RIP1 is a component of a TRAF2 complex, required for caspase-8 activation and tumor cell killing in response to ligand binding of CD40.
Abstract
CD40, a tumor necrosis factor (TNF) receptor family member, is widely recognized for its prominent role in the antitumor immune response. The immunostimulatory effects of CD40 ligation on malignant cells can be switched to apoptosis upon disruption of survival signals transduced by the binding of the adaptor protein TRAF6 to CD40. Apoptosis induction requires a TRAF2-interacting CD40 motif but is initiated within a cytosolic death-inducing signaling complex after mobilization of receptor-bound TRAF2 to the cytoplasm. We demonstrate that receptor-interacting protein 1 (RIP1) is an integral component of this complex and is required for CD40 ligand-induced caspase-8 activation and tumor cell killing. Degradation of the RIP1 K63 ubiquitin ligases cIAP1/2 amplifies the CD40-mediated cytotoxic effect, whereas inhibition of CYLD, a RIP1 K63 deubiquitinating enzyme, reduces it. This two-step mechanism of apoptosis induction expands our appreciation of commonalities in apoptosis regulatory pathways across the TNF receptor superfamily and provides a telling example of how TNF family receptors usurp alternative programs to fulfill distinct cellular functions.
Introduction
Receptor-interacting protein 1 (RIP1) is a death domain–containing kinase with diverse and context-specific roles in inflammation, cell survival, and apoptosis (Festjens et al., 2007; Galluzzi et al., 2009b). Genetic evidence has demonstrated that RIP1 is required for the pro-inflammatory and antiapoptotic functions of TNF receptor 1 (TNFR1) by mediating nuclear factor κB (NF-κB) and MAPK signaling (Kelliher et al., 1998; Vivarelli et al., 2004). whereas other studies have shown that RIP1 is an integral component of a cytoplasmic apoptosis-inducing signaling complex mediated by TNFR1 engagement (Micheau and Tschopp, 2003; Jin and El-Deiry, 2006; O’Donnell et al., 2007; Wang et al., 2008; Legarda-Addison et al., 2009). RIP1 is also required for caspase-8 activation within a Fas ligand (CD95L)-triggered death-inducing signaling complex in epithelial cells (Geserick et al., 2009; Morgan et al., 2009) and for necroptosis triggered by TNF-related apoptosis-inducing ligand (TRAIL), TNF, or anti-Fas Ab (Holler et al., 2000; Hitomi et al., 2008; Cho et al., 2009; Zhang et al., 2009).
CD40, a TNF family receptor, and its cognate ligand, CD154, have long been recognized for their prominent role in the regulation of the immune response (van Kooten and Banchereau, 2000). Humans with CD154 mutations develop a severe immune deficiency called hyper-IgM syndrome, which is clinically manifested by recurrent infections (Callard et al., 1993) and, interestingly, enhanced susceptibility to malignancy (Hayward et al., 1997). Accumulated experimental and clinical evidence suggests that activation of the CD40 pathway exerts tumor regression through a “two-hit” mechanism of action involving an indirect effect of immune activation and a direct cytotoxic effect on the tumor (Vonderheide, 2007; Loskog and Eliopoulos, 2009).
Similar to other TNF receptor family members, CD40 stimulates the activation of competing signals that influence malignant cell survival versus death. Thus, a recessive, death-inducing pathway emerges upon disruption of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase (ERK) survival signaling (Davies et al., 2004; Hill et al., 2005) or treatment with inhibitors of de novo protein synthesis such as cycloheximide (CHX), which target labile antiapoptotic proteins (Hess and Engelmann, 1996; Bugajska et al., 2002; Davies et al., 2004). However, the cytoplasmic tail of CD40 lacks a “death homology domain” that mediates death signals by the TNFR1, Fas, and TRAIL receptors, and so the nature of the CD40-triggered apoptotic pathway has been obscure. Data shown in this report reveal a novel role for RIP1 in linking CD40 to carcinoma cell death.
Results and discussion
The TRAF2/TRAF3-interacting domain of CD40 mediates death signals
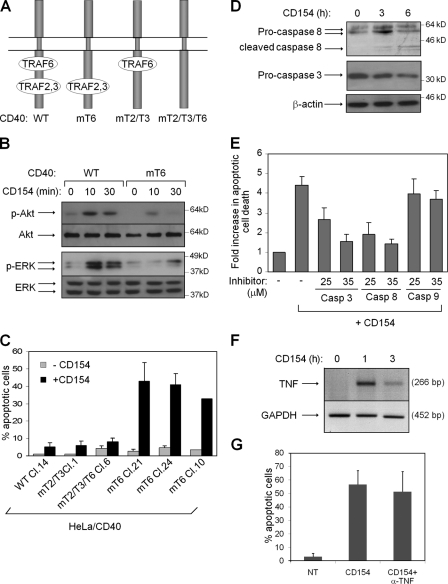
CD40 signals through TNF receptor-associated factor (TRAF) proteins (Bishop, 2004, 2007; Eliopoulos, 2008). Specifically, a membrane-proximal region of the receptor cytoplasmic C terminus binds TRAF6, whereas a membrane-distal domain recruits TRAF2 and TRAF3 (Fig. 1 A). To address the impact of specific CD40–TRAF interactions on apoptotic signaling, we used a panel of HeLa cell clones stably expressing wild-type or mutated CD40 sequences that were unable to directly associate with TRAF6 (CD40mT6), TRAF2/TRAF3 (CD40mT2/mT3), or all TRAFs (CD40mT2/T3/T6; Fig. 1 A; Tsukamoto et al., 1999; Jabara et al., 2002; Benson et al., 2006). We have previously used this cell system to demonstrate that the TRAF2/TRAF3-interacting domain of CD40 is primarily responsible for the engagement of NF-κB, JNK, and p38 cascades, whereas the TRAF6-binding region contributes to NF-κB signaling (Davies et al., 2005b).
Figure 1.
