Abstract
Numerous studies support the hypothesis that deficiency of insulin-like growth factor I (IGF-1) in adults contributes to depression, but direct evidence is limited. Many psychological and pro-cognitive effects have been attributed to IGF-1, but appropriate animal models of adult-onset IGF-1 deficiency are lacking. In this study, we use a viral-mediated Cre-loxP system to knockout the Igf1 gene in either the liver, neurons of the CA1 region of the hippocampus, or both. Knockout of liver Igf1 reduced serum IGF-1 levels by 40% and hippocampal IGF-1 levels by 26%. Knockout of Igf1 in CA1 reduced hippocampal IGF-1 levels by 13%. The most severe reduction in hippocampal IGF-1 occurred in the group with knockouts in both liver and CA1 (36% reduction), and was associated with a 3.5-fold increase in immobility in the forced swim test. Reduction of either circulating or hippocampal IGF-1 levels did not alter anxiety measured in an open field and elevated plus maze, nor locomotion in the open field. Furthermore, local compensation for deficiencies in circulating IGF-1 did not occur in the hippocampus, nor were serum levels of IGF-1 upregulated in response to the moderate decline of hippocampal IGF-1 caused by the knockouts in CA1. We conclude that adult-onset IGF-1 deficiency alone is sufficient to induce a depressive phenotype in mice. Furthermore, our results suggest that individuals with low brain levels of IGF-1 are at increased risk for depression and these behavioral effects are not ameliorated by increased local IGF-1 production or transport. Our study supports the hypothesis that the natural IGF-1 decline in aging humans may contribute to geriatric depression.
Keywords: insulin-like growth factor I, IGF-1, depression, conditional knockout, aging, hippocampus
1
Depression and anxiety in human populations are comorbid with, and influenced by, myriad illnesses and other stressors that can accumulate over a lifetime. Depression in older individuals, in particular, is frequently comorbid with cognitive impairment and dementia (Okura et al., 2010) and comes with a relatively high risk of successful suicide (Heisel & Duberstein, 2005). Indeed, depression in the elderly is associated with a reduction in life expectancy of 4 to 6 years, independent of the effects of other chronic diseases (Rapp et al., 2008; Reynolds et al., 2008). It is estimated that over 10 percent of the elderly who require home healthcare or hospital treatment develop major depression (Hybels & Blazer, 2003). Moreover, there are many older patients with subsyndromal depression, characterized as depressive symptoms that fall short of meeting the full diagnostic criteria for the disorder (Alexopoulos, 2000), who are at an increased risk of subsequently developing major depression (Horwath et al., 1992). In cases where depression arises very late in life, or is comorbid with executive dysfunction, treatment with antidepressants is less effective than in younger patients (Meyers & Jeste, 2010).
Although the etiology of depression in general, and specifically depression in older individuals, remains elusive, there is an extensive literature indicating that alterations in trophic factors and circulating hormones have the ability to influence the onset and progression of the disease (R. S. Duman, 2004). Insulin-like growth factor I (IGF-1) is a circulating anabolic hormone, produced largely in the liver under the control of growth hormone (GH), but also is produced locally in many cell types, including neurons and glia (Bondy et al., 1992; D’Ercole et al., 1984). Circulating IGF-1 levels decline with age in humans and many animal models of aging (Xu & Sonntag, 1996). Numerous studies demonstrate a potential role for IGF-1 in the amelioration of anxiety, depression, and memory deficits (Carro et al., 2000; Hoshaw et al., 2005; Lupien et al., 2003; Malberg et al., 2007; Markowska et al., 1998; Nieves-Martinez et al., 2010; Trejo et al., 2008) which may be due to the reported effects of IGF-1 on regulation of hippocampal neurogenesis (Lichtenwalner et al., 2001; Trejo et al., 2008), neurotransmission (Kar et al., 1997; Knusel & Hefti, 1991; Seto et al., 2002; Trejo et al., 2007), amyloid beta accumulation (Carro et al., 2002), and/or glucose metabolism (Sonntag et al., 2006). Levels of IGF-1 in the brain are not always correlated with blood levels (Adams et al., 2009), despite studies indicating that IGF-1 can cross the blood brain barrier (Armstrong et al., 2000; Carro et al., 2005; Pan & Kastin, 2000; Yu et al., 2006). In addition, uptake of IGF-1 into the brain can be increased by neuronal activity (Nishijima et al., 2010) or reduced by interfering with the transport protein megalin (Carro et al., 2005). Thus it is generally recognized that circulating levels of IGF-1 contribute, at least in part, to the levels of IGF-1 in brain and, as a result, the reported behavioral effects of the hormone (Carro et al., 2000; Lupien et al., 2003; Trejo et al., 2002; Trejo et al., 2008; Trejo et al., 2007). Nevertheless, the physiological contribution of circulating (endocrine) versus locally produced (paracrine) IGF-1 to brain function has not been clearly defined.
Several models of IGF-1 deficiency have been used in scientific studies, but most are limited by confounding variables that reduce their utility for assessing the consequences of age-related changes in levels of this hormone. For example, perinatal reductions of GH and IGF-1 present in Ames and Snell dwarf mice are accompanied by developmental abnormalities that result in deficiencies of several other important regulatory hormones (Carter et al., 2002). The liver IGF-1 deficient (LID) mouse exhibits an IGF-1 decline too late in development to cause a dwarf phenotype, but early enough to affect pubertal development (Tang et al., 2005), making it difficult to separate the developmental versus adult roles of IGF-1. Recently, an inducible LID mouse was developed (Wu et al., 2010), but prolonged studies relying on such tamoxifen-inducible Cre systems may risk early, unintentional recombination (Guo et al., 2002). Conversely, administration of high levels of GH or IGF-1 to normal animals has the ability to induce abnormal hormonal responses and an acromegaly-like syndrome. While physiological replacement of IGF-1 to aging animals is technically feasible, such studies cannot readily separate the effects of IGF-1 deficiency from other cellular and molecular changes that occur with age. Therefore, it is difficult to conclude whether a hormonal change contributes to, or results from, a particular aging phenotype. The aforementioned models are in stark contrast to the age-related decline in IGF-1 observed in humans and many animal models (Xu & Sonntag, 1996), and lack the ability to study the role of paracrine IGF-1 in isolation.
The present study addresses the potential role of both endocrine and paracrine IGF-1 in depression using a combination of conditional Igf1 knockout mice and viral vectors, achieving a high degree of precision in both temporal and tissue-specific manipulation of IGF-1 levels. By using recombinant adeno-associated viral (AAV) vectors specifically targeted to hepatocytes or CA1 neurons, with age-specific administration of the vectors, we create robust tissue- and age-specific models of IGF-1 deficiency. We find that these manipulations are sufficient to induce depression-like behaviors, but not anxiety or locomotor impairments in otherwise healthy adult mice. In particular, liver-derived IGF-1 appears to have a critical role in depression compared to IGF-1 produced locally in CA1 neurons of the dorsal hippocampus.
2. Experimental Procedures
2.1 Animals
Male mice homozygous for a floxed exon 4 of the Igf1 gene (Igf1f/f) were developed on a mixed 129Sv and C57BL/6 background (Liu et al., 1998). Briefly, these mice have the entirety of exon 4 of the Igf1 gene flanked by loxP sites, which allows for genomic excision of this exon when exposed to Cre recombinase. Transcripts of the altered Igf1 gene yield a protein upon translation that fails to bind the IGF receptor. The line was generated in embryonic stem cells from 129Sv mice. Correctly targeted clones were injected into C57BL/6 blastocysts and chimeric males were bred to C57BL/6 females. The line at the University of Oklahoma Health Sciences Center (OUHSC) was rederived at Charles River Laboratories in a C57BL/6 background for maintenance of the colony in a specific-pathogen-free rodent barrier facility. Animals were bred to homozygosity and housed in the Rodent Barrier Facility at OUHSC, on a 12h light/12h dark cycle, and given access to standard rodent chow (Purina Mills, Richmond, IN) and water ad libitum. Animals were assigned to treatment groups based upon results of ELISAs for serum IGF-1 (see below) to produce groups with equivalent serum IGF-1 levels prior to treatment. All procedures were approved by the Institutional Animal Care and Use Committee of OUHSC and performed during the animals’ light cycle.
