Abstract
Plants respond to elevated CO2 via carbonic anhydrases that mediate stomatal closing, but little is known about the early signalling mechanisms following the initial CO2 response. It remains unclear whether CO2, HCO3− or a combination activates downstream signalling. Here, we demonstrate that bicarbonate functions as a small-molecule activator of SLAC1 anion channels in guard cells. Elevated intracellular [HCO3−]i with low [CO2] and [H+] activated S-type anion currents, whereas low [HCO3−]i at high [CO2] and [H+] did not. Bicarbonate enhanced the intracellular Ca2+ sensitivity of S-type anion channel activation in wild-type and ht1-2 kinase mutant guard cells. ht1-2 mutant guard cells exhibited enhanced bicarbonate sensitivity of S-type anion channel activation. The OST1 protein kinase has been reported not to affect CO2 signalling. Unexpectedly, OST1 loss-of-function alleles showed strongly impaired CO2-induced stomatal closing and HCO3− activation of anion channels. Moreover, PYR/RCAR abscisic acid (ABA) receptor mutants slowed but did not abolish CO2/HCO3− signalling, redefining the convergence point of CO2 and ABA signalling. A new working model of the sequence of CO2 signalling events in gas exchange regulation is presented.
Keywords: abscisic acid, bicarbonate, calcium, carbon dioxide, stomatal conductance
Introduction
Plants control CO2 influx and water loss through stomatal pores, formed by guard cells in aerial tissues. Guard cells respond to abscisic acid (ABA), auxin, blue light, plant pathogens and CO2 and have been developed as a powerful system for plant cell signal transduction research (Blatt, 2000; Evans and Hetherington, 2001; Schroeder et al, 2001; Sirichandra et al, 2009). Elevated intercellular CO2 concentrations (Ci) that occur in leaves due to respiration in darkness and the continuing rise in atmospheric CO2 concentrations due to anthropogenic fossil fuel burning cause a reduction in stomatal apertures (Medlyn et al, 2001; Frommer, 2010).
Stomatal closing is regulated by ion channel-mediated anion and K+ efflux from guard cells and parallel organic solute metabolism (Schroeder et al, 1987; Schroeder and Hagiwara, 1989; Blatt and Armstrong, 1993; MacRobbie, 1998). Elevated CO2 activates anion channels and K+out efflux channels in Vicia faba guard cells (Brearley et al, 1997; Raschke et al, 2003; Roelfsema et al, 2004) and triggers chloride release from guard cells causing guard cell depolarization in leaves (Hanstein and Felle, 2002; Roelfsema et al, 2002). Furthermore, cytosolic pH does not change in response to physiological [CO2] shifts in V. faba guard cells (Brearley et al, 1997).
Recently, we have shown that the β-carbonic anhydrases, βCA1 and βCA4, function in CO2 regulation of stomatal movements. ca1;ca4 double-mutant plants show impaired CO2 induction of stomatal closing, whereas ABA-induced stomatal closing is functional (Hu et al, 2010). CO2 is reversibly catalysed by carbonic anhydrases (CAs) into bicarbonate ions and protons. Cytoplasmic high CO2 together with high HCO3− concentrations activates S-type anion channel currents in wild-type Arabidopsis guard cells (Hu et al, 2010). However, the mechanisms by which high CO2 and/or HCO3− mediate this response were not further investigated. Whether high [CO2] and [HCO3−] are able to activate anion channels in ca1;ca4 double-mutant plants remains unknown. The concentrations of HCO3− and CO2 required for channel regulation in patch-clamped guard cells are relatively high, necessitating genetic analyses to determine whether the high [CO2] plus [HCO3−] activation are physiologically relevant. Moreover, genetic mechanisms downstream of this high [HCO3−] plus [CO2] response and their position in the signalling cascade remain unknown and are dissected, with unexpected results, in the present study.
Activation of S-type anion channels at the plasma membrane of guard cells has been regarded as a critical step in stomatal closure (Schroeder and Hagiwara, 1989; Schmidt et al, 1995; Grabov et al, 1997; Pei et al, 1997). Mutations in the SLAC1 anion channel cause greatly reduced S-type anion current activities, whereas R-type anion channels and ABA-activated Ca2+ permeable channels remain intact in slac1 mutants (Negi et al, 2008; Vahisalu et al, 2008). SLAC1 functions as an anion channel with permeabilities to Cl− and NO3− when heterologously expressed in Xenopus oocytes (Geiger et al, 2009; Lee et al, 2009), consistent with in vivo Cl− and NO3− permeabilities of S-type anion channels (Schmidt and Schroeder, 1994).
The concentration of intracellular free calcium ions ([Ca2+]i) has been shown to function as a key signalling molecule in plants and mediates CO2 signal transduction in guard cells of several plant species (Schwartz, 1985; Webb et al, 1996; Hetherington and Woodward, 2003; Young et al, 2006; Kim et al, 2010). Elevation of [Ca2+]i in guard cells causes activation of S-type anion channels, downregulation of inward (rectifying) K+in channels and downregulation of proton ATPases, providing central mechanisms that mediate stomatal closing and inhibition of stomatal opening (Schroeder and Hagiwara, 1989; Kelly et al, 1995; Kinoshita et al, 1995; Grabov and Blatt, 1999; Siegel et al, 2009; Chen et al, 2010). Recent studies have suggested that the stomatal closing signals, CO2 and ABA, enhance the [Ca2+]i sensitivity of stomatal closing mechanisms (Young et al, 2006; Siegel et al, 2009) (for review see Hubbard et al, 2010). However, whether CO2 activation of S-type anion channels requires [Ca2+]i is not known. Furthermore, whether CO2 primes Ca2+ regulation of ion channels remains unknown and no genetic mutants and mechanisms are known that mediate CO2/HCO3− regulation of ion channels.
The HT1 protein kinase was identified as a major negative regulator of high CO2-induced stomatal closure (Hashimoto et al, 2006) and is genetically epistatic to βCA1 and βCA4 in CO2 response pathway (Hu et al, 2010). However, the cellular signalling mechanisms of HT1 have not yet been investigated and whether the HT1 kinase affects S-type anion channel regulation and/or Ca2+ signalling remains unknown.
The OST1 protein kinase, also named SnRK2.6 and SnRK2E, was identified as a key mechanism mediating ABA signal transduction (Mustilli et al, 2002; Yoshida et al, 2002; Vlad et al, 2009), but has no effect on low CO2-induced stomatal opening (Mustilli et al, 2002). Recent findings have shown that OST1 activates SLAC1 anion currents when OST1 and SLAC1 are coexpressed in Xenopus oocytes (Geiger et al, 2009; Lee et al, 2009).
In the present study, we show that elevated bicarbonate, more so than elevated CO2, acts as intracellular signalling molecule to activate SLAC1-mediated anion channels. Elevated bicarbonate enhances (primes) the [Ca2+]i sensitivity of SLAC1 channel activation. The ht1-2 kinase mutant is found to enhance the HCO3− sensitivity of anion channel activation but also requires cytosolic Ca2+ for S-type anion channel activation, further defining the placement of HT1 effects on the CO2 signalling cascade. Surprisingly, our analyses of OST1 on CO2 regulation of stomatal movements and anion channels demonstrate that the OST1 protein kinase is a major regulator of CO2-induced stomatal closing and CO2 activation of anion channels in guard cells, leading to a new model for CO2 control of gas exchange in plants.
Results
Bicarbonate activates S-type anion currents in ca1;ca4 double-mutant guard cell protoplasts
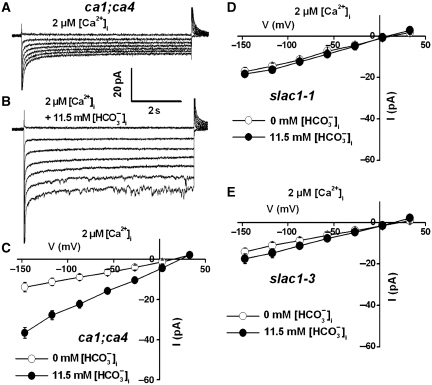
The βCA1 and βCA4 CAs act as upstream regulators in CO2-induced stomatal movements in guard cells (Hu et al, 2010). Elevated CO2 together with bicarbonate concentrations activate S-type anion channel currents in wild-type Arabidopsis guard cells. Previous studies of CO2 regulation of anion channels have only been pursued in wild-type guard cells (Brearley et al, 1997; Raschke et al, 2003; Hu et al, 2010). Therefore, we investigated whether elevated bicarbonate and intracellular CO2 can by-pass the ca1;ca4 mutant and activate S-type anion currents in ca1;ca4 mutant guard cells. Addition of 13.5 mM total bicarbonate to the pipette solution (equivalent to 11.5 mM free bicarbonate ([HCO3−]i)/2 mM free [CO2] at pH 7.1) activated anion currents in patch-clamped ca1;ca4 guard cells (Figure 1B and C), compared with control currents in the absence of added intracellular bicarbonate (Figure 1A). Free [HCO3−]i and [CO2] were calculated using the Henderson–Hasselbalch equation as described in Materials and methods. These findings are consistent with CAs acting as upstream regulators of CO2 signalling and that elevated bicarbonate and CO2 together can activate S-type anion channel in ca1;ca4 double-mutant guard cells.
