Abstract
Objective
The association between clinical symptoms and laboratory visceral sensitivity remains poorly defined and controversial. It has even been suggested that laboratory observations of visceral sensitivity are irrelevant to the clinical presentation of chronic visceral pain. To better understand this association, gastrointestinal and psychological features of pediatric patients’ clinical presentation were examined in relation to a laboratory-based measure of visceral sensitivity.
Patients and Methods
At the time of their medical evaluation, 101 patients with medically unexplained abdominal pain (ages 8–15 years) completed validated questionnaires assessing recent depressive symptoms, functional disability, pain efficacy beliefs, gastrointestinal (GI), and non-GI symptoms. These clinical features were examined in relation to visceral sensitivity assessed 2 months later in the laboratory. The measure of visceral sensitivity was based on increases in GI complaints in response to the water load symptom provocation task.
Results
More severe GI symptoms and functional disability in the weeks before patients’ clinical evaluation were associated with significantly greater increases in GI symptoms in the laboratory in response to the water load symptom provocation task (all P < 0.04). Patients believing that they had the ability to alleviate their pain (high problem-focused pain efficacy) had significantly lower laboratory visceral sensitivity (P < 0.01). Clinical depressive symptoms and non-GI symptoms were not associated with laboratory visceral sensitivity.
Conclusions
Clinical presentations of more severe GI symptoms and disability as well as low perceived pain efficacy are significant predictors of laboratory visceral sensitivity in children with functional abdominal pain. Depression does not account for the association between clinical presentation of GI symptoms and a laboratory measure of visceral sensitivity.
Keywords: Functional abdominal pain, Functional gastrointestinal disorders, Functional disability, Pain efficacy, Visceral hypersensitivity
Considerable research focuses on understanding the role of visceral sensitivity in medically unexplained functional abdominal pain (FAP) (1–7). Experimental paradigms such as barostat with intragastric balloon and the water load test are being used to assess gastric sensation and to identify pain sensitive phenotypes (4,7–11). These experimental models also are frequently used to make inferences about pathophysiology and potential treatment strategies for patients with functional gastrointestinal disorders (FGIDs). Despite the important implications, the association between experimental models of visceral sensitivity and symptoms in FGIDs is poorly defined. Indeed, it has even been suggested that laboratory observations of visceral sensitivity may be irrelevant to the clinical presentation and pathophysiology of chronic visceral pain in FGIDs (2,12,13).
The primary aim of this study was to evaluate whether the presence of laboratory visceral sensitivity was associated with clinical gastrointestinal (GI) symptom severity and psychological variables in pediatric patients with functional abdominal pain. We used the American Academy of Pediatrics definition of FAP defined as abdominal pain that occurs in the absence of anatomic abnormality, inflammation, or tissue damage (14). Current theory (15–18) and research (5,11) highlight the importance of perturbations in both the gut and central nervous system in visceral sensitivity. Although there are limited data on the role of psychological variables in pediatric visceral sensitivity, empirical evidence suggests that these variables may affect the severity and course of chronic abdominal pain (19). For example, stress, depression, and dysfunctional coping strategies have been associated with maintenance of abdominal complaints and disability (19,20). Thus, both GI and psychological features of children’s clinical presentation could be associated with laboratory visceral hypersensitivity.
In the present study, we examined the relation of laboratory visceral sensitivity to several clinical features of pediatric FAP, including severity of GI and non-GI symptoms, functional disability, depression, and perceived pain efficacy. The measure of visceral sensitivity was based on the increases in GI complaints in response to the water load symptom provocation task (WL-SPT) (7). We hypothesized that greater severity of clinical GI symptoms would predict higher visceral sensitivity. In addition, we hypothesized that higher clinical severity of non-GI symptoms, functional disability, and depression would predict greater increases in laboratory GI symptoms in response to the WL-SPT, and that beliefs of greater pain coping efficacy would predict lesser increases in laboratory GI symptoms in response to the WL-SPT. To assess whether patients’ clinical characteristics differentially predicted GI symptoms in the laboratory, we also assessed non-GI symptoms and negative emotions induced by the WL-SPT.
PATIENTS AND METHODS
Participants were consecutive new pediatric patients referred to the Pediatric Gastroenterology Clinic at Vanderbilt University Medical Center by their primary care provider for evaluation of abdominal pain. Parents of patients were identified by clinic staff and contacted by telephone several days before their pediatric gastroenterology appointment. Those who expressed interest were screened for eligibility and asked to arrive early for their appointment if they wished to participate in the clinic study. Patients were eligible for participation if they by parental report had 3 or more episodes of abdominal pain that interfered with activities during the previous 3 months, had no positive diagnosis for abdominal pain by the referring provider, had no chronic illness or disability by parent report, were living with a parent, and spoke English. The final sample included 101 patients.
Clinic Protocol
Approval was obtained from the Vanderbilt University Institutional Review Board before conducting this study. Before examination by the gastroenterologist, informed consent was obtained for participation in the research study by research staff. Each patient met with a trained interviewer in a private clinic room. Validated questionnaires were administered to assess GI symptoms, non-GI symptoms, depressive symptoms, and functional disability during the previous 2 weeks. Patients also completed a questionnaire assessing beliefs about their ability to cope with abdominal pain. The interviewer read each questionnaire item and allowed the child to select answers from a printed response sheet to ensure understanding and to standardize the procedure across participants.