2.2 Viral Vectors
To specifically target hepatocytes, pseudotyped AAV2/8 viruses were packaged with a MUP-iCRE transgene cassette consisting of the major urinary protein (MUP) promoter and iCre, a codon optimized Cre recombinase gene (Ho et al., 2008). While AAV8 is effective at transducing multiple tissues after intravenous delivery, including liver, the MUP promoter restricts expression solely to hepatocytes. For targeting hippocampal neurons, we generated a pseudotyped AAV2/10 virus in which expression of a Cre-GFP fusion protein is driven by the cytomegalovirus promoter (CMV). Neuronal specificity is achieved by the AAV10 virus, which preferentially transduces neurons when injected directly into the brain parenchyma. Control viruses encoding enhanced green fluorescent protein (EGFP) were also generated (MUP-EGFP-AAV8 and CMV-EGFP-AAV10).
Viruses were packaged according to a triple transfection protocol described previously (Ho et al., 2008). Briefly, the viruses were produced using AAV2 plasmids containing AAV2 inverted terminal repeats flanking a transgene cassette consisting of either the MUP or CMV promoter, the gene of interest (iCre, GFP-Cre, or EGFP), and an intron polyadenylation sequence derived from SV40. Triple transfection of AAV-293 cells with the AAV2 plasmid, pHelper (Stratagene, La Jolla, CA), and either AAV2/8 or AAV2/10 rep/cap plasmids (encoding AAV2 replicase and AAV8 or AAV10 capsid genes) was used to package the virus. Cells were harvested 72h after transfection, and clarified viral lysates were obtained. Lysates were titred by real-time PCR and stored at −80°C.
2.3 Viral Administration
At 4 months of age, mice received viral vectors targeting hepatocytes resulting in Cre-mediated recombination of the Igf1 gene, or vectors expressing EGFP as a control. Animals were anesthetized with ketamine/xylazine (100mg/kg/15mg/kg, respectively), and given retro-orbital injections of 1.5×1010 particles of either MUP-iCre-AAV8 or MUP-EGFP-AAV8 diluted in saline for a total injection volume of 100μl per animal. Dosages were determined empirically in preliminary studies. Specificity of MUP-AAV8 vectors for hepatocytes has been previously demonstrated (Ho et al., 2008).
One month later, animals were administered bilateral stereotaxic injections of either CMV-GFP-Cre-AAV10 or CMV-EGFP-AAV10, targeting the CA1 region of the dorsal hippocampus, with injection coordinates empirically determined for the strain and age of mouse (2.8mm posterior, 2.5mm lateral, 2.3mm ventral to bregma). Animals were anesthetized with ketamine/xylazine as before, mounted in the stereotaxic frame (Kopf Instruments, Tujunga, CA) and the dorsal surface of the head shaved and cleaned with disinfectant. An incision was made, and the dorsal surface of the skull was exposed. Holes were drilled in the skull at appropriate coordinates using a 1mm dental burr. Bilateral injections of 4×108 viral particles in an injection volume of 0.7μl per hippocampus were delivered with a 30G needle and 5μl Hamilton syringe (Hamilton Company, Reno, NV) mounted to the stereotaxic frame. Each injection was delivered over a period of six minutes. After injection, the holes in the skull were sealed with sterile bone wax, the wound was closed with silk suture, and the animal was allowed to recover in a heated cage. A separate cohort of Igf1f/f males of the same age, which had not received either AAV8 vector, also received these stereotaxic injections and was sacrificed 6 weeks later for immunohistochemical (IHC) verification of injection targeting, vector distribution, and vector specificity.
2.4 IGF-1 ELISA
Submandibular venous blood was collected into microcentrifuge tubes using a sterile lancet (Medipoint, Mineola, NY) according to the manufacturer’s instructions. Whole blood was centrifuged at 2500×g for 20 minutes at 4°C to collect serum, which was then stored at −80°C. Serum was processed for ELISA of IGF-1 (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol. Serum IGF-1 levels are reported in ng/ml. An IGF-1 control sample, with aliquots stored at −80°C, was included on each plate, and all data are reported using simple ratio normalization to the initial reading of the control sample.
Hippocampal tissue (with blood removed by transcardial perfusion, see Tissue Collection below) was processed for IGF-1 extraction using a protocol based on previously published work (Adams et al., 2009). Frozen hippocampi were thawed on ice in 15μl of homogenization buffer (1M sodium acetate, pH 3.6) per milligram of tissue. The tissue was sonicated in homogenization buffer at 4°C. Sonicated homogenates were shaken at 1400rpm for 2h at 4°C on a standard lab vortex mixer. Homogenates were centrifuged at 3000×g for 10min at 4°C, and the supernatants were collected on ice. Supernatants were frozen at −80°C until processed further. Frozen supernatants were dehydrated at 30°C in a centrifugal evaporator (Eppendorf, Hauppauge, NY) until completely dehydrated (approximately 2.5 h), and the dehydrated pellet was stored at −80°C until reconstitution for ELISA. Pellets were reconstituted in a volume of 0.1M HEPES buffer (pH 7.8) equal to the volume of supernatant collected from the homogenate. The pellets were vortexed until dissolved, then centrifuged at 3000×g for 10min at 4°C to remove insoluble material from the solution. The supernatant was assayed undiluted by ELISA, according to the manufacturer’s protocol. Tissue IGF-1 levels are reported in pg/mg tissue (wet weight), and are normalized in the same manner as the serum samples.
2.5 Behavioral Testing
Animals were tested for general activity and anxiety in an open field. Each mouse was given 10min to explore a white plastic, open box with dimensions 100cm × 100cm. EthoVision XT 7 software (Noldus, Wageningen, Netherlands) was used to record and analyze the movement of the animals. Time spent within 8cm of the wall (~30% of total field area), time spent in the central area of the field, and total distance traveled in 10min were measured. The field was cleaned with 70% ethanol between subjects.
The same mice were also assessed for anxiety using an elevated plus maze (BIOSEB, Vitrolles, France), having arms 35cm × 5cm, walls 25cm high on enclosed arms, and a 5cm × 5cm intersection area at the center of the arms. Mice were placed individually in the maze facing an open arm and allowed 5min to explore. Results from animals repeatedly leaving the maze were excluded from analysis. Time spent in open arms (center of body ≥ 2cm beyond the intersection area) and total distance traveled were analyzed using EthoVision XT. The maze was cleaned with 70% ethanol between trials.
A forced swim test was used to monitor depression-like behavior in each of these mice. Mice swam for 5min in a 4L beaker half full of 25°C water. Immobility was manually timed by investigators, and defined as time spent neither swimming nor attempting to climb the wall of the beaker, while allowing for slight movement to maintain balance. As a secondary measure of “despair” behavior, a tail suspension test was performed on these mice. Mice were suspended by their tail in an open-topped plastic box. Their tails were attached with electrical tape to plastic tubing stretched across the walls of the box, and the mice were observed for 5min. Time spent hanging relaxed, not trying to elevate their heads, was recorded as immobility time.
