ABSTRACT
Mucosal immunity to the enteric pathogen Shigella flexneri is mediated by secretory IgA (S-IgA) antibodies directed against the O-antigen (O-Ag) side chain of lipopolysaccharide. While secretory antibodies against the O-Ag are known to prevent bacterial invasion of the intestinal epithelium, the mechanisms by which this occurs are not fully understood. In this study, we report that the binding of a murine monoclonal IgA (IgAC5) to the O-Ag of S. flexneri serotype 5a suppresses activity of the type 3 secretion (T3S) system, which is necessary for S. flexneri to gain entry into intestinal epithelial cells. IgAC5’s effects on the T3S were rapid (5 to 15 min) and were coincident with a partial reduction in the bacterial membrane potential and a decrease in intracellular ATP levels. Activity of the T3S system returned to normal levels 45 to 90 min following antibody treatment, demonstrating that IgAC5’s effects were transient. Nonetheless, these data suggest a model in which the association of IgA with the O-Ag of S. flexneri partially de-energizes the T3S system and temporarily renders the bacterium incapable of invading intestinal epithelial cells.
IMPORTANCE
Secretory IgA (S-IgA) serves as the first line of defense against enteric infections. However, despite its well-recognized role in mucosal immunity, relatively little is known at the molecular level about how this class of antibody functions to prevent pathogenic bacteria from penetrating the epithelial barrier. It is generally assumed that S-IgA functions primarily by “immune exclusion,” a phenomenon in which the antibody binds to microbial surface antigens and thereby promotes bacterial agglutination, entrapment in mucus, and physical clearance from the gastrointestinal tract via peristalsis. The results of the present study suggest that in addition to serving as a physical barrier, S-IgA may have a direct impact on the ability of microbial pathogens to secrete virulence factors required for invasion of intestinal epithelial cells.
Introduction
Shigella flexneri is the causative agent of bacillary dysentery, an invasive disease of the colonic mucosa that afflicts more than a million children each year (1). Like Salmonella and other related Gram-negative bacterial pathogens, S. flexneri penetrates intestinal epithelial cells by virtue of a type 3 secretion (T3S) system, a macromolecular protein transport apparatus that is structurally and genetically related to bacterial flagella (2). Upon contact with epithelial cells, the T3S apparatus injects effector proteins, also known as the invasion plasmid antigens (e.g., IpaB, IpaC, and IpaD), into host cells, resulting in cytoskeletal rearrangements and bacterial macropinocytosis. Assembly and function of the T3S system are regulated in response to physical and environmental signals that the bacterium encounters in the gastrointestinal tract (3, 4).
In humans, protective immunity to S. flexneri is strongly correlated with secretory IgA (S-IgA) antibodies directed against the serotype-specific O-antigen (O-Ag) side chains of lipopolysaccharide (1, 5). In experimental models of shigellosis, it has been shown that passive mucosal administration of a murine monoclonal dimeric/polymeric IgA (MAb) known as IgAC5, directed against the O-Ag of S. flexneri serotype 5a, is sufficient to protect otherwise naive animals against infection (6, 7). Protection occurs at mucosal surfaces, as IgAC5-coated bacteria are demonstrably less invasive than their uncoated counterparts (7). Because IgAC5 is neither bactericidal nor bacteriostatic, IgAC5’s protective effects have been attributed in part to the ability of the antibody to promote bacterial entrapment in mucus, a phenomenon known as “immune exclusion” (7, 8). However, whether IgAC5 has additional effects on S. flexneri that contribute to protection of intestinal epithelial cells has not been determined.