Following completion of the research questionnaires, each participant was evaluated by the attending pediatric gastroenterologist. The medical evaluation included past medical history, review of records from the referring primary care provider, family and social history, review of systems, and complete physical examination. Specific laboratory tests and procedures were conducted as indicated, at the discretion of the attending physician. Physicians did not have access to the participants’ research responses. Following completion of the medical examination, patient charts were reviewed for clinical or laboratory evidence of organic disease. Patients whose evaluation lacked evidence of organic disease to explain the abdominal pain were invited to return to the medical center to participate in the laboratory study that is the focus of this article.
Clinic Measurements
Children’s Somatization Inventory
The Children’s Somatization Inventory is a validated self-report instrument to assess children’s nonspecific somatic symptoms (21). It contains 35 questions with the common structure, “How much were you bothered by (symptom)?” The response format is a 5-point scale ranging from “not at all” (0) to “a whole lot” (4). The standard time period for symptom report on the Children’s Somatization Inventory is 2 weeks to decrease the impact of brief minor illnesses on the scores. For the present study, GI and non-GI symptoms were averaged to create indices of GI and non-GI symptoms, respectively. The GI symptoms included items such as chest pain, difficulty swallowing, nausea, vomiting, food making you sick, constipation, diarrhea, stomach pain, and bloating. Non-GI symptoms included pain (eg, back pain, joint pain) and other miscellaneous complaints associated with somatization (eg, dizziness, heart racing, decreased energy). Alpha reliability of the GI and non-GI symptom index was .73 and .72, respectively.
Children’s Depression Inventory
Depressive symptoms were measured with the Children’s Depression Inventory, a validated child self-report instrument designed for children ages 7 to 17 years (22). Children rated the extent to which they experienced depressive symptoms during the previous 2 weeks using a 3-point scale. In the present sample, the inventory had an alpha reliability of .85.
Functional Disability Inventory
The Functional Disability Inventory (23,24) was used to assess children’s self-reported difficulty in physical and psychosocial functioning for the past 2 weeks because of physical health. (As an example, “In the last 2 weeks, I have had trouble doing things with friends because of my health.”) The validated self-report measure consisted of 15 items rated on a 5-point scale. Responses were summed to create a total score. Alpha reliability was .86.
Pain Beliefs Questionnaire
The Pain Beliefs Questionnaire (25) consists of 60 self-report items rated on a 5-point scale to assess children’s self-reported pain beliefs regarding the severity of their pain and their ability to cope with abdominal pain. It includes subscales to assess 2 types of perceived efficacy for coping with pain. Problem-focused pain efficacy refers to the belief that one can reduce or eliminate pain (eg, “When I have a bad stomachache I can feel better if I decide to”). Emotion-focused pain efficacy refers to the perceived ability to accommodate to pain regardless of severity (eg, “I can handle it no matter how bad my stomach hurts”). Alpha reliability was .65 for problem-focused pain efficacy and .63 for emotion-focused pain efficacy.
Laboratory Protocol
Around 1 to 2 months after evaluation in the gastroenterology clinic, eligible subjects returned to participate in the laboratory study. Children in the study completed the Laboratory Symptom and Emotion Report (7), which served as baseline assessment of current GI symptoms, non-GI symptoms, and negative affect immediately preceding the WL-SPT. Following the baseline assessment, the WL-SPT, a validated noninvasive laboratory analog task for inducing visceral discomfort, was administered (7). For the test, a water bag was filled with room temperature bottled water with 30 in. of plastic tubing (5/16 in. diameter) connected to the bag. The bag hung inside a large canvas backpack on the wall next to the participant with only the tube and the valve exposed to avoid visual monitoring of the water level as they drank. Participants were instructed to drink until “completely full” and were allowed to drink for a maximum of 15 minutes or until “completely full.” Immediately after completion of water ingestion, participants completed the posttest Laboratory Symptom and Emotion Report assessing GI symptoms, non-GI symptoms, and negative affect induced by the WL-SPT.
Laboratory Measurements
Indices of Laboratory GI and Non-GI Symptoms
A symptom checklist, the Laboratory Symptom and Emotion Report (7), was used to assess somatic symptoms experienced in the laboratory before and after the WL-SPT. Participants were asked to rate how much they currently felt each of 4 GI symptoms (stomachache, nausea/upset stomach, feel like throwing up, and sick). They were also asked to rate their current experience of 7 non-GI symptoms (dizzy, weak, tired, headache, heart beating fast, backache, and feel bad all over) on a 5-point numerical rating scale with responses ranging from “none” (coded “0”) to “a whole lot” (coded “4”). The GI and non-GI symptom ratings were summed and averaged to create the Laboratory GI Symptom Index and the Laboratory Non-GI Symptom Index. Alpha reliability was .73 for the Laboratory GI Symptom Index and .76 for the Laboratory Non-GI Symptom Index.
