Abstract
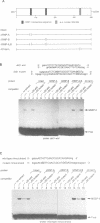
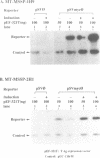
We have previously reported the human cDNA encoding MSSP-1, a sequence-specific double- and single-stranded DNA binding protein [Negishi, Nishita, Saëgusa, Kakizaki, Galli, Kihara, Tamai, Miyajima, Iguchi-Ariga and Ariga (1994) Oncogene, 9, 1133-1143]. MSSP-1 binds to a DNA replication origin/transcriptional enhancer of the human c-myc gene and has turned out to be identical with Scr2, a human protein which complements the defect of cdc2 kinase in S.pombe [Kataoka and Nojima (1994) Nucleic Acid Res., 22, 2687-2693]. We have cloned the cDNA for MSSP-2, another member of the MSSP family of proteins. The MSSP-2 cDNA shares highly homologous sequences with MSSP-1 cDNA, except for the insertion of 48 bp coding 16 amino acids near the C-terminus. Like MSSP-1, MSSP-2 has RNP-1 consensus sequences. The results of the experiments using bacterially expressed MSSP-2, and its deletion mutants, as histidine fusion proteins suggested that the binding specificity of MSSP-2 to double- and single-stranded DNA is the same as that of MSSP-1, and that the RNP consensus sequences are required for the DNA binding of the protein. MSSP-2 stimulated the DNA replication of an SV40-derived plasmid containing the binding sequence for MSSP-1 or -2. MSSP-2 is hence suggested to play an important role in regulation of DNA replication.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariga H., Imamura Y., Iguchi-Ariga S. M. DNA replication origin and transcriptional enhancer in c-myc gene share the c-myc protein binding sequences. EMBO J. 1989 Dec 20;8(13):4273–4279. doi: 10.1002/j.1460-2075.1989.tb08613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandziulis R. J., Swanson M. S., Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev. 1989 Apr;3(4):431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- Galli I., Iguchi-Ariga S. M., Ariga H. Mammalian genomic sequences can substitute for the SV40 AT stretch in sustaining replication of the SV40 origin of replication. FEBS Lett. 1993 Mar 8;318(3):335–340. doi: 10.1016/0014-5793(93)80541-2. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Iguchi-Ariga S. M., Ogawa N., Ariga H. Identification of the initiation region of DNA replication in the murine immunoglobulin heavy chain gene and possible function of the octamer motif as a putative DNA replication origin in mammalian cells. Biochim Biophys Acta. 1993 Feb 20;1172(1-2):73–81. doi: 10.1016/0167-4781(93)90271-e. [DOI] [PubMed] [Google Scholar]
- Iguchi-Ariga S. M., Okazaki T., Itani T., Ogata M., Sato Y., Ariga H. An initiation site of DNA replication with transcriptional enhancer activity present upstream of the c-myc gene. EMBO J. 1988 Oct;7(10):3135–3142. doi: 10.1002/j.1460-2075.1988.tb03180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaoka Y., Nojima H. SCR: novel human suppressors of cdc2/cdc13 mutants of Schizosaccharomyces pombe harbour motifs for RNA binding proteins. Nucleic Acids Res. 1994 Jul 11;22(13):2687–2693. doi: 10.1093/nar/22.13.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumano M., Nakagawa T., Imamura Y., Galli I., Ariga H., Iguchi-Ariga S. M. Stimulation of SV40 DNA replication by the human c-myc enhancer. FEBS Lett. 1992 Sep 7;309(2):146–152. doi: 10.1016/0014-5793(92)81083-x. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. New light on Myc and Myb. Part I. Myc. Genes Dev. 1990 Dec;4(12A):2025–2035. doi: 10.1101/gad.4.12a.2025. [DOI] [PubMed] [Google Scholar]
- Meichle A., Philipp A., Eilers M. The functions of Myc proteins. Biochim Biophys Acta. 1992 Dec 16;1114(2-3):129–146. doi: 10.1016/0304-419x(92)90011-m. [DOI] [PubMed] [Google Scholar]
- Negishi Y., Iguchi-Ariga S. M., Ariga H. Protein complexes bearing myc-like antigenicity recognize two distinct DNA sequences. Oncogene. 1992 Mar;7(3):543–548. [PubMed] [Google Scholar]
- Negishi Y., Nishita Y., Saëgusa Y., Kakizaki I., Galli I., Kihara F., Tamai K., Miyajima N., Iguchi-Ariga S. M., Ariga H. Identification and cDNA cloning of single-stranded DNA binding proteins that interact with the region upstream of the human c-myc gene. Oncogene. 1994 Apr;9(4):1133–1143. [PubMed] [Google Scholar]
- Norbury C., Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]