Figure 1.
High intracellular [CO2] and [HCO3−] activate S-type anion channel currents in Arabidopsis ca1;ca4 double-mutant guard cells but do not activate S-type anion currents in slac1 mutant guard cells with 2 μM [Ca2+]i. (A) Whole-cell currents without HCO3−/CO2 and (B) with 11.5 mM free [HCO3−]i/2 mM free CO2 in the pipette solution (pH 7.1) in ca1;ca4 double-mutant guard cells. (C) Steady-state current–voltage relationships of the whole-cell currents recorded in ca1;ca4 mutant guard cells as in (A) (open circles, n=4 guard cells) and (B) (filled circles, n=9 guard cells). (D) Steady-state current–voltage relationships of whole-cell currents recorded in slac1-1 mutant guard cells (open circles: 0 mM added [HCO3−]i, n=6; filled circles: 11.5 mM free [HCO3−]i and 2 mM free [CO2], n=6) and (E) in slac1-3 mutant guard cells (open circles: 0 mM added [HCO3−]i, n=4; filled circles: 11.5 mM free [HCO3−]i and 2 mM free [CO2], n=8). Liquid junction potential was +1 mV. Data are mean±s.e.
Bicarbonate-activated S-type anion currents are greatly impaired in slac1 mutant guard cell protoplasts
The reversal potential of CO2+HCO3− activated whole-cell currents was +24.0±3.6 mV (n=8), which was close to the imposed chloride equilibrium potential of +31.1 mV, supporting the hypothesis that CO2+HCO3− activate guard cell anion channels. The bicarbonate and CO2 concentrations used for anion current activation were very high (Figure 1B and C) (Hu et al, 2010), giving rise to the question whether these anion currents correspond to physiological guard cell anion channel currents. SLAC1 is required for Arabidopsis ABA and Ca2+ activation of guard cell S-type anion channel function (Vahisalu et al, 2008). Therefore, we analysed whether high bicarbonate- and CO2-activated anion currents are mediated by SLAC1. Guard cell protoplasts from the recessive slac1-1 and slac1-3 mutants displayed small anion currents in the presence of 11.5 mM free [HCO3−]i and 2 mM [CO2] in the pipette solution, similar to control currents in the absence of added bicarbonate (Figure 1D and E, P>0.05). These data suggest that the high intracellular [HCO3−]+[CO2]-mediated anion currents are largely mediated by the physiologically relevant SLAC1 anion channel (Figure 1).
Next, we analysed whether these anion currents show a clear HCO3− permeability in wild-type guard cells. The total bicarbonate was elevated to 50 mM in the pipette solution at pH 7.1 (corresponding to 43.4 mM free [HCO3−]i and 6.6 mM free [CO2]). Under such high [HCO3−], the reversal potential of whole-cell currents was +26.0±0.9 mV (Supplementary Figure S2, n=4). A relative permeability ratio of PHCO3−/PCl−=0.06±0.01 was estimated using the Goldman equation. This Cl− over HCO3− selectivity of whole-cell anion currents is consistent with the anion selectivity of SLAC1 channels found in heterologous expression experiments in Xenopus laevis oocytes (Geiger et al, 2009).
High [CO2] and protons do not activate S-type anion currents in the absence of high bicarbonate levels in guard cells
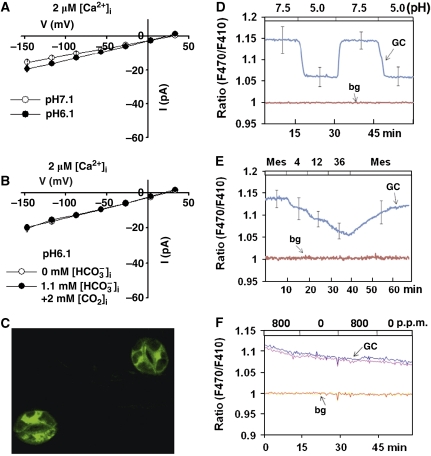
CAs reversibly catalyse the conversion of CO2 into bicarbonate ions and free protons (Supuran, 2008; Chandrashekar et al, 2009). Whether high [CO2], [HCO3−], [H+] or a combination of these mediates activation of S-type anion channels in Arabidopsis guard cells remains to be investigated (Hu et al, 2010). We investigated whether intracellular acidification is capable of activating S-type anion currents in wild-type guard cell protoplasts. Intracellular acidification at pH 6.1 alone did not significantly activate S-type anion channel currents compared with control recordings at pH 7.1 (Figure 2A, P>0.05, Student's t-test). Interestingly, when the intracellular free [CO2] was at a high concentration of 2 mM in the pipette solution (with 1.1 mM free [HCO3−]i) at pH 6.1, S-type anion channel currents were not activated in wild-type guard cell protoplasts, despite the applied high [CO2] and high proton concentrations (Figure 2B, P>0.05, Student's t-test).
Figure 2.
Elevated [H+] (pH 6.1) together with 2 mM intracellular free [CO2] did not activate S-type anion channel currents in wild-type Col-0 guard cells when bicarbonate levels were lower. (A) Steady-state current–voltage relationships of whole-cell currents recorded in guard cells at 2 μM [Ca2+]i without bicarbonate in the pipette solution at pH 7.1 (open circles, n=6) and pH 6.1 (filled circles, n=5). (B) Steady-state current–voltage relationships of whole-cell currents at pH 6.1 without bicarbonate (open circles, n=5) and with 2 mM intracellular free [CO2] and 1.1 mM free [HCO3−]i (filled circles, n=7) in the pipette solution. Data are mean±s.e. Liquid junction potential was +1 mV. (C) Example image of ratiometric pH sensitive Pt-GFP expressed guard cells. (D) Average fluorescence ratio time series of six guard cells expressing pH-sensitive reporter Pt-GFP during extracellular perfusion with buffers of different pH as indicated by the top bar. (E) Average fluorescence ratio time series of Pt-GFP expressed in guard cells perfused with MES buffer (10 mM MES, 10 mM KCl, 50 μM CaCl2, pH 5.6) and supplemented with sodium butyrate at mM concentrations as indicated by the top bar of the graph. Data are mean±s.e. The error bars presented in (D, E) were computed for the middle data points during each treatment, with the illustrated traces showing the averaged responses. (F) Fluorescence ratio time series of Pt-GFP-expressing guard cells perfused with extracellular buffers bubbled with 0 p.p.m. CO2 and 800 p.p.m. CO2. Raw data of two individual cells are depicted. GC denotes ratiometric fluorescence of guard cells and the ratio of non-guard cell background fluorescence (bg) is shown for the same experiments in (D–F).
Previous research has shown no intracellular pH shifts in V. faba guard cells in response to [CO2] changes (Brearley et al, 1997). To further investigate whether cytosolic pH is affected in Arabidopsis guard cells in response to [CO2] shifts, a ratiometric pH indicator Pt-GFP (Schulte et al, 2006) under the control of a strong guard cell preferential promoter pGC1 (Yang et al, 2008) was transformed into Arabidopsis guard cells (Figure 2C). In control experiments, in vivo recordings of pH in fluorescent pGC1::Pt-GFP transgenic guard cells showed clear reversible shifts in ratiometric intracellular pH fluorescence when the extracellular pH was repeatedly shifted from pH 5.0 to pH 7.5 and back (Figure 2D; Supplementary Figure S3). Weak acids can control intracellular pH while maintaining a constant extracellular pH (Blatt and Armstrong, 1993; Grabov and Blatt, 1997). Therefore, the weak acid sodium butyrate was used to analyse whether Pt-GFP can report intracellular pH. Ratiometric fluorescence recordings of Pt-GFP-expressing guard cells showed clear shifts, when intact plant epidermes were perfused with defined concentrations of sodium butyrate-containing MES buffers (Figure 2E), indicating intracellular pH changes were easily detected in guard cells (Figure 2D and E). However, no clear shifts in guard cell intracellular pH fluorescence were observed when the concentration of CO2 bubbled in the extracellular perfusion buffers was repeatedly shifted from 0 to 800 p.p.m. (Figure 2F), consistent with the findings in V. faba guard cells using a pH-sensitive dye (Brearley et al, 1997). Average changes in intracellular pH in response to extracellular pH changes appeared to be relatively rapid (Figure 2D), and slightly slower in response to weak acid treatments and without clearly discernable overshoots upon weak acid removal under the imposed conditions (Figure 2E). In conclusion, protons alone or in combination with elevated CO2 could not activate S-type anion channels (Figure 2A and B) and [CO2] changes did not cause measurable changes in intracellular pH of Arabidopsis guard cells (Figure 2F) (Brearley et al, 1997).