Laboratory Negative Affect Index
Negative affect was assessed before and after the WL-SPT. Children were asked to rate how much they currently felt scared, annoyed, nervous/worried, or upset on a 5-point numerical rating scale ranging from “none” coded as “0” to “a whole lot” coded as “4.” Scores were summed and averaged to create the Laboratory Negative Affect Index, which had an alpha reliability of .72.
Data Analysis
Means were calculated for each of the variables analyzed. Reliability for each of the clinic and laboratory measures was assessed using the Cronbach alpha. To examine demographic characteristics of the study population, frequency distributions were computed. Independent-samples t-test was used to compare mean scores between participants and those who did not participate in the study. Hierarchical multiple linear regression analysis was used to identify the association between clinical symptoms (GI and non-GI complaints, depressive symptoms, functional disability, and perceived coping efficacy) and laboratory symptoms (GI, non-GI, and negative affect). Both standardized (β) and unstandardized (B) coefficients were calculated. Significance was expressed at the P < 0.05 level. The sample effect size (ES) was evaluated using the multiple partial correlation test (26). Statistical analysis was performed using the Statistical Package for Social Sciences version 11.5 for Windows (SPSS, Chicago, IL).
RESULTS
Patient Characteristics
Of the 240 patients who participated in the initial clinical assessment, 36 were excluded due to positive evidence of organic disease found on medical evaluation. Of the remaining 204 patients who met eligibility criteria, 101 (49.5%) returned for the laboratory assessment. Of the children who did not participate, 4 patients were excluded due to incomplete medical evaluations during the period of recruitment, 11 families were unable to keep their scheduled appointments, and 73 families declined, primarily due to the distance from the medical center. Approximately 80% of participants lived outside of the county. Compared with those who did not participate, patients who participated did not differ with respect to duration of abdominal pain, age, sex, or clinical variables including GI symptoms, non-GI symptoms, depressive symptoms, functional disability, problem-focused coping, or emotion-focused coping.
Participants ranged in age from 8 to 15 years, and 60% were female. The mean age was 11.3 ± 2.1 years with 51% ages 8 to 11, and 49% ages 12 to 15. Participants were 94% white, 3% African American, 2% Asian, and 1% Hispanic (Table 1). On the Hollingshead 2 Factor Index of social status, the mean was 5.5 ± 1.6 (range 2–9).
TABLE 1.
Patient demographics
| Age, mean ± SD | 11.4 ± 2.1 |
| Ages 8–11, n (%) | 52 (51) |
| Ages 12–15, n (%) | 49 (49) |
| Sex, n (%) | |
| Female | 61 (60) |
| Male | 41 (40) |
| Race, n (%) | |
| White | 95 (94) |
| African American | 3 (3) |
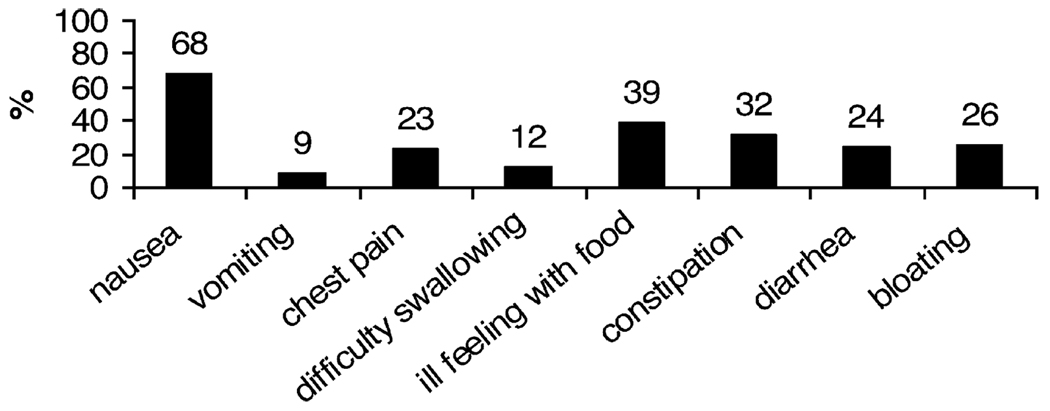
The frequency of pain during the 2 weeks before the medical evaluation was daily in 93% of participants. On a 10-point scale with 10 being the “most pain possible,” 73% of participants rated their pain intensity as 5 or greater (mean 5.6 ± 2.4). In addition to abdominal pain, 68% of patients reported nausea at the time of initial evaluation (Fig. 1).
FIG. 1.
Proportion of patients reporting selected gastrointestinal symptoms at baseline clinical evaluation.
