Abstract
Many human diseases result from mutations in specific genes. Once translated, the resulting aberrant proteins may be functionally competent and produced at near-normal levels. However, because of the mutations, the proteins are recognized by the quality control system of the endoplasmic reticulum and are not processed or trafficked correctly, ultimately leading to cellular dysfunction and disease. Pharmacological chaperones (PCs) are small molecules designed to mitigate this problem by selectively binding and stabilizing their target protein, thus reducing premature degradation, facilitating intracellular trafficking, and increasing cellular activity. Partial or complete restoration of normal function by PCs has been shown for numerous types of mutant proteins, including secreted proteins, transcription factors, ion channels, G protein-coupled receptors, and, importantly, lysosomal enzymes. Collectively, lysosomal storage disorders (LSDs) result from genetic mutations in the genes that encode specific lysosomal enzymes, leading to a deficiency in essential enzymatic activity and cellular accumulation of the respective substrate. To date, over 50 different LSDs have been identified, several of which are treated clinically with enzyme replacement therapy or substrate reduction therapy, although insufficiently in some cases. Importantly, a wide range of in vitro assays are now available to measure mutant lysosomal enzyme interaction with and stabilization by PCs, as well as subsequent increases in cellular enzyme levels and function. The application of these assays to the identification and characterization of candidate PCs for mutant lysosomal enzymes will be discussed in this review. In addition, considerations for the successful in vivo use and development of PCs to treat LSDs will be discussed.
Introduction
Lysosomes are membrane-bound acidic organelles that contain over 50 different acid hydrolases that are responsible for the catabolism of a wide range of different macromolecules, including glycosphingolipids, glycogen, mucopolysaccharides, oligosaccharides, cholesterol, peptides, and glycoproteins.1 The deficiency of any individual lysosomal enzyme leads to a lysosomal storage disorder (LSD), which is characterized by the pathological accumulation of the deficient enzyme's substrate in various cells, tissues, and organs throughout the body. To date, over 50 different LSDs have been identified.2,3 Substrate storage in lysosomes leads to chronic and progressive clinical syndromes that often display wide spectrums of severity that are unique to each LSD.2,3 The more severe, early-onset forms of these diseases are typically diagnosed in infancy through early childhood, and are characterized by little-to-no residual enzyme activity and severe clinical manifestations that may involve impairment of central nervous system (CNS) function.4 In contrast, the later-onset forms are typically diagnosed from adolescence through adulthood, are associated with significant residual enzyme activity, and show a more mild clinical presentation that is often restricted to peripheral pathology.4 Most LSDs are autosomal recessive, though a few have an X-linked inheritance pattern. The overall prevalence of LSDs is estimated to be 1:1,500–1:7,000 live births.5
The goal of all current therapies for LSDs, whether approved or experimental, is the reduction of accumulated substrate in lysosomes. Currently, enzyme replacement therapy (ERT) and small-molecule substrate reduction therapy (SRT) represent two of the primary treatment options that are approved for patients with some LSDs.6 ERT is based on the intravenous administration of a recombinant form of the deficient enzyme, and was first approved to treat the peripheral manifestations associated with the most common LSD, Gaucher disease.7,8 Thereafter, ERT products for Fabry disease,9–12 Pompe disease,13–16 and mucopolysaccharidoses (MPS) I,17,18 II,19 and VI20,21 followed (Table 1). In many cases, ERT leads to a reduction of lysosomal substrate load in a patient's cells and tissues, and improvements in clinical outcome. However, the CNS manifestations of these diseases do not respond well to ERT due to the inability of these exogenous enzymes to cross the blood–brain barrier.6,22 Similarly, delivery of infused enzyme to other disease-relevant cells, tissues, and organs is insufficient in certain cases.6,22 In addition, the infused enzyme can be immunogenic, which may limit efficacy23–28 and/or adversely affect tolerability.29–32 As these drugs are not orally available, lengthy periodic (typically biweekly) infusions are necessary, often in a hospital setting. In contrast, SRT drugs, only one of which is approved, have the potential for oral bioavailability, broad tissue distribution, and better CNS penetration, as the therapeutic agent is a small molecule. Zavesca® (N-butyl-1-deoxynojirimycin, miglustat) is currently approved for use in patients with mild-to-moderate Gaucher disease without CNS involvement.33–35 Zavesca reduces cellular substrate levels via inhibition of glucosyltransferase, the enzyme responsible for the synthesis of the substrate that accumulates in Gaucher disease, glucosylceramide (GlcCer). Zavesca has also been evaluated in neuronopathic Gaucher patients, though no significant benefit for the neurological manifestations in these patients was seen.36 Importantly, many patients treated with Zavesca experience side effects, including diarrhea, weight loss, tremor, and peripheral neuropathy,37 thus limiting broad clinical utility. More recently, Zavesca was approved in the European Union and several other countries as a treatment for the progressive neurological manifestations of another LSD, Niemann-Pick type C disease.38 In addition to Zavesca, a second-generation small molecule SRT drug for Gaucher disease has recently shown promise in Phase 2 studies, supporting further clinical development.39 Eliglustat tartrate is an orally active glucosyltransferase inhibitor for the potential treatment of Gaucher disease and other LSDs.39 Lastly, as an alternative to small molecule therapy, hematopoietic stem cell therapy has been successfully used for the treatment of some LSDs, including MPS I (Hurler disease), metachromatic leukodystrophy, Krabbe's disease (globoid cell leukodystropy), and α-mannosidosis.6
Table 1.
Approved Therapies and Pharmacological Chaperones for Lysosomal Storage Disorders
| |
|
|
Pharmacological Chaperones |
||
|---|---|---|---|---|---|
| Disease | Deficient Enzyme | Approved Drug(s)a | Name | Status | References |
| Fabry | α-Galactosidase A | Fabrazyme® (agalsidase beta)Replagal™ (agalsidase alpha) | Galactose | Preclinical | 164 |
| Case Study | 165 | ||||
| DGJ (AT1001; Amigal™) | Phase 3 | 40, 75, 131, 166 | |||
| Gaucher | Acid β-Glucosidase | Cerezyme® (imiglucerase)VPRIV™ (velaglucerase alfa) Zavesca® (miglustat; NB-DNJ) | α-allo-HNJ; α-galacto-HNJ; β-1-C-butyl-DGJ | Preclinical | 167 |
| DIA | Preclinical | 168 | |||
| NN-DNJ | Preclinical | 64, 126, 127, 169, 170 | |||
| N-(7-oxadecyl)DNJ | Preclinical | 127 | |||
| N-(n-octyl)DNJ | Preclinical | 127, 170 | |||
| NOV | Preclinical | 161 | |||
| Castanospermine; N-(n-octyl)IFG; PDMP; morpholine- and piperazine-substituted alkylated nitrogen heterocycles; N-octyl-2,5-dideoxy-2,5-imino-D-glucitol | Preclinical | 126 | |||
| CO-DNJ and CN-DNJ | Preclinical | 170 | |||
| N-hexanoic acid adamantyl amide DNJ | Preclinical | 64 | |||
| Calystegine derivatives; DIX | Preclinical | 171 | |||
| IFG (AT2101) | Phase 2 | 65, 70, 76 | |||
| 5-((4-methylphenyl)thio)-quinazoline 2,4-diamine | Preclinical | 108 | |||
| 5-(3,5-dichlorophenoxy)-N-(4-pyridinyl)-2-furamide | Preclinical | 108 | |||
| NOI-NJ, 6S-NOI-NJ, 6N-NOI-NJ, 6S-NOI-GNJ | Preclinical | 172 | |||
| Diltiazem | Preclinical | 107 | |||
| Ambroxol | Investigator-initiated pilot study | 61, 173, www.Gaucher.org | |||
| NB-DNJ, Aminocyclitol 1, Aminocyclitol 4 | Preclinical | 169 | |||
| Dansyl-capped N-substituted DNJ derivatives 10 and 11 | Preclinical | 173 | |||
| 6S-NDI-NJ | Preclinical | 174 | |||
| 2-O-alkly iminoxylitol derivatives | Preclinical | 175 | |||
| GM1 Gangliosidosis (Morquio B) | Acid β-Galactosidase | None | NOEV | Preclinical | 138, 176 |
| DGJ, NB-DGJ | Preclinical | 138, 177 | |||
| Galactose | Preclinical | 178 | |||
| DLHex-DGJ | Preclinical | 179 | |||
| DGJ derivatives (compounds 17, 18, 22) | Preclinical | 180 | |||
| Fluorous iminoalditols 6–8 | Preclinical | 181 | |||
| GM2 Gangliosidosis (Tay-Sachs / Sandhoff) | Acid β-Hexosaminidase | None | NGT | Preclinical | 59, 182 |
| AdDNJ; ADNJ; ACAS | Preclinical | 182 | |||
| M-22971 (nitro-indan-1-one); M-45373 (pyrrolo[3,4-d]pyridazin-1-one); M-31850 (bisnaphthalimide) | Preclinical | 59 | |||
| Pyrimethamine | Phase 2 | 58, 183 | |||
| N-benzyl LABNAc | Preclinical | 184 | |||
| Pompe | Acid α-Glucosidase | Myozyme® (alglucosidase alfa) Lumizyme® (alglucosidase alfa) | DNJ (AT2220) | Phase 2 | 62, 72, 73 |
| NB-DNJ (miglustat) | Preclinical | 72, 73 | |||
| NO-DNJ | Preclinical | 72 | |||
| Krabbe | Galactocerebrosidase | None | α-Lobeline | Preclinical | 185 |
| Batten | Palmitoyl:protein thioesterase | None | CS38 | Preclinical | 186 |
| MPS I (Hurler / Hurler-Scheie) | α-L-iduronidase | Aldurazyme® (laronidase) | None | ||
| MPS II (Hunter) | Iduronate sulphate sulphatase | Elaprase® (idursulfase) | None | ||
| MPS IIIC (Sanfilippo Syndrome type C) | Heparan sulfate acetyl-CoA: α-glucosaminidine N-acetyltransferase | None | Glucosamine | Preclinical | 187 |
| MPS VI (Maroteaux-Lamy) | N-acetylgalactosamine-4-sulfatase | Naglazyme® (galsulfase) | None | ||
All approved drugs are enzyme replacement therapies, with the exception of Zavesca, which is an substrate reduction therapy.
ACAS, 6-acetamido-6-deoxycastanospermine; AdDNJ, 2-acetamido-1,2-dideoxynojirimycin; ADNJ, 2-aceto-2-deoxynojirimycin; CN-DNJ, α-1-C-nonyl-1-deoxynojirimycin; CO-DNJ, α-1-C-octyl-1-deoxynojirimycin; DGJ, 1-deoxygalactonojirimycin; DIA, 2,5-dideoxy-2,5-imino-D-altritol; DIX, 1,5-dideoxy-1,5-iminoxylitol; DNJ, 1-deoxynojirimycin; HNJ, homonojirimycin; α-allo-HNJ, homoallonojirimycin; DLHex-DGJ, methyl 6-{[N2-(dansyl)-N6-(1,5-dideoxy-D-galactitol-1,5-diyl)-L-lysyl]amino} hexonate; IFG, isofagomine; LABNAc, 2-acetamido-1,4-imino-1,2,4-trideoxy-L-arabinitol; NB-DGJ, N-butyl-1-deoxygalactonojirimycin; NB-DNJ, N-butyl-1-deoxynojirimycin; NGT, N-acetyl-glucosamine-thiazoline; NN-DNJ, N-(n-nonly)-deoxynojirimicin; NO-DNJ, N-(7-oxadecyl) deoxynojirimycin; NOEV, N-octyl-4-epi-β-valienamine; 6S-NDI-NJ, 6-thio-(5N,6S)-[4-(N′-dansylamino)butylmethylidene]nojirimycin; 6S-NOI-GNJ, 5-N,6-thio-(N′-octyliminomethylidene)galactonojirimycin; NOI-NJ, 5-N,6-O-(N′-octyliminomethylidene)nojirimycin; 6N-NOI-NJ, 6-amino-6-deoxy-5,6-di-N-(N′-octyliminomethylidene)nojirimycin; 6S-NOI-NJ, 5-N,6-thio-(N′-octyliminomethylidene)nojirimycin; NOV, N-octyl-β-valienamine; PDMP, 1-phenyl-2-decanoylamino-3-morpholino-1-propanol; MPS, mucopolysaccharidoses.
Over the past decade, pharmacological chaperone (PC) therapy has been proposed and investigated as a potential treatment for many genetic diseases that result from misfolded and/or unstable proteins, including LSDs.4,40,41 Small molecule PCs are designed to selectively bind and stabilize mutant proteins, thereby facilitating proper folding and intracellular trafficking, and increasing total cellular levels and activity. Similar to SRT, PCs are low-molecular-weight molecules, and thus have the potential to be orally available with broad biodistribution, including the CNS. Proof of concept has now been established for numerous PCs at the cellular-, animal-, and clinical-level for the mutant lysosomal enzymes associated with a number of LSDs (Table 1). This review will first detail the proposed mechanism of action of PCs for lysosomal enzymes, followed by the strategies and assays that have been utilized to identify and characterize the pharmacological properties of these molecules both in vitro and in vivo, and will close with a discussion around considerations for their therapeutic use.
Mechanism of Action
Inherited mutations can alter the structure and function of lysosomal enzymes to varying degrees. Large deletions, insertions, truncations, or frameshift mutations often lead to the loss of entire protein domains that grossly alter structure and function, and may even result in the complete loss of expression. Similarly, splice site mutations can lead to incorrect processing of mRNA precursors, including exon skipping or splicing at cryptic splice points, resulting in gross structural and functional alterations. Small in-frame deletions and insertions, or missense mutations that result in a single base pair substitution in the coding sequence, can lead to more subtle changes in structure that may influence mRNA expression, protein folding, protein stability, intracellular trafficking, substrate binding, catalytic competency, and/or enzyme turnover rate. Many of the mutations in lysosomal enzymes that cause human LSDs are missense, and may result in less stable or trafficking-defective enzymes.42
Folding and maturation of lysosomal enzymes, like many other proteins, are monitored by the quality control system of the endoplasmic reticulum (ER).43 Only those proteins that are correctly folded and stable leave this cellular compartment efficiently and progress through the secretory pathway to their final destination in the lysosome.44 The primary quality control mechanisms in the ER rely on molecular chaperones and folding factors, such as BiP, calnexin, calreticulin, thiol-disulfide oxidoreductases, and protein disulfide isomerase. These molecular chaperones recognize common structural features, which may include exposed hydrophobic regions, unpaired cysteine residues, or aggregation, to distinguish stable, native protein conformations from unstable, non-native ones.45–47 In general, the mechanisms that distinguish native from non-native conformations and assist in folding of lysosomal enzymes have only begun to be elucidated.48,49 If, despite the action of the molecular chaperones, folding of the nascent protein fails, it is recognized by the ER quality control system as aberrant and is targeted for degradation. This ER-associated degradation involves polyubiquitination and translocation to the cytosol, where the less stable or misfolded enzymes are subjected to proteasomal degradation.50
While highly efficient, in some cases the ER quality control may recognize mutant enzymes that retain catalytic activity, or that have only modestly compromised function. As a consequence, slight modifications in protein stability or conformation, as is seen with many lysosomal enzymes that have missense mutations, may prevent release from the ER and result in premature degradation and a loss-of-function phenotype.4,51 Several types of interventions that have the potential to rescue mutant proteins from premature degradation and restore function have been investigated. Importantly, cell-permeant small molecules that selectively bind to their target mutant protein may confer enhanced thermodynamic stability and facilitate proper transport through the secretory pathway. These molecules appear to act primarily in the ER during biosynthesis, where they facilitate the release of the mutant proteins from the ER quality control mechanisms, preventing premature degradation, and promoting transport through the Golgi, and ultimately to the lysosome.4 Increased stability and restoration of normal cellular trafficking may also relieve stress on the ER that results from protein accumulation, and may minimize the toxic consequences of protein aggregation.52,53 Because these small molecules bind specifically to their target protein and promote proper cellular trafficking, they have been termed PCs.
PCs are distinct from small molecule chemical chaperones, such as glycerol, dimethylsulfoxide, and trimethylamine N-oxide, which can also stabilize mutant proteins and increase cellular levels. While both chemical and PCs have been shown to be very effective at promoting protein folding in the ER and subsequent trafficking through the secretory pathway in vitro, very high concentrations of chemical chaperones typically are required to see an effect. Further, chemical chaperones act nonspecifically on many proteins, raising the possibility that they could lead to premature ER release of folding intermediates for normal proteins, some of which could lack stability and have a propensity for aggregation and toxicity in the post-ER environment.54 PCs, on the other hand, are designed to specifically target the protein of interest, thereby eliciting little-to-no global perturbation of the ER quality control system and the general protein-folding environment. Because PCs specifically bind to their target proteins and can be selected to have suitably high affinity, concentrations that are lower than those used with chemical chaperones may be sufficient to lead to therapeutic benefit, reducing or preventing off-target side effects.55 In addition to lysosomal enzymes, restoration of partial or complete function by PCs has been shown for other types of mutated proteins, including G protein-coupled receptors, secreted proteins, transcription factors, ion channels, and transporters, that lead to such diseases as cystic fibrosis, hypercholesteremia, cataracts, Huntington's, Alzheimer's and Parkinson's diseases, retinitis pigmentosa, nephrogenic diabetes insipidus, and cancer. Importantly, a wide range of in vitro and in vivo assays is now available to measure mutant lysosomal enzyme interaction with and stabilization by PCs, as well as subsequent increases in cellular enzyme levels and function. The application of these assays for the identification and characterization of candidate PCs is shown in Figure 1, and will be discussed in this review. Additional considerations for the successful use and development of PCs are also provided.