Bicarbonate activates S-type anion currents at low free CO2 in guard cells
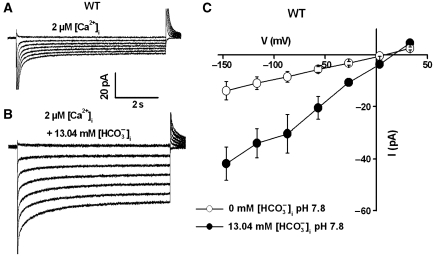
To analyse whether elevated intracellular [HCO3−] is sufficient to activate anion currents at low [H+] and low [CO2], 13.5 mM total CsHCO3 was applied to the pipette solution and the free [HCO3−] was calculated as 13.04 mM with 0.46 mM free [CO2] at pH 7.8. These analyses clearly showed that compared with the control recordings (Figure 3A), S-type anion currents were activated by the presence of high free HCO3− in the pipette solution (Figure 3B and C, P<0.05 at voltages from −146 to −26 mV, Student's t-test). Together, the above analyses show that elevated intracellular HCO3− is the main molecule that mediates activation of S-type anion currents in guard cells.
Figure 3.
High intracellular [HCO3−] at low [H+] and low free [CO2] activates S-type anion channel currents in wild-type Col-0 guard cells with 2 μM [Ca2+]i. (A) Typical recording of whole-cell currents in guard cell protoplasts without bicarbonate and (B) with 13.5 mM total bicarbonate (equivalent to 13.04 mM free [HCO3−]i/0.46 mM free [CO2]) added to the pipette solution at pH 7.8. (C) Average steady-state current–voltage relationships of whole-cell currents recorded as in (A) (open circles, n=3) and (B) (filled circles, n=5). Liquid junction potential was +1 mV. Data are mean±s.e.
Extracellular bicarbonate was next tested on activation of S-type anion currents in wild-type guard cells. After obtaining whole-cell recordings in wild-type guard cells, the bath solution (200 μl) was perfused for 2 min at 1 ml/min with a solution that contained 11.5 mM free [HCO3−]i and 2 mM [CO2] at pH 7.1 (Supplementary Figure S1A). No large S-type anion currents were activated (Supplementary Figure S1B and C). A small increase in average anion current magnitude was not statistically significant and was not comparable to the clear activation of S-type anion currents by the same concentration of applied intracellular HCO3− (Supplementary Figure S1B and C).
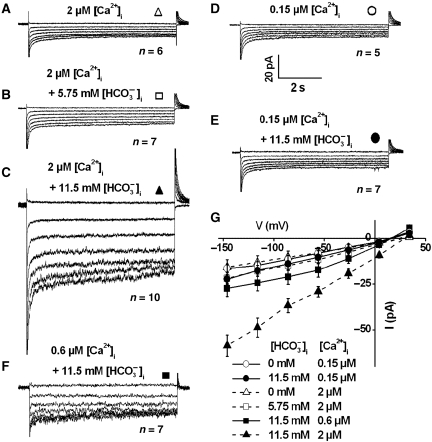
Elevated intracellular [Ca2+] is required for bicarbonate activation of S-type anion channel currents in guard cells
The above analyses of activation of S-type anion currents were all conducted at 2 μM cytosolic free Ca2+ ([Ca2+]i) (Figures 1, 2, 3). We investigated whether the elevated [Ca2+]i (2 μM) was necessary for bicarbonate activation of S-type anion channel currents in Arabidopsis guard cells. At 2 μM [Ca2+]i, anion currents were not strongly activated in the absence of added [HCO3−]i (Figure 4A and G), consistent with previous studies (Allen et al, 2002; Siegel et al, 2009). In contrast, 11.5 mM free [HCO3−]i activated strong S-type anion channels (Figure 4C and G, P<0.001), while an intermediate free [HCO3−]i of 5.75 mM did not activate significant S-type anion currents (Figure 4B and G, P>0.05, Student's t-test). When [Ca2+]i was buffered to a baseline level of 0.15 μM even with high 11.5 mM free [HCO3−]i and 2 mM free [CO2] in the pipette solution (pH 7.1), S-type anion currents were not activated (Figure 4E and G). There was no significant difference between the average amplitudes of current recordings at 0.15 μM free [Ca2+]i with or without added 11.5 mM free [HCO3−]i (Figure 4G, P>0.05, at voltages from −146 to +34 mV). In addition, an elevated cytosolic free [Ca2+]i of 0.6 μM together with high 11.5 mM free [HCO3−]i and 2 mM free [CO2] in the pipette solution (pH 7.1) activated anion currents of intermediate average amplitudes (Figure 4F and G). A summary of cytosolic free Ca2+ and HCO3− activation of S-type anion channels are shown in Supplementary Table I. These data demonstrate a requirement for an elevated [Ca2+]i in HCO3−-mediated activation of guard cell anion channels and provide direct and mechanistic evidence for the model that CO2-induced stomatal closing enhances the ability of [Ca2+]i to activate stomatal closing mechanisms (Young et al, 2006).
Figure 4.
Both [Ca2+]i and elevated bicarbonate are required for activation of S-type anion channel currents in wild-type (Col-0) guard cells. (A) Whole-cell currents in guard cell protoplasts at 2 μM [Ca2+]i without bicarbonate, (B) with 5.75 mM intracellular free [HCO3−]i/1 mM free [CO2] (6.75 mM total bicarbonate added) and (C) with 11.5 mM intracellular free [HCO3−]i/2 mM free [CO2] (13.5 mM total bicarbonate added) in the pipette solution at pH 7.1. (D) Whole-cell currents in guard cell protoplasts at 0.15 μM [Ca2+]i without bicarbonate, (E) with 11.5 mM free [HCO3−]i/2 mM free [CO2] (13.5 mM total bicarbonate) in the pipette solution at pH 7.1. (F) Whole-cell currents in guard cell protoplasts with 0.6 μM [Ca2+]i and 11.5 mM intracellular free [HCO3−]i/2 mM free [CO2] in the pipette solution at pH 7.1. (G) Steady-state current–voltage relationships of whole-cell currents as recorded in (A) (open triangles, n=6), (B) (open square, n=7), (C) (filled triangles, n=10), (D) (open circles, n=5), (E) (filled circles, n=7), and (F) (filled squares, n=7). Average data shown by dashed lines in (G) with or without of 5.75 mM and 11.5 mM free [HCO3−]i at 2 μM [Ca2+]i correspond to data reported in Hu et al (2010) and are included for comparison to 0.15 μM and 0.6 μM [Ca2+]i data. Liquid junction potential was +1 mV. Data are mean±s.e.
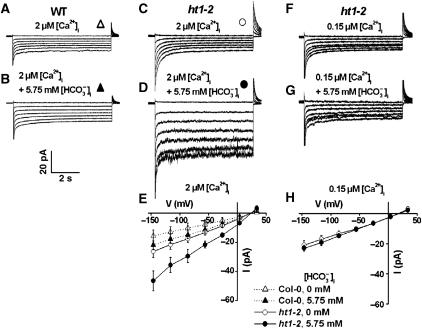
Lower [bicarbonate] is sufficient for activation of S-type anion channel currents in ht1-2 guard cells
The Arabidopsis HT1 protein kinase functions as a negative regulator of CO2-induced stomatal closing (Hashimoto et al, 2006). To test whether HT1 functions in the CO2/HCO3− SLAC1 signalling pathway (Figures 1, 2, 3), the effects of bicarbonate on S-type anion currents in recessive ht1-2 mutant guard cells were analysed. Whole-cell currents were recorded in guard cell protoplasts at lower intracellular [HCO3−]i, 5.75 mM free [HCO3−]i and 1 mM free [CO2] at pH 7.1, compared with the above experiments (Figure 5A and B). In wild-type control guard cells, these intermediate [HCO3−]i+[CO2] together with 2 μM free [Ca2+]i showed small whole-cell current amplitudes that were slightly larger than wild-type guard cells in the absence of added HCO3− (Figure 5A, B and E, P>0.05, Student's t-test) (Hu et al, 2010). However, significant activation of S-type anion currents by intracellular addition of 5.75 mM free [HCO3−]i and 1 mM free [CO2] (pH 7.1) was observed in ht1-2 guard cells (Figure 5D and E) compared with the control currents (Figure 5A–C and E, P<0.01 at voltages from −146 to −26 mV, Student's t-test). Note that 2 μM [Ca2+]i alone in ht1-2 guard cells was not sufficient to activate S-type anion currents (Figure 5C and E). While cytosolic [Ca2+]i was buffered to a typical resting level of 0.15 μM in ht1-2 guard cells, no significant S-type anion current activation was observed in the presence of 5.75 mM free [HCO3−]i (Figure 5F–H, P>0.05 at voltages from −146 to −26 mV, Student's t-test). Thus, ht1-2 guard cells show an enhanced sensitivity to intracellular HCO3−, but this enhanced activation cannot by-pass the requirement for [Ca2+]i in HCO3− activation of S-type anion currents.
Figure 5.
