Abstract
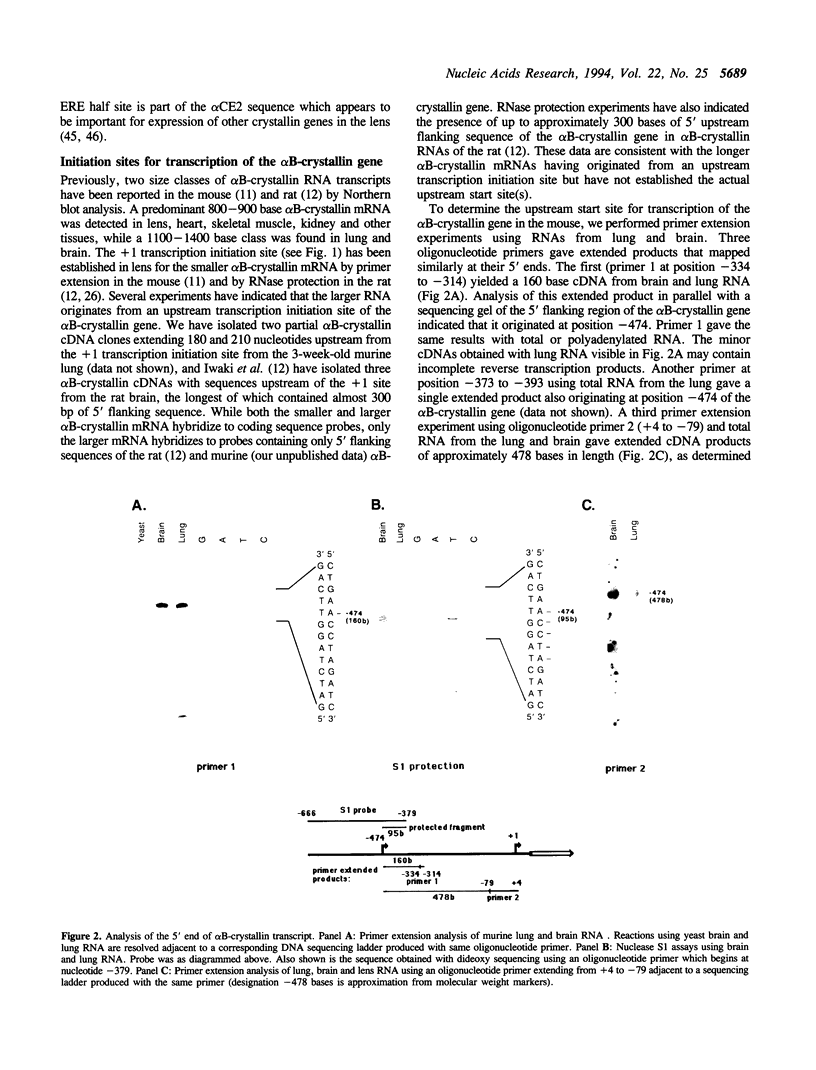
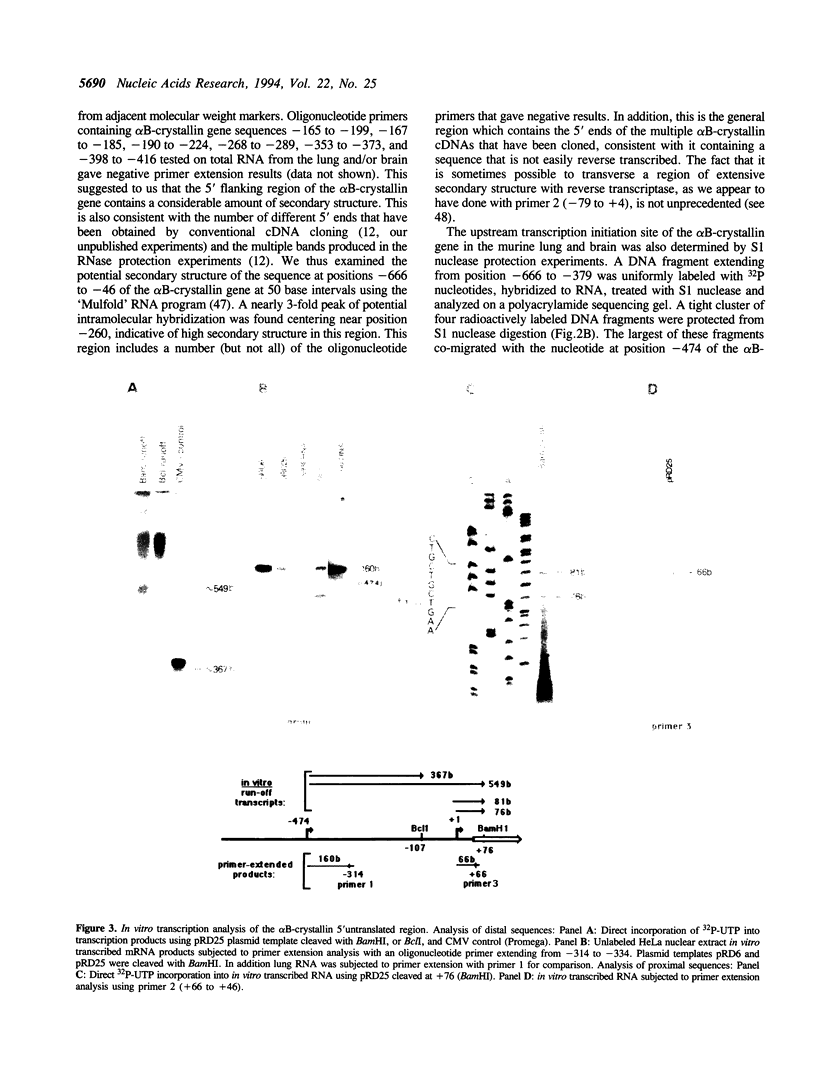
We have determined the complete nucleotide sequence (-865 to +3515) of the murine alpha B-crystallin/small heat shock protein gene, a major soluble protein of the vertebrate eye lens. Its 3 exon/2 intron structure is identical to that of the rat, hamster and human gene, with the exons being much more conserved than the introns. Previous reports indicated that there are two sizes of alpha B-crystallin mRNA; a larger alpha B-crystallin mRNA predominates in the lung and brain and is also found in low levels in most other tissues (except in lens and liver), while a smaller alpha B-crystallin mRNA exists at a high level in the lens and in variable amounts elsewhere. Sequence analysis suggests that secondary structure in the 5' untranslated sequence of the longer mRNA has led to difficulty in mapping the transcription initiation site of the longer transcript. Here we provide evidence by primer extension, S1 nuclease protection, and PCR (polymerase chain reaction) experiments for a transcription initiation site in the murine lung and brain at position -474. We also detected the utilization of the -474 initiation site in lens and of the +1 site in lung and brain, indicating that the tissue preference for these sites is not absolute. In vitro transcription experiments revealed that cell-free HeLa nuclear extracts specifically initiate transcription at the -474 and +1 sites. alpha B-crystallin was immunocytochemically localized to the bronchioles of the lung. Thus, regulation of alpha B-crystallin/small heat shock protein expression involves the utilization of tissue-preferred transcription initiation sites.
Full text
PDF



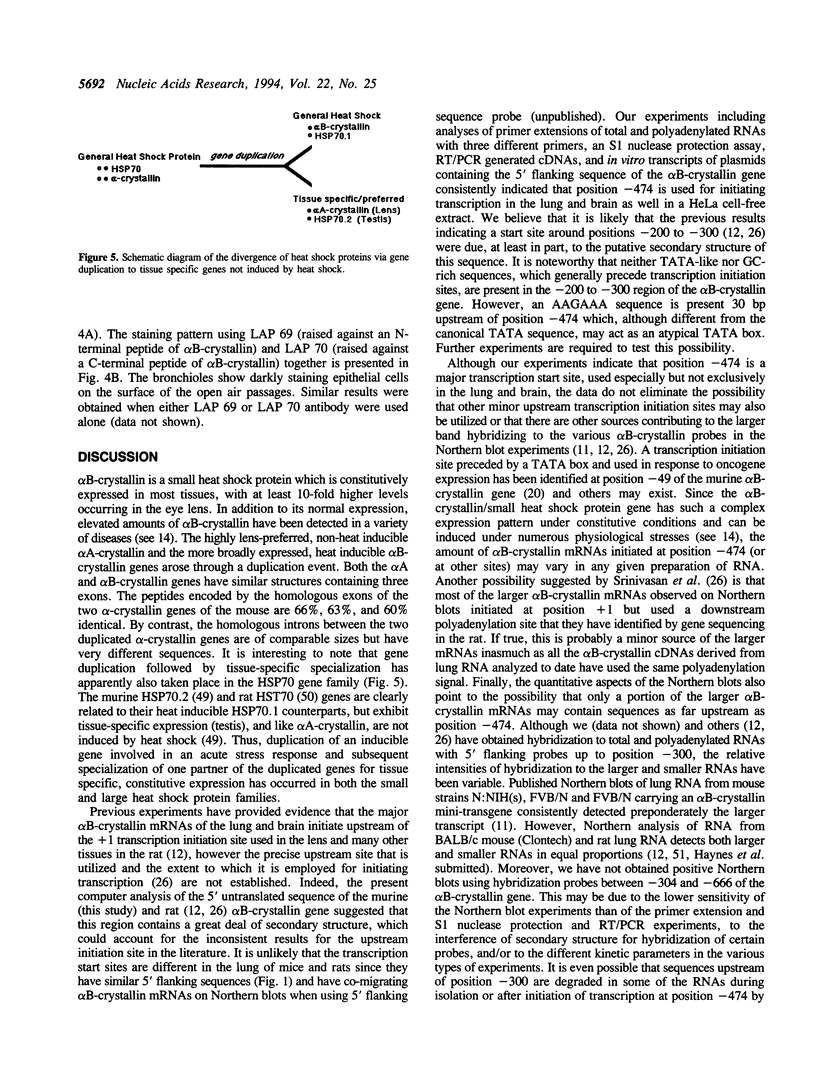


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett K. L., Hill R. E., Pietras D. F., Woodworth-Gutai M., Kane-Haas C., Houston J. M., Heath J. K., Hastie N. D. Most highly repeated dispersed DNA families in the mouse genome. Mol Cell Biol. 1984 Aug;4(8):1561–1571. doi: 10.1128/mcb.4.8.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bhat S. P., Nagineni C. N. alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989 Jan 16;158(1):319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Chou Q. Minimizing deletion mutagenesis artifact during Taq DNA polymerase PCR by E. coli SSB. Nucleic Acids Res. 1992 Aug 25;20(16):4371–4371. doi: 10.1093/nar/20.16.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin V., Maniatis T. The role of specific enhancer-promoter interactions in the Drosophila Adh promoter switch. Genes Dev. 1989 Dec;3(12B):2191–2120. doi: 10.1101/gad.3.12b.2191. [DOI] [PubMed] [Google Scholar]
