ABSTRACT
The immune response to Neisseria gonorrhoeae is poorly understood, but its extensive antigenic variability and resistance to complement are thought to allow it to evade destruction by the host’s immune defenses. We propose that N. gonorrhoeae also avoids inducing protective immune responses in the first place. We previously found that N. gonorrhoeae induces interleukin-17 (IL-17)-dependent innate responses in mice and suppresses Th1/Th2-dependent adaptive responses in murine cells in vitro through the induction of transforming growth factor β (TGF-β). In this study using a murine model of vaginal gonococcal infection, mice treated with anti-TGF-β antibody during primary infection showed accelerated clearance of N. gonorrhoeae, with incipient development of Th1 and Th2 responses and diminished Th17 responses in genital tract tissue. Upon secondary reinfection, mice that had been treated with anti-TGF-β during primary infection showed anamnestic recall of both Th1 and Th2 responses, with the development of antigonococcal antibodies in sera and secretions, and enhanced resistance to reinfection. In mouse knockout strains defective in Th1 or Th2 responses, accelerated clearance of primary infection due to anti-TGF-β treatment was dependent on Th1 activity but not Th2 activity, whereas resistance to secondary infection resulting from anti-TGF-β treatment during primary infection was due to both Th1- and Th2-dependent memory responses. We propose that N. gonorrhoeae proactively elicits Th17-driven innate responses that it can resist and concomitantly suppresses Th1/Th2-driven specific adaptive immunity that would protect the host. Blockade of TGF-β reverses this pattern of host immune responsiveness and facilitates the emergence of protective antigonococcal immunity.
IMPORTANCE
Pathogen-host interactions during infectious disease are conventionally thought of as two-way reactions, that of the host against the pathogen and vice versa, with the outcome dependent on which one ultimately prevails. We propose that Neisseria gonorrhoeae, a pathogen that has become extremely well adapted to its exclusive human host, proactively directs the manner in which the host responds in ways that are beneficial to its own survival but detrimental to the host. Gonorrhea is a widely prevalent sexually transmitted infection, and naturally occurring gonococcal strains are becoming resistant to most available antibiotics, yet no effective vaccine has been developed. These new insights into the immune response to N. gonorrhoeae should lead to novel therapeutic strategies and facilitate new approaches to vaccine development.
Introduction
The immune response to gonorrhea, an exclusively human sexually transmitted disease caused by Neisseria gonorrhoeae, is poorly understood. Because the infection can be acquired repeatedly, with no apparent diminution in severity or duration of disease, it is clear that recovery from each episode of infection does not leave a state of effective protective immunity against the organism (1, 2). However, N. gonorrhoeae possesses an extraordinary capacity for antigenic variation involving most of its major surface structures, including its lipooligosaccharide, pili, porin, and opacity (Opa) proteins, which are subject to various mechanisms of phase variation and recombinatorial gene expression, compounded by frequent horizontal gene transfer (reviewed in reference 3). In addition, N. gonorrhoeae utilizes several mechanisms for inhibiting the activation of human complement and resisting complement-mediated bacteriolysis (4–6). These factors undoubtedly contribute to the difficulty in generating an effective vaccine against gonorrhea (1). Thus, the conventional working hypothesis for explaining lack of immunity to gonorrhea holds that while specific immune responses may be generated to various individual gonococcal antigens, constant changes in antigenic structure coupled with resistance to a major bacteriolytic and opsonophagocytic defense mechanism enable N. gonorrhoeae to evade the consequences of host-adaptive immune responses.
However, antigonococcal antibodies can be detected in most individuals regardless of history of infection, and likely, the sharing or cross-reactivity of antigens with N. meningitidis, which is commonly carried in the nasopharynx, or with commensal species of Neisseria, accounts for these findings (7, 8). Yet, most symptomatic cases of gonococcal cervicitis or urethritis display an acute inflammatory response with mucopurulent discharge containing abundant neutrophils. We have therefore proposed that N. gonorrhoeae also has the capacity to avoid inducing, or possibly even to suppress, adaptive immune responses in the first place.
We have recently utilized the mouse model of genital tract gonococcal infection (9) to investigate interactions of N. gonorrhoeae with the cells of the immune system, both in vivo and in vitro. These studies have revealed that N. gonorrhoeae induces Th17 responses which are involved in the influx of neutrophils to the genital tract as well as the recruitment of other innate defense mechanisms (10). In contrast, N. gonorrhoeae does not induce strong Th1 or Th2 responses in the mouse model. Blockade of interleukin-17A (IL-17A) or deficiency of its principal receptor, IL-17 receptor A (IL-17RA), resulted in inhibition of the neutrophil influx and prolongation of the infection (10). Further in vitro studies on the underlying mechanisms have shown that the immunosuppressive cytokine, transforming growth factor β (TGF-β), is critically involved in these responses, both for the generation of Th17 innate responses and for the suppression of Th1- and Th2-driven adaptive immunity (Y. Liu, G. A. Jarvis, and M. W. Russell, submitted for publication). Moreover, blockade of TGF-β diverted the response of murine lymphocytes from Th17 to Th1 and Th2 modes. In the present study, we have applied these findings to the in vivo model and demonstrate a profound effect of anti-TGF-β antibody treatment on the outcome of vaginal infection with N. gonorrhoeae, including the generation of Th1/Th2 responses, memory, antibody responses, and protection against reinfection.
RESULTS
TGF-β production and the Th17 response in murine vaginal gonococcal infection.