Enhanced bicarbonate sensitivity of S-type anion channel activation in ht1-2 mutant guard cells only at elevated [Ca2+]i. (A) Whole-cell currents in wild-type Col-0 guard cells at 2 μM [Ca2+]i without bicarbonate and (B) with 6.75 mM total bicarbonate (equivalent to 5.75 mM free [HCO3−]i/1 mM free [CO2]) added to the pipette solution. (C) Whole-cell currents in ht1-2 mutant guard cells at 2 μM [Ca2+]i without bicarbonate and (D) with 6.75 mM bicarbonate (equivalent to 5.75 mM free [HCO3−]i/1 mM free [CO2]) in the pipette solution. (E) Average steady-state current–voltage relationships of whole-cell currents as recorded in (A) (open triangles, n=6), (B) (filled triangles, n=7), (C) (open circles, n=5) and (D) (filled circles, n=9). Average data for wild-type Col-0 controls (WT) shown by dashed lines in (E) with 0 and 6.75 mM total bicarbonate (5.75 mM free [HCO3−]) with 2 μM [Ca2+]i correspond to data reported in Hu et al (2010) and are included for comparison to ht1-2 mutant data. (F) Whole-cell currents in ht1-2 mutant guard cell protoplasts at low 0.15 μM [Ca2+]i without bicarbonate and (G) with 6.75 mM bicarbonate (equivalent to 5.75 mM free [HCO3−]i/1 mM free [CO2]) added to the pipette solution. (H) Average steady-state current–voltage relationships of whole-cell currents as recorded in (F) (open circles, n=5) and (G) (filled circles, n=5). Liquid junction potential was +1 mV. Data are mean±s.e.
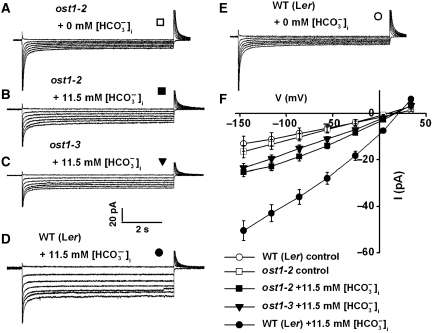
The OST1 kinase functions in bicarbonate activation of S-type anion currents in guard cell protoplasts and CO2-induced stomatal closure
The OST1 protein kinase was previously demonstrated to mediate ABA-induced stomatal closing. Recessive ost1 mutants disrupt ABA-induced stomatal closure as well as ABA inhibition of light-induced stomatal opening, but low CO2 induction of stomatal opening remained unaffected in the ost1-2 mutant, indicating that OST1 does not participate in CO2 signalling (Mustilli et al, 2002; Yoshida et al, 2002). Here, the effect of OST1 on bicarbonate activation of S-type anion channels was investigated. Using the same recording solutions as in Figure 1B, high [HCO3−]i (11.5 mM) and [CO2] (2 mM) activated only small S-type anion currents in Landsberg erecta (Ler) ost1-2 mutant guard cells (Figure 6A, B and F). Similar to Col-0 wild-type guard cells (Figures 1, 3 and 4), high HCO3− activated S-type anion channel currents in Ler wild-type guard cells (Figure 6D, E and F). While HCO3−-activated S-type anion currents in Ler wild-type guard cells were larger (I=−51±4.3 pA at a voltage of −146 mV, n=7) than that in ost1-2 mutant guard cells (I=−25.2±1.9 pA at a voltage of −146 mV, n=6) (Figure 6F, P<0.001, Student's t-test). Moreover, bicarbonate activation of S-type anion channels was also strongly impaired in Columbia based ost1-3 T-DNA insertion allele guard cells (Figure 6C and F) compared with Col-0 wild type (Figure 4C and G). At a voltage of −146 mV, the current amplitude activated by bicarbonate in ost1-3 mutant guard cells was −24±1.9 pA (Figure 6F, n=6), and in Col-0 wild type, it was −59±5.9 pA (Figure 4E, n=10, P<0.001, Student's t-test).
Figure 6.
HCO3−/CO2 activation S-type anion channel currents is disrupted in ost1-2 and ost1-3 mutant guard cells with 2 μM [Ca2+]i. (A) Whole-cell recording without and (B) with 13.5 mM total bicarbonate (11.5 mM free [HCO3−]i + 2 mM free [CO2]) added to the pipette solution in ost1-2 mutant guard cells. (C) Whole-cell recording with 13.5 mM total bicarbonate in the pipette solution in ost1-3 mutant guard cells. (D) Whole-cell currents with 13.5 mM total bicarbonate and (E) without bicarbonate added to the pipette solution in wild-type Ler guard cell protoplasts. (F) Steady-state current–voltage relationships of recordings as in (A) (open squares: ost1-2, –[HCO3−]i, n=5), (B) (filled squares: ost1-2, +[HCO3−]i, n=6), (C) (filled triangles: ost1-3, +[HCO3−]i, n=6), (D) (filled circles: wild-type Ler, +[HCO3−]i, n=7) and (E) (open circles: wild-type Ler, –[HCO3−]i, n=5). The pipette solution was adjusted to pH 7.1 in all the recordings. Liquid junction potential was +1 mV. Data are mean±s.e.
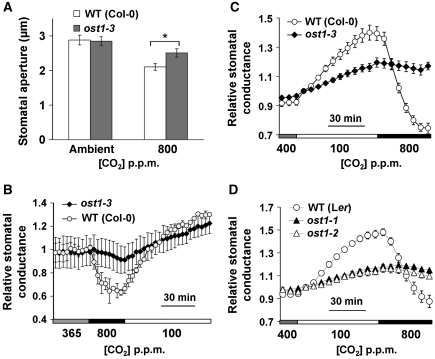
Elevated CO2-induced stomatal closure was also impaired in ost1-3 mutant leaf epidermes compared with wild-type controls in genotype-blind stomatal movement assays (Figure 7A, P<0.05 at 800 p.p.m. CO2, Student's t-test). We next analysed the stomatal conductance changes in intact ost1-3 mutant leaves in response to [CO2] shifts. Interestingly, stomatal conductance in ost1-3 mutant leaves showed a very strong CO2 insensitivity when the [CO2] was shifted to high concentrations (Figure 7B; Supplementary Figure S4A). To further investigate the unexpected strong CO2 insensitivity of ost1, whole intact plant gas exchange experiments were pursued and the strong CO2 insensitivity was observed in ost1-1, ost1-2 and ost1-3 plants (Figure 7C and D; Supplementary Figure S4B and C).
Figure 7.
CO2-induced stomatal closure is strongly impaired in ost1 mutants. (A) Stomatal closure is impaired in ost1-3 mutant leaves in response to elevated [CO2]. *P<0.05, Student's t-test. (B) Time-resolved relative stomatal conductance in responses to [CO2] in ost1-3 mutant and wild-type Col-0 intact leaves (n=4 for each genotype). (C) Patterns of whole-plant relative stomatal conductance in responses to changes in [CO2] in intact ost1-3 and Col-0 wild-type plants (n=8 for ost1-3, n=6 for WT) and (D) in intact ost1-1, ost1-2 and Ler wild-type plants (n=4 for each genotype). Imposed CO2 concentrations are shown at the bottom. Data are mean±s.e.
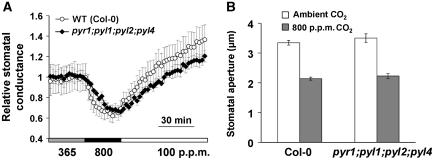
ABA receptor pyr1;pyl1;pyl2;pyl4 quadruple mutant maintains functional and slightly slower CO2 response
The PYR/RCAR ABA receptor family was recently identified in Arabidopsis as major ABA receptors (Ma et al, 2009; Park et al, 2009). Since these ABA receptors tightly regulate and form complexes with SnRK2 kinases including OST1 (Fujii et al, 2009; Ma et al, 2009; Park et al, 2009; Nishimura et al, 2010), CO2 regulation of gas exchange in intact pyr1;pyl1;pyl2;pyl4 quadruple mutant leaves was analysed to determine the requirement of ABA receptors for this CO2 response. Intact leaves of the pyr1;pyl1;pyl2;pyl4 quadruple mutant showed clear CO2 responses upon [CO2] changes (Figure 8A; Supplementary Figure S4D) and showed an average slight slowing of the CO2 response, observed in independent experimental sets that was not highly significant (P=0.1, Student's t-test) at 18 min after 365 to 800 p.p.m. CO2 transition. Upon shifting [CO2] from 365 to 800 p.p.m. for 30 min, the initial rates of stomatal conductance changes were −0.038±0.014 mmol H2O per m2 per s per min for Col-0 wild-type plants and −0.035±0.008 mmol H2O per m2 per s per min for pyr1;pyl1;pyl2;pyl4 mutant plants (P=0.24, Student's t-test). And during the first 30 min upon shifting [CO2] from 800 to 100 p.p.m., the initial rates were 0.042±0.013 mmol H2O per m2 per s per min for Col-0 wild-type plants and 0.022±0.002 mmol H2O per m2 per s per min for pyr1;pyl1;pyl2;pyl4 mutant plants (P=0.06, Student's t-test). Further, genotype-blind stomatal movement imaging analyses of individually mapped stomata showed that high CO2-induced stomatal closure was also retained in pyr1;pyl1;pyl2;pyl4 mutant leaf epidermes 30 min after CO2 elevation (Figure 8B).
Figure 8.
CO2-induced stomatal closure is slightly slowed in ABA receptor pyr1;pyl1;pyl2;pyl4 (Col based) quadruple mutant. (A) ABA receptor pyr1;pyl1;pyl2;pyl4 quadruple mutant does not abrogate but slows CO2 regulation of stomatal conductance in intact leaves (n=4 for each genotype). (B) pyr1;pyl1;pyl2;pyl4 quadruple leaf epidermes retained robust responses to elevated [CO2] (n=30 stomata for each genotype, genotype blind). For stomatal movement analyses in (B) individual stomata were imaged and tracked as previously reported (Siegel et al, 2009). Data are mean±s.e.