2.6 Tissue Collection
Animals were anesthetized with ketamine/xylazine as before, and perfused transcardially with PBS containing 5mM dextrose and 6U/ml sodium heparin. Immediately after perfusion, brains were removed from the skull and dissected on an ice-cooled aluminum block. Left hippocampi for IGF-1 ELISAs were weighed on an analytical balance tared to the empty weight of the collection tube. Right hippocampi were saved for gene expression analysis (comprehensive work to be described in a future study; select preliminary data included in this study’s Discussion). One animal had a brain tumor, and three others died prior to sacrifice from causes unrelated to experimental manipulation, and were excluded from hippocampal IGF-1 ELISA analysis. Brains from the cohort of animals used for validation of stereotaxic injections were hemisected sagittally, with one hemisphere kept intact for histology, and the other further dissected for ELISA.
2.7 PCR
Pellets of hippocampal homogenates (see IGF-1 ELISA above) from the vector-targeting cohort were processed in 500μl Trizol® (Invitrogen, Carlsbad, CA) to purify DNA. The manufacturer’s protocol was adjusted proportionally for the volume used. Approximately 1ng of DNA per 21μl PCR reaction was used, adjusting the dilution based on readings from a NanoDrop® spectrophotometer (Thermo Scientific, Wilmington, DE). Primers surrounding the floxed exon 4 of Igf1 (ES-1 and ID-3 (Liu et al., 1998), below) were used to amplify either the full-length transgenic exon 4 (>2000 bp PCR product) or the remaining sequence after Cre-mediated recombination (~250 bp). HotStarTaq (Qiagen, Valencia, CA) was used per the manufacturer’s recommendations in a master mix including RediLoad (Invitrogen) for visualization while running the 1% agarose gel. Contrast of the gel image was adjusted using Adobe Photoshop CS (Adobe Systems, San Jose, CA). PCR cycles were: 94°C for 15min; 35 cycles at 94°C for 45s, 57°C for 45s, 72°C for 2min; 72°C for 5min. Primers were (5′ to 3′):
ES-1: CTGTTAAAAGCCTCTCAACTAAGACAATA
ID-3: CCCACTAAGGAGTCTGTATTTGGAC
2.8 Immunohistochemistry
Sagittally hemisected, perfused brains were immersion fixed overnight at 4°C in 4% paraformaldehyde in 0.1M sodium phosphate buffer. Brains were briefly rinsed, then cryoprotected in 30% sucrose in phosphate-buffered saline at 4°C. Brains were embedded in CRYO-GEL™ embedding medium (Instrumedics, St. Louis, MO), frozen and cryosectioned. Coronal or sagittal sections through the entire hippocampus were cut at 30μm, free floated in 25% glycerol, 25% ethylene glycol, 50% 0.1M sodium phosphate buffer, pH 7.4, and stored at −20°C until stained. Floating sections were transferred to Tris-buffered saline with 0.25% Triton-X® 100 (TBS-X), briefly rinsed, then soaked for 10min in TBS-X to clean and permeabilize the sections. Sections were blocked with 10% normal donkey serum with 0.1mg/ml donkey anti-mouse IgG Fab fragments (both from Jackson ImmunoResearch, West Grove, PA) in TBS-X for 1h at room temperature. The blocking solution was removed and replaced with TBS-X containing mouse anti-NeuN (neuronal nuclei marker, Millipore, Billerica, MA) and rabbit anti-GFP (ab290 from Abcam, Cambridge, MA) at final dilutions of 1/250 and 1/1000, respectively. Sections were incubated in this solution overnight at 4°C. The following morning, sections were rinsed briefly in TBS-X, and then incubated 2h at room temperature with DyLight™549-conjugated donkey anti-mouse IgG and Cy2-conjugated donkey anti-rabbit IgG in TBS-X (both antibodies at a final dilution of 1/200, purchased from Jackson ImmunoResearch). Following this incubation, sections were briefly rinsed then incubated in 0.1mg/ml 4′,6-diamidino-2-phenylindole (DAPI; Sigma, St. Louis, MO) in TBS for 30 min at room temperature. Sections were rinsed briefly in TBS and floated onto positively charged slides. After drying, sections were dehydrated through an ethanol series, cleared in xylene, and then coverslips were mounted with CytoSeal 60 (Richard-Allen Scientific, Kalamazoo, MI).
2.9 Statistics
Data were entered into Sigma-Stat 3.5 (Systat Software, Chicago, IL) for analysis. Results were analyzed by two-way ANOVA using liver vector groups and brain vector groups as factors. Significant F-test results were further examined by pairwise post-hoc t-tests, with reported p-values adjusted using the Bonferonni method for multiple comparisons. Regression p-values are derived from F-tests comparing the contributions to variance from the regression model versus residual variance in the sample, and r2 values are presented unadjusted. Repeated measures analysis of serum IGF-1 data was performed with SAS 9.1 (SAS Institute, Cary, NC) using a mixed model, with a random intercept per subject (individual mice) but classifying viral treatments and time as fixed factors. Significance of fixed factors and their interactions was determined with Type III F-tests of each effect, with variance components estimated using the restricted maximum likelihood method. All results are presented as mean±SEM unless otherwise noted.
3. RESULTS
3.1 Viral Knockdown of Igf1
Targeting and efficacy of the neuronal viruses were validated in a separate cohort of animals using IHC against GFP and NeuN, and PCR for exon 4 of Igf-1. Representative coronal (Figure 1A) and sagittal (Figure 1B) sections taken at the approximate injection site demonstrate the extent and specificity of the CMV-GFP-Cre-AAV10 vector. All animals examined had the majority of CA1 neurons infected across the dorsal hippocampus, plus infection of a moderate number of CA2 neurons, with minimal to no infection of cortical, CA3, or dentate gyrus neurons. Anti-GFP staining colocalized with anti-NeuN staining, indicating neuronal specificity of the vector. All morphological indications were that nuclei with anti-GFP staining, but lacking anti-NeuN staining, were also neuronal. Those nuclei are likely from one of the neuron types that are known not to stain with the anti-NeuN antibody, such as developing neurons that have not yet exited the mitotic cycle (Mullen et al., 1992). Within the hippocampus, few nuclei outside the granular layer were anti-GFP positive, indicating a lack of significant astrocytic tropism. Though some anti-GFP staining was found in the cortex, staining was almost completely excluded from the corpus collosum, indicating a lack of oligodendrocytic tropism. These results are consistent with previous work demonstrating AAV10 viruses do not infect glia (Cearley & Wolfe, 2006). Furthermore, nuclei with anti-GFP staining were not visibly associated with blood vessels, ependymal cells, or choroid plexus.
Figure 1. Viral-mediated excision of Igf1 in CA1.
A & B: Representative IHC images to validate vector targeting in coronal (A) and sagittal (B) brain sections. Sections were stained with DAPI (blue), anti-NeuN (red), and anti-GFP (green). Nuclei of infected cells appear green or yellow. Images are from the approximate injection site, showing maximal spread of the vector. C: Gel electrophoresis of PCR products of Igf1, exon 4. Animals receiving CMV-GFP-Cre-AAV10, but not the control vector (CMV-EGFP-AAV10), show preferential amplification of a ~250 bp sequence (post-excision length) over the >2000bp product of the native floxed Igf1 exon 4. NT- no template control, +ctl – positive control from liver DNA of a mouse treated with MUP-iCre-AAV8. D: Whole hippocampus IGF-1 protein levels decrease as a result of Cre-loxP recombination in either CA1 or the liver. †Main effect of liver vector, p<0.001; ‡Main effect of brain vector, p=0.049; *p=0.018 versus group receiving both EGFP vectors; ***p<0.001 versus group receiving both EGFP vectors; #p=0.025 versus group receiving MUP-EGFP-AAV8 and CMV-GFP-Cre-AAV10. Data represent mean ± SEM.