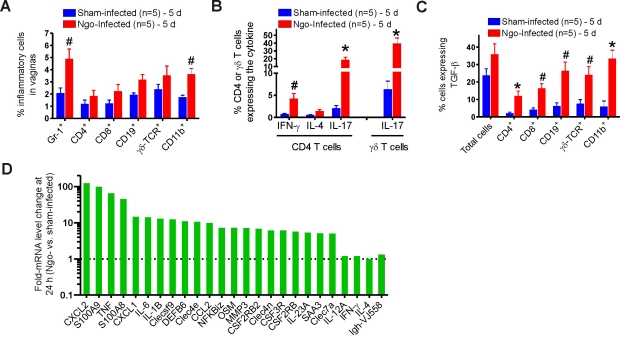
To characterize the local immune response to genital gonococcal infection, vaginas were removed from each of the 5 mice per group at 1, 3, 5, and 7 days after inoculation with N. gonorrhoeae or vehicle only. Single-cell suspensions, including the infiltrating leukocytes, were prepared from each specimen individually for evaluation by flow cytometry to detect intracellular cytokines gamma interferon (IFN-γ), IL-4, IL-17, and TGF-β. The infiltrating leukocytes included neutrophils (Gr-1+), CD4+, CD8+, CD19+, γδ T cell receptor (TCR), and CD11b+ cells, the numbers of which were elevated in infected mice relative to sham-infected mice (Fig. 1A). Starting on day 3 after inoculation, IL-17 production was observed, with production peaking at day 5 and continuing for the duration of infection. At day 5, 17.1% of CD4+ T cells present in the vaginas of infected mice were IL-17+, whereas only 3.7% were IFN-γ+ and few IL-4+ cells were detected (Fig. 1B). Even greater numbers and higher proportions of γδ T cells were positive for IL-17 (Fig. 1A and B), implying that γδ T cells were a more important source of IL-17 than CD4+ Th17 cells at the site of infection at this time point. TGF-β production in the vaginas was also elevated by 5 days of infection, with 11.6% to 34.2% of the isolated cell types being TGF-β+, significantly higher than the levels in sham-infected controls (Fig. 1C). Since infiltrating inflammatory cells accounted for <25% of the total isolated cells, whereas 35.6% or 23.3% of the total vaginal cells in infected and control mice, respectively, were TGF-β+ (Fig. 1C), this suggested that a large number of vaginal epithelial and stromal cells also produced TGF-β. Together, these data indicate that N. gonorrhoeae infection elicits a local IL-17 response in vivo in the murine model of genital tract gonococcal infection and that TGF-β production is an important component of this process.
FIG 1 .
Vaginal cell responses to gonococcal infection in BALB/c mice. (A) Profiles of vaginal cells isolated from N. gonorrhoeae (Ngo)-infected and sham-infected mice 5 days after inoculation, stained for phenotypic markers, and examined by flow cytometry. (B) Production of IFN-γ, IL-4, and IL-17 in CD4+ or γδ T cells isolated from the vaginal tissue of infected and sham-infected mice 5 days after infection and analyzed by flow cytometry. (C) TGF-β production by vaginal cells isolated from infected and sham-infected mice 5 days after infection and analyzed by flow cytometry. Panels A to C each show one of three independent experiments, giving essentially similar results and levels of significance. #, P < 0.05; *, P < 0.01 versus that of sham-infected mice. (D) Differential mRNA expression in vaginal tissue from 2 N. gonorrhoeae-infected mice versus that from 2 sham-infected mice determined by independent microarray analysis of tissues collected 24 hours after inoculation. Fold changes in mean mRNA expression in infected mice compared with those in sham-infected mice are shown.
To further define the immune response elicited by N. gonorrhoeae in vivo, we performed gene profiling analysis individually on vaginal mRNA from 2 infected and 2 sham-infected mice at 24 h after inoculation. Highly represented among many immune-associated genes were those associated with Th17 function or the targets of Th17 cytokines (Fig. 1D). Among the most strongly induced genes were S100A8 (45.2-fold), S100A9 (98.1-fold), CXCL1 (14.5-fold), CXCL2 (122.5-fold), CCL2 (9.8-fold), MMP3 (7.1-fold), SAA3 (5.0-fold), IL-1β (12.8-fold), IL-6 (14.1-fold), IL-23 (5.3-fold), tumor necrosis factor alpha (TNF-α; 65.3-fold), DEFB6 (β-defensin; 10.9-fold), and NF-κBiz (7.2-fold). All these are known Th17 signature genes. In contrast, characteristic Th1- or Th2-related genes, such as IL-12A, IFN-γ, and IL-4, were not upregulated (Fig. 1D).
Inhibition of TGF-β activity enhances the Th1/Th2 immune response in mouse vaginal gonococcal infection.
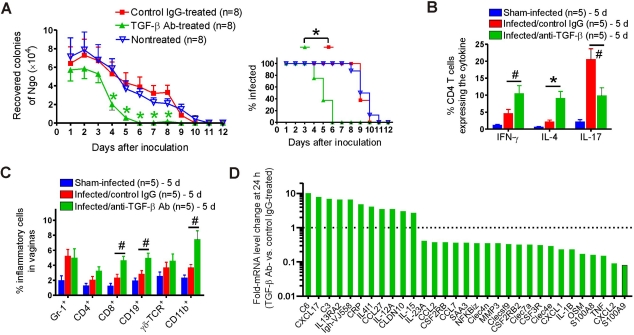
Given the known role of TGF-β in the generation of Th17 and suppression of Th1/Th2 responses, we hypothesized that blockade of TGF-β would divert the response to N. gonorrhoeae away from innate Th17-dominated responses and towards Th1- and Th2-adaptive responses. To test this, groups of 8 mice were treated on days −1 and 0 and every two days thereafter with 300 µg of anti-TGF-β antibody or control IgG1; a third group was not treated. All mice were inoculated intravaginally on day 0 with 5 × 106 CFU of N. gonorrhoeae, and the bacterial burden was monitored daily by vaginal swab culture. The duration of gonococcal infection in nontreated and control-treated mice was ~10 days (Fig. 2A). None of the infected mice in any group exhibited significant weight loss compared to that of sham-infected mice. Anti-TGF-β treatment significantly reduced the recoverable gonococcal load starting from day 4, and these mice cleared the infection by day 6, 4 days earlier than control-treated or untreated mice (F1,168 = 26.54; P < 0.001) (Fig. 2A). Anti-TGF-β treatment was also associated with enhanced Th1/Th2 and diminished Th17 immune responses, indicated by the increased numbers of IFN-γ+ (P < 0.05) and IL-4+ CD4+ (P < 0.01) T cells but decreased number of IL-17+ CD4+ (P < 0.05) T cells in vaginal tissue (Fig. 2B) from the treated mice in comparison to those in controls. Similar results were observed in iliac lymph nodes of the same mice (data not shown). Anti-TGF-β antibody treatment did not significantly affect Gr-1+ neutrophil influx into the genital tract, determined relative to total recovered vaginal cells (Fig. 2C), but it did significantly increase the infiltration of CD8+ (P < 0.05), CD19+ (P < 0.05), and CD11b+ (P < 0.05) cells (Fig. 2C).