Discussion
Elevated [CO2] in leaf intercellular spaces (Ci) and elevated atmospheric [CO2] cause closing of stomatal pores in diverse plant species (Medlyn et al, 2001). CAs have been identified that function early in CO2 signal transduction (Hu et al, 2010). However, major questions in CO2 signal transduction have arisen. Whether CO2 or bicarbonate ions or a combination of these function in CO2 signal transduction in guard cells remained unclear. The presented findings demonstrate that bicarbonate acts as an intracellular signalling molecule in CO2 signal transduction, by activating SLAC1-mediated S-type anion channels in guard cells. We further found a synergistic action of intracellular HCO3− with cytosolic Ca2+ that requires both of these small molecules for CO2 signalling to proceed. We also report the characterization of the cellular functions and relative positions within the CO2 signal transduction cascade of mutants that strongly affect CO2 control of stomatal movements, including ca1;ca4, slac1 and ht1-2. ht1-2 mutant guard cells show hypersensitivity to intracellularly applied HCO3−, but continue to require cytosolic Ca2+ for activation of SLAC1-dependent anion currents. In addition, we have unexpectedly found that loss-of-function mutations in the OST1 protein kinase cause a strong CO2 insensitivity of stomatal regulation by analyses of S-type anion channel regulation, stomatal movements and gas exchange in intact leaves and in whole plants, which leads to a new model for early CO2 signal transduction in guard cells.
Central function of the OST1 protein kinase in CO2 signal transduction
Previous stomatal movement assays indicated that the OST1 protein kinase may not function in CO2 inhibition of stomatal opening (Mustilli et al, 2002). We have found here that ost1 mutant guard cells in both Col-0 and Ler accessions show a dramatic impairment in CO2 regulation of stomatal conductance in intact leaves. Recent studies have shown that the OST1 kinase activates SLAC1 channels via phosphorylation (Geiger et al, 2009; Lee et al, 2009; Vahisalu et al, 2010). Together, our findings of impairment in bicarbonate activation of S-type anion currents in ost1-2 and ost1-3 mutant guard cells (Figure 6A, B and D) and the strong impairment in CO2-induced stomatal closing and stomatal conductance changes in intact leaves and in intact plants (Figure 7B–D) show that the OST1 protein kinase is a central transducer of CO2 signal transduction in guard cells.
The PYR/RCAR ABA receptors form a linear signal transduction module together with type 2C protein phosphatases and the OST1 protein kinase (Fujii et al, 2009; Ma et al, 2009; Park et al, 2009; Santiago et al, 2009; Umezawa et al, 2009; Nishimura et al, 2010). A quadruple mutant in four highly expressed guard cell ABA receptors pyr1;pyl1;pyl2;pyl4 shows a strong impairment in ABA-induced stomatal closing (Nishimura et al, 2010). In contrast CO2 regulation remained functional, albeit slowed, in intact leaves (Figure 8). These data lead to an updated model for early CO2 signal transduction in which the convergence point of CO2 and ABA signal transduction occurs earlier than previously thought at the level of the OST1 protein kinase or earlier (Figure 9). The CO2 response of pyr1;pyl1;pyl2;pyl4 quadruple mutant plants exhibited an average slowing compared with wild-type plants (Figure 8). This may be attributable to the early convergence of CO2 and ABA signalling at the level of the OST1 protein kinase as revealed here. Thus, a degree of cross-talk between ABA and CO2 signalling can be expected. Classical studies have shown that very low subthreshold concentrations of ABA do not cause an ABA response, but amplify CO2-induced stomatal closing (Raschke, 1975). Our findings provide a mechanistic basis for this classical observation, with both CO2 and ABA signal transduction occurring via the OST1 protein kinase (Figure 9), as ost1 mutant alleles show both strong CO2 (Figure 7) and ABA insensitivities (Mustilli et al, 2002; Yoshida et al, 2002).
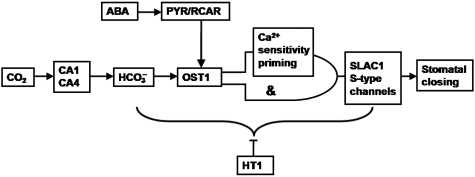
Figure 9.
Model for the mechanisms characterized in the present study showing the sequence of events that mediate CO2 regulation of S-type anion channels and stomatal closing. In this model, CA1/CA4, OST1 and SLAC1 function as positive mediators, and the HT1 protein kinase functions as a negative regulator of high CO2-incuded stomatal closing. [Ca2+]i sensitivity priming and [Ca2+]i-independent mechanisms are proposed to regulate SLAC1-dependent S-type anion currents in parallel via an ‘AND’-like gate. In this ‘AND’-like gate, one ‘input’ occurs via the OST1 pathway, and the other ‘input’ is mediated by the Ca2+ sensitivity priming pathway. Convergence with abscisic acid (ABA) signalling is also indicated here. CA, carbonic anhydrase; OST1, open stomata 1; HT1, high leaf temperature 1; SLAC1, slow anion channel; PYR/RCAR, ABA receptors.
The dominant protein phosphatase 2C (PP2C) mutants, abi1-1 and abi2-1, have been reported to conditionally affect CO2 signalling in guard cells (Webb and Hetherington, 1997; Leymarie et al, 1998a, 1998b). ABI1 interacts with the OST1 protein kinase (Belin et al, 2006; Yoshida et al, 2006; Umezawa et al, 2009; Vlad et al, 2009; Nishimura et al, 2010). Further research is needed to determine whether the wild-type PP2C-protein phosphatases control the CO2 response. The present study on CO2 signalling and research indicating ABA-independent activation of the OST1 protein kinase (Yoshida et al, 2006; Zheng et al, 2010) indicates that the early ABA signalling module consisting of ABA receptors (Ma et al, 2009; Park et al, 2009), PP2Cs and OST1/SnRK2 kinases may be more complex than the present models (Fujii et al, 2009).
Bicarbonate activates S-type anion channels
In mammalian cells, CO2 detection in olfactory receptor neurons require the expression of the receptor-type guanylate cyclase GC-D (Hu et al, 2007), which is activated by the CO2 metabolite bicarbonate (Guo et al, 2009; Sun et al, 2009), that is catalytically produced by CAs (Hu et al, 2007). Here, elevated bicarbonate activation of S-type anion currents in ca1;ca4 double-mutant guard cells (Figure 1) is consistent with the model that βCA1 and βCA4 act very early in the guard cell CO2 signal transduction pathway (Figure 9). S-type anion channel activation by bicarbonate reported here (Figure 3) shows similar properties to SLC26A9 channels in mammalian epithelial cells. SLC26A9 encodes a Cl− channel and is modulated by HCO3− (Loriol et al, 2008). Expression of SLC26A9 in X. laevis oocytes, produced Cl− currents that increased in magnitude in the presence of 24 mM HCO3− compared with 2.4 mM HCO3−. Furthermore, the SLC26A9 channel has no HCO3− permeability and is not regulated by intracellular pH (Loriol et al, 2008). In Arabidopsis hypocotyl cells, bicarbonate is permeable through voltage-dependent anion channels (R-type anion channels) with a relative permeability ratio PHCO3−/PCl− of 0.8 (Frachisse et al, 1999). Different from that, the SLAC1 channel is impermeable to HCO3− (Geiger et al, 2009), and our analyses of S-type anion currents also support this (Supplementary Figure S2). SLAC1 channels were not activated by bicarbonate when SLAC1 was heterologously expressed alone in X. laevis oocytes (Geiger et al, 2009). This can be explained by our findings that bicarbonate activation of S-type anion channel in planta requires other essential components, in particular the OST1 protein kinase and elevated [Ca2+]i, with the HT1 protein kinase functioning as a negative regulator within this module of the CO2 signal transduction cascade (Figures 4, 5, 6 and 9). Further research will be needed to identify the bicarbonate-binding proteins that mediate this response.
The intracellular concentrations of bicarbonate and CO2 used in patch-clamp experiments in the present study for S-type anion channel activation were higher than physiological concentrations in planta. Note that patch clamping of guard cells includes dialysis of the cytoplasm (Hamill et al, 1981) and it is possible that additional diluted small molecules or proteins are required for full sensitivity of this HCO3− response. Furthermore, typically high CO2 and HCO3− concentrations are used in electrophysiological studies, up to 72 mM HCO3− (Loriol et al, 2008; Chandrashekar et al, 2009; Yarmolinsky et al, 2009), although these experiments were conducted in different systems. The close correlation of high HCO3− regulation of S-type anion channels in the present study and the impaired CO2 response phenotypes in intact leaves of the Arabidopsis ca1;ca4, slac1, ht1-2 and ost1 mutants (Figures 6 and 7) and the [Ca2+]i sensitivity of this response (Figure 4) suggest that the analysed intracellular HCO3− regulation responses are physiologically relevant (Schwartz, 1985; Webb et al, 1996; Hashimoto et al, 2006; Young et al, 2006; Negi et al, 2008; Vahisalu et al, 2008; Hu et al, 2010).