The TRAF2/TRAF3 binding domain of CD40 mediates CD154-induced death signals. (A) Graphical representation of CD40 and its TRAF-binding domains. A double Q234E235 → AA mutation, yielding CD40mT6, selectively abolishes the interaction of TRAF6 with CD40, whereas a T254 → A mutation (CD40mT2/T3) inhibits TRAF2 and TRAF3 but not TRAF6 binding to CD40. CD40mT2/T3/T6 combines the aforementioned mutations and perturbs the binding of all TRAFs (Davies et al., 2005b). WT, wild type. (B) The TRAF6-interacting domain of CD40 transduces ERK and Akt signaling. Lysates from CD154-stimulated HeLa/CD40 and HeLa/CD40mT6 cells were analyzed for the expression of phosphorylated, active ERK and Akt, or the total proteins. (C) The TRAF2/TRAF3-interacting domain of CD40 mediates CD154-induced death signals. HeLa clones expressing the WT or mutated CD40 sequences described in A were stimulated with CD154 for 12 h before assessment of apoptosis. Mean values of percentage apoptotic cells from at least three independent experiments are shown with the exception of HeLa/CD40mT6 clone 10, where two determinations were performed. (D and E) The TRAF2/TRAF3-binding domain of CD40 mediates cell death via caspase-8 activation. HeLa/CD40mT6 clone 21 cells were stimulated with CD154 for the indicated time points, and lysates were analyzed for the expression of caspase-8, caspase-3, or β-actin (D). Inhibitors of caspase-8 and -3 but not caspase-9 protect HeLa/CD40mT6 cells from CD154-induced apoptosis assessed by cell death ELISA. Data are expressed as fold increase (±SD; n = 4) in apoptosis induced by CD154 relative to untreated cultures, which was given the arbitrary value of 1. (F) RT-PCR showing up-regulation of TNF mRNA after treatment of HeLa/CD40mT6 cells with CD154. GADPH, glyceraldehyde 3-phosphate dehydrogenase. (G) Early CD154-mediated death signals are independent of autocrine TNF production. HeLa/CD40mT6 cells were exposed to 0.5 µg/ml neutralizing anti-TNF mAb and then treated as described in C before assessment of apoptosis. Error bars indicate SD.
Other studies have shown that ligation of a CD40 mutant lacking a functional TRAF6 binding site is defective in activation of ERK and Akt in lymphoid cells (Mukundan et al., 2005; Benson et al., 2006) and that ectopic expression of TRAF6 but not TRAF2 or TRAF3 engages the ERK pathway in epithelial cells and fibroblasts (Kashiwada et al., 1998; Eliopoulos et al., 2003). Fig. 1 B shows that both ERK and Akt phosphorylation are significantly impaired in CD154-stimulated HeLa/CD40mT6 cells.
Akt and ERK are the survival signals that override CD154-induced apoptosis in carcinoma cells (Davies et al., 2004, 2005a). We therefore hypothesized that CD40mT6-expressing HeLa cells would be sensitive to the cytotoxic effects of CD40 ligation because of the defect in ERK/Akt activation. Indeed, treatment of HeLa/CD40mT6 cell clones with CD154 induced significant levels of apoptosis in the absence of protein synthesis inhibition (Fig. 1 C). In contrast, the stimulation of CD40mT2/mT3, CD40mT2/T3/T6, or wild-type CD40 HeLa clones with CD154 failed to increase apoptosis above background levels (Fig. 1 C).
Death signals transduced by the CD40 mutant lacking a functional TRAF6 binding site led to rapid caspase-8 and caspase-3 activation (Fig. 1 D). Caspase activation was required for apoptosis induction because the caspase-8 inhibitor peptide z-IETD.fmk or the caspase-3/7 inhibitor z-DEVD.fmk diminished the cytotoxicity of CD154, whereas the caspase-9 inhibitor z-LEHD.fmk had no effect (Fig. 1 E). Although TNF was up-regulated after CD40mT6 stimulation (Fig. 1 F), this early apoptotic response did not depend on autocrine TNF signaling, as viability remained unaffected by coculture with a TNF-neutralizing antibody (Fig. 1 G).
Together, these data reveal distinct roles for the TRAF-interacting CD40 domains in the regulation of carcinoma cell death. They demonstrate that upon CD154 stimulation, the TRAF6 binding site mediates survival signals that dominate over a caspase-dependent death pathway triggered by the TRAF2/TRAF3-interacting domain.
TRAF2 transduces CD40 death signals
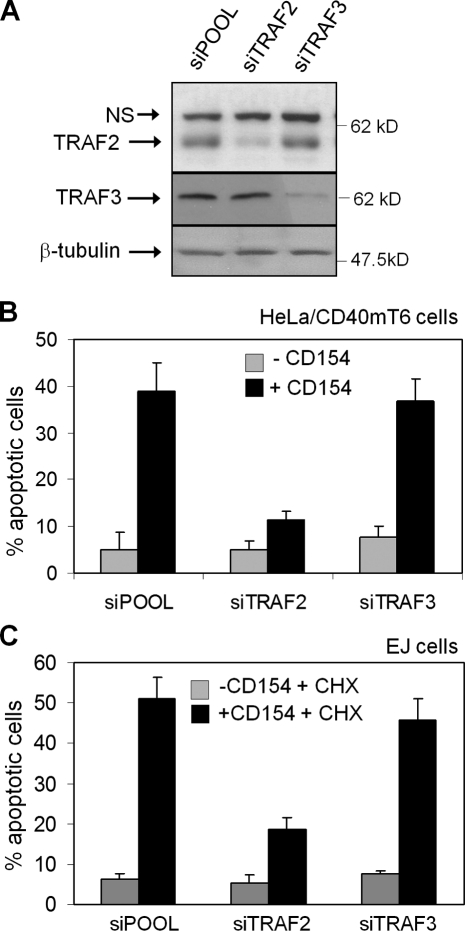
On the basis of the aforementioned findings, we used RNAi to define the relative contribution of TRAF2 versus TRAF3 to CD40-mediated apoptosis (Fig. 2 A). HeLa/CD40mT6 cells transfected with a control siRNA (siPOOL) responded to CD154 treatment with elevated apoptosis, which was reduced in TRAF2 siRNA-transfected cells (Fig. 2 B). In contrast, knockdown of TRAF3 did not have a significant effect.
Figure 2.
TRAF2 is required for the transduction of CD40 death signals. HeLa/CD40mT6 cells were transfected with TRAF2, TRAF3, or control siRNA and either lysed for the assessment of TRAF knockdown efficacy by immunoblotting (A) or exposed to 1 µg/ml soluble CD154 before evaluation of apoptosis (B). (C) Knockdown of TRAF2 in EJ bladder carcinoma cells confers resistance to apoptosis induced by soluble CD154 in the presence of 10 µg/ml CHX. Mean values (±SD) from three independent experiments are shown (error bars).
The role of TRAFs in CD154-induced apoptosis was also examined in EJ bladder carcinoma cells (Stamenkovic et al., 1989), which are responsive to CD40-mediated death signals in the presence of CHX (Davies et al., 2004). As shown in Fig. 2 C, TRAF2 knockdown significantly reduced the cytotoxic effects of CD154 and CHX combination treatment compared with TRAF3 or control siRNA. These data demonstrate a novel role for TRAF2 as a positive regulator of CD40-transduced death signals.