FIG 2 .
Effect of anti-TGF-β antibody treatment on primary gonococcal infection in BALB/c mice. (A) Time course of infection in anti-TGF-β antibody-treated, control IgG-treated, and nontreated control mice. (Left) Recovery of N. gonorrhoeae (Ngo) in vaginal swabs; (right) percentage of mice infected. (B) Cytokine expression in isolated vaginal cells from sham-infected and infected mice treated with anti-TGF-β antibody or control IgG. Expression of IFN-γ, IL-4, and IL-17 in CD4+ T cells at day 5 was analyzed by flow cytometry. (C) Phenotypic profile of vaginal cells isolated on day 5 from sham-infected and infected mice treated with anti-TGF-β antibody or control IgG. Panels A to C each show one of three independent experiments, giving essentially similar results and levels of significance. #, P < 0.05; *, P < 0.01 versus that of control IgG-treated mice. (D) Differential mRNA expression in vaginal tissue from 2 anti-TGF-β antibody-treated mice versus that from 2 control IgG-treated N. gonorrhoeae-infected mice determined by independent microarray analysis of tissues collected 24 hours after inoculation. Fold changes in mean mRNA expression in anti-TGF-β-treated mice compared with those in control mice are shown.
Microarray analysis of mRNA expression was performed on vaginas from 2 anti-TGF-β-treated and 2 control-treated mice 24 h after inoculation (Fig. 2D). In anti-TGF-β-treated mice, Th17-associated genes were downregulated compared to those in control mice. For example, neutralization of TGF-β led to diminished expression of S100A8 (8.5-fold), S100A9 (8.3-fold), SAA3 (2.7-fold), CXCL1 (4.1-fold), CXCL2 (16.1-fold), IL-1β (4.2-fold), IL-17A (2.5-fold), NF-κBiz (2.8-fold), and CSF3R (receptor for granulocyte colony-stimulating factor; 2.8-fold) (Fig. 2D). In contrast, anti-TGF-β treatment upregulated the Th1 and Th2 profile genes, which included IL-12A (2.9-fold), IL4i1 (4.1-fold), IL13RA2 (6.4-fold), IL-15 (2.7-fold), Igh-VJ558 (Ig heavy chain; 6.5-fold), and CCL27 (chemotactic for memory T cells; 3.5-fold) (Fig. 2D). The results further suggested that the effect of anti-TGF-β treatment on immune responses was multifaceted. For example, anti-TGF-β treatment also upregulated genes associated with innate immunity, such as C3 and C6 (6.9- and 10.1-fold, respectively), CRP (C-reactive protein; 4.8-fold), and CCL5 (chemotactic for T cells, eosinophils, and basophils; 3.1-fold) (Fig. 2D).
Treatment with anti-TGF-β antibody induces protective immunity against secondary gonococcal infection.
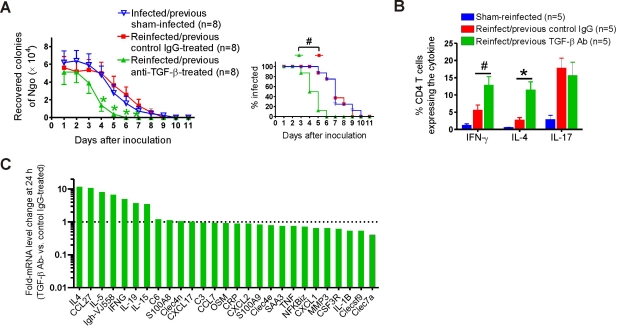
We further assessed whether the induction of Th1/Th2 adaptive responses to primary gonococcal infection by anti-TGF-β treatment resulted in the generation of immune memory and protective immunity. Reinfection studies were therefore performed in which groups of 8 mice treated with anti-TGF-β or control antibody were challenged with N. gonorrhoeae and then treated with ceftriaxone (300 µg intraperitoneally [i.p.]) on day 10 to ensure clearance of the infection. An additional group of untreated sham-infected mice served as naive controls. Four to five weeks later, all mice were inoculated with N. gonorrhoeae of the same strain without further anti-TGF-β or control IgG1 treatment. The results showed that primary infection of control-treated mice did not protect them against subsequent secondary challenge: there was no significance difference in either the duration of secondary infection or the average number of gonococci recovered compared to those of the simultaneous primary infection of age-matched naive control mice (F1,154 = 0.002; P = 0.96) (Fig. 3A). In contrast, anti-TGF-β treatment during primary infection induced protection against secondary infection: reinfected mice that had been treated with anti-TGF-β during the primary infection recovered significantly faster than controls (F1,154 = 13.31; P < 0.001) (Fig. 3A). Flow cytometric analysis of mouse vaginal (Fig. 3B) and iliac lymph node (data not shown) cells taken at day 5 of secondary infection showed higher Th1 (IFN-γ) and Th2 (IL-4) responses in the reinfected mice with previous anti-TGF-β treatment than in those receiving control treatment. Interestingly, the percentages of IL-17+ CD4+ cells were similar between these two groups (Fig. 3B), possibly because there was no anti-TGF-β treatment during the secondary infection. The enhanced Th1/Th2 immune responses in the reinfected mice treated previously with anti-TGF-β were further supported by microarray assays of vaginal mRNA. In comparison to those with previous control IgG treatment, mice with previous anti-TGF-β treatment expressed significantly higher levels of Th1- and Th2-related genes at 24 h after reinfection, including IFN-γ (4.9-fold), IL-4 (11.7-fold), IL-5 (8.1-fold), IL-13 (4.1-fold), Igh-VJ558 (6.6-fold), IL-19 (3.7-fold), and CCL27 (10.8-fold) (Fig. 3C). However, the expression levels of Th17-associated genes, such as S100A8, S100A9, CCL2, CXCL1, OSM, or NF-κBiz, were not significantly different between these two groups (Fig. 3C).