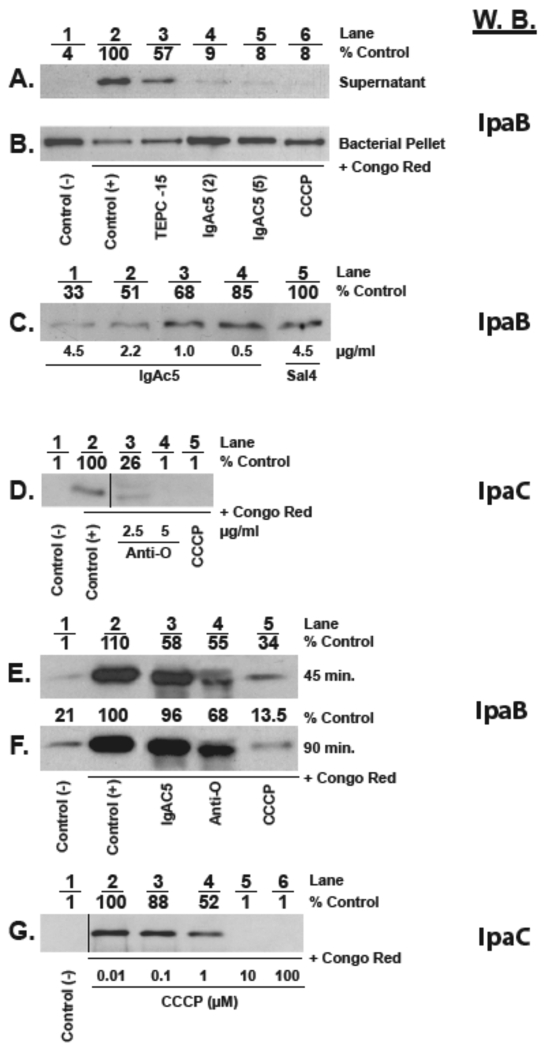
Recent work from our laboratory has suggested that exposure of a related bacterium, Salmonella enterica serovar Typhimurium, to an O-Ag-specific IgA under nonagglutinating conditions is sufficient to prevent bacterial invasion of intestinal epithelial cells, possibly through interference with the T3S system (9, 10). For this reason, we sought to examine the impact of IgAC5 on T3S system-mediated export of Ipa proteins by S. flexneri. Strain M90T (1 × 109 CFU per ml) was treated with IgAC5 (2 to 5 µg/ml) for 15 min prior to being exposed to Congo red (CR), a gratuitous inducer of S. flexneri type 3-mediated Ipa secretion (11). There was a marked reduction (>90%) in IpaB levels in the supernatants of cultures that had been treated with IgAC5 and CR, compared to supernatants from cells treated with CR only (Fig. 1A). Corresponding analysis of total cell lysates revealed that, following IgAC5 treatment, IpaB remained associated with the cell pellet (Fig. 1B). The reduction in IpaB secretion by IgAC5 was roughly equivalent to that achieved by treatment with the proton ionophore carbonyl cyanide m-chlorophenylhydrazone (CCCP; Sigma-Aldrich, St. Louis, MO) (Fig. 1A and B, lane 6). Surprisingly, however, we also noted that IpaB levels were markedly reduced (>45%) upon exposure of S. flexneri to several isotype control MAbs, like TEPC-15 (Fig. 1A and B, lane 3). This result suggests that IgA, irrespective of the variable region (Fv), interferes to some degree with T3S activity. This finding further expands a role for S-IgA in innate immunity at mucosal surfaces and may explain the observation by Wijburg and colleagues that T3S-mediated invasion of intestinal epithelial cells by S. Typhimurium is suppressed ~5-fold by IgA antibodies from naive mice (12, 13).
FIG 1 .