TRAF2 interacts with RIP1 in the context of CD40 signaling
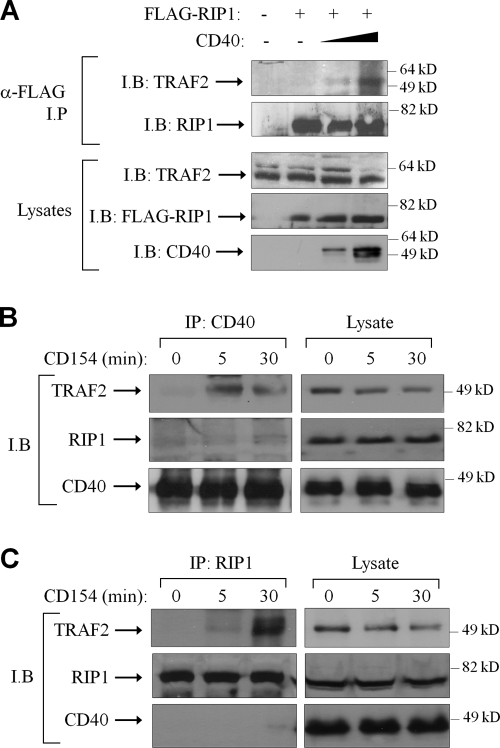
TRAF2 is a component of the cytosolic TNFR1 death-inducing signaling complex (Micheau and Tschopp, 2003), and binds RIP1 (Hsu et al., 1996). We thus reasoned that TRAF2 may channel CD40 signals to RIP1. We tested this hypothesis by performing coimmunoprecipitation experiments in lysates from CD40-negative 293 cells transfected with FLAG-tagged RIP1 in the presence or absence of a CD40 receptor expression vector. When overexpressed, CD40 stimulates signal activation through formation of receptor multimers and recruitment of TRAFs in a ligand-independent manner (Rothe et al., 1995; Pullen et al., 1999). As shown in Fig. 3 A, although very little endogenous TRAF2 coprecipitated with FLAG-RIP1 in the absence of CD40, this interaction dramatically increased upon transfection of increasing amounts of CD40 expression vector.
Figure 3.
TRAF2 and RIP1 interact in the context of CD40 signaling. (A) Ectopic expression of CD40 enhances the interaction of RIP1 with endogenous TRAF2. Two million 293 cells were transfected with 0.5 µg FLAG-tagged RIP1 in the presence of increasing amounts of a CD40 expression vector (0, 1, or 2.5 µg). Lysates were immunoprecipitated (IP) with anti-FLAG and immunoblotted (IB) with an anti-TRAF2 or RIP1 antibody as indicated. (B and C) CD40 ligation induces the association of endogenous RIP1 with TRAF2 but not CD40. HeLa/CD40mT6 cells were stimulated with CD154, then lysates were sequentially immunoprecipitated with anti-CD40 (B) and anti-RIP1 (C) and immunoblotted with TRAF2, RIP1, or CD40 antibodies.
The TRAF2-RIP1 link was further explored in physiological conditions under which endogenous proteins were analyzed. To this end, lysates were prepared from HeLa/CD40mT6 cells before and after stimulation with CD154 and sequentially immunoprecipitated with anti-CD40 and RIP1 antibodies. In anti-CD40 immunoprecipitates, no detectable association of endogenous CD40 and TRAF2 was observed in the absence of stimulation, but this interaction was rapidly induced after CD40 ligation, as described previously (Rothe et al., 1995; Matsuzawa et al., 2008). RIP1 did not coprecipitate with CD40 before or after stimulation (Fig. 3 B). Interestingly, TRAF2 was readily detected in anti-RIP1 immunoprecipitates from CD154-stimulated cultures (Fig. 3 C).
These data suggest that RIP1 is not a component of the CD40-bound TRAF2 signaling complex but forms a separate, cytoplasmic association with TRAF2 after CD40 ligation. We hypothesize that the absence of RIP1 in CD40-bound TRAF2 complexes is the result of the overlapping requirement of the C terminus of TRAF2 for binding to both CD40 (Rothe et al., 1995) and RIP1 (Liu et al., 1996). Once released from CD40, presumably after the CD154-mediated ubiquitination and degradation of TRAF3 (Matsuzawa et al., 2008; for review see Eliopoulos, 2008), the C terminus of TRAF2 could become accessible to RIP1, allowing the formation of a pro-apoptotic signaling complex. The difference in the kinetics of CD40–TRAF2 and RIP1–TRAF2 interactions after CD40 stimulation (Fig. 3, B and C) is compatible with this model.
RIP1 is required for CD40-induced death signaling in tumor cells
We next explored the functional role of RIP1 in CD40 death signaling by using geldanamycin, a compound which induces the degradation of RIP1 by disrupting the function of the RIP1-associating chaperone protein HSP90 (Lewis et al., 2000). Treatment of different tumor lines with geldanamycin induced a time-dependent decrease in RIP1 expression (Fig. S1 A) and protected them from CD154-induced apoptosis (Fig. S1 B).
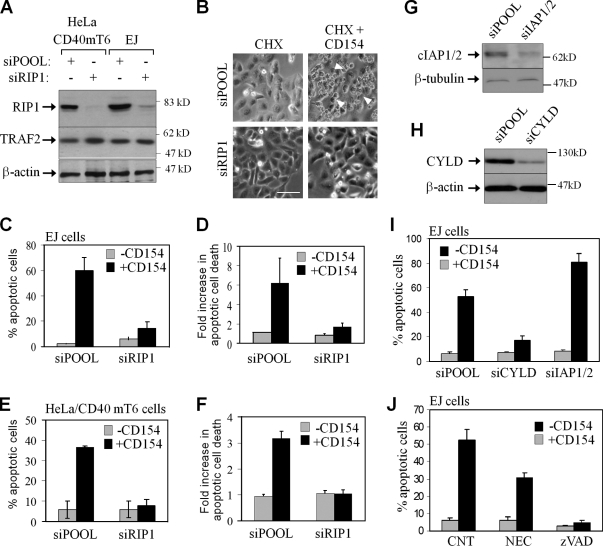
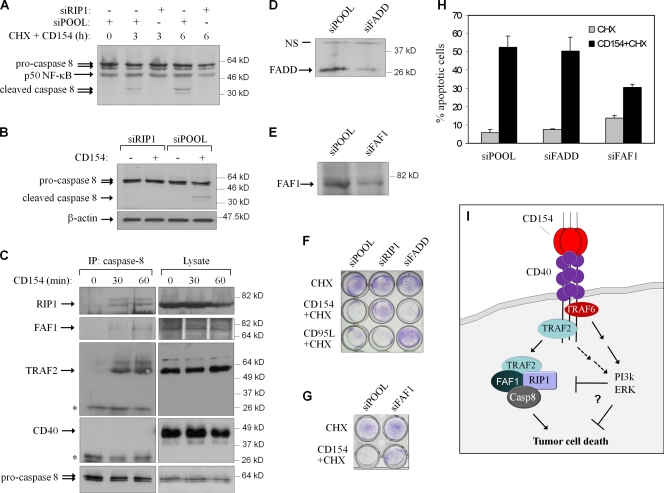
These results demonstrate a correlation between RIP1 protein levels, modulated by geldanamycin, and sensitivity to CD154-induced apoptosis but do not exclude the possibility that geldanamycin impacts on CD40-induced cell death through molecules other than RIP1. Therefore, we used RNAi to explore more specifically the role of RIP1 in death signaling. The knockdown of RIP1 in both EJ and HeLa/CD40mT6 (Fig. 4 A) dramatically reduced CD154-induced cytotoxicity, as determined by morphological changes (Fig. 4 B), nuclear condensation, and degradation detected by propidium iodide staining (Fig. 4, C and E), oligo-nucleosomal enrichment (Fig. 4, D and F), crystal violet staining (Fig. 5 F), or Annexin V staining and flow cytometry (not depicted). In contrast, diminished RIP1 did not affect CD95L-induced apoptosis (Fig. 5 F; Jin and El-Deiry, 2006).