Intracellular acidification activates slow anion channel currents in the plasma membrane of Arabidopsis hypocotyl cells (Colcombet et al, 2005). However, intracellular acidification did not activate S-type anion currents in Arabidopsis guard cells, even in the presence of elevated 2 μM free [Ca2+]i (Figure 2A). In animal chemosensitive neurons, intracellular pH is lowered in response to increasing CO2 levels from 10% up to 50% [CO2] (Putnam et al, 2004). Using the pH-sensitive dye BCECF (2′,7′-bis-(2-carboxyethyl)-5,6- carboxyflourescein) and fluorescence microphotometry to measure cytosolic pH in V. faba guard cells, no significant pH change was observed during transition from 0 to 1000 p.p.m. CO2 (Brearley et al, 1997). Our findings correlate with the previous study as no detectable pH changes were observed in guard cells expressing the ratiometric pH sensor Pt-GFP when intact leaf epidermes were perfused with buffers bubbled with 0 and 800 p.p.m. CO2 (Figure 2F). These data are also compatible with models proposing (Raschke et al, 1988) and findings from pH measurements showing (Grabov and Blatt, 1997) a high pH buffering capacity of V. faba guard cells.
CO2 enhances the [Ca2+]i sensitivity of S-type anion channel activation
Calcium is a second messenger that transduces diverse stimuli in plants (Sanders et al, 1999; Blatt, 2000; Hetherington and Brownlee, 2004; Kim et al, 2010; Kudla et al, 2010). Elevated CO2 caused an increase in [Ca2+]i in Commelina Communis guard cells (Webb et al, 1996). Furthermore, elevated CO2 caused a dampening of spontaneous repetitive [Ca2+]i transients, whereas low CO2 caused rapid [Ca2+]i transients in Arabidopsis guard cells (Young et al, 2006), which can be attributed to CO2-induced depolarization of guard cells (Grabov and Blatt, 1998; Staxen et al, 1999; Klusener et al, 2002). In both plant species, abolishment of [Ca2+]i elevations abolished CO2-induced stomatal closing (Schwartz, 1985; Webb et al, 1996; Young et al, 2006). Time-resolved [Ca2+]i imaging experiments led to the Ca2+ sensitivity priming hypothesis, in which CO2 was hypothesized to enhance (prime) the Ca2+ sensitivity of signalling mechanisms that relay CO2-induced stomatal closure (Young et al, 2006). However, additional and direct evidence for this CO2 signalling hypothesis has been lacking. Recent studies showed that ABA enhances (primes) the [Ca2+]i sensitivity of S-type anion channel and Kin+ channel regulation, strongly supporting the hypothesis that ABA primes [Ca2+]i signal transduction (Siegel et al, 2009).
ABA increases cytosolic Ca2+ concentration by activating plasma membrane Ca2+ channels in V. faba and Arabidopsis guard cells (Schroeder and Hagiwara, 1990; Grabov and Blatt, 1998; Hamilton et al, 2000; Pei et al, 2000; Murata et al, 2001). Cytosolic [Ca2+]i interacts with other signalling molecules including nitric oxide (Garcia-Mata et al, 2003) and cytosolic pHi (Grabov and Blatt, 1997) in ion channel regulation in guard cells. Recently, Chen et al (2010) showed that cytosolic free [Ca2+]i interacts with protein phosphorylation events during slow anion channel activation.
The present study shows that elevated bicarbonate enhances the [Ca2+]i sensitivity in S-type anion channel activation (Figure 4). ABA- and Ca2+-induced activation of S-type anion channels and stomatal closing are mediated by Ca2+-dependent protein kinases (CDPKs) (Mori et al, 2006; Zhu et al, 2007; Geiger et al, 2010). Heterologous reconstitution analysis has proposed that ABA activates anion channels by the OST1 protein kinase, in parallel through a Ca2+-dependent CDPK pathway (Geiger et al, 2010). Together with previous studies (Allen et al, 2002; Israelsson et al, 2006; Young et al, 2006; Siegel et al, 2009; Chen et al, 2010; Hu et al, 2010), the present findings provide strong evidence that Ca2+ sensitivity priming is a mechanism that controls both CO2 and ABA regulation on S-type anion channels (Figure 9). Interestingly, here patch-clamped guard cell protoplasts were exposed to elevated HCO3−/CO2 in the pipette solution for only ∼3 to 5 min prior to analysing [Ca2+] activation of S-type anion currents (Figure 4C and G), whereas ABA signalling studies tested 30 min ABA pre-incubation (Siegel et al, 2009). This rapid 3 to 5 min HCO3−/CO2−[Ca2+]i response provides first evidence that Ca2+ sensitivity priming is a rapid modification and that transcriptional and translational mechanisms may not mediate Ca2+ sensitivity priming.
ht1 kinase mutant enhances bicarbonate sensitivity but requires [Ca2+]i
The HT1 protein kinase functions as a negative regulator of CO2 signalling (Hashimoto et al, 2006) and our recent study showed that HT1 is epistatic to βCA1 and βCA4 in the CO2 response pathway (Hu et al, 2010). However, the role of HT1 within the guard cell signalling network had not been further analysed. The ht1-2 mutant exhibits a hypersensitive response in bicarbonate activation of S-type anion currents, confirming that the HT1 kinase functions as a negative regulator and demonstrating that HT1 affects CO2 signalling downstream of HCO3− production and upstream of anion channel activation (Figure 9). Cytosolic Ca2+ elevation is still required for S-type anion channel activation in ht1-2 mutant guard cells, showing that HT1 kinase-mediated CO2 signalling does not by-pass Ca2+ sensitivity priming (Figures 5 and 9).
In conclusion, the present study identifies the OST1 protein kinase and the synergistic roles of the intracellular small molecules HCO3− and Ca2+ in guard cell CO2 signal transduction and anion channel regulation. Furthermore, characterization of the positions and roles of OST1, the HT1 protein kinase, the βCA1 and βCA4 CAs, PYR/RCAR ABA receptors, and SLAC1 in CO2 regulation of S-type anion channels, leads to a revised model for CO2 signal transduction (Figure 9). During CO2-induced stomatal closing, CO2 is first catalysed by CAs into bicarbonate. Elevated bicarbonate activates S-type anion channels via an ‘AND’-like gate (Figure 9). In the ‘AND’-like gate, one ‘input’ occurs via the OST1 pathway, and the other ‘input’ is mediated by the Ca2+ sensitivity priming pathway. The HT1 kinase acts as a negative regulator in the CO2 signalling pathway downstream of HCO3− production and upstream of S-type anion channel activation, which continues to require [Ca2+]i. PYR/RCAR ABA receptors do not directly mediate guard cell CO2 signalling, but mutation slows CO2 responses, indicating that they function upstream of the convergence point of CO2 and ABA signalling, while affecting common downstream signalling mechanisms (Figure 8). The OST1 protein kinase is an essential mediator of guard cell CO2 signal transduction, providing evidence that mechanisms in addition to ABA can activate OST1-dependent signalling (Figures 6 and 7).
Materials and methods
Plant growth
The Arabidopsis mutant lines analysed in this study were ca1;ca4 (Hu et al, 2010), slac1-1, slac1-3 (Vahisalu et al, 2008), ht1-2 (Hashimoto et al, 2006), ost1-1, ost1-2 (Mustilli et al, 2002), ost1-3 (Yoshida et al, 2002) and pyr1;pyl1;pyl2;pyl4 in the backcrossed Col-0 background (Nishimura et al, 2010). Plants were grown in a Conviron growth chamber at 21°C, 65–85% humidity, except abi1-1 and abi2-1 were grown constantly at 75–85% humidity, and a 16-h light/8-h dark photoperiod regime at ∼75 μmol/m2 s.
Electrophysiology
Arabidopsis guard cell protoplasts were isolated as described previously (Leonhardt et al, 2004; Siegel et al, 2009). Whole-cell patch-clamp experiments were performed as described previously (Pei et al, 1997). During recordings of S-type anion currents, the membrane voltage was stepped to potentials starting at +35 to −145 mV for 7 s with −30 mV decrements and the holding potential was +30 mV. The interpulse period was 5 s. Liquid junction potentials were determined using Clampex 10.0. No leak subtraction was applied for all current–voltage curves. Steady-state currents were the average currents during the last 500 ms of pulses. Detail contents of solutions can be found in Supplementary data. Bicarbonate (CsHCO3) was freshly dissolved in the pipette solution before patch-clamp experiments and pH was adjusted to the indicated values. The pipette solution was stored using air-tight precision glass syringes during patch-clamp experiments to slow CO2 equilibration with the surrounding air and was not stored overnight. The concentrations of free CO2 and bicarbonate in solutions were calculated using the Henderson–Hasselbalch equation (pH=pK1+log[HCO3−]/[CO2]) (Hauser et al, 1995). [HCO3−] represents the free bicarbonate concentration; [CO2] represents the free CO2 concentration. A value, pK1=6.352, was used for calculations (Speight, 2005). To independently measure CO2 concentrations in the solutions at different pH values, an InPro 5000 CO2 sensor (Mettler Toledo 400, Mettler Toledo Inc.) was used for dissolved CO2. The significance of differences between data sets was assessed by noncoupled double-tailed Student's t-test analysis. Values of P<0.05 were considered statistically significant.