As verification that Cre recombinase was functional in infected cells, PCR using primers surrounding exon 4 of the Igf1 gene was performed on hippocampal DNA (Figure 1C). Animals administered CMV-EGFP-AAV10 exhibit only the full-length floxed Igf1 exon 4 (>2000 bp), while those administered CMV-GFP-Cre-AAV10 demonstrate excision of exon 4 (~250 bp product). The full-length product is also present in animals administered CMV-GFP-Cre-AAV10, but is barely detectable due to preferential amplification of the shorter product. This type of recombination in the brains and livers of the double-vector (behavioral) cohort resulted in altered protein levels of hippocampal IGF-1 between treatment groups (Figure 1D). Two-way ANOVA of ELISA data revealed main effects of both brain (F(1,30)=4.22, p=0.049) and liver (F(1,30)=20.10, p<0.001) vector-induced Igf1 excision on hippocampal IGF-1 levels, but no significant interactions of the deletions (F(1,30)=0.049, p=0.826). Specifically, the group having both hepatocyte and CA1 knockouts (n=9) had a highly significant reduction in hippocampal IGF-1 compared to the group administered both EGFP vectors (n=10; t=4.924, p<0.001), as well as a significant reduction compared to the group with focal Igf1 deletion in CA1 only (n=7; t=3.109, p=0.025). The group with liver knockouts only (n=8) also had significantly reduced hippocampal IGF-1 compared to the group administered both EGFP vectors (t=3.231, p=0.018).
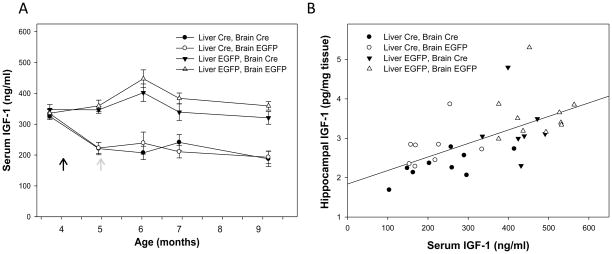
All groups had roughly equal serum IGF-1 levels prior to administration of liver-targeted viruses, but groups with hepatocyte-specific knockouts of Igf1 exhibited a significant decline (~40%) in serum IGF-1 compared to other groups across the experimental period (F(1,140)=89.29, p<0.0001; Figure 2A). Neither brain vector had a significant effect on serum IGF-1 levels during the course of the experimental studies (F(1,140)=1.12, p= 0.291). Serum IGF-1 levels of those receiving MUP-iCre-AAV8 decreased within 1 month of viral administration, and then remained essentially stable for the remainder of the study period. Hippocampal IGF-1 levels across groups showed a significant, positive correlation to serum IGF-1 levels at sacrifice (r2=0.375, F(1,32)=19.17, p<0.001; Figure 2B)
Figure 2. Serum IGF-1 is reduced by hepatocyte-specific knockout of Igf1, but not by focal knockout Igf1 in CA1.
A: Liver-targeted vectors were administered at the time indicated by the black arrow. Animals receiving liver-targeted Cre-expressing vectors (circles) exhibited lower serum IGF-1 compared to those receiving EGFP-expressing vectors (triangles) across the post-injection period (p<0.0001). Stereotaxic administration of neuron-targeted vectors (at the gray arrow) did not significantly alter serum IGF-1. Data represent mean ± SEM. B: Hippocampal IGF-1 protein levels had a significant, positive correlation to serum IGF-1 at sacrifice across the experimental cohort (r2=0.375, p<0.001), indicating a dependence upon circulating IGF-1 for the maintenance of local IGF-1 levels.
3.2 Open Field
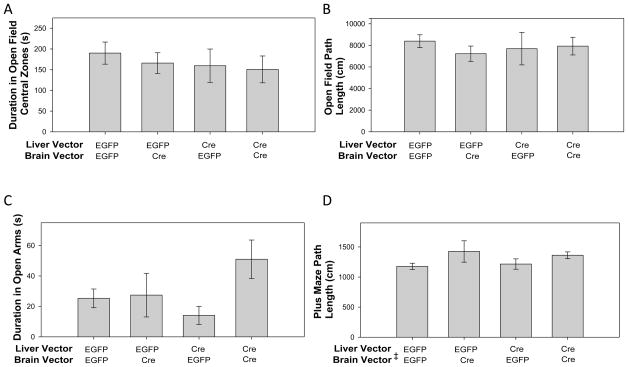
No differences between groups in open field activity in the central region of the field were observed (Figure 3A), indicating that IGF-1 deficiency did not influence anxiety levels. Two-way ANOVA of the central field data revealed no significant effect across treatment groups for liver knockouts (F(1,34)=0.513, p=0.479), brain knockouts (F(1,34)=0.268, p=0.608), nor interactions of the Igf1 deletions (F(1,34)=0.0568, p=0.813). Furthermore, basal mobility did not differ between groups in the open field, as assessed by total path length (Figure 3B). Two-way ANOVA examining effects of Igf1 deletion on mobility indicated no significant effect of the liver recombination (F(1,34)<0.0001, p=0.998), brain recombination (F(1,34)=0.253, p=0.618), nor interactions of the Igf1 deletions (F(1,34)=0.579, p=0.452).
Figure 3. Open field and elevated plus maze activity do not indicate anxiogenic or locomotor effects of IGF-1 deficiency.
A: Time spent in the central area of a novel open field was recorded as a measure of anxiety. No treatment significantly altered this measure. B: Total distance traveled in the open field demonstrated no differences in basal locomotion between groups. n(Liver EGFP, Brain EGFP)=11, n(Liver EGFP, Brain Cre)=8, n(Liver Cre, Brain EGFP)=8, n(Liver Cre, Brain Cre)=11. C: As a second measure of anxiety, time spent in the open arms of an elevated plus maze was examined. Two-way ANOVA revealed no main effect of viral vectors. D: Animals administered Cre-expressing vectors to CA1 have a marginally higher activity level as analyzed by Two-way ANOVA, but no significant differences were found between any pair of treatment groups, nor does such a trend appear in open field data. n(Liver EGFP, Brain EGFP)=11, n(Liver EGFP, Brain Cre)=5, n(Liver Cre, Brain EGFP)=8, n(Liver Cre, Brain Cre)=11. ‡Main effect of brain vector, p=0.024. Data represent mean ± SEM.
3.3 Elevated Plus Maze
Anxiety was also measured by time spent in the open arms of a plus maze and did not differ significantly by treatment group (Figure 3C). Two-way ANOVA on open-arm data revealed no significant effects across groups for liver vector (F(1,31)=0.335, p=0.567), brain vector (F(1,31)=3.335, p=0.077), or vector interactions (F(1,31)=2.650, p=0.114), though there was a trend for those animals with Igf1 excision in CA1 to spend more time in the open arms of the maze. Trends seen in the two-way ANOVA were partially due to an outlier 2.33 SD above the mean in the group receiving both Cre vectors. No correlations between open arm activity and serum IGF-1 or forced swim data (below) were observed (data not shown).