Figure 4.
RIP1 is required for CD40-mediated apoptosis in carcinoma cells. (A) Knockdown efficiency of RIP1 siRNA. (B) EJ cells treated with CD154 in the presence of CHX display morphology typical of apoptotic cells (see representative arrowheads), whereas RIP1 siRNA-transfected cultures remain unaffected. Bar, 25 µm. (C and D) RIP1 knockdown rescues EJ cells from CD154 and CHX-induced apoptosis, as determined by propidium iodide staining and immunofluorescence microscopy (C) or cell death ELISA (D). Mean values (±SD) from four independent experiments are shown. (E and F) RIP1 knockdown rescues HeLa/CD40mT6 cells from CD154-induced apoptosis, determined as described in the legend of Fig 4 C. (G and H) Expression of cIAP1/2 (G) and CYLD (H) before and after transfection with the respective siRNAs. (I) Effect of cIAP1/2 and CYLD knockdown on CD154-mediated EJ cell death. (J) Necrostatin (50 µM) inhibits and the pan-caspase inhibitor zVAD-fmk (15 µM) abolishes the pro-apoptotic effects of CD154 in EJ cells. Error bars indicate SD.
Figure 5.
RIP1 associates with caspase-8 and is required for its activation. (A and B) RIP1 knockdown suppresses CD154-induced caspase-8 cleavage in EJ (A) or HeLa/CD40mT6 (B) cells. Lysates were immunoblotted for caspase-8 or, as a control, β-actin or p50 NF-κB. (C) RIP1, TRAF2, FAF1, and caspase-8 interact upon CD40 stimulation. Cells were stimulated with CD154, and lysates were immunoprecipitated with a goat polyclonal against caspase-8. Immunoprecipitants were immunoblotted using rabbit polyclonal antibodies against RIP1, TRAF2, CD40, and FAF1 or a monoclonal anti-caspase-8, as indicated. Results are representative of three independent experiments. The Ig light chains are indicted by asterisks. (D and E) Expression of FADD (D) and FAF1 (E) before and after transfection with the respective siRNAs. (F and G) Crystal violet staining of RNAi-transfected EJ cells treated with CHX in the presence or absence of CD154 or CD95L. (H) FAF1 but not FADD knockdown protects from the pro-apoptotic effects of CD40 ligation. After knockdown, EJ cells were exposed to CD154 and CHX for 5 h before assessment of apoptosis. Error bars indicate SD. (I) Proposed model of CD40-induced death signaling in tumor cells. CD40-mediated apoptosis involves the formation of a secondary cytoplasmic complex of TRAF2, RIP1, FAF1 and caspase-8 (Casp-8). RIP1 is required for caspase-8 activation and cell death, whereas apoptosis is antagonized by survival signals predominantly mediated by the TRAF6-binding domain of CD40, which may operate at the level of the death-inducing signaling complex and/or downstream of it.
To confirm that RIP1 is a bona fide regulator of CD40 death signaling, we assessed the effect of RIP1 knockdown on apoptosis induced by CD154 transgene expression. Adenovirus-mediated delivery of the CD154 gene to carcinoma cells elicits pro-apoptotic effects through membrane-bound CD154 (Gomes et al., 2009; Vardouli et al., 2009), which is likely to mimic CD154 expressed on activated T lymphocytes. EJ cells transfected with control or RIP1 siRNA were transduced with recombinant adenovirus (RAd) expressing CD154 or, as a control, the lacZ gene, then analyzed for transgene expression (Fig. S1 C) or exposed to CHX before assessment of apoptosis (Fig. S1 D). It was found that RIP1 knockdown provided protection from the cytotoxic effect of RAd-CD154 and CHX treatment (Fig. S1 D). Collectively, these data provide compelling evidence that RIP1 has an essential role in the regulation of CD40-mediated death signaling in tumor cells.
RIP1 is critically involved in TNF-induced JNK, ERK, and NF-κB signaling in most (Kelliher et al., 1998; Devin et al., 2003; Lee et al., 2003) but not all settings (Wong et al., 2010). We have found that the knockdown of RIP1 did not significantly influence the CD154-mediated degradation of IκBα, a hallmark of canonical NF-κB signaling, ERK or JNK phosphorylation, or the processing of p100 NF-κB2 to p52 (Fig. S2).
CYLD and cellular inhibitors of apoptosis (cIAPs) are involved in CD40-mediated cell death
Ubiquitination and phosphorylation are two RIP1 posttranslational modifications that influence RIP1-mediated cell death. cIAP1/2 are required for TNF-induced K63-linked ubiquitination of RIP1 (Bertrand et al., 2008), which functions to inhibit TNF-induced apoptosis (O’Donnell et al., 2007), whereas RIP1 deubiquitination by CYLD facilitates its direct interaction with caspase-8 and initiation of cell death (Wang et al., 2008). Because cIAP1/2 are involved in CD40-mediated MAPK signaling (Matsuzawa et al., 2008), we hypothesized that they may also function in the CD40 death pathway. In line with this prediction, knockdown of cIAP1/2 increased EJ cell killing by CD154 and CHX treatment (Fig. 4, G and I), whereas the knockdown of CYLD inhibited it (Fig. 4, H and I).
