. McColl SR. Beard MR. Differential expression of the CXCR3 ligands in chronic hepatitis C virus (HCV) infection and their modulation by HCV in vitro. J Virol. 2009;83:836–846. doi: 10.1128/JVI.01388-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeremski M. Dimova R. Brown Q. Jacobson IM. Markatou M. Talal AH. Peripheral CXCR3-associated chemokines as biomarkers of fibrosis in chronic hepatitis C virus infection. J Infect Dis. 2009;200:1774–1780. doi: 10.1086/646614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.