Abstract
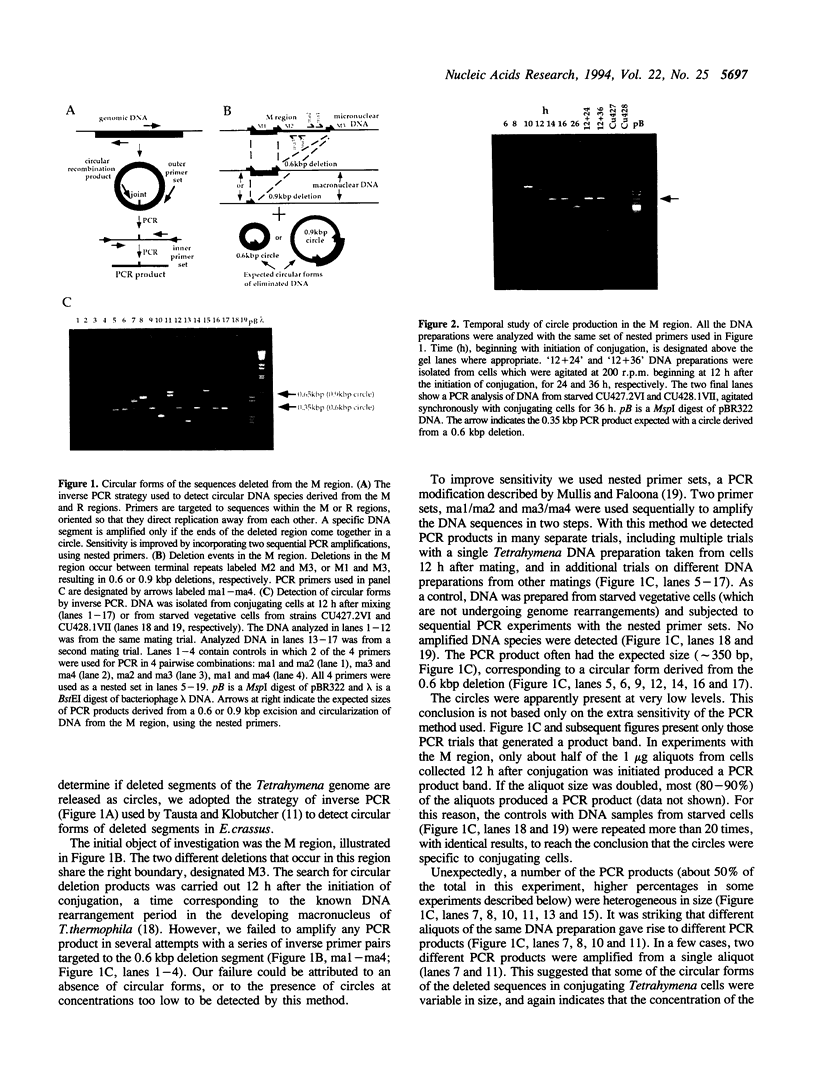
Thousands of DNA deletion events occur during macronuclear development in the ciliate Tetrahymena thermophila. In two deleted genomic regions, designated M and R, the eliminated sequences form circles that can be detected by PCR. However, the circles are not normal products of the reaction pathway. The circular forms occur at very low levels in conjugating cells, but are stable. Sequencing analysis showed that many of the circles (as many as 50% of those examined) reflected a precise deletion in the M and R regions. The remaining circles were either smaller or larger and contained varying lengths of sequences derived from the chromosomal DNA surrounding the eliminated region. The chromosomal junctions left behind after deletion were more precise, although deletions in either the M or R regions can generate any of several alternative junctions (1). Some new chromosomal junctions were detected in the present study. The results suggest that the deleted segment is released as a linear DNA species that is degraded rapidly. The species is only rarely converted to the stable circles we detect. The deletion mechanism is different from those proposed for deletion events in hypotrichous ciliates (2-4), and does not reflect a conservative site-specific recombination process such as that promoted by the bacteriophage lambda integrase (5).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheim N., Erlich H. Polymerase chain reaction strategy. Annu Rev Biochem. 1992;61:131–156. doi: 10.1146/annurev.bi.61.070192.001023. [DOI] [PubMed] [Google Scholar]
- Austerberry C. F., Allis C. D., Yao M. C. Specific DNA rearrangements in synchronously developing nuclei of Tetrahymena. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7383–7387. doi: 10.1073/pnas.81.23.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austerberry C. F., Snyder R. O., Yao M. C. Sequence microheterogeneity is generated at junctions of programmed DNA deletions in Tetrahymena thermophila. Nucleic Acids Res. 1989 Sep 25;17(18):7263–7272. doi: 10.1093/nar/17.18.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austerberry C. F., Yao M. C. Nucleotide sequence structure and consistency of a developmentally regulated DNA deletion in Tetrahymena thermophila. Mol Cell Biol. 1987 Jan;7(1):435–443. doi: 10.1128/mcb.7.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austerberry C. F., Yao M. C. Sequence structures of two developmentally regulated, alternative DNA deletion junctions in Tetrahymena thermophila. Mol Cell Biol. 1988 Sep;8(9):3947–3950. doi: 10.1128/mcb.8.9.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns P. J., Brussard T. B. Pair formation in tetrahymena pyriformis, an inducible developmental system. J Exp Zool. 1974 Jun;188(3):337–344. doi: 10.1002/jez.1401880309. [DOI] [PubMed] [Google Scholar]
