Abstract
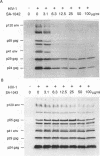
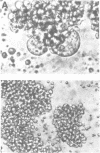
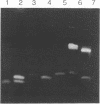
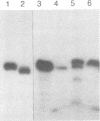
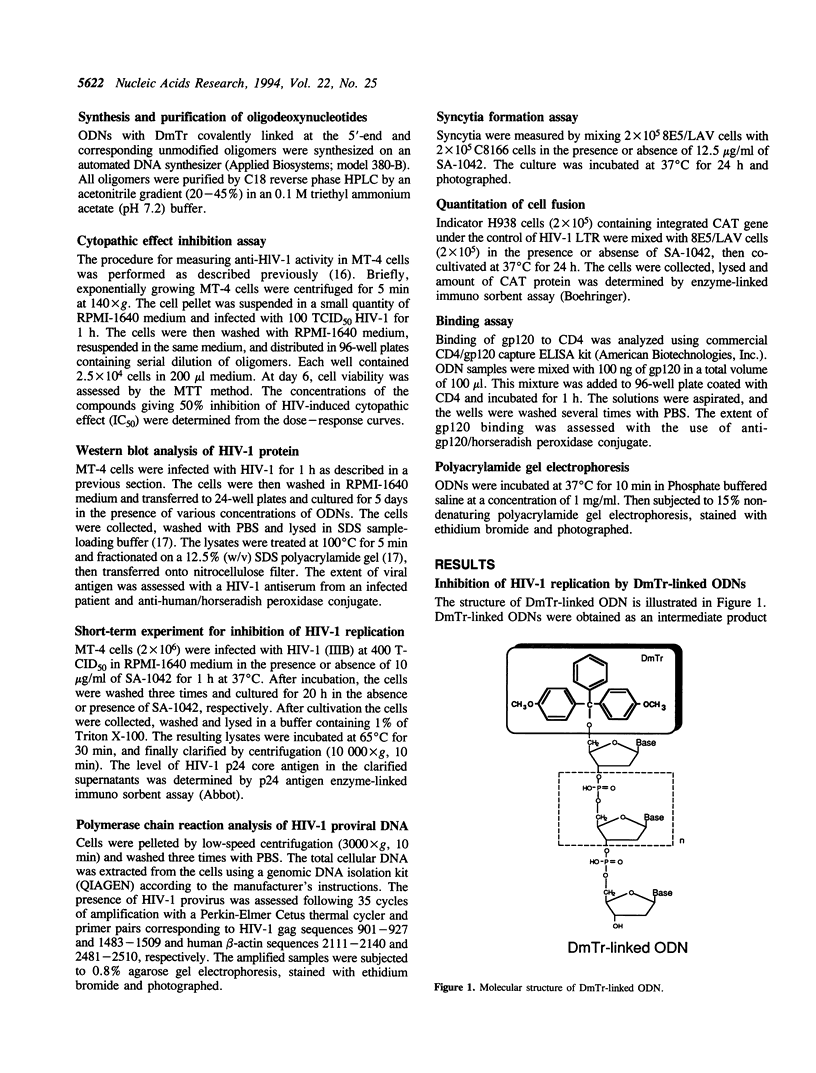
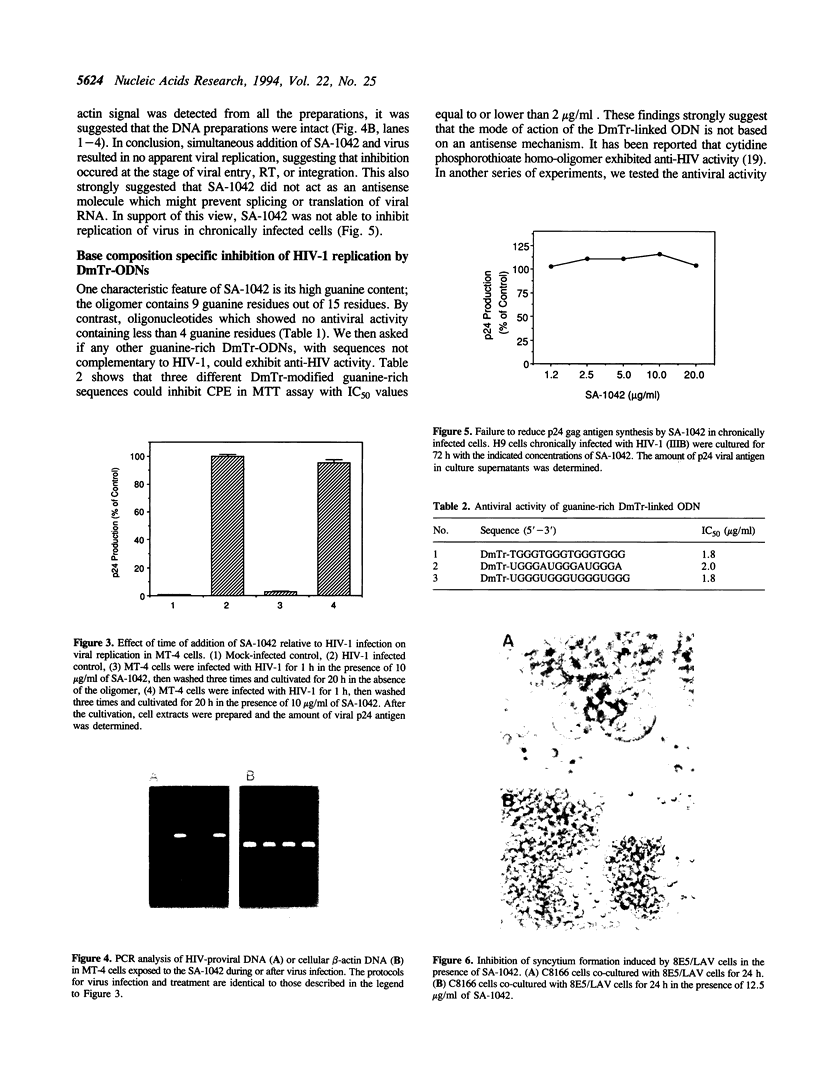
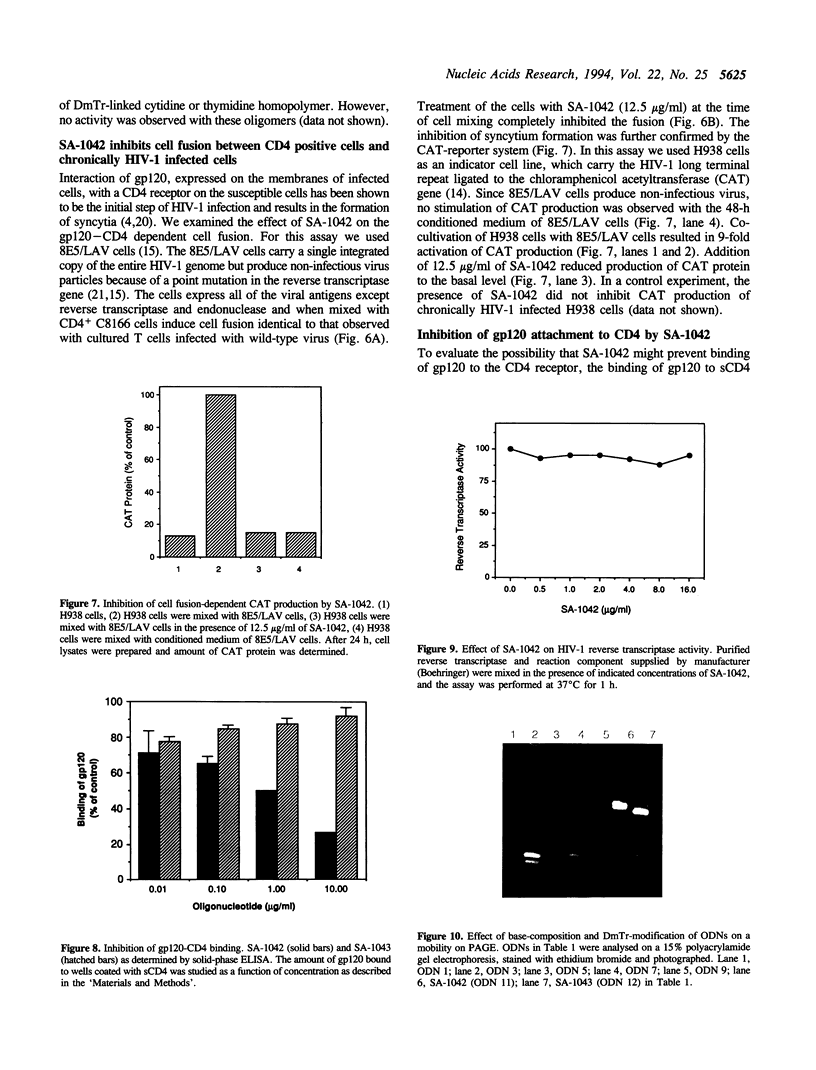
Oligodeoxyribonucleotides (ODN) linked at their 5'-end with dimethoxytrityl (DmTr) residue were examined for antiviral activities against human immunodeficiency virus type 1 (HIV-1). We found that guanine-rich oligonucleotides exhibit anti-HIV activity upon 5'-end modification with DmTr. One oligonucleotide, DmTr-TGGGAGGTGGGTCTG (SA-1042), showed potent anti-HIV activity in vitro. A greater than 95% reduction of infectivity was observed if the cells were treated with 10 micrograms/ml of SA-1042 at the time of viral infection, PCR analysis confirmed that there was a significant reduction of provirus in the cells exposed to virus in the presence of SA-1042. By contrast, no inhibition was observed if the cells were treated with the oligomer 1 h after virus adsorption. SA-1042 prevented syncytium formation between chronically infected cells and CD4 positive uninfected cells. Furthermore, the oligomer interfered the interaction of purified gp120 to the CD4 receptor. By contrast, SA-1042 had no inhibitory effect on chronically HIV-infected cells. These results strongly suggest that the DMTr-ODNs with appropriate base sequences antagonize HIV-1 infection during the stage of virus-cell interaction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaway G. P., Ryder A. M., Beaudry G. A., Maddon P. J. Synergistic inhibition of HIV-1 envelope-mediated cell fusion by CD4-based molecules in combination with antibodies to gp120 or gp41. AIDS Res Hum Retroviruses. 