IgAC5 suppresses secretion of Ipa proteins by S. flexneri. A total of 1 × 109 CFU per ml of mid-log-phase cultures of S. flexneri strain M90T was treated with IgAC5, control IgA MAbs (e.g., TEPC-15 and Sal4), O-Ag polyclonal rabbit antisera, or CCCP for the indicated times prior to the addition of Congo red (CR; 6 µg/ml) to trigger T3S system-mediated Ipa transport. Ten minutes later, the bacterial cultures were subjected to centrifugation (5 min at 8,000 relative centrifugal force [RCF]). The resulting supernatants were passed through a 0.2-µm filter to remove any contaminating bacterial cells, concentrated with trichloroacetic acid, solubilized in Laemmli sample buffer containing β-mercaptoethanol (β-ME; 5% [vol/vol]), and then analyzed by SDS-PAGE and Western blotting (W.B.) with murine IgG MAbs specific for IpaB or IpaC (as indicated) and chemiluminescent detection (Bio-Rad, Hercules, CA). The amount of IpaB or IpaC was determined by densitometric analysis of the Western blot films. IpaB and IpaC amounts below the level of detection were set to 1. For panel B only, the bacterial pellets were lysed by the addition of 2× Laemmli sample buffer with β-ME and subjected to SDS-PAGE and Western blotting, as indicated above. (A and B) Supernatants (A) or bacterial pellets (B) from cultures were treated as follows: lane 1, no treatment; lane 2, no treatment followed by CR; lane 3, TEPC-15 (5 µg/ml) for 15 min followed by CR; lane 4, IgAC5 (2 µg/ml) for 15 min followed by CR; lane 5, IgAC5 (5 µg/ml) for 15 min followed by CR; lane 6, CCCP (100 µM) for 15 min followed by CR. The relative amounts of IpaB in the cell supernatants are indicated across the top of the panel as “% control.” In this case, the control was the amount of IpaB associated with CR-treated cells (lane 2). (C) Dose-dependent inhibition of IpaB secretion by IgAC5. Shown are IpaB levels in the supernatants of cultures of S. flexneri M90T treated for 15 min with the indicated concentrations of IgAC5 or the S. Typhimurium-specific IgA MAb Sal4 prior to the addition of CR. Cells treated with SalIV and CR (lane 5) served as the positive control for this experiment, and the amounts of IpaB in the supernatants are expressed as a percentage of this control. (D) IpaC secretion is inhibited by O-Ag polyclonal antibodies (PAbs). Cultures of S. flexneri were treated with 2.5 or 5 µg/ml O-Ag PAbs or CCCP (100 µM) for 15 min prior to the addition of CR. Culture supernatants were prepared as indicated above and blotted with an IpaC-specific MAb. The vertical line between lanes 2 and 3 indicates that a lane(s) present in the original Western blot at this location was removed from the final image. The removed lane(s) represents experimental conditions that were not relevant to the figure. (E to F) IgAC5’s effects on Ipa secretion are transient. Cultures of S. flexneri were treated with IgAC5 (5 µg/ml), O-Ag PAbs (5 µg/ml), or CCCP (100 µM) for 45 min (E) or 90 min (F) prior to the addition of CR. Culture supernatants were then blotted with an IpaB-specific MAb. The elevated levels of IpaB in the negative control samples (lanes 1) in these experiments (compared to those described in panels A to D) reflect the baseline accumulation of the proteins in culture supernatants over the prolonged course of the experiments (45 and 90 min in panels E and F, compared to 15 min for experiments described in panels A to D). (G) Dose-dependent inhibition of Ipa secretion by CCCP. Cultures of S. flexneri were treated with indicated concentrations CCCP for 15 min prior to the addition of CR, after which cell supernatants were blotted for IpaC.
Therefore, to discern the specific contribution of the antibody-variable (Fv) region of IgAC5 on effector secretion by S. flexneri, IpaB levels were subsequently normalized to an isotype control MAb. A dose-response experiment revealed that IgAC5 reduced IpaB secretion by >65%, compared to a control MAb, thereby demonstrating the importance of O-Ag recognition in suppression of T3S activity (Fig. 1C). The contribution of the Fv region was further underscored by the observation that IpaB secretion into culture supernatants following CR treatment was reduced to baseline levels in the presence of rabbit polyclonal O-Ag-specific IgG antiserum (BD Diagnostic Systems, Sparks, MD) (Fig. 1D). Interestingly, the inhibitory effects of both IgAC5 and polyclonal O-Ag antiserum were transient, as secretion of IpaB levels returned to normal 45 min after IgAC5 treatment (Fig. 1E and F, lane 3) and ~90 min after polyclonal O-Ag antiserum exposure (Fig. 1E and F, lane 4). The prolonged blocking of Ipa secretion in the presence of antiserum might be due the diversity of epitopes recognized by the IgG antibody molecules, as opposed to the single epitope recognized by IgAC5.