FIG 3 .
Effect of anti-TGF-β antibody treatment during primary infection on secondary gonococcal infection in BALB/c mice. (A) Time course of secondary infection in mice treated with anti-TGF-β antibody or control IgG during primary infection or in previously sham-infected control mice. (Left) Recovery of N. gonorrhoeae (Ngo) in vaginal swabs; (right) percentage of mice infected. (B) Cytokine expression in isolated vaginal cells from sham-infected and reinfected mice treated previously with anti-TGF-β antibody or control IgG. Expression of IFN-γ, IL-4, and IL-17 in CD4+ T cells at day 5 was analyzed by flow cytometry. Panels A and B each show one of three independent experiments, giving essentially similar results and levels of significance. #, P < 0.05; *, P < 0.01 versus that of control IgG-treated mice. (C) Differential expression of mRNA in vaginal tissue from 2 reinfected mice treated previously with anti-TGF-β antibody versus that from 2 reinfected mice treated previously with control IgG determined by independent microarray analysis of tissues collected 24 hours after inoculation. Fold changes in mean mRNA expression in reinfected mice treated previously with anti-TGF-β antibody compared to those in reinfected mice treated previously with control IgG are shown.
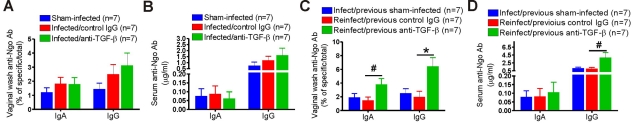
To test whether genital gonococcal infection induced an antibody response, we determined the levels of N. gonorrhoeae-specific IgA and IgG in vaginal wash, saliva, and serum from groups of 7 infected and sham-infected mice. IgM antibodies were also assayed but showed low levels and little difference between experimental groups. Total concentrations of IgA and IgG were also assayed in saliva and vaginal wash to compensate for individual variations in flow rate and the unknown dilution of vaginal wash. Primary N. gonorrhoeae infection of control-treated mice resulted in modestly increased gonococcus-specific IgA and IgG antibodies in vaginal washes (Fig. 4A) and weak gonococcus-specific serum IgG antibody responses in some animals at day 10 (Fig. 4B). However, the antibody levels were low and not statistically significant. No salivary gonococcus-specific antibody was detected (data not shown). There was no significant increase in gonococcus-specific IgA or IgG antibody response to primary infection after anti-TGF-β treatment (Fig. 4A and B). However, there was a robust specific secondary antibody response in anti-TGF-β-treated mice after they were reexposed to N. gonorrhoeae (Fig. 4C and D). Gonococcus-specific IgA (P < 0.05) and IgG (P < 0.01) antibodies in vaginal wash (Fig. 4C) and IgG (P < 0.05) antibody in serum (Fig. 4D) of reinfected mice with previous anti-TGF-β treatment were significantly higher than those of control groups. All these results demonstrated that inhibition of TGF-β activity during gonococcal infection overcomes the suppressive effect of the bacteria on Th1/Th2-mediated adaptive responses, permits the generation of immune memory and specific antibodies, and elicits protective immunity.
FIG 4 .
Antigonococcal antibody responses to primary and secondary infection in anti-TGF-β antibody-treated, control IgG-treated, or sham-infected BALB/c mice. (A) Vaginal IgA and IgG responses to primary infection. (B) Serum IgA and IgG responses to primary infection. (C) Vaginal IgA and IgG responses to secondary infection. (D) Serum IgA and IgG responses to secondary infection. Samples were collected 10 days after inoculation. Gonococcus-specific and total IgA and IgG in vaginal washes and sera were measured by ELISA. Data shown are from one experiment using 7 mice per group. #, P < 0.05; *, P < 0.01 versus that of control IgG-treated mice. Two more replicate experiments yielded essentially similar results and levels of significance.
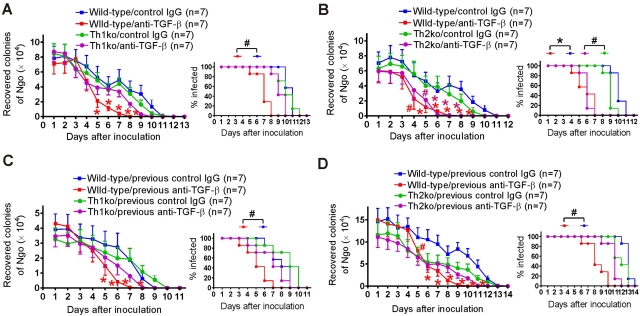
Th1 or Th2 deficiency does not affect the course of mouse vaginal gonococcal infection but diminishes the effect of anti-TGF-β treatment.