As in the open field, basal activity in the plus maze (assessed by total path length) did not vary significantly between any pair of treatment groups (Figure 3D). Two-way ANOVA showed no significant effect of liver recombination on path length across groups (F(1,31)=0.0218, p=0.884), although Igf1 deletion in CA1 had a significant influence on path length (F(1,31)=5.614, p=0.024), but there were no significant interactions (F(1,31)=0.383, p=0.541). Those animals receiving the brain Cre vector (n=16) had a moderately longer path length than those receiving the control vector (n=19; 1393±63cm vs 1196±54cm, respectively; t=2.369, p=0.024). Time spent in the open arms positively correlated with total path length across all groups (r2=0.236, F(1,33)=10.169, p=0.003) and this may partially explain why the groups with hippocampal knockouts of Igf1 exhibited a slight trend toward greater open arm time. This mobility influence (not observed in the open field), absence of trends in open field central time data, and outlier concerns in the plus maze data suggest that the trend for greater open arm time in animals with hippocampal Igf1 excision is not indicative of an anxiolytic effect.
3.4 Forced Swim and Tail Suspension
Immobility during the forced swim varied significantly between groups (Figure 4A). Two-way ANOVA revealed that animals with hepatocyte-specific knockouts of Igf1 exhibited greater immobility in the forced swim task than did animals receiving the liver-targeted EGFP vector, after accounting for group differences caused by brain recombination (F(1,34)=5.733, p=0.022). The effect of the CA1-targeted knockouts on forced swim performance was not statistically significant (F(1,34)=1.983, p=0.168), nor were there interactions of the two types of knockout affecting the forced swim measure (F(1,34)=0.0007, p=0.978). Examined individually, the group with both hippocampal and liver excision of Igf1 (n=11) had significantly higher immobility compared to the group receiving both EGFP vectors (n=11; t=2.930, p= 0.036), but no differences in the other pairs of groups reached significance.
Figure 4. Forced swim indices of depression increase with the severity of IGF-1 deficiency.
A: Immobility in the forced swim test was highest in the group receiving both Cre-expressing vectors, and lowest in the group receiving both EGFP vectors. n(Liver EGFP, Brain EGFP)=11, n(Liver EGFP, Brain Cre)=8, n(Liver Cre, Brain EGFP)=8, n(Liver Cre, Brain Cre)=11. †Main effect of liver vector, p=0.022; *p=0.036 versus group receiving both EGFP vectors. Data represent mean ± SEM. B: Immobility in the forced swim demonstrated an inverse correlation to hippocampal IGF-1 levels – i.e., lower IGF-1 levels predict a stronger depressive phenotype (r2=0.229, p=0.004; linear regression, solid line). The relationship between hippocampal IGF-1 and forced swim immobility is non-linear, and is modeled significantly better (p=0.009) by a second order polynomial regression (r2=0.382, p< 0.001; dashed line). Serum IGF-1 measured around the time of the forced swim test showed similar correlations (see text).
Linear regression of forced swim immobility data showed a strong negative correlation with hippocampal IGF-1 levels at sacrifice (r2=0.229, F(1,32)=9.494, p=0.004; Figure 4B, solid line). As the data suggested a non-linear decay of immobility with increasing hippocampal IGF-1 levels (or perhaps a threshold below which mice were more likely to be immobile), we examined the data using incremental polynomial regression. The fit of the regression model improved significantly with a second order polynomial regression (incremental r2= 0.153, F(1,32)=7.696, p=0.009), but further improvements were not significant with higher order polynomial regressions. The fit of the second order polynomial was highly significant (r2= 0.382, F(2,31)=9.589, p<0.001; Figure 4B, dashed line) and data conformed better to assumptions of normality and constant variance compared to the linear model. Serum IGF-1 measured 1 and 5 weeks before the forced swim test also had a modest, but statistically significant, negative linear correlation with forced swim immobility (r2=0.108, F(1,36)=4.257, p=0.047 and r2=0.197, F(1,36)=8.835, p=0.005, respectively – data not shown). There were no significant correlations between any measures of open field or plus maze activity and forced swim immobility (data not shown).
Tail suspension data from these mice showed qualitatively similar results to forced swim data, but did not reach statistical significance. Animals with hepatic Igf1 knockouts showed approximately 18% greater immobility than controls, and the group with both hepatic and CA1 knockouts of Igf1 had greater immobility than all other groups (data not shown).
4. DISCUSSION
In this study, we used adult-onset, tissue-specific knockouts of Igf1 to explore the endocrine and paracrine roles of IGF-1 in the genesis of depression. We confirmed that hippocampal IGF-1 protein levels are dependent upon serum IGF-1 levels in mice. Hippocampal IGF-1 levels have a significant effect on the forced swim measure of depressive behavior in mice, and differences in this test occurred in the absence of alterations in basal mobility. Furthermore, the open field test provided no evidence of differences in anxiety due to variation in IGF-1 levels, and this was also supported by data from the elevated plus maze. To our knowledge, this is the first demonstration that long-term IGF-1 deficiency initiated during adulthood is sufficient to induce depressive behavior.
Previous research examining the roles of IGF-1 in the genesis of depression has relied primarily on observational studies (Deuschle et al., 1997), exogenous supplementation of the protein (C. H. Duman et al., 2009; Hoshaw et al., 2005), or short-term blockade of either IGF-1 (C. H. Duman et al., 2009) or IGF-1 binding proteins (Malberg et al., 2007). Such studies provide valuable, but inconclusive, evidence for the role of IGF-1 deficiency in depressive disorders. Studies involving supplementation of growth factors or neurotrophins (including brain-derived neurotrophic factor, BDNF) provide strong evidence that such proteins ameliorate some types of depression. However, these studies do not provide direct evidence that prolonged deficiencies of those proteins contribute to depression. Previous work with 3 month-old LID (liver IGF-1 deficient) mice has demonstrated similar results in the forced swim test (Trejo et al., 2008), but it was unclear if the depressive phenotype was due to a primary IGF-1 deficiency during prepubertal development, secondary endocrine dysfunction, or if the phenotype was maintained into later adulthood. Our experimental design allows us to conclude that prolonged IGF-1 deficiency during adulthood contributes to depressive symptoms and that this effect is dependent on both serum and brain IGF-1 levels. Previous studies of short-term blockade of IGF-1 or its binding proteins provide compelling evidence that deficiencies of these proteins can affect mood within hours, but do not control for the possibility of homeostatic compensation over the course of weeks or months. Our study demonstrates that the brain does not compensate fully for adult-onset reduction in liver IGF-1 production by either increased IGF-1 production or transport of IGF-1 from the serum. Our methods do not exclude the possibility of other forms of compensation, but if present, they were insufficient to prevent the behavioral phenotype.
The possibility that other tissues may compensate for reductions in circulating IGF-1 is under considerable debate, which makes simultaneous control of endocrine and paracrine IGF-1 production an important experimental issue. Complete knockouts of Igf1 or its receptor in mice are generally lethal, and survivors are growth retarded and largely infertile (reviewed in (Liu et al., 2000)). Early life knockout in the liver only does not appear to have significant effects on growth or fertility (Yakar et al., 1999), and it was suggested that paracrine IGF-1, rather than circulating IGF-1 from the liver, exerted an important role in the growth compensation that occurs in this model. However, this conclusion was later challenged based on insufficient IGF-1 deficiency during an early critical growth period (Tang et al., 2005) and the demonstration that mice which produce IGF-1 only in their livers are fertile and have relatively mild growth phenotypes compared to their controls (Stratikopoulos et al., 2008). Together, these reports suggest that IGF-1 production by the liver is not necessary, but is sufficient to maintain tissue functions that are dependent upon IGF-1.