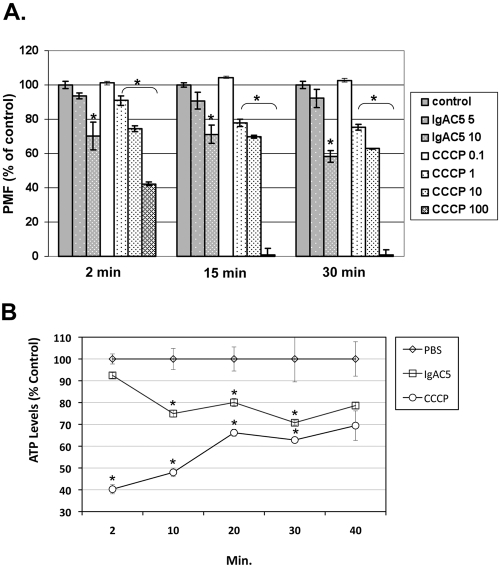
The observation that exposure of S. flexneri to IgAC5 resulted in a loss in T3S activity led us to postulate that antibody binding to the O-Ag may partially de-energize the electropotential of the bacterial membrane. We were particularly interested in the effect of IgAC5 on the proton motive force (PMF) and intracellular ATP levels, because the PMF and/or ATP levels have been implicated in powering the T3S machinery in several enterobacteria (14–17). To first define the relationship between Ipa secretion and PMF in S. flexneri, we subjected bacterial cells to escalating doses of the proton ionophore CCCP and then examined both IpaC secretion and PMF. PMF, or more specifically the membrane potential (ΔΨ) component of PMF, was estimated by using the cationic dye JC-1 (Invitrogen, Carlsbad, CA), essentially as done previously (18, 19). As expected, CCCP treatment resulted in a dose-dependent reduction in CR-mediated IpaC secretion (Fig. 1G). Correspondingly, CCCP treatment resulted in a time- and dose-dependent decrease in membrane potential (Fig. 2A). ATP levels also plummeted following CCCP treatment, although levels recovered with time, probably reflecting S. flexneri’s capacity to regenerate ATP in the absence of an intact PMF (Fig. 2B) (20).
FIG 2 .
Reduction in S. flexneri membrane potential and ATP levels following IgAC5 treatment. (A) A total of 1 × 108 CFU of S. flexneri SJF31 per ml was loaded with the dye JC-1, seeded in 96-well microtiter plates, and then treated with indicated concentrations of IgAC5 (µg/ml) or CCCP (µM). At 2, 15, and 30 min following IgAC5 or CCCP treatment, the relative fluorescence emission signal ratios (530 nm/590 nm) were determined by using a Synergy HT (Bio-Tek, Winooski, VT) fluorescent plate reader with dual excitation (485/20 and 530/25) and dual emission (528/20 and 590/35). The fluorescence emission ratio of control cells was set to 100%. The results are the average values (with standard error) from a representative experiment done in triplicate. The asterisks indicate a significant (P < 0.05) reduction in membrane potential relative to the control samples, as determined by using the Student t test (GraphPad Software, San Diego, CA). SJF31 (ΔtolC::kan), an isogenic derivative of strain M90T, was created using λ red-mediated recombination with pKD4 as the antibiotic resistance marker template (26). It was necessary to use a TolC mutant for these studies to avoid rapid expulsion of JC-1 from the cell cytoplasm (24). (B) A total of 1 × 108 CFU of mid-log-phase S. flexneri strain M90T per ml grown in minimal medium with succinate (0.5%) as the sole carbon source was treated with a phosphate-buffered saline (PBS), IgAC5 (10 µg/ml), or CCCP (100 µM). At the indicated time points, total cellular ATP levels were measured with the BacTiter-Glo (Promega, Madison, WI) luminescence assay. The asterisks indicate significant (P < 0.05) reduction in ATP levels relative to the control samples, as determined by using the Student t test.