To further determine the role of Th1 and Th2 responses in genital gonococcal infection and the importance of Th1/Th2 induction on the therapeutic effect of TGF-β neutralizing antibody, we repeated the experiment using IL-12 knockout (IL-12-ko; Th1-deficient) and IL-4-ko (Th2-deficient) mice. Groups of 7 C57BL/6 wild-type, IL-12-ko, or IL-4-ko mice were challenged with N. gonorrhoeae and sampled daily for recoverable gonococci until the infection was cleared. Consistent with the above-described data, the results showed that Th1 or Th2 deficiency did not in itself affect the course of vaginal gonococcal infection. IL-12-ko and IL-4-ko mice cleared the gonococci at the same rate as wild-type mice during either primary (Fig. 5A and B) or secondary (Fig. 5C and D) challenge. Similar to BALB/c mice, all infected mice in this experiment started to reduce the recoverable gonococcal load from day 4 and had cleared the infection by around day 11 (Fig. 5A to D).
FIG 5 .
Effect of anti-TGF-β antibody treatment during primary infection on the course of primary and secondary gonococcal infection in wild-type C57BL/6, Th1-deficient, and Th2-deficient mice. (A) Primary infection in wild-type and Th1-deficient mice. (B) Primary infection in wild-type and Th2-deficient mice. (C) Secondary infection in wild-type and Th1-deficient mice. (D) Secondary infection in wild-type and Th2-deficient mice. In each pair of panels, the left panel shows the recovery of N. gonorrhoeae in vaginal swabs, and the right panel shows the percentage of mice infected. Data shown are from one experiment using 7 mice per group. #, P < 0.05; *, P < 0.01 versus that of control IgG-treated mice.
We then examined whether Th1 or Th2 deficiency altered the effect of anti-TGF-β treatment on genital gonococcal infection. Although anti-TGF-β antibody was similarly effective in accelerating the clearance of the primary infection of IL-4-ko mice (F1,144 = 9.24; P < 0.001) and wild-type mice (F1,144 = 23.18; P < 0.001) (Fig. 5B), its therapeutic effect in IL-12-ko mice was dramatically diminished. Compared to wild-type mice (F1,144 = 16.89; P < 0.001), TGF-β neutralizing antibody did not significantly change the course of primary N. gonorrhoeae infection in IL-12-ko mice (F1,144 = 1.63; P = 0.20) (Fig. 5A). Thus, accelerated clearance of primary infection due to anti-TGF-β treatment appeared to depend on Th1 cells but not Th2 cells. However, both Th1 and Th2 deficiencies abrogated anti-TGF-β treatment-induced resistance to secondary infection. There was no significant difference in persistence of infection or recoverable gonococcal loads during secondary challenge among IL-12-ko (F1,132 = 1.23; P = 0.27) (Fig. 5C) or IL-4-ko (F1,168 = 1.42; P = 0.23) (Fig. 5D) mice with previous anti-TGF-β or control treatment. The data indicated that anti-TGF-β treatment increases resistance to gonococcal infection by enhancing both Th1- and Th2-dependent adaptive immune responses.
DISCUSSION
The present studies reveal the importance of TGF-β in the in vivo response to genital infection with N. gonorrhoeae in mice. These responses are predominantly Th17 driven (10), as revealed by the release of IL-17 by both Th17 CD4+ and innate γδ T cells, with very little IFN-γ or IL-4 production. We have further found that TGF-β is an integral component of the Th17 response to N. gonorrhoeae (Liu et al., submitted). Although TGF-β is constitutively present in genital tract tissue, gonococcal infection further enhanced vaginal TGF-β production. TGF-β is involved in both Th17 cell development (11) and the suppression of Th1 and Th2 responses directly, as well as induction of T regulatory cells (12). We find that treatment of mice with neutralizing anti-TGF-β antibody during primary infection with N. gonorrhoeae shifted the pattern of responses away from Th17 and towards Th1 and Th2, accelerated clearance of N. gonorrhoeae, and facilitated the establishment of Th1-dependent immune memory.
The effect of treatment with anti-TGF-β antibody during primary infection was further revealed upon secondary challenge of mice with N. gonorrhoeae. In control animals, secondary infection was cleared with the same kinetics as those used in primary infection, as reported also by Song et al. (13), implying that, as in humans, immune memory is not induced by gonococcal infection of mice. However, anti-TGF-β treatment during primary infection resulted in increased resistance to secondary infection, accompanied by strongly enhanced Th1 and Th2 cytokine responses, and the development of circulating and vaginal antigonococcal antibody responses, while Th17 responses were not impaired. Thus, we show for the first time that, when TGF-β is blocked, genital tract infection with N. gonorrhoeae can induce Th1/Th2-driven adaptive immune responses, including specific antibodies, memory, and protective immunity to reinfection. It is conceivable that anti-TGF-β treatment might enhance innate defenses because there is evidence that TGF-β can suppress neutrophil degranulation and NK cell activity (14, 15). However, such an effect would have to persist for >4 weeks to have an impact on secondary infection, and we found no evidence for increased neutrophil influx in anti-TGF-β-treated mice. Moreover, although microarray analysis revealed upregulation of mRNA for some innate response genes (e.g., C6, C3, and CRP) in primary infection under treatment with anti-TGF-β, this was not sustained into secondary infection 4 to 5 weeks later. Other innate defense genes, e.g., S100A8, S100A9, and SAA3, were downregulated during primary infection under anti-TGF-β treatment and were unaltered during secondary infection after anti-TGF-β treatment.