- Dasgupta S., Hohman T. C., Carper D. Hypertonic stress induces alpha B-crystallin expression. Exp Eye Res. 1992 Mar;54(3):461–470. doi: 10.1016/0014-4835(92)90058-z. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen H. P., Herbrink P., Bloemendal H., de Jong W. W. Primary structure of the bovine beta-crystallin Bp chain. Internal duplication and homology with gamma-crystallin. Eur J Biochem. 1981 Dec;121(1):83–91. doi: 10.1111/j.1432-1033.1981.tb06433.x. [DOI] [PubMed] [Google Scholar]
- Dubin R. A., Ally A. H., Chung S., Piatigorsky J. Human alpha B-crystallin gene and preferential promoter function in lens. Genomics. 1990 Aug;7(4):594–601. doi: 10.1016/0888-7543(90)90204-8. [DOI] [PubMed] [Google Scholar]
- Dubin R. A., Gopal-Srivastava R., Wawrousek E. F., Piatigorsky J. Expression of the murine alpha B-crystallin gene in lens and skeletal muscle: identification of a muscle-preferred enhancer. Mol Cell Biol. 1991 Sep;11(9):4340–4349. doi: 10.1128/mcb.11.9.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin R. A., Wawrousek E. F., Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989 Mar;9(3):1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. R., Rohwer R. G., Seed B. Isolation of cDNAs of scrapie-modulated RNAs by subtractive hybridization of a cDNA library. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5738–5742. doi: 10.1073/pnas.85.15.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua S. A., Blum-Salingaros M., McGuire W. L. Induction of the estrogen-regulated "24K" protein by heat shock. Cancer Res. 1989 Aug 1;49(15):4126–4129. [PubMed] [Google Scholar]
- Gaestel M., Gotthardt R., Müller T. Structure and organisation of a murine gene encoding small heat-shock protein Hsp25. Gene. 1993 Jun 30;128(2):279–283. doi: 10.1016/0378-1119(93)90575-n. [DOI] [PubMed] [Google Scholar]
- Gonzalez P., Rao P. V., Zigler J. S., Jr Molecular cloning and sequencing of zeta-crystallin/quinone reductase cDNA from human liver. Biochem Biophys Res Commun. 1993 Mar 31;191(3):902–907. doi: 10.1006/bbrc.1993.1302. [DOI] [PubMed] [Google Scholar]
- Goodwin L. O., Lees-Miller J. P., Leonard M. A., Cheley S. B., Helfman D. M. Four fibroblast tropomyosin isoforms are expressed from the rat alpha-tropomyosin gene via alternative RNA splicing and the use of two promoters. J Biol Chem. 1991 May 5;266(13):8408–8415. [PubMed] [Google Scholar]