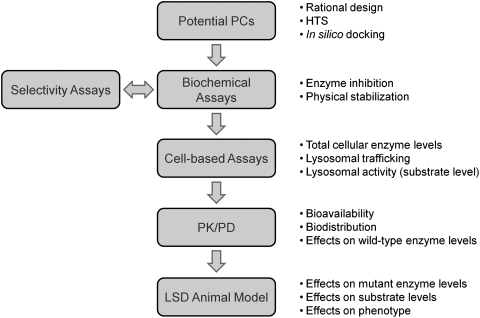
Fig. 1.
Schematic showing the key steps and activities that have been used to identify and characterize pharmacological chaperones (PCs) for lysosomal enzymes.
In Vitro Approaches to Identify and Characterize Pharmacological Chaperones for Lysosomal Enzymes
Identification of PCs for Lysosomal Enzymes
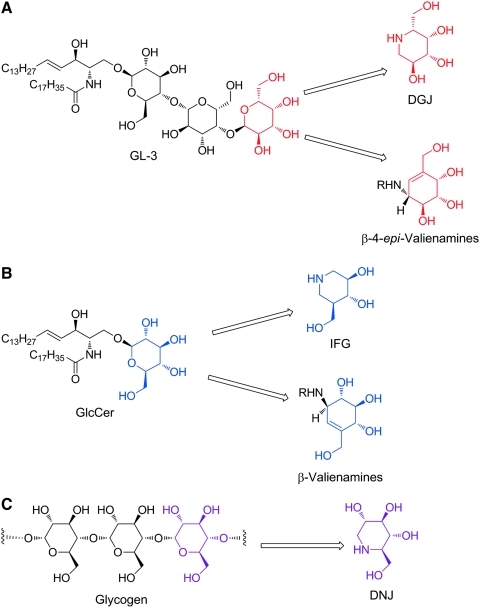
Rational drug design is a valuable strategy that has been instrumental in the identification of PCs for mutant lysosomal enzymes. Initially, Fan et al. rationalized that a small molecule mimic of the natural substrate might bind to and thereby stabilize the target enzyme, potentially acting as a PC (Fig. 2).40,56 More recently, crystallographic studies have indeed demonstrated that binding of some of these designed PCs to their target enzyme's active site is analogous to binding of the natural substrate.57 To date, all PCs that have been identified for LSDs (Table 1) have been shown, or are inferred based on structural similarity to key regions of the natural substrate, to interact with the active site of the target protein. Importantly, this rational design approach has led to the identification of three PC clinical candidates for Fabry, Gaucher, and Pompe disease (Fig. 2; Table 1),41 as well as providing starting points for further medicinal chemistry efforts, which may lead to second-generation compounds for these and other LSDs.
Fig. 2.
Rational drug design of PCs for lysosomal enzymes. (A) Structural homology of DGJ and GL-3. The iminosugar DGJ has a high level of structural homology with the terminal galactose of GL-3, the natural substrate of α-galactosidase (α-Gal A) that is deficient in Fabry disease. Based upon this homology, it was hypothesized that DGJ could bind to the active site of α-Gal A and act as a PC.40 For DGJ and related iminosugars, the nitrogen atom of the piperdine is analogous to the oxygen atom of galactose. The β-4-epi-valienamine carbasugars have also shown activity against α-galactosidases; however, this activity, as well as selectivity over other lysosomal enzymes, is generally poor, with higher activity toward β-galactosidases and β-glucosidases seen with increasing chain length (i.e., R>C8).159 (B) Structural homology of isofagomine (IFG) and glucosylceramide (GlcCer). The azasugar IFG is a mimic of GlcCer, the natural substrate of acid β-glucosidase (GCase) that is deficient in Gaucher disease. It has been proposed that the azasugar core of IFG (with the nitrogen atom replacing the anomeric carbon rather the oxygen) is responsible for the β selectivity of this compound, based on the geometries of transition states for the two possible glycosidic bond cleavages.160 Similarly, the carbasugar N-octyl valienamine (R=C8H17) acts as a PC for GCase.161 (C) Structural homology of 1-deoxynojirimycin (DNJ) and glycogen. Acid α-glucosidase (GAA), the enzyme that is deficient in Pompe disease, cleaves the α1–4, and to a lesser extent α1–6, glycosidic bonds of lysosomal glycogen to release glucose. The iminosugar DNJ contains a piperdine ring with an array of hydroxyl groups that closely resembles the terminal glucose unit of glycogen. This high level of structural similarity allows DNJ to bind and stabilize GAA. As with other iminosugars, the α- versus β-glucosidase activity of DNJ is based on a transition state mimetic in which the nitrogen atom is analogous to the oxygen atom of glucose.
As with any therapeutic target, high-throughput screening (HTS) of chemical libraries is also an important way to identify new lead compounds for medicinal chemistry development. This approach is particularly valuable because very often novel and unexpected structures are identified which can lead to both a better understanding of binding interactions in the active site and to the identification of structures that may have improved properties over those obtained from a rational design approach. Recently, HTS assays have been successfully utilized to identify novel PCs for a number of lysosomal enzymes.58–61 These assays have typically measured PC binding to the recombinant enzyme of interest using enzymatic inhibition assays or changes in physical stability.
Enzyme Inhibition Assays
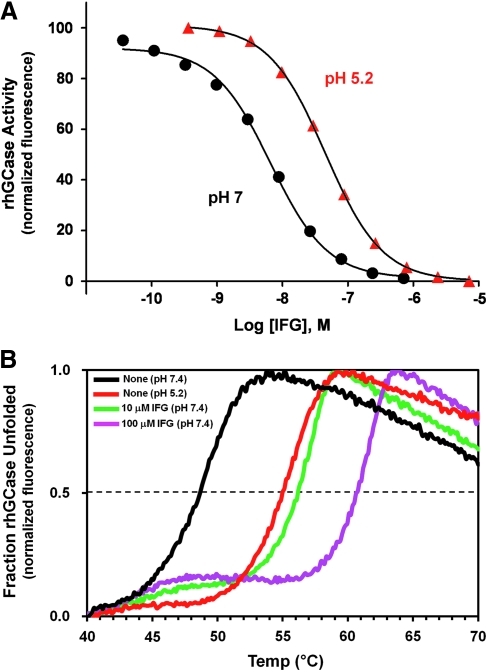
For LSDs, the preferred intracellular location for interaction between target hydrolases and small molecule PCs is in the ER rather than the lysosome. This distinction is important because all PCs identified to date are reversible, competitive inhibitors of their target enzymes. Ideally, a small molecule would bind with highest affinity in the neutral pH environment of the ER to promote PC activity (i.e., binding and stabilization), and would then bind with lower affinity in the acidic environment of the lysosome, thereby favoring dissociation and minimizing inhibition of the target enzyme. To this end, the effect of pH on target lysosomal enzyme binding affinity has now been described for a number of different PCs. For example, isofagomine (IFG), a PC that targets acid β-glucosidase (GCase), the enzyme that is deficient in Gaucher disease, binds with approximately sixfold higher affinity at pH 7.0 (concentration that yields 50% of maximal inhibition [IC50] value ∼7 nM) compared with pH 5.2 (IC50 value ∼44 nM) (Fig. 3A). Similarly, other PCs that target GCase, as well as PCs that target other lysosomal hydrolases (e.g., acid α-glucosidase, β-hexosaminidase, and α-galactosidase A) have been identified that demonstrate higher binding affinities at neutral pH compared with acidic pH.58,62,63 These enzyme inhibition assays can be configured using a variety of fluorogenic or chromogenic substrates that are now commercially available. When feasible, these assays should be conducted using purified recombinant enzymes, or lysates from cells that highly overexpress the enzyme of interest to minimize interference from related endogenous hydrolases that may be present in crude lysed-cell preparations and that have activity toward the artificial substrates. The selectivity of a PC can also be interrogated via the development of assays for hydrolases that have activities similar to the lysosomal enzyme of interest. While these approaches optimally would use mutant enzymes, the ability to express and purify large quantities of these proteins has proven difficult; hence, wild-type enzymes have traditionally been used for these purposes.
Fig. 3.
Enzyme inhibition and thermal stability assays can be used to characterize PCs for lysosomal enzymes. (A) Inhibition of recombinant human acid β-glucosidase (rhGCase) activity at pH 7 (endoplasmic reticulum pH) and pH 5.2 (lysosomal pH) as a function of IFG concentration. Inhibition of rhGCase activity by IFG was measured with the fluorogenic substrate 4-methylumbelliferyl-β-D-glucopyranoside (Sigma-Aldrich, St. Louis, MO). As seen in the enzyme inhibition curves, IFG binds with approximately sixfold higher affinity to rhGCase at neutral pH (circles) compared with acidic pH (triangles). (B) Thermal stability scans of rhGCase in the absence and presence of increasing concentrations of IFG. The unfolding of rhGCase was monitored using SYPRO Orange (Sigma-Aldrich). Binding of SYPRO Orange to exposed hydrophobic regions of a denatured protein results in increased fluorescence. rhGCase is physically more stable at acidic pH 5.2 (purple line) compared with neutral pH 7.4 (black line) as evident by a higher melting temperature at the lower pH. Likewise, as the concentration of IFG is increased at pH 7.4, rhGCase becomes more resistant to thermal denaturation (10 μM IFG, red line; 100 μM IFG, green line).
Assays to Measure Physical Stability
Various methodologies have been developed to monitor changes in the physical stability of lysosomal enzymes as a function of pH, temperature, and/or small molecule binding. For example, circular dichroism and activity assays were used by Kelly and colleagues to demonstrate the binding and stabilizing effects of small molecule inhibitors on GCase as a function of temperature.64 Similarly, Petsko and colleagues demonstrated small molecule-mediated stabilization of GCase and α-galactosidase A (α-Gal A), the enzyme that is deficient in Fabry disease, using differential scanning calorimetry.57 In addition, reporter dyes that fluoresce when bound to exposed hydrophobic amino acids have been used to monitor the degree of protein unfolding during thermal denaturation.62,65 In this assay, the fluorescence signal is proportional to the quantity of probe bound.66–68 Shifts toward higher melting temperatures (Tm) are seen with compounds that bind and stabilize the target proteins, as shown for GCase in the absence and presence to IFG (Fig. 3B). Any potential small molecule PCs that are identified in these thermal stability assays (i.e., via increases in the Tm) can subsequently be tested for enzyme inhibition, assuming that an in vitro activity assay is available.
While thermal stability assays report on global protein stability, hydrogen/deuterium exchange-mass spectrometery (H/D-MS), a technique that examines local protein structure, stability, and dynamics by monitoring the average rates of deuteration, has become a notable addition to drug discovery as improvements in the technique's throughput capability have been achieved.69 Recently, Mahuran and colleagues used H/D-MS to probe binding and stabilizing interactions of small molecule inhibitors of GCase.61 Although traditional enzyme inhibition-based methods were used to screen a 50,000 compound library for GCase inhibitors, H/D-MS was used as an orthogonal approach to gain insight into the binding regions that become stabilized upon ligand binding.
Assays to Measure Total Cellular Enzyme Levels
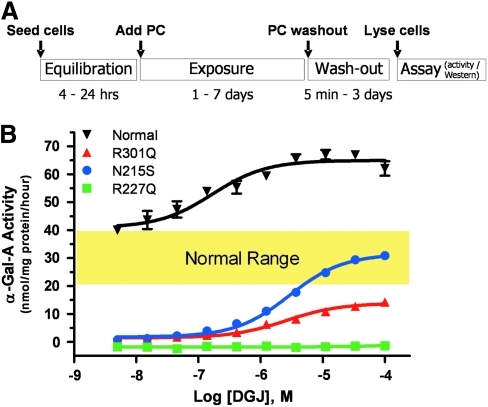
In addition to the properties described above, it is important to assess the effect of the PC at the cellular level. It is imperative that the PC readily penetrates both the plasma and ER membranes to allow interaction with the newly synthesized mutant enzymes during biosynthesis. The principle of the cell-based assay that has been widely used to show that incubation with a PC can increase the total cellular levels of the mutant enzyme (as measured by activity or quantification of the enzyme's protein levels in cell lysates) is outlined in Figure 4A. Cell-based assays that show increased total cellular levels of the enzyme after incubation with a PC indicate that at least the first steps in this process have been satisfied; that is, the PC has penetrated the plasma and ER membranes, and has bound and stabilized the mutant enzyme in the ER. These assays do not necessarily indicate that the mutant enzyme has trafficked to the lysosome and/or is able to metabolize endogenous substrate; for this, other assays are necessary (see below).
Fig. 4.
Assays to measure total cellular levels of lysosomal enzymes. (A) Principle of the cell-based assay. (B) DGJ increases α-galactosidase A (α-Gal A) levels in lymphoblastoid cell lines that were derived from normal human volunteers and Fabry patients. Representative α-Gal A activity in lysates from lymphoblasts (measured using 4-methylumbelliferyl-α-D-galactopyranoside [Sigma-Aldrich]) that were incubated with increasing concentrations of DGJ are shown. DGJ increased the levels of normal (inverted triangles), R301Q (triangles), and N215S (circles) α-Gal A, but had no effect on R227Q (squares) α-Gal A. These data demonstrate the varying degrees of correction that can be obtained for different mutant forms of a lysosomal enzyme, as well as the varying concentrations of PC that may be required. Color images available online at www.liebertonline.com/adt
Typically, primary fibroblasts or immortalized lymphoblastoid cell lines (LCLs) that were derived from individuals diagnosed with an LSD, such as Fabry, Gaucher, Pompe, Tay-Sachs, and Sandhoff disease, have been used to develop cell-based assays for PCs.40,62,65,70–76 While patient-derived dermal fibroblasts or LCLs often require a relatively long period to permanently establish (approximately 4–6 weeks for either cell type),77,78 they have been successfully used to identify new PCs via HTS, and to support lead optimization, as they are straightforward to maintain and manipulate in culture.74,79 Further, in patient-derived cells that are homo- or hemizygous for the disease-causing mutation, the effect of the PC on the endogenous mutant form of the deficient enzyme can be readily determined62,75 (Fig. 4B). Importantly, however, it is more challenging to identify PC-responsive mutant forms when using cells derived from patients that are heterozygous for two different mutant alleles, as it is difficult to interpret which mutant forms are expressed and responsive to the PC (see Pharmacogenetics section below).80
Two analytical methods have been extensively used to assess enzyme levels in the lysates from cells that were incubated with a PC, namely, western blotting and enzyme activity assays. Western blotting allows direct detection of the target enzyme protein levels based on molecular weight.81,82 The amount of total cell lysate, choice and concentration of antibodies, and choice of detection method (basic chemiluminescence, enhanced chemiluminescence, fluorescence, etc.) require optimization for sensitivity and for linearity of the concentration range of the target protein and the internal control protein. Importantly, the optimized conditions for measuring enzyme protein levels may be different for different mutant forms of an enzyme, or for the same form of the enzyme at baseline and after incubation with the PC. Western blotting has been used to clearly show increased total cellular levels of mutant forms of α-Gal A, GCase, acid α-glucosidase, β-hexosaminidase, and other enzymes after incubation with PCs in a variety of different cell types.59,62,71,75,76,83,84 More recently, different mutant forms of β-galactosidase were conjugated to dinoflagellate luciferase. Heterologous expression was then used to show PC-mediated increases in mutant enzyme levels via measurement of the associated luciferase activity in cell lysates. This approach has the potential to serve as an alternative to western blotting.85 These approaches do provide information on total cellular enzyme levels, but they do not provide any information as to whether these increased levels translate to increased activity.
The potential for a PC to have therapeutic efficacy in an LSD is minimally dictated by its ability to increase the total cellular activity of the mutant enzyme. For this purpose, the enzyme activity in lysates from cells incubated with the PC has commonly been measured using artificial, fluorogenic substrates (Fig. 4B).40,62,71,74–76 The enzyme activity is measured by mixing the cell lysate with exogenous substrate in a buffer that has been optimized for pH and other components necessary for the catalytic function of the target enzyme (as lysosomal hydrolases tend to have highest activity at acidic pH, these assays typically utilize low pH buffers to minimize metabolism of these artificial substrates by related cellular hydrolases that have higher pH optima). Importantly, lysate concentration and reaction time should be sufficiently sensitive to detect the often small quantities of product that are formed, and should be linear for product formation with respect to time and enzyme concentration. Again, the optimized conditions for measuring enzyme activity may be different for different mutant forms of an enzyme, or for the same form of the enzyme at baseline and after incubation with the PC. As such, studies that survey large numbers of mutant forms of a lysosomal enzyme and/or that investigate the concentration-dependence of the PC response may require the activity measurements to be conducted under several different conditions.86
It should also be noted that these cell-based assays alone cannot distinguish whether the elevated cellular enzyme levels are caused by a PC-mediated mechanism of action or via an alternate pathway or mechanism, thus necessitating the use of parallel assays such as thermostability or enzyme inhibition as discussed above.74 These complementary assays often utilize recombinant wild-type enzymes, as opposed to the cell-based assays that typically assess the effect of a PC on the endogenous mutant form of the enzyme. Hence, differences in compound affinity for wild-type versus mutant forms of a lysosomal enzyme can contribute to differences in the rank order of potency or efficacy between these assays. To circumvent these potential differences, the effects of PC incubation on fibroblasts or LCLs that were derived from normal individuals have been assessed in some cases. While the folding and trafficking of some wild-type lysosomal enzymes, like other proteins, can be inefficient,87 the relative increase in wild-type enzyme activity that can be typically achieved with a PC is often substantially lower than that achieved for the mutant enzymes (Fig. 4B),70,74,75 thereby limiting the sensitivity and signal-to-noise of this approach.