The gene expression profiling studies of vaginal tissue reflected the immunological and microbiological findings. Vaginally infected mice showed strong upregulation of genes associated with Th17 responses and innate defenses but not of those associated with adaptive immunity. This pattern was reversed in mice treated with anti-TGF-β antibody during primary infection, as follows: Th17-related genes were suppressed, and those associated with Th1/Th2-driven adaptive immune responses were upregulated. Th1/Th2 and adaptive immune response genes were also upregulated during secondary infection of mice that had been treated with anti-TGF-β antibody during primary infection, whereas innate response genes were not differentially affected by the earlier anti-TGF-β treatment.
The finding that anti-TGF-β treatment permitted the emergence of Th1 and Th2 responses revealed by the generation of IFN-γ and IL-4 after both primary and secondary infection prompted the question as to which was more important for protective immunity to N. gonorrhoeae. This was addressed by means of Th1- and Th2-deficient mice, i.e., IL-12p35-ko and IL-4-ko strains. Interestingly, these deficiencies in themselves had no impact on primary or secondary infection, implying that Th1- and Th2-dependent immunity normally has no role in the response to or clearance of gonococcal infection in mice. However, Th1 deficiency, but not Th2 deficiency, reversed the accelerating effect of anti-TGF-β treatment on clearance of primary infection, suggesting that Th1 responses were important for this. Both Th1 and Th2 deficiencies reversed the protective effect of anti-TGF-β treatment during primary infection against reinfection, suggesting that both Th1 and Th2 responses were required for the generation of protective immunity to repeated infection. However, caution is required because the Th1-deficient mice lacked IL-12p35, which is also a component of IL-35 (16), a cytokine involved in regulatory T (Treg) cell function (17), and the potential impact of this on gonococcal infection is unknown. Moreover, IL-4 deletion (in Th2-deficient mice) might also affect other cells besides Th2 cells. However, we have found that gonococcal infection does not upregulate IL-4, IL-12, or IFN-γ in normal mice (Liu et al., submitted; this study).
In vitro studies using mouse spleen cells have shown that Th17 responses are induced by gonococcal lipooligosaccharide acting through Toll-like receptor 4, whereas induction of TGF-β and suppression of Th1/Th2 responses involves gonococcal Opa proteins (Liu et al., submitted). In humans, most gonococcal Opa proteins bind to receptors of the carcinoembryonic antigen-related cell adhesion molecule (CEACAM) family (18), and interaction with CEACAM1 leads to inhibition of CD4+ T cell and B cell activation (19, 20), although this was not confirmed in another study (21). However, murine CEACAM receptors lack the residues required for Opa binding identified in human CEACAM (22), and the receptors involved in the Opa-dependent generation of TGF-β in mice remain to be identified.
Elevated IL-17 and IL-23 levels have recently been reported in the sera of mostly male patients with gonorrhea (23), and Th17 cells have been identified in the cervices of women infected with Chlamydia trachomatis (24). Moreover, IL-17 and Th17 responses have been found in numerous other human infections (25, 26) and are instrumental in recruiting potent innate antibacterial defense mechanisms, including neutrophils and the secretion of antimicrobial defense proteins, which are important for protection of mucosal surfaces against infection (27). The minimal antibody response of mice to gonococcal infection, reported also by Song et al. (13), accords with our previous findings in humans with uncomplicated gonorrhea (7, 8), yet in both species, there is typically a local inflammatory response in the genital tract with an influx of neutrophils. However, it is unlikely that neutrophils are the critical determining factor in clearance of the infection in mice, because suppression of the neutrophil response in IL-17RA-knockout mice delays, but does not prevent, clearance (10). Moreover, others have found that the extent of neutrophil influx varies considerably between mouse strains and does not correlate with resistance to infection (28). As the mouse is not a natural host for N. gonorrhoeae, many factors are probably involved in its clearance or failure to persist in the murine genital tract.
N. gonorrhoeae, as a result of its long association exclusively with humans, has become extremely well adapted to the human immune system. It exploits in particular an environment that is already rich in TGF-β, a pleiotropic cytokine with multiple functions, including immune regulation, and important for the control of immune responses to the developing fetus in the female reproductive tract (29). We propose that N. gonorrhoeae enhances TGF-β production and thereby promotes Th17-dependent responses and the deployment of innate defense mechanisms that it can largely survive while concomitantly suppressing the development of Th1- and Th2-driven adaptive immune responses, including specific antibodies, that might be capable of eliminating it. Thus, we hypothesize that N. gonorrhoeae is not merely reactive to the immune defenses that the host deploys against it—through antigenic variation and resistance to complement-mediated bacteriolysis—but also proactively elicits from the host the pattern of responses that it can cope with and suppresses those that would be inimical to it. This new concept in pathogenesis may be applicable to other pathogens that have become evolutionarily well adapted to their hosts.
In the context of gonorrhea, two corollaries emerge from this hypothesis. One is that therapeutic strategies might be devised to combat the ability of N. gonorrhoeae to subvert the host’s immune responses, perhaps by targeting TGF-β or its role in the suppression of Th1/Th2 responses. Second, efforts at vaccine development should be based on strategies of eliciting host responses, whether Th1 or Th2 dependent, against conserved or cross-reactive gonococcal antigens, sidestepping the ability of N. gonorrhoeae to subvert normal immune responsiveness.
MATERIALS AND METHODS
Mice.
BALB/c mice were purchased from Harlan Sprague Dawley (Frederick, MD). Wild-type C57BL/6 mice, B6.129S1-Il12atm1Jm/J (IL-12p35-ko, Th1-deficient), and B6.129P2-Il4tm1Cgn/J (IL-4-ko, Th2-deficient) mice on a C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were maintained under standard conditions in the Laboratory Animal Facility at the University at Buffalo. All animal use protocols were approved by the Institutional Animal Care and Use Committee.
Bacteria.