Smac mimetic compounds induce degradation of cIAPs and thus amplify death ligand–induced cancer cell killing (Li et al., 2004; Wang et al., 2008; Geserick et al., 2009). When EJ cells were treated with the Smac mimetic LBW242 (Gaither et al., 2007), cIAP1/2 were rapidly degraded (Fig. S3 A) and the cells became susceptible to CD154-induced apoptosis (Fig. S3 B). This effect was blocked by RIP1 knockdown or the pan-caspase inhibitor zVAD-fmk, further highlighting the critical involvement of the cIAP1/2–RIP1–caspase axis in the CD40 death pathway.
Moreover, the catalytic activity of RIP1 may also contribute to the CD40-trigerred death pathway, as necrostatin-1, an allosteric RIP1 kinase inhibitor (Degterev et al., 2008), inhibited CD40-mediated apoptosis by 40% (Fig. 4 J).
RIP1 associates with caspase-8 and is required for its activation
As caspase-8 activation is required for CD40 induced apoptosis (Fig. 1 E), we examined whether RIP1 functions upstream of caspase-8 in this pathway. Cells were depleted of RIP1 and treated with CD154 in the presence (EJ) or absence (HeLa/CD40mT6) of CHX before analysis of caspase-8 by immunoblotting. RIP1 knockdown was found to suppress caspase-8 activation in both cases (Fig. 5, A and B).
This observation prompted us to investigate the hypothesis that caspase-8 interacts with RIP1 and TRAF2. HeLa/CD40mT6 cells were stimulated with CD154, and lysates were subjected to immunoprecipitation using anti–caspase-8 antibody. In control lysates, neither RIP1 nor TRAF2 coprecipitated with caspase-8. However, both proteins (but not CD40) were found in complex with caspase-8 after exposure to CD154 (Fig. 5 C).
RIP1 has a death domain motif, and caspase-8 has a death effector domain (DED). Fas-associated death domain protein (FADD) possesses both motifs and has been proposed to link RIP1 and caspase-8 in death receptor signaling (Chinnaiyan et al., 1995; Kischkel et al., 2000; Sprick et al., 2000). Although FADD knockdown blocked CD95L-induced killing (Fig. 5, D and F), it did not impact on CD40-mediated apoptosis (Fig. 5, F and H). FADD knockdown also fails to influence TNF-induced, RIP1-mediated death signals in tumor cells (Jin and El-Deiry, 2006; Wang et al., 2008). FAF1 is a pro-apoptotic protein that possesses a DED-interacting domain responsible for association with the DED of caspase-8 and an atypical death domain (Ryu et al., 2003). Knockdown of FAF1 in EJ cells (Fig. 5 E) was found to partially reduce the cytotoxic effect of CD154 and CHX treatment (Fig. 5, G and H), and FAF1 is detected in the RIP1–caspase-8 complex (Fig. 5 C).
We have recently shown that TRAF2 is largely responsible for the CD154-mediated sequential activation of NF-κB and IRF1, which act in concert to ensure the synchronous synthesis of components of the antigen presentation machinery required for the engagement of antitumor immune responses (Moschonas et al., 2008). Results presented in this study demonstrate that TRAF2 is also required for CD40-mediated tumor cell killing (Figs. 1 and 2). Together, these observations suggest that TRAF2 is a master regulator of the antitumor functions of CD40 in malignant epithelial cells.
However, our data also show that apoptosis is not triggered at the level of the receptor, but requires the function of a cytosolic complex containing TRAF2, RIP1, FAF1, and caspase-8, which is antagonized by signals emanating from the TRAF6-binding domain of CD40 (Fig. 5 I). Considering the breadth of CD40 expression and the diversity of its roles, the identification of two signaling complexes regulating cell survival versus death could be exploited to fine-tune CD154-based anticancer strategies.
Materials and methods
Cell culture, adenovirus constructs, and reagents
The bladder carcinoma EJ, the cervical cell line HeLa, and CD40-expressing clones were maintained in RPMI medium supplemented with 10% FCS. The early passage ovarian AGE60 (Vardouli et al., 2009) and the human embryonic kidney (HEK) 293 cell line were cultured in Dulbecco’s modified Eagle medium supplemented with 10% FCS (Invitrogen). Parental 293 and HeLa cells are CD40-negative (Davies et al., 2005b). Human recombinant soluble CD40L was kindly provided by Amgen Inc., or purchased from Enzo Life Sciences, Inc. Geldanamycin, kinase, and caspase inhibitors were obtained from EMD and dissolved in dimethyl sulfoxide before use. RAds expressing CD154 and lacZ have been described previously (Vardouli et al., 2009). The Smac mimetic LBW242 was provided by L. Zawel (Novartis Institutes for Biomedical Research, Basel, Switzerland).
Antibodies, immunoprecipitations, and immunoblotting
Phospho-specific antibodies against JNK, ERK, Akt, and the corresponding antibodies that recognize both the phosphorylated and unphosphorylated forms, the FAF1, and monoclonal caspase Abs were purchased from Cell Signaling Technology and used at dilutions of 1:500–1:1000. The IκBα/MAD3 (C21), RIP1 (C20), TRAF2 (C20), CD40 (H120 and C20), and caspase-8 (C20) antibodies were obtained from Santa Cruz Biotechnology, Inc., the β-actin and FLAG M2 antibodies were obtained from Sigma-Aldrich, and the cIAP1/2 Ab was obtained from R&D Systems. Anti–rabbit IgG-HRP and anti–mouse IgG-HRP were obtained from Sigma-Aldrich. Immunoblotting was performed as described previously (Eliopoulos et al., 2003; Moschonas et al., 2008). For immunoprecipitation, cells (1–2 × 107) were lysed in 1 ml of DISC immunoprecipitation buffer (10 mM Tris, pH 7.5, 150 mM NaCl, 10% glycerol, 1 mM EDTA, and 1% Triton X-100) with protease inhibitor cocktail (Roche). Cell lysates (900 µl) were incubated overnight with 2–3 µg of antibody at 4°C. Complexes were precipitated by protein G–agarose (Millipore) and suspended in 50 µl of SDS sample buffer after three washes with DISC immunoprecipitation buffer. Immunoprecipitates were subjected to SDS-PAGE and Western blotting. The neutralizing anti-TNF mAb2101 was purchased from R&D Systems and used at 0.5 µg/ml.
Quantitative measurement of apoptosis
For the assessment of apoptosis, we took into consideration the guidelines for the use and interpretation of assays for monitoring cell death (Galluzzi et al., 2009a) and performed multiple, methodologically unrelated assays to quantify dead cells.
Cytochemical staining.