As all currently described PCs are reversible, competitive inhibitors of their target lysosomal enzymes, washout of a PC from cells may be necessary before cell lysis and assay to ensure that enzymatic activity is accurately measured. Carryover of residual PC into the enzymatic assay may interfere with the activity measurement, thereby masking the detection of increased enzyme levels. This phenomenon has been seen with wild-type and some mutant forms of α-Gal A, the enzyme deficient in Fabry disease, as well as with wild-type acid α-glucosidase (GAA), the enzyme deficient in Pompe disease, after incubation with high concentrations of the PCs 1-deoxygalactonojirimycin (DGJ) and 1-deoxynojirimycin (DNJ), respectively.62,83,84 Residual PC also was found to be a significant technical barrier to showing increased activity for mutant L444P GCase in Gaucher patient fibroblasts incubated with IFG.76 In the latter case, this hurdle was overcome with procedures designed to reduce IFG carryover into the enzymatic assay, including glycoprotein-enrichment, GCase-immunocapture, and overnight incubation of cells in IFG-free media before assay.76 Although the technically simplest approach is to provide an extended incubation time in the absence of PC to achieve a more complete washout, this timeframe can be limited by the half-life of the enzyme that has been chaperoned by the PC. As such, the PC washout time must be sufficiently shorter than the time required for the increased enzyme level to return to baseline. In many cases, the half-life of the mutant enzyme is shorter than the half-life of the wild-type enzyme.75,88 In addition, the half-life of the wild-type enzyme may vary somewhat from reported values due to differences in specific experimental conditions, such as cell type or cell growth conditions.88,89 Thus, to determine the PC responses of many different mutant forms of a lysosomal enzyme, evaluation of a range of PC concentrations and washout times was necessary to show a response to the PC.75 Even if the final assay to measure the cellular levels of the enzyme is not an activity assay, or the PC is designed to bind to an allosteric site to minimize inhibition of enzymatic activity (see section Considerations for the Therapeutics Use of Pharmacological Chaperones),90,91 excessive concentrations of PC should, nonetheless, be avoided. As with any small molecule that has pharmacological activity, exceedingly high concentrations may lead to nonspecific effects on other enzymes or cellular components, potentially affecting growth and viability of the cells, and thereby affecting the results of the experiment.
Assays to Measure Lysosomal Enzyme Trafficking
Subsequent to determining if a PC is able to elevate total cellular levels of its target enzyme, the ability of the PC to promote lysosomal trafficking requires evaluation. Screens for effective PCs should include assays for monitoring improvements in trafficking of target proteins, such as subcellular fractionation, proteolytic processing, glycan processing, and/or imaging-based subcellular localization.
Subcellular fractionation is the classical cell biological method for monitoring protein trafficking. The method involves homogenization of cells, isolation of organelle and membrane fractions (by ultracentrifugation, magnetic beads, etc.), and analysis of protein content by western blotting and/or enzyme activity coincident with established organelle-specific markers. Studies of mutant proteins in LSDs have used this approach to examine defects in trafficking, as well as PC-mediated improvements.40,49,70,76 However, the subcellular fractionation method can present technical challenges, such as the requirement for large amounts of cells (>106) and the high potential for incomplete separation of subcellular components. These challenges make adaptation into higher throughput formats difficult and often infeasible.
As an alternative, proteolytic processing of precursor target proteins into mature forms can be used as an indirect marker for protein trafficking, provided that the processing is coupled to trafficking. For example, GAA is synthesized in the ER as a 110-kDa glycoprotein precursor and is proteolytically processed into 70- and 76-kDa mature forms upon transport through the Golgi to lysosomes.92–94 Thus, this maturation process has been used to monitor (via western blotting) improvements in trafficking of GAA after incubation with PCs.62,72,73 Proteolytic processing has also been seen for a number of other lysosomal enzymes, including α-mannosidase,95 GCase,95 β-glucuronidase,96 α-fucosidase,97 β-hexosaminidase,98 and others,99–101 and thus could be used to monitor their trafficking as well. Monitoring proteolytic processing may also be adaptable to high-throughput methodologies. For example, antibodies specific to the mature forms of GAA102 could be used to develop high-throughput enzyme-linked immunosorbent assays that detect PC-mediated increases in the mature forms.
Similarly, glycan processing of lysosomal enzymes has been used as a marker for protein trafficking. As glycosylated proteins traffic through the secretory pathway, their glycan chains are modified and remodeled by resident glycosyltransferases and glycosidases.103 In principle, such changes can be detected by protein glycosylation analysis.104 For example, the glycan chains of GAA undergo extensive processing as the enzyme traffics through the secretory pathway; however, the glycan processing of GAA can be quite hetereogenous,93 making it difficult to unambiguously detect trafficked GAA. Nevertheless, this type of approach may also be adapted into high-throughput methods in theory, provided glycan processing of the target is relatively homogeneous. In that case, antibodies or lectins that recognize the processed glycan of interest could be used to develop plate-based methods to monitor enzyme trafficking in the absence or presence of potential PCs.
Perhaps the most robust method for monitoring protein trafficking is imaging-based subcellular localization. This method utilizes fluorescence-based microscopy to simultaneously monitor the target enzyme within the cell (via target-specific antibodies or genetically encoded tags) and organelle-specific markers. Studies of lysosomal enzyme trafficking typically monitor lysosome-associated membrane protein-1, a lysosomal-resident transmembrane protein,105 as a marker for colocalization. Moreover, many investigators have used this approach to examine aberrant trafficking of mutant enzymes and the subsequent restoration of normal trafficking after incubation of cells with candidate PCs.49,58,62,72,73,76,106–108 Recent technological and analytical advances, such as the development of several high-content imaging platforms capable of monitoring cells in 96- and 384-well formats, have made fluorescence-based microscopy amenable to screening large compound libraries for candidate PCs.109,110
Assays to Measure In Situ Lysosomal Enzyme Activity Using Artificial Substrates
The cell-based assays highlighted above indicate the cellular location of mutant enzymes before and after incubation with a PC. However, an important concern in the successful development of a PC is target inhibition by candidate compounds. In an ideal situation, PCs would act purely as agonists or activators, stabilizing mutant enzymes, correcting folding defects, and stimulating activity. However, all PCs identified to date bind to the active sites of their target enzymes and act as reversible, competitive inhibitors (described above). Selecting for compounds that are effective chaperones but weak inhibitors in vitro would greatly aid in the development of good development candidates.
As discussed above, one approach to reduce chaperone inhibition in the lysosome is to select for compounds that have higher affinity for their target enzyme in the ER, compared with the affinity in the lysosome. An alternate and complementary approach is to select for compounds that rapidly leave the lysosome (and the cell) after the mutant enzyme has trafficked to the lysosome. An enzyme assay that measures activity in the lysosomes of intact, living cells (i.e., in situ) can be employed for both of these approaches. With an in situ enzyme activity assay, the potency of inhibition can be measured for potential PCs in the lysosome, with candidates that show lower potency for inhibition receiving a higher priority during the selection process. An in situ enzyme assay can also measure inhibition as a function of time after compound removal. This application of the assay can provide a surrogate measure of compound efflux from the lysosome, with candidates that show faster efflux rates also receiving a higher priority during screening.
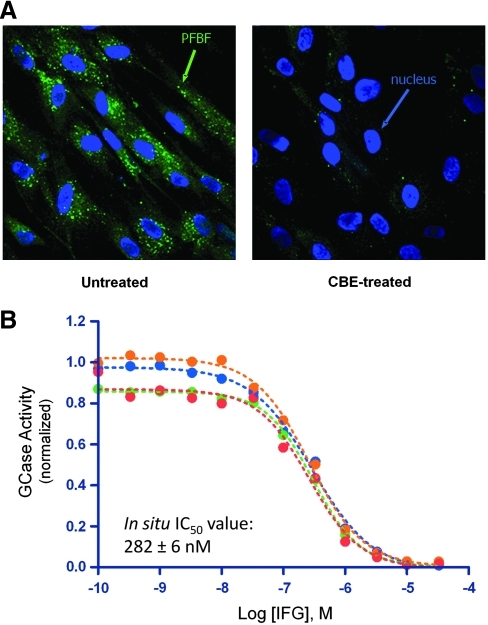
To date, in situ enzyme activity assays have been developed for a number of lysosomal enzymes, including GCase,111–117 α-Gal A,118,119 β-hexosaminidase,120,121 and β-galactosidase.122 For GCase, in situ assays have been developed using a number of different substrates.111–117 Most of these assays use fluorogenic substrates that enter the cell, presumably through fluid phase endocytosis, and are hydrolyzed by GCase within the endosomal/lysosomal system. These substrates were developed as tools for measuring enzyme activity in cells isolated from Gaucher patients, and have also been used to determine postinfusion uptake levels of recombinant human GCase during ERT.123 An in situ activity assay was also recently developed to measure the rate of lysosomal efflux of the Gaucher PC IFG utilizing the substrate 5-(pentafluorobenzoylamino)fluorescein-di-β-D-glucopyranoside (PFBF-β-glucose).70 The advantage of this substrate is that the enzymatically liberated PFBF fluorophore is rapidly conjugated to thiol groups124 and remains trapped inside the cell during the assay period.113 This trapping permits detection of activity within lysosomes by fluorescence microscopy (Fig. 5A), as well as within cells by conventional fluorescence plate readers and flow cytometry.
Fig. 5.
In situ assay for GCase activity reveals inhibition of lysosomal GCase by IFG. (A) The in situ substrate 5-(pentafluorobenzoylamino)fluorescein-di-β-D-glucopyranoside (PFBF-β-glucose; Invitrogen, Carlsbad, CA) is specifically hydrolyzed by lysosomal GCase within the intact cell. Human skin fibroblasts with wild-type levels of GCase were incubated with PFBF-β-glucose (500 μg/mL for 1 h at 37°C) in the absence or presence of conduritol-B-epoxide (0.25 μg/mL; Sigma-Aldrich), a selective, irreversible GCase inhibitor. Liberated PFBF fluorophore is evident as puncta throughout the cell (green) in untreated cells and is almost completely absent in cells incubated with conduritol-B-epoxide; nuclei are stained with Hoescht 33342 (Invitrogen) (blue). (B) IFG acts as a potent inhibitor of GCase. Lysosomal GCase activity was measured in situ in normal human skin fibroblasts incubated for 18–24 h with increasing concentrations of IFG, followed by PFBF-β-glucose (500 μg/mL for 1 h at 37°C). Four independent concentration–inhibition experiments are shown; the mean in situ concentration that yields 50% of maximal inhibition±SEM value for all experiments is indicated.
In addition to measuring efflux rates, the in situ GCase assay has also been used to characterize the potency of lysosomal GCase inhibition by IFG. IFG inhibits lysosomal GCase activity with an IC50 value of ∼280 nM (Fig. 5B). This is substantially lower affinity than discussed above using purified enzyme in a cell-free GCase inhibition assay (∼44 nM at pH 5.2), suggesting the potential for even less IFG-mediated inhibiton in an intact cell. As mentioned earlier, screens for future PC candidates for Gaucher disease can use in situ assays to select for compounds with reduced potency of lysosomal GCase inhibition. Compounds with lower potency would be preferred for further development.
Several studies have used an alternative method to measure GCase activity in intact cells.125–127 Unlike the in situ methods described above that use physiological culture media as vehicle for the fluorogenic substrate, this method calls for the incubation of cells with a 1:1 (v/v) mixture of PBS (pH 7.2) and 0.2 M acetate (pH 4.0) followed by addition of 4-methylumbelliferyl-β-glucopyranoside.127 This method most likely reflects enzyme activity that has been liberated by cell lysis rather than activity within intact cells, since the original description of the method showed that acetate buffer treatment of fibroblasts gave similar levels of enzyme activity as the use of freeze/thaw or detergent lysis methods.128 Similarly, low pH can reduce viability and membrane integrity of dermal fibroblasts.129
In addition, investigators have developed in situ assays for measuring the lysosomal activity of α-Gal A,118,119 β-hexosaminidase,121 and β-galactosidase,120,122 the enzymes deficient in Fabry disease, GM2 gangliosidosis, and GM1 gangliosidosis, respectively. For β-galactosidase in particular, a number of substrates that are suitable for the development of in situ assays are commercially available (www.invitrogen.com/site/us/en/home.html). Such assays could also be useful for disease diagnostics and as screening tools for candidate PCs for each of these targets.
As a cell-based fluorescence method, an in situ enzyme activity assay would be highly amenable to adaptation for HTS, depending on the enzyme levels in the cell type used and on whether inhibition or efflux is the desired endpoint. For cell types with high enzyme levels, in situ assays can be performed with most conventional fluorescence plate readers. For cell types with lower enzyme levels, high-content imaging platforms would be more appropriate for measuring correspondingly low activity levels. Moreover, high-content platforms can also assess the subcellular location of activity if organelle markers (such as Lysotracker Red; Molecular Probes, Eugene, OR) are also present. Inhibition assays would be readily adaptable as well, since they can be configured as homogeneous assays. Efflux assays present more of a challenge for high-throughput adaptation, since PC-containing medium needs to be removed and the cells incubated for some length of time post-removal.70
Assays to Measure Endogenous Substrate Levels
As an alternative to the development of an in situ assay that utilizes an exogenous artificial substrate, the ability of a PC to reduce endogenous substrate that has accumulated in patient-derived cells has also been investigated. Due to the complexities involved with this type of in situ cell-based assay, decreased levels of endogenous substrate in cultured cells in response to a PC have been shown only for the deficient enzymes associated with two LSDs, namely, Fabry and Gaucher disease.
In fibroblasts isolated from Fabry patients that express different mutant forms of α-Gal A, lysosomal levels of the substrate globotriaosylceramide (GL-3) were measured by double-labeling immunofluorescence confocal microscopy and quantification using monoclonal antibodies specific for GL-3 and mouse lysosome-associated membrane protein-1.49,130 Continuous incubation with DGJ (20 μM) decreased lysosomal GL-3 staining over time, with ∼50% maximum reductions seen after 6 days that were maintained for up to 100 days. Surprisingly, DGJ washout was not required to show this effect. Importantly, two of the cell lines that showed reduced GL-3 levels in response to DGJ expressed the R301Q and T194I missense mutant forms of α-Gal A, both of which have also shown increased cellular activity after incubation with DGJ.40,75,130 Curiously, the same effect was also seen in Fabry fibroblasts with nonsense mutations (V390fsX8 and Q357X) that do not show increased cellular α-Gal A activity after incubation with DGJ.49,130 Additionally, transient transfection of HEK-293 cells with cDNAs for these same two nonsense mutations showed no measureable baseline α-Gal A activity and no α-Gal A response (unpublished results). While encouraging for DGJ as a potential treatment for Fabry disease, these results, particularly those in the nonsense mutant cell lines, are difficult to interpret as a genuine PC effect that leads to restored lysosomal α-Gal A activity in these cells. In separate studies, total cellular GL-3 levels in Fabry fibroblasts seeded in 96-well microtiter plates were semiquantified using dual-immunolabel infrared fluorescence imaging and were normalized to actin levels.75 In fibroblasts with the DGJ-responsive mutant forms R301Q and L300P, 7-day incubation with DGJ followed by 3-day washout resulted in concentration-dependent maximal reductions in GL-3 levels of 45% and 38%, respectively. In contrast, continuous 10-day incubation with DGJ did not significantly reduce GL-3 levels in these cells. As expected, Fabry fibroblasts with a nonresponsive mutant form, C52S, also showed no reduction in GL-3 levels after DGJ incubation with or without washout. Thus, although the requirements for achieving decreased GL-3 levels in cells from the two studies differed, particularly with respect to the need for PC washout and the expression of a DGJ-responsive mutant form of α-Gal A, the combined results do indicate that incubation of some Fabry patient cell lines with DGJ can restore lysosomal α-Gal A function.
Similarly, levels of the endogenous substrate GlcCer were measured from cell pellets after solid phase extraction and liquid chromatography mass spectrometry in four different Gaucher patient cell lines that were homozygous for L444P GCase.76 All four cell lines showed elevated baseline levels of GlcCer as compared with normal control cells as well as increased cellular GCase activity after incubation with IFG. Glucosylceramide levels were reduced 23% to 50% after 7-day incubation with IFG (30 μM) and 3-day washout. Incubation with IFG for 10 days did not reduce GlcCer levels, whereas the positive control SRT, Zavesca, significantly decreased GlcCer levels up to 75%. Importantly, this study provided the first proof of concept for a PC-mediated restoration of lysosomal GCase function on endogenous substrate in cells derived from a Gaucher patient. Hopefully, these cell-based results for GCase, as well as for α-Gal A, will be extended to other mutant forms of these enzymes in the future and may serve as basic starting points for the design of new cell-based assays to assess the effects of PCs on endogenous substrate levels in cell lines derived from patients with other LSDs.
Assays that show decreased levels of the endogenous substrate in enzyme-deficient cells after incubation with a PC indicate that the function of the enzyme in the lysosome has been successfully restored. In combination with supportive results from the assays described above, restoration of in situ lysosomal enzyme function adds further support that the molecule acts as a PC for its intended enzyme target. Such results would thus warrant further evaluation of the bonafide PC in preclinical animal models, and ultimately in patients with the LSD.