N. gonorrhoeae FA1090 (streptomycin resistant) (30) was kindly provided by Janne Cannon (University of North Carolina at Chapel Hill). Bacteria were cultured on GC agar supplemented with hemoglobin and IsoVitaleX (BD Diagnostic Systems, Franklin Lakes, NJ) in an atmosphere with 5% CO2 at 37°C, and the resultant growth was checked for colony morphology consistent with those of Opa protein and pilus expression. Bacteria were harvested from plates after 18 to 24 h of growth and suspended in phosphate-buffered saline (PBS). Bacterial cell density was determined by testing the optical density at 600 nm and reference to a previously determined calibration curve.
Mouse vaginal infection model.
Female mice between 7 and 9 weeks old were infected with live N. gonorrhoeae FA1090 as previously described (9), with the modification that water-soluble estradiol was administered on days −2, 0, and 2 (10, 13). On day 0, mice were inoculated intravaginally with 5 × 106 CFU of N. gonorrhoeae or PBS. Vaginal mucus was quantitatively cultured on GC agar daily to determine the bacterial colonization loads. In some experiments, mice were treated with TGF-β neutralizing monoclonal antibody or control mouse IgG1 purchased from Bio X Cell (West Lebanon, NH). Each experiment utilized 7 or 8 mice per group and was repeated independently on at least 3 occasions for verification, except as noted.
Vaginal cell isolation.
Mice were sacrificed, the vaginas were excised, and single-cell suspensions were prepared from each mouse separately as described in reference 31, with slight modifications. Briefly, the vagina was cut into small pieces, incubated in Hanks’ buffered salt solution containing 5 mM EDTA for 30 minutes at 37°C, washed, and incubated in Dulbecco modified Eagle medium with 5% fetal bovine serum, 1.5 mg/ml collagenase D (Sigma-Aldrich, St. Louis, MO), 0.5 mg/ml dispase II (Sigma-Aldrich), and 40 U/ml DNase I (Sigma-Aldrich) for 1 h at 37°C. The digestion was repeated until little tissue remained. Single cells were collected by centrifugation in 35% Percoll (Sigma-Aldrich).
Flow cytometry.
Isolated cells were washed with staining buffer twice and then incubated with the indicated antibodies for 30 minutes on ice, washed twice, and analyzed on a FACSCalibur cytometer. For determination of intracellular cytokine expression, cells were restimulated with phorbol myristate acetate (PMA)-ionomycin-GolgiStop (BD Biosciences, Bedford, MA) for 4 to 6 h. Antibodies to mouse Gr-1, CD4, CD8, CD19, γδ TCR, CD11b, IL-17A, IFN-γ, and IL-4 conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or allophycocyanin (APC) were purchased from BD Biosciences or eBioscience (San Diego, CA). PE-conjugated anti-TGF-β antibody was obtained from Cedarlane Laboratories (Burlington, NC).
Gene expression analysis.
Whole vaginas from control- or anti-TGF-β antibody-treated mice were harvested at baseline or 24 h after N. gonorrhoeae inoculation and preserved in RNAlater (Qiagen Sciences, Valencia, CA). Total RNA of the tissue was isolated with RNeasy minikits (Qiagen). Analysis was performed by the Roswell Park Cancer Institute Gene Expression Resource Facility (Buffalo, NY) using MouseWG-6 v2.0 Expression BeadChip kits (Illumina Inc., San Diego, CA).
Analyses of serum and mucosal antibodies.
Samples of saliva, vaginal wash, and serum were collected from individual mice on day 10 postinoculation (13, 32). Gonococcus-specific IgA, IgG, and IgM in saliva, sera, and vaginal washes were measured by enzyme-linked immunosorbent assay (ELISA) as described previously (13) on plates coated with gonococcal (FA1090) outer membrane vesicle preparation (10). Total IgA, IgG, and IgM concentrations in secretions were assayed by ELISA on plates coated with anti-IgA, -IgG, or -IgM antibodies (Southern Biotech, Birmingham, AL). H5 mouse monoclonal antibody (specific for FA1090 porin; kindly provided by Ann E. Jerse, Uniformed Services University of the Health Sciences) (13) or affinity-purified mouse IgA, IgG, and IgM (Southern Biotech) were used to establish standard curves. Bound antibodies were detected by alkaline phosphatase-conjugated goat anti-mouse IgA, IgG, or IgM antibody (Southern Biotech) and p-nitrophenylphosphate substrate (Southern Biotech). Plates were read in a VersaMax microplate reader (Molecular Devices, Sunnyvale, CA), and calibration curves for interpolation of unknowns were constructed using SoftMax software (Molecular Devices).
Statistical analysis.
Data are expressed as the means ± standard errors of the means (SEM) of results obtained for each group of mice in independent experiments. Data on the effect of anti-TGF-β versus control IgG treatments on mouse vaginal N. gonorrhoeae infection were analyzed using two-way analysis of variance (ANOVA) with Fisher’s protected least significant difference post hoc tests for multiple comparisons. Unpaired two-tailed t tests were used to compare the mean values between two groups. P values of <0.05 were considered statistically significant.
Microarray data accession numbers.
The original microarray data have been deposited in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/projects/geo/). All data are MIAME (minimum information about a microarray experiment) compliant and are available under GEO series accession number GSE28055 (GEO accession numbers GSM693975 to GSM693986).
ACKNOWLEDGMENTS
We acknowledge the assistance of the Confocal Microscope and Flow Cytometry Facility at the School of Medicine and Biomedical Sciences, University at Buffalo. We thank Ann E. Jerse for helpful advice and valuable discussions during the course of this work.
This work was supported by U.S. Public Health Service grant AI074791 from the National Institute of Allergy and Infectious Diseases and by a grant from the John R. Oishei Foundation, Buffalo, NY.