In the absence of liver IGF-1, paracrine mechanisms may compensate for the reduction in circulating IGF-1. Indeed, some of the few phenotypes detected in LID mice disappear by two years of age, and/or are sexually dimorphic (Tang et al., 2005). These results emphasize the need to consider the role of paracrine IGF-1 separately for different tissues and functions. However, analysis of Igf1 gene expression alone is insufficient to determine local IGF-1 deficiency, as the circulating protein may simply be transported into tissues at a greater rate. In this study, we clearly observed that a reduction in circulating IGF-1 decreases IGF-1 protein levels in the hippocampus, with little or no compensation even after 7 months. Additionally, Igf1 knockouts in CA1 neurons equivalently in all animals, independent of circulating IGF-1 knockdowns (two-way ANOVA test of interactions, p=0.826), demonstrating that there was little or no compensatory IGF-1 production by CA1 neurons. These results are in qualitative agreement with gene expression data from 6-week-old LID mice, which do not show detectable increases in Igf1 expression in fat, spleen, heart, muscle, or kidney (Yakar et al., 1999). It is possible (albeit unlikely) that such compensation may appear later in life; nevertheless the appearance of other phenotypes of aging may confound interpretation in a more prolonged experiment. For our model, we note that lack of detectable paracrine compensation for endocrine IGF-1 deficiency (either through IGF-1 production or transport), and lack of detectable endocrine compensation for paracrine deficiency should not be construed to represent limited transport of IGF-1 across the blood-brain barrier. We doubt we could create a severe enough neuronal knockout of Igf1 to produce a detectable change in serum IGF-1 levels using our current methods, particularly if endocrine compensation occurs. Furthermore, the robust positive correlation between hippocampal and serum IGF-1 levels observed in this study largely precludes a hypothesis of limited IGF-1 transport. Though this correlation existed across experimental groups, our methods do not allow a direct measure of IGF-1 transport into or out of the brain, and further work is necessary to determine whether if IGF-1 transport is altered in IGF-1 deficient animals.
In the present study we were able to substantially reduce paracrine IGF-1 in the neurons of the CA1 region of the hippocampus. We chose this region because magnetic resonance imaging (Ballmaier et al., 2008; Cho et al., 2010; Cole et al., 2010), physiological (Marchetti et al., 2010), and ultrastructural (Hajszan et al., 2009; Hajszan et al., 2005) studies of depressed humans and animals have demonstrated changes in CA1 compared to controls. As tissue volume and neuronal/synaptic development (Kar et al., 1997; Knusel & Hefti, 1991; Lichtenwalner et al., 2001; Seto et al., 2002; Trejo et al., 2008; Trejo et al., 2007) are affected by IGF-1, we hypothesized that the effects of depression on the CA1 region may be related to local IGF-1 production. Furthermore, CA1 (and the hippocampus in general) has an important role in memory formation and maintenance. Since depression, reduced cognitive function, and IGF-1 decline in the geriatric population exhibit frequent coincidence, we reasoned that the effects of IGF-1 within various regions of the hippocampus should be examined experimentally. Our results are consistent with the hypothesis that the low IGF-1 levels present in older animals and humans have the potential to contribute to depressive disorders. The present study also complements studies of GH deficiency and GH replacement therapy, which associate GH deficiency with depression and reduced quality of life, and GH replacement with improved quality of life (Abe et al., 2009; Deijen & Arwert, 2006; Kelly et al., 2006). It appears likely that these reported effects of GH may be largely due to IGF-1, but further study with other models is necessary to determine the contribution of IGF-1 independent effects of GH. For example, the IGF-2 pathway interacts with the GH pathway, and is known to have behavioral effects in the hippocampus (Chen et al., 2011).
The degree of serum IGF-1 deficiency in the present study (roughly a 40% reduction from control levels) appears to be translationally relevant to the aging human phenotype (see, e.g., (Rudman et al., 1981)). Taken together with studies of IGF-1 supplementation, our results suggest that depression in at least a subset of elderly humans may be due to the age-related decline in circulating IGF-1. Additionally, the effect of IGF-1 deficiency on the forced swim measure of depression was non-linear in this study, implying that there may be a threshold IGF-1 level below which humans are at greater risk for depression. Conversely, low IGF-1 was insufficient in this study to induce anxiety. It is possible that targeting different regions of the brain (or more extreme knockdowns of circulating IGF-1) could induce anxiety behaviors. However, we observed a highly significant correlation of depressive behavior with serum and hippocampal IGF-1 levels. Not even a modest correlation between anxiety measures and IGF-1 measures was observed. It is possible that IGF-1 deficiency may only compromise the anxiolytic effects of other interventions such as exercise (Trejo et al., 2008), without directly modulating anxiety. Both explanations suggest that comorbidity of anxiety and IGF-1 related depression may occur due to unrelated factors.
The molecular mechanisms by which IGF-1 deficiency causes depression are unclear, but several possibilities have been identified. Previous studies indicate that blocking hippocampal IGF-1 receptors reduces or abolishes exercise-induced increases in hippocampal BDNF mRNA and protein (Ding et al., 2006). As many studies have described a role for BDNF in the action of antidepressant medications, electroconvulsive shock therapy, and the effects of exercise on depression (reviewed in (R. S. Duman, 2004)), reductions of IGF-1 in the hippocampus may lead to depression by interrupting already identified BDNF pathways. An additional, parallel action of IGF-1 deficiency may involve regulation of serotonin levels in the hippocampus. IGF-1 administration has been shown to increase extracellular serotonin levels in rat hippocampus in approximately the same time course that it ameliorates depression (measured by the forced swim test). In the same study, it was also demonstrated that depletion of serotonin blocks the antidepressant effects of IGF-1 administration (Hoshaw et al., 2008). Yet, very late-onset depression in elderly humans (who have relatively low IGF-1 levels) can be resistant to antidepressants (Meyers & Jeste, 2010). Furthermore, older rodents show a decreased behavioral and neurogenic response to SSRIs (selective serotonin reuptake inhibitors) (Herrera-Perez et al., 2010), providing evidence that depression may be regulated through multiple pathways. Also, growth hormone is produced in the hippocampus (Donahue et al., 2006), and its pathways may interact and/or have IGF-1 independent effects on mood. These issues emphasize the need for clinical studies of the interactions of serotonin, growth factors, and neurotrophins in geriatric depression. Our preliminary hippocampal gene expression data suggest no changes in Igf1, Igf1r (the IGF-1 receptor), Igf2 (insulin-like growth factor II), Igf2r (insulin-like growth factor II receptor), Gh, Ghr (growth hormone receptor), or Ghrh (growth hormone releasing hormone; unpublished observations). It should be noted that in a similar model, the LID mouse, GH overexpression and other phenotypes disappear with age (Tang et al., 2005), and a cross-sectional study through the lifespan of our model would be required to conclude these pathways were never affected by knockout of Igf1. However, in our model Bdnf gene expression appears to be upregulated in the hippocampi of hepatic, hippocampal, and double knockouts of Igf1, and serotonergic pathways may be altered as well (unpublished observations). If these are compensatory changes, they were insufficient to prevent the behavioral effects of IGF-1 deficiency, and suggest that Bdnf regulation in IGF-1 deficient animals is dominated by factors other than IGF-1.
Future studies will determine whether it is more important for neurons to produce their own IGF-1 (i.e., autocrine IGF-1), or if other local cell types (e.g., glia and vasculature) support IGF-1 production in the hippocampus. As the hippocampal circuitry is only a part of the putative depression pathway, the techniques used in this study have the potential to be applied to other brain regions as well. Additionally, IGF-1 production and IGF-1 cell signaling are interdependent issues in both experimental design and translational medicine. It is necessary to know which cells are most important for IGF-1 production in the aged brain in order to design viable treatments for paracrine IGF-1 deficiency. However, similar experiments targeting the IGF-1 receptor are necessary to understand the IGF-1-mediated depression pathway. It is likely that depressive effects caused by IGF-1 deficiency are not solely due to reduced IGF-1 receptor activation in neurons, but reduced activation of the receptor in other cell types. Animals from all such experiments should be tested for effects on learning and memory, and examined for physiological, genomic, and proteomic correlates to their behavioral profiles.