To estimate apoptosis based on nuclear morphology, the fluorescent DNA staining dye propidium iodide (Sigma-Aldrich) was used. Approximately 2 × 104 cells in 25 µl were stained by adding 1 µl of 100 µg/ml propidium iodide. Uptake of the dye was examined by fluorescence microscopy. To estimate the apoptotic index, a minimum of 300 cells was examined by at least two independent investigators and quantified by recording the relative number of cells showing condensed or fragmented chromatin. Unless stated otherwise, data shown depict apoptosis measurements using this assay, with error bars representing SD of n independent experiments indicated in the figure legends.
ELISA-based nucleosomal DNA fragmentation enrichment assay.
Apoptosis was quantified by direct determination of nucleosomal DNA fragmentation using the Cell Death Detection Elisa Plus kit (Roche), an assay designed to provide relative measurements of apoptosis rather than absolute numbers of dead cells. Cells were plated in 96-well plates at an initial concentration 5–7 × 103 cells/well depending on the cell line. The following day, cells were treated with CD154 in the presence or absence of CHX. Alternatively, cells were infected with adenoviruses at MOI 200 and allowed to express the transgene for 16 h before application of 10 µg/ml CHX. Cells were then processed according to the manufacturer’s instructions. The mono- and oligonucleosomes contained in the cell lysates were determined using the anti–histone-biotin Ab. The concentration of the mono- and oligonucleosomes was determined photometrically using an anti–histone-biotin Ab, followed by incubation with 2,2’-azino-di(3-ethylbenzthiazolin-sulfonate) (ABTS) as substrate for the antibody. The OD was read on a microplate reader (Bio-Rad Laboratories) at a wavelength of 405 nm, and the ratio of OD of the sample to the OD of cells without treatment was estimated to give the fold increase in apoptosis.
Annexin V staining.
Cells were lightly trypsinized and incubated with Annexin V-FITC (BD) and propidium iodide for 15 min before assessment of fluorescence intensity on a flow cytometer (BD).
RNAi
For the delivery of siRNAs, 5 × 104 EJ or HeLa/CD40mT6 cells were plated into each well of a 24-well plate (Costar), and two rounds of transfection with siRNA duplexes were performed as described previously (Davies et al., 2005b; Moschonas et al., 2008). The sequences of the siRNAs used were as follows. RIP1 siRNA, 5′-GUACUCCGCUUUCUGUAAA-3′; TRAF2 siRNA, Dharmacon siGENOME SMARTpool M-005198 (Thermo Fischer Scientific); TRAF3 siRNA, Dharmacon siGENOME SMARTpool M-005252 (Thermo Fischer Scientific); FADD siRNA, Dharmacon siGENOME SMARTpool M-003800 (Thermo Fischer Scientific); and FAF1 siRNA, Dharmacon siGENOME SMARTpool M-009106 (Thermo Fischer Scientific). The siRNA sequences for cIAP1, cIAP2, and CYLD were as described previously (Wang et al., 2008). cIAP1 and cIAP2 were knocked down simultaneously.
Light microscopy
Morphological changes related to apoptosis were observed using an inverted microscope (DMIRE2; Leica) equipped with a digital camera (DFC300 FX; Leica). Camera image acquisition was controlled by IM50 software (Leica), and single images were exported as TIFF files. Individual frames were prepared for presentation using Photoshop (Adobe). Cells were seeded into 4-well, chambered coverglass units with coverslip-quality glass bottoms (Laboratory-Tek; Thermo Fischer Scientific) and, after treatment, were examined with a 63× dry objective lens.
Online supplemental material
Fig. S1 shows data supporting the involvement of RIP1 in CD40-mediated apoptosis. Fig. S2 illustrates that RIP1 is dispensable for CD154-stimulated NF-κB and MAPK signaling. Fig. S3 shows that RIP1 is required for apoptosis induced by a Smac mimetic and CD154 combination treatment. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201003087/DC1.
Acknowledgments
The authors declare no conflict of interest.
This work was supported by grants from the Association for International Cancer Research (UK; grant No. 06-324) and the European Commission FP6 program Apotherapy (EC contract No. 037344) to A.G. Eliopoulos; part of the work was initiated at the University of Birmingham, UK, before the relocation of the Eliopoulos laboratory to the University of Crete, Greece.
Footnotes
Abbreviations used in this paper:
- CHX
- cycloheximide
- cIAP
- cellular inhibitor of apoptosis
- DED
- death effector domain
- ERK
- extracellular signal-regulated kinase
- FADD
- Fas-associated death domain protein
- NF-κB
- nuclear factor κB
- RAd
- recombinant adenovirus
- RIP1
- receptor-interacting protein 1
- TRAF
- TNF receptor-associated factor
References
- Benson R.J., Hostager B.S., Bishop G.A. 2006. Rapid CD40-mediated rescue from CD95-induced apoptosis requires TNFR-associated factor-6 and PI3K. Eur. J. Immunol. 36:2535–2543 10.1002/eji.200535483 [DOI] [PubMed] [Google Scholar]
- Bertrand M.J., Milutinovic S., Dickson K.M., Ho W.C., Boudreault A., Durkin J., Gillard J.W., Jaquith J.B., Morris S.J., Barker P.A. 2008. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell. 30:689–700 10.1016/j.molcel.2008.05.014 [DOI] [PubMed] [Google Scholar]
- Bishop G.A. 2004. The multifaceted roles of TRAFs in the regulation of B-cell function. Nat. Rev. Immunol. 4:775–786 10.1038/nri1462 [DOI] [PubMed] [Google Scholar]
- Bishop G.A., Moore C.R., Xie P., Stunz L.L., Kraus Z.J. 2007. TRAF proteins in CD40 signaling. Adv. Exp. Med. Biol. 597:131–151 10.1007/978-0-387-70630-6_11 [DOI] [PubMed] [Google Scholar]
- Bugajska U., Georgopoulos N.T., Southgate J., Johnson P.W., Graber P., Gordon J., Selby P.J., Trejdosiewicz L.K. 2002. The effects of malignant transformation on susceptibility of human urothelial cells to CD40-mediated apoptosis. J. Natl. Cancer Inst. 94:1381–1395 [DOI] [PubMed] [Google Scholar]
- Callard R.E., Armitage R.J., Fanslow W.C., Spriggs M.K. 1993. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol. Today. 14:559–564 10.1016/0167-5699(93)90188-Q [DOI] [PubMed] [Google Scholar]