Having established a correlation between S. flexneri’s bioenergetic state and Ipa secretion, we next examined the effect of IgAC5 on PMF and ATP levels. We observed that treatment of S. flexneri with IgAC5 was accompanied by a dose- and time-dependent reduction in membrane potential (Fig. 2A). For example, exposure of cells to 10 µg/ml IgAC5 resulted in a reduction in ΔΨ ranging from 25 to 40% and was accompanied by a coincident decrease (20 to 25%) in ATP levels (Fig. 2B), supporting our hypothesis that IgAC5 affects the electropotential of the S. flexneri cell envelope. However, treatment of cells with 5 µg/ml IgAC5 only marginally reduced ΔΨ (5 to 10%), despite the fact that 5 µg/ml IgAC5 was previously shown to be sufficient to suppress Ipa secretion to virtually undetectable levels (Fig. 1). We postulate that this apparent inconsistency is due to the relatively high threshold required to promote JC-1 aggregation inside cells (21) and the exquisite sensitivity of the Ipa T3S system to the physiologic state of the cell (3). On the other hand, we cannot rule out the possibility that IgAC5 not only perturbs membrane potential but also interferes with the T3S system by an additional mechanism related to its ability to physically associate with the O-Ag. Indeed, the observation that 1 µM CCCP reduced bacterial membrane potential by >20% but only reduced IpaC secretion by 50% (compare Fig. 1G and 2A) reveals that bioenergetics alone may not explain IgAC5’s full impact on T3S.
Although it remains unclear from this study exactly how the association of IgAC5 with the O-Ag leads to alterations in Ipa secretion and changes in membrane potential, there are signal transduction pathways and mechanosensitive channels localized in the periplasm and inner membrane dedicated to “sensing” changes at the cell surface (22, 23). IgAC5, by virtue of its ability to bind and cross-link the O-Ag, may induce a type of outer membrane “stress” that effectively destabilizes the outer leaflet of the outer membrane (24). Triggering of membrane stress response pathways (23) and/or mechanosensitive channels (22) could directly or indirectly impact the PMF and activity of the T3S system. In support of an antibody-mediated membrane stress model, we have recently described distinct ultrastructural changes in the outer membrane of S. Typhimurium associated with exposure to a monoclonal IgA against the O-Ag (10). We have also observed that IgAC5 treatment results in slightly elevated levels of the periplasmic protein alkaline phosphatase (AP) in S. flexneri culture supernatants (S. Forbes and N. Mantis, unpublished results), consistent with antibody-induced alterations in outer membrane integrity and leakage of periplasmic proteins into the external medium.
While IgAC5’s effects on T3S and bioenergetics were only transient, they could represent a significant component of IgA-mediated immunity to S. flexneri at epithelial surfaces. We propose, for example, that IgAC5, upon first encountering S. flexneri in vivo, may temporarily render the bacteria noninvasive. This would then enable antibody-mediated agglutination to take place, as well as subsequent entrapment of IgA-immune complexes in mucus (8, 25). On another level, antibody-mediated damage to the outer membrane could potentially make S. flexneri susceptible to normally sublethal concentrations of antimicrobial factors present in intestinal secretions, thereby providing a link between the innate and adaptive immune systems at mucosal surfaces.
ACKNOWLEDGMENTS
We thank Edwin V. Oaks (The Walter Reed Army Institute of Research) for providing us with mouse monoclonal antibodies against the Ipa proteins. We also acknowledge the Wadsworth Center’s Immunology core facility for providing instrumentation used in this study.
This work was supported in part by grant R01HD061916 to N.J.M. from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). T.B. was supported in part by the Wadsworth Center’s Research Experience for Undergraduates (REU) program sponsored by the National Science Foundation.
Footnotes
Citation Forbes SJ, Bumpus T, McCarthy E, Corthésy B, Mantis NJ. 2011. Transient suppression of Shigella flexneri type 3 secretion by a protective O-antigen-specific monoclonal IgA. mBio 2(3)e00042-11. doi:10.1128/mBio.00042-11.