Footnotes
Citation Liu Y, Russell MW. 2011. Diversion of the immune response to Neisseria gonorrhoeae from Th17 to Th1/Th2 by treatment with anti-transforming growth factor β antibody generates immunological memory and protective immunity. mBio 2(3):e00095-11. doi:10.1128/mBio.00095-11.
REFERENCES
- 1. Russell MW, Hook EW., III 2009. Gonorrhea, p. 963–981. In Barrett ADT, Stanberry LR, Vaccines for biodefense and emerging and neglected diseases, 1st ed Academic Press, London, United Kingdom [Google Scholar]
- 2. Fox KK, et al. 1999. Longitudinal evaluation of serovar-specific immunity to Neisseria gonorrhoeae. Am. J. Epidemiol. 149:353–358 [DOI] [PubMed] [Google Scholar]
- 3. Liu Y, Feinen B, Russell MW. 2011. New concepts in immunity to Neisseria gonorrhoeae: innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front. Microbiol. 2:52 doi: 10.3389/fmicb.2011.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ram S, et al. 2001. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J. Exp. Med. 193:281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith H, Parsons NJ, Cole JA. 1995. Sialylation of neisserial lipopolysaccharide: a major influence on pathogenicity. Microb. Pathog. 19:365–377 [DOI] [PubMed] [Google Scholar]
- 6. Lewis LA, Burrowes E, Rice PA, Ram S. 2010. Interactions of Neisseria with complement, p. 123–144. In Genco CA, Wetzler L, Neisseria: molecular mechanisms of pathogenesis. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 7. Hedges SR, Sibley DA, Mayo MS, Hook EW, III, Russell MW. 1998. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J. Infect. Dis. 178:742–751 [DOI] [PubMed] [Google Scholar]
- 8. Hedges SR, Mayo J, Mestecky EW, Hook EW, III, Russell MW. 1999. Limited local and systemic antibody responses to Neisseria gonorrhoeae during uncomplicated genital infections. Infect. Immun. 67:3937–3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jerse AE. 1999. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect. Immun. 67:5699–5708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feinen B, Jerse AE, Gaffen SL, Russell MW. 2010. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 3:312–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Korn T, Bettelli E, Oukka M, Kuchroo VK. 2009. IL-17 and Th17 cells. Annu. Rev. Immunol. 27:485–517 [DOI] [PubMed] [Google Scholar]
- 12. Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. 2006. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 24:99–146 [DOI] [PubMed] [Google Scholar]
- 13. Song W, et al. 2008. Local and humoral immune responses against primary and repeat Neisseria gonorrhoeae genital tract infections of 17beta-estradiol-treated mice. Vaccine 26:5741–5751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shen L, et al. 2007. Inhibition of human neutrophil degranulation by transforming growth factor-beta1. Clin. Exp. Immunol. 149:155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eriksson M, Meadows SK, Wira CR, Sentman CL. 2006. Endogenous transforming growth factor-beta inhibits toll-like receptor mediated activation of human uterine natural killer cells. Am. J. Reprod. Immunol. 56:321–328 [DOI] [PubMed] [Google Scholar]
- 16. Collison LW, Vignali DA. 2008. Interleukin-35: odd one out or part of the family? Immunol. Rev. 226:248–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collison LW, et al. 2010. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 11:1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dehio C, Gray-Owen SD, Meyer TF. 1998. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 6:489–495 [DOI] [PubMed] [Google Scholar]
- 19. Boulton IC, Gray-Owen SD. 2002. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol. 3:229–236 [DOI] [PubMed] [Google Scholar]
- 20. Pantelic M, et al. 2005. Neisseria gonorrhoeae kills carcinoembryonic antigen-related cellular adhesion molecule 1 (CD66a)-expressing human B cells and inhibits antibody production. Infect. Immun. 73:4171–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Youssef AR, et al. 2009. Opa+ and Opa− isolates of Neisseria meningitidis and Neisseria gonorrhoeae induce sustained proliferative responses in human CD4+ T cells. Infect. Immun. 77:5170–5180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Virji M, et al. 1999. Critical determinants of host receptor targeting by Neisseria meningitidis and Neisseria gonorrhoeae: identification of Opa adhesiotopes on the N-domain of CD66 molecules. Mol. Microbiol. 34:538–551 [DOI] [PubMed] [Google Scholar]
- 23. Gagliardi MC, et al. 2010. Circulating levels of interleukin-17A and interleukin-23 are increased in patients with gonococcal infection. FEMS Immunol. Med. Microbiol. 61:129–132 [DOI] [PubMed] [Google Scholar]
- 24. Jha R, et al. 2011. Spontaneous secretion of interleukin-17 and -22 by human cervical cells in Chlamydia trachomatis infection. Microbes Infect. 13:167–178 [DOI] [PubMed] [Google Scholar]
- 25. Curtis MM, Way SS. 2009. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology 126:177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khader SA, Gaffen SL, Kolls JK. 2009. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aujla SJ, Dubin PJ, Kolls JK. 2007. Th17 cells and mucosal host defense. Semin. Immunol. 19:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Packiam M, Veit SJ, Anderson DJ, Ingalls RR, Jerse AE. 2010. Mouse strain-dependent differences in susceptibility to Neisseria gonorrhoeae infection and induction of innate immune responses. Infect. Immun. 78:433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wira CR, et al. 2010. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am. J. Reprod. Immunol. 63:544–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen MS, et al. 1994. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169:532–537 [DOI] [PubMed] [Google Scholar]
- 31. Weigmann B, et al. 2007. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2:2307–2311 [DOI] [PubMed] [Google Scholar]
- 32. Nawar HF, Arce S, Russell MW, Connell TD. 2005. Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities. Infect. Immun. 73:1330–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]