1993 Jul;9(7):581–587. doi: 10.1089/aid.1993.9.581. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Pavlakis G. N. A quantitative bioassay for HIV-1 based on trans-activation. Science. 1988 Jan 8;239(4836):184–187. doi: 10.1126/science.3422113. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Powell D., Lightfoote M., Koenig S., Fauci A. S., Benn S., Rabson A., Daugherty D., Gendelman H. E., Hoggan M. D. Biological and biochemical characterization of a cloned Leu-3- cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986 Jul 1;164(1):280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELLERT M., LIPSETT M. N., DAVIES D. R. Helix formation by guanylic acid. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2013–2018. doi: 10.1073/pnas.48.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Theodore T. S., Willey R., McCoy J., Adachi A., Mervis R. J., Venkatesan S., Martin M. A. Molecular characterization of a polymerase mutant human immunodeficiency virus. Virology. 1987 Oct;160(2):323–329. doi: 10.1016/0042-6822(87)90002-x. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederman S., Gulick R., Chess L. Dextran sulfate and heparin interact with CD4 molecules to inhibit the binding of coat protein (gp120) of HIV. J Immunol. 1989 Aug 15;143(4):1149–1154. [PubMed] [Google Scholar]
- Lifson J. D., Feinberg M. B., Reyes G. R., Rabin L., Banapour B., Chakrabarti S., Moss B., Wong-Staal F., Steimer K. S., Engleman E. G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986 Oct 23;323(6090):725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- Majumdar C., Stein C. A., Cohen J. S., Broder S., Wilson S. H. Stepwise mechanism of HIV reverse transcriptase: primer function of phosphorothioate oligodeoxynucleotide. Biochemistry. 1989 Feb 7;28(3):1340–1346. doi: 10.1021/bi00429a060. [DOI] [PubMed] [Google Scholar]
- Matsukura M., Shinozuka K., Zon G., Mitsuya H., Reitz M., Cohen J. S., Broder S. Phosphorothioate analogs of oligodeoxynucleotides: inhibitors of replication and cytopathic effects of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7706–7710. doi: 10.1073/pnas.84.21.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya H., Looney D. J., Kuno S., Ueno R., Wong-Staal F., Broder S. Dextran sulfate suppression of viruses in the HIV family: inhibition of virion binding to CD4+ cells. Science. 1988 Apr 29;240(4852):646–649. doi: 10.1126/science.2452480. [DOI] [PubMed] [Google Scholar]
- Montefiori D. C., Graham B. S., Zhou J., Zhou J., Bucco R. A., Schwartz D. H., Cavacini L. A., Posner M. R. V3-specific neutralizing antibodies in sera from HIV-1 gp160-immunized volunteers block virus fusion and act synergistically with human monoclonal antibody to the conformation-dependent CD4 binding site of gp120. NIH-NIAID AIDS Vaccine Clinical Trials Network. J Clin Invest. 1993 Aug;92(2):840–847. doi: 10.1172/JCI116658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Ojwang J., Elbaggari A., Marshall H. B., Jayaraman K., McGrath M. S., Rando R. F. Inhibition of human immunodeficiency virus type 1 activity in vitro by oligonucleotides composed entirely of guanosine and thymidine. J Acquir Immune Defic Syndr. 1994 Jun;7(6):560–570. [PubMed] [Google Scholar]