Clinical Predictors of Laboratory GI Symptoms, Non-GI Symptoms, and Negative Affect
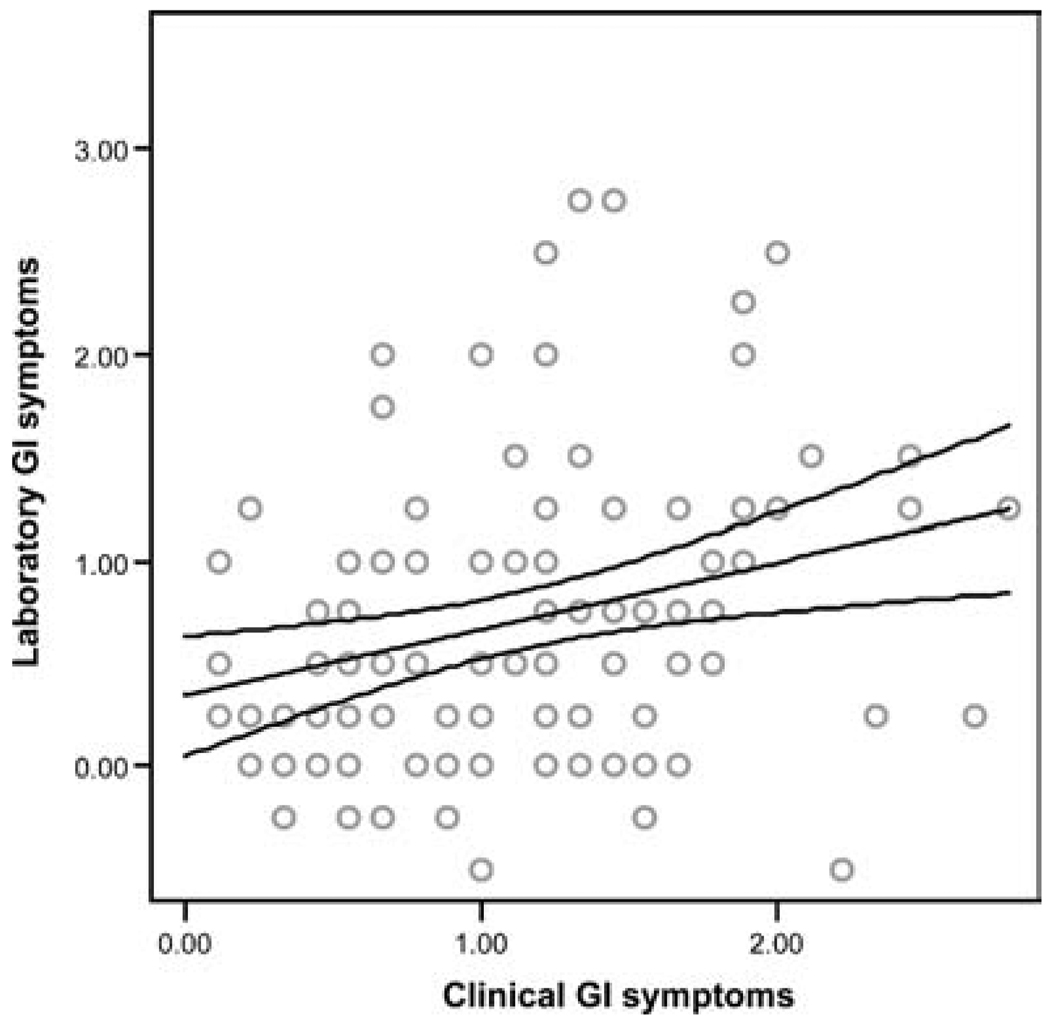
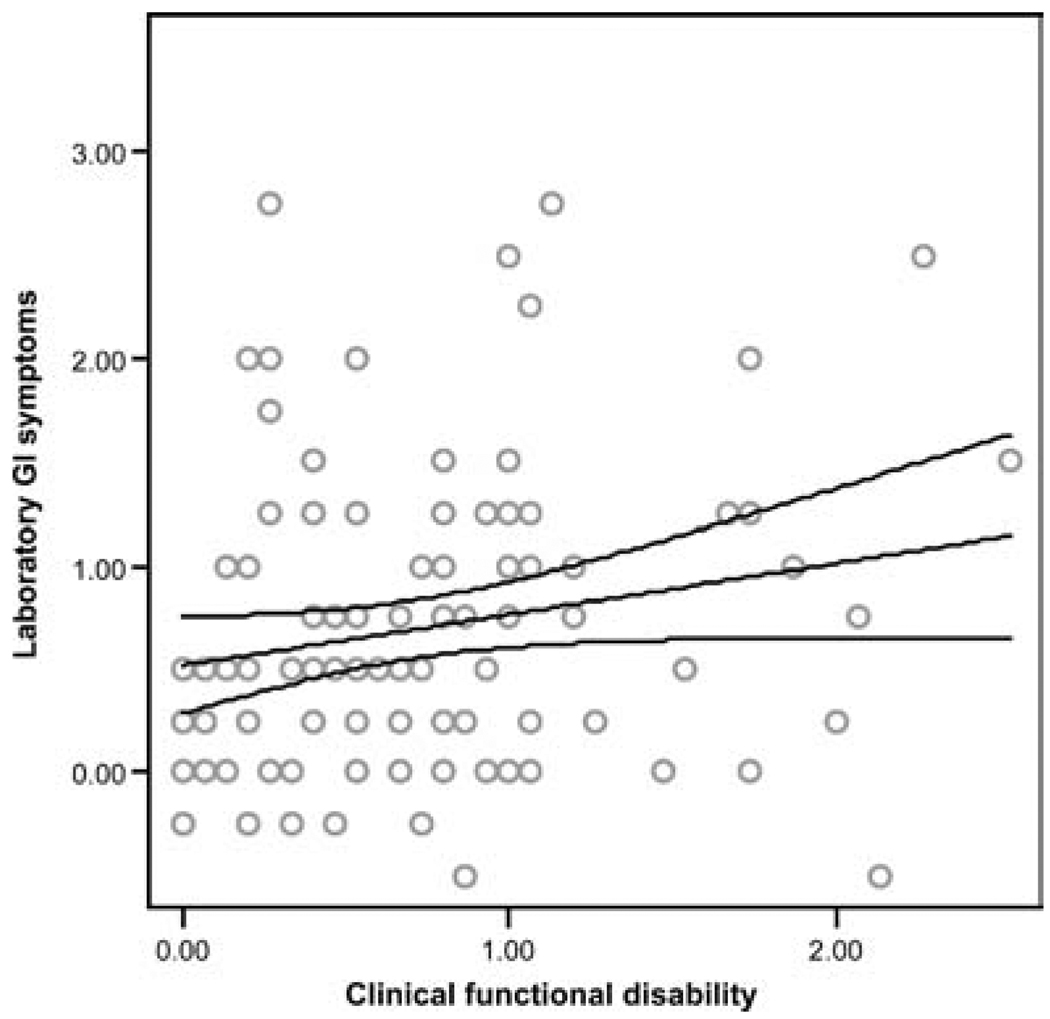
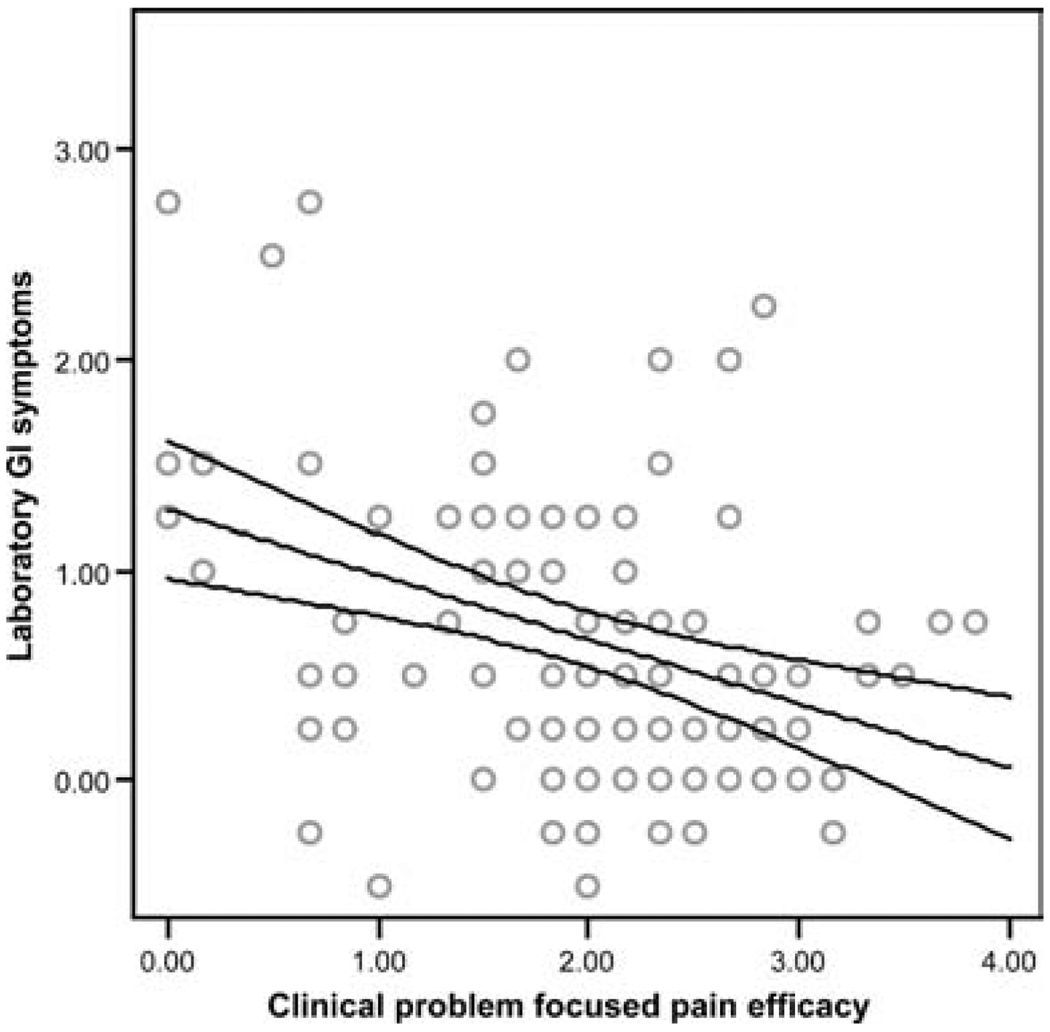
Analysis was performed using hierarchical multiple regression, controlling for sex and laboratory baseline symptoms before the WL-SPT. Patients with higher levels of clinical GI symptoms consistently demonstrated greater laboratory GI symptoms in response to the WL-SPT (β = .23, P < 0.01, Fig. 2). In addition, children with higher levels of clinical functional disability showed significantly higher levels of laboratory GI symptoms in response to the WL-SPT (β = .18, P = 0.04, Fig. 3). In contrast, patients believing that they had the ability to reduce their pain (ie, high problem-focused coping efficacy) reported significantly smaller increases in laboratory GI symptoms in response to the WL-SPT (β = −.29, P < 0.01, Fig. 4). Clinical non-GI symptoms, clinical depressive symptoms, and clinical emotion-focused pain efficacy were not significant predictors of laboratory GI symptoms.