- Gopal-Srivastava R., Piatigorsky J. Identification of a lens-specific regulatory region (LSR) of the murine alpha B-crystallin gene. Nucleic Acids Res. 1994 Apr 11;22(7):1281–1286. doi: 10.1093/nar/22.7.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal-Srivastava R., Piatigorsky J. The murine alpha B-crystallin/small heat shock protein enhancer: identification of alpha BE-1, alpha BE-2, alpha BE-3, and MRF control elements. Mol Cell Biol. 1993 Nov;13(11):7144–7152. doi: 10.1128/mcb.13.11.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G., Wolf M., Katyal S. L., Singh G., Beato M., Suske G. Tissue-specific expression, hormonal regulation and 5'-flanking gene region of the rat Clara cell 10 kDa protein: comparison to rabbit uteroglobin. Nucleic Acids Res. 1990 May 25;18(10):2939–2946. doi: 10.1093/nar/18.10.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head M. W., Corbin E., Goldman J. E. Coordinate and independent regulation of alpha B-crystallin and hsp27 expression in response to physiological stress. J Cell Physiol. 1994 Apr;159(1):41–50. doi: 10.1002/jcp.1041590107. [DOI] [PubMed] [Google Scholar]
- Hodin J., Wistow G. 5'-RACE PCR of mRNA for three taxon-specific crystallins: for each gene one promoter controls both lens and non-lens expression. Biochem Biophys Res Commun. 1993 Jan 29;190(2):391–396. doi: 10.1006/bbrc.1993.1060. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki A., Iwaki T., Goldman J. E., Liem R. K. Multiple mRNAs of rat brain alpha-crystallin B chain result from alternative transcriptional initiation. J Biol Chem. 1990 Dec 25;265(36):22197–22203. [PubMed] [Google Scholar]
- Iwaki T., Kume-Iwaki A., Goldman J. E. Cellular distribution of alpha B-crystallin in non-lenticular tissues. J Histochem Cytochem. 1990 Jan;38(1):31–39. doi: 10.1177/38.1.2294148. [DOI] [PubMed] [Google Scholar]
- Iwaki T., Kume-Iwaki A., Liem R. K., Goldman J. E. Alpha B-crystallin is expressed in non-lenticular tissues and accumulates in Alexander's disease brain. Cell. 1989 Apr 7;57(1):71–78. doi: 10.1016/0092-8674(89)90173-6. [DOI] [PubMed] [Google Scholar]
- Jaworski C. J., Chepelinsky A. B., Piatigorsky J. The alpha A-crystallin gene: conserved features of the 5'-flanking regions in human, mouse, and chicken. J Mol Evol. 1991 Dec;33(6):495–505. doi: 10.1007/BF02102802. [DOI] [PubMed] [Google Scholar]
- Kato K., Shinohara H., Kurobe N., Goto S., Inaguma Y., Ohshima K. Immunoreactive alpha A crystallin in rat non-lenticular tissues detected with a sensitive immunoassay method. Biochim Biophys Acta. 1991 Oct 25;1080(2):173–180. doi: 10.1016/0167-4838(91)90146-q. [DOI] [PubMed] [Google Scholar]