In conclusion, we have generated a novel model of tissue and age-specific IGF-1 deficiency, and demonstrated robust control over the production of both circulating and locally produced IGF-1. Using this system, we have demonstrated that IGF-1 deficiency alone is sufficient to induce depression-like behaviors in normally developed adult mice, and that no sufficient compensatory mechanisms appear within a timeframe of several months. Our results suggest that depression in a subset of the geriatric population may be due to the age-related decline in IGF-1 production.
Acknowledgments
The authors wish to thank Dr. S. Yakar for generously donating the founders of the Igf1f/f mouse colony. This study was supported by the National Institute on Aging grants P01 AG11370 and R01 AG26607 (to WES), National Institute on Drug Abuse grant DA024763 (to CEB), and the Donald W. Reynolds Foundation.
Abbreviations
- AAV
adeno-associated virus
- ANOVA
analysis of variance
- BDNF
brain-derived neurotrophic factor
- CA1
Cornu Ammonis I
- CMV
cytomegalovirus
- DAPI – 4′
6-diamidino-2-phenylindole
- DNA
deoxyribonucleic acid
- EGFP
enhanced green fluorescent protein
- ELISA
Enzyme-linked immunosorbant assay
- GFP
green fluorescent protein
- GH
growth hormone
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IGF-1
Insulin-like growth factor I (protein)
- Igf1
Insulin-like growth factor I (gene)
- IHC
immunohistochemistry
- LID
liver IGF-1 deficient
- MUP
major urinary protein
- OUHSC
The University of Oklahoma Health Sciences Center
- PCR
polymerase chain reaction
- SSRI
selective serotonin reuptake inhibitor
- TBS-X
Tris-buffered saline with 0.25% Triton-X® 100
Footnotes
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe S, Okumura A, Mukae T, Nakazawa T, Niijima S, Yamashiro Y, et al. Depressive tendency in children with growth hormone deficiency. Journal of paediatrics and child health. 2009;45(11):636–640. doi: 10.1111/j.1440-1754.2009.01586.x. [DOI] [PubMed] [Google Scholar]
- Adams MM, Elizabeth Forbes M, Constance Linville M, Riddle DR, Sonntag WE, Brunso-Bechtold JK. Stability of local brain levels of insulin-like growth factor-I in two well-characterized models of decreased plasma IGF-I. Growth Factors. 2009;27(3):181–188. doi: 10.1080/08977190902863639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS. Mood disorders. In: Sadock BJ, Sadock VA, editors. Comprehensive Textbook of Psychiatry. 7. Vol. 2. Baltimore: Williams and Wilkins; 2000. [Google Scholar]
- Armstrong CS, Wuarin L, Ishii DN. Uptake of circulating insulin-like growth factor-I into the cerebrospinal fluid of normal and diabetic rats and normalization of IGF-II mRNA content in diabetic rat brain. Journal of Neuroscience Research. 2000;59(5):649–660. doi: 10.1002/(SICI)1097-4547(20000301)59:5<649::AID-JNR8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Narr KL, Toga AW, Elderkin-Thompson V, Thompson PM, Hamilton L, et al. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am J Psychiatry. 2008;165(2):229–237. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy C, Werner H, Roberts CT, LeRoith D. Cellular pattern of type-I insulin-like growth factor receptor gene expression during maturation of the rat brain: comparison with insulin-like growth factors I and II. Neuroscience. 1992;46(4):909–923. doi: 10.1016/0306-4522(92)90193-6. [DOI] [PubMed] [Google Scholar]
- Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. The Journal of Neuroscience. 2000;20(8):2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Spuch C, Trejo JL, Antequera D, Torres-Aleman I. Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. The Journal of Neuroscience. 2005;25(47):10884–10893. doi: 10.1523/JNEUROSCI.2909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nature Medicine. 2002;8(12):1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and IGF-1 in aging and lifespan. Trends in genetics : TIG. 2002;18(6):295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Molecular Therapy. 2006;13(3):528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, et al. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469(7331):491–497. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho ZH, Kim YB, Han JY, Kim NB, Hwang SI, Kim SJ, et al. Altered T2* relaxation time of the hippocampus in major depressive disorder: Implications of ultra-high field magnetic resonance imaging. J Psychiatr Res. 2010 doi: 10.1016/j.jpsychires.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Cole J, Toga AW, Hojatkashani C, Thompson P, Costafreda SG, Cleare AJ, et al. Subregional hippocampal deformations in major depressive disorder. J Affect Disord. 2010 doi: 10.1016/j.jad.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ercole AJ, Stiles AD, Underwood LE. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(3):935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deijen JB, Arwert LI. Impaired quality of life in hypopituitary adults with growth hormone deficiency : can somatropin replacement therapy help? Treatments in endocrinology. 2006;5(4):243–250. doi: 10.2165/00024677-200605040-00005. [DOI] [PubMed] [Google Scholar]
- Deuschle M, Blum WF, Strasburger CJ, Schweiger U, Weber B, Korner A, et al. Insulin-like growth factor-I (IGF-I) plasma concentrations are increased in depressed patients. Psychoneuroendocrinology. 1997;22(7):493–503. doi: 10.1016/s0306-4530(97)00046-2. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140(3):823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Donahue CP, Kosik KS, Shors TJ. Growth hormone is produced within the hippocampus where it responds to age, sex, and stress. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(15):6031–6036. doi: 10.1073/pnas.0507776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Terwilliger R, Russell DS, Newton SS, Duman RS. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav Brain Res. 2009;198(2):366–371. doi: 10.1016/j.bbr.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular medicine. 2004;5(1):11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Guo C, Yang W, Lobe CG. A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis (New York, NY: 2000) 2002;32(1):8–18. doi: 10.1002/gene.10021. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Dow A, Warner-Schmidt JL, Szigeti-Buck K, Sallam NL, Parducz A, et al. Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol Psychiatry. 2009;65(5):392–400. doi: 10.1016/j.biopsych.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci. 2005;21(5):1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- Heisel MJ, Duberstein PR. Suicide prevention in older adults. Clinical Psychology: Science and Practice. 2005;12(3):242–259. [Google Scholar]
- Herrera-Perez JJ, Martinez-Mota L, Fernandez-Guasti A. Aging impairs the antidepressant-like response to citalopram in male rats. European journal of pharmacology. 2010;633(1–3):39–43. doi: 10.1016/j.ejphar.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Ho KJ, Bass CE, Kroemer AH, Ma C, Terwilliger E, Karp SJ. Optimized adeno-associated virus 8 produces hepatocyte-specific Cre-mediated recombination without toxicity or affecting liver regeneration. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2008;295(2):G412–419. doi: 10.1152/ajpgi.00590.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwath E, Johnson J, Klerman GL, Weissman MM. Depressive symptoms as relative and attributable risk factors for first-onset major depression. Arch Gen Psychiatry. 1992;49(10):817–823. doi: 10.1001/archpsyc.1992.01820100061011. [DOI] [PubMed] [Google Scholar]