FIG. 2.
Regression line with 95% confidence intervals showing the relation between clinical gastrointestinal (GI) symptoms and increase in laboratory GI symptoms following the water load symptom provocation task (R2 = .33, β = .23, P = 0.01).
FIG. 3.
Regression line with 95% confidence intervals showing the relation between clinical functional disability and increase in laboratory gastrointestinal (GI) symptoms following the water load symptom provocation task (R2 = .31, β = .18, P = 0.04).
FIG. 4.
Regression line with 95% confidence intervals showing the relation between clinical laboratory problem-focused pain efficacy and decrease in laboratory gastrointestinal (GI) symptoms following the water load symptom provocation task (R2 = .40, β = −.29, P < 0.01).
We also examined the relation of clinical variables to laboratory non-GI symptoms and negative affect induced by the WL-SPT. None of the clinical variables significantly predicted changes in non-GI symptoms or negative affect in response to the WL-SPT.
Finally, we tested the possibility that clinical depressive symptoms may mediate the observed association between clinical GI symptoms and laboratory visceral sensitivity. Using the approach to mediation analysis recommended by Baron and Kenny (27), there was no evidence that depressive symptoms accounted for the significant association between clinical GI symptoms and laboratory visceral sensitivity.
Clinical Effect Sizes
The sample ES was judged as small, medium, or large by Cohen criteria (26). For this test, the H0 was d = 0 and the small, medium, and large ES were d = .02, .15, and .35, respectively (26). Three clinical variables had effects of large magnitude on laboratory GI symptoms induced by the WL-SPT. These significant clinical predictor variables included GI symptoms (ES = .497), functional disability (ES = .454), and problem-focused pain efficacy (ES = .675).
DISCUSSION
Experimental models of visceral hypersensitivity often are used to make inferences about the pathophysiology and potential treatment for patients with FGIDs. It is important to identify which features of patients’ clinical presentation, if any, are associated with laboratory visceral hypersensitivity because these may be promising targets for treatment. Consistent with the view of FGIDs as disorders of brain–gut interaction, we examined both gastrointestinal and psychological features of patients’ clinical presentation in relation to a laboratory-based measure of visceral sensitivity. We operationalized visceral sensitivity as the increase in GI complaints in response to the WL-SPT (6). As hypothesized, more severe clinical GI symptoms (eg, nausea, food intolerance) in the weeks before the patients’ clinical evaluation were associated with significantly greater visceral sensitivity in the laboratory.
Similar to our findings, adult studies also have reported an association between clinical severity of GI symptoms and laboratory visceral hypersensitivity using both the barostat (1,28) and water load test (9) as experimental paradigms. The lack of association between clinical non-GI symptoms and laboratory symptoms suggests that the utility of the WL-SPT as a symptom provocation paradigm is specific to GI symptoms.
In addition to demonstrating the association between clinical GI symptoms and visceral sensitivity, we also found that the degree of patients’ functional disability, assessed by questionnaire in the clinic, significantly predicted the level of visceral sensitivity exhibited in the laboratory. Similar findings were reported by Jones et al. (9), who found that the physical and social functioning subscales of the SF-36 were significantly associated with visceral hypersensitivity in a laboratory study using the water load test.
We extended the literature by investigating the relation of a cognitive variable (perceived coping ability) to laboratory visceral hypersensitivity. Folkman et al’s research (29) on stress appraisal and coping describes 2 major types of pain coping beliefs. Problem-focused pain efficacy refers to the belief that one has the ability to alleviate or eliminate one’s pain. Emotion-focused pain efficacy refers to the belief that one can accept or adjust to pain, even if it is not relieved (29). In our study, patients believing they could alter circumstances to modify their pain (high problem-focused pain efficacy) demonstrated significantly less laboratory visceral sensitivity than those believing they could do little to control their abdominal discomfort. This finding suggests that beliefs about one’s coping efficacy can influence the response to a stressor such as physical discomfort or pain (29).
To our knowledge, this is the first pediatric study to examine the relation of perceived coping efficacy to laboratory visceral hypersensitivity. Salomons et al (30) showed that perceived controllability modulates the neural response to experimental pain. Examination of the potential clinical implications of perceived pain efficacy in patients with FAP is an important area for further study. For example, it is possible that the mechanism accounting for the benefits of hypnosis (31) and cognitive behavioral therapy (32) in the treatment of FGIDs could be the increased perception of control that patients experience with these treatments in comparison to treatments that simply provide reassurance regarding the absence of significant organic disease.