- Chinnaiyan A.M., O’Rourke K., Tewari M., Dixit V.M. 1995. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 81:505–512 10.1016/0092-8674(95)90071-3 [DOI] [PubMed] [Google Scholar]
- Cho Y.S., Challa S., Moquin D., Genga R., Ray T.D., Guildford M., Chan F.K. 2009. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 137:1112–1123 10.1016/j.cell.2009.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C.C., Mason J., Wakelam M.J., Young L.S., Eliopoulos A.G. 2004. Inhibition of phosphatidylinositol 3-kinase- and ERK MAPK-regulated protein synthesis reveals the pro-apoptotic properties of CD40 ligation in carcinoma cells. J. Biol. Chem. 279:1010–1019 10.1074/jbc.M303820200 [DOI] [PubMed] [Google Scholar]
- Davies C.C., Bem D., Young L.S., Eliopoulos A.G. 2005a. NF-kappaB overrides the apoptotic program of TNF receptor 1 but not CD40 in carcinoma cells. Cell. Signal. 17:729–738 10.1016/j.cellsig.2004.10.014 [DOI] [PubMed] [Google Scholar]
- Davies C.C., Mak T.W., Young L.S., Eliopoulos A.G. 2005b. TRAF6 is required for TRAF2-dependent CD40 signal transduction in nonhemopoietic cells. Mol. Cell. Biol. 25:9806–9819 10.1128/MCB.25.22.9806-9819.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A., Hitomi J., Germscheid M., Ch’en I.L., Korkina O., Teng X., Abbott D., Cuny G.D., Yuan C., Wagner G., et al. 2008. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 4:313–321 10.1038/nchembio.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devin A., Lin Y., Liu Z.G. 2003. The role of the death-domain kinase RIP in tumour-necrosis-factor-induced activation of mitogen-activated protein kinases. EMBO Rep. 4:623–627 10.1038/sj.embor.embor854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos A.G. 2008. Cell signaling. “Make and brake” in signaling. Science. 321:648–649 10.1126/science.1162212 [DOI] [PubMed] [Google Scholar]
- Eliopoulos A.G., Wang C.C., Dumitru C.D., Tsichlis P.N. 2003. Tpl2 transduces CD40 and TNF signals that activate ERK and regulates IgE induction by CD40. EMBO J. 22:3855–3864 10.1093/emboj/cdg386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festjens N., Vanden Berghe T., Cornelis S., Vandenabeele P. 2007. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ. 14:400–410 10.1038/sj.cdd.4402085 [DOI] [PubMed] [Google Scholar]
- Gaither A., Porter D., Yao Y., Borawski J., Yang G., Donovan J., Sage D., Slisz J., Tran M., Straub C., et al. 2007. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res. 67:11493–11498 10.1158/0008-5472.CAN-07-5173 [DOI] [PubMed] [Google Scholar]
- Galluzzi L., Aaronson S.A., Abrams J., Alnemri E.S., Andrews D.W., Baehrecke E.H., Bazan N.G., Blagosklonny M.V., Blomgren K., Borner C., et al. 2009a. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 16:1093–1107 10.1038/cdd.2009.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Kepp O., Kroemer G. 2009b. RIP kinases initiate programmed necrosis. J. Mol. Cell. Biol. 1:8–10 10.1093/jmcb/mjp007 [DOI] [PubMed] [Google Scholar]
- Geserick P., Hupe M., Moulin M., Wong W.W., Feoktistova M., Kellert B., Gollnick H., Silke J., Leverkus M. 2009. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J. Cell Biol. 187:1037–1054 10.1083/jcb.200904158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes E.M., Rodrigues M.S., Phadke A.P., Butcher L.D., Starling C., Chen S., Chang D., Hernandez-Alcoceba R., Newman J.T., Stone M.J., Tong A.W. 2009. Antitumor activity of an oncolytic adenoviral-CD40 ligand (CD154) transgene construct in human breast cancer cells. Clin. Cancer Res. 15:1317–1325 10.1158/1078-0432.CCR-08-1360 [DOI] [PubMed] [Google Scholar]
- Hayward A.R., Levy J., Facchetti F., Notarangelo L., Ochs H.D., Etzioni A., Bonnefoy J.Y., Cosyns M., Weinberg A. 1997. Cholangiopathy and tumors of the pancreas, liver, and biliary tree in boys with X-linked immunodeficiency with hyper-IgM. J. Immunol. 158:977–983 [PubMed] [Google Scholar]
- Hess S., Engelmann H. 1996. A novel function of CD40: induction of cell death in transformed cells. J. Exp. Med. 183:159–167 10.1084/jem.183.1.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S.C., Youde S.J., Man S., Teale G.R., Baxendale A.J., Hislop A., Davies C.C., Luesley D.M., Blom A.M., Rickinson A.B., et al. 2005. Activation of CD40 in cervical carcinoma cells facilitates CTL responses and augments chemotherapy-induced apoptosis. J. Immunol. 174:41–50 [DOI] [PubMed] [Google Scholar]
- Hitomi J., Christofferson D.E., Ng A., Yao J., Degterev A., Xavier R.J., Yuan J. 2008. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 135:1311–1323 10.1016/j.cell.2008.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler N., Zaru R., Micheau O., Thome M., Attinger A., Valitutti S., Bodmer J.L., Schneider P., Seed B., Tschopp J. 2000. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1:489–495 10.1038/82732 [DOI] [PubMed] [Google Scholar]
- Hsu H., Huang J., Shu H.B., Baichwal V., Goeddel D.V. 1996. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 4:387–396 10.1016/S1074-7613(00)80252-6 [DOI] [PubMed] [Google Scholar]
- Jabara H., Laouini D., Tsitsikov E., Mizoguchi E., Bhan A., Castigli E., Dedeoglu F., Pivniouk V., Brodeur S., Geha R. 2002. The binding site for TRAF2 and TRAF3 but not for TRAF6 is essential for CD40-mediated immunoglobulin class switching. Immunity. 17:265–276 10.1016/S1074-7613(02)00394-1 [DOI] [PubMed] [Google Scholar]
- Jin Z., El-Deiry W.S. 2006. Distinct signaling pathways in TRAIL- versus tumor necrosis factor-induced apoptosis. Mol. Cell. Biol. 26:8136–8148 10.1128/MCB.00257-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwada M., Shirakata Y., Inoue J.-I., Nakano H., Okazaki K., Okumura K., Yamamoto T., Nagaoka H., Takemori T. 1998. Tumor necrosis factor receptor-associated factor 6 (TRAF6) stimulates extracellular signal-regulated kinase (ERK) activity in CD40 signaling along a ras-independent pathway. J. Exp. Med. 187:237–244 10.1084/jem.187.2.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher M.A., Grimm S., Ishida Y., Kuo F., Stanger B.Z., Leder P. 1998. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 8:297–303 10.1016/S1074-7613(00)80535-X [DOI] [PubMed] [Google Scholar]