- Craig N. L. The mechanism of conservative site-specific recombination. Annu Rev Genet. 1988;22:77–105. doi: 10.1146/annurev.ge.22.120188.000453. [DOI] [PubMed] [Google Scholar]
- Godiska R., James C., Yao M. C. A distant 10-bp sequence specifies the boundaries of a programmed DNA deletion in Tetrahymena. Genes Dev. 1993 Dec;7(12A):2357–2365. doi: 10.1101/gad.7.12a.2357. [DOI] [PubMed] [Google Scholar]
- Godiska R., Yao M. C. A programmed site-specific DNA rearrangement in Tetrahymena thermophila requires flanking polypurine tracts. Cell. 1990 Jun 29;61(7):1237–1246. doi: 10.1016/0092-8674(90)90688-b. [DOI] [PubMed] [Google Scholar]
- Jahn C. L., Krikau M. F., Shyman S. Developmentally coordinated en masse excision of a highly repetitive element in E. crassus. Cell. 1989 Dec 22;59(6):1009–1018. doi: 10.1016/0092-8674(89)90757-5. [DOI] [PubMed] [Google Scholar]
- Jaraczewski J. W., Jahn C. L. Elimination of Tec elements involves a novel excision process. Genes Dev. 1993 Jan;7(1):95–105. doi: 10.1101/gad.7.1.95. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Turner L. R., LaPlante J. Circular forms of developmentally excised DNA in Euplotes crassus have a heteroduplex junction. Genes Dev. 1993 Jan;7(1):84–94. doi: 10.1101/gad.7.1.84. [DOI] [PubMed] [Google Scholar]
- Krikau M. F., Jahn C. L. Tec2, a second transposon-like element demonstrating developmentally programmed excision in Euplotes crassus. Mol Cell Biol. 1991 Sep;11(9):4751–4759. doi: 10.1128/mcb.11.9.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Tausta S. L., Klobutcher L. A. Detection of circular forms of eliminated DNA during macronuclear development in E. crassus. Cell. 1989 Dec 22;59(6):1019–1026. doi: 10.1016/0092-8674(89)90758-7. [DOI] [PubMed] [Google Scholar]
- Williams K., Doak T. G., Herrick G. Developmental precise excision of Oxytricha trifallax telomere-bearing elements and formation of circles closed by a copy of the flanking target duplication. EMBO J. 1993 Dec;12(12):4593–4601. doi: 10.1002/j.1460-2075.1993.tb06148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H. Detection of circular excised DNA deletion elements in Tetrahymena thermophila during development. Nucleic Acids Res. 1994 Dec 25;22(25):5702–5708. doi: 10.1093/nar/22.25.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]