Children with FGIDs often have concurrent depressive symptoms (33,34) and somatic complaints such as headache, decreased energy, or pain in non-GI locations (35). It is known that negative affect, such as depression, can induce response bias that in some cases may account for associations between self-reports of various health-related and psychological symptoms (36). The importance of response bias is highlighted by a recent study by Dorn et al (5), in which differences in laboratory visceral pain thresholds between irritable bowel syndrome patients and controls were explained primarily by an increased tendency to report pain rather than increased neural sensitivity. In our study, patients’ depressive symptoms and somatic complaints failed to predict visceral sensitivity in the laboratory, suggesting that visceral sensitivity was not simply a reflection of depressive symptoms or somatization. Moreover, the pattern of correlations in our study indicated that depressive symptoms did not mediate the observed relation between clinical presentation and laboratory visceral sensitivity. Thus, our findings are unlikely to be because of reporting bias. The relation between clinical presentation and visceral sensitivity may reflect differences in nociceptive processing or central processing such as hypervigilance to gastrointestinal sensations that may be exacerbated in patients with low perceived pain efficacy (19).
Our results are congruent with other studies that have been unable to demonstrate a strong correlation between depression scores and thresholds for visceral pain in patients with irritable bowel syndrome or other FGIDs (1,4,8,37). For example, Posserud et al (1) recently demonstrated that altered rectal perception in irritable bowel syndrome patients is not merely a reflection of patient anxiety or depression. Similarly, we have shown that visceral sensitivity in pediatric FAP patients does not reflect patient depression or general somatic distress.
A potential limitation of this study was the inability to stratify our data analysis based on the Rome criteria. The ethical considerations associated with a pediatric sample prevented us from using more invasive procedures to evaluate the physiological component of visceral sensitivity. An additional limitation was the inability to control for treatment offered after the initial clinical evaluation. Although pediatric GI providers often use a combination of counseling and education after diagnosing an FGID, it is possible that variations existed among providers.
In summary, our study demonstrates that clinical GI symptoms, disability, and pain coping efficacy are useful predictors of laboratory-induced visceral sensitivity in children with FAP. Importantly, depressive symptoms did not account for the association between clinical presentation and laboratory visceral sensitivity. Future research should investigate the relation of perceived pain efficacy to visceral and central pain processes.
Acknowledgments
Supported, in part, by National Institute on Child Health and Development grant HD23264 (L.S.W.) and core grant HD15052 to the John F. Kennedy Center at Vanderbilt University, by Vanderbilt Digestive Disease Research Center grant DK058404, and by National Center for Research Resources of National Institutes of Health grant M01 RR-00095 to Vanderbilt University Medical Center.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Posserud I, Syrous A, Linstrom L, et al. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133:1113–1123. doi: 10.1053/j.gastro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Aziz A. Visceral hypersensitivity: fact or fiction. Gastroenterology. 2006;131:661–670. doi: 10.1053/j.gastro.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 3.Delvaux M. Alterations of sensori-motor functions of the digestive tract in the pathophysiology of irritable bowel syndrome. Best Pract Res Clin Gastroenterol. 2004;18:747–771. doi: 10.1016/j.bpg.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Di Lorenzo C, Youssef N, Sigurdsson L, et al. Visceral hyperalgesia in children with functional abdominal pain. J Pediatr. 2001;139:838–843. doi: 10.1067/mpd.2001.118883. [DOI] [PubMed] [Google Scholar]
- 5.Dorn S, Palsson O, Syed T, et al. Increased colonic pain sensitivity in irritable bowel syndrome is the result of increased tendency to report pain rather than increased neurosensory sensitivity. Gut. 2007;56:1202–1206. doi: 10.1136/gut.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mertz H, Fullerton S, Naliboff B, et al. Symptoms and visceral perception in severe functional and organic dyspepsia. Gut. 1998;42:814–822. doi: 10.1136/gut.42.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker L, Williams S, Smith C, et al. Validation of a symptom provocation test for laboratory studies of abdominal pain and discomfort in children and adolescents. J Pediatr Psychol. 2006;31:703–713. doi: 10.1093/jpepsy/jsj062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones M, Roth L, Crowell M. Symptom reporting by functional dyspeptics during the water load test. Am J Gastroenterol. 2005;100:1334–1339. doi: 10.1111/j.1572-0241.2005.40802.x. [DOI] [PubMed] [Google Scholar]