Expression of pH sensor Pt-GFP in Arabidopsis guard cells
The Pt-GFP cDNA was amplified with the primers PGF (5′-AACCATGGCGCAGACCCTTCCTCTAT-3′, with NcoI site) and PGR (5′-AACTGCAGAGGCGTCTCGCATATCTC-3′, with PstI site) from the construct pART7-Pt-GFP (Schulte et al, 2006), kindly provided by Dr Christoph Plieth. The sequenced PCR product was digested with NcoI and PstI and then subcloned into the binary expression vector pGreenII 0179-pGCP(D1)-terminator under the control of guard cell specific promoter pGC1 (Yang et al, 2008). The construct pGC1::Pt-GFP was transformed to the Agrobacterium strain GV3101-containing helper plasmid pSOUP and then was introduced into Arabidopsis (Col-0) by the floral dip method (Clough and Bent, 1998).
Fluorescence imaging of guard cells expressing Pt-GFP
Fluorescence imaging was performed with a TE300 inverted microscope using a TE-FM Epi-Fluorescence attachment (Nikon) as previously described (Allen et al, 2000). Fluorescence images at excitation wavelengths of 470 and 440 nm were taken every 2 s using light from a 75-W xenon short arc lamp (Osram, Germany). In all, 32′ neutral density filters were used to reduce bleaching of fluorescent reporter. Metafluor software (MDS, Inc.) was used to control filter wheels, shutter and CoolSNAP CCD camera from Photomerics when recording and also processing raw data. The fluorescence ratio F470/F440 of Pt-GFP was analysed as a detection of pH shifts. Intact epidermes from pGC1::Pt-GFP-expressing leaves were prepared and affixed to glass Coverslips using Medical Adhesive (Hollister Incorporated Libertyville, IL) and then adhered to a glass slide with a hole in the middle generating a well, as described (Young et al, 2006; Siegel et al, 2009; Hu et al, 2010).
For recording intracellular Pt-GFP fluorescence in response to changes in extracellular pH incubation buffers, the pH of incubation buffers containing 10 mM MES, 10 mM KCl and 50 μM CaCl2 at 5.0 and 7.5 was adjusted by adding Tris–HCl. The well was perfused with incubation buffer at pH 5.0 (or pH 4.5) for 15 min to obtain a background value and subsequently perfused with buffer at pH 7.5 for 15 min and returned to pH 5.0 (or pH 4.5) again. For recording intracellular Pt-GFP fluorescence in response to constant extracellular pH and added weak acid, the perfusion buffers contained 10 mM MES, 10 mM KCl and 50 μM CaCl2, pH 5.6 supplemented with the indicated concentrations of sodium butyrate. For recording the Pt-GFP fluorescence of guard cells in response to CO2 changes, the incubation buffer (10 mM MES, 10 mM KCl and 50 μM CaCl2, pH 6.15) was continually bubbled with 800 p.p.m. CO2 or bubbled with air through soda lime, which was considered as nominal 0 p.p.m. CO2 inside the buffer. Note that the final CO2 concentrations to which leaf epidermes were exposed were as reported previously using the same experimental set up and conditions (Young et al, 2006). The well was perfused with buffers shifting from 800 to 0 p.p.m. CO2 via a peristaltic pump and teflon tubing. Background fluorescence intensities at 470 nm were measured in regions lacking guard cells and are also shown for the corresponding experiments.
Supplementary Material
Acknowledgments
We thank Dr Cawas Engineer and Dr Rama Vaidyanathan for critical reading of the manuscript and Dr Kristiina Laanements for confirmation of whole-plant gas exchange data in the Ler ost1 mutants. We also thank Drs Nan Sang, Yanxi Pei and Shuqing Zhao for help during revision of the manuscript. This work was supported by grants from the National Science Foundation (MCB0918220), the National Institutes of Health (GM060396), Bayer Crop Sciences, the Chemical Sciences, Geosciences, and Biosciences Division of the Office of Basic Energy Sciences at the US Department of Energy (DOE-DE-FG02-03ER15449) and the Human Frontiers in Science Program to JIS and a fellowship from the King Abdullah University of Science and Technology (KAUST; No. KUS-F1-021-31) to HH, and in part supported by grants from the National Science Foundation of China (20701028) to SX and from the Estonian Science Foundation 7763 and SF0180071S07 to HK.
Author contributions: Experiments were conceived by JIS and designed by JIS, SX and HH. SX and HH performed most of the experiments at UCSD and contributed equally to this work. Some experiments requested by the reviewers were conducted by SX at Shanxi University. Whole-plant gas exchange analyses of ost1 mutants were performed by EM and HK at University of Tartu. SX, HH and JIS wrote the paper.
Footnotes
UCSD has submitted a patent application based on some of the findings in this study.
References
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, Chory J, Schroeder JI (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289: 2338–2342 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Murata Y, Chu SP, Nafisi M, Schroeder JI (2002) Hypersensitivity of abscisic acid-induced cytosolic calcium increases in the Arabidopsis farnesyltransferase mutant era1-2. Plant Cell 14: 1649–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin C, de Franco PO, Bourbousse C, Chaignepain S, Schmitter JM, Vavasseur A, Giraudat J, Barbier-Brygoo H, Thomine S (2006) Identification of features regulating OST1 kinase activity and OST1 function in guard cells. Plant Physiol 141: 1316–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR (2000) Cellular signaling and volume control in stomatal movements in plants. Annu Rev Cell Dev Biol 16: 221–241 [DOI] [PubMed] [Google Scholar]
- Blatt MR, Armstrong F (1993) K+ channels of stomatal guard cells: abscisic acid-evoked control of the outward rectifier mediated by cytoplasmic pH. Planta 191: 330–341 [Google Scholar]
- Brearley J, Venis MA, Blatt MR (1997) The effect of elevated CO2 concentrations on K+ and anion channels of Vicia faba L. guard cells. Planta 203: 145–154 [Google Scholar]
- Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, Zuker CS (2009) The taste of carbonation. Science 326: 443–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Hills A, Lim CK, Blatt MR (2010) Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant J 61: 816–825 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Colcombet J, Lelievre F, Thomine S, Barbier-Brygoo H, Frachisse JM (2005) Distinct pH regulation of slow and rapid anion channels at the plasma membrane of Arabidopsis thaliana hypocotyl cells. J Exp Bot 56: 1897–1903 [DOI] [PubMed] [Google Scholar]
- Evans NH, Hetherington AM (2001) Plant physiology: the ups and downs of guard cell signalling. Curr Biol 11: R92–R94 [DOI] [PubMed] [Google Scholar]
- Frachisse JM, Thomine S, Colcombet J, Guern J, Barbier-Brygoo H (1999) Sulfate is both a substrate and an activator of the voltage-dependent anion channel of Arabidopsis hypocotyl cells. Plant Physiol 121: 253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer WB (2010) CO2mmon sense. Science 327: 275–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK (2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462: 660–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR (2003) Nitric oxide regulates K+ and Cl− channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci USA 100: 11116–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, Liese A, Wellmann C, Al-Rasheid KAS, Grill E, Romeis T, Hedrich R (2010) Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci USA 107: 8023–8028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KA, Romeis T, Hedrich R (2009) Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci USA 106: 21425–21430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Blatt MR (1997) Parallel control of the inward-rectifier K+ channel by cytosolic free Ca2+ and pH in Vicia guard cells. Planta 201: 84–95 [Google Scholar]
- Grabov A, Blatt MR (1998) Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc Natl Acad Sci USA 95: 4778–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Blatt MR (1999) A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiol 119: 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Leung J, Giraudat J, Blatt MR (1997) Alteration of anion channel kinetics in wild type and abi1-1 transgenic Nicotiana benthamiana guard cells by abscisic acid. Plant J 12: 203–213 [DOI] [PubMed] [Google Scholar]
- Guo D, Zhang JJ, Huang XY (2009) Stimulation of guanylyl cyclase-D by bicarbonate. Biochemistry 48: 4417–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391: 85–100 [DOI] [PubMed] [Google Scholar]
- Hamilton DW, Hills A, Kohler B, Blatt MR (2000) Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc Natl Acad Sci USA 97: 4967–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanstein SM, Felle HH (2002) CO2-triggered chloride release from guard cells in intact fava bean leaves. Kinetics of the onset of stomatal closure. Plant Physiol 130: 940–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Negi J, Young J, Israelsson M, Schroeder JI, Iba K (2006) Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat Cell Biol 8: 391–397 [DOI] [PubMed] [Google Scholar]
- Hauser M, Eichelmann H, Heber U, Laisk A (1995) Chloroplast pH values and buffer capacities in darkened leaves as revealed by CO2 solubilization in-vivo. Planta 196: 199–204 [Google Scholar]
- Hetherington AM, Brownlee C (2004) The generation of Ca2+ signals in plants. Annu Rev Plant Biol 55: 401–427 [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424: 901–908 [DOI] [PubMed] [Google Scholar]
- Hu H, Boisson-Dernier A, Israelsson-Nordstrom M, Bohmer M, Xue S, Ries A, Godoski J, Kuhn JM, Schroeder JI (2010) Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat Cell Biol 12: 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M (2007) Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science 317: 953–957 [DOI] [PubMed] [Google Scholar]
- Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI (2010) Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev 24: 1695–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelsson M, Siegel RS, Young J, Hashimoto M, Iba K, Schroeder JI (2006) Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr Opin Plant Biol 9: 654–663 [DOI] [PubMed] [Google Scholar]
- Kelly WB, Esser JE, Schroeder JI (1995) Effects of cytosolic calcium and limited, possible dual, effects of G protein modulators on guard cell inward potassium channels. Plant J 8: 479–489 [Google Scholar]
- Kim TH, Bohmer M, Hu H, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61: 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Nishimura M, Shimazaki K (1995) Cytosolic concentration of Ca2+ regulates the plasma membrane H+-ATPase in guard cells of Fava Bean. Plant Cell 7: 1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusener B, Young JJ, Murata Y, Allen GJ, Mori IC, Hugouvieux V, Schroeder JI (2002) Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol 130: 2152–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J, Batistic O, Hashimoto K (2010) Calcium signals: the lead currency of plant information processing. Plant Cell 22: 541–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S (2009) A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA 106: 21419–21424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt N, Kwak JM, Robert N, Waner D, Leonhardt G, Schroeder JI (2004) Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell 16: 596–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leymarie J, Lasceve G, Vavasseur A (1998a) Interaction of stomatal responses to ABA and CO2 in Arabidopsis thaliana. Aust J Plant Physiol 25: 785–791 [Google Scholar]
- Leymarie J, Vavasseur A, Lasceve G (1998b) CO2 sensing in stomata of abi1-1 and abi2-1 mutants of Arabidopsis thaliana. Plant Physiol Biochem 36: 539–543 [Google Scholar]
- Loriol C, Dulong S, Avella M, Gabillat N, Boulukos K, Borgese F, Ehrenfeld J (2008) Characterization of SLC26A9, facilitation of Cl− transport by bicarbonate. Cell Physiol Biochem 22: 15–30 [DOI] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- MacRobbie EA (1998) Signal transduction and ion channels in guard cells. Philos Trans R Soc Lond 353: 1475–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlyn BE, Barton CVM, Broadmeadow MSJ, Ceulemans R, De Angelis P, Forstreuter M, Freeman M, Jackson SB, Kellomaki S, Laitat E, Rey A, Roberntz P, Sigurdsson BD, Strassemeyer J, Wang K, Curtis PS, Jarvis PG (2001) Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytol 149: 247–264 [DOI] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang Y, Munemasa S, Wang YF, Andreoli S, Tiriac H, Alonso JM, Harper JF, Ecker JR, Kwak JM, Schroeder JI (2006) CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol 4: 1749–1752, (e327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder J (2001) Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K (2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486 [DOI] [PubMed] [Google Scholar]
- Nishimura N, Sarkeshik A, Nito K, Park SY, Wang A, Carvalho PC, Lee S, Caddell DF, Cutler SR, Chory J, Yates JR, Schroeder JI (2010) PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. Plant J 61: 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, Alfred SE, Bonetta D, Finkelstein R, Provart NJ, Desveaux D, Rodriguez PL, McCourt P, Zhu JK, Schroeder JI, Volkman BF et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI (1997) Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild type and abi1 and abi2 mutants. Plant Cell 9: 409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA (2004) Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526 [DOI] [PubMed] [Google Scholar]
- Raschke K (1975) Simultaneous requirement of carbon dioxide and abscisic acid for stomatal closing in Xanthium strumarium L. Planta 125: 243–259 [DOI] [PubMed] [Google Scholar]
- Raschke K, Hedrich R, Reckmann U, Schroeder JI (1988) Exploring biophysical and biochemical components of the osmotic motor that drives stomatal movements. Bot Acta 101: 283–294 [Google Scholar]
- Raschke K, Shabahang M, Wolf R (2003) The slow and the quick anion conductance in whole guard cells: their voltage-dependent alternation, and the modulation of their activities by abscisic acid and CO2. Planta 217: 639–650 [DOI] [PubMed] [Google Scholar]
- Roelfsema MR, Hanstein S, Felle HH, Hedrich R (2002) CO2 provides an intermediate link in the red light response of guard cells. Plant J 32: 65–75 [DOI] [PubMed] [Google Scholar]
- Roelfsema MR, Levchenko V, Hedrich R (2004) ABA depolarizes guard cells in intact plants, through a transient activation of R- and S-type anion channels. Plant J 37: 578–588 [DOI] [PubMed] [Google Scholar]
- Sanders D, Brownlee C, Harper JF (1999) Communicating with calcium. Plant Cell 11: 691–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J, Rodrigues A, Saez A, Rubio S, Antoni R, Dupeux F, Park SY, Marquez JA, Cutler SR, Rodriguez PL (2009) Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. Plant J 60: 575–588 [DOI] [PubMed] [Google Scholar]
- Schmidt C, Schelle I, Liao YJ, Schroeder JI (1995) Strong regulation of slow anion channels and abscisic acid signaling in guard cells by phosphorylation and dephosphorylation events. Proc Natl Acad Sci USA 92: 9535–9539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Schroeder JI (1994) Anion selectivity of slow anion channels in the plasma membrane of guard cells (large nitrate permeability). Plant Physiol 106: 383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S (1989) Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338: 427–430 [Google Scholar]
- Schroeder JI, Hagiwara S (1990) Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels. Proc Natl Acad Sci USA 87: 9305–9309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Raschke K, Neher E (1987) Voltage dependence of K+ channels in guard-cell protoplasts. Proc Natl Acad Sci USA 84: 4108–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte A, Lorenzen I, Bottcher M, Plieth C (2006) A novel fluorescent pH probe for expression in plants. Pl Methods 2: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A (1985) Role of Ca2+ and EGTA on stomatal movements in Commelina Communis L. Plant Physiol 79: 1003–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RS, Xue S, Murata Y, Yang Y, Nishimura N, Wang A, Schroeder JI (2009) Calcium elevation-dependent and attenuated resting calcium-dependent abscisic acid induction of stomatal closure and abscisic acid-induced enhancement of calcium sensitivities of S-type anion and inward-rectifying K+ channels in Arabidopsis guard cells. Plant J 59: 207–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichandra C, Wasilewska A, Vlad F, Valon C, Leung J (2009) The guard cell as a single-cell model towards understanding drought tolerance and abscisic acid action. J Exp Bot 60: 1439–1463 [DOI] [PubMed] [Google Scholar]
- Speight JG (2005) Lange's Handbook of Chemistry. 16th edn New York, USA: McGraw-Hill Press [Google Scholar]
- Staxen I, Pical C, Montgomery LT, Gray JE, Hetherington AM, McAinsh MR (1999) Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc Natl Acad Sci USA 96: 1779–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wang H, Hu J, Han J, Matsunami H, Luo M (2009) Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci USA 106: 2041–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supuran CT (2008) Carbonic anhydrases--an overview. Curr Pharm Des 14: 603–614 [DOI] [PubMed] [Google Scholar]
- Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K (2009) Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci USA 106: 17588–17593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmaki A, Brosche M, Moldau H, Desikan R, Schroeder JI, Kangasjarvi J (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Puzorjova I, Brosche M, Valk E, Lepiku M, Moldau H, Pechter P, Wang YS, Lindgren O, Salojarvi J, Loog M, Kangasjarvi J, Kollist H (2010) Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J 62: 442–453 [DOI] [PubMed] [Google Scholar]
- Vlad F, Rubio S, Rodrigues A, Sirichandra C, Belin C, Robert N, Leung J, Rodriguez PL, Lauriere C, Merlot S (2009) Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell 21: 3170–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AA, Hetherington AM (1997) Convergence of the abscisic acid, CO2, and extracellular calcium signal transduction pathways in stomatal guard cells. Plant Physiol 114: 1557–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AAR, McAinsh MR, Mansfield TA, Hetherington AM (1996) Carbon dioxide induces increases in guard cell cytosolic free calcium. Plant J 9: 297–304 [Google Scholar]
- Yang Y, Costa A, Leonhardt N, Siegel RS, Schroeder JI (2008) Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Pl Methods 4: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky DA, Zuker CS, Ryba NJ (2009) Common sense about taste: from mammals to insects. Cell 139: 234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K (2002) ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol 43: 1473–1483 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K (2006) The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J Biol Chem 281: 5310–5318 [DOI] [PubMed] [Google Scholar]
- Young JJ, Mehta S, Israelsson M, Godoski J, Grill E, Schroeder JI (2006) CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proc Natl Acad Sci USA 103: 7506–7511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Xu X, Crosley RA, Greenwalt SA, Sun Y, Blakeslee B, Wang L, Ni W, Sopko MS, Yao C, Yau K, Burton S, Zhuang M, McCaskill DG, Gachotte D, Thompson M, Greene TW (2010) The protein kinase SnRK2.6 mediates the regulation of sucrose metabolism and plant growth in Arabidopsis. Plant Physiol 153: 99–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SY, Yu XC, Wang XJ, Zhao R, Li Y, Fan RC, Shang Y, Du SY, Wang XF, Wu FQ, Xu YH, Zhang XY, Zhang DP (2007) Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 19: 3019–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.