Preclinical in Vivo Assessment of Pharmacological Chaperone Activity
Pharmacokinetics, Tissue Distribution, and Effects on Wild-Type Enzymes
In contrast to ERT, PCs have the potential for oral bioavailability and broad tissue distribution, including the CNS. Subsequent to the in vitro mechanistic and pharmacological studies described above, the pharmacokinetic properties and importantly the tissue distribution profile should be assessed to determine if the PC is orally available, and to ensure that it distributes to and achieves sufficient levels in tissues relevant to the LSD being treated. Importantly, administration of the PC at doses that achieve clinically relevant exposures in animals should result in cellular and tissue concentrations that allow binding to the mutant form of the hydrolase. In addition, the PC should be cleared from these cells and tissues in a relatively short period (preferably hours). Sensitive analytical techniques, such as mass spectrometry, have been used to measure the tissue concentrations of two PCs, IFG and DGJ, in wild-type mice.76,131 Both molecules showed high oral bioavailability and broad tissue distribution including the CNS. In addition, high tissue concentrations were achieved for both molecules after oral administration, indicating the potential for interaction with their intended targets in vivo. Lastly, both molecules were cleared from tissues with half-lives of less than 6 h, an important parameter to ensure that the PC quickly attains low tissue levels and dissociates from the enzyme, thereby allowing the enzyme to interact with and turn over accumulated substrate (see below).
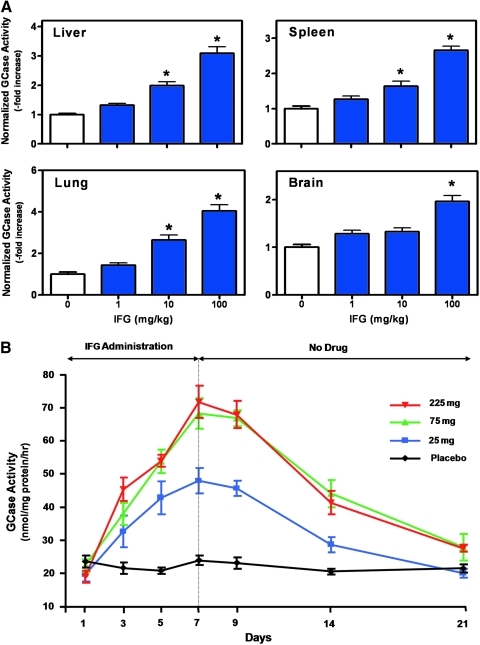
In some circumstances, wild-type mice can be used to investigate whether a PC interacts with its intended target in vivo. The synthesis and folding of some wild-type lysosomal enzymes in the ER and subsequent trafficking through the secretory pathway are not 100% efficient (i.e., only a fraction of the total synthesized enzyme is ultimately delivered to lysosomes).87 In these cases, a PC may stabilize the wild-type enzyme, thereby increasing tissue levels in vivo, a situation that is analogous to that described above for normal cells (Fig. 4B). This approach can also be used to indirectly monitor the tissue distribution of a PC, as elevated wild-type enzyme levels can provide a surrogate readout for biodistribution of the small molecule. As an example, the effects of IFG were investigated on wild-type GCase levels using C57BL/6 mice (Fig. 6A). Administration of IFG for 4 weeks resulted in dose-dependent and significant increases in GCase activity in tissues relevant to Gaucher disease, including liver, spleen, lung, and brain. Importantly, IFG administration did not affect the tissue levels of two other lysosomal hydrolases, α-Gal A and GAA (data not shown). Taken together, these data clearly indicate that IFG is orally available, has broad tissue distribution, including the brain, and interacts specifically with wild-type GCase in vivo. Similar results with DGJ and DNJ on α-Gal A63 and GAA,132 respectively, have been seen in wild-type mice. In cases where animal models for particular LSDs are unavailable, inappropriate, or are limited in number (lack of fertility, longevity, inability to thrive, etc.), the use of wild-type mice may provide a quick, reliable, and cost-effective way to evaluate the pharmacodynamic effects of a PC.
Fig. 6.
IFG increases wild-type GCase levels in animals and in humans. (A) Effect of IFG on wild-type mouse GCase. Eight-week-old male C57BL/6 mice were administered IFG ad libitum in drinking water for 4 weeks at the indicated doses. Animals were sacrificed and GCase activity was measured in tissue lysates as previously described using 4-methylumbelliferyl-β-D-glucopyranoside (Sigma-Aldrich).76 Dose-dependent and significant increases in GCase activity (*P<0.05 vs. untreated, t-test) were seen in all four disease-relevant tissues. The data presented have been normalized to untreated levels and represent the mean±SEM of 7 mice per group. Baseline tissue GCase activities were 70±4, 80±5, 37±2, and 40±42 nmol 4-methylumbelliferone/mg protein/h, in liver, spleen, lung, and brain, respectively. Animal husbandry and all in vivo experiments in mice were conducted under Institutional Animal Care and Use Committee–approved protocols. (B) Effect of IFG on normal human GCase. IFG was orally administered to healthy human volunteers once daily for 7 days at 25 mg (squares), 75 mg (triangles), or 225 mg (inverted triangles) as indicated. Blood was drawn on days 1 (predose), 3, 5, 7, 9, 14, and 21 for preparation of mononuclear cells. Cell lysates were used to measure GCase activity via 4-methylumbelliferyl-β-D-glucopyranoside hydrolysis. A time- and concentration-dependent increase in cellular GCase levels was observed, which persisted for up to 1 week after IFG withdrawal. Each point on the graph represents the mean±SD from 6 subjects administered IFG, or 2 subjects administered placebo (circles). Institutional review board approval was obtained for all centers involved in the human studies and all subjects gave written informed consent to participate. Color images available online at www.liebertonline.com/adt
Similarly, the ability to monitor the cellular levels of wild-type lysosomal enzymes has been instrumental in demonstrating a pharmacodynamic effect for PCs in the clinical setting. In Phase 1 studies, administration of IFG to healthy volunteers once daily for 7 days resulted in a time- and dose-dependent increase in GCase levels in peripheral blood mononuclear cells that were collected via routine blood draws periodically during the treatment period (Fig. 6B).133 GCase levels were elevated up to 3.5-fold compared with baseline levels, and remained elevated for up to 7 days after withdrawal of IFG. These data clearly indicate that IFG can interact with its intended target in humans, and that this interaction results in stabilization and increased GCase levels in vivo. Similar results have been demonstrated in Phase 1 studies with DGJ.134
Animal Models and Dose Optimization
In addition to the properties described above, the ability of a PC to restore enzymatic activity in the lysosomes of disease-relevant tissues also needs to be evaluated. Several mouse models for LSDs have been generated that can be used for testing and optimizing administration regimens for PCs. Importantly, these models express missense mutant forms of particular lysosomal enzymes that have been previously shown to respond to PC incubation in vitro. Further, these models express the mutant proteins on genetic backgrounds that lack endogenous, wild-type murine enzymes. Preferably, the expressed enzyme is a mutant form associated with human disease, and most preferably the model shows disease pathology similar to that seen in patients.4 Unfortunately, however, some of the mouse models for LSDs that are useful for evaluating PCs (i.e., express a missense mutant form of the enzyme) do not recapitulate the hallmarks associated with human disease. However, these can still serve as excellent biochemical models for monitoring the effects of the PC on enzyme and/or substrate levels in disease-relevant tissues.76,131,135,136 Mouse models that are currently available and relevant to the study of PCs for LSDs are presented in Table 2.
Table 2.
Animal Models of LSDs Amenable to Evaluating Pharmacological Chaperones
| |
|
Animal Model |
|
|
|---|---|---|---|---|
| Disease | Deficient Enzyme | Mutation | Species | References |
| Fabry disease | α-Galactosidase A | hR301Q TgM | GLA KO mouse | 136 |
| hR301Q Tg | GLA KO mouse | 131 | ||
| hR301Q TgG3S(+/−)M(+/−) | GLA KO mouse+GB3 synthase | 188 | ||
| Gaucher disease | β-Glucocerebrosidase | mL444P | KI mouse | 189, 190 |
| mV394L | KI mouse | 191 | ||
| mD409H | KI mouse | 191 | ||
| M4L/PS-NA | mV394L KI mouse crossed with prosaposin KO mouse (PS-NA) | 192 | ||
| M9H/PS-NA | mD409H KI mouse crossed with prosaposin KO mouse | 192 | ||
| C381Y/P467L | Sheep (spontaneous mutation) | 193 | ||
| Pompe disease | Acid α-glucosidase | hP545L Tg | GAA KO mouse | 132 |
| 1639delG | Japanese quail (spontaneous mutation) | 194 | ||
| Tay Sach's disease | β-Hexosaminidase A | P4694L | Flamingo (spontaneous mutation) | 195 |
| GM1 gangliosidosis (Morquio B) | β-Galactosidase | hR201C Tg | GLB1 KO mouse | 138 |
| Krabbe disease | Galactocerebrosidase | mH168C | Mouse (spontaneous mutation) | 196 |
| cC158S | Dog (spontaneous mutation) | 197 | ||
| MPS IIIA (Sanfillippo disease) | α-N-Acetylglucosaminidase | mD31N | Mouse (spontaneous mutation) | 198, 199 |
| MPS VI (Maroteaux-Lamy) | N-Acetylgalactosamine-4-sulfatase | fL476P | Cat (spontaneous mutation) | 200 |
KO, knockout; KI, knockin; Tg, transgenic.
Given that the current set of PCs compete with endogenous substrates for binding to the active sites of lysosomal enzymes, it is necessary to optimize the administration regimen to provide periods of binding and stabilization for chaperoning, followed by “off” periods for dissociation and clearance of the PC to maximize lysosomal enzyme in situ activity and substrate turnover. This is partially achieved by the reduced binding affinity between the PC and lysosomal enzyme as it traffics from the ER to the acidified lysosome (discussed above). In addition, high lysosomal concentrations of stored substrate help prevent reassociation of the PC to the enzyme once it has dissociated. Also important, however, is the difference in the tissue half-lives of the PC and the rescued mutant lysosomal enzyme. While PCs typically have relatively short tissue half-lives (on the order of hours), many mutant lysosomal enzymes are stable once delivered to the lysosome, often having lysosomal half-lives that can be similar to wild-type enzymes (typically days).75,88 Hence, detailed tissue distribution studies as described above are necessary to determine the quantity of PC delivered to disease-relevant tissues and the kinetics for distribution and clearance. Further, determination of the tissue half-life of the rescued lysosomal enzyme can be conducted in patient-derived cells, or ideally in relevant animal models. Taken together, this information can be used to design administration regimens that maximize lysosomal enzyme activity and substrate reduction.
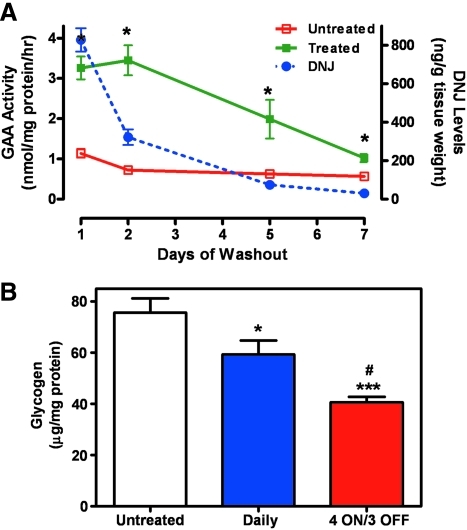
To this end, we recently generated a mouse model that expresses low levels of a PC-responsive mutant form of human GAA, P545L62,72,73 on a GAA knock-out background (hP545L GAA transgenic [Tg]/knockout [KO] mice).132 Importantly, these mice show progressive accumulation of glycogen in tissues that are relevant to Pompe disease, including the heart and skeletal muscles.137 Daily administration of the small molecule PC DNJ for 4 weeks to these mice significantly increased mutant GAA levels in skeletal muscle, which remained elevated above baseline levels for up to 7 days after DNJ withdrawal (Fig. 7A). The measured half-life of elevated hP545L GAA in these mice was significantly longer than that of DNJ, indicating that elevated hP545L GAA is stable in the absence of the PC. This difference was used to devise administration regimens that could produce large net gains in lysosomal GAA activity, as measured by the reduction in tissue glycogen levels. In this case, daily administration of DNJ for four consecutive days provided an “ON” period during which time enhanced protein stabilization and trafficking to lysosomes occurs, followed by a 3-day “OFF” period during which time DNJ dissociates from the enzyme and is cleared from the tissue to provide maximal in situ enzyme activity. Four cycles of this “4 ON/3 OFF” regimen yielded a twofold greater reduction in tissue glycogen levels as compared with daily administration (Fig. 7B), and importantly, utilized ∼40% less drug. In previous studies, similar strategies were used to maximize the in situ activity of mutant α-Gal A by DGJ in Fabry patient-derived cells75 and in a mouse model of Fabry mouse,131 as well as mutant GCase by IFG in Gaucher patient-derived cells.76
Fig. 7.
Optimization of PC administration to mice to maximize in situ enzyme activity and substrate reduction. (A) The half-life of elevated mutant GAA is significantly longer than the PC DNJ. Eight-week-old male hP545L GAA Tg/KO mice were administered drinking water (open rectangle) or DNJ (300 mg/kg per day (solid rectangle)) ad libitum in drinking water for 4 weeks, followed by a washout period (drinking water only) of up to 7 days. Groups of mice were then euthanized on day 1, 2, 5, or 7 after DNJ withdrawal. Gastrocnemius muscle was isolated at each time point and GAA activity was measured using 4-methylumbelliferyl-α-D-glucopyranoside (Sigma-Aldrich) as described previously.162 In addition, DNJ levels (dotted line) were measured in tissue lysates by liquid chromatography mass spectrometry. Mutant GAA activity was significantly increased (*P<0.05 vs. untreated, t-test) after 4-week DNJ administration, and was sustained above baseline for up to 7 days after DNJ withdrawal. The half-lives of elevated mutant GAA and DNJ in muscle were estimated to be 2.0 and 0.6 days, respectively. For the GAA activity and DNJ levels, each data point represents the mean±SEM of 7–8 mice/time point. The limit of quantitation for DNJ was 10 ng/g. (B) Less-frequent DNJ administration results in greater glycogen reduction. Eight-week-old male hP545L GAA Tg/KO mice were administered DNJ (100 mg/kg per day) ad libitum in drinking water for 4 weeks either daily or less-frequently using four cycles of a “4 ON/3 OFF” regimen. Glycogen levels were subsequently measured in lysates prepared from gastrocnemius as described previously.163 Significant reductions in glycogen levels were seen both with daily and “4 ON/3 OFF” regimens (*P<0.05 and ***P<0.001 vs. untreated, t-test), though reduction was significantly greater with the less-frequent regimen (approximately 30% reduction versus 60% reduction; #P<0.05 daily vs. “4 ON/3 OFF,” t-test). The data presented represent two independent experiments with the mean±SEM of 14–15 mice/group. Gycogen levels in gastocnemisus isolated from wild-type littermate mice were 20±2 μg/mg protein. Animal husbandry and all in vivo experiments in mice were conducted under Institutional Animal Care and Use Committee–approved protocols. Color images available online at www.liebertonline.com/adt
In the models described above, phenotypic similarity to human clinical presentation is lacking. However, in the case of GM1 gangliosidosis, a mouse model that expresses a hR201C missense mutation on a β-galactosidase-deficient background does show some neurological and behavioral deficits that are similar to those seen in patients.138 Administration of increasing doses of the β-galactosidase-selective PC N-octyl-4-epi-β-valienamine (NOEV) for 7 days to these mice increased β-galactosidase activity and decreased GM1 ganglioside levels in brain. Most importantly, 3- to 5-month administration of NOEV to 2-month-old mice prevented neurological deterioration as measured by a series of assays, including gait, posture (forelimb, hindlimb, trunk, and tail), avoidance response, rolling over, parachute reflex, and horizontal/vertical wire netting.139
In addition to mouse models, a number of other small and large animal models of LSDs have arisen from spontaneous mutations (Table 2). While the large animal models may be advantageous for the study of PCs due to their heterogeneous genetic backgrounds and phenotypes that often more closely mimic human disease, as well as their size and longevity that allow multiple and frequent samples to be drawn from the same animals over long periods, they generally are not practical for the types of preclinical dose optimization studies described above, as these studies typically require large numbers of animals and broad dose ranges (that necessitate significant quantities of drug). However, we believe that the knowledge gained through the dose optimization studies using mouse models of Pompe and Fabry disease could be applied to mouse models of other LSDs, and could be extended to larger species in more focused studies designed to evaluate the long-term effects of PCs on clinically-relevant endpoints.