- 9.Jones M, Hoffman S, Shah D, et al. The water load test: observations from healthy controls and patients with functional dyspepsia. Am J Physiol Gastrointest Liver Physiol. 2003;284:G896–G904. doi: 10.1152/ajpgi.00361.2002. [DOI] [PubMed] [Google Scholar]
- 10.Mayer E. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 11.Coffin B, Azpiroz F, Guarner F, et al. Selective gastric hypersensitivity and reflex hyporeactivity in functional dyspepsia. Gastroenterology. 1994;107:1345–1351. doi: 10.1016/0016-5085(94)90536-3. [DOI] [PubMed] [Google Scholar]
- 12.Boeckxstaens G, Hirsch D, Kuiken S. The proximal stomach and postprandial symptoms in functional dyspeptics. Am J Gastroenterol. 2002;97:40–48. doi: 10.1111/j.1572-0241.2002.05421.x. [DOI] [PubMed] [Google Scholar]
- 13.Rhee P, Kim Y, Son H, et al. Evaluation of individual symptoms cannot predict presence of gastric hypersensitivity in functional dyspepsia. Dig Dis Sci. 2000;45:1680–1684. doi: 10.1023/a:1005550019308. [DOI] [PubMed] [Google Scholar]
- 14.American Academy of Pediatrics. Chronic abdominal pain in children. Technical Report. Pediatrics. 2005;115:e370–e381. doi: 10.1542/peds.2004-2523. [DOI] [PubMed] [Google Scholar]
- 15.Delvaux M. Role of visceral sensitivity in the pathophysiology of irritable bowel syndrome. Gut. 2002;51:i67–i71. doi: 10.1136/gut.51.suppl_1.i67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer E, Collins S. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122:2032–2048. doi: 10.1053/gast.2002.33584. [DOI] [PubMed] [Google Scholar]
- 17.Moshiree B, Zhou Q, Price D, et al. Central sensitization in visceral pain disorders. Gut. 2006;55:905–908. doi: 10.1136/gut.2005.078287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price D, Zhou Q, Moshiree B, et al. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain. 2006;7:529–535. doi: 10.1016/j.jpain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Walker L, Garber J, Greene J. Somatic complaints in pediatric patients: a prospective study of the role of negative life events, child social and academic competence, and parental somatic symptoms. J Consult Clin Psychol. 1994;62:1213–1221. doi: 10.1037//0022-006x.62.6.1213. [DOI] [PubMed] [Google Scholar]
- 20.Whitehead W, Palsson O, Jones K. Systematic review of the co-morbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 21.Walker L, Greene J. Children with recurrent abdominal pain and their parents: more somatic complaints, anxiety, and depression than other patient families? J Pediatr Psychol. 1989;14:231–243. doi: 10.1093/jpepsy/14.2.231. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatr. 1981;46:305–315. [PubMed] [Google Scholar]
- 23.Claar R, Walker L. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker L, Greene J. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 25.Walker L, Smith C, Garber J, et al. Testing a model of pain appraisal and coping in children with chronic abdominal pain. Health Psychol. 2005;24:364–374. doi: 10.1037/0278-6133.24.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 27.Baron R, Kenny D. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 28.Tack J, Caenepeel B, Fischler B, et al. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121:526–535. doi: 10.1053/gast.2001.27180. [DOI] [PubMed] [Google Scholar]
- 29.Folkman S, Lazarus R, Dunkel-Schetter C, et al. Dynamics of a stressful encounter: cognitive appraisal, coping, and encounter outcomes. J Pers Soc Psychol. 1986;50:992–1003. doi: 10.1037//0022-3514.50.5.992. [DOI] [PubMed] [Google Scholar]
- 30.Salomons T, Johnstone T, Backonja M. Perceived controllability modulates the neural response to pain. J Neurosci. 2004;24:7199–7203. doi: 10.1523/JNEUROSCI.1315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitehead W. Hypnosis for irritable bowel syndrome: the empirical evidence of therapeutic effects. Int J Clin Exp Hypn. 2006;54:7–20. doi: 10.1080/00207140500328708. [DOI] [PubMed] [Google Scholar]
- 32.Youssef N, Rosh J, Loughram M, et al. Treatment of functional abdominal pain in childhood with cognitive behavioral strategies. J Pediatr Gastroenterol Nutr. 2004;39:192–196. doi: 10.1097/00005176-200408000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Campo J, Bridge J, Ehmann M, et al. Recurrent abdominal pain, anxiety, and depression in primary care. Pediatrics. 2004;113:817–824. doi: 10.1542/peds.113.4.817. [DOI] [PubMed] [Google Scholar]
- 34.Hyams J, Burke G, Davis P, et al. Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. J Pediatr. 1996;129:220–226. doi: 10.1016/s0022-3476(96)70246-9. [DOI] [PubMed] [Google Scholar]
- 35.Little C, Williams S, Puzanovova M, et al. Multiple somatic symptoms linked to positive screen for depression in pediatric patients with chronic abdominal pain. J Pediatr Gastroenterol Nutr. 2007;44:58–62. doi: 10.1097/01.mpg.0000243423.93968.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa P, McCrae R. Neuroticism, somatic complaints, and disease: is the bark worse than the bite? J Pers. 1987;55:299–316. doi: 10.1111/j.1467-6494.1987.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 37.Faure C, Wieckowska A. Somatic referral of visceral sensations and rectal sensory threshold for pain in children with functional gastrointestinal disorders. J Pediatr. 2007;150:66–71. doi: 10.1016/j.jpeds.2006.08.072. [DOI] [PubMed] [Google Scholar]