REFERENCES
- 1. Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. 2007. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat. Rev. Microbiol. 5:540–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galán JE, Wolf-Watz H. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444:567–573 [DOI] [PubMed] [Google Scholar]
- 3. Marteyn B, et al. 2010. Modulation of Shigella virulence in response to available oxygen in vivo. Nature 465:355–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. West NP, et al. 2005. Optimization of virulence functions through glucosylation of Shigella LPS. Science 307:1313–1317 [DOI] [PubMed] [Google Scholar]
- 5. Rasolofo-Razanamparany V, Cassel-Beraud AM, Roux J, Sansonetti PJ, Phalipon A. 2001. Predominance of serotype-specific mucosal antibody response in Shigella flexneri-infected humans living in an area of endemicity. Infect. Immun. 69:5230–5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boullier S, et al. 2009. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J. Immunol. 183:5879–5885 [DOI] [PubMed] [Google Scholar]
- 7. Phalipon A, et al. 1995. Monoclonal immunoglobulin A antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J. Exp. Med. 182:769–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phalipon A, et al. 2002. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17:107–115 [DOI] [PubMed] [Google Scholar]
- 9. Forbes SJ, Eschmann M, Mantis NJ. 2008. Inhibition of Salmonella enterica serovar Typhimurium motility and entry into epithelial cells by a protective antilipopolysaccharide monoclonal immunoglobulin A antibody. Infect. Immun. 76:4137–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mantis NJ, Forbes SJ. 2010. Secretory IgA: arresting microbial pathogens at epithelial borders. Immunol. Invest. 39:383–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bahrani FK, Sansonetti PJ, Parsot C. 1997. Secretion of Ipa proteins by Shigella flexneri: inducer molecules and kinetics of activation. Infect. Immun. 65:4005–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strugnell RA, Wijburg OL. 2010. The role of secretory antibodies in infection immunity. Nat. Rev. Microbiol. 8:656–667 [DOI] [PubMed] [Google Scholar]
- 13. Wijburg OL, et al. 2006. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J. Exp. Med. 203:21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berg HC. 2003. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72:19–54 [DOI] [PubMed] [Google Scholar]
- 15. Galán JE. 2008. Energizing type III secretion machines: what is the fuel? Nat. Struct. Mol. Biol. 15:127–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rathinavelan T, et al. 2010. A repulsive electrostatic mechanism for protein export through the type III secretion apparatus. Biophys. J. 98:452–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilharm G, et al. 2004. Yersinia enterocolitica type III secretion depends on the proton motive force but not on the flagellar motor components MotA and MotB. Infect. Immun. 72:4004–4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Becker LA, Bang IS, Crouch ML, Fang FC. 2005. Compensatory role of PspA, a member of the phage shock protein operon, in rpoE mutant Salmonella enterica serovar Typhimurium. Mol. Microbiol. 56:1004–1016 [DOI] [PubMed] [Google Scholar]
- 19. Jovanovic G, Lloyd LJ, Stumpf MP, Mayhew AJ, Buck M. 2006. Induction and function of the phage shock protein extracytoplasmic stress response in Escherichia coli. J. Biol. Chem. 281:21147–21161 [DOI] [PubMed] [Google Scholar]
- 20. Kinoshita N, Unemoto T, Kobayashi H. 1984. Proton motive force is not obligatory for growth of Escherichia coli. J. Bacteriol. 160:1074–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smiley ST, et al. 1991. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl. Acad. Sci. U. S. A. 88:3671–3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Booth IR, Edwards MD, Black S, Schumann U, Miller S. 2007. Mechanosensitive channels in bacteria: signs of closure? Nat. Rev. Microbiol. 5:431–440 [DOI] [PubMed] [Google Scholar]
- 23. Rowley G, Spector M, Kormanec J, Roberts M. 2006. Pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nat. Rev. Microbiol. 4:383–394 [DOI] [PubMed] [Google Scholar]
- 24. Nikaido H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phalipon A, Corthésy B. 2003. Novel functions of the polymeric Ig receptor: well beyond transport of immunoglobulins. Trends Immunol. 24:55–58 [DOI] [PubMed] [Google Scholar]
- 26. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]