Considerations for the Therapeutic use of Pharmacological Chaperones
Pharmacogenetics
Mutational heterogeneity represents a significant barrier to the development of therapies for inherited diseases. In many instances, the outcome of the genetic mutation on the transcribed protein is fairly easy to predict. For example, the presence of a premature stop codon early in the transcript could lead to a truncated enzyme that, even if delivered to the lysosome, would be unlikely to bind and metabolize substrate. In contrast, missense mutations that result in the substitution of a single amino acid in the encoded protein theoretically could have a number of effects: (1) loss of stability or misfolding, thus preventing folding into the native state and/or interfering with normal trafficking to the lysosome; (2) compromised formation or structure of the active site, thus substantially lowering affinity for, or completely abolishing the ability to bind, substrate; (3) compromised activity, thus preventing or substantially reducing the ability to metabolize substrate; (4) altered protein structure outside the active site, thus affecting domains that interact with other subunits and/or cofactors necessary for function. Mutant lysosomal enzymes that have compromised stability, but that retain catalytic competency, are most likely to be responsive to a PC. Mutant forms that are catalytically inactive, unable to bind substrate, cannot be effectively stabilized by a small molecule due to severe folding deficiencies, or that have gross alterations in structure, are unlikely to respond to a PC. Before testing a PC in a clinical setting, it is important to first clearly understand the nature of the molecular defect and its functional consequences in vitro. Efforts should then be focused on those mutant forms that are trafficking-defective and functionally rescuable, and hence define the potentially responsive patient population. In recent years, this has been accomplished using several approaches.
Patient-derived cells that are either homo- or hemizygous for a disease-causing mutation have been utilized to monitor the effects of PCs on the single mutant form that is expressed. For instance, a cell line that was derived from a Pompe patient homozygous for P545L GAA was responsive to the PC DNJ.62 Similarly, in cell lines derived from male Fabry patients, the measured α-Gal A responses to a PC are straightforward to interpret because Fabry disease is X-linked and males are hemizygous for the disease-causing mutation (i.e., only one mutant form is expressed) (Fig. 4B).75 Technically, however, patient-derived cell lines often require a relatively long period of time to establish (approximately 4–6 weeks),77,78 making this an inefficient ex vivo screening tool to quickly select patients for treatment with a PC. In contrast, differentiated T-cell cultures derived from a patient's peripheral blood mononuclear cells require only 4–10 days to establish and 3 days to test; thus, this approach has been successfully used to support Fabry patient selection for treatment with DGJ in Phase 2 clinical trials.83,84
While cell lines derived from patients that are heterozygous for two different mutant alleles have proven useful to investigate the effects of PCs, it is not possible to determine which of the two expressed mutant forms is responsive. This is problematic because many of the patients with diseases that are autosomal recessive, such as Gaucher, have complex genotypes with two different mutant alleles.80 In cell lines derived from these patients it is difficult to interpret which mutant form is responsive to the PC without additional information from other patient cell lines that are homozygous for one of the mutations. Similarly, this can be problematic in female patients with X-linked diseases. For instance, cell lines derived from most female Fabry patients cannot be used to investigate the effects of a PC due to random X-chromosome inactivation, which results in a mixture of cells that express either the wild-type or the mutant form of α-Gal. A. In these cell lines, the wild-type enzyme often dominates the α-Gal A activity that is measured. Further, wild-type α-Gal A is responsive to PCs (Fig. 4B), which tends to mask any PC activity on the coexpressed mutant form of α-Gal A.
To overcome the complexity of using patient-derived cells to investigate the effect of PCs on the cellular levels of mutant lysosomal enzymes, heterologous expression systems that use cell lines such as COS-7 or HEK-293 have been developed. In this case, mutant forms of a lysosomal enzyme are individually introduced via transient transfection and incubated in the absence or presence of a PC. The response of the mutant form is then monitored. Importantly, this approach requires that the mutant enzyme expression level after transfection or PC incubation exceeds that of the endogenous wild-type enzyme level in the host cell line.71,140 The endogenous mutant enzyme level at baseline or after PC incubation can then be subtracted, allowing the PC response of the heterologously expressed mutant form to be determined.140 Using this approach, at least 16 mutant forms of GAA were shown be responsive to DNJ in vitro.62,72,73 Similarly, 19 missense mutant forms of α-Gal A were shown to have increased protein levels and total cellular activity after expression in COS-7 cells and incubation with DGJ.71 Importantly, the responses to DGJ were consistent with those seen in cell lines derived from male Fabry patients that expressed the same mutant forms. Similar results were shown for many of these as well as other mutant forms of α-Gal A in additional heterologous expression studies.141,142 Most recently, a more comprehensive survey of the response to DGJ of more than 400 known Fabry disease-associated α-Gal A mutant forms heterologously expressed in HEK-293 cells was completed.143 The effects of DGJ on missense mutant forms (>90% of those tested) and several small in-frame insertion or deletion mutations were investigated. More than 60% of these mutant forms were responsive. Importantly, the HEK-293 cell responses of a small subset of missense mutant forms were generally consistent with the α-Gal A responses observed after oral administration of DGJ to male Fabry patients in Phase 2 clinical trials that expressed the same subset of mutant forms.86 Taken together, these results suggest that the in vitro α-Gal A response in HEK-293 cells may aid in the identification of Fabry patients who express mutant forms of α-Gal A that can respond to DGJ in vivo. Phase 3 clinical studies with DGJ are currently ongoing to further assess its safety and efficacy in Fabry patients that express mutant forms of α-Gal A that respond to DGJ in vitro.
Restoring Lysosmal Enzyme Function: How Much Activity Is Required for Clinical Response?
The age of onset, progression, and severity of an LSD is dependent to some extent on the rate at which the substrate accumulates, which in turn is partially dictated by the amount of enzymatic activity in lysosomes (determined by total enzyme level and catalytic competency).144 Complete lack of residual activity may lead to the rapid accumulation of substrate, and a more severe form of the disease (often early onset with rapid progression). In contrast, small quantities of residual activity may be sufficient to degrade a large proportion of the substrate, and would thus substantially slow the storage process, leading to a more mild form of the disease (later onset and slower progression). Further, the rate at which a substrate is delivered to the lysosome may influence the rate at which it accumlates, and may be different for different cell types, tissues, and organs.144 Considering these factors, it is anticipated that even modest increases in enzymatic activity may be sufficient to attenuate a severe clinical phenotype. In most LSDs, 1% to 6% of normal activity has been estimated to be sufficient to delay or prevent disease onset or to yield a more mild form of the disease. For instance, 1%–2% of normal α-iduronidase activity has been reported in mild cases of MPS I145; <1% of normal GCase activity has been reported in mild Gaucher disease146; 6% of normal β-galactosidase activity has been reported in late-onset GM1 gangliosidosis147; 2–4% of normal β-hexosaminidase activity has been reported in the adult form of GM2 gangliosidosis148; and 10% of normal enzyme activity seems to prevent GM1 and GM2 gangliosidoses altogether.147,149,150 Further, healthy individuals who are carriers of arylsulfatase A151–153 or β-hexosaminidase deficiency154,155 have been identified with extremely low residual enzyme activities that are typically seen in severely affected patients. These data suggest that small increases in activity could have a significant impact on substrate levels, and hence disease severity and the rate of disease progression.
It is important also to consider the relationship of the in vitro and in vivo responses of a mutant lysosomal enzyme to a PC with respect to clinical outcome. It is expected that a mutant lysosomal enzyme that shows no response in vitro would also not respond in vivo. However, predicting the in vivo response of a mutant lysosomal enzyme that does respond in vitro is more difficult, as cultured cells and/or animals are not perfect representatives of what occurs in the body of an LSD patient. For example, age, sex, treatment history, and disease status at the time of therapy initiation may affect the clinical outcome, with patients that have been severely affected for a prolonged period of time expected to have the lowest likelihood of therapeutic benefit. As discussed above, the pharmacokinetic profile of a PC, including its tissue distribution and clearance rate, may also influence the net response, especially if the elimination half-life is long or tissue penetration is poor. Further, given that mutant enzymes are often less stable than their wild-type counterparts, faster turnover rates could limit the time that a rescued enzyme is available to metabolize substrate.
The Next Generation of Pharmacological Chaperones?
The success of a PC depends critically on biophysical properties that dictate affinity, specificity, and reversibility of target binding, as well as cell permeability. For LSDs, the preferred intracellular location for interaction between target hydrolases and small molecule PCs is within the neutral environment of the ER rather than the acidic milieu of the lysosome, an important consideration given that all PCs identified to date are reversible, competitive inhibitors of their target enzymes. The pH differences of these compartments together with the pKa values of relevant PCs may provide a better understanding of the physical properties that are important for the binding interactions. For instance, whether a PC interacts with its target enzyme as a protonated amine or a neutral species, or whether a protonated species promotes dissociation of the enzyme/PC complex, may be important parameters to consider. In turn, this information would be expected to be of vital importance in guiding medicinal chemistry programs aimed at optimizing PC activity for new therapeutic candidates. While there are numerous approaches available for calculating pKa values, these do not accurately predict these values for some of the most common chemical classes of lysosomal enzyme PCs identified to date, such as iminosugars and azasugars. However, Bols and colleagues have developed an empirical formula that has proven useful in calculating pKa values for these compound classes.156 Hence, knowledge of the small molecule pKa values together with measurements of the affinity for the target enzyme at various relevant pH values may allow the identification of PCs that have much lower affinities for their target hydrolases in acidified lysosomes.
Similarly, another important area of current PC research involves the identification of allosteric sites that may be available for binding and stabilization of target proteins. Importantly, an allosteric ligand could stabilize the enzyme in the ER and lysosome while not inhibiting enzyme activity, in contrast to ligands that bind to the active site. To this end, it has been suggested that α-Gal A contains a second site that selectively binds the β-anomer over the α-anomer of D-galactose when a mixture of the two is soaked into the crystals.157 Further, using multiple solvent crystal structures, Ringe and coworkers identified a number of regions on the protein surface of GCase that colocalize with multiple solvent molecules such as methanol, glycerol, and phenol.158 A complementary computational fragment-based method (FTmap) was also employed by this group to detect hot spots on the protein surface of GCase. Results from both methods were then used to form a consensus for the existence of potential new binding regions that could be screened in silico to identify nonactive site PCs.158 It should be noted that in silico screening has been conducted on models of fully folded wild-type enzymes, which may be different than the mutant enzyme forms associated with the LSDs. Hence, it is unclear if these potential allosteric sites exist in an unfolded or partially folded mutant enzyme. It is also unknown if the occupation of these sites would preserve catalytic activity, as well as provide significant stability to the enzyme as seen with active site-binding PCs. Lastly, it is unclear if PCs can be identified that bind to these sites with sufficient affinity and specificity to prevent unwanted off-target effects. While identification of such compounds could lead to a significant advancement for the treatment of these genetic diseases, there are several important outstanding questions that remain unanswered.
Concluding Remarks
Collectively, LSDs represent a large and growing unmet medical need, with therapies either unavailable or insufficient to treat all of the underlying molecular defects and associated symptoms. Compared with ERT and SRT, PCs represent an alternative therapeutic strategy that aims to restore activity to mutant lysosomal enzymes naturally produced in the body that are often functionally competent but not trafficked in sufficient amounts to their intended physiological location, the lysosome. Compelling evidence for the utility of this approach has now been provided in vitro, in vivo, and in the clinical setting for several LSDs. However, to exert their beneficial effects on enzyme stability and trafficking, current PCs bind to the same sites as their endogenous substrates, potentially hindering full therapeutic benefit due to competitive inhibition. As such, this strategy presents challenges for clinical development that necessitate a thorough understanding and appropriate utilization of the pharmacological properties of the PCs, as well as the biological properties of the rescued enzymes. Importantly, many different approaches now exist to fully characterize the interactions of these molecules with their intended targets, to better understand the biological consequences of the restored activity, and to guide the use of these molecules in a clinical setting to maximize lysosomal activity and substrate reduction in key tissues and organs. It is anticipated that some of these challenges may be reduced or eliminated in the future via the identification of nonactive site-binding PCs. We are hopeful that PCs will soon deliver much-needed therapies to many of the patients that suffer with these debilitating, and in many cases, life-threatening diseases.
Abbreviations
- α-Gal A
α-galactosidase A
- CNS
central nervous system
- DGJ
1-deoxygalactonojirimycin
- DNJ
1-deoxynojirimycin
- ER
endoplasmic reticulum
- ERT
enzyme replacement therapy
- GAA
acid α-glucosidase
- GCase
acid β-glucosidase
- GL-3
globotriaosylceramide
- GlcCer
glucosylceramide
- H/D-MS
hydrogen/deuterium exchange-mass spectrometery
- HTS
high-throughput screening
- IC50
concentration that yields 50% of maximal inhibition
- IFG
isofagomine
- KO
knockout
- LCLs
lymphoblastoid cell lines
- LSD
lysosomal storage disorder
- MPS
mucopolysaccharidoses
- NB-DNJ
N-butyl-1-deoxynojirimycin
- NOEV
N-octyl-4-epi-β-valienamine
- PC
pharmacological chaperone
- PFBF
5-(pentafluorobenzoylamino)fluorescein
- rhGCase
recombinant human GCase
- SRT
substrate reduction therapy
- Tg
transgenic
- Tm
melting temperature
Acknowledgments
The authors wish to thank Tammy Allen for her assistance in the assembly of this article, and David Lockhart for his critical review and helpful suggestions.
Author Disclosure Statement
K.J.V., R.K., J.J.F., R.B., G.L., and E.R.B. are employees and shareholders of Amicus Therapeutics. A.C.P. is an employee and shareholder of ArunA Biomedical, Inc.