- Klement J. F., Cvekl A., Piatigorsky J. Functional elements DE2A, DE2B, and DE1A and the TATA box are required for activity of the chicken alpha A-crystallin gene in transfected lens epithelial cells. J Biol Chem. 1993 Mar 25;268(9):6777–6784. [PubMed] [Google Scholar]
- Klement J. F., Wawrousek E. F., Piatigorsky J. Tissue-specific expression of the chicken alpha A-crystallin gene in cultured lens epithelia and transgenic mice. J Biol Chem. 1989 Nov 25;264(33):19837–19844. [PubMed] [Google Scholar]
- Klemenz R., Fröhli E., Aoyama A., Hoffmann S., Simpson R. J., Moritz R. L., Schäfer R. Alpha B crystallin accumulation is a specific response to Ha-ras and v-mos oncogene expression in mouse NIH 3T3 fibroblasts. Mol Cell Biol. 1991 Feb;11(2):803–812. doi: 10.1128/mcb.11.2.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Fröhli E., Steiger R. H., Schäfer R., Aoyama A. Alpha B-crystallin is a small heat shock protein. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krayev A. S., Markusheva T. V., Kramerov D. A., Ryskov A. P., Skryabin K. G., Bayev A. A., Georgiev G. P. Ubiquitous transposon-like repeats B1 and B2 of the mouse genome: B2 sequencing. Nucleic Acids Res. 1982 Dec 11;10(23):7461–7475. doi: 10.1093/nar/10.23.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockett T. J., Ashburner M. Temporal and spatial utilization of the alcohol dehydrogenase gene promoters during the development of Drosophila melanogaster. Dev Biol. 1989 Aug;134(2):430–437. doi: 10.1016/0012-1606(89)90115-2. [DOI] [PubMed] [Google Scholar]
- Lok S., Stevens W., Breitman M. L., Tsui L. C. Multiple regulatory elements of the murine gamma 2-crystallin promoter. Nucleic Acids Res. 1989 May 11;17(9):3563–3582. doi: 10.1093/nar/17.9.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubsen N. H., Aarts H. J., Schoenmakers J. G. The evolution of lenticular proteins: the beta- and gamma-crystallin super gene family. Prog Biophys Mol Biol. 1988;51(1):47–76. doi: 10.1016/0079-6107(88)90010-7. [DOI] [PubMed] [Google Scholar]
- Mahendroo M. S., Means G. D., Mendelson C. R., Simpson E. R. Tissue-specific expression of human P-450AROM. The promoter responsible for expression in adipose tissue is different from that utilized in placenta. J Biol Chem. 1991 Jun 15;266(17):11276–11281. [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo I., Yasuda K. The cooperative interaction between two motifs of an enhancer element of the chicken alpha A-crystallin gene, alpha CE1 and alpha CE2, confers lens-specific expression. Nucleic Acids Res. 1992 Jul 25;20(14):3701–3712. doi: 10.1093/nar/20.14.