- Hoshaw BA, Hill TI, Crowley JJ, Malberg JE, Khawaja X, Rosenzweig-Lipson S, et al. Antidepressant-like behavioral effects of IGF-I produced by enhanced serotonin transmission. European journal of pharmacology. 2008;594(1–3):109–116. doi: 10.1016/j.ejphar.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Research. 2005;1037(1–2):204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Hybels CF, Blazer DG. Epidemiology of late-life mental disorders. Clin Geriatr Med. 2003;19(4):663–696. v. doi: 10.1016/s0749-0690(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Kar S, Seto D, Dore S, Hanisch U, Quirion R. Insulin-like growth factors-I and -II differentially regulate endogenous acetylcholine release from the rat hippocampal formation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):14054–14059. doi: 10.1073/pnas.94.25.14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DF, McArthur DL, Levin H, Swimmer S, Dusick JR, Cohan P, et al. Neurobehavioral and quality of life changes associated with growth hormone insufficiency after complicated mild, moderate, or severe traumatic brain injury. Journal of neurotrauma. 2006;23(6):928–942. doi: 10.1089/neu.2006.23.928. [DOI] [PubMed] [Google Scholar]
- Knusel B, Hefti F. Trophic actions of IGF-I, IGF-II and insulin on cholinergic and dopaminergic brain neurons. Adv Exp Med Biol. 1991;293:351–360. doi: 10.1007/978-1-4684-5949-4_31. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107(4):603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Liu JL, Grinberg A, Westphal H, Sauer B, Accili D, Karas M, et al. Insulin-like growth factor-I affects perinatal lethality and postnatal development in a gene dosage-dependent manner: manipulation using the Cre/loxP system in transgenic mice. Molecular Endocrinology. 1998;12(9):1452–1462. doi: 10.1210/mend.12.9.0162. [DOI] [PubMed] [Google Scholar]
- Liu JL, Yakar S, LeRoith D. Conditional knockout of mouse insulin-like growth factor-1 gene using the Cre/loxP system. Proceedings of the Society for Experimental Biology and Medicine. 2000;223(4):344–351. doi: 10.1046/j.1525-1373.2000.22349.x. [DOI] [PubMed] [Google Scholar]
- Lupien SB, Bluhm EJ, Ishii DN. Systemic insulin-like growth factor-I administration prevents cognitive impairment in diabetic rats, and brain IGF regulates learning/memory in normal adult rats. Journal of Neuroscience Research. 2003;74(4):512–523. doi: 10.1002/jnr.10791. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Platt B, Rizzo SJ, Ring RH, Lucki I, Schechter LE, et al. Increasing the levels of insulin-like growth factor-I by an IGF binding protein inhibitor produces anxiolytic and antidepressant-like effects. Neuropsychopharmacology. 2007;32(11):2360–2368. doi: 10.1038/sj.npp.1301358. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Tafi E, Middei S, Rubinacci MA, Restivo L, Ammassari-Teule M, et al. Synaptic adaptations of CA1 pyramidal neurons induced by a highly effective combinational antidepressant therapy. Biol Psychiatry. 2010;67(2):146–154. doi: 10.1016/j.biopsych.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87(3):559–569. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Meyers BS, Jeste DV. Geriatric psychopharmacology: evolution of a discipline. The Journal of clinical psychiatry. 2010;71(11):1416–1424. doi: 10.4088/JCP.10r06485gry. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116(1):201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nieves-Martinez E, Sonntag WE, Wilson A, Donahue A, Molina DP, Brunso-Bechtold J, et al. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. The Journal of Endocrinology. 2010;204(1):31–36. doi: 10.1677/JOE-09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima T, Piriz J, Duflot S, Fernandez AM, Gaitan G, Gomez-Pinedo U, et al. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron. 2010;67(5):834–846. doi: 10.1016/j.neuron.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Okura T, Plassman BL, Steffens DC, Llewellyn DJ, Potter GG, Langa KM. Prevalence of neuropsychiatric symptoms and their association with functional limitations in older adults in the United States: the aging, demographics, and memory study. Journal of the American Geriatrics Society. 2010;58(2):330–337. doi: 10.1111/j.1532-5415.2009.02680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Interactions of IGF-1 with the blood-brain barrier in vivo and in situ. Neuroendocrinology. 2000;72(3):171–178. doi: 10.1159/000054584. [DOI] [PubMed] [Google Scholar]
- Rapp MA, Gerstorf D, Helmchen H, Smith J. Depression predicts mortality in the young old, but not in the oldest old: results from the Berlin Aging Study. Am J Geriatr Psychiatry. 2008;16(10):844–852. doi: 10.1097/JGP.0b013e31818254eb. [DOI] [PubMed] [Google Scholar]
- Reynolds SL, Haley WE, Kozlenko N. The impact of depressive symptoms and chronic diseases on active life expectancy in older Americans. Am J Geriatr Psychiatry. 2008;16(5):425–432. doi: 10.1097/JGP.0b013e31816ff32e. [DOI] [PubMed] [Google Scholar]
- Rudman D, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP. Impaired growth hormone secretion in the adult population: relation to age and adiposity. J Clin Invest. 1981;67(5):1361–1369. doi: 10.1172/JCI110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto D, Zheng WH, McNicoll A, Collier B, Quirion R, Kar S. Insulin-like growth factor-I inhibits endogenous acetylcholine release from the rat hippocampal formation: possible involvement of GABA in mediating the effects. Neuroscience. 2002;115(2):603–612. doi: 10.1016/s0306-4522(02)00450-5. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Bennett C, Ingram R, Donahue A, Ingraham J, Chen H, et al. Growth hormone and IGF-I modulate local cerebral glucose utilization and ATP levels in a model of adult-onset growth hormone deficiency. American Journal of Physiology. Endocrinology and Metabolism. 2006;291(3):E604–610. doi: 10.1152/ajpendo.00012.2006. [DOI] [PubMed] [Google Scholar]
- Stratikopoulos E, Szabolcs M, Dragatsis I, Klinakis A, Efstratiadis A. The hormonal action of IGF1 in postnatal mouse growth. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(49):19378–19383. doi: 10.1073/pnas.0809223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Yu R, Lu Y, Parlow AF, Liu JL. Age-dependent onset of liver-specific IGF-I gene deficiency and its persistence in old age: implications for postnatal growth and insulin resistance in LID mice. American Journal of Physiology. Endocrinology and Metabolism. 2005;289(2):E288–295. doi: 10.1152/ajpendo.00494.2004. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Nunez A, Torres-Aleman I. Sedentary life impairs self-reparative processes in the brain: the role of serum insulin-like growth factor-I. Reviews in the Neurosciences. 2002;13(4):365–374. doi: 10.1515/revneuro.2002.13.4.365. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Molecular and Cellular Neurosciences. 2008;37(2):402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Piriz J, Llorens-Martin MV, Fernandez AM, Bolos M, LeRoith D, et al. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Molecular Psychiatry. 2007;12(12):1118–1128. doi: 10.1038/sj.mp.4002076. [DOI] [PubMed] [Google Scholar]
- Wu Y, Brodt P, Sun H, Mejia W, Novosyadlyy R, Nunez N, et al. Insulin-like growth factor-I regulates the liver microenvironment in obese mice and promotes liver metastasis. Cancer research. 2010;70(1):57–67. doi: 10.1158/0008-5472.CAN-09-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Sonntag WE. Growth hormone and aging: Regulation, signal transduction and replacement therapy. Trends in Endocrinology and Metabolism. 1996;7(4):145–150. doi: 10.1016/1043-2760(96)00043-4. [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(13):7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Kastin AJ, Pan WCINEJ, Pmid Reciprocal interactions of insulin and insulin-like growth factor I in receptor-mediated transport across the blood-brain barrier. Endocrinology. 2006;147(6):2611–2615. doi: 10.1210/en.2006-0020. [DOI] [PubMed] [Google Scholar]