References
- 1.Hopwood J. Brooks D. An introduction to the basic science, biology of the lysosome, storage diseases. In: Applegarth D, editor; Dimmick J, editor; Hall J, editor. Organelle Diseases. Chapman & Hall Medical; New York: 1997. pp. 7–35. [Google Scholar]
- 2.Schopfer K. Miebach E. Beck M. Pitz S. Lysosomal storage diseases—update and new therapeutic options. Klin Monbl Augenheilkd. 2011;228:144–160. doi: 10.1055/s-0028-1109958. [DOI] [PubMed] [Google Scholar]
- 3.Parkinson-Lawrence EJ. Shandala T. Prodoehl M. Plew R. Borlace GN. Brooks DA. Lysosomal storage disease: revealing lysosomal function and physiology. Physiology. 2010;25:102–115. doi: 10.1152/physiol.00041.2009. [DOI] [PubMed] [Google Scholar]
- 4.Fan JQ. A counterintuitive approach to treat enzyme deficiencies: use of enzyme inhibitors for restoring mutant enzyme activity. Biol Chem. 2008;389:1–11. doi: 10.1515/BC.2008.009. [DOI] [PubMed] [Google Scholar]
- 5.Staretz-Chacham O. Lang TC. LaMarca ME. Krasnewich D. Sidransky E. Lysosomal storage disorders in the newborn. Pediatrics. 2009;123:1191–1207. doi: 10.1542/peds.2008-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck M. Therapy for lysosomal storage disorders. IUBMB Life. 2010;62:33–40. doi: 10.1002/iub.284. [DOI] [PubMed] [Google Scholar]
- 7.Figueroa ML. Rosenbloom BE. Kay AC. Garver P. Thurston DW. Koziol JA, et al. A less costly regimen of alglucerase to treat Gaucher's disease. N Engl J Med. 1992;327:1632–1636. doi: 10.1056/NEJM199212033272304. [DOI] [PubMed] [Google Scholar]
- 8.Barton NW. Brady RO. Dambrosia JM. Di Bisceglie AM. Doppelt SH. Hill SC, et al. Replacement therapy for inherited enzyme deficiency—macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med. 1991;324:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- 9.Eng CM. Banikazemi M. Gordon RE. Goldman M. Phelps R. Kim L, et al. A phase 1/2 clinical trial of enzyme replacement in fabry disease: pharmacokinetic, substrate clearance, and safety studies. Am J Hum Genet. 2001;68:711–722. doi: 10.1086/318809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiffmann R. Floeter MK. Dambrosia JM. Gupta S. Moore DF. Sharabi Y, et al. Enzyme replacement therapy improves peripheral nerve and sweat function in Fabry disease. Muscle Nerve. 2003;28:703–710. doi: 10.1002/mus.10497. [DOI] [PubMed] [Google Scholar]
- 11.Schiffmann R. Kopp JB. Austin Iii HA. Sabnis S. Moore DF. Weibel T, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285:2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- 12.West M. Nicholls K. Mehta A. Clarke JTR. Steiner R. Beck M, et al. Agalsidase alfa and kidney dysfunction in Fabry disease. J Am Soc Nephrol. 2009;20:1132–1139. doi: 10.1681/ASN.2008080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L-R. Chen C-A. Chiu S-N. Chien Y-H. Lee N-C. Lin M-T, et al. Reversal of cardiac dysfunction after enzyme replacement in patients with infantile-onset Pompe disease. J Pediatr. 2009;155:271–275. doi: 10.1016/j.jpeds.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Klinge L. Straub V. Neudorf U. Schaper J. Bosbach T. Gorlinger K, et al. Safety and efficacy of recombinant acid alpha-glucosidase (rhGAA) in patients with classical infantile Pompe disease: results of a phase II clinical trial. Neuromuscul Disord. 2005;15:24–31. doi: 10.1016/j.nmd.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Strothotte S. Strigl-Pill N. Grunert B. Kornblum C. Eger K. Wessig C, et al. Enzyme replacement therapy with alglucosidase alfa in 44 patients with late-onset glycogen storage disease type 2: 12-month results of an observational clinical trial. J Neurol. 2010;257:91–97. doi: 10.1007/s00415-009-5275-3. [DOI] [PubMed] [Google Scholar]
- 16.Thurberg BL. Lynch Maloney C. Vaccaro C. Afonso K. Tsai AC-H. Bossen E, et al. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for pompe disease. Lab Invest. 2006;86:1208–1220. doi: 10.1038/labinvest.3700484. [DOI] [PubMed] [Google Scholar]
- 17.Clarke LA. Wraith JE. Beck M. Kolodny EH. Pastores GM. Muenzer J, et al. Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I. Pediatrics. 2009;123:229–240. doi: 10.1542/peds.2007-3847. [DOI] [PubMed] [Google Scholar]
- 18.Wraith JE. Beck M. Lane R. van der Ploeg A. Shapiro E. Xue Y, et al. Enzyme replacement therapy in patients who have mucopolysaccharidosis I and are younger than 5 years: results of a multinational study of recombinant human α-L-iduronidase (laronidase) Pediatrics. 2007;120:e37–e46. doi: 10.1542/peds.2006-2156. [DOI] [PubMed] [Google Scholar]
- 19.Muenzer J. Gucsavas-Calikoglu M. McCandless SE. Schuetz TJ. Kimura A. A Phase I/II clinical trial of enzyme replacement therapy in mucopolysaccharidosis II (Hunter syndrome) Mol Genet Metab. 2007;90:329–337. doi: 10.1016/j.ymgme.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Harmatz P. Giugliani R. Schwartz D. Guffon N. Teles EL. Miranda MCS, et al. Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase. Mol Genet Metab. 2008;94:469–475. doi: 10.1016/j.ymgme.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Harmatz P. Giugliani R. Schwartz I. Guffon N. Teles EL. Miranda MC, et al. Enzyme replacement therapy for mucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J Pediatr. 2006;148:533–539. doi: 10.1016/j.jpeds.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Lim-Melia ER. Kronn DF. Current enzyme replacement therapy for the treatment of lysosomal storage diseases. Pediatr Ann. 2009;38:448–455. doi: 10.3928/00904481-20090723-09. [DOI] [PubMed] [Google Scholar]
- 23.Beck M. Alglucosidase alfa: long term use in the treatment of patients with Pompe disease. Ther Clin Risk Manag. 2009;5:767–772. doi: 10.2147/tcrm.s5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bénichou B. Goyal S. Sung C. Norfleet AM. O'Brien F. A retrospective analysis of the potential impact of IgG antibodies to agalsidase b on efficacy during enzyme replacement therapy for Fabry disease. Mol Genet Metab. 2009;96:4–12. doi: 10.1016/j.ymgme.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 25.de Vries JM. van der Beek NAME. Kroos MA. Özkan L. van Doorn PA. Richards SM, et al. High antibody titer in an adult with Pompe disease affects treatment with alglucosidase alfa. Mol Genet Metab. 2010;101:338–345. doi: 10.1016/j.ymgme.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Hollak CEM. Linthorst GE. Immune response to enzyme replacement therapy in Fabry disease: impact on clinical outcome? Mol Genet Metab. 2009;96:1–3. doi: 10.1016/j.ymgme.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Kishnani PS. Goldenberg PC. DeArmey SL. Heller J. Benjamin D. Young S, et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab. 2010;99:26–33. doi: 10.1016/j.ymgme.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Breemen MJ. Rombach SM. Dekker N. Poorthuis BJ. Linthorst GE. Zwinderman AH, et al. Reduction of elevated plasma globotriaosylsphingosine in patients with classic Fabry disease following enzyme replacement therapy. Biochim Biophys Acta. 2011;1812:70–76. doi: 10.1016/j.bbadis.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Bodensteiner D. Scott CR. Sims KB. Shepherd GM. Cintron RD. Germain DP. Successful reinstitution of agalsidase beta therapy in Fabry disease patients with previous IgE-antibody or skin-test reactivity to the recombinant enzyme. Genet Med. 2008;10:353–358. doi: 10.1097/GIM.0b013e318170f868. [DOI] [PubMed] [Google Scholar]
- 30.Lipinski SE. Lipinski MJ. Burnette A. Platts-Mills TA. Wilson WG. Desensitization of an adult patient with Pompe disease and a history of anaphylaxis to alglucosidase alfa. Mol Genet Metab. 2009;98:319–321. doi: 10.1016/j.ymgme.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Mohrenschlager M. Ollert M. Ring J. A study on serum IgE and clinical symptomatology of atopy in patients suffering from the lysosomal storage disorder Fabry disease. J Eur Acad Dermatol Venereol. 2008;22:692–695. doi: 10.1111/j.1468-3083.2008.02638.x. [DOI] [PubMed] [Google Scholar]
- 32.Tesmoingt C. Lidove O. Reberga A. Thetis M. Ackaert C. Nicaise P, et al. Enzyme therapy in Fabry disease: severe adverse events associated with anti-agalsidase cross-reactive IgG antibodies. Br J Clin Pharmacol. 2009;68:765–769. doi: 10.1111/j.1365-2125.2009.03501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox T. Lachmann R. Hollak C. Aerts J. van Weely S. Hrebicek M, et al. Novel oral treatment of Gaucher's disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. The Lancet. 2000;355:1481–1485. doi: 10.1016/S0140-6736(00)02161-9. [DOI] [PubMed] [Google Scholar]
- 34.Platt FM. Jeyakumar M. Andersson U. Priestman DA. Dwek RA. Butters TD, et al. Inhibition of substrate synthesis as a strategy for glycolipid lysosomal storage disease therapy. J Inherit Metab Dis. 2001;24:275–290. doi: 10.1023/a:1010335505357. [DOI] [PubMed] [Google Scholar]
- 35.Tifft CJ. Proia RL. Stemming the tide: glycosphingolipid synthesis inhibitors as therapy for storage diseases. Glycobiology. 2000;10:1249–1258. doi: 10.1093/glycob/10.12.1249. [DOI] [PubMed] [Google Scholar]
- 36.Schiffmann R. FitzGibbon EJ. Harris C. DeVile C. Davies EH. Abel L, et al. Randomized, controlled trial of miglustat in Gaucher's disease type 3. Ann Neurol. 2008;64:514–522. doi: 10.1002/ana.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimran A. Elstein D. Gaucher disease and the clinical experience with substrate reduction therapy. Philos Trans R Soc Lond B Biol Sci. 2003;358:961–966. doi: 10.1098/rstb.2003.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wraith JE. Vecchio D. Jacklin E. Abel L. Chadha-Boreham H. Luzy C, et al. Miglustat in adult and juvenile patients with Niemann-Pick disease type C: Long-term data from a clinical trial. Mol Genet Metab. 2010;99:351–357. doi: 10.1016/j.ymgme.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Cox TM. Eliglustat tartrate, an orally active glucocerebroside synthase inhibitor for the potential treatment of Gaucher disease and other lysosomal storage diseases. Curr Opin Investig Drugs Oct. 2010;11:1169–1181. [PubMed] [Google Scholar]
- 40.Fan J-Q. Ishii S. Asano N. Suzuki Y. Accelerated transport and maturation of lysosomal α-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat Med. 1999;5:112–115. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- 41.Parenti G. Treating lysosomal storage diseases with pharmacological chaperones: from concept to clinics. EMBO Mol Med. 2009;1:268–279. doi: 10.1002/emmm.200900036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Germain DP. Fan JQ. Pharmacological chaperone therapy by active-site-specific chaperones in Fabry disease: in vitro and preclinical studies. Int J Clin Pharmacol Ther. 2009;47:S111–S117. [PubMed] [Google Scholar]
- 43.Ellgaard L. Helenius A. ER quality control: towards an understanding at the molecular level. Curr Opin Cell Biol. 2001;13:431–437. doi: 10.1016/s0955-0674(00)00233-7. [DOI] [PubMed] [Google Scholar]
- 44.Brooks DA. Protein processing: A role in the pathophysiology of genetics disease. FEBS Lett. 1997;409:115–120. doi: 10.1016/s0014-5793(97)00423-7. [DOI] [PubMed] [Google Scholar]
- 45.Anelli T. Sitia R. Protein quality control in the early secretory pathway. EMBO J. 2008;27:315–327. doi: 10.1038/sj.emboj.7601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hebert DN. Molinari M. In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol Rev. 2007;87:1377–1408. doi: 10.1152/physrev.00050.2006. [DOI] [PubMed] [Google Scholar]
- 47.Ushioda R. Hoseki J. Araki K. Jansen G. Thomas DY. Nagata K. ERdj5 is required as a disulfide reductase for degradation of misfolded proteins in the ER. Science. 2008;321:569–572. doi: 10.1126/science.1159293. [DOI] [PubMed] [Google Scholar]
- 48.Ron I. Horowitz M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum Mol Genet. 2005;14:2387–2398. doi: 10.1093/hmg/ddi240. [DOI] [PubMed] [Google Scholar]
- 49.Yam GH. Zuber C. Roth J. A synthetic chaperone corrects the trafficking defect and disease phenotype in a protein misfolding disorder. FASEB J. 2005;19:12–18. doi: 10.1096/fj.04-2375com. [DOI] [PubMed] [Google Scholar]
- 50.Bonifacino JS. Weissman AM. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu Rev Cell Dev Biol. 1998;14:19–57. doi: 10.1146/annurev.cellbio.14.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beck M. New therapeutic options for lysosomal storage disorders: enzyme replacement, small molecules and gene therapy. Hum Genet. 2007;121:1–22. doi: 10.1007/s00439-006-0280-4. [DOI] [PubMed] [Google Scholar]
- 52.Chaudhuri TK. Paul S. Protein-misfolding diseases and chaperone-based therapeutic approaches. FEBS J. 2006;273:1331–1349. doi: 10.1111/j.1742-4658.2006.05181.x. [DOI] [PubMed] [Google Scholar]
- 53.Wei H. Kim S-J. Zhang Z. Tsai P-C. Wisniewski KE. Mukherjee AB. ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum Mol Genet. 2008;17:469–477. doi: 10.1093/hmg/ddm324. [DOI] [PubMed] [Google Scholar]
- 54.Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- 55.Conn PM. Ulloa-Aguirre A. Ito J. Janovick JA. G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev. 2007;59:225–250. doi: 10.1124/pr.59.3.2. [DOI] [PubMed] [Google Scholar]
- 56.Zhu X. Sheth KA. Li S. Chang HH. Fan JQ. Rational design and synthesis of highly potent β-glucocerebrosidase inhibitors. Angew Chem Int Ed Engl. 2005;44:7450–7453. doi: 10.1002/anie.200502662. [DOI] [PubMed] [Google Scholar]
- 57.Lieberman RL. D'Aquino JA. Ringe D. Petsko GA. The effects of pH and iminosugar pharmacological chaperones on lysosomal glycosidase structure and stability. Biochemistry (Mosc) 2009;48:4816–4827. doi: 10.1021/bi9002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maegawa GHB. Tropak M. Buttner J. Stockley T. Kok F. Clarke JTR, et al. Pyrimethamine as a potential pharmacological chaperone for late-onset forms of GM2 gangliosidosis. J Biol Chem. 2007;282:9150–9161. doi: 10.1074/jbc.M609304200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tropak MB. Blanchard JE. Withers SG. Brown ED. Mahuran D. High-throughput screening for human lysosomal β-N-acetyl hexosaminidase inhibitors acting as pharmacological chaperones. Chem Biol. 2007;14:153–164. doi: 10.1016/j.chembiol.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng W. Padia J. Urban DJ. Jadhav A. Goker-Alpan O. Simeonov A, et al. Three classes of glucocerebrosidase inhibitors identified by quantitative high-throughput screening are chaperone leads for Gaucher disease. Proc Natl Acad of Sci U S A. 2007;104:13192–13197. doi: 10.1073/pnas.0705637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maegawa GHB. Tropak MB. Buttner JD. Rigat BA. Fuller M. Pandit D, et al. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J Biol Chem. 2009;284:23502–23516. doi: 10.1074/jbc.M109.012393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flanagan JJ. Rossi B. Tang K. Wu X. Mascioli K. Donaudy F, et al. The pharmacological chaperone 1-deoxynojirimycin increases the activity and lysosomal trafficking of multiple mutant forms of acid alpha-glucosidase. Hum Mutat. 2009;30:1683–1692. doi: 10.1002/humu.21121. [DOI] [PubMed] [Google Scholar]
- 63.Valenzano K. Khanna R. Flanagan J. Chang HH. Soska R. Schilling A, et al. The pharmacological chaperone AT1001 increases the levels of wild-type α-galactosidase A, the enzyme deficient in Fabry disease in vitro, in vivo. Manuscript in preparation.
- 64.Sawkar AR. Schmitz M. Zimmer K. Reczek D. Edmunds T. Balch WE, et al. Chemical chaperones and permissive temperatures alter localization of Gaucher disease associated glucocerebrosidase variants. ACS Chem Biol. 2006;1:235–251. doi: 10.1021/cb600187q. [DOI] [PubMed] [Google Scholar]
- 65.Kornhaber GJ. Tropak MB. Maegawa GH. Tuske SJ. Coales SJ. Mahuran DJ, et al. Isofagomine induced stabilization of glucocerebrosidase. Chembiochem. 2008;9:2643–2649. doi: 10.1002/cbic.200800249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pantoliano MW. Petrella EC. Kwasnoski JD. Lobanov VS. Myslik J. Graf E, et al. High-density miniaturized thermal shift assays as a general strategy for drug discovery. J Biomol Screen. 2001;6:429–440. doi: 10.1177/108705710100600609. [DOI] [PubMed] [Google Scholar]