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murano S., Thweatt R., Shmookler Reis R. J., Jones R. A., Moerman E. J., Goldstein S. Diverse gene sequences are overexpressed in werner syndrome fibroblasts undergoing premature replicative senescence. Mol Cell Biol. 1991 Aug;11(8):3905–3914. doi: 10.1128/mcb.11.8.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazábal U. E., Pfaff D. W., Mobbs C. V. Sex differences in the regulation of heat shock protein 70 kDa and 90 kDa in the rat ventromedial hypothalamus by estrogen. Brain Res. 1992 Nov 20;596(1-2):311–314. doi: 10.1016/0006-8993(92)91563-t. [DOI] [PubMed] [Google Scholar]
- Overbeek P. A., Chepelinsky A. B., Khillan J. S., Piatigorsky J., Westphal H. Lens-specific expression and developmental regulation of the bacterial chloramphenicol acetyltransferase gene driven by the murine alpha A-crystallin promoter in transgenic mice. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7815–7819. doi: 10.1073/pnas.82.23.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quax-Jeuken Y., Quax W., van Rens G., Khan P. M., Bloemendal H. Complete structure of the alpha B-crystallin gene: conservation of the exon-intron distribution in the two nonlinked alpha-crystallin genes. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5819–5823. doi: 10.1073/pnas.82.17.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkawek K., de Jong W. W., Merck K. B., Frenken C. W., van Workum F. P., Bosman G. J. alpha B-crystallin is present in reactive glia in Creutzfeldt-Jakob disease. Acta Neuropathol. 1992;83(3):324–327. doi: 10.1007/BF00296796. [DOI] [PubMed] [Google Scholar]
- Sax C. M., Piatigorsky J. Expression of the alpha-crystallin/small heat-shock protein/molecular chaperone genes in the lens and other tissues. Adv Enzymol Relat Areas Mol Biol. 1994;69:155–201. doi: 10.1002/9780470123157.ch5. [DOI] [PubMed] [Google Scholar]
- Schibler U., Sierra F. Alternative promoters in developmental gene expression. Annu Rev Genet. 1987;21:237–257. doi: 10.1146/annurev.ge.21.120187.001321. [DOI] [PubMed] [Google Scholar]
- Srinivasan A. N., Bhat S. P. Complete structure and expression of the rat alpha B-crystallin gene. DNA Cell Biol. 1994 Jun;13(6):651–661. doi: 10.1089/dna.1994.13.651. [DOI] [PubMed] [Google Scholar]
- Srinivasan A. N., Nagineni C. N., Bhat S. P. alpha A-crystallin is expressed in non-ocular tissues. J Biol Chem. 1992 Nov 15;267(32):23337–23341. [PubMed] [Google Scholar]
- Stripp B. R., Sawaya P. L., Luse D. S., Wikenheiser K. A., Wert S. E., Huffman J. A., Lattier D. L., Singh G., Katyal S. L., Whitsett J. A. cis-acting elements that confer lung epithelial cell expression of the CC10 gene. J Biol Chem. 1992 Jul 25;267(21):14703–14712. [PubMed] [Google Scholar]