- Kischkel F.C., Lawrence D.A., Chuntharapai A., Schow P., Kim K.J., Ashkenazi A. 2000. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 12:611–620 10.1016/S1074-7613(00)80212-5 [DOI] [PubMed] [Google Scholar]
- Lee T.H., Huang Q., Oikemus S., Shank J., Ventura J.J., Cusson N., Vaillancourt R.R., Su B., Davis R.J., Kelliher M.A. 2003. The death domain kinase RIP1 is essential for tumor necrosis factor alpha signaling to p38 mitogen-activated protein kinase. Mol. Cell. Biol. 23:8377–8385 10.1128/MCB.23.22.8377-8385.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legarda-Addison D., Hase H., O’Donnell M.A., Ting A.T. 2009. NEMO/IKKgamma regulates an early NF-kappaB-independent cell-death checkpoint during TNF signaling. Cell Death Differ. 16:1279–1288 10.1038/cdd.2009.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J., Devin A., Miller A., Lin Y., Rodriguez Y., Neckers L., Liu Z.G. 2000. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J. Biol. Chem. 275:10519–10526 10.1074/jbc.275.14.10519 [DOI] [PubMed] [Google Scholar]
- Li L., Thomas R.M., Suzuki H., De Brabander J.K., Wang X., Harran P.G. 2004. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 305:1471–1474 10.1126/science.1098231 [DOI] [PubMed] [Google Scholar]
- Liu Z.G., Hsu H., Goeddel D.V., Karin M. 1996. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 87:565–576 10.1016/S0092-8674(00)81375-6 [DOI] [PubMed] [Google Scholar]
- Loskog A.S., Eliopoulos A.G. 2009. The Janus faces of CD40 in cancer. Semin. Immunol. 21:301–307 10.1016/j.smim.2009.07.001 [DOI] [PubMed] [Google Scholar]
- Matsuzawa A., Tseng P.H., Vallabhapurapu S., Luo J.L., Zhang W., Wang H., Vignali D.A., Gallagher E., Karin M. 2008. Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex. Science. 321:663–668 10.1126/science.1157340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheau O., Tschopp J. 2003. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 114:181–190 10.1016/S0092-8674(03)00521-X [DOI] [PubMed] [Google Scholar]
- Morgan M.J., Kim Y.S., Liu Z.G. 2009. Membrane-bound Fas ligand requires RIP1 for efficient activation of caspase-8 within the death-inducing signaling complex. J. Immunol. 183:3278–3284 10.4049/jimmunol.0803428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschonas A., Kouraki M., Knox P.G., Thymiakou E., Kardassis D., Eliopoulos A.G. 2008. CD40 induces antigen transporter and immunoproteasome gene expression in carcinomas via the coordinated action of NF-kappaB and of NF-kappaB-mediated de novo synthesis of IRF-1. Mol. Cell. Biol. 28:6208–6222 10.1128/MCB.00611-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukundan L., Bishop G.A., Head K.Z., Zhang L., Wahl L.M., Suttles J. 2005. TNF receptor-associated factor 6 is an essential mediator of CD40-activated proinflammatory pathways in monocytes and macrophages. J. Immunol. 174:1081–1090 [DOI] [PubMed] [Google Scholar]
- O’Donnell M.A., Legarda-Addison D., Skountzos P., Yeh W.C., Ting A.T. 2007. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr. Biol. 17:418–424 10.1016/j.cub.2007.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen S.S., Labadia M.E., Ingraham R.H., McWhirter S.M., Everdeen D.S., Alber T., Crute J.J., Kehry M.R. 1999. High-affinity interactions of tumor necrosis factor receptor-associated factors (TRAFs) and CD40 require TRAF trimerization and CD40 multimerization. Biochemistry. 38:10168–10177 10.1021/bi9909905 [DOI] [PubMed] [Google Scholar]
- Rothe M., Sarma V., Dixit V.M., Goeddel D.V. 1995. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 269:1424–1427 10.1126/science.7544915 [DOI] [PubMed] [Google Scholar]
- Ryu S.W., Lee S.J., Park M.Y., Jun J.I., Jung Y.K., Kim E. 2003. Fas-associated factor 1, FAF1, is a member of Fas death-inducing signaling complex. J. Biol. Chem. 278:24003–24010 10.1074/jbc.M302200200 [DOI] [PubMed] [Google Scholar]
- Sprick M.R., Weigand M.A., Rieser E., Rauch C.T., Juo P., Blenis J., Krammer P.H., Walczak H. 2000. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 12:599–609 10.1016/S1074-7613(00)80211-3 [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Clark E.A., Seed B. 1989. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J. 8:1403–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto N., Kobayashi N., Azuma S., Yamamoto T., Inoue J. 1999. Two differently regulated nuclear factor kappaB activation pathways triggered by the cytoplasmic tail of CD40. Proc. Natl. Acad. Sci. USA. 96:1234–1239 10.1073/pnas.96.4.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooten C., Banchereau J. 2000. CD40-CD40 ligand. J. Leukoc. Biol. 67:2–17 [DOI] [PubMed] [Google Scholar]
- Vardouli L., Lindqvist C., Vlahou K., Loskog A.S., Eliopoulos A.G. 2009. Adenovirus delivery of human CD40 ligand gene confers direct therapeutic effects on carcinomas. Cancer Gene Ther. 16:848–860 10.1038/cgt.2009.31 [DOI] [PubMed] [Google Scholar]
- Vivarelli M.S., McDonald D., Miller M., Cusson N., Kelliher M., Geha R.S. 2004. RIP links TLR4 to Akt and is essential for cell survival in response to LPS stimulation. J. Exp. Med. 200:399–404 10.1084/jem.20040446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheide R.H. 2007. Prospect of targeting the CD40 pathway for cancer therapy. Clin. Cancer Res. 13:1083–1088 10.1158/1078-0432.CCR-06-1893 [DOI] [PubMed] [Google Scholar]
- Wang L., Du F., Wang X. 2008. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 133:693–703 10.1016/j.cell.2008.03.036 [DOI] [PubMed] [Google Scholar]
- Wong W.W., Gentle I.E., Nachbur U., Anderton H., Vaux D.L., Silke J. 2010. RIPK1 is not essential for TNFR1-induced activation of NF-kappaB. Cell Death Differ. 17:482–487 10.1038/cdd.2009.178 [DOI] [PubMed] [Google Scholar]
- Zhang D.W., Shao J., Lin J., Zhang N., Lu B.J., Lin S.C., Dong M.Q., Han J. 2009. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 325:332–336 10.1126/science.1172308 [DOI] [PubMed] [Google Scholar]