- 67.Vedadi M. Niesen FH. Allali-Hassani A. Fedorov OY. Finerty PJ., Jr. Wasney GA, et al. Chemical screening methods to identify ligands that promote protein stability, protein crystallization, and structure determination. Proc Natl Acad Sci U S A. 2006;103:15835–15840. doi: 10.1073/pnas.0605224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niesen FH. Berglund H. Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protocols. 2007;2:2212–2221. doi: 10.1038/nprot.2007.321. [DOI] [PubMed] [Google Scholar]
- 69.Hamuro Y. Coales SJ. Southern MR. Nemeth-Cawley JF. Stranz DD. Griffin PR. Rapid analysis of protein structure and dynamics by hydrogen/deuterium exchange mass spectrometry. J Biomol Tech. 2003;14:171–182. [PMC free article] [PubMed] [Google Scholar]
- 70.Steet RA. Chung S. Wustman B. Powe A. Do H. Kornfeld SA. The iminosugar isofagomine increases the activity of N370S mutant acid β-glucosidase in Gaucher fibroblasts by several mechanisms. Proc Natl Acad Sci U S A. 2006;109:13813–13818. doi: 10.1073/pnas.0605928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ishii S. Chang HH. Kawasaki K. Yasuda K. Wu HL. Garman SC, et al. Mutant α-galacatosidase A enzymes identified in Fabry patients with residual enzyme activity: Biochemical characterization and restoration of normal intracellular processing by 1-deoxygalactonojirimycin. Biochem J. 2007;406:285–295. doi: 10.1042/BJ20070479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okumiya T. Kroos MA. Vliet LV. Takeuchi H. Van der Ploeg AT. Reuser AJ. Chemical chaperones improve transport and enhance stability of mutant α-glucosidases in glycogen storage disease type II. Mol Genet Metab. 2007;90:49–57. doi: 10.1016/j.ymgme.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 73.Parenti G. Zuppaldi A. Gabriela Pittis M. Rosaria Tuzzi M. Annunziata I. Meroni G, et al. Pharmacological enhancement of mutated α-glucosidase activity in fibroblasts from patients with Pompe disease. Mol Ther. 2007;15:508–514. doi: 10.1038/sj.mt.6300074. [DOI] [PubMed] [Google Scholar]
- 74.Tropak MB. Mahuran D. Lending a helping hand, screening chemical libraries for compounds that enhance β-hexosaminidase A activity in GM2 gangliosidosis cells. FEBS J. 2007;274:4951–4961. doi: 10.1111/j.1742-4658.2007.06040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benjamin E. Flanagan J. Schilling A. Chang H. Agarwal L. Katz E, et al. The pharmacological chaperone 1-deoxygalactonojirimycin increases α-galactosidase A levels in Fabry patient cell lines. J Inherit Metab Dis. 2009;32:424–440. doi: 10.1007/s10545-009-1077-0. [DOI] [PubMed] [Google Scholar]
- 76.Khanna R. Benjamin ER. Pellegrino L. Schilling A. Rigat BA. Soska R, et al. The pharmacological chaperone isofagomine increases the activity of the Gaucher disease L444P mutant form of β-glucosidase. FEBS J. 2010;277:1618–1638. doi: 10.1111/j.1742-4658.2010.07588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rüdiger HW. Chapter 2. Methods to culture diploid fibroblasts on a large scale. In: David MP, editor. Methods in Cell Biology. Vol. 9. Academic Press; Watham, MA: 1975. pp. 13–23. [DOI] [PubMed] [Google Scholar]
- 78.Neitzel H. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum Genet. 1986;73:320–326. doi: 10.1007/BF00279094. [DOI] [PubMed] [Google Scholar]
- 79.Wang G-N. Reinkensmeier G. Zhang S-W. Zhou J. Zhang L-R. Zhang L-H, et al. Rational design and synthesis of highly potent pharmacological chaperones for treatment of N370S mutant Gaucher disease. J Med Chem. 2009;52:3146–3149. doi: 10.1021/jm801506m. [DOI] [PubMed] [Google Scholar]
- 80.Grabowski GA. Gaucher disease: Gene frequencies and genotype/phenotype correlations. Genet Test. 1997;1:5–12. doi: 10.1089/gte.1997.1.5. [DOI] [PubMed] [Google Scholar]
- 81.Towbin H. Staehelin T. Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burnette WN. “Western Blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 83.Shin S-H. Murray GJ. Kluepfel-Stahl S. Cooney AM. Quirk JM. Schiffmann R, et al. Screening for pharmacological chaperones in Fabry disease. Biochem Biophys Res Commun. 2007;359:168–173. doi: 10.1016/j.bbrc.2007.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shin SH. Kluepfel-Stahl S. Cooney AM. Kaneski CR. Quirk JM. Schiffmann R, et al. Prediction of response of mutated α-galactosidase A to a pharmacological chaperone. Pharmacogenet Genomics. 2008;18:773–780. doi: 10.1097/FPC.0b013e32830500f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li L. Higaki K. Ninomiya H. Luan Z. Iida M. Ogawa S, et al. Chemical chaperone therapy: luciferase assay for screening of β-galactosidase mutations. Mol Genet Metab. 2010;101:364–369. doi: 10.1016/j.ymgme.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 86.Wu X. Katz E. Della Valle C. Mascioli K. Flanagan J. Castelli J, et al. A pharmacogenetic approach to identify mutant forms of α-galactosidase A that respond to a pharmacological chaperone for Fabry disease. Hum Mutat. doi: 10.1002/humu.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yewdell JW. Not such a dismal science: the economics of protein synthesis, folding, degradation and antigen processing. Trends Cell Biol. 2001;11:294–297. doi: 10.1016/s0962-8924(01)02030-x. [DOI] [PubMed] [Google Scholar]
- 88.Lemansky P. Bishop DF. Desnick RJ. Hasilik A. von Figura K. Synthesis and processing of α-galactosidase A in human fibroblasts. Evidence for different mutations in Fabry disease. J Biol Chem. 1987;262:2062–2065. [PubMed] [Google Scholar]
- 89.LeDonne NCJ. Fairley JL. Sweeley CC. Biosynthesis of α-galactosidase A in cultured Chang liver cells. Arch Biochem Biophys. 1983;224:186–195. doi: 10.1016/0003-9861(83)90203-5. [DOI] [PubMed] [Google Scholar]
- 90.Marugan JJ. Zheng W. Motabar O. Southall N. Goldin E. Sidransky E, et al. Evaluation of 2-thioxo-2,3,5,6,7,8-hexahydropyrimido[4,5-d]pyrimidin-4(1H)-one analogues as GAA activators. Eur J Med Chem. 2010;45:1880–1897. doi: 10.1016/j.ejmech.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ringe D. Petsko G. Q&A: what are pharmacological chaperones and why are they interesting? J Biol. 2009;8:80. doi: 10.1186/jbiol186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hasilik A. Neufeld EF. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980;255:4946–4950. [PubMed] [Google Scholar]
- 93.Moreland RJ. Jin X. Zhang XK. Decker RW. Albee KL. Lee KL, et al. Lysosomal acid α-glucosidase consists of four different peptides processed from a single chain precursor. J Biol Chem. 2005;280:6780–6791. doi: 10.1074/jbc.M404008200. [DOI] [PubMed] [Google Scholar]
- 94.Wisselaar HA. Kroos MA. Hermans MM. van Beeumen J. Reuser AJ. Structural and functional changes of lysosomal acid α-glucosidase during intracellular transport and maturation. J Biol Chem. 1993;268:2223–2231. [PubMed] [Google Scholar]
- 95.Richardson JM. Woychik NA. Ebert DL. Dimond RL. Cardelli JA. Inhibition of early but not late proteolytic processing events leads to the missorting and oversecretion of precursor forms of lysosomal enzymes in Dictyostelium discoideum. J Cell Biol. 1988;107:2097–2107. doi: 10.1083/jcb.107.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Erickson AH. Blobel G. Carboxyl-terminal proteolytic processing during biosynthesis of the lysosomal enzymes .beta.-glucuronidase and cathepsin D. Biochemistry (Mosc) 1983;22:5201–5205. doi: 10.1021/bi00291a021. [DOI] [PubMed] [Google Scholar]
- 97.Leibold DM. Robinson CB. Scanlin TF. Glick MC. Lack of proteolytic processing of α-L-fucosidase in human skin fibroblasts. J Cell Physiol. 1988;137:411–420. doi: 10.1002/jcp.1041370304. [DOI] [PubMed] [Google Scholar]
- 98.Quon DV. Proia RL. Fowler AV. Bleibaum J. Neufeld EF. Proteolytic processing of the β-subunit of the lysosomal enzyme, beta-hexosaminidase, in normal human fibroblasts. J Biol Chem. 1989;264:3380–3384. [PubMed] [Google Scholar]
- 99.Kornfeld S. Trafficking of lysosomal enzymes in normal and disease states. J Clin Invest. 1986;77:1–6. doi: 10.1172/JCI112262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kornfeld S. Trafficking of lysosomal enzymes. FASEB J. 1987;1:462–468. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- 101.Neufeld EF. Lysosomal storage diseases. Annu Rev Biochem. 1991;60:257–280. doi: 10.1146/annurev.bi.60.070191.001353. [DOI] [PubMed] [Google Scholar]
- 102.Oude Elferink RP. Strijland A. Surya I. Brouwer-Kelder EM. Kroos M. Hilkens J, et al. Use of a monoclonal antibody to distinguish between precursor and mature forms of human lysosomal α-glucosidase. Eur J Biochem. 1984;139:497–502. doi: 10.1111/j.1432-1033.1984.tb08033.x. [DOI] [PubMed] [Google Scholar]
- 103.Kornfeld R. Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 104.Merry T. Current techniques in protein glycosylation analysis. A guide to their application. Acta Biochim Pol. 1999;46:303–314. [PubMed] [Google Scholar]
- 105.Fukuda M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem. 1991;266:21327–21330. [PubMed] [Google Scholar]
- 106.Lieberman RL. Wustman BA. Huertas P. Powe AC., Jr. Pine CW. Khanna R, et al. Structure of acid β-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat Chem Biol. 2007;3:101–107. doi: 10.1038/nchembio850. [DOI] [PubMed] [Google Scholar]
- 107.Rigat B. Mahuran D. Diltiazem, a L-type Ca2+ channel blocker, also acts as a pharmacological chaperone in Gaucher patient cells. Mol Genet Metab. 2009;96:225–232. doi: 10.1016/j.ymgme.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tropak MB. Kornhaber GJ. Rigat BA. Maegawa GH. Buttner JD. Blanchard JE, et al. Identification of pharmacological chaperones for Gaucher disease and characterization of their effects on β-glucocerebrosidase by hydrogen/deuterium exchange mass spectrometry. ChemBioChem. 2008;9:2650–2662. doi: 10.1002/cbic.200800304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Starkuviene V. Pepperkok R. The potential of high-content high-throughput microscopy in drug discovery. Br J Pharmacol. 2007;152:62–71. doi: 10.1038/sj.bjp.0707346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zanella F. Lorens JB. Link W. High content screening: seeing is believing. Trends Biotechnol. 2010;28:237–245. doi: 10.1016/j.tibtech.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 111.Agmon V. Cherbu S. Dagan A. Grace M. Grabowski GA. Gatt S. Synthesis and use of novel fluorescent glycosphingolipids for estimating β-glucosidase activity in vitro in the absence of detergents and subtyping Gaucher disease variants following administration into intact cells. Biochim Biophys Acta. 1993;1170:72–79. doi: 10.1016/0005-2760(93)90177-b. [DOI] [PubMed] [Google Scholar]
- 112.Kohen E. Kohen C. Hirschberg JG. Santus R. Grabowski G. Mangel W, et al. An in situ study of β-glucosidase activity in normal and Gaucher fibroblasts with fluorogenic probes. Cell Biochem Funct. 1993;11:167–177. doi: 10.1002/cbf.290110304. [DOI] [PubMed] [Google Scholar]
- 113.Lorincz M. Herzenberg LA. Diwu Z. Barranger JA. Kerr WG. Detection and isolation of gene-corrected cells in Gaucher disease via a fluorescence-activated cell sorter assay for lysosomal glucocerebrosidase activity. Blood. 1997;89:3412–3420. [PubMed] [Google Scholar]
- 114.Madar-Shapiro L. Pasmanik-Chor M. Dinur T. Dagan A. Gatt S. Horowitz M. Intracellular degradation of fluorescent glycolipids by lysosomal enzymes and their activators. J Inherit Metab Dis. 1999;22:623–637. doi: 10.1023/a:1005573812430. [DOI] [PubMed] [Google Scholar]
- 115.Rudensky B. Paz E. Altarescu G. Raveh D. Elstein D. Zimran A. Fluorescent flow cytometric assay: a new diagnostic tool for measuring β-glucocerebrosidase activity in Gaucher disease. Blood Cells Mol Dis. 2003;30:97–99. doi: 10.1016/s1079-9796(03)00010-x. [DOI] [PubMed] [Google Scholar]
- 116.Sasagasako N. Kobayashi T. Yamaguchi Y. Shinnoh N. Goto I. Glucosylceramide and glucosylsphingosine metabolism in cultured fibroblasts deficient in acid β-glucosidase activity. J Biochem. 1994;115:113–119. doi: 10.1093/oxfordjournals.jbchem.a124284. [DOI] [PubMed] [Google Scholar]
- 117.van Es HH. Veldwijk M. Havenga M. Valerio D. A flow cytometric assay for lysosomal glucocerebrosidase. Anal Biochem. 1997;247:268–271. doi: 10.1006/abio.1997.2090. [DOI] [PubMed] [Google Scholar]
- 118.Hölzl MA. Gärtner M. Kovarik JJ. Hofer J. Bernheimer H. Sunder-Plassmann G, et al. Quantification of α-galactosidase activity in intact leukocytes. Clin Chim Acta. 2010;411:1666–1670. doi: 10.1016/j.cca.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 119.Kaneski CR. Schiffmann R. Brady RO. Murray GJ. Use of lissamine rhodamine ceramide trihexoside as a functional assay for α-galactosidase A in intact cells. J Lipid Res. 2010;51:2808–2817. doi: 10.1194/jlr.D007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marchesini S. Demasi L. Cestone P. Preti A. Agmon V. Dagan A, et al. Sulforhodamine GM1-ganglioside: synthesis and physico-chemical properties. Chem Phys Lipids. 1994;72:143–152. doi: 10.1016/0009-3084(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 121.Tropak MB. Bukovac SW. Rigat BA. Yonekawa S. Wakarchuk W. Mahuran DJ. A sensitive fluorescence-based assay for monitoring GM2 ganglioside hydrolysis in live patient cells and their lysates. Glycobiology. 2010;20:356–365. doi: 10.1093/glycob/cwp183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaneski CR. French SA. Brescia MR. Harbour MJ. Miller SP. Hydrolysis of a novel lysosomotropic enzyme substrate for β-galactosidase within intact cells. J Lipid Res. 1994;35:1441–1451. [PubMed] [Google Scholar]
- 123.Chan KW. Waire J. Simons B. Karey K. Fung J. Copeland D, et al. Measurement of lysosomal glucocerebrosidase activity in mouse liver using a fluorescence-activated cell sorter assay. Anal Biochem. 2004;334:227–233. doi: 10.1016/j.ab.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 124.Arttamangkul S. Bhalgat MK. Haugland RP. Diwu Z. Liu J. Klaubert DH, et al. 5-(Pentafluorobenzoylamino)fluorescein: a selective substrate for the determination of glutathione concentration and glutathione S-transferase activity. Anal Biochem. 1999;269:410–417. doi: 10.1006/abio.1999.4044. [DOI] [PubMed] [Google Scholar]
- 125.Mu T-W. Fowler DM. Kelly JW. Partial restoration of mutant enzyme homeostasis in three distinct lysosomal storage disease cell lines by altering calcium homeostasis. PLoS Biology. 2008;6:e26. doi: 10.1371/journal.pbio.0060026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sawkar AR. Adamski-Werner SL. Cheng WC. Wong CH. Beutler E. Zimmer KP, et al. Gaucher disease-associated glucocerebrosidases show mutation-dependent chemical chaperoning profiles. Chem Biol. 2005;12:1235–1244. doi: 10.1016/j.chembiol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 127.Sawkar AR. Cheng WC. Beutler E. Wong CH. Balch WE. Kelly JW. Chemical chaperones increase the cellular activity of N370S β-glucosidase: A therapeutic strategy for Gaucher disease. Proc Natl Acad Sci U S A. 2002;99:15428–15433. doi: 10.1073/pnas.192582899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Beutler E. Kuhl W. Trinidad F. Teplitz R. Nadler H. β-glucosidase activity in fibroblasts from homozygotes and heterozygotes for Gaucher's disease. Am J Hum Genet. 1971;23:62–66. [PMC free article] [PubMed] [Google Scholar]
- 129.Zungu IL. Evans DH. Houreld N. Abrahamse H. Biological responses of injured human skin fibroblasts to assess the efficacy of in vitro models for cell stress studies. Afr J Biochem Res. 2007;1:060–071. [Google Scholar]
- 130.Yam GH. Bosshard N. Zuber C. Steinmann B. Roth J. Pharmacological chaperone corrects lysosomal storage in Fabry disease caused by trafficking-incompetent variants. Am J Physiol Cell Physiol. 2006;290:C1076–C1082. doi: 10.1152/ajpcell.00426.2005. [DOI] [PubMed] [Google Scholar]
- 131.Khanna R. Soska R. Lun Y. Feng J. Frascella M. Young B, et al. The pharmacological chaperone 1-deoxygalactonojirimycin reduces tissue globotriaosylceramide levels in a mouse model of Fabry disease. Mol Ther. 2010;18:23–33. doi: 10.1038/mt.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khanna R. Flanagan J. Feng J. Frascella M. Soska R. Lun Y, et al. The pharmacological chaperone 1-deoxynojirimycin reduces glycogen levels in a mouse model of Pompe disease. Manuscript in preparation.