- Panyutin I. G., Kovalsky O. I., Budowsky E. I., Dickerson R. E., Rikhirev M. E., Lipanov A. A. G-DNA: a twice-folded DNA structure adopted by single-stranded oligo(dG) and its implications for telomeres. Proc Natl Acad Sci U S A. 1990 Feb;87(3):867–870. doi: 10.1073/pnas.87.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988 Aug;20(4):309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Cleary A. M., Yakubov L., Lederman S. Phosphorothioate oligodeoxynucleotides bind to the third variable loop domain (v3) of human immunodeficiency virus type 1 gp120. Antisense Res Dev. 1993 Spring;3(1):19–31. doi: 10.1089/ard.1993.3.19. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Pal R., DeVico A. L., Hoke G., Mumbauer S., Kinstler O., Sarngadharan M. G., Letsinger R. L. Mode of action of 5'-linked cholesteryl phosphorothioate oligodeoxynucleotides in inhibiting syncytia formation and infection by HIV-1 and HIV-2 in vitro. Biochemistry. 1991 Mar 5;30(9):2439–2444. doi: 10.1021/bi00223a020. [DOI] [PubMed] [Google Scholar]
- Thiele D., Guschlbauer W. Protonated polynucleotide structures. IX. Disproportionation of poly (G)-poly (C) in acid medium. Biopolymers. 1971;10(1):143–157. doi: 10.1002/bip.360100111. [DOI] [PubMed] [Google Scholar]
- Wyatt J. R., Vickers T. A., Roberson J. L., Buckheit R. W., Jr, Klimkait T., DeBaets E., Davis P. W., Rayner B., Imbach J. L., Ecker D. J. Combinatorially selected guanosine-quartet structure is a potent inhibitor of human immunodeficiency virus envelope-mediated cell fusion. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1356–1360. doi: 10.1073/pnas.91.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]