- Summers L., Slingsby C., White H., Narebor M., Moss D., Miller L., Mahadevan D., Lindley P., Driessen H., Blundell T. The molecular structures and interactions of bovine and human gamma-crystallins. Ciba Found Symp. 1984;106:219–236. doi: 10.1002/9780470720875.ch13. [DOI] [PubMed] [Google Scholar]
- Tora L., Gaub M. P., Mader S., Dierich A., Bellard M., Chambon P. Cell-specific activity of a GGTCA half-palindromic oestrogen-responsive element in the chicken ovalbumin gene promoter. EMBO J. 1988 Dec 1;7(12):3771–3778. doi: 10.1002/j.1460-2075.1988.tb03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrousek E. F., Chepelinsky A. B., McDermott J. B., Piatigorsky J. Regulation of the murine alpha A-crystallin promoter in transgenic mice. Dev Biol. 1990 Jan;137(1):68–76. doi: 10.1016/0012-1606(90)90008-7. [DOI] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Wistow G. Evolution of a protein superfamily: relationships between vertebrate lens crystallins and microorganism dormancy proteins. J Mol Evol. 1990 Feb;30(2):140–145. doi: 10.1007/BF02099940. [DOI] [PubMed] [Google Scholar]
- Wiśniewski J., Kordula T., Krawczyk Z. Isolation and nucleotide sequence analysis of the rat testis-specific major heat-shock protein (HSP70)-related gene. Biochim Biophys Acta. 1990 Jan 30;1048(1):93–99. doi: 10.1016/0167-4781(90)90027-y. [DOI] [PubMed] [Google Scholar]
- Xiao H., Perisic O., Lis J. T. Cooperative binding of Drosophila heat shock factor to arrays of a conserved 5 bp unit. Cell. 1991 Feb 8;64(3):585–593. doi: 10.1016/0092-8674(91)90242-q. [DOI] [PubMed] [Google Scholar]
- Zelent A., Mendelsohn C., Kastner P., Krust A., Garnier J. M., Ruffenach F., Leroy P., Chambon P. Differentially expressed isoforms of the mouse retinoic acid receptor beta generated by usage of two promoters and alternative splicing. EMBO J. 1991 Jan;10(1):71–81. doi: 10.1002/j.1460-2075.1991.tb07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenzie-Gregory B., O'Shea-Greenfield A., Smale S. T. Similar mechanisms for transcription initiation mediated through a TATA box or an initiator element. J Biol Chem. 1992 Feb 5;267(4):2823–2830. [PubMed] [Google Scholar]
- Zuker M. On finding all suboptimal foldings of an RNA molecule. Science. 1989 Apr 7;244(4900):48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Leunissen J. A., Voorter C. E. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol. 1993 Jan;10(1):103–126. doi: 10.1093/oxfordjournals.molbev.a039992. [DOI] [PubMed] [Google Scholar]