- 133.Wustman B. Pine C. Ranes B. Flanagan J. Palling D. Do H, et al. 114. Pharmacological chaperone therapy for Gaucher disease: mechanism of action, a survey of responsive mutations and phase I clinical trial results. Mol Genet Metab. 2008;93:44. [Google Scholar]
- 134.Khanna R. Benjamin ER. Soska R. Lun Y. Sitaraman SA. Palling DJ, et al. 50. The pharmacological chaperone AT1001 and treatment of Fabry disease. Mol Genet Metab. 2008;93:26. [Google Scholar]
- 135.Ishii S. Chang H-H. Yoshioka H. Shimada T. Mannen K. Higuchi Y, et al. Preclinical dfficacy and safety of 1-deoxygalactonojirimycin in mice for Fabry disease. J Pharmacol Exp Ther. 2009;328:723–731. doi: 10.1124/jpet.108.149054. [DOI] [PubMed] [Google Scholar]
- 136.Ishii S. Yoshioka H. Mannen K. Kulkarni AB. Fan JQ. Transgenic mouse expressing human mutant alpha-galactosidase A in an endogenous enzyme deficient background: a biochemical animal model for studying active-site specific chaperone therapy for Fabry disease. Biochim Biophys Acta. 2004;1690:250–257. doi: 10.1016/j.bbadis.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 137.Raben N. Plotz P. Byrne BJ. Acid α-glucosidase deficiency (glycogenosis type II, Pompe disease) Curr Mol Med. 2002;2:145–166. doi: 10.2174/1566524024605789. [DOI] [PubMed] [Google Scholar]
- 138.Matsuda J. Suzuki O. Oshima A. Yamamoto Y. Noguchi A. Takimoto K, et al. Chemical chaperone therapy for brain pathology in GM1-gangliosidosis. Proc Natl Acad of Sci U S A. 2003;100:15912–15917. doi: 10.1073/pnas.2536657100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Suzuki Y. Ichinomiya S. Kurosawa M. Ohkubo M. Watanabe H. Iwasaki H, et al. Chemical chaperone therapy: clinical effect in murine GM1-gangliosidosis. Ann Neurol. 2007;62:671–675. doi: 10.1002/ana.21284. [DOI] [PubMed] [Google Scholar]
- 140.Wu X. Mascioli K. Katz E. Chang H-H. Shin S-H. Kluepfel-Stahl S, et al. 113. Identification of Fabry disease-causing mutations that are responsive to the pharmacological chaperone AT1001. Mol Genet Metab. 2008;93:43–44. [Google Scholar]
- 141.Park JY. Kim GH. Kim SS. Ko JM. Lee JJ. Yoo HW. Effects of a chemical chaperone on genetic mutations in α-galactosidase A in Korean patients with Fabry disease. Exp Mol Med. 2009;41:1–7. doi: 10.3858/emm.2009.41.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shimotori M. Maruyama H. Nakamura G. Suyama T. Sakamoto F. Itoh M, et al. Novel mutations of the GLA gene in Japanese patients with Fabry disease and their functional characterization by active site specific chaperone. Hum Mutat. 2008;29:331. doi: 10.1002/humu.9520. [DOI] [PubMed] [Google Scholar]
- 143.Wu X. A pharmacogenetic approach to the selection of Fabry patients for pharmacological chaperone therapy. Presented at the Annual Symposium of the Society for the Study of Inborn Errors of Metabolism; Istanbul, Turkey. Sep, 2010. [Google Scholar]
- 144.Conzelmann E. Sandhoff K. Partial enzyme deficiencies: residual activities and the development of neurological disorders. Dev Neurosci. 1983;6:58–71. doi: 10.1159/000112332. [DOI] [PubMed] [Google Scholar]
- 145.Hopwood JJ. Muller V. Biochemical discrimination of Hurler and Scheie syndromes. Clin Sci (Lond) 1979;57:265–272. doi: 10.1042/cs0570265. [DOI] [PubMed] [Google Scholar]
- 146.Desnick RJ. Fan JQ. Pharmacological chaperone therapy for lysosomal disease. In: Futerman AH, editor; Zimran A, editor. Gaucher Disease. CRC Press; Boca Raton, FL: 2006. p. 544. [Google Scholar]
- 147.Suzuki Y. Ogawa S. Sakakibara Y. Chaperone therapy for neuronopathic lysosomal diseases: competitive inhibitors as chemical chaperones for enhancement of mutant enzyme activities. Perspect Med Chem. 2009;3:7–19. doi: 10.4137/pmc.s2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Conzelmann E. Kytzia HJ. Navon R. Sandhoff K. Ganglioside GM2 N-acetyl-β-D-galactosaminidase activity in cultured fibroblasts of late-infantile and adult GM2 gangliosidosis patients and of healthy probands with low hexosaminidase level. Am J Hum Genet. 1983;35:900–913. [PMC free article] [PubMed] [Google Scholar]
- 149.Leinekugel P. Michel S. Conzelmann E. Sandhoff K. Quantitative correlation between the residual activity of β-hexosaminidase A and arylsulfatase A and the severity of the resulting lysosomal storage disease. Hum Genet. 1992;88:513–523. doi: 10.1007/BF00219337. [DOI] [PubMed] [Google Scholar]
- 150.Mahuran DJ. Biochemical consequences of mutations causing the GM2 gangliosidoses. Biochim Biophys Acta. 1999;1455:105–138. doi: 10.1016/s0925-4439(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 151.Dubois G. Harzer K. Baumann N. Very low arylsulfatase A and cerebroside sulfatase activities in leukocytes of healthy members of metachromatic leukodystrophy family. Am J Hum Genet. 1977;29:191–194. [PMC free article] [PubMed] [Google Scholar]
- 152.Kolodny E. Raghavan S. Spielvogel C. Gajewski A. Lacson A. Jungalwala F, et al. Genetic heterogeneity in aryl sulfatase A (ASA) deficiency. Neurology. 1979;29:576. [Google Scholar]
- 153.Lott IT. Dulaney JT. Milunsky A. Hoefnagel D. Moser HW. Apparent biochemical homozygosity in two obligatory heterozygotes for metachromatic leukodystrophy. J Pediatr. 1976;89:438–440. doi: 10.1016/s0022-3476(76)80546-x. [DOI] [PubMed] [Google Scholar]
- 154.Dreyfus J-C. Poenaru L. Svennerholm L. Absence of hexosaminidase A and B in a normal adult. N Engl J Med. 1975;292:61–63. doi: 10.1056/NEJM197501092920201. [DOI] [PubMed] [Google Scholar]
- 155.Kelly TE. Reynolds LW. O'Brien JS. Segregation within a family of two mutant alleles for hexosaminidase A. Clin Genet. 1976;9:540–543. doi: 10.1111/j.1399-0004.1976.tb01609.x. [DOI] [PubMed] [Google Scholar]
- 156.Jensen HH. Lyngbye L. Jensen A. Bols M. Stereoelectronic substituent effects in polyhydroxylated piperidines and hexahydropyridazines. Chemistry. 2002;8:1218–1226. doi: 10.1002/1521-3765(20020301)8:5<1218::aid-chem1218>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 157.Guce AI. Clark NE. Salgado EN. Ivanen DR. Kulminskaya AA. Brumer H, et al. The catalytic mechanism of human α-galactosidase. J Biol Chem. 2010;285:3625–3632. doi: 10.1074/jbc.M109.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Landon M. Lieberman R. Hoang Q. Ju S. Caaveiro J. Orwig S, et al. Detection of ligand binding hot spots on protein surfaces via fragment-based methods: application to DJ-1 and glucocerebrosidase. J Comput Aided Mol Des. 2009;23:491–500. doi: 10.1007/s10822-009-9283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ogawa S. Sakata Y. Ito N. Watanabe M. Kabayama K. Itoh M, et al. Convenient synthesis and evaluation of glycosidase inhibitory activity of α- and β-galactose-type valienamines, and some N-alkyl derivatives. Bioorg Med Chem. 2004;12:995–1002. doi: 10.1016/j.bmc.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 160.Ichikawa Y. Igarashi Y. Ichikawa M. Suhara Y. 1-N-Iminosugars: potent and selective inhibitors of α-glycosidases. J Am Chem Soc. 1998;120:3007–3018. [Google Scholar]
- 161.Lin H. Sugimoto Y. Ohsaki Y. Ninomiya H. Oka A. Taniguchi M, et al. N-octyl-β-valienamine up-regulates activity of F213I mutant β-glucosidase in cultured cells: a potential chemical chaperone therapy for Gaucher disease. Biochim Biophys Acta. 2004;1689:219–228. doi: 10.1016/j.bbadis.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 162.Raben N. Nagaraju K. Lee E. Kessler P. Byrne B. Lee L, et al. Targeted disruption of the acid α-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem. 1998;273:19086–19092. doi: 10.1074/jbc.273.30.19086. [DOI] [PubMed] [Google Scholar]
- 163.Amalfitano A. McVie-Wylie AJ. Hu H. Dawson TL. Raben N. Plotz P, et al. Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-alpha-glucosidase. Proc Natl Acad Sci U S A. 1999;96:8861–8866. doi: 10.1073/pnas.96.16.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Okumiya T. Ishii S. Takenaka T. Kase R. Kamei S. Sakuraba H, et al. Galactose stabilizes various missense mutants of α-galactosidase in Fabry disease. Biochem Biophys Res Commun. 1995;214:1219–1224. doi: 10.1006/bbrc.1995.2416. [DOI] [PubMed] [Google Scholar]
- 165.Frustaci A. Chimenti C. Ricci R. Natale L. Russo MA. Pieroni M, et al. Improvement in cardiac function in the cardiac variant of Fabry's disease with galactose-infusion therapy. N Engl J Med. 2001;345:25–32. doi: 10.1056/NEJM200107053450104. [DOI] [PubMed] [Google Scholar]
- 166.Schiffmann R. Germain DP. Castelli J. Shenker A. Lockhart DJ. 768/T. Phase 2 clinical trials of the pharmacological chaperone AT1001 for the treatment of Fabry disease. Presented at the American Society of Human Genetics Conference; Philadelphia. Nov 11–15, 2008. [Google Scholar]
- 167.Asano N. Ishii S. Kizu H. Ikeda K. Yasuda K. Kato A, et al. In vitro inhibition and intracellular enhancement of lysosomal α-galactosidase A activity in Fabry lymphoblasts by 1-deoxygalactonojirimycin and its derivatives. Eur J Biochem. 2000;267:4179–4186. doi: 10.1046/j.1432-1327.2000.01457.x. [DOI] [PubMed] [Google Scholar]
- 168.Kato A. Yamashita Y. Nakagawa S. Koike Y. Adachi I. Hollinshead J, et al. 2,5-Dideoxy-2,5-imino-d-altritol as a new class of pharmacological chaperone for Fabry disease. Bioorg Med Chem. 2010;18:3790–3794. doi: 10.1016/j.bmc.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 169.Sánchez-Ollé G. Duque J. Egido-Gabás M. Casas J. Lluch M. Chabás A, et al. Promising results of the chaperone effect caused by iminosugars and aminocyclitol derivatives on mutant glucocerebrosidases causing Gaucher disease. Blood Cells Mol Dis. 2009;42:159–166. doi: 10.1016/j.bcmd.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 170.Yu L. Ikeda K. Kato A. Adachi I. Godin G. Compain P, et al. α-1-C-Octyl-1-deoxynojirimycin as a pharmacological chaperone for Gaucher disease. Bioorg Med Chem. 2006;14:7736–7744. doi: 10.1016/j.bmc.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 171.Chang HH. Asano N. Ishii S. Ichikawa Y. Fan JQ. Hydrophilic iminosugar active-site-specific chaperones increase residual glucocerebrosidase activity in fibroblasts from Gaucher patients. FEBS J. 2006;273:4082–4092. doi: 10.1111/j.1742-4658.2006.05410.x. [DOI] [PubMed] [Google Scholar]
- 172.Luan Z. Higaki K. Aguilar-Moncayo M. Ninomiya H. Kousaku O. García-Moreno MI, et al. Chaperone activity of bicyclic nojirimycin analogues for Gaucher mutations in comparison with N-(n-nonyl)deoxynojirimycin. Chembiochem. 2009;10:2780–2792. doi: 10.1002/cbic.200900442. [DOI] [PubMed] [Google Scholar]
- 173.Fröhlich RFG. Furneaux RH. Mahuran DJ. Rigat BA. Stütz AE. Tropak MB, et al. 1-Deoxynojirimycins with dansyl capped N-substituents as probes for Morbus Gaucher affected cell lines. Carbohydr Res. 2010;345:1371–1376. doi: 10.1016/j.carres.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Luan Z. Higaki K. Aguilar-Moncayo M. Li L. Ninomiya H. Nanba E, et al. A fluorescent sp2-iminosugar with pharmacological chaperone activity for Gaucher disease: synthesis and intracellular distribution studies. Chembiochem. 2010;11:2453–2464. doi: 10.1002/cbic.201000323. [DOI] [PubMed] [Google Scholar]
- 175.Oulaïdi F. Front-Deschamps S. Gallienne E. Lesellier E. Ikeda K. Asano N, et al. Second-generation iminoxylitol-based pharmacological chaperones for the treatment of Gaucher disease. Chemmedchem. 2011;6:353–361. doi: 10.1002/cmdc.201000469. [DOI] [PubMed] [Google Scholar]
- 176.Iwasaki H. Watanabe H. Iida M. Ogawa S. Tabe M. Higaki K, et al. Fibroblast screening for chaperone therapy in β-galactosidosis. Brain Dev. 2006;28:482–486. doi: 10.1016/j.braindev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 177.Tominaga L. Ogawa Y. Taniguchi M. Ohno K. Matsuda J. Oshima A, et al. Galactonojirimycin derivatives restore mutant human β-galactosidase activities expressed in fibroblasts from enzyme-deficient knockout mouse. Brain Dev. 2001;23:284–287. doi: 10.1016/s0387-7604(01)00216-9. [DOI] [PubMed] [Google Scholar]
- 178.Caciotti A. Donati MA. d'Azzo A. Salvioli R. Guerrini R. Zammarchi E, et al. The potential action of galactose as a “chemical chaperone”: increase of beta galactosidase activity in fibroblasts from an adult GM1-gangliosidosis patient. Eur J Paediatr Neurol. 2009;13:160–164. doi: 10.1016/j.ejpn.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 179.Fantur K. Hofer D. Schitter G. Steiner AJ. Pabst BM. Wrodnigg TM, et al. DLHex-DGJ, a novel derivative of 1-deoxygalactonojirimycin with pharmacological chaperone activity in human GM1-gangliosidosis fibroblasts. Mol Genet Metab. 2010;100:262–268. doi: 10.1016/j.ymgme.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 180.Schitter G. Scheucher E. Steiner AJ. Stutz AE. Thonhofer M. Tarling CA, et al. Synthesis of lipophilic 1-deoxygalactonojirimycin derivatives as D-galactosidase inhibitors. Beilstein J Org Chem. 2010;6:21–27. doi: 10.3762/bjoc.6.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schitter G. Steiner AJ. Pototschnig G. Scheucher E. Thonhofer M. Tarling CA, et al. Fluorous iminoalditols: a new family of glycosidase inhibitors and pharmacological chaperones. Chembiochem. 2010;11:2026–2033. doi: 10.1002/cbic.201000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tropak MB. Reid SP. Guiral M. Withers SG. Mahuran D. Pharmacological enhancement of β-hexosaminidase activity in fibroblasts from adult Tay-Sachs and Sandhoff patients. J Biol Chem. 2004;279:13478–13487. doi: 10.1074/jbc.M308523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Clarke JTR. Mahuran DJ. Sathe S. Kolodny EH. Rigat BA. Raiman JA, et al. An open-label phase I/II clinical trial of pyrimethamine for the treatment of patients affected with chronic GM2 gangliosidosis (Tay-Sachs or Sandhoff variants) Mol Genet Metab. 2011;102:6–12. doi: 10.1016/j.ymgme.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rountree JSS. Butters Terry D. Wormald Mark R. Boomkamp Stephanie D. Dwek Raymond A. Asano N, et al. Design, synthesis, and biological evaluation of enantiomeric beta-N-acetylhexosaminidase inhibitors LABNAc and DABNAc as potential agents against Tay-Sachs and Sandhoff disease. Chemmedchem. 2009;4:378–392. doi: 10.1002/cmdc.200800350. [DOI] [PubMed] [Google Scholar]
- 185.Lee WC. Kang D. Causevic E. Herdt AR. Eckman EA. Eckman CB. Molecular characterization of mutations that cause globoid cell leukodystrophy and pharmacological rescue using small molecule chemical chaperones. J Neurosci. 2010;30:5489–5497. doi: 10.1523/JNEUROSCI.6383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dawson G. Schroeder C. Dawson PE. Palmitoyl:protein thioesterase (PPT1) inhibitors can act as pharmacological chaperones in infantile Batten disease. Biochem Biophys Res Commun. 2010;395:66–69. doi: 10.1016/j.bbrc.2010.03.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Feldhammer M. Durand S. Pshezhetsky AV. Protein misfolding as an underlying molecular defect in mucopolysaccharidosis III type C. PLoS ONE. 2009;4:e7434. doi: 10.1371/journal.pone.0007434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shiozuka C. Taguchi A. Matsuda J. Noguchi Y. Kunieda T. Uchio-Yamada K, et al. Increased globotriaosylceramide levels in a transgenic mouse expressing human α1,4-galactosyltransferase and a mouse model for treating Fabry disease. J Biochem. 2011;149:161–170. doi: 10.1093/jb/mvq125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu Y. Suzuki K. Reed JD. Grinberg A. Westphal H. Hoffmann A, et al. Mice with type 2 and 3 Gaucher disease point mutations generated by a single insertion mutagenesis procedure. Proc Natl Acad Sci U S A. 1998;95:2503–2508. doi: 10.1073/pnas.95.5.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mizukami H. Mi Y. Wada R. Kono M. Yamashita T. Liu Y, et al. Systemic inflammation in glucocerebrosidase-deficient mice with minimal glucosylceramide storage. J Clin Invest. 2002;109:1215–1221. doi: 10.1172/JCI14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xu YH. Quinn B. Witte D. Grabowski GA. Viable mouse models of acid β-glucosidase deficiency: the defect in Gaucher disease. Am J Pathol. 2003;163:2093–2101. doi: 10.1016/s0002-9440(10)63566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sun Y. Quinn B. Witte DP. Grabowski GA. Gaucher disease mouse models: point mutations at the acid β-glucosidase locus combined with low-level prosaposin expression lead to disease variants. J Lipid Res. 2005;46:2102–2113. doi: 10.1194/jlr.M500202-JLR200. [DOI] [PubMed] [Google Scholar]
- 193.Karageorgos L. Lancaster M. Nimmo J. Hopwood J. Gaucher disease in sheep. J Inherit Metab Dis. 2011;34:209–215. doi: 10.1007/s10545-010-9230-3. [DOI] [PubMed] [Google Scholar]
- 194.Nakabayashi O. Setsuie R. Sekine M. Kunita R. Mizutani M. Kikuchi T. A single base-pair deletion in exon 7 in qgaa1 gene responsible for acid maltase deficiency in Japanese quail (Coturnix Coturnix Japonica) Basic Appl Myol. 2005;15:19–21. [Google Scholar]
- 195.Zeng BJ. Torres PA. Viner TC. Wang ZH. Raghavan SS. Alroy J, et al. Spontaneous appearance of Tay-Sachs disease in an animal model. Mol Genet Metab. 2008;95:59–65. doi: 10.1016/j.ymgme.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 196.Luzi P. Rafi MA. Zaka M. Curtis M. Vanier MT. Wenger DA. Generation of a mouse with low galactocerebrosidase activity by gene targeting: a new model of globoid cell leukodystrophy (Krabbe disease) Mol Genet Metab. 2001;73:211–223. doi: 10.1006/mgme.2001.3194. [DOI] [PubMed] [Google Scholar]
- 197.Victoria T. Rafi MA. Wenger DA. Cloning of the canine GALC cDNA and identification of the mutation causing globoid cell leukodystrophy in West Highland White and Cairn Terriers. Genomics. 1996;33:457–462. doi: 10.1006/geno.1996.0220. [DOI] [PubMed] [Google Scholar]
- 198.Bhattacharyya R. Gliddon B. Beccari T. Hopwood JJ. Stanley P. A novel missense mutation in lysosomal sulfamidase is the basis of MPS III A in a spontaneous mouse mutant. Glycobiology. 2001;11:99–103. doi: 10.1093/glycob/11.1.99. [DOI] [PubMed] [Google Scholar]
- 199.Bhaumik M. Muller VJ. Rozaklis T. Johnson L. Dobrenis K. Bhattacharyya R, et al. A mouse model for mucopolysaccharidosis type III A (Sanfilippo syndrome) Glycobiology. 1999;9:1389–1396. doi: 10.1093/glycob/9.12.1389. [DOI] [PubMed] [Google Scholar]
- 200.Yogalingam G. Litjens T. Bielicki J. Crawley AC. Muller V. Anson DS, et al. Feline mucopolysaccharidosis type VI. J Biol Chem. 1996;271:27259–27265. doi: 10.1074/jbc.271.44.27259. [DOI] [PubMed] [Google Scholar]